Abstract
Young women with breast cancer experience unique treatment and survivorship issues centering on treatment-related amenorrhea (TRA), including fertility preservation and management of ovarian function as endocrine therapy. The Young Women’s Breast Cancer Study (YWS) is a multi-center, prospective cohort study of women diagnosed at age ≤40, enrolled from 2006 to 2016. Menstrual outcomes were self-reported on serial surveys. We evaluated factors associated with TRA using logistic regression. One year post-diagnosis, 286/789 (36.2%) experienced TRA, yet most resumed menses (2-year TRA: 120/699; 17.2%). Features associated with 1-year TRA included older age (OR≤30vs36-40 = 0.29 (0.17–0.48), OR31-35vs36-40 = 0.67 (0.46–0.94), p = 0.02); normal body mass index (BMI) (OR≥25vs18.5-24. =0.59 (0.41–0.83), p < 0.01); chemotherapy (ORchemo vs no chemo = 5.55 (3.60–8.82), p < 0.01); and tamoxifen (OR = 1.55 (1.11–2.16), p = 0.01). TRA rates were similar across most standard regimens (docetaxel/carboplatin/trastuzumab +/− pertuzumab: 55.6%; docetaxel/cyclophosphamide +/− trastuzumab/pertuzumab: 41.8%; doxorubicin/cyclophosphamide/paclitaxel +/− trastuzumab/pertuzumab: 44.1%; but numerically lower with AC alone (25%) or paclitaxel/trastuzumab (11.1%). Among young women with breast cancer, lower BMI appears to be an independent predictor of TRA. This finding has important implications for interpretation of prior studies, future research, and patient care in our increasingly obese population. Additionally, these data describe TRA associated with use of docetaxel/cyclophosphamide, which is increasingly being used in lieu of anthracycline-containing regimens. Collectively, these data can be used to inform use of fertility preservation strategies for women who need to undergo treatment as well as the potential need for ovarian suppression following modern chemotherapy for young women with estrogen-receptor-positive breast cancer.
Clinical trial registration: www.clinicaltrials.gov, NCT01468246.
Similar content being viewed by others
Introduction
Young women with breast cancer, defined as those diagnosed at age ≤40 years, represent a small minority of breast cancer patients overall1. However, the optimization of breast cancer outcomes and breast cancer survivorship for young women requires attention to several unique issues, many of which center around ovarian function at and following diagnosis2. Young age has historically been considered a risk factor for disease recurrence3,4 and recent data have demonstrated that this risk appears to be predominantly among those with luminal A and luminal B breast cancer subtypes5. To what extent this risk stems from the biology of premenopausal breast cancer versus suboptimal endocrine therapy remains poorly understood. Ovarian function suppression (OFS) is a well-recognized therapeutic strategy for premenopausal women with hormone-receptor-positive (HR+) breast cancer and those who experience chemotherapy-associated amenorrhea similarly experience improved risk of recurrence6,7. Recent data from Suppression of Ovarian Function Trial (SOFT) and Tamoxifen vs Exemestane Trial (TEXT) have identified substantial improvements in survival outcomes with the addition of OFS to tamoxifen, with particular benefit among women age <356,8. However, chemotherapy-related amenorrhea and OFS may have many negative impacts, including infertility or subfertility, vasomotor symptoms, impaired sexual functioning, and long-term medical sequelae including bone loss9.
The ability to predict which patients will experience transient or permanent loss of menstrual function is important in counseling patients on the risks of chemotherapy, potential benefits of fertility preservation, and the management of ovarian function during adjuvant endocrine therapy. The vast majority of studies examining rates of treatment-related amenorrhea (TRA) were completed prior to the use of modern regimens, including docetaxel/cyclophosphamide (TC) and docetaxel/carboplatin/trastuzumab (TCH), and there are limited data regarding TRA following dose-dense doxorubicin/cyclophosphamide (AC) regimens7,10,11,12,13. Here we describe patient-reported menstrual function outcomes, including development of TRA and resumption of menses from a large, prospective cohort of young women with early breast cancer.
