Abstract
Enrollment in metastatic breast cancer trials usually requires measurable lesions, but patients with invasive lobular carcinoma (ILC) tend to form diffuse disease. We found that the proportion of patients with metastatic ILC enrolled in clinical trials at our institution was significantly lower than that of patients with invasive ductal carcinoma (IDC). Possible links between requiring measurable disease and decreased enrollment of ILC patients require further study to ensure equitable trial access.
Similar content being viewed by others
The need for unified criteria and a common language to evaluate treatment efficacy in oncologic clinical trials led to recommendations for standardized definitions from the World Health Organization1. These recommendations subsequently formed the basis for the Response Evaluation Criteria in Solid Tumors (RECIST), now one of the most common means of objectively measuring tumor response in therapeutic clinical trials2,3. Despite its widespread use, some have criticized RECIST because change in tumor size, the main response endpoint utilized, may not translate to overall survival difference4. Others have identified particular tumor types for which RECIST may not optimally capture treatment response5. ILC of the breast may be one such tumor type. ILC is the second most common type of breast cancer after IDC, accounting for 10–15% of all cases6,7. In the metastatic setting, ILC forms diffuse lesions in the gastrointestinal tract, peritoneal lining, leptomeninges, or pleura, with its characteristic lack of adhesion protein E-cadherin likely contributing to its diffuse growth pattern6.
Other tumor types for which investigators have identified challenges with RECIST utilize alternative disease-specific factors in clinical trial eligibility. For example, metastatic prostate cancer frequently presents with unmeasurable bone metastases. The Prostate Cancer Clinical Trials Working Group recommends utilization of disease-specific caveats to RECIST and investigation into surrogate biomarkers8,9. Similarly, modified RECIST criteria have been proposed for malignant mesothelioma, which has an ill-defined, diffuse growth pattern10,11. Currently, there are no special provisions for ILC in clinical trial enrollment criteria. In this report, we characterize the utilization patterns of RECIST or comparable measurable disease criteria among therapeutic, metastatic breast cancer trials registered in clinicaltrials.gov. We then employ a retrospective analysis to assess clinical trial enrollment patterns by breast histology and stage using the University of California, San Francisco (UCSF) Cancer Registry and an institutional clinical trials registry (OnCore clinical trials management system [CTMS]).
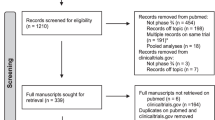
There were 160 actively recruiting, interventional clinical trials for stage IV breast cancer in clinicaltrials.gov. Fourteen behavioral and dietary supplement studies were excluded. Of the 146 remaining studies, 119 (81.5%) were drug trials. Overall, 104 (71.2%) required measurable disease for study participation. Of these 104 studies, 29 (27.9%) utilized RECIST in inclusion criteria; 22 (21.5%) utilized RECIST as an outcome measure, and 48 (46.2%) utilized RECIST in both inclusion criteria and outcome measures. Five (4.8%) studies used measurable or alternative size criteria. No trials explicitly restricted the study population by histology.
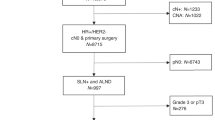
The UCSF Cancer Registry included 8679 patients, including 7320 (84.3%) IDC patients and 1359 (15.6%) ILC or mixed ILC/IDC patients. Most patients had stage I–III disease (n = 8187, 94.3%), whereas 492 (5.7%) had metastatic disease. There were 1511 individuals enrolled in OnCore CTMS, including 1304 (86.3%) patients with IDC and 207 (13.7%) patients with ILC or mixed ILC/IDC. Of these 1337 (88.5%) had stage I–III disease, and 174 (11.5%) had metastatic disease. In patients with early-stage disease, where RECIST is not typically used, there was no difference in the proportion of ILC patients enrolled in clinical trials versus in the cancer registry (Fig. 1). However, among those with stage IV disease, there was a significantly lower proportion of ILC patients in the OnCore CTMS than the cancer registry (9.2% versus 17.9%, p = 0.005). In contrast, patients with metastatic IDC were overrepresented in clinical trials compared with the cancer registry.
Overall, we found that the majority of stage IV breast cancer trials utilize RECIST and that patients with metastatic ILC are significantly less likely to be enrolled in clinical trials in an institutional database. These findings are important because clinical trial participation is associated with improved overall survival12,13. Although we cannot conclude from this work that high use of RECIST contributes to lower clinical trial enrollment rates for ILC patients, our findings warrant attention to this potential issue and further examination of larger datasets. We do hypothesize that ILC may represent another tumor type for which modified RECIST criteria may be useful. Apart from lacking E-cadherin, investigators have reported differences in tumor stroma between ILC and IDC tumors, with fewer CD34-positive fibroblasts, lower levels of tumor-infiltrating lymphocytes, and reduced desmoplastic reaction in ILC. Such differences may contribute to the lower sensitivity of imaging studies for ILC14,15,16. Alternative endpoints for treatment response in ILC could rely upon novel imaging tools targeting the high levels of estrogen receptor positivity, such as [18]F-fluoroestradiol PET imaging17. Patients with metastatic ILC have also been shown to have higher absolute levels of circulating tumor cells than those with metastatic IDC, suggesting that liquid biopsy might be a good indicator of disease response18.
Our analysis of CTMS and the cancer registry are restricted to a single institution, limiting its generalizability. Owing to its retrospective nature, our study lacked patient-level data regarding why individuals were not enrolled in trials, such as tumor receptor subtype. Although we cannot conclude from these analyses that lack of measurable disease resulted in decreased enrollment, these findings certainly should compel further investigation to ensure equal clinical trial opportunities for patients with ILC. As with other cancer types that form diffuse disease, modifications to RECIST or alternative endpoints specifically for ILC may be warranted.
