Abstract
Invasive lobular breast cancer (ILC) differs from invasive breast cancer of no special type in many ways. Evidence on treatment efficacy for ILC is, however, lacking. We studied the degree of documentation and representation of ILC in phase III/IV clinical trials for novel breast cancer treatments. Trials were identified on Pubmed and clinicaltrials.gov. Inclusion/exclusion criteria were reviewed for requirements on histological subtype and tumor measurability. Documentation of ILC was assessed and ILC inclusion rate, central pathology and subgroup analyses were evaluated. Inclusion restrictions concerning tumor measurability were found in 39/93 manuscripts. Inclusion rates for ILC were documented in 13/93 manuscripts and varied between 2.0 and 26.0%. No central pathology for ILC was reported and 3/13 manuscripts had ILC sub-analyses. ILC is largely disregarded in most trials with poor representation and documentation. The current inclusion criteria using RECIST v1.1, fall short in recognizing the unique non-measurable metastatic infiltration of ILC.
Similar content being viewed by others
Introduction
Invasive lobular breast cancer (ILC) is the second most common histological type of breast cancer (BC) and differentiates itself from invasive breast cancer of no special type (IBC-NST) at the clinical, histological and molecular level1,2,3,4,5. The majority of ILC belong to a luminal surrogate intrinsic subtype since >90% are hormone sensitive and lack HER2 amplification6. The pathological diagnosis of ILC relies, according to the World Health Organization’s (WHO) Classification of Breast Tumors, on the non-cohesive nature and single file or targetoid pattern of tumor cells observed on routine histological examination7. This definition seems however insufficient since several retrospective analyses revealed only a limited agreement between local and central pathology in diagnosing ILC8,9.
In current clinical practice, implications of ILC histology on treatment choices are limited5. Evidence on the efficacy of novel BC treatments in patients with ILC is often missing5,10. Differential responses to the long-used pillars of BC treatment, endocrine therapy and chemotherapy, have been demonstrated in early stage between patients with ILC and patients with IBC-NST11,12,13. The use of (neo-)adjuvant chemotherapy regimens is believed to be less beneficial in patients with ILC compared to patients with IBC-NST, even when correcting for surrogate intrinsic subtypes12,13. Data from the BIG 1-98 trial suggested improved outcomes for the adjuvant use of aromatase inhibitors (AI) over tamoxifen only in patients with ILC11. Recent data concluded that the use of AI is also preferred in patients with IBC-NST however the magnitude of outcome improvement might still be greater for patients with ILC14.
Survival of patients with BC has steadily improved with the introduction of several novel BC treatments15. The landscape of BC treatment has broadened with the development of CDK4/6 inhibitors, oral selective estrogen receptor degrader (SERDs), immune checkpoint inhibitors (ICI), antibody-drug conjugates (ADCs) and other novel drug classes. While pure (i.e. not mixed with IBC-NST) lobular histology is found in approximately 15% of all BC16, the efficacy of these novel drugs for patients with ILC is currently understudied5. Abel and colleagues provided some first quantitative insights on this topic by retrospectively assessing the enrollment of patients with ILC in clinical drug trials conducted in their institution17. They reported that while 17.9% of all stage IV BC treated in that center had ILC, only 9.2% of all patients with BC included in stage IV clinical drug trials were patients with ILC.
One hypothesis to explain lower enrollment of patients with ILC is that the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1)18,19 is not accurately reflecting the metastatic spread and disease progression of ILC17. The RECIST v1.1 guideline is used by the majority of clinical trials to evaluate tumor progression by relying on the measurability of tumoral lesions19. By these RECIST criteria, bone lesions are seen as non-measurable with the exception of lytic or mixed lytic-blastic bone lesions of ≥10 mm on computed tomography (CT) and magnetic resonance imaging (MRI). A number of trials are allowing the inclusion of patients with bone only disease (e.g. patients having bone metastases without evidence of any metastases in other organs) alongside patients with measurable disease according to RECIST v1.1 criteria20,21,22,23,24.
