Abstract
Grain size is a key component trait of grain weight and yield. Numbers of quantitative trait loci (QTLs) have been identified in various bioprocesses, but there is still little known about how metabolism-related QTLs influence grain size and yield. The current study report GS3.1, a QTL that regulates rice grain size via metabolic flux allocation between two branches of phenylpropanoid metabolism. GS3.1 encodes a MATE (multidrug and toxic compounds extrusion) transporter that regulates grain size by directing the transport of p-coumaric acid from the p-coumaric acid biosynthetic metabolon to the flavonoid biosynthetic metabolon. A natural allele of GS3.1 was identified from an African rice with enlarged grains, reduced flavonoid content and increased lignin content in the panicles. Notably, the natural allele of GS3.1 caused no alterations in other tissues and did not affect stress tolerance, revealing an ideal candidate for breeding efforts. This study uncovers insights into the regulation of grain size though metabolic-flux distribution. In this way, it supports a strategy of enhancing crop yield without introducing deleterious side effects on stress tolerance mechanisms.
Similar content being viewed by others
Introduction
Rice, as one of the most important global staple crops, provides sustenance for more than half of the world’s population. Rice yield has been determined to be a quantitative trait regulated by a series of QTLs1,2,3. Many yield-related genes were discovered by map-based cloning and mutant identification in the past studies, including genes involved in multiple agricultural traits such as effective tiller number, grain number per panicle, grain weight, and even stress resistance4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. Large numbers of genes were identified and characterized in rice that influence grain weight, which were attached to some important biological pathways4,5,6,9,14,16,18,23,24,25,26,27,28. GW2 (LOC_Os02g14720) was reportedly involved in the ubiquitin-proteasome pathway regulating grain width26. GS3 (Os03g0407400) encodes a protein considered to take part in the G-protein signaling pathway9,23,27,28. GSN1 (LOC_Os05g02500) is a mitogen-activated protein kinase phosphatase that plays a role in the mitogen-activated protein kinase signaling pathway24. GL3.1 (LOC_Os03g44500) regulates grain length by regulating the cell cycle5,14,25. Phytohormones also play a vital role in regulating grain size. For example, GS2, also referred to as OsGRF4 (LOC_Os02g47280), is a transcriptional regulator and participates in the brassinolide pathway4,16,18.
The phenylpropanoid metabolic pathway, identified to synthesize flavonoids, lignin, and anthocyanins in plants, is important for plant growth and stress tolerance. Increased flavonoids content can enhance stress tolerance such as drought tolerance and UV tolerance in plants29,30. NARROW AND ROLLED LEAF 2 (NRL2, LOC_Os03g19520) mutants exhibit a narrow leaf and reduced grain size and lignin content31. SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 (SPL9, LOC_Os05g33810) regulates stress tolerance by controlling anthocyanin metabolism32. Recent study indicated that a UDP-glucosyltransferase, Grain Size and Abiotic stress tolerance 1 (GSA1, LOC_Os03g55040), synergistically regulated grain size and stress tolerance by controlling flavonoid and lignin content6. Nevertheless, the knowledge about QTLs that influence how metabolism, especially phenylpropanoid metabolism, influence grain size and yield is still much to be known.
Multidrug and toxic compound extrusion (MATE) transporters were reported to transport a variety of compounds and ions via membrane transport, and to regulate diverse bioprocesses in plants33. The best-known process to be regulated by the MATE transporter was stress tolerance, in which large numbers of MATEs were indicated to take part in refs. 34,35,36,37,38. For example, EDS5 is involved in salicylic acid (SA) biosynthesis, and DTX50 and its rice ortholog DG1 function as an abscisic acid (ABA) transporter34,35,39. Some MATEs were also reported to regulate plant development such as lateral organ size and initiation rate40. MATE transporter substrates and the mechanism by which they regulate plant development still needs more research. Meanwhile, MATEs encoded within QTLs to influence grain size and yield can be further explored.
The current study cloned and characterized a QTL in rice that regulates flavonoid and lignin metabolic fluxes via the allocation of the metabolic intermediate, p-coumaric acid, which finally influenced grain size and yield in rice.
Results
GS3.1 is a QTL for grain size
One of the chromosome segment substitution lines (CSSLs; the donor parent: HP1, HP for short, Oryza glaberrima; the recurrent parent: HJX74, HJX for short, Oryza sativa indica), HPC078, was identified exhibiting difference in grain size when compared to the recurrent parent, HJX (Supplementary Fig. 1a). A QTL was mapped to the short arm of chromosome 3 and was named GS3.1 for further characterization. GS3.1 showed the effect on grain size, which was determined by 1000-grain weight, grain length, and grain width. The results showed that GS3.1 explained 33.2% of the phenotypic variation for 1000-grain weight, 23.6% of the phenotypic variation for grain length and 23.5% of the phenotypic variation for grain width (Supplementary Table 1).
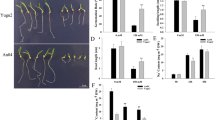
To clone the individual gene controlling grain size, high-resolution mapping was performed and the GS3.1 locus was narrowed to a 9.45 kbp region (Fig. 1a), and a nearly isogenic line (NIL) of GS3.1, NIL-GS3.1HP was constructed together with a NIL-GS3.1HJX as control (Supplementary Fig. 1b, c). Examining the rice genome database, there was one transcript corresponding to Loc_Os03g12790 that was annotated as a MATE transporter family protein in the GS3.1 region. Sequence comparison between the HJX allele and the HP allele indicated the presence of five nucleotide substitutions and one insertion (G159T, A279G, C921T, C1881T, A1891C, and 1727_1728 ins TGCGGCTGC), which caused one amino acid substitution (N631H) and one insertion (576_577 ins CGC) in Loc_Os03g12790 (Fig. 1a).
a GS3.1 was preliminarily mapped to the interval between the markers 3-27.208 and 3-28.502 on the short arm of chromosome 3 and then narrowed to a 9.45 kbp region containing one gene. The numbers of recombinant individuals are shown between the marker positions. 1000-grain weight is shown on the right of each representative recombinant line. Recombinant lines R1 to R6 originated from recombinant line C2. Values represent the mean ± s.d. (n ≥ 23 plants). Different letters indicate significant differences (P < 0.05) as determined by a Tukey’s test. Single nucleotide mutations and the corresponding amino acid changes in NIL-GS3.1HP are shown on the schematic illustration of the GS3.1 gene. Syn, synonymous variations. b Mature grains of GS3.1 knock out lines KO-GS3.1#1, KO-GS3.1#2, KO-GS3.1#6, and the wild-type control. Scale bar =1 cm. Comparison of 1000-grain weight (c), grain length (d), and grain width (e) between GS3.1 knock out lines and the wild-type control (n ≥ 7 plants). f Mature grains of GS3.1 complementation lines COMP-GS3.1 #3, COMP-GS3.1 #5, and NIL-GS3.1HP wild-type control. Scale bar =1 cm. Comparison of 1000-grain weight (g), grain length (h), and grain width (i) between GS3.1 complementation lines and NIL-GS3.1HP wild-type control (n ≥ 7 plants). The values in c–e and g–i represent the mean ± s.d.. *P < 0.05 and **P < 0.01 indicate significant differences compared to the control in two-tailed Student’s t-tests. The source data underlying Fig. 1a, c–e and g–i are provided as Source data file.
