Abstract
Extended adjuvant endocrine therapy (eET) improves outcomes in breast cancer survivors. Most studies however have been limited to postmenopausal women, and optimal eET for young survivors is uncertain. We report eET use among participants in the Young Women’s Breast Cancer Study (YWS), a multicenter prospective cohort of women age ≤40 newly diagnosed with breast cancer enrolled between 2006–2016. Women with stage I–III hormone receptor-positive breast cancer, ≥6 years from diagnosis without recurrence were considered eET candidates. Use of eET was elicited from annual surveys sent years 6–8 after diagnosis, censoring for recurrence/death. 663 women were identified as eET candidates with 73.9% (490/663) having surveys eligible for analysis. Among eligible participants, mean age was 35.5 (±3.9), 85.9% were non-Hispanic white, and 59.6% reported eET use. Tamoxifen monotherapy was the most reported eET (77.4%), followed by aromatase inhibitor (AI) monotherapy (21.9%), AI-ovarian function suppression (AI-OFS) (6.8%) and tamoxifen-OFS (3.1%). In multivariable analysis, increasing age (per year odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.04–1.16), stage (II v. I: OR: 2.86, 95% CI: 1.81–4.51; III v. I: OR: 3.73, 95%CI: 1.87–7.44) and receipt of chemotherapy (OR: 3.66, 95% CI: 2.16–6.21) were significantly associated with eET use. Many young breast cancer survivors receive eET despite limited data regarding utility in this population. While some factors associated with eET use reflect appropriate risk-based care, potential sociodemographic disparities in uptake warrants further investigation in more diverse populations.
Similar content being viewed by others
Introduction
Adjuvant endocrine therapy (ET) is the cornerstone of systemic treatment for hormone receptor (HR)-positive early breast cancer. Five years of tamoxifen reduces the risk of recurrence by ~40% and breast cancer-related mortality by a third through 15 years post-diagnosis1. In postmenopausal women, further improvement in both outcomes has been achieved with aromatase inhibitors (AI), whether administered upfront or following 2–3 years of tamoxifen2. Similarly, for premenopausal women, adding ovarian function suppression (OFS) to tamoxifen has been shown to improve disease-free and overall survival, with a further reduction in disease-free survival and distant recurrences achieved with an AI compared to tamoxifen3. An overall survival benefit from AI with OFS, compared to tamoxifen alone, has yet to be observed.
Despite 5 years of adjuvant ET, the substantial risk of recurrence of HR-positive breast cancers continues at a steady rate for at least another 15 years following treatment discontinuation4. This observation has motivated the study of extended endocrine therapy (eET), defined as continuing ET beyond 5 years. The first such trials, ATLAS and later aTTom, showed a survival benefit from 5 additional years of tamoxifen, following an initial 5 years5,6. More recently, multiple trials have evaluated the extension of AI therapy in postmenopausal patients, and although superior disease-free survival (DFS) has been observed, improved overall survival (OS) has not been shown7. Furthermore, optimal sequencing and duration of therapy remains unclear8. Patient decisions regarding eET are therefore complicated by uncertainty regarding individual risks and benefits, as well as resignation to the presence of ongoing side effects or practice of side effect management9. Little data describing real-life eET uptake is available.
Young women with early-stage, HR-positive breast cancer have a particularly increased risk for late recurrence and breast cancer-related death, and thus may benefit from eET4,10. In the MA17 trial, among women who were postmenopausal following 5 years of tamoxifen, letrozole eET was significantly more effective in improving DFS among women who were premenopausal, compared to postmenopausal, at diagnosis11. For those remaining premenopausal at 5 years follow-up, the benefit has only been demonstrated for 5 years of additional tamoxifen, after an initial 5 years of tamoxifen5,6. No data are available to inform risks and benefits of eET among premenopausal women treated with initial AI-OFS, despite the widespread adoption of this regimen for very young and high-risk premenopausal women3. Thus, for young breast cancer survivors, there is even greater uncertainty regarding optimal eET12. We aim to determine eET uptake and evaluate patient and clinical characteristics associated with uptake in young women with HR-positive early breast cancer in the Young Women’s Breast Cancer Study (YWS, NCT01468246).
