Abstract
Quality of life (QoL) is a complex, ordinal endpoint with multiple conditioning factors. A predictive model of QoL after adjuvant chemotherapy can support decision making or the communication of information about the range of treatment options available. Patients with localized breast cancer (n = 219) were prospectively recruited at 17 centers. Participants completed the EORTC QLQ-C30 questionnaire. The primary aim was to predict health status upon completion of adjuvant chemotherapy adjusted for multiple covariates. We developed a Bayesian model with six covariates (chemotherapy regimen, TNM stage, axillary lymph node dissection, perceived risk of recurrence, age, type of surgery, and baseline EORTC scores). This model allows both prediction and causal inference. The patients with mastectomy reported a discrete decline on all QoL scores. The effect of surgery depended on the interaction with age. Women with ages on either end of the range displayed worse scores, especially with mastectomy. The perceived risk of recurrence had a striking effect on health status. In conclusion, we have developed a predictive model of health status in patients with early breast cancer based on the individual’s profile.
Similar content being viewed by others
Introduction
In recent decades, we have witnessed an upsurge in the use of patient-reported outcomes (PROs), such as quality of life (QoL), when designing and interpreting clinical trials. These endpoints, together with physician-reported outcomes, are beginning to be taken into account vis-à-vis regulations, as well as therapeutic decision making1,2. Thus, the European Society of Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS), a tool that classifies the magnitude of benefit that can be expected from anticancer treatments, integrates QoL estimations in addition to efficacy and toxicity variables3.
In women with breast cancer, QoL has numerous interwoven determinants with mutual, complex, interactions, such as type of surgery, socio-economic status, psychological factors, age, or aspects ranging from body image to fear of recurrence4,5. This multiplicity of factors affecting QoL makes this a complex endpoint, which often poses an analytical challenge1,6,7. By and large, simplified analyses with bivariate methods, such as Wilcoxon signed-rank tests, on the basis of the variables of interest (e.g., type of surgery) are used to compare QoL. Likewise, in clinical trials longitudinal models are routinely used that focus on time to health-related QoL score deterioration, depending on the study arms8,9.
Such univariate analyses have two fundamental drawbacks: (1) their inability to make individual predictions on QoL, conditioned by complex clinical profiles, and (2) the impossibility of pondering interactions a priori deemed relevant2. Evidence of these problems is a recent meta-analysis that concluded that modified radical mastectomy (MRM) results in greater decline on several QoL domains than breast-conserving surgery (BCS)10. Nevertheless, this methodology is not able to identify the modulating factors of interest, leaving meaningful questions, such as interaction with age, up in the air. Thus, when the inference is made about average subjects, it is not possible to clarify key aspects, for instance, whether seniors experience the same impact on their QoL with a mastectomy as much as younger women10. Overall, these limitations impede conveying information regarding the spectrum of treatment options, hinders decision making, and disallows PROs’ support for statements regarding therapeutic effects7.
One of the reasons for this imprecision in the literature is that QoL is an ordered categorical variable, which requires the use of specific ordinal regression methods8,11,12,13. The most straightforward is the proportional odds (PO) model14, which assumes that the effect of each predictor is similar across all levels of the endpoint14. In the real world, it is easy to find examples in which this PO assumption is not met, since the relation with the predictor is not similar for each endpoint category and neighboring values. This conditions the not-so-trivial need to allow for a nonhomogenous effect of a subgroup of predictors across QoL thresholds, treating the variables as nominal, thereby increasing the random error and complexity of analysis15. As an intermediate solution, Peterson & Harrell introduced the constrained partial PO (CPPO) model that assumes a linear, monotonic constriction for the coefficients of the variables that diverge from the PO assumption15,16. CPPO models are an appealing solution to model QoL based on multiple covariates, by joining accuracy and parsimony. Despite the above, thus far, this method has been uncommon in QoL studies12,13,17 or in cohorts of oncological patients9,18 given the absence of available software. However, the Coronavirus Disease 2019 (COVID-19) pandemic has prompted the development of a software program, the R rmsb package, that enables Bayesian CPPO models to be simply and efficiently fitted19. The reason is that the evolution of viral diseases, similar to QoL, is better captured with ordinal endpoints20.
In this situation, the key idea has been to illustrate the complexity, as well as the opportunities that rich modeling of QoL poses by means of this family of ordinal models. Thus, the Bayesian CPPO model is an ideal instrument for both drawing causal inferences and specifying those covariates that predict QoL. As proof of concept with respect to the cross-sectional usefulness of this tool to model these two facets of health status, we have applied these ideas in patients with breast cancer. The primary aim of this study was to predict the health status upon completion of adjuvant chemotherapy adjusted for multiple covariates. Secondary objectives comprised to (1) develop an online calculator to implement this model with potential usefulness for individualized prediction, integrating R with NET; (2) estimate the effect of the type of surgery, based on the individual’s age and profile, and (3) evaluate the incremental benefit of CPPO models versus other alternatives available in this context.
