Abstract
NARROW LEAF1 (NAL1) exerts a multifaceted influence on leaf morphology and crop yield. Recent crystal study proposed that histidine 233 (H233) is part of the catalytic triad. Here we report that unlike suggested previously, H234 instead of H233 is a component of the catalytic triad alongside residues D291 and S385 in NAL1. Remarkably, residue 233 unexpectedly plays a pivotal role in regulating NAL1’s proteolytic activity. These findings establish a strong foundation for utilizing NAL1 in breeding programs aimed at improving crop yield.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Accession codes: The atomic coordinate and structure factors have been deposited in the Protein Data Bank (www.wwpdb.org) with the following accession numbers: 8PME, 8PMG, 8PMI, 8PML, 8PMM, 8PN1 and 8PN2. The cryo-EM maps of NAL1SPIKE and NAL1IR64 have been deposited in the EM Database with accession code EMD-17765 and EMD-17766. Source data for Figs. 1c–e and 2a,b and Extended Data Figs. 1a and 5a–d are provided with this paper. Source data are provided with this paper. The other data supporting this study’s findings are available from the corresponding author on request.
References
Li, W. et al. Serine protease NAL1 exerts pleiotropic functions through degradation of TOPLESS-related corepressor in rice. Nat. Plants 9, 1130–1142 (2023).
Qi, J. et al. Mutation of the rice Narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiol. 147, 1947–1959 (2008).
Takai, T. et al. A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Sci. Rep. 3, 2149 (2013).
Ding, X., Li, X. & Xiong, L. Evaluation of near-isogenic lines for drought resistance QTL and fine mapping of a locus affecting flag leaf width, spikelet number, and root volume in rice. Theor. Appl. Genet. 123, 815–826 (2011).
Huang, Y. et al. Variation in the regulatory region of FZP causes increases in secondary inflorescence branching and grain yield in rice domestication. Plant J. 96, 716–733 (2018).
Fujita, D. et al. NAL1 allele from a rice landrace greatly increases yield in modern indica cultivars. Proc. Natl Acad. Sci. USA 110, 20431–20436 (2013).
Zhang, G. H. et al. LSCHL4 from Japonica Cultivar, which is allelic to NAL1, increases yield of Indica super rice 93-11. Mol. Plant 7, 1350–1364 (2014).
Sauer, R. T. & Baker, T. A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 (2011).
Hedstrom, L. J. C. R. Serine protease mechanism and specificity. Chem. Rev. 102, 4501–4524 (2002).
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010).
Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. Automated structure solution with autoSHARP. Methods Mol. Biol. 364, 215–230 (2007).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Zivanov, J., Nakane, T. & Scheres, S. H. J. I. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Acknowledgements
This Article is dedicated to the late Wei-Fei Chen. We thank beamline scientists at BL17U1 of the Shanghai Synchrotron Radiation Facility (China) and at BL18U1 and BL19U1 of the National Center for Protein Sciences, Shanghai, for assistance with data collection. We are grateful for access to the Electron Microscopy Centre of Lanzhou University for cryo-EM data collection. We extend our gratitude to K.-M. Chen (Northwest A&F University) for his insightful discussion. This study was supported by the National Natural Science Foundation of China (32071291, 32201042, 32071225, 31870788 and 32171300), Centre national de la recherche scientifique-Laboratoire International Associé (‘Helicase-mediated G-quadruplex DNA unwinding and genome stability’) and Fundamental Research Funds for the Central Universities (number lzujbky-2021-ct05).
Author information
Authors and Affiliations
Contributions
L.-Y.H., N.-N.L., W.-F.C., S.R. and X.-G.X. designed the experiments and analysed the data. L.-Y.H. carried out protein purification, proteolytic activity assays and crystallization. D.-S.L. and Z.-L.Z. collected and analysed cryo-EM data. L.-Y.H., N.-N.L. and W.-F.C. collected crystal diffraction data. S.R., P.F. and O.M. determined the crystal structures and performed molecular dynamics simulations. L.-Y.H. and S.R. contributed to comparing the crystal structures. X.A., H.-H.L. and X.-M.H. conducted additional protein purification. X.-G.X. interpreted the data and wrote the paper with input from other authors. All authors commented on the results and contributed to the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Hyun Kyu Song and Junfeng Liu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 NAL1 demonstrates substrate preference and offers a structural comparison of protease domains in the serine protease family.
a, NAL1 selectively and partially hydrolyzes model substrates, which include α-Casein, β-Casein, and α-Lactalbumin. For each assay, 300 μM of the respective substrate was introduced into a reaction chamber containing 50 μL of reaction buffer (comprising 25 mM Tris-HCl at pH 7.5, 10 mM NaCl, 2 mM MgCl2, and 0.1% NP-40), along with 600 μM of NAL1 proteins. The reaction mixtures were then incubated for 6 hours at 37 °C, after which substrate degradation was evaluated through 15% SDS-PAGE analysis. Experiments were independently repeated three times with similar results obtained. Uncropped gel image is available as source data. b, Overall crystal structure of NAL1IR64(88-458) (PDB: 8PME) trimer. c-d, The protease domain of NAL1 exhibits homology to Streptogrisin B (SGPB, PDB: 2QAA) and Alpha-lytic protease (PDB: 4PRO), as revealed through Dali search. Structural superposition of the protease domain of NAL1SPIKE(31-458) (PDB: 8PMM) is shown in light orange, Streptogrisin B in pale green, with an r.m.s.d. of 1.76 Å in (c). In (d), a similar structural superposition is presented, with the protease domain of NAL1SPIKE(31-458) in light orange and Alpha-lytic protease in pale cyan, demonstrating an r.m.s.d. of 2.40 Å.