To verify that Loc_Os03g12790 was the specific gene that regulated grain size within the GS3.1 locus, Loc_Os03g12790 was knocked out using CRISPR/Cas9 in WYJ7 (9522, Oryza sativa japonica) and the recurrent parent HJX background. The current study observed a significant increase in 1000-grain weight (33.36%), grain length (11.59%), and grain width (14.47%) in HJX background mutants without noticeable changes to plant height and tiller number. Similar phenotypes were observed on grain size in the 9522 background (Fig. 1b–e; Supplementary Fig. 2a–e and Supplementary Table 2). Furthermore, a genetic complementation assay was performed by transforming the Loc_Os03g12790 genic region from HJX with its upstream 2.5 kbp promoter region and 1.5 kbp downstream region into NIL-GS3.1HP. The transgenic construct lines showed a decrease in 1000-grain weight (−9.35%), grain length (−2.03%), and plant height (−5.03%), but there were no significant differences in grain width and tiller number compared to NIL-GS3.1HP controls (Fig. 1f-i and Supplementary Fig. 2f, g). Overexpressing the Loc_Os03g12790 gene region using the rice ubiquitin promoter caused a decrease in grain length together with plant height (Supplementary Fig. 2h–j). These data indicate that Loc_Os03g12790 negatively regulated grain size in the GS3.1 locus of the rice genome.
GS3.1 enlarges grain size and enhances rice yield by influencing cell expansion
To further characterize this QTL, the agronomic phenotypes were evaluated. NIL-GS3.1HP exhibited an increase in 1000-grain weight (6.2%), grain length (1.9%), and grain width (2.5%) compared to NIL-GS3.1HJX (Fig. 2a–d, Supplementary Fig. 3a, b, and Supplementary Table 3). Moreover, the current study observed more tillers but no difference in spikelet number or plant height in NIL-GS3.1HP compared to NIL-GS3.1HJX (Fig. 2e, f, Supplementary Fig. 3c, d and Supplementary Table 3). These factors led to an increase in grain yield per plant (16.7%), grain yield per panicle (11.5%) and ultimately plot yield (8.7%) compared to NIL-GS3.1HJX (Fig. 2g–i and Supplementary Table 3).
a Mature grains of NIL-GS3.1HJX and NIL-GS3.1HP. Scale bar =1 cm. Comparison of 1000-grain weight (b), grain length (c), grain width (d), tiller number (e), spikelet number (f), yield per plant (g) and yield per panicle (h) between NIL-GS3.1HJX and NIL-GS3.1HP (n = 30 plants). i Comparison of plot yield between NIL-GS3.1HJX and NIL-GS3.1HP (n = 3 plots, about 2 square meters per plot). j Time-course of the change in caryopsis wet weight and dry weight for NIL-GS3.1HJX and NIL-GS3.1HP (n = 3 plants, ≥20 caryopses per plant). k Scanning electron micrographs of the outer epidermal cells of spikelet hulls just before heading. Scale bar =1 mm. The red rectangle border shows the area for measuring the cell length. Comparison of the outer epidermal cell length (l) and cell number in the grain-length direction (m) between NIL-GS3.1HJX and NIL-GS3.1HP (n = 24 spikelet hulls, 10 cells per spikelet hull for cell length measuring). n Micrographs of the cross-section of spikelet hulls just before heading in the grain width direction. Scale bar = 1 mm. The red rectangle border shows the area used for measuring the cell width. Comparison of the outer epidermal cell width (o) and cell number in the grain-width direction (p) between NIL-GS3.1HJX and NIL-GS3.1HP (n ≥ 3 spikelet hulls, n = 45 cells). The values in b–h, j, l, m, o, p represent the mean ± s.d. *P < 0.05 and **P < 0.01 indicate significant differences compared with NIL-GS3.1HJX in two-tailed Student’s t-tests. The values in i represent the mean ± s.d.. *P < 0.1 indicate significant differences compared to NIL-GS3.1HJX in two-tailed Student’s t tests. The source data underlying Fig. 2b–j, l, m, o and p are provided as Source data file.
Next, the current study investigated grain milk filling rate in NIL-GS3.1HP and NIL-GS3.1HJX, and found that there were significant differences between NIL-GS3.1HP and NIL-GS3.1HJX in both wet weight and dry weight of caryopses in the late filling stage. However, there was no significant difference in grain weight from the flowering day to 15 days after flowering (Fig. 2j). These results indicate that the difference in 1000-grain weight might mainly come from an increase in dry matter resulting from a larger spikelet hull.
To assess the contribution of the spikelet hull to grain weight, the cell number and size in young and mature spikelet hulls were investigated by measuring the size of the outer epidermal cells. Scanning electron micrographs showed that the cell number was similar between NIL-GS3.1HP and NIL-GS3.1HJX in the grain-length direction, but the cell length of spikelet hulls of NIL-GS3.1HP was significantly increased compared to NIL-GS3.1HJX (Fig. 2k–m and Supplementary Table 4). Concerning cell width, similar trends were found as the cell number showed no significant difference but the cell width increased in the cross-sections of spikelet hulls (Fig. 2n–p and Supplementary Table 4). Scanning electron micrograph analysis was also performed on the knockout lines of GS3.1, KO-GS3.1, and the wild-type control. KO-GS3.1 revealed an increased cell length and unchanged cell number in the grain-length direction (Supplementary Fig. 4). These results suggested that GS3.1 enhanced the cell expansion and enlarged grains. Relying on the enhanced grain size, NIL-GS3.1HP plants had an increase in yield compared to NIL-GS3.1HJX.
GS3.1 is expressed in the panicles and the phloem of plant leaves and culms
A GS3.1 promoter-driven GUS construct was transformed into ZH11 (Oryza sativa japonica) and used to analyze the expression pattern of GS3.1. The panicles, spikelet hulls, leaves, and culms were stained blue after the incubation with X-gluc (Fig. 3a–c, and Supplementary Fig. 5a–c). Upon further observation, the current study found that the blue stain in culms, leaves and late-stage panicles had a consistent localization within the vasculature. So that, cross-sections of stained young leaves were observed and the phloem was found to be stained (Fig. 3d, and Supplementary Fig. 5d). Then, the current study examined the tissue-specific expression pattern of GS3.1 using qRT-RCR and revealed a similar pattern as the GUS staining assay. GS3.1 was wildly expressed in various organs including panicles, leaves, culms and culm nodes, but barely expressed in roots. The relative expression levels of GS3.1 in NIL-GS3.1HJX were significantly higher compared to that of NIL-GS3.1HP in young panicles (3–5 cm) and were slightly higher in culms but showed no significant difference in other tissues (Fig. 3e).
GUS staining of Pro GS3.1HJX:GUS of a young 1 cm-long panicle (a), culm (b), and leaf (c). Scale bar = 1 mm. d Micrograph of a cross-section of a young leaf. The red arrow shows the stained phloem. Scale bar = 50 μm. e The relative expression levels of GS3.1 in NIL-GS3.1HJX and NIL-GS3.1HP leaves (L), roots (R), culm nodes (CN), culms (C), and panicles (P, numbers indicate the length of young panicles, cm) as determined by qRT-PCR (n = 3 biological replicates). The actin gene was used for normalization. The values in e represent the mean ± s.d.. *P < 0.05 and **P < 0.01 indicate significant differences compared to NIL-GS3.1HJX in two-tailed Student’s t-tests. The source data underlying Fig. 3e are provided as Source data file.