ILC is less likely to be mass-forming and has a propensity to spread to non-measurable areas such as the peritoneum25,26,27. Therefore, disease burden of ILC is likely to be underestimated by conventional imaging5. The greater likelihood of non-measurable disease in patients with metastatic ILC might thus lead to decreased enrollment in clinical drug trials for stage IV BC17. This systematic review, therefore, aims at identifying the magnitude of the gaps in ILC documentation and representation in clinical drug trials.
Methods
Literature search
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to conduct the literature search and study design (Supplementary Table 1)28. Two reviewers (KVB and JVC) searched independently in PubMed and in the clinicaltrials.gov database to identify phase III and IV clinical trials on novel BC therapies by screening titles and abstracts. Novel drug categories were identified from reviews on novel BC treatments29,30,31. The predefined categories considered were: CDK4/6 inhibitors, ADCs, oral SERDs, PARP inhibitors, tyrosine kinase inhibitors (TKI), mTOR inhibitors, ICIs, PI3K/AKT/PTEN-inhibitors and ‘others’. Medical subject heading terms (MeSH) related to the treatments and treatment categories were used as well as names of individual drugs in the search together with the MeSH-term ‘Breast Neoplasms’ (Supplementary Table 2).
Study selection
Records were excluded when title, abstract or information on clinicaltrials.gov showed that it was not a phase III/IV trial. In case of multiple records on the same trial, only the record on the main publication was considered. An exception was made for basket trials investigating multiple drugs where 1 record per study arm was allowed. Pooled analyses using results of multiple trials were excluded as well as trials that were not investigating the efficacy of drugs associated with the used search term (records off topic). The PRISMA-diagram of study selection for clinical trials is shown in Fig. 1. Only trials with a full manuscript available on 31 July 2023 were considered. Therefore, records of which no available publication could be retrieved or with only an abstract or poster available at 31 July 2023, were excluded. Duplicates found on both Pubmed and clinicaltrials.gov were removed. In case there were disagreements between the reviewers, these were resolved by discussion and consensus.
Data extraction
Inclusion and exclusion criteria reported in the manuscript and on clinicaltrials.gov were examined to see if histological subtype and measurability of disease were used as criteria. Furthermore, full manuscripts and accompanying supplementary data were analyzed to see if the number or percentage of patients with ILC was reported. In cases where the inclusion of patients with ILC was documented in the manuscript or supplementary data, the percentage of patients with ILC included was collected, the performance of central pathology for histological subtype was checked as well as the performance of specific subgroup analyses for ILC. These manuscripts were also analyzed to see if they reported on the inclusion rates of different races. Other data collected included the pharmaceutical company linked to the trial, investigated treatment arms, primary endpoints, secondary endpoints, restrictions on therapy lines and samples needed for inclusion.
Secondary analyses
In a second stage a search was done on PubMed to see if any secondary analyses specific for patients with ILC were done for all included trials. The names and classes of the drugs as well as the names of the different trials were used together with the MeSH-term ‘Carcinoma, Lobular’ to see if any additional analyses had been done. In this stage, pooled analyses of the included trials were also searched to see if they reported on sub-analyses for patients with ILC.
Results are shown in a descriptive manner. No statistical analyses have been performed.
Results
Identification of the trials
In total, 93 manuscripts were included in this systematic review. The majority of the clinical trials were conducted for patients with stage IV disease (Table 1). The categories for which more than 10 manuscripts were found were CDK4/6 inhibitors, ADCs, TKIs and mTOR inhibitors. Novartis, Roche and Pfizer were each involved in ≥10 of the included trials. Supplementary Table 3 gives an overview of the acquired data per manuscript.