Extended Data Fig. 2 The loop spanning residues 173–184 in the NAL1IR64(36-458)-FZP190-198 complex undergoes a notable reorganization in response to substrate binding.
An overview of the loop 173–184 in the structure of the NAL1IR64(36-458)-FZP190-198 (PDB: 8PMI) complex reveals its proximity to the catalytic triad (as shown in the right panel).
Extended Data Fig. 3 Molecular dynamics simulation of NAL1 and FZP peptides complex.
a-b, Detailed structural view and schematic diagram of the interactions between NAL1 with FZP peptide in NAL1SPIKE(31-458)-FZP114-121 modeled complex structure (a) and NAL1SPIKE(31-458)-FZP192-199 modeled complex structure (b). c, A close-up view of the catalytic triad of the modeled NAL1SPIKE-FZP114-121 complex. d, A close-up view of the catalytic triad of the modeled NAL1SPIKE-FZP192-199 complex. e, A closeup view of the catalytic triad of the modeled NAL1IR64-FZP114-121 complex. f, A close-up view of the catalytic triad of the modeled NAL1IR64-FZP192-199 complex.
Extended Data Fig. 4 Structural and primary sequence comparison of NAL1SPIKE and NAL1IR64.
a, Structural comparison of NAL1SPIKE(31-458) (slate, PDB: 8PMM) and NAL1IR64(36-458) (pink, PDB: 8PMG). Superposition of hexamer forms of NAL1SPIKE(31-458) (slate) and NAL1IR64(36-458) (pink) (left panel) with an rmsd of 1.40 Å, and superposition of monomer forms of NAL1SPIKE(31-458) (slate) and NAL1IR64(36-458) (pink) (right panel) with an rmsd of 0.53 Å. b, Primary sequence alignment between NAL1SPIKE and NAL1IR64. The white boxes mark the difference amino acids between NAL1SPIKE and NAL1IR64. Arrows and cylinders represent predicted β-strands and α-helices, respectively. The domains are coloured as in Fig. 1a.
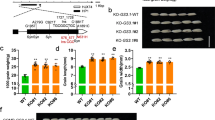
Extended Data Fig. 5 Regulation of NAL1 Proteolytic Activity: The Dual Impact of Residue 233.
a-d, Proteolytic activity assays were performed with a series of H233 mutants of NAL1SPIKE. The left panel displays a comparison of proteolytic activity between the wild type (NAL1SPIKE) and the mutants, while the right panel presents a histogram representing the residual content (%) of the substrate (FZP51-244). The integrated densities of various bands were scanned and calculated using Quantity One software from Bio-Rad. Data show mean ± SD from three independent experiments. Black circles represent individual data points. All differences between each mutant and the wild-type (NAL1SPIKE) were analysed within the same degradation time by two-tailed paired t-tests. Means with p < 0.05 are indicated. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. All proteolytic activity assays were carried out according to the procedures outlined in the Methods section, utilizing three different degradation time intervals, including 1 h, 3 h, and 6 h. CK represents the substrate (FZP51-244) alone. The triangle symbol indicates a decrease in the substrate (FZP51-244), while the round symbol signifies an increase in the proteolyzed substrate. Uncropped gel images are available as source data. The H233 mutants are categorized as follows: (a) H233R and H233K (positively charged residues), (b) H233D and H233E (negatively charged residues), (c) H233N and H233Q (polar uncharged residues), and (d) H233Y and H233W (hydrophobic residues).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Crystallographic Data 1
The crystallographic data of NAL1IR64(88-458) (PDB 8PME).
Crystallographic Data 2
The crystallographic data of NAL1IR64(36-458) (PDB 8PMG).
Crystallographic Data 3
The crystallographic data of NAL1IR64(36-458)-FZP190-198 (PDB 8PMI).
Crystallographic Data 4
The crystallographic data of NAL1SPIKE(46-458) (PDB 8PML).
Crystallographic Data 5
The crystallographic data of NAL1SPIKE(31-458) (PDB 8PMM).
Cryo-EM Data 1
The Cryo-EM data of NAL1SPIKE (PDB 8PN1).
Cryo-EM Data 2
The Cryo-EM data of NAL1IR64 (PDB 8PN2).
Source data
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 2
Unprocessed gels and statistical source data.
Source Data Fig. 2
Unprocessed gels and statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 5
Unprocessed gels and statistical source data.
Source Data Extended Data Fig. 5
Unprocessed gels and statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, LY., Liu, NN., Chen, WF. et al. The catalytic triad of rice NARROW LEAF1 involves H234. Nat. Plants (2024). https://doi.org/10.1038/s41477-024-01668-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41477-024-01668-1