Results
Population characteristics
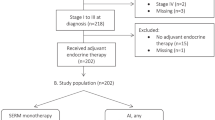
Of the 1297 eligible women enrolled in the YWS, 774 were diagnosed with stage I–III HR-positive breast cancer and received surveys including questions regarding ET use (Fig. 1). Following the exclusion of 111 women with a documented new primary breast cancer, breast cancer recurrence or death in the first 6 years post-diagnosis, 663 remaining participants were considered potential eET candidates. After censoring new primary breast cancers and recurrences occurring after 6 years, 73.9% (490/663) of eET candidates returned at least one survey between years 6–8 and were eligible for analysis. One participant returned a survey but did not respond to the question on ET use and was categorized as a non-responder. Compared to those without a 6–8-year survey (n = 173), the analytic cohort (n = 490) tended to be non-Hispanic white (85.9 vs. 77.5%, p = 0.010, Chi-square test), financially comfortable (54.0 vs. 39.5%, p = 0.05, Chi-square test), have a college or above education (85.5 vs. 72.2%, p < 0.001, Chi-square test), have children pre-diagnosis (59.6 vs. 41.6%, <0.001, Chi-square test), and to have received chemotherapy (77.0 vs. 69.0%, p = 0.039, Chi-square test) or any ET in the first 5 years post-diagnosis (93.6 vs. 83.3%, p < 0.001, Chi-square test). (Supplementary Table 1).
eET use
Overall, 59.6% (292/490) of the analytic cohort reported eET. The proportion of participants using eET declined from 56.8% (263/463) to 51.3% (217/423) to 47.1% (161/342) at years 6, 7, and 8 post-diagnosis, respectively (Fig. 2). Among eET users (n = 292), tamoxifen monotherapy was the most commonly reported approach (77.4%, 226/292), followed by AI monotherapy (21.9%, 64/292), AI-OFS (6.8%, 20/292) and tamoxifen-OFS (3.1%, 9/292). Women diagnosed in 2012 or later were as likely to receive eET as those diagnosed prior to 2012 (59.0%, 115/195 vs. 60.0%, 177/295, p = 0.821, Chi-square test); however, eET more often included AI (40.0% vs. 18.6%, p < 0.001, Chi-square test) or OFS (17.4 vs. 7.3%, p = 0.008, Chi-square test) in later years.
eET users vs. eET non-users
A comparison of eET users to non-users is presented in Table 1. eET users were older at diagnosis (mean age 35.9 vs. 35.0, p = 0.008, t-test), more likely to be non-Hispanic white (88.7 vs.81.8%, p = 0.032, Chi-square test), partnered at baseline (83.4 vs. 68.8%, p < 0.001, Chi-square test) and parous pre-diagnosis (63.7 vs. 53.5% p = 0.025, Chi-square test). Users compared to non-users, respectively, had been diagnosed with more advanced stage breast cancer (stage I: 27.4 vs. 61.1%, stage II: 54.1 vs. 31.3%, stage III: 18.5 vs. 7.6%, p = <0.001, Chi-square test), and accordingly more frequently underwent mastectomy (lumpectomy: 27.1 vs. 40.4%, unilateral mastectomy: 26.0 vs. 23.7%, bilateral mastectomy: 46.9 vs. 35.9%, p = 0.006, Chi-square test), and received radiotherapy (69.4 vs. 59.4%, p = 0.022, Chi-square test) and/or chemotherapy (88.6 vs. 60.1%, p < 0.001, Chi-square test) as part of their primary breast cancer treatment.
All but one eET user reported initiation of any ET in their first 5 years post-diagnosis (99.7 vs. 84.5%, p = <0.001, Chi-square test). As part of their initial 5-year ET, eET users vs. non-users more often received an AI (65/288, 22.6% vs. 22/164, 13.4%, p = 0.018, Chi-square test) and/or OFS (90/288, 31.3% vs. 33/164, 20.1%, p = 0.011, Chi-square test), while tamoxifen use was similar (273/288, 94.8% vs. 156/164, 95.1%, p = 0.878, Chi-square test). Users were less likely to have reported fertility concerns impacting their ET decisions on any survey during the first 5 years post-diagnosis (33.2 vs. 43.8%, p = 0.018, Chi-square test). Pregnancy rates in years 1–5 did not differ statistically between eET users and non-users (9.7 vs. 8.8%, p = 0.732, Chi-square test). Most participants (63.2%) were premenopausal during the period of interest (36.8% were postmenopausal). A greater proportion of eET users compared to non-users were postmenopausal (45.4 vs. 24.5%, p < 0.001, Chi-square test). Postmenopausal eET users more often received an AI as part of their eET compared to premenopausal eET users (±OFS), (50/123, 40.7% vs. 27/148, 18.2%, p < 0.001, Chi-square test).