Results
Patients
The database comprises 339 patients with breast cancer, 219 of whom were eligible for this analysis, having completed the questionnaires at baseline and upon completion of adjuvant treatment. The recruitment process is shown in Supplementary Fig. 1. The baseline characteristics of the sample are summarized in Table 1, with no substantial differences between participants with or without questionnaires after completing adjuvant chemotherapy (approximately 6 months later). All participants received adjuvant chemotherapy. Sixty-four percent (64.2%) of the HER2-positive and 69.3% of the HER2-negative subjects received postoperative radiotherapy. Supplementary Table 2 displays baseline characteristics based on type of surgery, BCS (n = 126, 58%), and total mastectomy (n = 93, 42%). In this case, relevant clinical–pathological differences can be seen, with the total mastectomy group presenting a more advanced TNM stage (stage II–III, 78% vs 63%), fewer HER2 + cancers (15% vs 31%), and more ALND (33% vs 16%) than the BSC group. Other sociodemographic characteristics were similar across both groups.
The most common occupational status was unemployed, housewife, temporary worker, or retired in 41% of the cases. Close to 86% (85.9%) reported that breast cancer had caused them no or very little economic hardship. The distribution of health status is shown in Supplementary Fig. 2, prevailing good or very good scores (e.g., summatory score >50%). Most women perceive risk of recurrence as high and respond very similarly in stages I and II (Supplementary Fig. 3A). The perception of risk of recurrence does not differ on the basis of type of surgery (Supplementary Fig. 3B).
Effect of surgery on QoL and its domains
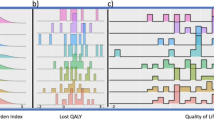
Figure 1A and B illustrates box plots with the distribution of scores depending on type of surgery. At baseline, the physical status following total mastectomy was only slightly worse compared to BSC, mean 84.6 vs 88.6, respectively (p = 0.007). In contrast, upon completion of adjuvant chemotherapy, women who underwent total mastectomy reported a slight increase in symptoms (e.g., mean sum score of 19 vs 24, p = 0.002), as well as significant decline on all QoL scores, such as global health status, physical/ role/ emotional functioning, fatigue, and nausea & vomiting (p < 0.05) (Supplementary Table 3). For instance, mean global health status scores at the end were 62 vs 70 for total mastectomy vs BCS, respectively (p = 0.004).
(A) quality-of-life domain scores pre-chemotherapy; (A) and after concluding systemic adjuvant therapy (B). Note: The graph presents the median, the first and third quartile (25th and 75th percentiles), maximum scores, and individual points that were outside the extremes of the whiskers. The hormonal status of the six males with breast cancer was coded as unknown. BSC breast-conserving surgery, QoL quality of life, HE health status, PH physical domain, RO role domain, EF emotional functioning, SO social domain, CO cognitive domain.
Prediction of global health status upon completion of adjuvant chemotherapy
The frequentist PO model for global health status at 6 months is illustrated in Supplementary Fig. 4. With this approach, total mastectomy was associated with worse scores with OR 0.53 (95% confidence interval, 0.27–1.05). The PO assumption was evaluated separately for each of its predictors (Supplementary Fig. 5). The PO holds up well for categorical variables (surgery, perceived risk of recurrence, ALND, chemotherapy, and stage). However, a deviation is seen with respect to quantitative variables (age, EORTC QLQ-C30 sum score), critical if we are to comprehend the individualized effect as a function of them. The multinomial formulation (complete or partial) generates abstruse, implausible models (Supplementary Fig. 6).
To allow departures from the PO, a Peterson–Harrell’s CPPO model was fitted. The coefficients of this more parsimonious model are displayed in Supplementary Fig. 7. The model has an acceptable discriminatory ability, c-index 0.65 (95% highest posterior density interval [HPDI], 0.63–0.67) and a Brier score of 0.21 (95% HPDI 0.19–0.28). Other performance measures are shown in Supplementary Fig. 8.
The analysis of partial effects on the log odds reveals the nonlinear influence of age on global health status (worse on both ends), as well as the risk of worse scores following total mastectomy or with high perceived risk of recurrence (Fig. 2). The consequence of assuming constrained parameters in certain covariates is apparent in the OR plot (Fig. 3). Thus, the magnitude of association between type of surgery and health status gradually decreases if the cutoff increases (e.g., OR 0.30, 0.49, and 0.57, for thresholds of ≥50%, ≥75%, and ≥83%, respectively), denoting a nonhomogenous effect on the spectrum of this domain. This trend is similar when comparing young and middle-aged women. Indeed, the most relevant variables are baseline QoL, extreme age (young or old), and perceived risk of relapse. In a sensitivity analysis, the model was fitted including the tumor stage; this covariate was not associated with QoL, while the other coefficients remained unaltered (data not shown).