Results
Patient characteristics
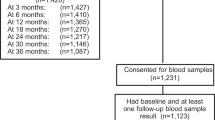
Among 954 eligible participants, 789 women were evaluable at the 1-year timepoint, among whom 109 (13.8%) were at age ≤30 at diagnosis, 216 (27.4%) at age 31–35, and 464 (58.8%) at age 36–40, with a median age of 36. The majority (592/782, 75.0%) received chemotherapy (Table 1), including 16 women who received less than 50% of the standard total dose of an agent. No dose reductions for obesity were identified. Patient, disease, and treatment factors for patients excluded because of missing surveys at baseline/6-month surveys or 1-year surveys are described in Supplementary Table 1.
Menstrual patterns
The proportions of evaluable participants with active menstrual function, defined as having a period within the prior 12 months, at each annual timepoint within the first five years following diagnosis were: 1 year: 710/789 (90.0%); 2 year: 537/699 (76.8%); 3 year: 444/592 (75.0%); 4 year: 391/511 (76.5%); 5 year: 314/430 (73.0%). To acknowledge that some women experience bleeding in the first few months after initiating treatment before ovarian function is impacted, we evaluated TRA at 1 year as the proportion with no period within 6 months prior (286/789; 36.2%) and at 2 years as the proportion with no period within the 18 months prior (120/699; 17.2%).
Among the participants who had TRA at the 1-year and 2-year timepoints, 183/286 (63.9%) and 13/120 (10.8%), respectively, had resumption of menstrual function reported on a subsequent survey within 5 years following diagnosis.
Of the participants who had a last menstrual period (LMP) within 12 months of the 2-year, 3-year, and 4-year timepoints, the proportions who became amenorrheic at the subsequent timepoints were: 2 year: 43/537 (8.0%); 3 year: 27/444 (6.1%); 4 year: 18/391 (4.6%).
Patient and treatment characteristics associated with TRA
The proportions of participants at age ≤30, 31–35, and 36–40 who experienced TRA at 1 year were 23/109 (21.1%), 140/216 (35.2%), and 187/464 (40.3%) and at 2 years were 10/82 (12.2%), 25/149 (16.8%), and 53/307 (17.3%), respectively. Among 80 participants across all age groups who received neither tamoxifen nor chemotherapy, 5 (6.3%) experienced 1-year TRA. Among 115 participants who received tamoxifen but no chemotherapy, 23 (20.0%) experienced 1-year TRA. Of 592 participants who received chemotherapy, 258 (43.6%) experienced 1-year TRA vs 28/197 (14.2%) of those who did not receive chemotherapy.
Characteristics associated with 1-year TRA in univariable analysis included older age, normal body mass index (BMI) (compared to higher), chemotherapy use, and tamoxifen use, whereas race and smoking were not (Table 2). In the multivariable logistic regression model, the following characteristics remained associated with TRA: age (OR≤30 vs 36-40 = 0.29 (0.17–0.48), OR31-35 vs 36-40 = 0.67 (0.46–0.94), p = 0.02); BMI (OR≥25 vs 18.5-24.9 = 0.59 (0.41–0.83), p < 0.01; OR<18.5 vs 18.5-24.9 = 0.90 (0.41–1.89), p = 0.78); chemotherapy use (vs not; OR = 5.55 (3.60–8.82), p < 0.01; and tamoxifen use (vs not; OR = 1.55 (1.11–2.16), p = 0.01). There appeared to be a consistent association between BMI ≥ 25 and a lower odds of 1-year TRA among each age group and by receipt or non-receipt of chemotherapy and tamoxifen (Fig. 1). To further explore the association between BMI and 1-year TRA, participants with a BMI ≥ 25 were divided into overweight (BMI 25.0–29.9) (n = 155) and obese (BMI ≥ 30) (n = 86) in a univariable model. Rates of 1-year TRA were 45/155 (29.0%) and 28/86 (32.6%), respectively, and the direction and magnitude of the associations with TRA were similar (OR25-29.9 vs 18.5-24.9 = 0.64 (0.43–0.94), p = 0.025; OR≥30 vs 18.5-24.9 = 0.75 (0.46–1.21), p = 0.25), though not statistically significant in the obese (vs normal BMI) patients.