Inclusion and exclusion criteria
ILC subtype was used as an exclusion criterion in only one trial: the NeoTRIP32 trial, which investigated the use of atezolizumab in the neoadjuvant setting. Measurability of disease was more often considered in the eligibility criteria. Patients with non-measurable disease were excluded in 20/93 (21.5%) trials, in 2 out of the 14 (14.3%) neoadjuvant and in 18 out of the 68 (26.5%) metastatic trials. In the metastatic setting, 19/68 (27.9%) trials additionally excluded non-measurable disease, however making an exception for patients with bone-only lesions which could be included. Therefore, restrictions based on measurability of lesions were found in 54.4% of the trials conducted in the metastatic setting.
ILC documentation
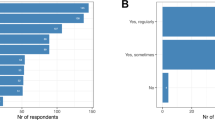
For a total of 13/93 (14.0%) of the included phase III and IV clinical drug trials, the number or percentage of included patients with ILC could be found in the manuscript or accompanying supplementary data. In the neoadjuvant setting, 5/14 (35.7%) trials documented the inclusion of ILC. For adjuvant and metastatic trials, documentation on ILC was only found in 1/11 (9.1%) and 7/68 (10.3%) of the trials, respectively. Patients with luminal hormone receptor-positive (HR+) HER2 negative BC were of interest in 49/93 (52.7%) manuscripts (Supplementary Table 3). In 10/49 (20.4%) of these manuscripts there was documentation on ILC inclusion. In Fig. 2, the documentation on ILC per drug category is shown for neoadjuvant, adjuvant and metastatic drug trials. Several drug categories had no documentation on ILC in any of the published manuscripts. These categories included oral SERDs, ADCs and PARP inhibitors.
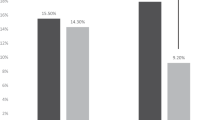
In 83/93 (89.2%) trials, at least one pharmaceutical company was involved. In 3 trials, 2 companies were involved. Figure 3 gives an overview on how many trials per pharmaceutical company reported on ILC inclusion in their original publications. For the majority of companies, no manuscript reported on ILC. Pfizer had a reporting rate of 30% (3/10 trials), Novartis of 11.5% (3/26 trials) and Roche of 7.1% (1/14 trials).
ILC central pathology, representation, and sub-analyses
The 13 manuscripts with documentation on ILC are summarized in Table 2. The performance of central pathology to review the histological subtype, was not reported in any of these manuscripts. Non-measurable disease was an exclusion criterion in two of the trials33,34 and non-measurable disease with exception of bone-only disease was excluded in two other trials23,24. The inclusion rate of patients with ILC varied between 2.0 and 26.0%. In the case of a population with HR + HER2- BC, the inclusion rate varied between 3.8 and 26.0%. Specific sub-analyses for patients with ILC were only performed in 3/13 (23.1%) trials35,36,37. Only 6/13 (46.2%) had reporting on the race of included patients23,24,33,34,37,38. However, 3 of these manuscripts only differentiated between Asian race and ‘others’24,34,37.
Secondary analyses
Secondary analyses specific for patients with ILC were not found for any of the included trials. The search on the included drugs names and classes neither uncovered any secondary analyses for ILC that were using trial data. Pooled analyses with comparison of efficacy for ILC vs. IBC-NST were only found for CDK4/6 inhibitors39,40.
Discussion
This systematic review demonstrates that ILC is largely disregarded in many clinical trials since only 13 out of 93 manuscripts (14.0%) reported how many patients with ILC were included. Trials including patients with luminal breast cancer did a little better with 20.4% of the manuscripts reporting on ILC inclusion. Specific sub-analyses for ILC on prospective data to evaluate the efficacy of novel BC treatments are seldom performed. Future reporting of the proportion of patients with ILC included will not guarantee the possibility of performing sub-analyses in specific clinical trials, since the subset might be too small. However, it is still valuable to collect data on histological subtypes and report the proportion of ILC since it will indicate if patients with ILC were underrepresented or well represented in the clinical trial. Furthermore, pooled analyses on different histological subtypes can only be performed if the data is collected and made available.