Univariable and multivariable analyses of demographic and clinical characteristics associated with eET use are presented in Table 2. In multivariable analysis, increasing age (per year odds ratio [OR]: 1.10, 95% confidence interval [CI]: 1.04–1.16, p < 0.001, logistic regression), higher stage (II vs. I: OR: 2.86, 95% CI: 1.81–4.51, p < 0.001; III vs. I: OR: 3.73, 95% CI: 1.87–7.44, p < 0.001, logistic regression) and receipt of chemotherapy (OR: 3.66, 95% CI: 2.16–6.21, p < 0.001, logistic regression) were significantly associated with eET use, while women not of non-Hispanic white ethnicity were less likely to report eET use (OR: 0.49, 95% CI: 0.28–0.87, p = 0.015, logistic regression).
Breast Cancer Prevention Trial (BCPT) scores, ascertained from the last available year 1–5 survey when concomitant ET use was reported, were available for 99.6% (450/452) of the sub-group who initiated ET in the first five years (Table 3). Endocrine symptom scores, including hot flashes, weight problems, musculoskeletal symptoms, and vaginal problems, were not significantly different between eET users and non-users. The largest difference was observed for hot flash scores, which were modestly higher among eET users (1.30 vs. 1.07, p = 0.050, t-test). Distributions of symptom severity were similar with the proportions of patients unbothered by symptoms in the first 5 years (score = 0) or extremely bothered by symptoms (score = 3.5–4) comparable between eET users and non-users.
Discussion
Multiple prospective clinical trials have evaluated the role of eET in breast cancer and its use is currently supported by society guidelines for women with breast cancer at high risk for late recurrence8. However, sparse data are available describing the adoption of eET in clinical practice. This paucity is particularly worrisome for young premenopausal women, who were excluded from most eET clinical trials, and greater uncertainty exists regarding appropriate eET in this population. Our finding that a majority (59.6%) of eligible young breast cancer survivors received eET is striking, given the limited data regarding its utility in this population.
While higher stage and receipt of chemotherapy represent risk-based factors associated with eET use, we also observed that younger women and women not of non-Hispanic white ethnicity were independently less likely to report eET use. Prior limited data also suggest biases in the receipt of eET. Myrick et al reported an eET eligibility rate of 48.3% in an institutional cohort of 286 women treated in Switzerland who had initiated adjuvant ET between 1999–200513. A recommendation for eET was given to 64.5% of eligible women, who tended to have a higher-stage disease and more often had received chemotherapy. General practitioners were less likely to recommend eET than oncologists and younger patient age was associated with offering eET (61 vs. 68 years). However, this study excluded women still premenopausal following 5 years of ET, and accordingly, the population was overall much older than our cohort. Notably, in our study, we did not ask women whether they were recommended eET or not.
Underuse of adjuvant ET in non-white women in the first 5 years following a breast cancer diagnosis is well-documented, spanning non-initiation, non-adherence, and/or non-persistence. Studies show that Black women are at increased risk for ET underuse, with more mixed findings for other minorities14. While only 14% of the analytic cohort were from diverse racial and ethnic backgrounds, the association we observed between non-white non-Hispanic ethnicity and eET underuse may indicate that this disparity extends to the eET setting. While some barriers to care may be shared by all racial/ethnic and socioeconomic subgroups, others may vary between subgroups. For example, in one study, Hispanic women most commonly reported concerns regarding side effects as a barrier, while Black women most commonly cited the lack of a recommendation for ET15. Interactions between race/ethnicity, socioeconomic status, and out-of-pocket medication costs also contribute to disparities in ET adherence16. We did not find an association between a self-reported assessment of financial comfort and eET use. Further investigation of eET use in Black and Hispanic/Latina breast cancer survivors would provide additional insight regarding whether these women experience unique barriers and facilitators to uptake in the setting of eET.
