Abstract
Reversal or mitigation against an age-related decline in NAD+ has likely benefits, and this premise has driven academic and commercial endeavour to develop dietary supplements that achieve this outcome. We used a systems-based approach to improve on current supplements by targeting multiple points in the NAD+ salvage pathway. In a double-blind, randomised, crossover trial, the supplement – Nuchido TIME+® (NT) - increased NAD+ concentration in whole blood. This was associated with an increase in SIRT1 and an increase in nicotinamide phosphoribosyltransferase (NAMPT) in peripheral blood mononucleocytes, lower concentrations of pro-inflammatory cytokines in plasma, including a reduction in interleukin 2 (IL2), a reduction in glycated serum protein and a shift in the glycosylation profile of immunoglobulin G (IgG) toward a younger biological age, all of which are likely to promote a healthier ageing trajectory.
Similar content being viewed by others
Introduction
Changes in many interrelated cellular processes and metabolic functions contribute to ageing. The key changes identified as underpinning cellular ageing are collectively known as the hallmarks of ageing1. Cellular senescence, mitochondrial dysfunction and deregulated nutrient sensing are among those studied most intensively, and chronically low NAD+ (nicotinamide adenine dinucleotide) has been identified as a central mediator of multiple hallmarks of ageing2. Interventions to prevent or reverse ageing-related changes in these variables, processes and pathways, or to stimulate counteracting cellular pathways, are made with the aim to slow or reverse the process of ageing and thus increase years of healthy life.
NAD+ is an attractive target point of intervention because, as the conduit of reducing power between the fundamental metabolic pathways of glycolysis, the TCA cycle and the mitochondrial electron transport chain, it plays a central role in the generation of cellular energy (ATP). Also, a sizable body of data provides evidence for an age-related decline in NAD+ levels in tissues including plasma and muscle, though this is not a universally consistent observation (reviewed in ref. 3). Also, NAD+ depletion is a feature of some diseases of accelerated ageing, including Ataxia Telangiectasia (AT), Xeroderma Pigmentosum group A (XPA), and Cockayne Syndrome (CS) (reviewed in ref. 4). NAD+ is also a cofactor for a number of enzymes, including enzymes with functions that impinge on cellular processes that have a role in ageing. Among these, the sirtuins are of likely particular importance. These enzymes, of which there are seven human members, are a family of deacylases and ADP-ribosyltransferases. SIRT1, the first named of the mammalian sirtuin family and the most extensively studied, catalyses the deacetylation of protein substrates at lysine residues in a reaction in which NAD+ is cleaved to release nicotinamide (NAM; see ref. 5 for recent review), and, like some other members of the family, catalyses the deacetylation of a range of substrates that have functions in a myriad of processes that impinge on ageing (see ref. 6 for a recent review). These include the transcription factor PGC1α (peroxisome proliferator-activated receptor-γ coactivator), which is activated as a result to stimulate mitochondrial biogenesis among other cellular processes, members of the FOXO family of transcription factors, resulting in downstream beneficial effects on glucose metabolism and insulin signalling, and NF-κβ, with consequent tempering of pro-inflammatory pathways7.
Among other enzymes that cleave NAD+ and appear to affect cellular ageing either directly or as a consequence of the knock-on effects of its consumption (for example reduced sirtuin action) are the PARPs (poly ADP-ribose polymerases) and CD38 (cluster of differentiation 38). PARP action involves cleavage of NAD+ at the N-glycosidic bond to generate ADP ribose. This is then added as a monomer or as a polymerised chain to proteins involved in a number of cellular functions, which include the response to DNA damage by base excision repair of single strand breaks8. PARP activity has been associated correlatively with longer lifespan and slower ageing, which is likely attributable in part to this role in the DNA damage response and thus to genome stability (e.g., ref. 9). However, PARP activity is something of a double-edged sword because the consumption of NAD+ reduces availability for the generation of ATP and for the action of enzymes, including the sirtuins, that afford protection against ageing.
CD38 has been described as the principal NAD+ hydrolase in the cell. It has been shown to have multiple functions, among which is a role in cell signalling by generating, from NAD+, the second messenger molecule, cyclic ADP ribose (cADPR). cADPR plays a role in intracellular Ca2+ signalling through activation of ryanodine receptors to mobilise Ca2+ from the endoplasmic reticulum and also has a role in multiple aspects of the inflammatory response10. The protection against features of ageing afforded by the pharmacological inhibition or knockout of CD38 in mice, such as protection against obesity11, improved glucose tolerance, muscle function, exercise capacity and cardiac function12 and increased lifespan13, have been attributed to the NAD+-sparing effect of these interventions.