Even when ILC is reported, no details are given on the specific ILC subtypes that the included patients were diagnosed with. Classic ILC only represents ~55% of all ILC cases and worse outcomes have been described for patients with non-classic types ILC compared to classic ILC1,41. At present, no clear histological criteria have been defined to recognize and diagnose the different ILC subtypes7.
This review shows that these prospective trials do not perform central pathology to confirm histological subtypes. However, previous studies have revealed that 34-40% of the tumors diagnosed as ILC locally are not confirmed by central pathology review, while ~2% of the tumors that are locally subtyped as non-ILC are centrally reclassified as ILC8,9. This could be explained by the great variability in the definition of pathological diagnosis of ILC1, which was recently documented in a worldwide survey42. Efforts to harmonize and facilitate the pathological diagnosis of ILC are currently being undertaken by the pathology working group of the European Lobular Breast Cancer Consortium (www.elbcc.org).
Since patients with ILC are rarely considered in phase III/IV clinical drug trials, patients with ILC and their treating physicians are mainly relying on results in the global BC population and limited retrospective analyses in ILC populations to estimate the clinical benefit. Concerning CDK4/6 inhibitors, Gao et al. reported in their pooled analyses that CDK4/6 inhibitors were as effective for patients with ILC as for patients with IBC-NST in the metastatic setting39,40. Unfortunately, histological subtyping was missing for more than half of the patients in these pooled analyses, and as a result, the analyses for ILC were performed only on a small number of patients (n = 269). Several retrospective studies came to a similar conclusion that the benefit of CDK4/6 inhibitors is similar for metastatic ILC in comparison to IBC-NST43,44.
Limited data exist that there is no significant difference in the benefit of everolimus between patients with metastatic ILC vs. IBC-NST43. A retrospective study concluded that patients with ILC benefit more from the combination of exemestane with everolimus than the combination of palbociclib with fulvestrant when used in second line of metastatic treatment in patients with hormone-resistant disease45. However, no comparison with patients with IBC-NST was made and inclusion was limited to 48 patients receiving palbociclib with fulvestrant and 26 receiving everolimus with exemestane. In one study, no difference in progression-free survival for alpelisib between patients with ILC and IBC-NST was observed, however, only 9 patients with ILC were included in this analysis43. To our knowledge, no additional retrospective analyses evaluating other novel BC treatments for patients with ILC are available. Several drugs included in this systematic review are still under investigation and not commercially available. It is highly regrettable that patients with ILC need to wait until real-world data with enough follow-up are available to evaluate the benefit of the different novel BC treatments.
Some prospective trials that were either specifically designed for patients with ILC or have a clear aim to include patients with ILC for retrospective sub-analyses, are currently ongoing5. The majority of these trials are phase II and were thus not included in this systematic review. ROS1-inhibitors which exhibit synthetic lethality with CDH1 mutations are currently under investigation in the neoadjuvant and metastatic setting46,47. Other trials in the metastatic setting target BC with HER2 and HER3 mutations which are more common in ILC compared to IBC-NST2,48,49. In the phase 2 MutHER trial, 42.5% of the included patients were diagnosed with ILC49. A higher clinical benefit rate (61.5% vs 18.2%) of neratinib was described for patients with ILC in comparison to patients with IBC-NST. The use of atezolizumab for patients with ILC was investigated in the GELATO trial50, which was terminated prematurely due to short-lived treatment responses. The responses they observed were mostly in patients with triple-negative ILC. Clearly, there is a need for phase 3 and 4 trials dedicated to study drug efficiency in patients with ILC.
Concerning the manuscripts reporting on ILC, the percentage of ILC patients varied between 2.0% and 26.0%. However, if we consider the clinical subtype (HR+, triple negative or HER2+) that was targeted in the trials, the representation of ILC in these trials was quite similar to what is seen in clinical practice. Triple negative and HER2 positive tumors are found in 2.0–9.0% and 3.0–13.0% of ILC, respectively1. A noteworthy exception was however seen in PALOMA 4, which included only 3.8% patients with ILC although this trial focused on HR+ HER2− tumors24. This study was conducted in an Asian population and ILC has been reported to be less often diagnosed in Asia as compared to Europe51.