The raw proportions of participants who experienced 1-year TRA and 2-year TRA by age and the most common chemotherapy regimens are reported in Tables 3 and 4 and for less common regimens in Supplementary Table 2. Overall, 1-year TRA rates associated with AC-paclitaxel (AC-T) (primarily administered dose dense), TC, and TCH regimens were similar (44.1%, 41.8%, and 55.6%, respectively), but the TRA rate was lower with AC (25%) and even lower with weekly paclitaxel and trastuzumab (11.1%). Among women receiving AC-T, the most commonly used regimen, rates of 1-year TRA among women at age ≤30, 31–35, and 36–40 were 11/59 (18.6%), 43/105 (41.0%), and 103/192 (53.6%), respectively.
Discussion
Over the past several years, the critical implications of ovarian function on the management and outcomes of young women with breast cancer have become well-recognized. In NSABP B30, amenorrhea lasting 6 months or longer following chemotherapy was associated with improved disease-free and overall survival7. Similarly, the addition of OFS to tamoxifen also improves disease-free and overall survival6,14. On the other hand, loss of ovarian function increases the risk of menopausal symptoms and medical comorbidities and results in infertility9. The ability to accurately predict which young patients will experience temporary or permanent amenorrhea, and which patients will not, is needed upon diagnosis and in follow-up to inform the optimal treatment plan. Our data demonstrate that, in a large prospective cohort of young women treated with modern chemotherapy regimens, short episodes of TRA are common, but the vast majority will maintain or regain menstrual function.
The ability to predict loss of ovarian function may be most critical in informing the decision to use fertility preservation, including embryo and oocyte cryopreservation. While TRA is an imperfect surrogate of future fertility—those who regain menstrual function may still be infertile or subfertile—and any risk of treatment-related infertility may be enough to warrant fertility preservation for some women, our data help inform which patients may be at very low risk of TRA and, therefore, less likely to need fertility preservation. For instance, only 20% of women at age 30 or younger experienced even a short course of amenorrhea. Similarly, very low rates of TRA were reported following paclitaxel/trastuzumab, which omits alkylating chemotherapy, the class most strongly associated with gonadotoxicity. Therefore, these patients may be less likely to need banked embryos or oocytes to conceive in the future, though additional research is needed to better stratify risk of infertility and pre-treatment biomarkers such as AMH are being explored. Rates of 1-year TRA were more moderate for patients at ages 31–35 and 36–40, yet the majority had subsequent resumption of menstrual function by the 2-year timepoint, supporting the 2-year cutoff for defining TRA15. While fertility preservation is important for many women, it may not be feasible for many others due to cost, availability, or the need to urgently initiate treatment for symptomatic disease. Additionally, while controlled ovarian stimulation for fertility preservation appears safe16,17, the available data are somewhat limited, particularly for patients receiving neoadjuvant chemotherapy18,19 and for whom delays in care could be more impactful than those who undergo fertility preservation following resection of the tumor. For young women who face such barriers to fertility preservation use and are at low risk of TRA, our finding that they are likely to maintain or regain ovarian function in the years following diagnosis may be reassuring.
Another common treatment decision for young breast cancer patients completing chemotherapy for HR+ breast cancer is whether and when to initiate therapeutic OFS. These data support initiation of OFS prior to resumption of menses following chemotherapy for women at age 40 or younger, given that fewer than 20% of women at age 35–40 had TRA 2 years following diagnosis. This approach prevents women from undergoing a second menopausal transition with initiation of OFS following resumption of menses. Additionally, this approach addresses the common challenge of how to incorporate use of aromatase inhibitors (AI) for young women following chemotherapy, due to the concern that resumption of menstrual function would interrupt effective endocrine therapy if an AI alone were given in this setting. Recognizing that the routine use of GnRH agonist in this setting would lead to overtreatment of a small proportion of young women who do experience permanent menopause, this decision should be tailored to the individual patient’s risk of long-term amenorrhea, breast cancer risk features, and concerns regarding side effects, out-of-pocket costs, and the burden of injection visits.