The most common form of eET among young women in our sample was tamoxifen alone. This approach is supported by the aTTom and ATLAS trials, which currently represent the only available data for eET in women who remain premenopausal following 5 years of ET5,6. As to be expected, given the young age of this cohort, most (59.6%) women remained premenopausal at 6 years post-diagnosis and were less likely to receive eET. Those who were postmenopausal at 6 years post-diagnosis were more likely to receive eET, which more often included an AI. For women who were premenopausal at diagnosis and who have transitioned to a postmenopausal state following their initial ET, whether due to age-related menopause, chemotherapy-related menopause, or oophorectomy, switching to an AI may be advantageous. In MA17, following 5 years of tamoxifen, letrozole eET was associated with a larger improvement in DFS and distant DFS in this population when compared to women who were postmenopausal at diagnosis11. While the current standard of care ET for many women in our population would initially include OFS, there are no data to support extending OFS beyond 5 years, and the potential long-term toxicities associated with extended OFS are concerning12. Nevertheless, OFS was a part of eET in 10% of cases, highlighting the need for additional research and guidance regarding this approach.
While all women included in this analysis were at least 6 years post-diagnosis, some had not reached 7-year, 8-year, or later landmarks, and although we observed a decreasing proportion of eET use at 6 vs. 8 years post-diagnosis, we cannot reliably comment on adherence with eET. In the aforementioned study by Myrick et al. among the 64 patients who initiated eET, 28.1% were determined to be non-persistent13. Lee et al. reported that among 398 women who started letrozole eET following 5 years of tamoxifen, the cumulative discontinuation rate of eET through 5 years was 45.5%, with 16.1% discontinuing treatment during the first year17. Both analyses were conducted in populations largely diagnosed prior to 2006; thus, participants in those studies initiated eET prior to the publication of contemporary eET clinical trials. Similar findings have been reported from prospective trials. For example, in ABCSG-16, comparing 2 to 5 years of anastrozole following 5 years of initial ET, discontinuation rates of 20 and 33%, respectively, were reported18.
Treatment-related toxicities and fertility concerns have been reported as important drivers of non-adherence within the first 5 years of ET among young breast cancer survivors19. Although side effects are very common with ET, experiencing side effects does not necessarily lead to poor adherence or discontinuation through the first 5 years20. In the current analysis, ET-related symptoms reported while taking ET at years 1–5 were not associated with eET use. In YWS, a third of women reported that fertility concerns affected their ET decisions, and this was most significantly related to nulliparity at diagnosis21. In the current subset, 36% reported that fertility concerns had impacted their ET decision-making during their first 5 years post-diagnosis, and this was significantly associated with a decreased likelihood to take eET. Similarly, parity prior to diagnosis was associated with taking eET on univariable analysis; however, this did not persist in the multivariable model, and pregnancy during years 1–5, reported by ~10% of participants, was similarly unassociated with eET use.
To date, eET uptake has been sparsely reported. Using a large and well-annotated prospective cohort, we were able to describe trends in a young population for which particular uncertainty exists. Our observations, however, should be considered in light of several limitations. We ascertained eET use from surveys, thus relying on self-report. Given the scope of our surveys, we could not comment on whether all eligible women were offered eET. Surveys were available for ~70% of the eligible population, with several demographic and clinical differences observed between groups with and without available surveys. Most participants were non-Hispanic white, considered themselves financially comfortable and were treated in academic cancer centers. Women who were ineligible due to missing surveys, tended to belong to racial and ethnic minorities, reported less financial comfort and lower education at diagnosis, and less often received chemotherapy for their primary breast cancer. As discussed, these characteristics may be associated with non-adherence to ET, and their enrichment in the excluded sub-population may limit the generalizability of our findings and underscores the need for additional reports from other populations.