Although representation in those 13 manuscripts seemed to reflect the relative incidence of ILC compared to IBC-NST in clinical practice, only 4 of these trials had inclusion restrictions based on measurability of lesions. In total, 39/93(41.9%) manuscripts had inclusion and exclusion criteria involving measurability. Since documentation on ILC was so poor in these trials, no conclusion can be made if the presence of inclusion or exclusion criteria on measurability affects the inclusion rate of patients with ILC. Conventional imaging has its limitations in quantifying the disease burden in the case of metastatic ILC. [18F]2-fluoro-2-deoxy-D-glucose (18F-FDG)–positron emission tomography (PET)/CT sensitivity is limited by the decreased uptake of glucose by the metabolically less active ILC lesions52. Although whole-body diffusion-weighted magnetic resonance imaging (WB-DWI/MRI) has superiority over other imaging techniques to detect peritoneal metastases, this infiltration is often diffuse and not measurable53. Additionally, WB-DWI/MRI is not widely available and routinely used in clinical trials. Current RECIST criteria put the emphasis on measurable lesions of at least 10 mm for non-nodal and 15 mm for nodal lesions18. This does not reflect the unique metastatic pattern and diffuse infiltration of ILC25,26. Artificial intelligence might help in quantifying the total disease burden in patients with BC and therefore evaluating progression, even in cases of non-measurable disease54.
This systematic review has several limitations. Only clinical trials registered in Pubmed or clinicaltrials.gov were included. Data on clinicaltrials.gov depend for a large part on updates from the clinical trial groups involved and could therefore have been incomplete at the time of our search. Only the main publication was included per trial. Information on patients with ILC might have been available in other reports on these trials. We therefore also performed a secondary search to identify secondary analyses done for patients with ILC.
As shown in this systematic review, patients with ILC are often neglected by clinical investigators and pharmaceutical industries. This leads to a significant unmet clinical need, as the global age-standardized incidence (ASI) of BC, according to GLOBOCAN 2020 was 47.8 per 100.000 women55 and estimating that ILC represents approximately 15% of all BC16, the incidence of ILC would be around 7.2 per 100.000 women. This is comparable to the number of women affected by stomach cancer (ASI 7.0 per 100.000) and even higher as compared to the incidence of ovarian (ASI 6.6 per 100.000) and liver cancers (ASI 5.2 per 100.000) in women55. Other rarer histological subtypes are most probably even less documented in clinical trials.
The problem of unsatisfactory inclusion of other important patient subgroups is reflected by the underrepresentation of e.g., racial minorities, elderly patients, and male patients56,57,58. For patients belonging to multiple minority subgroups, the treatment benefit of the different drugs is even more unclear. Considering the manuscripts with documentation on ILC, less than half of them (46.2%) reported inclusion rates per race24,34,37. As a result, women diagnosed with ILC belonging to a racial minority group are highly unsure of the treatment benefit in case novel drugs are implemented in their treatment.
It is important to acknowledge the poor documentation and underrepresentation of ILC in clinical trials since it impedes the personalized treatment of all patients diagnosed with ILC. Clinical investigators and pharmaceutical industries should increase their efforts to include patients with ILC in trials. Inclusion and exclusion based on RECIST criteria need to be reevaluated for that purpose. Furthermore, all clinical trials should aim to do prospective analyses dedicated to ILC, so that these patients do not have to rely solely on limited retrospective analyses. In peer-reviewed journals, one could insist on reporting of the percentage of patients with ILC involved in these trials. It is clear that urgent efforts are needed to accommodate patients with ILC.