Our data demonstrate similar rates of TRA across the most frequently used modern multi-agent chemotherapy regimens AC-T +/− H +/− P, TC +/− H +/− P, and TCH +/− P. The number of important confounders (age, tamoxifen use, BMI) and variety of chemotherapy regimens precludes the evaluation of an association between specific regimens and TRA. However, the higher numerical rate of TRA associated with AC-T relative to AC is consistent with prior studies demonstrating that taxanes contribute to amenorrhea11,20,21,22. As noted previously, the rate of TRA among patients receiving weekly paclitaxel/trastuzumab was low, consistent with prior studies23. Only very limited data are available describing TRA associated with use of platinum chemotherapy for young breast cancer patients, and in other settings as well. In a small retrospective study, the rate of TRA following carboplatin/taxane chemotherapy was only 13%24. On the other hand, an analysis of the ALTTO trial found that the TRA rate was higher following TCH than anthracycline-taxane chemotherapy, though comparisons may have been limited by differences in the taxanes used21. In the Childhood Cancer Survivor Study, one of the largest studies to evaluate the impact of chemotherapy on menstrual function, albeit among children rather than adults, cisplatin use was not associated with long-term fertility outcomes25. The rate of TRA associated with docetaxel-carboplatin-trastuzumab (TCH) in this study was numerically higher than that of patients treated with docetaxel-cyclophosphamide. While these data are unadjusted and there are differences in standard number of cycles (6 vs 4, respectively), cyclophosphamide is generally regarded as the main driver of TRA among breast cancer patients and the high rates of TRA associated with TCH suggest that carboplatin does contribute to TRA and warrant further attention in future studies.
Our finding that overweight and obese women treated with chemotherapy in this study experienced a 40% lower risk of TRA than those with a normal body weight is striking. The association between overweight/obesity and a lower odds of TRA appeared consistent across subpopulations. In particular, the association seemed similar among women who did vs did not receive chemotherapy, suggesting that the lower odds of TRA among overweight/obese women is not solely mediated by differences in chemotherapy pharmacokinetics by body mass. Most studies evaluating TRA among young women have found no association between BMI and TRA20,21,23,26. A prior prospective study of young women found that overweight women were more likely to experience amenorrheic episodes, but no association was identified between obesity and TRA11. Our study is the largest to prospectively evaluate menstrual function among breast cancer patients, potentially contributing to the ability to detect an association that was obscured by prior smaller studies. In the POSH study, obese women at age ≤40 years with estrogen-receptor-positive (ER+) breast cancer experienced significantly inferior distant disease-free interval and overall survival relative to those with normal weights27. Only a small proportion of patients had received OFS and the analysis did not adjust for TRA. While the relationship between obesity and breast cancer recurrence risk is complex, the consistent association between amenorrhea and improved breast cancer outcomes7,21,28 raises the question of whether the lower risk of TRA observed might contribute to the inferior outcomes experienced by obese patients. Even among young women with HR+ breast cancer treated with OFS with a GnRH agonist, obese patients are more likely to experience elevated estradiol levels, and incomplete OFS may underlie a higher recurrence risk in this setting as well29,30. Obese postmenopausal women with ER+ breast cancer treated with AIs in the adjuvant setting also experience inferior breast cancer outcomes, possibly due to inadequate suppression of aromatase, which is produced in adipose tissue31,32,33 and this has been observed among premenopausal women as well33. These accumulating data have implications for future research and patient care, especially as the proportion of women in many populations who are overweight or obese is increasing.