Previous studies showed that the ortholog of GS3.1 in Arabidopsis (ABS4) had five paralogs (ABS3, ABS3L1, ABS3L2, ABS3L3, and ABS3L4) that might have similar function41. A maximum-likelihood phylogenetic tree was constructed with the amino acid sequences of all six Arabidopsis genes (ABS3, ABS3L1, ABS3L2, ABS3L3, ABS3L4, and ABS4), their corresponding orthologs in rice (Loc_Os06g36330, Loc_Os04g48290, and GS3.1), and an Arabidopsis MATE gene (DTX14) as the outgroup (Supplementary Fig. 6a). All nine genes (excluding the outgroup) were divided into four classes, and three rice paralogs separated into three classes, indicating that these three rice paralogs including GS3.1 may have functional redundancy in rice. Relative expression levels of these genes were determined using qRT-PCR to examine the expression pattern, and Loc_Os04g48290 was the most highly expressed among the three MATE paralogs. The expression level of Loc_Os04g48290 was over 10 times higher compared to GS3.1 in other tissues except in panicles, indicating that Loc_Os04g48290 had a more important role in these tissues (Supplementary Fig. 6b). When it comes to panicles, Loc_Os04g48290 and GS3.1 showed a comparable expression level which indicated that GS3.1 contributed a relatively more important role in panicles than in other tissues (Supplementary Fig. 6b). The expression level of Loc_Os06g36330 was not detected in any of the analyzed tissues (Supplementary Fig. 6b). With the evidence above, the current study concluded that GS3.1 was expressed in various tissues including panicles, leaves, and culms, but only at a minimal level in roots. In mature tissues, GS3.1 was expressed specifically in the phloem. NIL-GS3.1HP showed a decreased expression level of GS3.1 only in young panicles compared to NIL-GS3.1HJX, indicating that GS3.1 had a more prominent function in young panicles to regulate grain size. Two paralogs of GS3.1 might functionally complement GS3.1, but in panicles, GS3.1 likely to serve a more prominent role because of the lower relative expression level of its paralogs compared to their relative expression in the other tissues.
GS3.1 functions as a MATE transporter and might transport p-coumaric acid in rice
As GS3.1 is annotated as a MATE transporter, an E. coli growth complementation assay was used to affirm that GS3.1 functioned as a multidrug efflux to complement the function of Acrb42. The current study expressed the GS3.1 full-length CDS by a pMAL-c5x vector (pMAL) in an acrb loss-of-function E. coli mutant, BW25113Δacrb, and observed its growth on the medium supplemented with the antibiotic norfloxacin, on which the control stain transformed pMAL could not grow, confirming that GS3.1 has similar function with its ortholog MATE transporter acrB in E. coli. (Fig. 4a and Supplementary Fig. 7).
a Growth complementation assays of the E. coli ΔacrB mutant expressing GS3.1HJX on medium supplemented with the antibiotic norfloxacin. The E. coli ΔacrB mutant expressing pMAL empty vector and the wild-type E. coli BW25113 were used as control strains, and mediums without norfloxacin were used as the growth condition control. Scale bar = 1 cm. b A schematic of the predicted GS3.1 structure showing 12 transmembrane helixes. Numbers indicate the sequence number of amino acid residues. c Predicted GS3.1 model docked with p-coumaric acid (red). The α-helixes interacting with p-coumaric acid were colored purple. The amino residues interacting with p-coumaric were colored yellow. The red arrow indicates the key α-helix for its transporter function.
Comparing to some MATE transporters with solved crystal structures, such as DTX14 in Arabidopsis, the structure of GS3.1 was predicted. Similar to those known MATE proteins, GS3.1 had twelve transmembrane helixes (TM1-12) that formed an internal cavity. Twelve helixes arranged into two bundles (TM1-6 and TM7-12), forming a typical V-shape conformation. TM7 is in the middle of this conformation and was supposed to have a major effect on the predicted transporting function (Fig. 4b, c)43. As the GS3.1 transmembrane domain above-mentioned is similar to those structurally available MATEs, the transmembrane domain (amino acids 45-496) of the predicted structure was chosen for molecular docking studies with candidate compounds that might be transported by GS3.1. Finally, p-coumaric acid was considered to be a top candidate compound. The binding free energy of p-coumaric acid was calculated to be –91.36 kcal·mol−1 docking with the transmembrane domain of GS3.1. Six amino acid residues in TM7, TM10, and TM11 were important for establishing the interaction of p-coumaric acid for binding free energy calculated under −4 kcal·mol−1 (Fig. 4c). To conclude, GS3.1 works as a MATE transporter, and it is predicted to transport p-coumaric acid as one of its substrates.
GS3.1 regulates grain size by modulating flavonoid and lignin biosynthesis
To further illuminate the effect of GS3.1, widely-targeted metabolomic analysis was applied on KO-GS3.1 (9522) and the wild-type control. P-coumaric acid was proved to be the common substrate of flavonoid and lignin biosynthesis. P-coumaric acid can be transformed to p-coumaroyl-CoA by 4CLs, and then to naringenin chalcone by the catalysis of CHS. Naringenin chalcone can then be transformed into naringenin and other flavonoids44,45. On the other side, p-coumaric acid can also be transformed into a set of phenolic acids such as caffeic acid and ferulic acid. Then, these can be transformed into coniferyl alcohol and lignans, which is a substrate for lignin biosynthesis46. The widely-targeted metabolome results showed that in the panicles of KO-GS3.1, p-coumaric acid content was reduced along with one main flavonoid, naringenin, but levels for caffeic acid and ferulic acid were elevated along with their downstream product coniferyl alcohol and one kind of lignans, secoisolariciresinol-4-glucoside (Fig. 5a–f). Further tests illustrated that in KO-GS3.1 the flavonoid content was downregulated and lignan content was upregulated on the whole (Fig. 5g, h). This tendency was also consistent in the comparison of NIL-GS3.1HP and NIL-GS3.1HJX (Supplementary Fig. 8). These data indicated that GS3.1 might be involved in flavonoid biosynthesis by transporting p-coumaric acid. Consequently, downregulation of GS3.1 caused the suppression of flavonoid biosynthesis, meanwhile, lignin biosynthesis was accelerated.
Comparison of the relative content of p-coumaric acid (a), naringenin (b), caffeic acid (c), ferulic acid (d), coniferyl alcohol (e), and secoisolariciresinol 4-O-glucoside (f) between the GS3.1 knock-out line KO-GS3.1 panicles and wild-type control panicles (n = 3 biological replicates, 4 plants per biological replicate). Heat maps of the relative content of flavonoids (g) and lignans (h) in KO-GS3.1 panicles and wild-type control panicles determined using metabolomics. Standard-scores (Z-scores) were used as the numerical metrics to evaluate the standard deviations from the mean of the corresponding samples. i Comparison of lignin content between the leaves of GS3.1 overexpression lines OE-GS3.1 and wild-type controls (n = 9 leaves from 3 plants for wildtype, n = 7 leaves from 3 plants for OE-GS3.1). j The relative expression of flavonoid and lignin biosynthesis-related genes and auxin-related genes in GS3.1 knock-out line KO-GS3.1 panicles and wild-type control panicles determined by qRT-PCR (n = 3 biological replicates). The values in a–f, i and j represent the mean ± s.d. *P < 0.05 and **P < 0.01 indicate significant differences compared to the wild-type control in two-tailed Student’s t-tests. The source data underlying Fig. 5a–j are provided as Source data file.
