Abstract
Eukaryotic membrane fusion requires trans-SNARE complexes bridging the gap between adjacent membranes1. Fusion between a transport vesicle and its target membrane transforms the trans- into a cis-SNARE complex. The latter interacts with the hexameric AAA+-ATPase N-ethylmaleimide-sensitive factor (NSF) and its co-factor alpha-soluble NSF attachment protein (αSNAP), forming a 20S complex2,3. ATPase activity disassembles the SNAP receptor (SNARE) complex into Qa-SNARE, which folds back onto itself, and its partners4,5. The fusion of identical membranes has a different sequence of events6. The fusion partners each have cis-SNARE complexes to be broken up by NSF and αSNAP. The Qa-SNARE monomers are then stabilized by interaction with Sec1/Munc18-type regulators (SM proteins) to form trans-SNARE complexes, as shown for the yeast vacuole7. Membrane fusion in Arabidopsis cytokinesis is formally akin to vacuolar fusion8. Membrane vesicles fuse with one another to form the partitioning membrane known as the cell plate. Cis-SNARE complexes of cytokinesis-specific Qa-SNARE KNOLLE and its SNARE partners are assembled at the endoplasmic reticulum and delivered by traffic via the Golgi/trans-Golgi network to the cell division plane9. The SM protein KEULE is required for the formation of trans-SNARE complexes between adjacent membrane vesicles10. Here we identify NSF and its adaptor αSNAP2 as necessary for the disassembly of KNOLLE cis-SNARE complexes, which is a prerequisite for KNOLLE–KEULE interaction in cytokinesis. In addition, we show that NSF is required for other trafficking pathways and interacts with the respective Q-SNAREs. The SNARE complex disassembly machinery is conserved in plants and plays a unique essential role in cytokinesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All materials are available from the corresponding author upon request. All data generated or analysed during this study are included in this Letter. Source data are provided with this paper.
References
Jahn, R. & Scheller, R. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006).
Zhou, Q. et al. Cryo-EM structure of SNAP–SNARE assembly in 20S particle. Cell Res. 25, 551–560 (2015).
Zhao, M. & Brunger, A. Recent advances in deciphering the structure and molecular mechanism of the AAA+ ATPase N-ethylmaleimide-sensitive factor (NSF). J. Mol. Biol. 428, 1912–1926 (2016).
Huang, X. et al. Mechanistic insights into the SNARE complex disassembly. Sci. Adv. 5, eaau8164 (2019).
Kim, C. et al. Extreme parsimony in ATP consumption by 20S complexes in the global disassembly of single SNARE complexes. Nat. Commun. 12, 3206 (2021).
Baker, R. & Hughson, F. Chaperoning SNARE assembly and disassembly. Nat. Rev. Mol. Cell Biol. 17, 465–479 (2016).
Baker, R. et al. A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science 349, 1111–1114 (2015).
Müller, S. & Jürgens, G. Plant cytokinesis—no ring, no constriction but centrifugal construction of the partitioning membrane. Semin. Cell Dev. Biol. 53, 10–18 (2016).
Karnahl, M., Park, M., Mayer, U., Hiller, U. & Jürgens, G. ER assembly of SNARE complexes mediating formation of partitioning membrane in Arabidopsis cytokinesis. eLife 19, e25327 (2017).
Park, M., Touihri, S., Müller, I., Mayer, U. & Jürgens, G. Sec1/Munc18 protein stabilizes fusion-competent syntaxin for membrane fusion in Arabidopsis cytokinesis. Dev. Cell 22, 989–1000 (2012).
Novick, P., Field, C. & Schekman, R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 (1980).
Block, M., Glick, B., Wilcox, C. & Rothman, J. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl Acad. Sci. USA 85, 7852–7856 (1988).
Clary, D., Griff, I. & Rothman, J. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell 61, 709–721 (1990).
Fujiwara, M. et al. Interactomics of Qa-SNARE in Arabidopsis thaliana. Plant Cell Physiol. 55, 781–789 (2014).
Rancour, D., Dickey, C., Park, S. & Bednarek, S. Characterization of AtCDC48: evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol. 130, 1241–1253 (2002).