This study should be interpreted in the context of its limitations. Some subgroups by age, BMI, and chemotherapy regimen contain small numbers of participants, limiting comparisons and the ability to incorporate specific subgroups into the multivariable analysis. Menstrual function and BMI were evaluated by patient report and potentially subject to recall and other biases. The proportion of participants with BMI ≥ 25 may have been higher among those excluded due to missing 1-year survey information, though TRA data were not available for these patients, limiting analysis of how this could impact the findings. While TRA is an important surrogate for ovarian function, some women may have residual ovarian estradiol production without menstruating. Future analyses will evaluate serum FSH and estradiol levels at serial timepoints to further examine menstrual function among young women treated for early breast cancer. Additionally, TRA is also an imperfect surrogate for ovarian reserve damage and fertility and additional analyses are warranted to evaluate pregnancy and birth outcomes.
In conclusion, the management of young women with breast cancer should be informed by accurate information regarding the risk of TRA with modern treatment to optimize disease outcomes and preserve fertility when desired. Women at age ≤40 diagnosed with breast cancer experience moderate rates of early TRA but most will resume menstrual function within 2 years and this varies by patient factors including age and body mass. Future research to confirm and further understand the relationship between BMI, TRA, and breast cancer outcomes as well as to incorporate novel biomarkers to improve the prediction of TRA for young patients is clearly warranted.
Methods
Study design
The Young Women’s Breast Cancer Study (YWS) is a multi-center, prospective cohort study of women diagnosed with breast cancer at age ≤40. Participants were enrolled from 12 sites in the United States and Canada from 2006 to 2016 within 6 months of diagnosis. Those who were able to respond to questionnaires in English were eligible. Potential participants at Dana-Farber/Harvard Cancer Center (DF/HCC) sites were identified by the Rapid Case Identification Core through pathology record review and elsewhere through systematic review of clinic lists. Women who did not return the baseline survey during the recruitment period and neither consented nor declined participation were invited 1 year after diagnosis to participate with abbreviated “short form” surveys. Participants at Sunnybrook Health Sciences Centre completed abbreviated surveys at all timepoints. Follow-up surveys were sent every 6 months for 3 years (with the third survey timed to be answered approximately 1 year from diagnosis and subsequent survey timepoints based on that reset timing) and are sent annually thereafter. Participants provided written informed consent, authorizing medical record review, participant questionnaires, and biospecimen collection. IRB approval for the study was obtained through DF/HCC and other participating centers.
Study population
This analysis describes patient-reported menstrual outcomes for participants with stage 0–III breast cancer (Fig. 2). Participants who did not complete surveys administered at baseline or 6 months after diagnosis (n = 135), or the menstrual history question on these surveys (n = 40), were excluded, as were participants who reported having undergone hysterectomy (n = 17), unilateral oophorectomy (n = 18), or bilateral oophorectomy (n = 40) at baseline or 6 months. Participants who reported a LMP more than 1 year prior to diagnosis (n = 30) were classified as postmenopausal and excluded. Participants with stage IV disease at diagnosis (n = 58) or with recurrence within 1 year (n = 5) were excluded given substantial differences in treatment patterns. Participants who later reported being pregnant or receiving a gonadotropin receptor hormone (GnRH) agonist within 1 year were censored at those timepoints but eligible at subsequent timepoints. Participants who later underwent a hysterectomy, unilateral oophorectomy, or bilateral oophorectomy, or experienced a disease recurrence were censored at all subsequent timepoints.
Outcomes
We evaluated menstrual outcomes in the 5 years following diagnosis. The proportion with active menstrual function, defined as the number of participants with LMP within 1 year of completing the survey over the number who were evaluable, was calculated at annual timepoints. TRA at 1 year was defined as the number of eligible participants whose LMP was greater than 6 months prior to completing the survey, and TRA at 2 years as the proportion whose LMP was greater than 18 months prior to completing the survey, to recognize that amenorrhea may not occur immediately upon treatment initiation and, therefore, TRA should not be defined using menstrual function in the first 6 months after diagnosis. Resumption of menses was defined as the proportion of women with TRA at 1 and 2 years who reported a period on a subsequent survey through the 5-year timepoint. We also assessed the development of amenorrhea, defined as having one or more amenorrheic periods of 1 year or longer, among women who did not have TRA at 1 year.