Methods
Data sets
The clinicaltrials.gov registry, a web-based registry maintained by the National Library of Medicine and National Institutes of Health, was queried to identify trials from inception to July 2019. We used the search function to select the following factors: “stage IV breast cancer”, “actively recruiting”, and “interventional studies (clinical trials)”. Utilization of measurable disease criteria was defined as either (1) the explicit use of RECIST, (2) the definition of measurable disease by RECIST (imaging or physical exam that shows at least one measurable lesion with a minimum size in at least one diameter of ≥10 mm for lesions and ≥15 mm for lymph nodes), or (3) the explicit requirement for measurable disease without further qualification.
The University of California, San Francisco (UCSF) Cancer Registry of the Helen Diller Family Comprehensive Cancer Center was used to capture individuals with invasive breast cancer who received care at UCSF between 1 January 2000 to 31 December 2018. In addition, we utilized the OnCore CTMS to identify patients with invasive breast cancer who were enrolled in interventional, therapeutic clinical trials at UCSF during the same timeframe. Only patients with documented IDC, ILC, or mixed ILC/IDC histology were included. To assess differences between registry and clinical trial enrollment, we used two-sample tests of proportions stratified by histology (IDC versus ILC) and stage (I–III versus IV). Data were analyzed in Stata 14.2 (StataCorp LLC, College Station, TX, USA). The study was approved by the UCSF Institutional Review Board.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Aggregate data are available upon reasonable request with appropriate institutional review board approval.
Code availability
Analysis codes are available from the corresponding author upon reasonable request.
References
Miller, A. B., Hoogstraten, B., Staquet, M. & Winkler, A. Reporting results of cancer treatment. Cancer 47, 207–214 (1981).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 92, 205–216 (2000).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Twombly, R. Criticism of tumor response criteria raises trial design questions. J. Natl. Cancer Inst. 98, 232–234 (2006).
Villaruz, L. C. & Socinski, M. A. The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin. Cancer Res. 19, 2629–2636 (2013).
Sledge, G. W., Chagpar, A. & Perou, C. Collective wisdom: lobular carcinoma of the breast. Am. Soc. Clin. Oncol. Educ. B. 35, 18–21 (2016).
Mamtani, A. & King, T. A. Lobular breast cancer: different disease, different algorithms? Surg. Oncol. Clin. N. Am. 27, 81–94 (2018).
Brown, M. S. et al. Quantitative bone scan lesion area as an early surrogate outcome measure indicative of overall survival in metastatic prostate cancer. J. Med. Imaging 5, 011017 (2018).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 34, 1402–1418 (2016).
Van Klaveren, R. J. et al. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer 43, 63–69 (2004).
Byrne, M. J. & Nowak, A. K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann. Oncol. 15, 257–260 (2004).
Unger, J. M. et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J. Natl. Cancer Inst. 106, dju002 (2014).
Chow, C. J. et al. Does enrollment in cancer trials improve survival? J. Am. Coll. Surg. 216, 774–780 (2013).
Westhoff, C. C. et al. Prognostic relevance of the loss of stromal CD34 positive fibroblasts in invasive lobular carcinoma of the breast. Virchows Arch. 477, 717–724 (2020).
Desmedt, C. et al. Immune infiltration in invasive lobular breast cancer. J. Natl. Cancer Inst. 110, 768–776 (2018).
Winchester, D. J. et al. A comparative analysis of lobular and ductal carcinoma of the breast: presentation, treatment, and outcomes. J. Am. Coll. Surg. 186, 416–422 (1998).
Venema, C. et al. 18F-FES PET has added value in staging and therapy decision making in patients with disseminated lobular breast cancer. Clin. Nucl. Med. 42, 612–614 (2017).
Narbe, U. et al. The distribution of circulating tumor cells is different in metastatic lobular compared to ductal carcinoma of the breast-long-term prognostic significance. Cells 9, 1718 (2020).
Author information
Authors and Affiliations
Contributions
Concept and design: Abel, Borno, Mukhtar. Acquisition, analysis, or interpretation of data: Abel, Griffin, McGuire, Mukhtar. Drafting of the manuscript: Abel, Mukhtar. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Abel, Mukhtar. Obtained funding: N/A. Administrative, technical, or material support: Mukhtar. Supervision: Borno, Mukhtar.
Corresponding author
Ethics declarations
Competing interests
Dr. Melisko receives research funding from Astra Zeneca, Novartis, KCRN Research, and Puma and consulting fees from Biotheranostics. Dr. Rugo receives research support for clinical trials through the University of California from Pfizer, Merck, Novartis, Lilly, Genentech, OBI, Odonate, Daiichi, Seattle Genetics, Eisai, Macrogenics, Sermonix, Immunomedics, and AstraZeneca. She has also received travel support from Daiichi, Mylan, Pfizer, Merck, AstraZeneca, Novartis, and Macrogenics and honoraria from Puma, Mylan, and Samsung. Dr. Chien receives research funding from Merck, Puma, Amgen, and Seattle Genetics. Dr. Esserman is an unpaid member of the board of directors of Quantum Leap Healthcare Collaborative and receives research funding from QLHC for the I-SPY TRIAL. She is a member of the Blue Cross/Blue Shield Medical Advisory Panel and receives honoraria and travel funding. She has a grant from Merck for an Investigator-initiated trial of DCIS. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abel, M.K., Melisko, M.E., Rugo, H.S. et al. Decreased enrollment in breast cancer trials by histologic subtype: does invasive lobular carcinoma resist RECIST?. npj Breast Cancer 7, 139 (2021). https://doi.org/10.1038/s41523-021-00348-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00348-z