Partial effect plots are shown with 0.95 highest posterior density intervals. Point estimates are posterior modes. BCS breast-conserving surgery, M mastectomy, ALND axillary lymph node dissection, EORTC European Organisation for Research and Treatment of Cancer, TNM tumor-node-metastases, ML medium-low, H high, VH very high. Note: the EORTC variable refers to the sum score.
HDI highest density interval, ALND axillary lymph node dissection, TNM tumor-node-metastases, OR odds ratio. Note: odds ratios are based on posterior mode parameters, also displaying the 0.95 highest posterior density intervals. The shades of blue represent different cutoffs of the response variable (health status at 6 months). The plot reveals the linear constrained effect on the predictors. We can see that the CPPO model assumes proportional odds for all variables, except for baseline QoL and age (that interacts with surgery). The model imposes a linear restriction on the coefficients of these variables that do not meet the proportional odds assumption with respect to cutoffs. In contrast, the variables that do meet the proportional odds assumption have a single odds ratio that is valid for the entire range of possible cutoffs.
In the frequentist formulation, neither ALND nor locally advanced stages had a relevant impact after completing adjuvant chemotherapy (Supplementary Fig. 4). Nevertheless, the Bayesian approach estimates 78% and 81% of posterior probability of harm, respectively (Table 2). Likewise, total mastectomy worsened the health status (cutoff ≥ 83) with OR 0.57 (95% HPDI, 0.28–1.23) and this translated as a probability of associated harm of 93%, 84%, and 70% for any magnitude of detriment, effect size >15% and >30%, respectively. Figure 4 illustrates the model’s ability to predict health status across four patient profiles, BSC, or total mastectomy in younger or older women. Finally, we have designed an online calculator (https://www.prognostictools.es/neoBreast/inicio.aspx) to make individual predictions. The posterior probability of decline with mastectomy clearly differs based on age (e.g., the older and younger women suffer greater detriment). Alternative models (e.g., detailing chemotherapy regimens, adding anthracyclines or different nonlinear effects, etc.) were explored but did not fit the data-generating process much better.
Discussion
In this study, we have fit a CPPO model to probe the factors that predict global health status in women with breast cancer who undergo surgery and complete systemic adjuvant chemotherapy. This model was put forth by Peterson & Harrell in 1990 to model ordinal endpoints, easing the PO assumption, often unmet in analysis15. These predictions are tricky, since they entail a trade-off between parsimony and adequate model fit (e.g., the need to broaden parameters involves increased random error). Among the compromise solutions, the model used here imposes a linear constriction on certain selected covariates, which preserves simplicity15. Despite being deemed a valuable method, the absence of software has prevented it from being more widely used12,21. A package recently written to examine ordinal endpoints using this approach in COVID-19 trials affords us an opportunity for studies on QoL in cancer patients22,23.
As previously reported in the literature, the CPPO model confirms worse health status following total mastectomy vs BSC10, although it contributes nuances and additional insights. Firstly, while total mastectomy increases the probability of QoL scores in the lowest range, conserving the breast does not increase the odds of higher scores to the same degree, which must be taken into account when making predictions. Secondly, age is a predictive factor per se, albeit it is also a modifier of the effect of surgery. This interaction is clearly nonlinear, such that both extremes (younger and older women) fare worse. Nonlinear effects are adequately captured here using a spline (a function defined piecewise by polynomials). Thirdly, long-term predictions require that the starting point be taken into account, which cannot be done with bivariate methods. The baseline EORTC QLQ-C30 sum score is therefore germane and, likewise, nonlinear. This is in line with other data that point toward the existence of different trajectories in the evolution of QoL, depending on specific clinical profiles24.
The initial perception of risk of recurrence modulates the evolution of health status at completion of systemic therapy, in keeping with other series5,25. Regardless of evaluating participants’ perception of risk after their appointment with their oncologist, the correlation with TNM stage is discrete, given that women measure it similarly when facing stages I and II. The same holds true for the type of surgery. This indicates that after being informed, some women perceive a level of risk typically deemed ‘low’ compared to advanced stages or other neoplasms, as being ‘high’. A plausible explanation is that the use of adjuvant chemotherapy as an eligibility criterion in this study may have led many patients to consider that their risk, even with stage I, exceeds their actual probability of relapse. This perception is far from trivial, as it is projected on health status for at least the following six months and, therefore, has an identifiable clinical impact. Nonetheless, the reader must be cognizant of the subtlety that the woman was asked with respect to her subjective perception and not about her quantitative prognosis. Be that as it may, it is not clear that there is a component of misunderstanding or insufficient explanation.