There is currently much interest academically and commercially in approaches to boost NAD+ as a potential anti-ageing therapy. In vivo NAD+ restoration has been investigated extensively and demonstrates whole body benefits, recently reviewed in detail14. The instability and low bioavailability to most cell types of NAD+ render direct oral supplementation inefficacious. Dietary supplements intended to increase NAD+ in human subjects feed in at points in the process of biosynthesis, of which there are three pathways (see ref. 4 for a full description). Of these, the salvage pathway is the dominant pathway. This pathway recycles NAM released by the cleavage of NAD+ through the action of NAD+-consuming enzymes including the sirtuins, PARPs and CD38. Most dietary supplements are based on providing a supply of precursors to the salvage pathway, most commonly NR or NMN (nicotinamide mononucleotide). We summarise the outcomes of 10 we identified, which all provided NR, in a recent review article4. Of these, seven measured NAD+ in blood (whole blood or peripheral blood mononucleocytes (PBMCs)) and three measured NAD+ in skeletal muscle. Significant but variable increases in NAD+ are reported. Modest increases (40–59%) were achieved using the dose of 300 mg/d approved by the FDA and EFSA, with larger increases (more than a doubling of the sample mean or doubling of concentration in the highest responder) requiring doses far in excess (1000–2000 mg/d). Even at these very high doses, the three studies that measured NAD+ in skeletal muscle detected no increase15,16,17. Nine published studies that provided NMN report similarly variable outcomes. Increases in NAD+ in whole blood, plasma, serum or PBMCs were measured in six of these18,19,20,21,22,23, which include a study that achieved a 5.7-fold mean increase after 60 d at 600 mg/d23, but three achieved no increase, despite use of similar doses24,25,26. Factors such as duration of the trial period, participant demographics, physical properties of NR or NMN administered (e.g., particle size and crystalline form), methods of sample collection and NAD+ measurement likely account for some of this variability. However, we argue that a key factor that limits efficacy is likely to be changes to the NAD+-metabolising machinery in the cell that occur as the cell gets older, which themselves curtail the ability of the cell to generate NAD+ from NR, NMN and other precursors or to consume NAD+ at a higher rate. Notably, the enzyme nicotinamide phosphoribosyltransferase (NAMPT), which catalyses the rate-limiting step in the NAD+ salvage pathway, has been shown to decline with age in mice and rats27,28,29,30,31,32,33, as well as humans27,34. There is good evidence that CD38, the major cellular NAD+ hydrolase and mediator of various aspects of the inflammatory response, becomes overexpressed during ageing, accompanying the high level of inflammation frequently reported in older tissues (referred to as ‘inflammaging’)35,36. Increased expression of the methylating enzyme nicotinamide-N-methyltransferase (NNMT) also leads to the methylation and excretion of NAM as methyl nicotinamide (MeNAM) further limiting the recycling of NAM to NAD+ by the salvage pathway37. We argue that a significant limitation of simple, single precursor supplements is that, while providing a supply of substrate into the salvage pathway, this cannot be optimally converted to NAD+ because of the aforementioned issues that prevent optimal NAD+ conversion and usage. Figure 1a shows the age-related changes to the NAD+ salvage pathway and enzymes that contribute to depletion of NAD+. Thus, we took a systems approach to administer participants with a supplement (NT) targeting multiple enzymes involved in NAD+ metabolism whilst also providing NAD+ precursor (Fig. 1b). Table 1 lists the components of NT and specifies the basis (intended action) for including them in the supplement38,39,40,41,42,43,44,45,46,47,48,49,50.
a shows impairment of the pathway by age-associated changes. b shows points of redress through a multitarget approach that forms the basis of the NT dietary supplement. a CD38 becomes overexpressed during ageing due to chronic activation from persistent low level ‘inflammaging’. CD38 cleaves NAD+ to produce nicotinamide (NAM). Increased consumption of NAD+ by CD38 leads to an accumulation of cellular NAM. Aged cells exhibit lower levels of NAMPT, the key rate-limiting enzyme for adequate NAD+ recycling from NAM, resulting in reduced NAD+ production and an accumulation of NAM. NNMT is recruited to methylate and excrete excess NAM as methyl nicotinamide (MeNAM). b The NT dietary supplement contains multiple ingredients to target the root causes of NAD+ decline. Rutin and alpha lipoic acid (ALA) act to increase expression of the rate-limiting enzyme NAMPT. ALA induces NAMPT expression indirectly by activating the cellular energy sensor AMPK which is known to increase NAMPT expression. ALA also increases expression of NQO1 which increases NAD+ by oxidation of NADH, pushing the NAD+:NADH ratio in a more favourable direction. Parsley extract contains a high proportion of natural apigenin which acts to inhibit CD38. EGCG acts to inhibit NNMT to promote recycling of NAM into NAD+ by the Salvage pathway rather than its methylation and excretion. NAM is used as an NAD+ precursor as it is the most bioavailable of all the NAD+ precursors.
Results
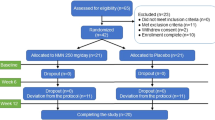
We conducted a double-blind placebo-controlled trial (Fig. 2) to determine if the dietary supplement NT (Nuchido TIME+®) designed using a systems approach to address the root causes of NAD+ decline, was efficacious in increasing the NAD+ concentration in whole blood. We recruited 33 human participants, spread across an age range of 20–80 y, of whom 26 (18 female) completed the study. One drop out, before attending, was due to personal reasons and three failed to attend to provide blood samples. One participant withdrew at the first visit to provide a baseline sample (arm 1) and another withdrew at the beginning of arm 2. We were unable to obtain a blood sample at the beginning of arm 2 for the sixth non-completer. All participants were asked about adverse effects. Very few were reported; two participants reported trouble sleeping when taking NT, and one reported stomach irritation. One participant reported feeling hyperactive on the placebo arm. No other adverse effects were reported.
Toxicity
The activity of alanine aminotransferase, the activity of alkaline phosphatase, and the concentration of albumin in plasma were unaffected by either NT or the placebo (Supplementary Table 1). Thus, we found no indication that the supplement induced any level of hepatic toxicity.