Tang, L. P. et al. AtNSF regulates leaf serration by modulating intracellular trafficking of PIN1 in Arabidopsis thaliana. J. Integr. Plant Biol. 63, 737–755 (2020).
Tanabashi, S. et al. A missense mutation in the NSF gene causes abnormal Golgi morphology in Arabidopsis thaliana. Cell Struct. Funct. 43, 41–51 (2018).
Liu, F. et al. The canonical alpha-SNAP is essential for gametophytic development in Arabidopsis. PLoS Genet. 17, e1009505 (2021).
Whiteheart, S. et al. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 129, 945–954 (1994).
Dalal, S., Rosser, M., Cyr, D. & Hanson, P. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell 15, 637–648 (2004).
Barnard, R., Morgan, A. & Burgoyne, R. Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J. Cell Biol. 139, 875–883 (1997).
Weijers, D., Van Hamburg, J. P., Van Rijn, E., Hooykaas, P. J. & Offringa, R. Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol. 133, 1882–1892 (2003).
Zuo, J., Niu, Q. & Chua, N. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24, 265–273 (2000).
Alexandersson, E., Saalbach, G., Larsson, C. & Kjellbom, P. Arabidopsis plasma membrane proteomics identifies components of transport, signal transduction and membrane trafficking. Plant Cell Physiol. 45, 1543–1556 (2004).
Richter, S. et al. Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. eLife 8, e02131 (2014).
Geldner, N., Friml, J., Stierhof, Y., Jürgens, G. & Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428 (2001).
Shirakawa, M. et al. Arabidopsis Qa-SNARE SYP2 proteins localized to different subcellular regions function redundantly in vacuolar protein sorting and plant development. Plant J. 64, 924–935 (2010).
Uemura, T. & Ueda, T. Plant vacuolar trafficking driven by RAB and SNARE proteins. Curr. Opin. Plant Biol. 22, 116–121 (2014).
Samuels, A., Giddings, T. J. & Staehelin, L. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J. Cell Biol. 130, 1345–1357 (1995).
Waizenegger, I. et al. The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr. Biol. 10, 1371–1374 (2000).
Lukowitz, W., Mayer, U. & Jürgens, G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84, 61–71 (1996).
Lauber, M. et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 139, 1485–1493 (1997).
Hong, Z., Delauney, A. & Verma, D. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 13, 755–768 (2001).
Thiele, K. et al. The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J. 58, 13–26 (2009).
El Kasmi, F. et al. SNARE complexes of different composition jointly mediate membrane fusion in Arabidopsis cytokinesis. Mol. Biol. Cell 24, 1593–1601 (2013).
Park, M. et al. Concerted action of evolutionarily ancient and novel SNARE complexes in flowering-plant cytokinesis. Dev. Cell 44, 500–511.e504 (2018).
Kwon, C. et al. Co-option of a default secretory pathway for plant immune responses. Nature 451, 835–840 (2008).
Vietri, M., Radulovic, M. & Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 21, 25–42 (2020).
Frémont, S. & Echard, A. Membrane traffic in the late steps of cytokinesis. Curr. Biol. 28, R458–R470 (2018).
Chang, L. et al. Structural characterization of full-length NSF and 20S particles. Nat. Struct. Mol. Biol. 9, 268–275 (2012).
Jiao, J. et al. Munc18-1 catalyzes neuronal SNARE assembly by templating SNARE association. eLife 7, e41771 (2018).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Dettmer, J., Hong-Hermesdorf, A., Stierhof, Y. D. & Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18, 715–730 (2006).
Karnahl, M. et al. Functional diversification of Arabidopsis SEC1-related SM proteins in cytokinetic and secretory membrane fusion. Proc. Natl Acad. Sci. USA 115, 6309–6314 (2018).
Müller, I. et al. Syntaxin specificity of cytokinesis in Arabidopsis. Nat. Cell Biol. 5, 531–534 (2003).
Musielak, T., Schenkel, L., Kolb, M., Henschen, A. & Bayer, M. A simple and versatile cell wall staining protocol to study plant reproduction. Plant Reprod. 28, 161–169 (2015).