Fourthly, the Bayesian formulation enables gradual results and actionable probabilities to be obtained. Thus, constrained by the small sample size, the evidence is limited for ALND or TNM stage III tumors, although the model suggests that a possible negative effect of these factors is highly probable. Depending on the external context, clinicians must determine the degree to which the differences in probability are clinically relevant in each case. This makes the Bayesian approach an appealing, pragmatic method. In this line, Spiegelhalter et al. have proposed that the post hoc Bayesian interpretation be routinely used in clinical trial analyses26. The Bayesian CPPO model brings together the requirements to meet this function in terms of interpreting gradual QoL outcomes. Fifthly, in addition to causal inference, the complete Bayesian model can be used for patient profile-based predictions. Thus, the tool enables nomograms or online calculators to be built, factoring in the aforementioned considerations. This allows clinical questions to be answered, such as: What is my probability of achieving a certain level of QoL according to my specific characteristics? On the other hand, the bivariate methods so assiduously applied, are unable to capture these nuances, or do so only partially10.
The web calculator is potentially useful, and its relevance must be put into context. BCS was developed more than 40 years ago as an alternative to modified radical mastectomy (MRM) in T1-2 tumors27,28. Survival following BCS and radiotherapy is comparable to survival following MRM29,30, with better esthetic outcomes. Over time, the indication of BCS was extended to T3 tumors or tumors with node involvement (N + ), after responding to neoadjuvant chemotherapy27,31. Given that survival outcomes with BCS and MRM are commensurate when BSC is indicated, knowing the impact on mid-term QoL acquires special interest in decision making4. Several studies have reported differences in specific QoL domains related to type of surgery32,33,34,35,36. A recent meta-analysis confirmed that BCS improved certain aspects of QoL (e.g., body image, future perspectives, or treatment sequelae), but not all of them10, requiring more prospective studies to elucidate the impact37. After half a century of clinical research in this field, it is striking that important aspects such as the modification of the effect on QoL according to age, or individualized prediction, are still seldom explored10.
Our study has various limitations. First of all, the specific EORTC module for women with breast cancer, the QLQ-BR23, was not administered38. The use of the global health status detects generic changes and accounts for three quarters of the variability of the complete score, but may be insensitive to certain aspects. Secondly, QoL is a dynamic construct that varies based on time since interventions. However, some trajectories are stable according to individual clinical profile24. Here, the response variable was the health status evaluated at the end of systemic chemotherapy since this is the only post-baseline timepoint contemplated. The QoL recorded prior to chemotherapy was used as a covariate (baseline measurement taken as the starting point). However, it is plausible that the progressive disappearance of adverse effects would accentuate the differences between BCS and mastectomy at a later stage of evolution (for instance, 2 years). Therefore, assessing QoL at several timepoints, over a minimum of 1–2 years of follow-up might be more relevant. If more measures were available, the CPPO model allows random effects from longitudinal data to be integrated. Moreover, the reader should be aware that baseline QoL scores were obtained post surgery and may be worse than pre-surgery scores. As in other studies, NEOCOPING has questionnaires that were not completed at the 6-month timepoint. Nonetheless, the clinical scenario is adjuvancy, and most are confirmed missing at random and not due to clinical decline. In any case, the library makes it possible to work with multiple imputations of these data, if necessary22. Thirdly, other clinical elements that can affect QoL, such as early breast reconstruction or radiotherapy, have not been completed. These factors do indeed modulate QoL in some patients. Other uncommon variables or those not supported by the model, such as level of education, occupation, marital status, or specific comorbidities, might also be relevant for some patients. Fourth, the model was causally specified to explore the effect of surgery; consequently, the interpretation of the remaining effect estimates, including ‘secondary exposures’ such as perceived risk of recurrence, can be challenging39. Fifth, the self-report subjective measures may have limitations, such as response bias (social desirability, inaccurate memory, etc.) and difficulty in fully comprehending the questionnaire. Finally, the model must be independently and prospectively validated for its clinical implementation.
Bearing these limitations in mind, the conditions of applicability must always be verified. Consequently, the conclusions are generalizable to women who have undergone surgery for breast cancer for whom adjuvant chemotherapy initiation is being contemplated. Despite the fact that this limits the sphere of application of the model and the gradual decrease in chemotherapy notwithstanding thanks to predictive genomic tests, chemotherapy continues to be part of many women’s experience of cancer (e.g., luminal breast cancer in premenopausal or postmenopausal women with positive nodes and high-risk negative nodes, as well as in most triple-negative or HER2-positive tumors)40. One could speculate that the differences between mastectomy and BCS might be different in women without chemotherapy, given that the impact of surgical sequelae and changes in self-image would not have been diluted by the full burden of post-chemotherapy side effects.