Effect of NT on blood NAD+, NAM and NMN concentration
Figure 3 shows the profile of NAD+ concentration measured using a commercial fluorometric assay in whole blood over each arm of the trial period for each participant. Two-way, repeated measures ANOVA showed a significant main effect of NT with time on NAD+ concentration (P = 0.007), and Bonferroni post-hoc testing showed a significant increase compared with baseline (mean 66.55 ng/ml; SD 36.97) at weeks 2 (mean 82.59 ng/ml; SD 49.56, P = 0.008) and 4 (mean 84.17 ng/ml; SD 54.82, P = 0.029; Fig. 4b). These data show that NT increased NAD+ in whole blood at week 4 by an average (mean) of 26.5%. However, as is seen from Fig. 3, there was considerable inter-individual variability in response size. In the three individuals where the response was greatest, increases of 105%, 73% and 65% were achieved. There was no significant effect of the placebo (Fig. 4a). Analysis of the data at each time point by Student’s paired t-test showed that the concentration of NAD+ in whole blood was significantly greater on NT than on placebo at all points measured (Fig. 4c).
We also measured NAD+ in whole blood, along with NAM and NMN, in 17 of the participants at baseline, 21 days and 28 days by LC–MS. Use of the subsample was a pragmatic measure enforced by the availability of sufficient remaining sample following measurement using the fluorometric assay. These data revealed the same trend (compare Fig. 5a) (placebo) with Fig. 5b (NT) but this did not reach statistical significance when data were analysed by 2-way repeated measures ANOVA. To determine if the results of the two different measurement techniques were discordant, or if the reduced power of the analysis accounted for the loss of a significant effect of NT on NAD+ measured by LC–MS, the equivalent reduced dataset (same 17 participants, baseline, 21 days and 28 days only) obtained using the commercial fluorometric kit were re-analysed by 2-way repeated measures ANOVA. This analysis did not reveal a significant effect of either NT or placebo on NAD+. Thus, the reduced power of the analysis, due to the smaller sample set, may have obscured a true significant effect of NT to increase NAD+ in whole blood, which was detectable with the larger sample. However, at the point of making the measurements by LC–MS, samples had been stored at −80 °C for several months due to interruption of work by the COVID-19 pandemic, meaning that NAD+ degradation may also have a contributory factor. Moreover, as can be seen from Fig. 3, four study participants showed a notably large increase in blood NAD+ concentration across the sampling time points measured using the fluorometric enzymatic assay, and one showed an increase at day 28 that approximated the increase of the other four high responders at this point. Of these participants (numbers 11, 17, 23, 26; 18 (late responder), Supplementary Table 1) only two of the four high responders (numbers 11, 23) plus the late responder (number 18) were included in the group for which measurements of NAD+ were made by LC/MS, which will have influenced the statistical analysis.
Analysis by one-way repeated measures ANOVA showed an effect of NT but not the placebo, on NAM (Fig. 5c, d). This was revealed by Bonferroni post-hoc testing to be a significant (P = 0.016) increase in the mean concentration compared with baseline (mean 186.96 ng/100 μl, SD 27.60) at 21 days (mean 236.88 ng/100 μl; SD 73.22). There was a trend (P = 0.067) towards an increase also at 28 days (mean 214.19 ng/100 μl; SD 50.90). There was no significant change in NMN (Fig. 5e, f).
Effect of NT on expression of selected proteins
Our trial was designed primarily to determine if NT was efficacious in increasing NAD+ in whole blood. However, we measured a number of other secondary outcome variables to explore if there was any evidence of beneficial consequent (or parallel) effects. Thus, we measured SIRT1, NAMPT, PGC1α and CD38 in PBMCs by western blotting at all sample time points in 18–21 participants for each antibody (as determined by sample availability). The four participants who showed the highest increases in whole blood NAD+ concentration (numbers 11, 17, 23, 26; Fig. 3 and Supplementary Table 1), as well as the late responder (number 18), were included in all western blot assays. For these measurements, signal intensities were expressed relative to the signal for ACTB. To enable combination of data across blots, these ratios were then normalised against the mean ratio value for the two baseline measurements. This analysis showed that NT increased SIRT1 significantly (P < 0.05) at 14 and 28 days (Fig. 6a, b) and increased NAMPT (P < 0.05) at 28 days (Fig. 6c, d). We measured no changes in PGC1α or CD38 (Fig. 6e–h). Figure 6I shows examples of blots for each protein antigen. Uncropped blots for SIRT1 and NAMPT, and corresponding ACTB loading controls are provided as Supplementary Figure 2.
a, c, e, g Placebo. b, d, f, h NT. Data are expressed as mean ± SEM; n = 18–21 and are relative to corresponding signal for ACTB and normalised to the measurement at baseline, which was used and is plotted as the mean of the two baseline samples (BL1 and BL2) collected at the same time of day in the week preceding the intervention. Gels for each primary antibody used derive from the same experiment and were processed in parallel. The four participants who showed the highest increases in whole blood NAD+ concentration (numbers 11, 17, 23, 26; Fig. 3 and Supplementary Table 1), as well as the late responder (number 18), were included in all western blot assays. *P < 0.05 by Student’s t-test. i Representative western blots for each antibody, as labelled, with corresponding signals for ACTB.