Heese, M. et al. Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J. Cell Biol. 155, 239–249 (2001).
Zheng, H. et al. NPSN11 is a cell plate-associated SNARE protein that interacts with the syntaxin KNOLLE. Plant Physiol. 129, 530–539 (2002).
Sanderfoot, A., Kovaleva, V., Bassham, D. & Raikhel, N. Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol. Biol. Cell 12, 3733–3743 (2001).
Zhang, Z. et al. A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J. 49, 302–312 (2007).
Sanderfoot, A., Kovaleva, V., Zheng, H. & Raikhel, N. The t-SNARE AtVAM3p resides on the prevacuolar compartment in Arabidopsis root cells. Plant Physiol. 121, 929–938 (1999).
Bubeck, J. et al. The syntaxins SYP31 and SYP81 control ER–Golgi trafficking in the plant secretory pathway. Traffic 9, 1629–1652 (2008).
Singh, M. et al. A single class of ARF GTPase activated by several pathway-specific ARF–GEFs regulates essential membrane traffic in Arabidopsis. PLoS Genet. 14, e1007795 (2018).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Use R) 1–212 (Springer Press, 2009).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing http://www.R-project.org/ (2020).
Acknowledgements
We thank K. Romanova for the initial mutant screening, T. Nürnberger and C. Hua for introducing us to size-exclusion chromatography and providing the equipment, R. Stahl and L. Henneberg for electron microscopy technical assistance, the Tübingen Structural Microscopy facility for providing access to their high-pressure freezing machine (EXC 2124-390838134), S. Bednarek (University of Wisconsin–Madison) for anti-SYP31 antiserum, K. Schumacher (COS, Heidelberg University) for anti-SYP81 antiserum, T. Uemura (Ochanomizu University) and A. Nakano (RIKEN Center, Waco) for sharing T-DNA lines, and M. Bayer and F. El Kasmi for critical reading. This work was funded by the Deutsche Forschungsgemeinschaft (DFG JU 179/24-1 to G.J.). Additional DFG funding numbers: Leica TCS-SP8, INST 37/819-1 FUGG; TEM Electron microscope, INST 37/900-1 FUGG.
Author information
Authors and Affiliations
Contributions
M.P. and G.J. conceptualized the project and wrote the original draft of the manuscript. M.P. and U.M. devised the methodology and conducted the investigation. S.R. conducted the electron microscopy analysis. M.P., U.M., S.R. and G.J. reviewed and edited the manuscript. G.J. acquired the funding, provided the resources and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Panagiotis Moschou, Yiqun Bao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sequence alignment and predicted protein structures.
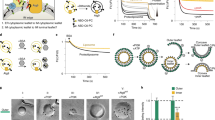
(a) Alignment of C-terminal sequences of αSNAP-related proteins. Arabidopsis thaliana αSNAP1 (AT3G56450) and αSNAP2 (AT3G56190) were aligned with αSNAP from human (Homo sapiens, GenBank NP_003818), cattle (Bos taurus, GenBank AAB25812) and yeast (Saccharomyces cerevisiae, GenBank NP_009503) in the CLC Main Workbench program. Note that αSNAP1 is larger than the other αSNAPs and less similar to bovine αSNAP (E value 1e-29 vs. 2e-71 for αSNAP2). Asterisk marks the conserved leucine residue L288 that was mutated to alanine in this study. Numbers indicate protein lengths (amino acid residues). (b-e) Structural models of NSF and αSNAP2. Predicted structures of wild type NSF (grey, b and c) and αSNAP2 (grey, d and e) are superimposed with those of dominant-negative NSFEQ (green, b and c) and αSNAP2LA (blue, d and e), respectively. The substituted residues are highlighted in red (b-e). (c and e) Boxed areas in (b and d) at higher magnification.
Extended Data Fig. 2 Schematic of NSF and αSNAP2 and impact of their dominant-negative variants on Arabidopsis embryo development.