To further verify the effects on lignin production caused by GS3.1, the lignin content was examined using a GS3.1 overexpression line, OE-GS3.1, and the lignin content of OE-GS3.1 was observed to be decreased compared to its wild-type control (Fig. 5i). The relative expression levels of genes participating in the biosynthesis pathway mentioned above were examined in the panicles. The current study found that in KO-GS3.1, flavonoid-biosynthesis-related genes (CHS, F3H, and F3’H) were downregulated, as well as genes participating in both flavonoid and lignan biosynthesis (4CL1, 4CL3, 4CL4, and 4CL5) compared to the wild-type control (Fig. 5j). Previous studies indicated that flavonoids could suppress auxin transport, biosynthesis, and the subsequent signal transduction47, this phenomenon could also be observed in KO-GS3.1 which had a lower flavonoid level and showed higher expression of the auxin transporter PIN1b, auxin synthase YUCCA7, auxin response factors (ARFs; ARF15 and ARF24) and a lower expression level of the auxin inactivation protein GH3.8. GA signaling is considered to be activated by auxin by promoting its synthesis and suppressing its inactivation48, so one GA inactivation enzyme GA2ox10, and two grain size-related GA downstream genes, GRF1 and GRF6 were also be examined. The results showed that GA2ox10 was downregulated and GRFs were upregulated in KO-GS3.1 compared to the wild-type control (Supplementary Fig. 9a). The same assay was examined in NIL-GS3.1HP and NIL-GS3.1HJX, and the tendency of the above-mentioned marker genes was consistent with the comparison of KO-GS3.1 and the wild-type control (Supplementary Fig. 9b, c). All of the above results indicated that GS3.1 might participate in flavonoid and lignin biosynthesis by regulating the allocation of p-coumaric acid. The altered flavonoid and lignin biosynthesis influence downstream auxin and GA signaling pathways and cell wall synthesis, thereby resulting in a change of cell size and grain size.
Discussion
Enlarged grain size can directly enhance rice yield. As such, grain size became one of the most-studied yield traits over the past few decades. There were many QTLs discovered that regulated grain size though various pathways4,5,6,9,14,16,18,23,24,25,26,27,28. For example, QTLs involved in grain size has been linked to the ubiquitin-proteasome pathway, G-protein signaling pathway, mitogen-activated protein kinase signaling pathway, phytohormone pathways, and transcriptional regulation2. In recent years, some metabolism-related genes were linked to grain size such as Os02g57760 and GSA1. The former regulated grain size by affecting the trigonelline pathway and the latter by the phenylpropanoid pathway6,49. These two genes functioned as enzymes that directly catalyze their substrates, but another way to influence metabolism is to change the distribution of metabolic intermediates. For example, EDS5 transports isochorismate from plastids to the cytosol for further biosynthesis of SA34. The present study identified a QTL, GS3.1, which functioned as a MATE transporter that may transport a critical metabolic intermediate, p-coumaric acid, and control the metabolic fluxes of flavonoid and lignin biosynthesis by regulating the allocation of their common substrate, p-coumaric acid.
Previous studies indicated that auxin played a vital role in grain formation8,12. Auxin regulation includes auxin biosynthesis, transport, and signal transduction, which is considered to affect cell expansion8,12. Big Grain1 (BG1) is a positive regulator of primary auxin response and auxin transport12. Overexpression of BG1 resulted in larger grains by altering indole-3-acetic acid (IAA) distribution and transport. The mutant of TILLERING AND SMALL GRAIN 1 (TSG1) exhibited small grains by controlling local auxin biosynthesis8. ARFs such as OsARF24 were reported to regulate cell elongation in rice50. When it comes to GS3.1, some auxin-related genes such as OsYUCCA7, OsPIN1b, and OsARF24 were upregulated in KO-GS3.1, indicating that the auxin pathway was active in the absence of GS3.1 (Fig. 5j).
For studying the relationship between flavonoid synthesis and auxin transport, previous studies used a series of mutants named transparent testa (tt) in Arabidopsis47,51,52,53. Some of the tt mutants (tt3, tt4, tt5, tt6, and tt7) were known to be related to flavonoid biosynthesis, and tt4 was the most wildly-studied because it was a loss-of-function mutant of a key enzyme in flavonoid biosynthesis, chalcone synthase51,52,54. The tt4 mutant lacked flavonoids and showed a high rate of auxin transport, which was restored to a normal rate by treating with a flavonoid (naringenin), or flavonoid analog (1-N-naphthylphthalamic acid, NPA)52,55,56. The current study showed that in KO-GS3.1, the naringenin and total flavonoid content were downregulated compared to the wild-type (Fig. 5b, g). This study supposed that the activation of the auxin pathway is due to the reduced flavonoid content caused by the reduction of GS3.1 function in KO-GS3.1 and NIL-GS3.1HP. The decrease in flavonoid content maybe weakened the suppression of auxin transportation in panicles, which further activated the auxin signaling pathway, and finally led to enlarged cell and grain size.
Lignin is considered to be related to plant growth and stress resistance and has been shown to affect plant height, leaf size, and grain size31,57,58,59,60. Expression of a lignin biosynthetic gene, CCoAOMT from jute (Corchorus capsularis) in Arabidopsis resulted in increased plant height and silique length relative to non-transgenic plants59. NRL2 regulates lignin content and the loss-of-function mutant had narrow leaves and small grains compared to the wild-type31. GSA1 is a QTL that was associated with reduced grain size and lower lignin content6. In KO-GS3.1 the content of the lignin precursor was increased, and the grain size became larger than its respective control (Fig. 5e, f, h and Fig. 1b–e). On the other hand, lignin content was decreased in OE-GS3.1 which had a reduced grain size compared to its control (Fig. 5i and Supplementary Fig. 2j).
MATE was first characterized as a xenobiotic efflux pump, which was related to multidrug resistance. A series of MATE structures were resolved, within which was a crystal structure of AtDTX14 (c5y50A). AtDTX14 was determined to be the most similar to GS3.143,61,62. Mechanistic studies of AtDTX14 showed that AtDTX14 bonded its substrate at TM7, TM8, and TM10, and that TM7 played a vital role in its transport function43. Previous studies have described a large number of MATEs in plants, and clarified diverse secondary metabolites as substrates, including flavonoids, phytohormones, and their precursors, ions, nicotine, proanthocyanidin precursors, and toxic compounds34,35,37,38,63,64,65,66,67,68. Interestingly, in this study, p-coumaric acid was predicted to bond GS3.1 at TM7, TM10, and TM11 similarly to the AtDTX14 binding site. Thus, the current study predicted that GS3.1 likely functions as a MATE transporter for p-coumaric acid in rice (Fig. 4c).
Previous studies revealed that part of the phenylpropanoid biosynthetic pathway, including the enzymes producing p-coumaric acid from phenylalanine, transforming p-coumaric acid to naringenin (a flavonoid) and transforming p-coumaric acid to coniferyl alcohol (a monolignol) were localized to the cytoplasm69. Further studies indicated that some enzymes in these processes such as hydroxylcinnamoyl transferase (HCT) and cinnamate-4-hydroxylase (C4H) were anchored on the rough endoplasmic reticulum (rER) and some related cytoplasmic enzymes interacted with them to form complexes. For example, phenylalanine ammonia-lyase (PAL), the key enzyme for p-coumaric acid synthesis, interacted with C4H during p-coumaric acid biosynthesis process70,71,72,73,74,75. Additional evidence suggested that the rate-limiting enzyme for lignan biosynthesis, coumarate-3-hydroxylase (C3H) interacted with C4H76. Early flavonoid and iso-flavonoid biosynthesis enzymes were also reportedly located at the rER70. There is a theory that the cooperating enzymes in the phenylpropanoid pathway organized into complexes called metabolons in order to promote activation efficiency73,74,77. Based on the aforementioned evidence, the current study considers that the processes of phenylalanine catalysis into p-coumaric acid, p-coumaric acid catalysis into naringenin, and p-coumaric acid catalysis into coniferyl alcohol were separated into three metabolons, and the forepart of lignans biosynthesis metabolon might be more closely associated with the p-coumaric acid biosynthesis metabolon compared to flavonoid biosynthesis metabolon because of the direct interaction of C3H and C4H.