Effect of NT on expression of cytokines
We also measured a panel of 14 cytokines at baseline and 28 days in the plasma of 16 participants (Fig. 7) and detected a reduction (P < 0.05) in the mean value compared with baseline in IL2 after 28 days on NT. Lower mean values at 28 days on NT compared with baseline for IL4, IL5 and IL23 were also close to reaching statistical significance (P = 0.055, 0.079 and 0.073, respectively). In contrast, there were no changes in any of the cytokines measured after 28 days on the placebo (P > 0.28 for every cytokine). The four participants who showed the highest increases in whole blood NAD+ concentration (numbers 11, 17, 23, 26; Fig. 3 and Supplementary Table 1), as well as the late responder (number 18), were included in the group of participants in whose samples we measured cytokines.
a IL2; b IL4; c IL5; d IL23. Data are expressed as mean ± SEM as relative measures of assay signal (fluorescence intensity); n = 16. The four participants who showed the highest increases in whole blood NAD+ concentration (numbers 11, 17, 23, 26; Fig. 3 and Supplementary Table 1), as well as the late responder (number 18), were included in all *P < 0.05 by Student’s paired t-test.
Effect of NT on biological age
Measurement of changes to IgG glycosylation patterns, as an indicator of ‘biological age’, revealed an average difference between baseline and 28 days of +0.36 y on placebo and −1.26 y on NT (Fig. 8a).
a Changes to IgG glycosylation profiles measured then extrapolated as ‘biological age’ through commercial service (GlycanAge, UK) expressed as difference from baseline at 28 d; mean with 95% CI (n = 24). b Serum protein glycation (Jinfiniti, Augusta, USA) at baseline and 28 d expressed as mean ± SEM (n = 24). *P < 0.05 by Student’s t-test.
Effect of NT on serum protein glycation
Glycation of serum protein was also measured and found to be reduced on NT, but not placebo (P < 0.05) (Fig. 8b).
Discussion
We aimed to improve on current approaches to increasing systemic NAD+ levels by formulating a dietary supplement (NT) based on targeting multiple points in the network of NAD+ metabolism. We provided the substrate for NAD+ synthesis though the salvage pathway as NAM, a compound present in a normal diet. Uniquely, we aimed to overcome limitations with current approaches that fail to address the root causes of NAD+ decline, namely a reported age-related decline in NAMPT, the rate-limiting enzyme essential for endogenous NAD+ recycling4. Our approach to achieving this was to include in NT alpha lipoic acid and rutin, both of which have been reported to activate NAMPT38,47. Significantly, a measured increase in the expression of NAMPT in PBMCs of participants while taking NT, but not placebo, demonstrated the efficacy of the supplement to achieve this key, target outcome.
Our trial was afforded particular statistical power by being of crossover design. Compared with placebo, NT increased NAD+ concentration in whole blood at each of 4 weekly sampling time points. Also, changes in established ageing-related markers, including SIRT1 protein in PBMCs and pro-inflammatory cytokines in plasma1,51 in a direction favourable to slower ageing were observed in parallel.
Participants in this study spanned an age range of 21–72 y. While not designed to test an effect of age on response, we nonetheless analysed the data for any evidence that age had any effect on the increase in whole blood NAD+ concentration achieved. We found no statistically significant correlation between participant age and the size of the increase in NAD+ in response to NT (R = 1.64; p = 0.229 by Spearman’s rank correlation). Also, analysis by Student’s unpaired t-test does not show that the age profile of the 4 participants who showed the biggest increases in whole blood NAD+ concentration (40 y, 59 y, 61 y, 66 y -mean 56.5 ± 11.4 years (mean ± SD)) differs significantly from that of the other participants (46 ± 15.0 years (mean ± SD)) (p = 0.20).
Variations between studies in daily precursor dose and other features mean that direct comparison with increases achieved, or not, in other studies - which have yielded inconsistent results - has limitations.
To further increase efficacy through counteracting age-related changes in NAD+ metabolism in the cell, we included agents aimed to simultaneously reduce NAD+ breakdown by the NAD+ hydrolase CD38. The observed reduction in pro-inflammatory cytokines would be commensurate with a reduction in CD38 activity, as CD38 has been shown to facilitate signalling pathways that lead to the production of pro-inflammatory cytokines52. NT includes parsley leaf extract as a natural source of apigenin, a known inhibitor of CD3849, with the aim of achieving this benefit. An alternative or additional contributory factor to the reduction in pro-inflammatory cytokines could be increased SIRT1 activity, which has been shown to have this effect53.
After taking NT, there was also an average reduction in ‘biological age’ across the study participants of 1.26 y, measured as a change to IgG glycosylation patterns, which has been identified as a biomarker and developed into a commercial assay by GlycanAge. In large population cohorts, changes to the IgG glycome have been found to be a highly effective indicator of an individual’s biological age, lifestyle and risk of age-related disease54,55. Furthermore, the plasticity of IgG glycosylation makes this a biomarker that is responsive to intervention.
The measured reduction in serum protein glycation is also indicative of an improvement in glucose tolerance elicited by NT. Low NAD+ is associated with metabolic dysfunction, which can be attenuated by an NAD+-dependent increase in SIRT1 activity56. Indeed, SIRT1 activation is a major target of interest for diabetic neuropathy, which is associated with high levels of serum protein glycation57,58.
In addition to the primary reason for which components of the supplement were selected - reported action at specific points in the cellular processes that affect NAD+ levels - some also have antioxidant and other actions that could potentially augment any efficacy of the supplement to slow the ageing process. Specifically, the catechins, included primarily as inhibitors of NNMT, are powerful antioxidants59, as is the polyphenol troxerutin60, which was incorporated with the aim that its reported action to increase NAMPT expression would stimulate the salvage pathway of NAD+ biosynthesis47,48. Moreover, an added potential, incidental benefit of deriving apigenin, EGCG and quercetin - intended to influence cellular NAD+ content - from the natural extracts of parsley leaf, green tea leaf and rutin respectively (included in the supplement as a mixed ‘botanical blend’ of extracts), is that these are also rich in additional natural antioxidants and polyphenols. Further potential benefits of these components include stimulation of the removal of senescent cells (senolysis) by quercetin61, and suppression by rutin of inflammation associated with cellular senescence (senomorphic activity)62.