In conclusion, the majority of eligible young breast cancer survivors in the YWS have received eET despite limited data regarding its utility in this population, particularly among women who received anything but tamoxifen in the first 5 years and remain premenopausal. Heterogeneity exists regarding strategies utilized, and while some factors associated with eET use appear to reflect appropriate risk-based care, such as stage and receipt of chemotherapy, others, including age and race, warrant further investigation and may be mediated by differences in communication, access and/or patient preferences. Prospective clinical studies are needed to inform the optimal approach to eET for young women with a history of early-stage, HR-positive breast cancer who remain premenopausal following 5 years of ET. Further, ensuring equitable uptake of available risk-reducing treatment should be a priority.
Methods
Setting
The YWS is a multicenter prospective cohort that enrolled women with newly diagnosed breast cancer at age ≤40 years between 2006–2016. Study sites included academic and community hospitals in Massachusetts and academic sites in Colorado, Minnesota, and Toronto, Canada. Potential participants at Dana-Farber/Harvard Cancer Center (DF/HCC) sites were identified by the Rapid Case Identification Core through pathology record review and elsewhere through a systematic review of clinic lists. Following written informed consent, participants were administered a baseline survey (median: 4.6 months post-diagnosis) and then surveyed twice a year for the first three years following diagnosis and annually thereafter. The study was approved by the DF/HCC (06-169) and participating site Institutional Review Boards (Mayo Clinic: PR13-006386, Sunnybrook: 2463, University of Colorado: 08-1222).
Study population and measures
YWS participants diagnosed with stage I–III HR-positive (estrogen and/or progesterone receptor) breast cancer and alive at least 6 years post-diagnosis, without having experienced a new primary breast cancer or breast cancer recurrence, were considered candidates for eET. Use of eET was elicited on surveys completed at 6, 7, and/or 8 years post-diagnosis, and those completing at least one survey in years 6–8 were eligible for analysis. If a woman had a new primary breast cancer, recurrence, death, or had yet to reach the timepoint, she was censored prior to that timepoint. On each survey, women were asked whether they were currently taking tamoxifen, an AI, and/or OFS injections. Those reporting taking any ET on at least one of the available year 6–8 surveys were considered eET users, while those reporting no ET use were considered non-users. For each survey, eET was categorized as follows: tamoxifen alone, AI alone, tamoxifen-OFS, AI-OFS, or OFS alone. Participants sent an abbreviated survey excluding these questions, including all participants enrolled in Toronto, Canada, were excluded from this analysis (n = 90). Surveys will be made available, on request, from the corresponding author.
Participants self-reported their sociodemographic characteristics, including race and ethnicity, marital status, parity, education and financial comfort at baseline1,2. Financial comfort was assessed using a single-item measure and dichotomized as financially comfortable (after paying the bills, enough money for special things) vs. not financially comfortable (money to pay the bills, but little spare money to buy extra or special things/money to pay the bills, but only because you cut back on things/difficulty paying the bills, no matter what you do)22,23,24. When unavailable by self-report, race and ethnicity were collected from the medical record. Race and ethnicity variables are categorized into the following ethnic combinations: non-Hispanic white, non-Hispanic Black, Hispanic, Asian, Multiracial, or Unknown/other. White and Black participants who did not report ethnicity were defined as non-Hispanic, We ascertained participants’ stage and receptor status from pathology reports and medical record review and obtained treatment-related information and breast cancer new primary/recurrence events through a combination of survey data and medical record review. We extracted information regarding participants’ post-diagnosis pregnancies, ET use, and endocrine-related symptoms from year 1–5 post-diagnosis surveys. Symptoms were assessed using the BCPT symptom scales encompassing eight physical symptoms associated with breast cancer treatment, of which we focused on 4 ET-related symptoms: hot flashes, vaginal problems, musculoskeletal pain, and weight problems25. Average severity scores were calculated for each multi-item scale, based on the reported degree of symptomatic bother during the prior 4 weeks using a five-point scale: 0-not at all; 1-slightly; 2-moderately; 3-quite a bit; 4-extremely. Scores between 3.5–4 were considered to reflect extreme bother, as previously defined26. Among the subset of patients who initiated ET in the first 5 years, we used BCPT scores reported on the last available year 1–5 survey, during which a participant also endorsed currently taking ET in order to define endocrine symptom burden. The impact of fertility concerns on ET decision-making was assessed at baseline, 6 months post-baseline, and annually from 1 to 5 years post-diagnosis. Women who responded that they chose not to take ET or chose/may choose to take less than 5 years of ET due to fertility concerns on any survey during these timepoints were classified as having ET decisions impacted by fertility concerns. Menopausal status during the potential eET period was defined based on participants’ first available year 6–8 survey responses. Women were considered postmenopausal if they reported a history of bilateral oophorectomy or absence of menses in the 12 months prior to the survey, and premenopausal if they reported current OFS use or menses within 12 months of the survey. If a prior hysterectomy was reported or if none of these conditions were met, then menopausal status was considered unknown.