Other covariates
Disease and treatment information, including stage, HR expression, and chemotherapy use was obtained through medical record review, including each dose of chemotherapy and doses reduced, held, or delayed. Race, smoking status, and BMI were self-reported on the baseline survey. Chemotherapy regimens were categorized based upon the entirety of treatment received for (neo)adjuvant therapy. Receipt of trastuzumab and/or pertuzumab vs not was collapsed within the cytotoxic chemotherapy regimen given prior data finding no impact of trastuzumab on TRA20. Dose reduction >50% was characterized as receipt of any drug at 50% or less of the standard total dose, including held and reduced doses. Endocrine therapy use, including oral endocrine therapies and GnRH agonists, at serial timepoints was obtained from completed surveys and tamoxifen use at 1 year is reported.
Statistical analysis
We identified patient and treatment characteristics potentially associated with TRA. Those which were significantly associated with 1-year TRA in univariable analysis (p < 0.20) were evaluated in a multivariable logistic regression model34. To evaluate the association between BMI and TRA and interactions with other relevant patient and treatment factors, univariable analyses were performed within relevant subpopulations.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data generated and analyzed during this study are described in the following data record: https://doi.org/10.6084/m9.figshare.1482829835. The data underlying the final analyses are contained in 52.csv files. These files are not publicly available as the IRB-approved research protocol specified that all data must be collected, coded, and stored at the Dana-Farber Cancer Institute and be limited-access and password-protected in the Partners system, in order to protect the identity of respondents. Requests can be made to share data privately. However, any data sharing will require a formal data transfer agreement between the Dana-Farber Cancer Institute and the other party. Requests to this effect should be directed to the corresponding author.
References
American Cancer Society. Breast Cancer Facts & Figures 2017–2018 (American Cancer Society, 2017).
Paluch-Shimon, S. et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast 35, 203–217 (2017).
Han, W. et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer 4, 82 (2004).
Gnerlich, J. L. et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J. Am. Coll. Surg. 208, 341–347 (2009).
Partridge, A. H. et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J. Clin. Oncol. 34, 3308–3314 (2016).
Francis, P. A. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med 379, 122–137 (2018).
Swain, S. M. et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N. Engl. J. Med 362, 2053–2065 (2010).
Saha, P. et al. Treatment efficacy, adherence and quality-of life among very young women (age <35 years) in the IBCSG TEXT and SOFT Adjuvant Endocrine Therapy Trials. J. Clin. Oncol. 35, 3113–3122 (2017).
Howard-Anderson, J., Ganz, P. A., Bower, J. E. & Stanton, A. L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J. Natl. Cancer Inst. 104, 386–405 (2012).
Petrek, J. A. et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J. Clin. Oncol. 24, 1045–1051 (2006).
Sukumvanich, P. et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer 116, 3102–3111 (2010).
Han, H. S. et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res. Treat. 115, 335–342 (2009).
Lambertini, M. et al. Dose-dense adjuvant chemotherapy in premenopausal breast cancer patients: A pooled analysis of the MIG1 and GIM2 phase III studies. Eur. J. Cancer 71, 34–42 (2017).
Kim, H. A. et al. Premenopausal breast cancer: a randomized phase III trial. J. Clin. Oncol. 38, 434–443 (2020).
Paluch-Shimon, S. et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4).Ann. Oncol. 31, 674–696 (2020).
Kim, J., Turan, V. & Oktay, K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J. Clin. Endocrinol. Metab. 101, 1364–1371 (2016).
Oktay, K. et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 36, 1994–2001 (2018).
Chien, A. J. et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage II-III breast cancer receiving neoadjuvant therapy. Breast Cancer Res. Treat. 165, 151–159 (2017).
Letourneau, J. M. et al. Fertility preservation before breast cancer treatment appears unlikely to affect disease-free survival at a median follow-up of 43 months after fertility-preservation consultation. Cancer 126, 487–495 (2020).