Previous studies had indicated that membrane-less compartments were formed based on liquid-liquid phase separation (LLPS), which relies on protein-protein interaction or some other noncovalent interactions to form biomolecular condensates78,79. An Autophagy-related protein, ATG8 (AUTOPHAGY-RELATED PROTEIN 8), recognizes and takes part in the protein condensate formation by LLPS80. The above-mentioned GS3.1 ortholog in Arabidopsis, ABS4, reportedly interacts with ATG841. This result was also verified using GS3.1 and OsATG8b by yeast two-hybrid and luciferase complementation assays (Supplementary Fig. 10). There is evidence that ABS4 localizes to late endosomes81, and these endosomes were observed to interact with protein condensates formed by LLPS82. Taken together, the p-coumaric acid biosynthetic metabolon, the lignans biosynthetic metabolon, and the flavonoid biosynthetic metabolon might be separated as three protein condensates formed by LLPS. The lignan biosynthetic metabolon might be closely functionally associated with the upstream p-coumaric acid biosynthetic metabolon. However, for flavonoid biosynthetic metabolon, p-coumaric acid might be transported to the flavonoid biosynthetic metabolon by GS3.1 with the assist of proteins like ATG8.
GS3.1 expression was significantly different between NIL-GS3.1HJX and NIL-GS3.1HP in panicles compared to other tissues, which indicated that GS3.1 might be regulated by a panicle-specific transcriptional factor. Meanwhile, the cis-element of this transcriptional factor differed between the promoters of NIL-GS3.1HJX and NIL-GS3.1HP alleles. Furthermore, the redundant paralogs of GS3.1 exhibited a different expression pattern compared to that of GS3.1, indicating that GS3.1 played a relatively more important role in panicles than other tissues such as leaves (Supplementary Fig. 6b). The phenylpropanoid pathway affects both plant growth and stress tolerance6,29,30,31,32. Reduced flavonoid content causes enhanced plant growth but also causes the reduction of stress tolerance as a side effect. The current study found that NIL-GS3.1HP has decreased GS3.1 expression only in young panicles but shows no difference in other tissues. Furthermore, there was no observed changes in flavonoid biosynthesis in other tissues and consequently not to cause the stress tolerance reduction (Supplementary Figs. 11 and 12).
To summarize the evidence above, a conceptual model was proposed for the regulation of grain size by modulating the allocation of p-coumaric acid between flavonoid and lignin anabolism. Compared to NIL-GS3.1HJX, the expression level of GS3.1 in NIL-GS3.1HP is decreased, which decreased the rate of p-coumaric acid transport in the panicle. Thus, p-coumaric acid is more readily available for the lignan biosynthetic metabolon, which leads to increased lignin biosynthesis and suppression of flavonoid biosynthesis in the panicle. Then, the reduced flavonoid levels weaken the inhibition of auxin transport, biosynthesis, and downstream signal transduction. The activated auxin pathway, subsequent activation of the GA pathway, and the increased lignin content encourage cell expansion, thereby enlarging grains and enhancing the rice yield (Fig. 6). Moreover, in NIL-GS3.1HP, there was no change in flavonoid biosynthesis in other tissues such as leaves and consequently not to result the stress tolerance reduction (Supplementary Figs. 11 and 12).
In HP young panicles, a lower GS3.1 expression level leads to reduced GS3.1 protein. As a result, less p-coumaric acid is transported to the flavonoid biosynthetic metabolon, which reduces flavonoid biosynthesis and instead increases p-coumaric acid availability for lignan biosynthesis. The decreased flavonoid content weakens the suppression of the auxin transport, biosynthesis and signal transduction, which in combination with subsequent activation of the gibberellin signaling and the increased lignin content results in a larger grain size in rice.
To conclude, the current study reported the identification and characterization of a QTL, GRAIN SIZE 3.1 (GS3.1), which encodes a MATE transporter protein. GS3.1 was found to mediate the distribution of p-coumaric acid between flavonoid biosynthesis and lignin biosynthesis, which led to the differences in flavonoid and lignin content between the panicles of natural allelic variants. Furthermore, flavonoid biosynthesis influenced the downstream auxin pathway and the gibberellic acid (GA) pathway. These alterations together with lignin content influenced grain size. Meanwhile, this study also discovered a gene resource by which rice yield can be enhanced without reducing stress tolerance.
Methods
Plant materials and growth conditions
A set of CSSLs (Chromosome Segment Substitution Lines) was constructed for identifying and characterizing grain size QTLs. An African rice variety, HP1 (HP, O. glaberrima, IRGC100127, International Rice Research Institute), was used as the donor parent and an Asian rice variety, HUA-JING-XIAN-74 (HJX74, HJX, O. sativa indica, CNA20050527.0, South China Agricultural University), was used as the recurrent parent. One CSSL named HPC078 was selected and backcrossed to HJX for mapping the QTL of grain size, and the QTL was named as GS3.1. The current study then consecutively selected plants that contained the GS3.1 locus and almost all other regions were homozygous for HJX for several rice harvests by marker-assisted selection. Finally, we selected a line that had the smallest region homozygous for HP that also contained GS3.1, referred to as NIL-GS3.1HP, and its isogenic control, referred to as NIL-GS3.1HJX. All rice plants were cultivated in an experimental field in Shanghai (121°E, 30°N, alt. 4.5 m) during the summer (May–October, 2014–2020, max temperature: 38 °C, min temperature: 17 °C, rainfall: 723.1 mm) and in Hainan (109°E, 18°N, alt. 7 m) during the spring (November– April of the following year, 2015–2020, max temperature: 33 °C, min temperature: 18 °C, rainfall: 152.3 mm) under natural growth conditions. The soil type was waterloggogenic paddy soil, and the soil pH was 6.65 in Shanghai and 5.94 in Hainan. The rice was transplanted 25 days after seeded and harvested at ripening stage (about 110 days after transplanting). Seedlings were fertilized thrice and applied pesticide five times during the grown period.
Fine mapping of the QTL of grain size
Grain length, grain width, and 1000-grain weight were measured to characterize grain size during fine mapping of GS3.1. Six molecular markers (3-2.934, 3-10.249, 3-20.52, 3-27.208, 3-28.502, 3-30.667 on chromosome 3) and 200 F2 plants of HPC078 backcrossed to HJX were used for fine mapping and GS3.1 was mapped to the interval between 3-27.208 and 3-28.502. Then, four new molecular markers (3-27.412, 3-27.426, 3-37.51, 27.822 on chromosome 3) in the aforementioned interval were designed and used for detecting recombinants in 6552 F2 plants. GS3.1 was mapped in the interval between the molecular markers 3-27.412 and 3-27.510 and then narrowed to a 9.45 kbp region on the short arm of chromosome 3 by sequencing. The candidate gene, Loc_Os03g12790, was amplified using HPC078 and HJX genomic DNA, sequenced and compared. The PCR primers used in this study are listed in Supplementary Data 2. Linkage maps were constructed using MAPMAKER/EXP 3.0 software83. QTLs were identified using the MAPMAKER/QTL program84.