Comparison of baseline NAD+ concentration in blood before each arm of the trial in those participants randomised to take NT in arm 1 and placebo in arm 2 did not reveal a statistically significant difference, indicating that the 28-day washout period was sufficient and also that the elevation achieved was transient. However, this does not exclude the possibility that other effects may persist - in particular epigenetic effects as a result of the activation of sirtuins leading to histone deacetylation. It would be of interest in further work to measure such potential epigenetic changes.
Further to the increase in SIRT1, reduction in pro-inflammatory cytokines and favourable changes to serum protein glycation and biological age observed in the current study, we aim in future work to investigate if these changes and the increase in NAD+ we measured translate to more tangible physiological changes of more obvious benefit as individuals get older. In this work, we will focus in particular on the skin as there is growing evidence that NAD+ plays a critical role in the biology of skin ageing63. Key variables to measure will be skin appearance, including levels of wrinkling, pigmentation, and elasticity. Although arguably of cosmetic importance, the changes in these measures that accompany ageing are reflective of a decline in barrier function, thinning of the dermis and epidermis, and an increase in fragility and susceptibility to sunburn. Maintenance of barrier function is important to reduce water loss and in thermoregulation. Skin resilience to physical damage from trauma and sunburn is important to reduce the likelihood of skin injury, which in older people particularly can lead to infection. Moreover, barrier immunity is reduced in aged skin, which in part appears to contribute to the increase in skin cancer observed in older people64.
Limitations of the study in relation to determining efficacy of NT to increase NAD+ in blood include that a single sample was taken from each participant to make each of the two baseline measurements and at each of the four weekly visits during each arm of the intervention. NAD+ concentrations show circadian fluctuation65, and it was not pragmatic to take samples from every participant at the same point in the day. Thus, there is likely to have been a level of noise in the data relating to this variability. However, our crossover design afforded the opportunity to counteract this variability with regard to statistical analysis, to an extent, by having each participant attend to provide samples at times in the day as closely aligned as possible for that individual. Another limitation is the relatively short treatment period. It would be optimal to measure the effects of NT over many months, and whether the increases in NAD+ and secondary outcomes (cytokine profile, protein glycation, IgG glycosylation) are sustained, enhanced or reduced during long-term supplementation. For the purpose of this study however it was not practical to conduct the study over such a long period.
In summary, this intervention study demonstrates efficacy of a dietary supplement designed using a systems approach to address the root causes of NAD+ decline to increase systemic NAD+ levels and indicates that co-administered components of the supplement contribute to this positive effect. Furthermore, we report a positive change in multiple healthy-ageing biomarkers (besides NAD+) in response to an NAD+ targeting supplement in a human cohort. Hence, targeting multiple points in the complex network of NAD+ metabolism represents a promising strategy for the new development of new ‘healthy-ageing’ interventions.
Methods
Human intervention study
Ethical approval for the study – a double-blind, randomised crossover trial – was granted by the Northumbria University Research Ethics Committee (reference 16845). All subjects recruited provided written informed consent to participate in the study.
Thirty-three human participants, age 20–80 y (8 male), were recruited in September 2019. Measurements of NAD+ in whole blood after the consumption of NT by 2 human subjects in a preliminary test gave an average effect size of 1.3 SD of mean, detection of which at 80% power and P < 0.05 (2 tailed) would require 12 participants, and for which the 33 participants provided 99% power.
Volunteers were excluded from participation on the basis of meeting any of the following criteria: Regular consumption of supplements containing niacin (vitamin B3), niacinamide nicotinamide riboside or nicotinamide mononucleotide; Food allergies or sensitivities; Currently taking blood pressure medication; Currently taking blood thinning medication (e.g. aspirin, warfarin, heparin); History or current diagnosis of drug/alcohol abuse; History of kidney or liver disease, or other severe diseases of the gastrointestinal; History of neurological or psychiatric illness (excluding depressive illness and anxiety); Blood disorders (e.g., anaemia, haemophilia, thrombocytosis); Heart disorder, or vascular illness; Chronic gastrointestinal problems (e.g., Inflammatory Bowel Disease, Irritable Bowel Syndrome, coeliac disease); Any known active infection; Diagnosed with or may be at risk of having syphilis, hepatitis, the Human T-lymphotropic virus, the Human Immunodeficiency Virus or any other infectious blood-borne disease; Health condition that would prevent fulfilment of the study requirements; Currently participating in or in the past 3 weeks participated in other clinical or nutrition intervention studies. For each arm of the trial, each participant attended on 2 separate occasions at the same time of day in the week preceding administration of the test intervention to provide a baseline sample of blood. NT or placebo (microcrystalline cellulose) was taken as a daily dose of 6 capsules – 3 with breakfast and 3 with lunch - for a period of 28 days. Participants were asked to keep their weekly diet and sleep patterns as constant as possible and to avoid any drinks or supplements containing niacin, nicotinamide or nicotinamide riboside. Participants attended on days 7, 14, 21 and 28 of the intervention period to provide a blood sample, collected as for the baseline samples. Each arm of the trial was separated by a washout period of 28 days. Figure 2 shows the study design.
The coding used to mark the active supplement (NT) and placebo (microcrystalline cellulose) was revealed only after the intervention trial, measurement of samples and statistical analysis was complete.