To conclude, patients with ILC are often overlooked in clinical drug trials. Documentation on the number of patients with ILC included is poor. The few trials reporting on ILC inclusion lack specific sub-analyses on ILC and do not report on central pathology to confirm histological subtype. Eligibility criteria and definitions of treatment response need to be re-evaluated to better reflect the unique biology of ILC. It is critical that patients with ILC are considered by clinical investigators and pharmaceutical industries.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data has been made available in the manuscript and supplementary material.
Code availability
Given the descriptive nature of the manuscript, no code was used.
References
Christgen, M. et al. Lobular breast cancer: histomorphology and different concepts of a special spectrum of tumors. Cancers 13, 3695 (2021).
Desmedt, C. et al. Genomic characterization of primary invasive lobular breast cancer. J. Clin. Oncol. 34, 1872–1880.
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Richard, F. et al. Characterization of stromal tumor-infiltrating lymphocytes and genomic alterations in metastatic lobular breast cancer. Clin. Cancer Res. 26, 6254–6265 (2020).
Van Baelen, K. et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol. https://doi.org/10.1016/J.ANNONC.2022.05.006 (2022).
Christgen, M. et al. Lobular breast cancer: clinical, molecular and morphological characteristics. Pathol. Res Pract. 212, 583–597 (2016).
Tan, P. H. et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 77, 181–185 (2020).
Christgen, M. et al. Differential impact of prognostic parameters in hormone receptor–positive lobular breast cancer. Cancer 126, 4847–4858 (2020).
Metzger, O. et al. Clinical utility of MammaPrint testing in invasive lobular carcinoma: results from the MINDACT phase III trial. Eur. J. Cancer 138, S5–S6 (2020).
Mouabbi, J. A., Hassan, A., Lim, B., Hortobagyi, G. N., Tripathy, D. & Layman, R. M. Invasive lobular carcinoma: an understudied emergent subtype of breast cancer. Breast Cancer Res. Treat. 193, 253–264 (2022).
Metzger, O. et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. J. Clin. Oncol. 33, 2772–U85 (2015).
Timbres, J. et al. Survival outcomes in invasive lobular carcinoma compared to oestrogen receptor-positive invasive ductal carcinoma. Cancers 13, 3036 (2021).
Trapani, D. et al. Benefit of adjuvant chemotherapy in patients with lobular breast cancer: a systematic review of the literature and metanalysis. Cancer Treat. Rev. 97, 102205 (2021).
Bradley, R. et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 23, 382 (2022).
Harbeck, N. et al. Breast cancer. Nat. Rev. Dis. Prim. 5, 1–31 (2019).
McCart Reed, A. E., Kutasovic, J. R., Lakhani, S. R. & Simpson, P. T. Invasive lobular carcinoma of the breast: morphology, biomarkers and’omics. Breast Cancer Res. 17. https://doi.org/10.1186/s13058-015-0519-x (2015).
Abel, M. K. et al. Decreased enrollment of patients with advanced lobular breast cancer compared to ductal breast cancer in interventional clinical trials. J. Clin. Oncol. 39, 1092–1092 (2021).
Schwartz, L. H. et al. RECIST 1.1 - Standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur. J. Cancer 62, 138–145 (2016).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). https://doi.org/10.1016/j.ejca.2008.10.026.
Sledge, G. W. et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 35, 2875–2884 (2017).
Zhang, Q. Y. et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2– advanced breast cancer: the multinational randomized phase III study. Ther. Adv. Med. Oncol. 12. https://doi.org/10.1177/1758835920963925 (2020).
Johnston, S. et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 5 (2019).
Finn, R. S. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL + LET) versus placebo plus letrozole (PBO + LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J. Clin. Oncol. 40 LBA1003–LBA1003 (2022).
Xu, B. et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer: Primary results from PALOMA-4. Eur. J. Cancer 175, 236–245 (2022).
Ferlicot, S. et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur. J. Cancer 40, 336–341 (2004).
Vincent-Salomon, A. et al. Lobular phenotype related to occult-metastatic spread in axillary sentinel node and/or bone marrow in breast carcinoma. Eur. J. Cancer 45, 1979–1986 (2009).