RNA extraction and qRT-PCR
Total RNA was extracted using the E.Z.N.A Plant RNA Kit(Omega). Reverse transcription and cDNA synthesis were performed using the Evo M-MLV RT Kit with gDNA Clean for qPCR (Accurate Biotechnology) from 500 ng total RNA. The ABI 7300 Real-Time PCR System, Fast Start Universal SYBR Green Master MIX with ROX (Roche), and SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology) were used to perform quantitative RT-PCR analysis. An Applied Biosystems 7300 Real-Time PCR System Software (Version 1.4.0) was used for data collection and the 2−ΔΔCT method and Microsoft Excel 2019 were used for data analysis. Actin (LOC_Os03g50885) was used for normalization and either three or four biological replicates were used for one analysis. PCR primers for qRT-PCR are listed in Supplementary Data 2.
Plasmid construction
The CRISPR/Cas9 gene-editing construct of GS3.1 was designed and constructed as per the previous study85. Two designed gRNAs of GS3.1 were ligated to the OsU3 and OsU6a promoters and transferred into the pYLCRISPR/Cas9-MTmono vector. The plasmids for split-ubiquitin assay were constructed using T4 DNA ligase (New England Biolabs). The CDS fragments were prepared by PCR amplification and both fragments and vectors (pBT3-N and pPR3-N) were digested by SfiI. Other plasmids were all constructed using NEBuilder HIFI DNA Assembly Master Mix (New England Biolabs). The fragments were also prepared by PCR amplification and vectors were linearized by restriction endonuclease double digestion.
Full length GS3.1 genome sequence from the translation start codon to the last codon before the stop codon from HJX were cloned into pCAMBIA1300 under the control of rice ubiquitin promoter to create the GS3.1 overexpression construct. The sequence from 2.5 kbp upstream of the translation start codon of GS3.1 to 1.5 kbp downstream of the stop codon of GS3.1 from HJX was cloned into pCAMBIA1300 for the genomic complementation construct. The GUS plus gene promoted by GS3.1 promoter region (from 2.5 kbp upstream to the last base-pair before the translation start codon of GS3.1) of HJX allele was cloned to pCAMBIA1300 for the ProGS3.1HJX:GUS construct.
All plasmids used in this study were confirmed by sequencing. PCR primers for fragment amplification were listed in Supplementary Data 2 together with restriction endonuclease digestion sites.
Plant transformation
Agrobacterium tumefaciens-mediated transformation of rice calli was performed using strain EHA10586. Constructs were transferred into EHA105 Agrobacterium cells, which were grown in Yeast Mannitol Broth (YEB) medium at 30 °C overnight and then co-cultivated with rice calli for three days. The transgenic plants were then screened on 25 μg·mL−1 hygromycin. All transgenic plants were identified by PCR amplification of the hygromycin resistant gene and GS3.1 CRISPR/Cas9 transgenic plants were additionally identified by sequencing of the GS3.1 target region.
Histochemical analysis and electron microscopy24,87,88
Spikelet hulls before heading were fixed in FAA (50% ethanol, 5% glacial acetic acid, and 5% formaldehyde) and held in vacuo for 15 min for three to four times, then dehydrated using an ethanol series (30%, 50%, 70%, 80%, 90%, 95%, 100%, and 100% ethanol for 15 min each). Tissues were transferred to acetone and then embedded in Eponate 12 Resin (TED PELLA) and sliced into 8-μm or 6-μm thin sections with a rotary microtome (Leica). Sections of spikelet hulls were stained by Toluidine Blue (TBO), and the prepared tissue sections were observed under a light microscope (Carl Zeiss). For electron microscopy scanning, the spikelet hulls before heading were dried in a critical point drier (Leica) after dehydration, and mature grains were dried at 42 °C for 3–5 d after harvesting. Then the spikelet hulls were gold sputter coated, and imaged under a scanning electron microscope (JEOL and Carl Zeiss). Image J software was used to measure cell size and cell number89,90.
GUS staining
GUS staining was performed according to the previous method91. Tissues were soaked in acetone at 4 °C for 20 min and washed in PBS (50 mM, pH 7.0), then transferred to GUS staining buffer (PBS, 0.5 mM K-Fe-Nc, 0.1% Triton X-100, 20% methanol, and 0.6 mg·mL−1 X-Gluc) and held in vacuo for 15 min for twice. After that, samples were stained overnight in GUS staining buffer at 37 °C, and cleared in 95% ethanol to remove chlorophyll. The prepared tissues were then observed under a stereomicroscope and Leica Application Suite (Version V4.2) was used for image collection. For observing sections, cleared tissues were then sectioned and imaged as indicated above.
Phylogenetic relationship analysis
Multiple sequence alignments were performed in MEGA6 by MUSCLE under default parameters92. Phylogenetic analysis was carried out using the maximum-likelihood method in MEGA. LG with frequencies and gamma distributed (G) model and a complement deletion treatment for missing data (gaps) with a 1000 replications bootstrap analysis were used.
Prediction of GS3.1 three-dimensional (3D) structure and molecular docking
Three-dimensional (3D) structural models of GS3.1 were produced by Phyre2 mainly according to the previously described AtDTX14 structure (c5y50A) using default parameters43,93. The prediction of ligand-binding sites for the interaction with target molecules was made by molecular docking using the GEMDOCK software (BioXGEM) under the default parameters for stable docking94.
E. coli mutant growth complementation assay
The growth complementation assay using the drug-sensitive E. coli strain (BW25113Δacrb) was performed as described previously41,42. The full-length coding sequence of GS3.1 was cloned into pMAL-c5X (pMAL, New England Biolabs). The constructed pMAL-GS3.1 and pMAL were used to transform the Δacrb strain. Transformants and the wild-type BW25113 strain were streaked on LB plates containing 0.25 mM isopropyl β -D-thiogalactoside (IPTG), and 0.035 μg·mL−1 norfloxacin (Sigma) was added to the plate for drug resistance testing.
Widely-targeted metabolomics assay
Widely-targeted metabolomics was performed by Metware (Wuhan, China) as previously described6. The freeze-dried plant samples were ground using a mixer mill (MM 400, Retsch) with zirconia beads at 30 Hz for 1.5 min. The powder was weighed (100 mg) and extracted with 1 mL 70% aqueous methanol overnight at 4 °C. After centrifugation at 10,000 × g for 10 min, the sample extracts were absorbed (CNWBOND Carbon-GCB SPE Cartridge, 250 mg, 3 ml; ANPEL, Shanghai, China) and filtered (SCAA-104, 0.22 μm pore size; ANPEL). Then the extracts were analyzed using an LC-ESI-MS/MS system.
Lignin content measurement
For measuring lignin content, the cell walls were first isolated and then lignin was extracted from the isolated cell walls as previously described95,96. In brief, tissue powder was extracted with 70% ethanol at 70 °C for 1 h 3 times, the supernatant was discarded, and the residue was dried at 50 °C to a consistent weight. The alcohol-insoluble residue (largely cell wall material) was weighed (10–15 mg) and hydrolyzed with 0.3 mL thioglycolic acid and 1.5 mL 2 M HCl at 95 °C for 4 h. Centrifugations at 15,000 × g for 15 min were performed for precipitate collection and the material was washed three times with distilled water. Then the precipitate was extracted with 0.5 M NaOH at 20 °C with shaking for 16 h twice (1 mL and 400 μL NaOH). The supernatants were combined after centrifugation at 15,000 × g for 15 min each time. The combined supernatant was acidified with 400 μL concentrate HCl at 4 °C for 4 h, collected by centrifugation at 15,000 × g for 20 min and dissolved in 0.5 M NaOH. The absorbance of samples was measured at 280 nm (OD280) against a 0.5 M NaOH blank using a spectrometer (Beckman Coulter). The relative content of lignin was determined as OD280·g−1 of the weighed cell wall powder.