Statistical analysis
Frequencies and proportions were calculated for categorical variables. Means and standard deviations were calculated for continuous covariates. We hypothesized that results from the SOFT/TEXT trials, which showed an OS benefit for OFS and first reported in 2017, may affect ET choices over time and therefore compared eET use with McNemar’s test among those diagnosed between 2006–2011 vs. 2012–2015. We compared patient and disease characteristics between eET users and non-users using Chi-square statistics and compared mean BCPT scores between eET users and non-users using the Student’s t-test. Univariable and multivariable logistic regression with backward model selection were used to evaluate factors associated with eET use. All variables and non-missing data were entered into the final multivariable models and then backward eliminated until all remaining factors were significantly associated with eET use. Two-sided p values <0.05 were considered significant throughout. We conducted analyses using Microsoft Excel for Microsoft 365 MSO Version 2108 (Redmond, WA) and SAS Software, Version 9.4 (SAS Institute, Cary, NC).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data underlying this analysis are not publicly available as the Institutional Review Board approved research protocol specified that all data must be collected, coded, and stored at the Dana-Farber Cancer Institute and be limited-access and password-protected in the Partners system, in order to protect the identity of respondents. Requests can be made to share data privately. However, any data sharing will require a formal data transfer agreement between the Dana-Farber Cancer Institute and the other party. Requests to this effect should be directed to the corresponding author.
References
Early Breast Cancer Trialists’ Collaborative, G. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378, 771–784 (2011).
Early Breast Cancer Trialists’ Collaborative, G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386, 1341–1352 (2015).
Francis, P. A. et al. Tailoring aadjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379, 122–137 (2018).
Pan, H. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Gray, R. G. et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J. Clin. Oncol. 31, 5–5 (2013).
Walsh, E. M., Nunes, R., Wilkinson, M. J. & Santa-Maria, C. A. Extended endocrine therapy for early-stage breast cancer: how do we decide? Curr. Oncol. Rep. 22, 123 (2020).
Burstein, H. J., Lacchetti, C. & Griggs, J. J. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J. Oncol. Pract. 15, 106–107 (2019).
Eraso, Y., Stefler, D., Moon, Z., Rossi, L. & Assefa, S. Extending adjuvant endocrine therapy for 10 years: a mixed-methods analysis of women’s decision making in an online breast cancer forum. Healthcare https://doi.org/10.3390/healthcare9060688 (2021).
Partridge, A. H. et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J. Clin. Oncol. 34, 3308–3314 (2016).
Goss, P. E. et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann. Oncol. 24, 355–361 (2013).
Sella, T., Ruddy, K. J., Carey, L. A. & Partridge, A. H. Optimal endocrine therapy in premenopausal women: a pragmatic approach to unanswered questions. JCO Oncol. Pract. 18, 211–216 (2022).
Myrick, M. E., Schmid, S. M., Kilic, N. & Guth, U. Eligibility, compliance and persistence of extended adjuvant endocrine therapy for breast cancer. Acta Oncol. 51, 247–253 (2012).
Roberts, M. C., Wheeler, S. B. & Reeder-Hayes, K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am. J. Public Health 105, e4–e15 (2015).
Livaudais, J. C. et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the women’s health initiative. Cancer Epidemiol. Biomarkers Prev. 22, 365–373 (2013).
Farias, A. J. & Du, X. L. Association between out-of-pocket costs, race/ethnicity, and adjuvant endocrine therapy adherence among medicare patients with breast cancer. J. Clin. Oncol. 35, 86–95 (2017).