As for the generalizability of the method itself, the Bayesian CPPO model comprises a new, extremely versatile, and flexible tool to investigate QoL associated with cancer in multiple scenarios, facilitating the obtention of rich, individualized descriptions of patients’ evolution.
In short, we have fit a Bayesian ordinal model using software programmed for COVID-19 trials to illustrate its usefulness in analyzing QoL in oncological patients. The model makes it possible to overcome certain issues associated with QoL analyses with assumable complexity and accurately capture the main factors that affect global health status (type of surgery, interaction with age, or perceived risk of recurrence). The study demonstrates the feasibility of post hoc Bayesian analysis with QoL data that can be implemented in clinical trials. This Bayesian model is also a potentially useful tool in making decisions grounded in the foreseeable evolution of QoL, when facing therapies of comparable benefit.
Methods
Patients and study design
The data are from a prospective cohort of individuals with early and locally advanced breast cancer from the NEOCOPING multicohort study. This study was promoted by the Continuous Care Group of the Spanish Society of Medical Oncology (SEOM, for its acronym in Spanish) and was conducted at 17 Spanish hospitals between 2016 and 2019 (Supplementary Table 1)41,42,43,44,45. The participating centers are tertiary university hospitals distributed all over Spain. The participants had undergone surgery with curative intent for nonadvanced cancers for which clinical guidelines report adjuvant chemotherapy as a valid alternative. The study sought to explore biopsychosocial and pathological aspects that affected the quality of life, coping, and oncologist–patient communication.
For this analysis, women ≥18 years were chosen, with a histologically confirmed diagnosis of breast cancer, stages TNM I–III, and indication of adjuvant chemotherapy. All participants were recruited during the interval between surgery and initiation of adjuvant chemotherapy. The patients included were those who consulted with Medical Oncology and were selected consecutively and prospectively by the medical oncologist. Exclusion criteria included receiving neoadjuvant therapy and scheduled to receive adjuvant hormone therapy alone or radiotherapy without chemotherapy.
Ethical statement
This study was performed in accordance with the ethical standards of the Declaration of Helsinki and its subsequent amendments. This observational, noninterventional trial was approved by the Research Ethics Committee of the Principality of Asturias (19 January 2015) and by the Spanish Agency of Medicines and Medical Devices (AEMPS) (number: L34LM-MM2GH-Y925U-RJDHQ).
Informed consent statement
All subjects signed informed consent forms and agreed to participate prior to completing baseline questionnaires.
Consent for publication
Informed consent and approval by the competent national authorities includes permission for publication and dissemination of the data.
Measures and variables
Participants completed the self-report European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ-C30) scale46. This instrument can be downloaded in English and Spanish at https://qol.eortc.org/. This questionnaire is a cancer-specific QOL instrument, psychometrically validated for most tumors, and the most widely used tool to quantify cancer patients’ QoL38,47,48. It is a 30-item, self-report questionnaire that covers five QoL dimensions: physical (5 items), role (2), emotional (4), social (2), and cognitive (2); an overall health status assessment (2), and the following specific symptoms: pain (2), fatigue (3), and nausea and vomiting (2 items). It contains six items to appraise financial impact, as well as other symptoms (such as constipation, sleep problems, hyporexia, etc.). The score is expressed on a scale of 0 to 100 (the higher the score, the better the QoL or more symptoms according to the item)49 and takes less than 15 min to complete50. The reliability of these scales denotes suitable internal consistency (Cronbach’s alpha ≈0.67–0.92)51. The information from the questionnaire was agglutinated into a sum score and evaluated in line with the scoring manual recommended by the EORTC QoL Group49,52. The scale has been translated and validated in Spanish53.
Since the questionnaire has items that are prima facie scantly applicable to subjects with few symptoms (e.g., diarrhea, nausea, fatigue…), the calculator uses the global health status (average of items 29 and 30) at 6 months as the response variable. Health status correlated closely to the EORTC sum score (Spearman, ρ = 0.88) and accounted for 75% of its variability by means of only two items: “How would you rate your overall health during the past week?” and “How would you rate your overall quality of life during the past week?”.
When selecting covariates, the limiting sample size was contemplated to evaluate an ordinal response variable, as well as the need to nonlinearly model some variables54. Taking into account the maximum number of parameters the model can support, the covariates were selected after a bibliographic review and at the researchers’ discretion. These predictors were age (continuous, nonlinear), TNM classification of malignant tumors, 8th edition (stage I, II, III), patient’s perceived risk of recurrence (4-point Likert scale: low/intermediate/high/very high risk), type of surgery on the primary tumor (total mastectomy vs BCS), axillary surgery (axillary lymph node dissection (ALND) [yes vs no]), the pre-chemotherapy EORTC QLQ-C30 sum score, and planned chemotherapy regimen (taxane-containing regimens and use of anthracyclines). Other clinical or sociodemographic variables (e.g., social status, number of children, etc.) were considered for descriptive purposes.