Split-ubiquitin assay
The split-ubiquitin assay was performed using the DUALmembrane pairwise interaction kit (Dualsystems Biotech)16,66. The membrane protein GS3.1 was cloned into the pBT3-N bait vector and OsATG8b was cloned into the pPR3-N prey vector. The yeast reporter strain NMY51 was used and pOst1-NubI was used as the positive control prey vector.
Luciferase complementation assay
The luciferase complementation assay was conducted as described previously97. GS3.1 was fused to the N-terminus of luciferase (nLUC) and OsATG8b was fused with C-terminus of luciferase (cLUC). Agrobacterium tumefaciens-mediated transient transformation was performed by injection into leaves of N. tabacum using strain GV3101. Luciferin (Promega) and UVP ChemStudio PLUS (Analytik Jena) were used for fluorescence excitation and imaging.
Open resource data acquisition
Rice genomic information was acquired from RGAP (http://rice.plantbiology.msu.edu/). Arabidopsis genomic information was retrieved from TAIR (https://www.arabidopsis.org/). Compound structures were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/).
Statistics and reproducibility
The difference between two groups were assessed by Student’s t-tests. The values represented in mean ± s.d. Statistically significant differences among multiple groups were evaluated by ANOVA followed by a Tukey’s test. Details of each statistical test are indicated in the figure legends.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Zuo, J. & Li, J. Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 48, 99–118 (2014).
Li, N., Xu, R. & Li, Y. Molecular networks of seed size control in plants. Annu. Rev. Plant Biol. 70, 435–463 (2019).
Xing, Y. & Zhang, Q. Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 61, 421–442 (2010).
Che, R. et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2, 15195 (2016).
Qi, P. et al. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res. 22, 1666–1680 (2012).
Dong, N. Q. et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 11, 2629 (2020).
Shi, C. L. et al. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 103, 1174–1188 (2020).
Guo, T. et al. Tillering and small grain 1 dominates the tryptophan aminotransferase family required for local auxin biosynthesis in rice. J. Integr. Plant Biol. 62, 581–600 (2020).
Liu, Q. et al. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 9, 1–12 (2018).
Miura, K. et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42, 545–549 (2010).
Ashikari, M. et al. Plant science: cytokinin oxidase regulates rice grain production. Science 309, 741–745 (2005).
Liu, L. et al. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc. Natl Acad. Sci. U SA 112, 11102–11107 (2015).
Tong, H. et al. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24, 2562–2577 (2012).
Zhang, X. et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl Acad. Sci. USA 109, 21534–21539 (2012).
Huang, X. et al. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41, 494–497 (2009).
Duan, P. et al. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat. Plants 1, 15203 (2015).
Si, L. et al. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 48, 447–456 (2016).
Hu, J. et al. A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant 8, 1455–1465 (2015).
Wang, S. et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954 (2012).
Wang, Y. et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 47, 944–948 (2015).
Zhang, L. et al. A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat. Commun. 8, 14789 (2017).
Jiao, Y. et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544 (2010).
Fan, C. et al. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171 (2006).
Guo, T. et al. GRAIN SIZE AND NUMBER1 Negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell 30, 871–888 (2018).
Hu, Z. et al. A kelch motif-containing serine/threonine protein phosphatase determines the large grain QTL trait in rice. J. Integr. Plant Biol. 54, 979–990 (2012).
Song, X.-J., Huang, W., Shi, M., Zhu, M.-Z. & Lin, H.-X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 39, 623–630 (2007).
Sun, S. et al. A G-protein pathway determines grain size in rice. Nat. Commun. 9, 851 (2018).
Mao, H. et al. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl Acad. Sci. USA 107, 19579–19584 (2010).
Peng, M. et al. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 8, 1975 (2017).
Nakabayashi, R. et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 77, 367–379 (2014).
Zhao, S. et al. NARROW AND ROLLED LEAF 2 regulates leaf shape, male fertility, and seed size in rice. J. Integr. Plant Biol. 58, 983–996 (2016).
Cui, L. G., Shan, J. X., Shi, M., Gao, J. P. & Lin, H. X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 80, 1108–1117 (2014).
Upadhyay, N. et al. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 70, 4643–4656 (2019).
Rekhter, D. et al. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365, 498–502 (2019).
Zhang, H. et al. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 7, 1522–1532 (2014).
Magalhaes, J. V. How a microbial drug transporter became essential for crop cultivation on acid soils: aluminium tolerance conferred by the multidrug and toxic compound extrusion (MATE) family. Ann. Bot. 106, 199–203 (2010).
Li, L., He, Z., Pandey, G. K., Tsuchiya, T. & Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 277, 5360–5368 (2002).
Zhang, H. et al. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E2036–E2045 (2017).
Qin, P. et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 7, eabc8873 (2021).
Suzuki, M., Sato, Y., Wu, S., Kang, B. H. & McCarty, D. R. Conserved functions of the MATE transporter BIG EMBRYO1 in regulation of lateral organ size and initiation rate. Plant Cell 27, 2288–2300 (2015).
Jia, M. et al. Noncanonical ATG8–ABS3 interaction controls senescence in plants. Nat. Plants 5, 212–224 (2019).
Tanaka, Y. et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature 496, 247–251 (2013).
Miyauchi, H. et al. Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat. Commun. 8, 1633 (2017).
Dixon, R. A. & Steele, C. L. Flavonoids and isoflavonoids—a gold mine for metabolic engineering. Trends Plant Sci. 4, 394–400 (1999).
Dong, N. Q. & Lin, H. X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 63, 180–209 (2021).
Whetten, R. W., MacKay, J. J. & Sederoff, R. R. Recent advances in understanding lignin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 585–609 (1998).
Peer, W. A. & Murphy, A. S. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12, 556–563 (2007).
Dorcey, E., Urbez, C., Blázquez, M. A., Carbonell, J. & Perez-Amador, M. A. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 58, 318–332 (2009).
Chen, W. et al. Comparative and parallel genome-wide association studies for metabolic and agronomic traits in cereals. Nat. Commun. 7, 12767 (2016).
Li, G. et al. Rice actin-binding protein RMD is a key link in the auxin-actin regulatory loop that controls cell growth. Proc. Natl Acad. Sci. USA 111, 10377–10382 (2014).
Shirley, B. W. et al. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 8, 659–671 (1995).
Brown, D. E. et al. Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol. 126, 524–535 (2001).
Buer, C. S. & Djordjevic, M. A. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J. Exp. Bot. 60, 751–763 (2009).
Ward, J. M., Cufr, C. A., Denzel, M. A. & Neff, M. M. Corrections. Plant Cell 17, 2614–2614 (2005).
Murphy, A., Peer, W. A. & Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211, 315–324 (2000).
Peer, W. A. et al. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with auxin transport in Arabidopsis thaliana. Plant Cell 16, 1898–1911 (2004).
Liu, Q., Luo, L. & Zheng, L. Lignins: biosynthesis and biological functions in plants. Int. J. Mol. Sci. 19, 335 (2018).
Vanholme, R. et al. A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell 24, 3506–3529 (2012).