Johnson, K., Sarma, D. & Hwang, E. S. Lobular breast cancer series: Imaging. Breast Cancer Res. 17, 94 (2015).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189 (2021).
Nagayama, A., Vidula, N. & Bardia, A. Novel therapies for metastatic triple-negative breast cancer: spotlight on immunotherapy and antibody-drug conjugates. Oncology (Williston Park) 35, 249–254 (2021).
Elliott, M. J. & Cescon, D. W. Development of novel agents for the treatment of early estrogen receptor positive breast cancer. Breast 62, S34 (2022).
Martin, M. & López-Tarruella, S. Emerging therapeutic options for HER2-positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book 35, e64–e70 (2016).
Gianni, L. et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann. Oncol. 33, 534–543 (2022).
Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100 (2020).
Guan, Z. et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2–overexpressing metastatic breast cancer. J. Clin. Oncol. 31, 1947–1953 (2013).
von Minckwitz, G. et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto). Ann. Oncol. 25, 2363–2372 (2014).
Untch, M. et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 13, 135–144 (2012).
Decker, T. et al. Final results from IMPROVE: a randomized, controlled, open-label, two-arm, cross-over phase IV study to determine patients’ preference for everolimus in combination with exemestane or capecitabine in combination with bevacizumab in advanced HR-positive, HER2-negative breast cancer. BMC Cancer 20, 286 (2020).
Baselga, J. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 904 (2017).
Gao, J. J. et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 21, 250–260 (2020).
Gao, J. J. et al. Overall survival in patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer treated with a cyclin-dependent kinase 4/6 inhibitor plus fulvestrant: a US Food and Drug Administration pooled analysis. Lancet Oncol. 22, 1573–1581 (2021).
Iorfida, M. et al. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res Treat. 133, 713–723 (2012).
De Schepper, M. et al. Results of a worldwide survey on the currently used histopathological diagnostic criteria for invasive lobular breast cancer. Mod. Pathol. https://doi.org/10.1038/S41379-022-01135-2 (2022).
Mouabbi, J. A. et al. Histology-based survival outcomes in hormone receptor-positive metastatic breast cancer treated with targeted therapies. NPJ Breast Cancer 8, 131 (2022).
Agostinetto, E. et al. Clinico-molecular characteristics associated with outcomes in breast cancer patients treated with CDK4/6 inhibitors: results from the AURORA Molecular Screening Initiative. J. Clin. Oncol. 41, 1019–1019 (2023).
Orlandi, A. et al. Palbociclib plus fulvestrant or everolimus plus exemestane for pretreated advanced breast cancer with lobular histotype in ER+/HER2− patients: a propensity score-matched analysis of a multicenter retrospective patient series. J. Pers. Med. 10, 1–11 (2020).
Agostinetto, E. et al. ROSALINE: a phase II, neoadjuvant study targeting ROS1 in combination with endocrine therapy in invasive lobular carcinoma of the breast. Future Oncol. 18, 2383–2392 (2022).
Crizotinib in Lobular Breast, Diffuse Gastric and Triple Negative Lobular Breast Cancer or CDH1-mutated Solid Tumours—Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03620643?cond=NCT03620643&draw=2&rank=1 (accessed 27 Apr 2021).
Hyman, D. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Ma, C. X. et al. The phase II MutHER study of neratinib alone and in combination with fulvestrant in HER2 mutated, non-amplified metastatic breast cancer. Clin. Cancer Res. clincanres.CCR-21-3418-E.2021 (2022).
Voorwerk, L. et al. PD-L1 blockade in combination with carboplatin as immune induction in metastatic lobular breast cancer: the GELATO trial. Nat. Cancer 4, 535 (2023).