Procedures
The QoL evaluations were performed twice, once after the first appointment with the oncologist, approximately 1 month following surgery for the primary tumor and 1 week prior to initiating adjuvant therapy, and again, during the month following completion of adjuvant chemotherapy, some 6 months after initiation and prior to undertaking adjuvant radiotherapy/endocrine therapy when necessary. Baseline questionnaires were completed by the participants themselves after their visit to the oncologist, during which they were informed about the risk of relapse and indication of adjuvant therapy.
Statistical analysis
The rmsb package enables Bayesian CPPO models to be fitted. Vague priors (noninformative) were used here for coefficients. As the response variable, the health status endpoint is an ordinal variable with 13 levels, scaled from 0-100. The CPPO model determines the probability that the participants would have a health status Y ≥ j, with j being each of the 13 levels. The PO assumption was assessed separately for each predictor computing logits for each proportion of the form Y ≥ j, with j being the thresholds for the ordinal response variable. When the PO is maintained, the difference of logits between various j values should be similar for different levels of the predictor54. Bearing this in mind, we fitted a CPPO model as per the formulation proposed by Peterson & Harrell15. Here, this model assumes PO for all variables, except for age and baseline EORTC QLQ-C30 sum score. Nonlinear relations were assessed by means of restricted cubic splines (age) or adding quadratic terms to the equation (baseline EORTC QLQ-C30 summary score). Moreover, the model contemplates the interaction between surgery and age. We run a Markov chain Monte Carlo (MCMC) method with 4 chains, 2000 iterations, and a burn-in of 1000 samples, in each one. The c-index and the Brier score have been used as measures of model performance. For comparison between alternative models (e.g., with or without the covariate ‘anthracyclines’), we used the widely applicable information criterion (WAIC) for parsimony55. Odds ratios (OR) < 1 indicate worse QoL scores in the presence of a binary variable. In the variables that deviate from the PO assumption, the model imposes a linear restriction on the coefficients (e.g., the variable has a different OR for each QoL score cutoff, but all of them are linearly related). In contrast, those variables that do meet the proportional odds assumption have a single odds ratio that is applicable to the entire range of possible cutoffs. To illustrate these concepts, the prediction has been visually depicted for the different levels, odds ratios, and partial effects, and it has been contrasted with the evaluation of average effects stratified for a single variable. The global health status predictive model was depicted graphically by a web calculator programmed in .NET and R.
For comparative purposes, a frequentist PO model and a multinomial model were also fitted. QoL scores according to type of surgery were also compared by means of two-samples Wilcoxon tests (α = 0.05, two-tailed tests).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data generated and analyzed during this study are described in the following data record: https://doi.org/10.6084/m9.figshare.1468127456. The breast cancer quality of life datasets are openly available as part of the data record in the files ‘breast_cppo.xlsx’ (underlying Figs. 2–4, Tables 1–2, Supp Figs. 2–8, Supp Tab 2 of the related article), ‘breast_scores.xlsx’ (underlying Fig. 1) and ‘breast_qlq_c30.xlsx’ (underlying Supp Tab 3).
References
Bottomley, A., Jones, D. & Claassens, L. Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency. Eur. J. Cancer 45, 347–353 (2009).
Bonnetain, F. et al. How health-related quality of life assessment should be used in advanced colorectal cancer clinical trials. Ann. Oncol. 28, 2077–2085 (2017).
Cherny, N. I. et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann. Oncol. 28, 2340–2366 (2017).
Fehlauer, F., Tribius, S., Mehnert, A. & Rades, D. Health-related quality of life in long term breast cancer survivors treated with breast conserving therapy: impact of age at therapy. Breast Cancer Res. Treat. 92, 217–222 (2005).
Lasry, J. M. & Margolese, R. G. Fear of recurrence, breast‐conserving surgery, and the trade‐off hypothesis. Cancer 69, 2111–2115 (1992).
Kyte, D. G. et al. Patient reported outcomes (PROs) in clinical trials: is ‘in-trial’guidance lacking? a systematic review. PLoS ONE 8, e60684 (2013).
Smith, A. B., Cocks, K., Parry, D. & Taylor, M. Reporting of health-related quality of life (HRQOL) data in oncology trials: a comparison of the European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy-General (FACT-G). Qual. Life Res. 23, 971–976 (2014).
Anota, A. et al. Comparison of three longitudinal analysis models for the health-related quality of life in oncology: a simulation study. Health Qual. Life Outcomes 12, 192 (2014).