Zhang, G. et al. The CCoAOMT1 gene from jute (Corchorus capsularis L.) is involved in lignin biosynthesis in Arabidopsis thaliana. Gene 546, 398–402 (2014).
Ahn, C. S., Ahn, H. K. & Pai, H. S. Overexpression of the PP2A regulatory subunit Tap46 leads to enhanced plant growth through stimulation of the TOR signalling pathway. J. Exp. Bot. 66, 827–840 (2015).
Lu, M. et al. Structures of a Na+-coupled, substrate-bound MATE multidrug transporter. Proc. Natl Acad. Sci. USA 110, 2099–2104 (2013).
He, X. et al. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature 467, 991–994 (2010).
Thompson, E. P., Wilkins, C., Demidchik, V., Davies, J. M. & Glover, B. J. An Arabidopsis flavonoid transporter is required for anther dehiscence and pollen development. J. Exp. Bot. 61, 439–451 (2010).
Chen, G., Liang, H., Zhao, Q., Wu, A. & Wang, B. Exploiting MATE efflux proteins to improve flavonoid accumulation in Camellia sinensis in silico. Int. J. Biol. Macromol. 143, 732–743 (2020).
Tegli, S., Bini, L., Calamai, S., Cerboneschi, M. & Biancalani, C. A MATE transporter is involved in pathogenicity and IAA homeostasis in the hyperplastic plant pathogen Pseudomonas savastanoi pv. nerii. Microorganisms 8, 156 (2020).
Tian, W. et al. A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 6, 6057 (2015).
Morita, M. et al. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl Acad. Sci. USA 106, 2447–2452 (2009).
Gonzalez, A. et al. TTG2 controls the developmental regulation of seed coat tannins in Arabidopsis by regulating vacuolar transport steps in the proanthocyanidin pathway. Dev. Biol. 419, 54–63 (2016).
Barber, M. S. & Mitchell, H. J. Regulation of phenylpropanoid metabolism in relation to lignin biosynthesis in plants. Int. Rev. Cytol. 172, 243–293 (1997).
Saslowsky, D. & Winkel-Shirley, B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 27, 37–48 (2001).
Bassard, J. E. et al. Protein-protein and protein-membrane associations in the lignin pathwayw oa. Plant Cell 24, 4465–4482 (2012).
Yan, X. et al. CAD1 and CCR2 protein complex formation in monolignol biosynthesis in Populus trichocarpa. N. Phytol. 222, 244–260 (2019).
Wang, J. P., Liu, B., Sun, Y., Chiang, V. L. & Sederoff, R. R. Enzyme-enzyme interactions in monolignol biosynthesis. Front. Plant Sci. 9, 1–7 (2019).
Biała, W. & Jasiński, M. The phenylpropanoid case—it is transport that matters. Front. Plant Sci. 9, 1–8 (2018).
Achnine, L., Blancaflor, E. B., Rasmussen, S. & Dixon, R. A. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16, 3098–3109 (2004).
Chen, H. C. et al. Membrane protein complexes catalyze both 4- and 3-hydroxylation of cinnamic acid derivatives in monolignol biosynthesis. Proc. Natl Acad. Sci. USA 108, 21253–21258 (2011).
Sweetlove, L. J. & Fernie, A. R. The spatial organization of metabolism within the plant cell. Annu. Rev. Plant Biol. 64, 723–746 (2013).
Xie, D. et al. Phase separation of SERRATE drives dicing body assembly and promotes miRNA processing in Arabidopsis. Nat. Cell Biol. 23, 32–39 (2021).
Wunder, T. & Mueller-Cajar, O. Biomolecular condensates in photosynthesis and metabolism. Curr. Opin. Plant Biol. 58, 1–7 (2020).
Wilfling, F. et al. A selective autophagy pathway for phase-separated endocytic protein deposits. Mol. Cell 80, 764–778.e7 (2020).
Wang, R. et al. A subgroup of MATE transporter genes regulates hypocotyl cell elongation in Arabidopsis. J. Exp. Bot. 66, 6327–6343 (2015).
Zhao, Y. G. & Zhang, H. Phase separation in membrane biology: the interplay between membrane-bound organelles and membraneless condensates. Dev. Cell 55, 30–44 (2020).
Lander, E. S. et al. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1, 174–181 (1987).
Lander, E. S. & Botstein, D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121, 185–199 (1989).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282 (1994).
Guo, T. et al. A SAC phosphoinositide phosphatase controls rice development via hydrolyzing PI4P and PI(4,5)P 2. Plant Physiol. 182, 1346–1358 (2020).
Luft, J. H. Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol. 9, 409–414 (1961).
Schindelin, J., Rueden, C. T., Hiner, M. C. & Eliceiri, K. W. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518–529.
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907 (1987).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. Trabajo práctico No 13. Varianzas en función de variable independiente categórica. Nat. Protoc. 10, 845–858 (2016).
Yang, J. M. & Chen, C. C. GEMDOCK: a generic evolutionary method for molecular docking. Proteins Struct. Funct. Genet. 55, 288–304 (2004).
Musel, C. et al. Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical immunological probes. Planta 201, 146–159 (1997).
Turner, S. R. & Somerville, C. R. Collapsed xylem phenotype of arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9, 689–701 (1997).
Zhou, Z., Bi, G. & Zhou, J. M. Luciferase complementation assay for protein-protein interactions in plants. Curr. Protoc. plant Biol. 3, 42–50 (2018).
Acknowledgements
We thank Professor Guiquan Zhang (South China Agricultural University) for providing CSSL plant materials. We thank Min Shi (CAS Center for Excellence in Molecular Plant Sciences, Shanghai Institute of Plant Physiology and Ecology, CAS) for technical supports of transgenic assay. We thank Jiqin Li and Zhiping Zhang (CAS Center for Excellence in Molecular Plant Sciences, Shanghai Institute of Plant Physiology and Ecology, CAS) for technical supports of histochemical analysis and electron microscopy scanning. We thank Professor Yaoguang Liu (South China Agriculture University) for donation of CRISPR/Cas9 plasmids. We thank Professor Fei Yu (Northwest A & F University) for donation of BW25113Δacrb and BW25113 E. coli strain. This work was supported by the grants from Laboratory of Lingnan Modem Agriculture Project (NT2021002), Chinese Academy of Sciences (XDB27010104, QYZDY-SSW-SMC023, 159231KYSB20200008), National Natural Science Foundation of China (31788103), the Shanghai Science and Technology Development (18JC1415000), CAS-Croucher Funding Scheme for Joint Laboratories and National Key Laboratory of Plant Molecular Genetics.
Author information
Authors and Affiliations
Contributions
H.X.L. conceived and supervised the project, and H.X.L. and Y.M.Z. designed the experiments. Y.M.Z. performed most of the experiments. H.X.Y., W.W.Y., J.X.S., N.Q.D., T.G., Y.K., Y.H.X., H.Z., Y.B.Y., Y.C.L., H.Y.Z., Z.Q.L., S.Q.G., J.J.L., B.L., X.R.M., Y.J.C., J.J.Y., and H.X.L. performed some of the experiments. Y.M.Z. and H.X.L. analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks Jitendra Kumar and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Leena Tripathi and Caitlin Karniski. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, YM., Yu, HX., Ye, WW. et al. A rice QTL GS3.1 regulates grain size through metabolic-flux distribution between flavonoid and lignin metabolons without affecting stress tolerance. Commun Biol 4, 1171 (2021). https://doi.org/10.1038/s42003-021-02686-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02686-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.