Abstract
Although SWI/SNF chromatin remodelling complexes are known to regulate diverse biological functions in plants, the classification, compositions and functional mechanisms of the complexes remain to be determined. Here we comprehensively characterized SWI/SNF complexes by affinity purification and mass spectrometry in Arabidopsis thaliana, and found three classes of SWI/SNF complexes, which we termed BAS, SAS and MAS (BRM-, SYD- and MINU1/2-associated SWI/SNF complexes). By investigating multiple developmental phenotypes of SWI/SNF mutants, we found that three classes of SWI/SNF complexes have both overlapping and specific functions in regulating development. To investigate how the three classes of SWI/SNF complexes differentially regulate development, we mapped different SWI/SNF components on chromatin at the whole-genome level and determined their effects on chromatin accessibility. While all three classes of SWI/SNF complexes regulate chromatin accessibility at proximal promoter regions, SAS is a major SWI/SNF complex that is responsible for mediating chromatin accessibility at distal promoter regions and intergenic regions. Histone modifications are related to both the association of SWI/SNF complexes with chromatin and the SWI/SNF-dependent chromatin accessibility. Three classes of SWI/SNF-dependent accessibility may enable different sets of transcription factors to access chromatin. These findings lay a foundation for further investigation of the function of three classes of SWI/SNF complexes in plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
A reporting summary for this paper is available as a Supplementary Information file. Raw data of RNA-seq, ChIP–seq and ATAC–seq results have been deposited in GEO (accession number GSE193397). The accession numbers of genes reported in this study are as follows: AT3G01890 (SWP73A), AT5G14170 (SWP73B), AT4G22320 (BCL7A), AT5G55210 (BCL7B), AT1G18450 (ARP4), AT3G60830 (ARP7), AT2G46020 (BRM), AT1G21700 (SWI3C), AT1G20670 (BRD1), AT1G76380 (BRD2), AT5G55040 (BRD13), AT3G03460 (BRIP1), AT5G17510 (BRIP2), AT5G28640 (AN3), AT1G01160 (GIF2), AT4G00850 (GIF3), AT2G28290 (SYD), AT4G34430 (SWI3D), AT5G07940 (SYS1), AT5G07970 (SYS2), AT5G07980 (SYS3), AT3G22990 (LFR), AT3G06010 (MINU1), AT5G19310 (MINU2), AT2G47620 (SWI3A), AT2G33610 (SWI3B), AT3G17590 (BSH), AT3G18380 (SHH2), AT1G50620 (PMS1A), AT3G20280 (PMS1B), AT3G08020 (PMS2A), AT3G52100 (PMS2B), AT1G58025 (BRD5), AT1G32730 (MIS), AT1G06500 (SSM), Os09g0284300 (OsBCL7), Os08g0137200 (OsARP4), Os03g0783000 (OsARP7), Os04g0382100 (OsSWIB), Os02g0114033 (OsCHR707), Os11g0183700 (OsSWI3C), Os12g0176700 (OsCHB701), Os03g0130800 (OsBRD1), Os09g0550000 (OsBRD2), Os12g0465700 (OsDEC), Os03g0733600 (OsGIF1), Os11g0615200 (OsGIF2), Os12g0496900 (OsGIF3), Os06g0255200 (OsCHR720), Os04g0110300 (OsCHB704), Os03g0213300 (OsSYS), Os07g0609766 (OsLFR), Os05g0144300 (OsCHR719), Os04g0480300 (OsCHB703), Os02g0194000 (OsCHB702), Os02g0723700 (OsBSH), Os06g0485100 (OsSHH2), Os06g0309000 (OsPMS1), Os12g0527800 (OsPMS2), Os08g0109500 (OsBRD5), Os08g0129500 (OsMIS) and Os01g0246500 (OsSSM). Source data are provided with this paper.
Change history
10 May 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41477-023-01428-7
References
Clapier, C. R., Iwasa, J., Cairns, B. R. & Peterson, C. L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 18, 407–422 (2017).
Hodges, C., Kirkland, J. G. & Crabtree, G. R. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med. 6, a026930 (2016).
Michel, B. C. et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat. Cell Biol. 20, 1410–1420 (2018).
Mashtalir, N. et al. Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell 175, 1272–1288 (2018).
Verbsky, M. L. & Richards, E. J. Chromatin remodeling in plants. Curr. Opin. Plant Biol. 4, 494–500 (2001).
Han, S.-K., Wu, M.-F., Cui, S. & Wagner, D. Roles and activities of chromatin remodeling ATPases in plants. Plant J. 83, 62–77 (2015).
Jerzmanowski, A. SWI/SNF chromatin remodeling and linker histones in plants. Biochim. Biophys. Acta Gene Struct. Expr. 1769, 330–345 (2007).