Barbieri, A. et al. Item response models for the longitudinal analysis of health-related quality of life in cancer clinical trials. BMC Med. Res. Methodol. 17, 148 (2017).
Ng, E. T. et al. Comparing quality of life in breast cancer patients who underwent mastectomy versus breast-conserving surgery: a meta-analysis. Int. J. Environ. Res. Public Health 16, 4970 (2019).
Kahler, E., Rogausch, A., Brunner, E. & Himmel, W. A parametric analysis of ordinal quality-of-life data can lead to erroneous results. J. Clin. Epidemiol. 61, 475–480 (2008).
Abreu, M. N. S., Siqueira, A. L., Cardoso, C. S. & Caiaffa, W. T. Ordinal logistic regression models: application in quality of life studies. Cad. Saude Publica 24, s581–s591 (2008).
Lall, R., Campbell, M. J., Walters, S. J., Morgan, K. & Co-operative, M. R. C. C. A review of ordinal regression models applied on health-related quality of life assessments. Stat. Methods Med. Res. 11, 49–67 (2002).
McCullagh, P. Regression models for ordinal data. J. R. Stat. Soc. Ser. B 42, 109–127 (1980).
Peterson, B. & Harrell, F. E.Jr Partial proportional odds models for ordinal response variables. J. R. Stat. Soc. Ser. C Appl. Stat. 39, 205–217 (1990).
Fullerton, A. S. & Xu, J. The proportional odds with partial proportionality constraints model for ordinal response variables. Soc. Sci. Res. 41, 182–198 (2012).
Espinosa, J. & Hennig, C. A constrained regression model for an ordinal response with ordinal predictors. Stat. Comput. 29, 869–890 (2019).
Smith, A. W. et al. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol. Prev. Biomark. 18, 656–663 (2009).
Self, W. H. et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA 324, 2165–2176 (2020).
Beigel, J. H. et al. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir. Med. 5, 500–511 (2017).
Scott, S. C., Goldberg, M. S. & Mayo, N. E. Statistical assessment of ordinal outcomes in comparative studies. J. Clin. Epidemiol. 50, 45–55 (1997).
Harrell, F. E. The R rmbs package. https://hbiostat.org/R/rmsb/ (2021).
Harrell, F. E. rmsb: Bayesian Regression Modeling Strategies. Available at: https://cran.r-project.org/web/packages/rmsb/index.html.
Avis, N. E., Levine, B., Marshall, S. A. & Ip, E. H. Longitudinal examination of symptom profiles among breast cancer survivors. J. Pain. Symptom Manag. 53, 703–710 (2017).
Moyer, A. Psychosocial outcomes of breast-conserving surgery versus mastectomy: a meta-analytic review. Health Psychology. 16, 284–298 (1997).
Spiegelhalter, D. J., Freedman, L. S. & Parmar, M. K. B. Applying Bayesian ideas in drug development and clinical trials. Stat. Med. 12, 1501–1511 (1993).
Veronesi, U. et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 347, 1227–1232 (2002).
Fletcher, S. W. Breast cancer screening: a 35-year perspective. Epidemiol. Rev. 33, 165–175 (2011).
Saadatmand, S., Bretveld, R., Siesling, S. & Tilanus-Linthorst, M. M. A. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173 797 patients. BMJ 351, h4901 (2015).
Hwang, E. S., Lichtensztajn, D. Y., Gomez, S. L., Fowble, B. & Clarke, C. A. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 119, 1402–1411 (2013).
Puig, C. A., Hoskin, T. L., Day, C. N., Habermann, E. B. & Boughey, J. C. National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: a National Cancer Data Base study. Ann. Surg. Oncol. 24, 1242–1250 (2017).
Sun, Y. et al. Comparison of quality of life based on surgical technique in patients with breast cancer. Jpn. J. Clin. Oncol. 44, 22–27 (2014).
Acil, H. & Cavdar, I. Comparison of quality of life of Turkish breast cancer patients receiving breast conserving surgery or modified radical mastectomy. Asian Pac. J. Cancer Prev. 15, 5377–5381 (2014).
Lagendijk, M. et al. Patient reported outcome measures in breast cancer patients. Eur. J. Surg. Oncol. 44, 963–968 (2018).
Jendrian, S. et al. Quality of life in patients with recurrent breast cancer after second breast-conserving therapy in comparison with mastectomy: the German experience. Breast Cancer Res. Treat. 163, 517–526 (2017).
Tsai, H.-Y., Kuo, R. N.-C. & Chung, K. Quality of life of breast cancer survivors following breast-conserving therapy versus mastectomy: a multicenter study in Taiwan. Jpn. J. Clin. Oncol. 47, 909–918 (2017).