Blood collection, preparation and storage
Blood (2 × 8 ml) was collected via venepuncture from the basilic, cephalic or median cubital vein into a vacutainer (Sodium heparin, BD), which was inverted 2–3 times then placed on ice. Two millilitres of whole blood was then immediately transferred to storage at −80 °C in a cryovial and PBMCs and plasma were prepared from the remainder of the sample. For this, all steps were performed on ice, except centrifugation (3 °C) and completed within 1 h of blood collection. The sample was diluted 3-fold in 2% FBS/1 × PBS then added on top of 15 ml Lymphoprep™ (STEM CELL Technologies) in a 50 ml Falcon tube. This was then centrifuged at 1000 × g, for 12 min at 3 °C. The upper plasma layer was then removed using a Pasteur pipette, transferred to 3 cryovials (~2 ml each) and stored at −80 °C. The buffy layer of PBMCs was pipetted into a 15 ml Falcon tube and washed with ice-cold 2% FBS/1× PBS by filling the tube then collecting the pellet generated by centrifugation at 500 × g for 8 min at 3 °C. The supernatant was discarded and the PBMC pellet was resuspended in 500 μl of ice-cold 1 × PBS, aliquoted equally into 3x cryovials and stored in liquid nitrogen.
Measurement of markers of hepatic and renal function
The activity of alanine aminotransferase and alkaline phosphatase and the concentration of albumin was measured in an aliquot of all plasma samples to indicate liver and kidney function by Jinfiniti Precision Medicine, Augusta, USA.
Measurement of NAD+ and selected, related metabolites
NAD+ in whole blood was measured using 2 techniques – a commercial kit that detects total NAD+ plus NADH as a luminescent signal as luciferin is generated from pro-luciferin by an NADH-dependent reaction (NAD+/NADH-Glo Assay, Promega), and also, along with nicotinamide mononucleotide (NMN) and nicotinamide (NAM), by LC–MS. Measurement using the NAD+/NADH-Glo Assay was in accordance with the manufacturer’s instructions. Measurement by LC–MS was through a commercial service provided by Northumbria University, Newcastle upon Tyne, UK. Samples were prepared by addition of 13C tryptophan as an internal standard to 100 μl whole blood, followed by 700 μl 2:1 chloroform/methanol and, after sonication on ice, 400 μl LC/MS grade water. Following further sonication on ice, the upper layer separated by centrifugation was dried down under vacuum and reconstituted in 75 μl LC/MS grade water then filtered through a 0.22 μm column. Separation by LC was on a Waters Acquity UPLC BEH amide column (2.1 × 150 mm with particle size of 1.7 μm) at 65 °C using a binary buffer system: 95%/5% (LC/MS grade water/acetonitrile) and 90/10% (acetonitrile/water). MS data were acquired using a Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific) with orbitrap detector operating parameters of: mass resolution 30 K; mass scan range 100–700 m/z; RF lens 35%; normalised AGC target 25% (100% = 3e6); intensity threshold 2e4. All data were acquired in profile mode.
Western blotting
Western blotting was used to measure SIRT1 (mouse monoclonal antibody, Cat. No. 60303-1-Ig, Proteintech), NAMPT (rabbit polyclonal antibody, Cat. No. 11776-1-AP, Proteintech), PGC1α (mouse monoclonal antibody, Cat. No. 66369-1-Ig, Proteintech) and CD38 (mouse monoclonal antibody, Cat. No. 60006-1-Ig, Proteintech) in PBMCs, relative to ACTB as loading and transfer standard (mouse monoclonal antibody, Cat. No. 60008-1-Ig, Proteintech). Samples (10 μg protein, quantified using a BCA assay (Micro BCA, Pierce) and Bovine serum albumin (BSA) as a series of standards) were separated by electrophoresis through a 10% polyacrylamide gel then transferred to PDVF membrane using the wet transfer method. Tris-buffered saline, containing 0.15 Tween (TBST) and 5% BSA or, for NAMPT, 5% milk powder, was used for blocking (45–60 min at room temperature), incubation with specific primary antibody (all used at 1:500 dilution, 4 °C overnight), incubation with ACTB antibody (1:10,000, 4 °C overnight) and for incubation with secondary antibody (Polyclonal Goat Anti-Mouse Immunoglobulin/HRP, Cat. No. P044701-2, Dako or, for NAMPT, Polyclonal Goat Anti-Rabbit Immunoglobulin/HRP, Cat. No. P044801-2, Dako) used at 1:2000, except to detect the ACTB primary antibody for which it was used at 1:5000, 1 h at room temperature. Washing between incubations was in TBST. Supplementary Table 5 summarises all antibody sources and dilutions. Secondary antibody was detected using ECL plus or ECL regular (Pierce) and a Syngene G:BOX system. Images were saved as TIF files then densitometry measurements were made using ImageJ software. Band intensities were expressed relative to the corresponding sample intensity on the same blot for ACTB and, to enable pooling of data across different blots for statistical analysis, then normalised to the mean of the corresponding baseline (pre-supplement) measures (relative to ACTB).
Measurement of cytokines in plasma
Selected cytokines were measured in plasma as a service provided by Eve Technologies, Calgary, Canada using the Human High Sensitivity T-Cell 14-plex Discovery Assay® Array (HDHSTC14).
Measurement of glycated serum protein
Levels of serum protein glycation in plasma samples at baseline and 28 days for both arms of the trial was measured by Jinfiniti Precision Medicine, Augusta, USA.