Kadys, A., Gremke, N., Schnetter, L., Kostev, K. & Kalder, M. Intercontinental comparison of women with breast cancer treated by oncologists in Europe, Asia, and Latin America: a retrospective study of 99,571 patients. J. Cancer Res. Clin. Oncol. 149, 7319–7326 (2023).
Hogan, M. P. et al. Comparison of 18F-FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J. Nucl. Med. 56, 1674–1680 (2015).
Zugni, F. et al. The added value of whole-body magnetic resonance imaging in the management of patients with advanced breast cancer. PLoS ONE 13, e0205251 (2018).
Bi, W. L. et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J. Clin. 69, 127–157 (2019).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249 (2021).
Javier-DesLoges, J. et al. Disparities and trends in the participation of minorities, women, and the elderly in breast, colorectal, lung, and prostate cancer clinical trials. Cancer 128, 770–777 (2022).
Sedrak, M. S. et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J. Clin. 71, 78–92 (2021).
Corrigan, K. L. et al. Exclusion of men from randomized phase III breast cancer clinical trials. Oncologist 25, e990–e992 (2020).
Alsaleh, K. et al. Neoadjuvant endocrine therapy with or without palbociclib in low-risk patients: a phase III randomized double-blind SAFIA trial. J. Cancer Res. Clin. Oncol. 149, 6171–6179 (2023).
Bundred, N. et al. Combined perioperative lapatinib and trastuzumab in early HER2-positive breast cancer identifies early responders: randomized UK EPHOS-B trial long-term results. Clin. Cancer Res. 28, 1323 (2022).
Guarneri, V. et al. Everolimus plus aromatase inhibitors as maintenance therapy after first-line chemotherapy: final results of the phase III randomised MAIN-A (MAINtenance Afinitor) trial. Eur. J. Cancer 154, 21–29 (2021).
Fehm, T. et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 124, 403–412 (2010).
Willemsen, A. E. C. A. B. et al. Everolimus exposure and early metabolic response as predictors of treatment outcomes in breast cancer patients treated with everolimus and exemestane. Target Oncol. 13, 641–648 (2018).
Acknowledgements
This work is dedicated to Leigh Pate and Deborah Mueller, two patient advocates who are greatly missed. Leigh dedicated her life to setting up patient advocacy for ILC, making sure that patients had a strong voice in the ILC community and inspiring many other patients, but also clinicians and researchers. Deborah Mueller brought together many of the included co-authors which started up the discussion on lack of documentation of ILC in clinical trials. Both of them were devoted to improving ILC research. This article/publication is based upon work from the European Lobular Breast Cancer Consortium (ELBCC) and the COST Action LOBSTERPOT CA19138, supported by COST (European Cooperation in Science and Technology, http://www.cost.eu/). This study is funded by the Belgian Foundation against Cancer (C/2022/2046). KVB is funded by the Conquer Cancer—Lobular Breast Cancer Alliance Young Investigator Award for Invasive Lobular Carcinoma Research, supported by Lobular Breast Cancer Alliance. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology® or Conquer Cancer®, or Lobular Breast Cancer Alliance. JVC is funded the KU Leuven Fund Nadine de Beauffort.
Author information
Authors and Affiliations
Contributions
Conception and design: K.V.B., J.V.C., M.M., A.C., M.-C.C., V.F., L.H., J.K.L., R.C.J. and C.D.; Collection and/or assembly of data: K.V.B. and J.V.C.; Data analysis and interpretation: K.V.B., J.V.C., M.M. and C.D.; Manuscript writing: K.V.B., J.V.C., M.M. and C.D.; Critical revision and final approval of manuscript: all authors; Accountability for all aspects of the work: K.V.B., J.V.C., M.M. and C.D. Given the equal contribution of K.V.B. and J.V.C., they are considered as co-first authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Baelen, K., Van Cauwenberge, J., Maetens, M. et al. Reporting on invasive lobular breast cancer in clinical trials: a systematic review. npj Breast Cancer 10, 23 (2024). https://doi.org/10.1038/s41523-024-00627-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00627-5