Asgeirsson, K. S., Rasheed, T., McCulley, S. J. & Macmillan, R. D. Oncological and cosmetic outcomes of oncoplastic breast conserving surgery. Eur. J. Surg. Oncol. 31, 817–823 (2005).
Tan, M. L. et al. Validation of EORTC QLQ-C30 and QLQ-BR23 questionnaires in the measurement of quality of life of breast cancer patients in Singapore. Asia Pac. J. Oncol. Nurs. 1, 22–32 (2014).
Westreich, D. & Greenland, S. The Table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am. J. Epidemiol. 177, 292–298 (2013).
Sparano, J. A. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 379, 111–121 (2018).
Calderon, C. et al. Incidence of sleep problems and their mediating role on depression and anxious preoccupation in patients with resected, non-advanced cancer: data from NEOcoping study. Clin. Transl. Oncol. 21, 1104–1107 (2019).
Jimenez-Fonseca, P. et al. Factors associated with anxiety and depression in cancer patients prior to initiating adjuvant therapy. Clin. Transl. Oncol. (2018). https://doi.org/10.1007/s12094-018-1873-9 (2018).
Calderon, C. et al. Validation of SDM-Q-Doc Questionnaire to measure shared decision-making physician’s perspective in oncology practice. Clin. Transl. Oncol. https://doi.org/10.1007/s12094-017-1671-9 (2017).
Jimenez-Fonseca, P. et al. The mediating role of spirituality (meaning, peace, faith) between psychological distress and mental adjustment in cancer patients. Support. Care Cancer https://doi.org/10.1007/s00520-017-3969-0 (2017).
Calderón, C. et al. Quality of life, coping, and psychological and physical symptoms after surgery for non-metastatic digestive tract cancer. Surg. Oncol. 31, 26–32 (2019).
Aaronson, N. K. et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 85, 365–376 (1993).
Jiménez-Fonseca, P. et al. Health-related quality of life in well-differentiated metastatic gastroenteropancreatic neuroendocrine tumors. Cancer Metastasis Rev. 34, 381–400 (2015).
Groenvold, M., Klee, M. C., Sprangers, M. A. G. & Aaronson, N. K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 50, 441–450 (1997).
Fayers, P., Aaronson, N. K., Bjordal, K. & Sullivan, M. EORTC QLQ–C30 Scoring Manual (European Organisation for Research and Treatment of Cancer, 1995).
Bredart, A. et al. An international prospective study of the EORTC cancer in-patient satisfaction with care measure (EORTC IN-PATSAT32). Eur. J. Cancer 41, 2120–2131 (2005).
Kaasa, S. et al. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur. J. Cancer 31, 2260–2263 (1995).
Giesinger, J. M. et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 69, 79–88 (2016).
Cerezo, O. et al. Validation of the Mexican‐Spanish version of the EORTC QLQ‐C30 and BR23 questionnaires to assess health‐related quality of life in Mexican women with breast cancer. Eur. J. Cancer Care 21, 684–691 (2012).
Harrell, F. Regression Modeling Strategies: with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
Carmona-Bayonas, A. et al. Metadata Record for the Article: prediction of Quality of Life in Early Breast Cancer upon Completion of Adjuvant Chemotherapy. Figshare https://doi.org/10.6084/m9.figshare.14681274 (2021).
R Core Team. R: a Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014).
Harrell Jr, F., Frank, E. & Maintaner Frank, E. Package ‘rms’. 229 (2015). http://cran.r-project.org/web/packages/rms/index.html (2020)
Custodio, A. et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br. J. Cancer 116, 1526–1535 (2017).
Acknowledgements
This study was supported by the FSEOM-Onvida for Projects on Long Survivors and Quality of Life, SEOM (Spanish Society of Medical Oncology) 2015 grant. The sponsor of this research has not participated in data collection, analysis, or interpretation, in writing the report, or in the decision to submit the article for publication.
The authors would like to thank the investigators of the NEOcoping study, the Supportive Care Working Group of the Spanish Society of Medical Oncology (SEOM), Priscilla Chase Duran for editing the manuscript and Natalia Cateriano, Miguel Vaquero, IRICOM S.A. for supporting the registry website. Gustavo Reporte has helped in the interpretation of the mathematics of the CPPO model.
Author information
Authors and Affiliations
Contributions
A.C.B., C.C. and P.J.F. developed the project, analyzed the data and drafted the manuscript. The other authors recruited patients and provided clinical information, comments, and improvements to the manuscript. All authors participated in the interpretation and discussion of data, and the critical review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carmona-Bayonas, A., Calderón, C., Hernández, R. et al. Prediction of quality of life in early breast cancer upon completion of adjuvant chemotherapy. npj Breast Cancer 7, 92 (2021). https://doi.org/10.1038/s41523-021-00296-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00296-8