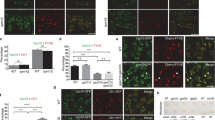
(a) Schematic of NSF and αSNAP2. NSF domains indicated; N domain, interaction with αSNAP2. Numbers, amino acid positions. Residues altered in dominant-negative variants highlighted in red: E326 (NSF), L288 (αSNAP2). (b-m) Embryo abnormalities caused by RPS5A (R)»UAS two-component expression of NSFEQ:G (GFP-tagged dominant-negative NSFE326Q, b-g) or R:αS2LA (RFP-tagged dominant-negative αSNAP2L188A, h-m). Non-transgenic wild-type control embryo (n-p); (b, e, h, k, n) cell-wall staining with Renaissance blue 2200; (c, f, i, l, o) FP, fluorescent fusion proteins (c and f, GFP; i and l, RFP; o, no FP fluorescence); (d, g, j, m, p) bright-field Images; scale bars, 10 µm. Note that the mutants were clearly discernible around the heart stage, developing severe abnormalities thereafter (e-g, k-m). Number of embryos analysed, ≥300.
Extended Data Fig. 3 Interaction of NSF with αSNAP2 and impact of their dominant-negative variants on Arabidopsis seedling development.
(a-d) Seedling abnormalities caused by estradiol (EST)-induced expression of NSFEQ:G (a) or R:αS2LA (b) were analyzed 5 days after germination (5DAG) on EST plates. Below: Boxed areas at higher magnification. C, non-transformed wild-type; two transgenic lines (1, 2) each for the wild-type (NSF:G, R:αS2) and dominant-negative (NSFEQ:G, R:αS2LA) constructs. (c, d) Control seedlings grown in estradiol solvent DMSO for 5 days. Scale bars, 1 cm. Number of seedlings analysed, ≥200 (a-d). (e-f) Expression of transgene-encoded proteins. Protein extracts from seedlings of two transgenic lines (1, 2) incubated with EST (inducer) or DMSO (solvent control) for 24 hours were separated of SDS-PAGE gels and immunoblotted (IB) with anti-GFP (e) and anti-RFP (f) antibodies. C, non-transformed wild-type; two transgenic lines (1, 2) each for the wild-type (NSF:G in e, R:αS2 in f) and dominant-negative (NSFEQ:G in e, R:αS2LA in f) constructs. Molecular marker size on the left (kDa, kilodalton); Pon S, Ponceau S-stained membrane as loading control. (g) Co-immunoprecipitation of NSF:G and R:αS2. T, total extract; U, unbound; IP, immunoprecipitate (IP-GFP, anti-GFP beads). IB, immunoblot with antibody indicated (right); Molecular marker size on the left (kDa, kilodalton) NSF:G/R:αS2, doubly transgenic; NSF:G or R:αS2, singly transgenic.
Extended Data Fig. 4 Subcellular localization of NSF and αSNAP2 in seedling roots.
(a-b) Live imaging of wild type NSF:GFP (a) and dominant-negative NSFEQ:GFP (b). (c-e) Live imaging of NSF:GFP (NSF:G) and wild type RFP:αSNAP2 (R:αS2) in seedling roots. (g-n) Localization of NSF at TGN/early endosomes. Live imaging of NSF:GFP (g-i) and NSFEQ:GFP (k-m) against TGN marker a1-RFP. Arrowheads indicate merged punctate signals of NSF:GFP (i) or NSFEQ:GFP (m) with a1-RFP. (f, j, n) Signal intensities of the scanned lines in (e, i, m). f, NSF:G (green) to R:αS2 (red); j, NSF:G (green) to a1-RFP (red); n, NSFEQ:G (green) to a1-RFP (red). Arrowheads indicate merged signals (e, i, m). Double arrowheads indicate the cell division plane positively labeled with NSF:G (e and i) or NSFEQ:G (b). Scale bars, 5 µm (a, b, e, i, m). Images were taken after 24 hours of EST induction. Numbers of seedlings analysed: >100 (a and b); 12 (c-e); 22 (g-i); 23 (k-m).
Extended Data Fig. 5 Effect of dominant-negative NSF on endocytosis.