Measurement of biological age using IgG glycosylation profiling
Changes to IgG glycosylation patterns were used as a measure of ‘biological age’ in plasma samples at baseline and 28 days for both arms of the trial, using a commercial service provided by Glycanage Ltd., UK.
Statistical analysis
Measurements of NAD+, NAM and NMN in whole blood (multiple time points) were expressed as difference from the mean of the 2 baseline measurements and analysed using repeated measures 2-way ANOVA, followed by Bonferroni post-hoc test (SPSS). Measurements of cytokines in plasma at 28 days were expressed as difference from the mean of the 2 baseline measurements and analysed by Student’s paired t-test. Western blotting densitometric data for SIRT1, NAMPT, PGC1α and CD38 was normalised to ACTB, then expressed as a percentage of the mean of the baseline measurements and analysed by Student’s t-test. Measurements of IgG glycosylation and serum protein was analysed by Student’s paired t-test.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Sharma, R. & Ramanathan, A. The aging metabolome-biomarkers to hub metabolites. Proteomics 20, e1800407 (2020).
McReynolds, M. R., Chellappa, K. & Baur, J. A. Age-related NAD(+) decline. Exp. Gerontol. 134, 110888 (2020).
Conlon, N. & Ford, D. A systems-approach to NAD+ restoration. Biochem. Pharmacol. 198, 114946 (2022).
Sharma, A., Mahur, P., Muthukumaran, J., Singh, A. K. & Jain, M. Shedding light on structure, function and regulation of human sirtuins: a comprehensive review. 3 Biotech 13, 29 (2023).
Zietara P., Dziewiecka M., Augustyniak M. Why is longevity still a scientific mystery? Sirtuins-past, present and future. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24010728 (2022).
Yang, H. et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS ONE 7, e46364 (2012).
Alemasova, E. E. & Lavrik, O. I. Poly(ADP-ribosyl)ation by PARP1: reaction mechanism and regulatory proteins. Nucleic Acids Res. 47, 3811–3827 (2019).
Burkle, A., Diefenbach, J., Brabeck, C. & Beneke, S. Ageing and PARP. Pharmacol. Res. 52, 93–99 (2005).
Horenstein A. L. et al. The circular life of human CD38: from basic science to clinics and back. Molecules https://doi.org/10.3390/molecules25204844 (2020).
Barbosa, M. T. et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 21, 3629–3639 (2007).
Tarrago, M. G. et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD(+) decline. Cell Metab. 27, 1081–1095.e10 (2018).
Peclat, T. R. et al. CD38 inhibitor 78c increases mice lifespan and healthspan in a model of chronological aging. Aging Cell 21, e13589 (2022).
Reiten, O. K., Wilvang, M. A., Mitchell, S. J., Hu, Z. & Fang, E. F. Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing. Mech. Ageing Dev. 199, 111567 (2021).
Remie, C. M. E. et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 112, 413–426 (2020).
Elhassan, Y. S. et al. Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 28, 1717–1728.e6 (2019).
Dollerup, O. L. et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J. Physiol. 598, 731–754 (2020).
Yoshino, M. et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 372, 1224–1229 (2021).
Morita, Y. et al. Clinical evaluation of changes in biomarkers by oral intake of NMN. Glycative Stress Res. 9, 33–41 (2022).
Igarashi, M. et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging 8, 5 (2022).
Okabe, K. et al. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front. Nutr. 9, 868640 (2022).
Pencina, K. M. et al. MIB-626, an oral formulation of a microcrystalline unique polymorph of beta-nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle-aged and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 78, 90–96 (2023).
Yi, L. et al. The efficacy and safety of beta-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. Geroscience 45, 29–43 (2023).
Irie, J. et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr. J. 67, 153–160 (2020).
Katayoshi, T. et al. Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: a randomized, double-blind, placebo-controlled trial. Sci. Rep. 13, 2786 (2023).
Huang, H. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Front. Aging 3, 851698 (2022).
Zhou, C. C. et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br. J. Pharmacol. 173, 2352–2368 (2016).
Koltai, E. et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 131, 21–28 (2010).
Ma, C. et al. Nampt expression decreases age-related senescence in rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS ONE 12, e0170930 (2017).
Liu, L. Y. et al. Nicotinamide phosphoribosyltransferase may be involved in age-related brain diseases. PLoS ONE 7, e44933 (2012).
Jadeja, R. N. et al. Loss of NAMPT in aging retinal pigment epithelium reduces NAD(+) availability and promotes cellular senescence. Aging 10, 1306–1323 (2018).
Xing, S., Hu, Y., Huang, X., Shen, D. & Chen, C. Nicotinamide phosphoribosyltransferase‑related signaling pathway in early Alzheimer’s disease mouse models. Mol. Med. Rep. 20, 5163–5171 (2019).
Stein, L. R. & Imai, S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 33, 1321–1340 (2014).
de Guia, R. M. et al. Aerobic and resistance exercise training reverses age-dependent decline in NAD(+) salvage capacity in human skeletal muscle. Physiol. Rep. 7, e14139 (2019).
Polzonetti, V. et al. Population variability in CD38 activity: correlation with age and significant effect of TNF-alpha -308G>A and CD38 184C>G SNPs. Mol. Genet. Metab. 105, 502–507 (2012).
Amici, S. A. et al. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front. Immunol. 9, 1593 (2018).
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021).
Zhang, T. et al. Alpha-lipoic acid activates AMPK to protect against oxidative stress and apoptosis in rats with diabetic peripheral neuropathy. Hormones 22, 95–105 (2023).