Farrona, S., Hurtado, L., Bowman, J. L. & Reyes, J. C. The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development 131, 4965–4975 (2004).
Kwon, C. S. et al. A role for chromatin remodeling in regulation of CUC gene expression in the Arabidopsis cotyledon boundary. Development 133, 3223–3230 (2006).
Wagner, D. & Meyerowitz, E. M. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 12, 85–94 (2002).
Kwon, C. S., Chen, C. B. & Wagner, D. WUSCHEL is a primary target for transcriptional regulation by SPLAYED in dynamic control of stem cell fate in Arabidopsis. Genes Dev. 19, 992–1003 (2005).
Li, C. et al. Concerted genomic targeting of H3K27 demethylase REF6 and chromatin-remodeling ATPase BRM in Arabidopsis. Nat. Genet. 48, 687–693 (2016).
Shu, J. et al. Genome-wide occupancy of Arabidopsis SWI/SNF chromatin remodeler SPLAYED provides insights into its interplay with its close homolog BRAHMA and Polycomb proteins. Plant J. 106, 200–213 (2021).
Bezhani, S. et al. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell 19, 403–416 (2007).
Mlynarova, L., Nap, J.-P. & Bisseling, T. The SWI/SNF chromatin-remodeling gene AtCHR12 mediates temporary growth arrest in Arabidopsis thaliana upon perceiving environmental stress. Plant J. 51, 874–885 (2007).
Li, C., Zhao, J., Gao, Y. & Cui, S. Two putative chromatin-remodeling ATPases play redundant roles in seed and embryo development of Arabidopsis. Plant Physiol. J. 48, 1084–1090 (2012).
Sang, Y. et al. Mutations in two non-canonical Arabidopsis SWI2/SNF2 chromatin remodeling ATPases cause embryogenesis and stem cell maintenance defects. Plant J. 72, 1000–1014 (2012).
Brzeski, J., Podstolski, W., Olczak, K. & Jerzmanowski, A. Identification and analysis of the Arabidopsis thaliana BSH gene, a member of the SNF5 gene family. Nucleic Acids Res. 27, 2393–2399 (1999).
Sarnowski, T. J., Swiezewski, S., Pawlikowska, K., Kaczanowski, S. & Jerzmanowski, A. AtSWI3B, an Arabidopsis homolog of SWI3, a core subunit of yeast Swi/Snf chromatin remodeling complex, interacts with FCA, a regulator of flowering time. Nucleic Acids Res. 30, 3412–3421 (2002).
Vercruyssen, L. et al. ANGUSTIFOLIA3 binds to SWI/SNF chromatin remodeling complexes to regulate transcription during Arabidopsis leaf development. Plant Cell 26, 210–229 (2014).
Wang, Z. et al. LFR, which encodes a novel nuclear-localized Armadillo-repeat protein, affects multiple developmental processes in the aerial organs in Arabidopsis. Plant Mol. Biol. 69, 121 (2009).
Yu, Y. et al. BRAHMA-interacting proteins BRIP1 and BRIP2 are core subunits of Arabidopsis SWI/SNF complexes. Nat. Plants 6, 996–1007 (2020).
Yu, Y. et al. Bromodomain-containing proteins BRD1, BRD2, and BRD13 are core subunits of SWI/SNF complexes and vital for their genomic targeting in Arabidopsis. Mol. Plant 14, 888–904 (2021).
Jaronczyk, K. et al. Bromodomain-containing subunits BRD1, BRD2, and BRD13 are required for proper functioning of SWI/SNF complexes in Arabidopsis. Plant Commun. 2, 100174 (2021).
Shang, J. Y. et al. COMPASS functions as a module of the INO80 chromatin remodeling complex to mediate histone H3K4 methylation in Arabidopsis. Plant Cell 33, 3250–3271 (2021).
Luo, Y.-X. et al. A plant-specific SWR1 chromatin-remodeling complex couples histone H2A.Z deposition with nucleosome sliding. EMBO J. 39, e102008 (2020).
Tan, L. M. et al. The PEAT protein complexes are required for histone deacetylation and heterochromatin silencing. EMBO J. 37, e98770 (2018).
Bieluszewski, T. et al. AtEAF1 is a potential platform protein for Arabidopsis NuA4 acetyltransferase complex. BMC Plant Biol. 15, 75 (2015).
Qi, D. et al. OsLFR is essential for early endosperm and embryo development by interacting with SWI/SNF complex members in Oryza sativa. Plant J. 104, 901–916 (2020).
Hurtado, L., Farrona, S. & Reyes, J. C. The putative SWI/SNF complex subunit BRAHMA activates flower homeotic genes in Arabidopsis thaliana. Plant Mol. Biol. 62, 291–304 (2006).