Abusief, M. E., Missmer, S. A., Ginsburg, E. S., Weeks, J. C. & Partridge, A. H. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer 116, 791–798 (2010).
Lambertini, M. et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J. Natl. Cancer Inst. 111, 86–94 (2019).
Lambertini, M. et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-mullerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. 9, 575 (2019).
Ruddy, K. J. et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res. Treat. 151, 589–596 (2015).
Gast, K. C. et al. Regimen-specific rates of chemotherapy-related amenorrhea in breast cancer survivors. JNCI Cancer Spectr. 3, 1–3 (2019).
Green, D. M. et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 27, 2677–2685 (2009).
Najafi, S. et al. Taxane-based regimens as a risk factor for chemotherapy-induced amenorrhea. Menopause 18, 208–212 (2011).
Copson, E. R. et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann. Oncol. 26, 101–112 (2015).
Parulekar, W. R. et al. Incidence and prognostic impact of amenorrhea during adjuvant therapy in high-risk premenopausal breast cancer: analysis of a National Cancer Institute of Canada Clinical Trials Group Study–NCIC CTG MA.5. J. Clin. Oncol. 23, 6002–6008 (2005).
Bellet, M. et al. Twelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST substudy. J. Clin. Oncol. 34, 1584–1593 (2016).
Pfeiler, G. et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J. Clin. Oncol. 29, 2653–2659 (2011).
Ioannides, S. J., Barlow, P. L., Elwood, J. M. & Porter, D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res. Treat. 147, 237–248 (2014).
Sestak, I. et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J. Clin. Oncol. 28, 3411–3415 (2010).
Gnant, M. et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG-6a trial. Br. J. Cancer 109, 589–596 (2013).
Bursac, Z., Gauss, C. H., Williams, D. K. & Hosmer, D. W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 3, 17 (2008).
Poorvu, P. D. et al. Metadata record for the article: Treatment-related amenorrhea in a modern, prospective cohort study of young women with breast cancer. figshare https://doi.org/10.6084/m9.figshare.14828298 (2021).
Acknowledgements
This research was supported by Susan G. Komen and the Breast Cancer Research Foundation (BCRF). Data included in this manuscript were presented at the 51st Annual American Society of Clinical Oncology Meeting, Chicago, IL, May 29–June 2, 2015.
Author information
Authors and Affiliations
Contributions
Conceptualization: P.D.P., R.M.T., and A.H.P. Data curation: P.D.P., J.H., Y.Z., S.I.G., S.M.R., and A.H.P. Formal analysis: P.D.P., J.H., Y.Z., S.I.G., S.M.R., and A.H.P. Funding acquisition: A.H.P. Investigation: All authors. Methodology: P.D.P., J.H., Y.Z., S.I.G., K.J.R., R.M.T., M.L., S.M.R., and A.H.P. Project administration: All authors. Resources: K.J.R., J.P., L.S., V.F.B., S.E.C., E.W., and A.H.P. Software: H.H., Y.Z., and S.I.G. Supervision: S.M.R. and A.H.P. Validation: All authors. Visualization: P.D.P., J.H., Y.Z. and S.I.G. Writing – original draft: P.D.P. Writing – review & editing: All authors.
Corresponding author
Ethics declarations
Competing interests
P.D.P. reports personal fees from WebMD, outside the submitted work. J.P. reports personal fees from GlaxoSmithKline, Athenex and Abbott Labs, and grants from Pfizer, outside the submitted work. M.L. reports personal fees from Roche, Novartis, Pfizer, Lilly, Takeda, AstraZeneca, and Sandoz, outside the submitted work. All other authors report no disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poorvu, P.D., Hu, J., Zheng, Y. et al. Treatment-related amenorrhea in a modern, prospective cohort study of young women with breast cancer. npj Breast Cancer 7, 99 (2021). https://doi.org/10.1038/s41523-021-00307-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00307-8
This article is cited by
-
Risk of recurrence and pregnancy outcomes in young women with breast cancer who do and do not undergo fertility preservation
Breast Cancer Research and Treatment (2022)