(a-l) Live imaging of NSF:G (a-c) or NSFEQ:G (d-f) and endocytic tracer SynaptoRedTM C2 in seedling roots after 24 hours of EST induction. (g-l) Imaging of NSF:G (g-i) or NSFEQ:G (j-l) and SynaptoRedTM C2 upon brefeldin A (BFA) application. (m) Cytosolic intensity quantification of SynaptoRedTM C2 signals. Cytosolic area in a single cell was selected for quantification. The P value was calculated using two-tailed Welch’s t-test by comparing NSF:G to NSFEQ:G. The center line of the box plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. Data points are shown as jitter. n, number of counted cells.
Extended Data Fig. 6 Effect of dominant-negative NSF on vacuolar traffic.
(a-f) Live imaging of NSF:G (a-c) or NSFEQ:G (d-f) and vacuolar soluble cargo marker AFVY:RFP in seedling roots after 24 hours of EST induction. (g) Intensity quantification of AFVY:RFP. Vacuolar area in each single cell was selected for quantification. The P value was calculated using unpaired Student’s t-test (two-tailed) by comparing wild type NSF:G to NSFEQ:G. The center line of the box plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. Data points are shown as jitter. n, the number of counted cells. Scale bars, 5 µm (c, f).
Extended Data Fig. 7 Semi-quantitative analysis of coimmunoprecipitation assay.
(a-b) Group box plots of semi-quantitative analysis. Signal intensities of the immunoprecipitate (IP) fractions with anti-GFP (a) and anti-RFP (b) beads were measured from the two or three immunoblots that were probed with Q-SNARE antisera as in Fig. 1i,j: SYP132, SYP121, SYP61, SYP31, SYP21, SYP81. Band intensity values were normalized with NSF:G and R:αS2 set at 100; A.U, arbitrary units. For simplicity, IP fractions from the non-transformed wild type extract were not shown. The P values were calculated using unpaired Student’s t-test (two-tailed) by comparing the respective wild type to its dominant negative mutant. The center line of the box plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. n, number of the counted immunoblots. Note that P value was not calculated (n.c) for n≤2.
Extended Data Fig. 8 Cytokinesis defects caused by expression of dominant-negative αSNAP2 dominant negative variant.
Dividing cells from embryos (a-f) or seedling roots (h-m) expressing wild type R:αS2 (a-c, h-j) or dominant-negative R:αS2LA (d-f, k-m) counterstained with anti-KNOLLE (KN) (a-f) and anti-callose (h-m) antisera. (a, d, h, k) RFP; (b, e, i, l) KNOLLE; (j, m) callose; (c, f) merged images and nuclei stained with DAPI (blue); (j, m) Insets, merged images of GFP, anti-callose (cyan) and anti-KNOLLE (green); double arrowheads, cell division plane region (a, d, h, k); scale bars, 5 µm (c, f, j, m). (a-f) RPS5A (R)::GAL4»UAS expression; (h-m) estradiol induction. Double arrowheads, plane of cell division region (a, d, h, k). (g, n) Width quantification of KNOLLE (g) or callose (n) label in the cell division region. The P values were calculated using two-tailed Welch’s t-test by comparing wild type R:αS2 (pale green, g; pale blue, n) to dominant-negative R:αS2LA (green, g; blue, n). The center line of the box plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. Data points are shown as jitter. n, number of counted cells.
Extended Data Fig. 9 Semi-quantitative analysis of coimmunoprecipitation assay, interaction of NSF and KNOLLE and chromatography analysis of NSF and KNOLLE complexes.