Canto, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Oh, G. S. et al. Increased cellular NAD(+) level through NQO1 enzymatic action has protective effects on bleomycin-induced lung fibrosis in mice. Tuberc. Respir. Dis. 79, 257–266 (2016).
Elangovan, S. & Hsieh, T. C. Control of cellular redox status and upregulation of quinone reductase NQO1 via Nrf2 activation by alpha-lipoic acid in human leukemia HL-60 cells. Int. J. Oncol. 33, 833–838 (2008).
Packer, L., Witt, E. H. & Tritschler, H. J. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 19, 227–250 (1995).
Carr A. C., Maggini S. Vitamin C and immune function. Nutrients https://doi.org/10.3390/nu9111211 (2017).
Wessels I., Maywald M. & Rink L. Zinc as a gatekeeper of immune function. Nutrients https://doi.org/10.3390/nu9121286 (2017).
Wu, F. et al. Epigallocatechin-3-gallate (EGCG) protects against chromate-induced toxicity in vitro. Toxicol. Appl. Pharmacol. 258, 166–175 (2012).
Gao, Y., Martin, N. I. & van Haren, M. J. Nicotinamide N-methyl transferase (NNMT): an emerging therapeutic target. Drug Discov. Today 26, 2699–2706 (2021).
Zhang, Z. F. et al. Troxerutin protects against 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD(+)-depletion. J. Hazard. Mater. 283, 98–109 (2015).
Zhang, Z. F. et al. Troxerutin improves hepatic lipid homeostasis by restoring NAD(+)-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem. Pharmacol. 91, 74–86 (2014).
Escande, C. et al. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62, 1084–1093 (2013).
Srinivasan, K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 47, 735–748 (2007).
Hirata, T. et al. Associations of cardiovascular biomarkers and plasma albumin with exceptional survival to the highest ages. Nat. Commun. 11, 3820 (2020).
Kar A., Mehrotra S. & Chatterjee S. CD38: T cell immuno-metabolic modulator. Cells https://doi.org/10.3390/cells9071716 (2020).
Yang, Y. et al. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 13, 831168 (2022).
Kristic, J. et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 69, 779–789 (2014).
Shkunnikova, S. et al. IgG glycans in health and disease: prediction, intervention, prognosis, and therapy. Biotechnol. Adv. 67, 108169 (2023).
Higgins, C. B. et al. SIRT1 selectively exerts the metabolic protective effects of hepatocyte nicotinamide phosphoribosyltransferase. Nat. Commun. 13, 1074 (2022).
Wang, N., Guo, C., Han, P. & Li, T. Glycated albumin indicates peripheral diabetic neuropathy. Acta Diabetol. 53, 973–979 (2016).
Wang, W., Sun, W., Cheng, Y., Xu, Z. & Cai, L. Role of sirtuin-1 in diabetic nephropathy. J. Mol. Med. 97, 291–309 (2019).
Singh, B. N., Shankar, S. & Srivastava, R. K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 82, 1807–1821 (2011).
Elangovan, P., Jalaludeen, A. M., Ramakrishnan, R., Amutha, K. & Pari, L. In-vivo and in-vitro antioxidant activity of troxerutin on nickel induced toxicity in experimental rats. Iran J. Pharm. Res. 19, 89–97 (2020).
Zoico, E. et al. Senolytic effects of quercetin in an in vitro model of pre-adipocytes and adipocytes induced senescence. Sci. Rep. 11, 23237 (2021).
Liu, H. et al. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell https://doi.org/10.1111/acel.13921 (2023).
Conlon, N. J. The role of NAD+ in regenerative medicine. Plast. Reconstr. Surg. 150, 41S–48S (2022).
Chambers, E. S. & Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 160, 116–125 (2020).
Basse, A. L. et al. NAMPT-dependent NAD(+) biosynthesis controls circadian metabolism in a tissue-specific manner. Proc. Natl Acad. Sci. USA 120, e2220102120 (2023).
Acknowledgements
The authors thank Dr Willian Cheung, Northumbria University, for carrying out analysis of samples by LC–MS. The study was co-funded by Northumbria University (studentships to J.D.H. and S.N.Z.Q. and infrastructure support) and Nuchido Ltd. (contribution to studentship for S.N.Z.Q., consumables costs, staff costs of S.S.C.).
Author information
Authors and Affiliations
Contributions
J.D.H. ran the clinical trial and set up and conducted the procedure for measurement of NAD+. S.N.Z.Q. assisted in running the trial, carried out measurements on participant samples and conducted statistical analysis. J.D.H. and S.N.Z.Q. are co-first author since these contributions were equal. S.S.C. set up and optimised western blotting, carried out measurements on participant samples and conducted statistical analysis. N.C. provided input into the design of the clinical trial, directed the analysis of participant samples, provided advice to S.N.Z.Q., produced the figures and contributed to the manuscript. D.F. designed the clinical trial in consultation with N.C., provided supervision to S.N.Z.Q., produced the first draft of the manuscript and took responsibility for completion.
Corresponding authors
Ethics declarations
Competing interests
Authors J.D.H., S.N.Z.Q. and D.F. declare no financial or non-financial competing interests. Author N.C. is Founder and Chief Executive Officer of Nuchido Ltd. Author S.S.C. is an employee of Nuchido Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henderson, J.D., Quigley, S.N.Z., Chachra, S.S. et al. The use of a systems approach to increase NAD+ in human participants. npj Aging 10, 7 (2024). https://doi.org/10.1038/s41514-023-00134-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-023-00134-0