Lee, H. S. et al. Low adherence to upfront and extended adjuvant letrozole therapy among early breast cancer patients in a clinical practice setting. Oncology 86, 340–349 (2014).
Gnant, M. et al. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N. Engl. J. Med. 385, 395–405 (2021).
Llarena, N. C., Estevez, S. L., Tucker, S. L. & Jeruss, J. S. Impact of fertility concerns on tamoxifen initiation and persistence. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djv202 (2015).
Toivonen, K. I., Williamson, T. M., Carlson, L. E., Walker, L. M. & Campbell, T. S. Potentially modifiable factors associated with adherence to adjuvant endocrine therapy among breast cancer survivors: a systematic review. Cancers https://doi.org/10.3390/cancers13010107 (2020).
Sella, T. et al. Impact of fertility concerns on endocrine therapy decisions in young breast cancer survivors. Cancer 127, 2888–2894 (2021).
Gierisch, J. M., Earp, J. A., Brewer, N. T. & Rimer, B. K. Longitudinal predictors of nonadherence to maintenance of mammography. Cancer Epidemiol. Biomarkers Prev. 19, 1103–1111 (2010).
Ruddy, K. J. et al. Breast cancer presentation and diagnostic delays in young women. Cancer 120, 20–25 (2014).
Williams, R. B. et al. Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease. JAMA 267, 520–524 (1992).
Stanton, A. L., Bernaards, C. A. & Ganz, P. A. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J. Natl Cancer Inst. 97, 448–456 (2005).
Ganz, P. A., Petersen, L., Bower, J. E. & Crespi, C. M. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J. Clin. Oncol. 34, 816–824 (2016).
Acknowledgements
The Young Women’s Breast Cancer Study is funded by the Breast Cancer Research Foundation (A.H.P.) and Komen (A.H.P.). Tal Sella is supported by the Pinchas Borenstein Talpiot Medical Leadership Program, Chaim Sheba Medical Center, Tel HaShomer, Israel. The authors would also like to thank Kate Bifolck and Valerie Hope Goldstein for their assistance with editing and manuscript submission. The study was previously presented as a spotlight poster at the 2021 San Antonio Breast Cancer Symposium, Dec 7–10, 2021.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.S., S.M.R., and A.H.P. Data curation: T.S., S.M.R., and A.H.P. Formal analysis: T.S., Y.Z., S.M.R., and A.H.P. Funding acquisition: A.H.P. Investigation: all authors. Methodology: T.S., Y.Z., S.M.R., and A.H.P. Project administration: all authors. Resources: K.J.R., J.M.P., L.S., V.F.B., S.E.C., E.P.W., and A.H.P. Software: T.S. and Y.Z. Supervision: S.M.R. and A.H.P. Validation: all authors. Visualization: T.S., Y.Z., and S.I.G. Writing—original draft: T.S. Writing—review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
TS received honorarium from Roche and travel expenses for Gilead. SMR reports research funding from Pfizer/Conquer Cancer. JMP reports institutional research funding from Outcomes4me, serving as a consultant for Abbott Labs and spousal employment by GlaxoSmithKline. LS reports serving as an advisor to Blue Note Therapeutics and Rubedo Life Sciences and serving as a consultant for Novartis. VFB reports institutional research funding from SeaGen, AstraZeneca, Olema, and Oncosec, serving as a consultant for SeaGen and AstraZeneca, and serving as founder/stock ownership in PerlaTx. LAC reports institutional research funding from Syndax, Novartis, NanoString Technologies, SeaGen, VeraCyte, and AstraZeneca. EPW served over the past 3 years as a consultant to and received honoraria from Carrick Therapeutics, Genentech/Roche, and Jounce Therapeutics. He is an uncompensated member of the Scientific Advisory Boards for Leap Therapeutics. AHP reports royalties for co-authoring the Breast Cancer Survivorship section of UpToDate. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sella, T., Zheng, Y., Rosenberg, S.M. et al. Extended adjuvant endocrine therapy in a longitudinal cohort of young breast cancer survivors. npj Breast Cancer 9, 31 (2023). https://doi.org/10.1038/s41523-023-00529-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00529-y