(a-b) Group box plots of semi-quantitative analysis. Signal intensities of the immunoprecipitate (IP) fractions with anti-GFP (a) and anti-RFP (b) beads were measured from two or three immunoblots probed with SNARE antisera as in Fig. 3a,b: KNOLLE, NPSN11, SYP33, SYP71, VAMP721/722 (VAMP721). Band intensity values were normalized with wild type NSF and αSNAP2 set at 100; A.U, arbitrary units. For simplicity, immunoprecipitate from the non-transformed wild type extract was not included. The P values were calculated using unpaired Student’s t-test (two-tailed) by comparing the respective wild type to its dominant negative mutant. The center line of the whisker plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. n, number of the counted immunoblots. (c-d) Coimmunoprecipitation of wild type NSF:G or dominant-negative NSFEQ:G with Myc:KNOLLE (Myc:KN). Extracts from non-transformed wild-type (C) or transgenic seedlings coexpressing Myc:KN and NSF:G or NSFEQ:G were immunoprecipitated (IP) with anti-Myc beads. Protein blots were immunoblotted (IB) with antibodies indicated on the right. Myc:KN, immunoprecipitate of extract from transgenic seedlings expressing only Myc:KNOLLE. Band intensity values were normalized with wild type NSF:G in IP fraction set at 100%. Molecular marker size on the left (kDa, kilodalton); Total, total extract; IP, immunoprecipitate. (d) Semi-quantitative analysis of (c). Signal intensities of the immunoprecipitate (IP) were measured from two immunoblots. Band intensity values were normalized with wild type NSF:G in IP fraction set at 100; A.U, arbitrary units. For simplicity, immunoprecipitate from the non-transformed wild type extract was not included. The center line of the box plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. n, number of the counted immunoblots. Note that P value was not calculated (n.c) for n≤2. (e) Immunoblots from size-exclusion chromatography of NSF and KNOLLE complexes shown in Fig. 3c. Continuous fractions of NSF:G and NSFEQ:G in the high molecular mass range were immunoblotted (IB) with the indicated antisera anti-GFP, anti-KNOLLE (KN), and anti-VAMP721/722 (V721). INPUT, filtered protein extracts before running size exclusion chromatography; C, non-transformed wild-type protein extracts. Peaks of the standard markers are indicated (669 kDa, thyroglobulin; 443 kDa, ferritin).
Extended Data Fig. 10 Interference with KEULE localization at the cell division plane by αSNAP2 dominant negative variant.
(a-h) KNOLLE (KN) and KEULE localization in dividing cells of seedling roots after estradiol induction of wild-type R:αS2 (red, a-d) or dominant-negative αS2LA (red, e-h). (b, f) anti-KN antiserum (blue); (c, g) vYFP (vY):KEULE (green); same images as shown in Fig. 3d–i; (a, e) RFP channels; (d, h) merged images; scale bars, 5 µm (d, h). Double arrowheads, cell division plane region (a, e). (i) Localization efficiency of KEULE at the cell division plane (CDP). The P value was calculated using two-tailed Welch’s t-test by comparing R:αS2 to R:αS2LA. The center line of the box plots is the median, the bottom and top lines represent the lower quartile and upper quartile, respectively. Data points are shown as jitter. n, number of images comprising 40 cytokinetic cells in R:αS2 and 119 cells in R:αS2LA that were analysed in total, respectively. Numbers of seedlings analysed: 24 (a-d); 40 (e-h).
Supplementary information
Supplementary Information
Supplementary Table 1.
Supplementary Data 1
List of primers used for cloning.
Supplementary Table 1
Seed viability for Supplementary Table 1.
Source data
Source Data Fig. 1
Unprocessed immunoblots for Fig. 1.
Source Data Fig. 3
Unprocessed immunoblots for Fig. 3.
Source Data Extended Data Fig. 2
Width measurements of KNOLLE and callose for Extended Data Fig. 2g,n.
Source Data Extended Data Fig. 3
Unprocessed immunoblots for Extended Data Fig. 3.
Source Data Extended Data Fig. 5
Cytosolic signal intensity of SynaptoRed C2 for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Signal intensity of AFVY–RFP for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Signal intensity of co-immunoprecipitation for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Width measurements of KNOLLE and callose for Extended Data Fig. 8g,n.
Source Data Extended Data Fig. 9
Unprocessed immunoblots for Extended Data Fig. 9.
Source Data Extended Data Fig. 9
Signal intensity of co-immunoprecipitation for Extended Data Fig. 9a,b,d.
Source Data Extended Data Fig. 10
Localization efficiency of KEULE at the cell division plane for Extended Data Fig. 10i.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, M., Mayer, U., Richter, S. et al. NSF/αSNAP2-mediated cis-SNARE complex disassembly precedes vesicle fusion in Arabidopsis cytokinesis. Nat. Plants 9, 889–897 (2023). https://doi.org/10.1038/s41477-023-01427-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01427-8