Sacharowski, S. P. et al. SWP73 subunits of Arabidopsis SWI/SNF chromatin remodeling complexes play distinct roles in leaf and flower development. Plant Cell 27, 1889–1906 (2015).
Sarnowski, T. J. et al. SWI3 subunits of putative SWI/SNF chromatin-remodeling complexes play distinct roles during Arabidopsis development. Plant Cell 17, 2454–2472 (2005).
Lin, X. et al. LFR physically and genetically interacts with SWI/SNF component SWI3B to regulate leaf blade development in Arabidopsis. Front. Plant Sci. 12, 717649 (2021).
Wagner, F. R. et al. Structure of SWI/SNF chromatin remodeller RSC bound to a nucleosome. Nature 579, 448–451 (2020).
Ye, Y. et al. Structure of the RSC complex bound to the nucleosome. Science 366, 838–843 (2019).
He, S. et al. Structure of nucleosome-bound human BAF complex. Science 367, 875–881 (2020).
Su, Y. H. et al. The N-terminal ATPase AT-hook-containing region of the Arabidopsis chromatin-remodeling protein SPLAYED is sufficient for biological activity. Plant J. 46, 685–699 (2006).
Han, Y., Reyes, A. A., Malik, S. & He, Y. Cryo-EM structure of SWI/SNF complex bound to a nucleosome. Nature 579, 452–455 (2020).
Mashtalir, N. et al. A structural model of the endogenous human BAF complex informs disease mechanisms. Cell 183, 802–817 (2020).
Han, W. et al. The SWI/SNF subunit SWI3B regulates IAMT1 expression via chromatin remodeling in Arabidopsis leaf development. Plant Sci. 271, 127–132 (2018).
Lin, X. et al. LFR is functionally associated with AS2 to mediate leaf development in Arabidopsis. Plant J. 95, 598–612 (2018).
Li, C. et al. The Arabidopsis SWI2/SNF2 chromatin remodeler BRAHMA regulates polycomb function during vegetative development and directly activates the flowering repressor gene SVP. PLoS Genet. 11, e1004944 (2015).
Farrona, S. et al. Brahma is required for proper expression of the floral repressor FLC in Arabidopsis. PLoS ONE 6, e17997 (2011).
Archacki, R. et al. Arabidopsis SWI/SNF chromatin remodeling complex binds both promoters and terminators to regulate gene expression. Nucleic Acids Res. 45, 3116–3129 (2017).
Guo, J. et al. The CBP/p300 histone acetyltransferases function as plant‐specific MEDIATOR subunits in Arabidopsis. J. Integr. Plant Biol. 63, 755–771 (2020).
Zhao, N. et al. The RNA recognition motif‐containing protein UBA2c prevents early flowering by promoting transcription of the flowering repressor FLM in Arabidopsis. N. Phytol. 233, 751–765 (2022).
Eberharter, A. & Becker, P. B. Histone acetylation: a switch between repressive and permissive chromatin. EMBO Rep. 3, 224–229 (2002).
Zhang, X., Bernatavichute, Y. V., Cokus, S., Pellegrini, M. & Jacobsen, S. E. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 10, R62 (2009).
Zhang, X. et al. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 5, e129 (2007).
Zhao, S., Zhang, B., Yang, M., Zhu, J. & Li, H. Systematic profiling of histone readers in Arabidopsis thaliana. Cell Rep. 22, 1090–1102 (2018).
Tan, L.-M. et al. Dual recognition of H3K4me3 and DNA by the ISWI component ARID5 regulates the floral transition in Arabidopsis. Plant Cell. 32, 2178–2195 (2020).
Yang, Z. et al. EBS is a bivalent histone reader that regulates floral phase transition in Arabidopsis. Nat. Genet. 50, 1247–1253 (2018).
Qian, S. et al. Dual recognition of H3K4me3 and H3K27me3 by a plant histone reader SHL. Nat. Commun. 9, 2425 (2018).
López-González, L. et al. Chromatin-dependent repression of the Arabidopsis floral integrator genes involves plant specific PHD-containing proteins. Plant Cell 26, 3922–3938 (2014).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Torres, E. S. & Deal, R. B. The histone variant H2A.Z and chromatin remodeler BRAHMA act coordinately and antagonistically to regulate transcription and nucleosome dynamics in Arabidopsis. Plant J. 99, 144–162 (2019).
Hernández-García, J. et al. Comprehensive identification of SWI/SNF complex subunits underpins deep eukaryotic ancestry and reveals new plant components. Commun. Biol. 5, 549 (2022).
Chatterjee, N. et al. Histone acetylation near the nucleosome dyad axis enhances nucleosome disassembly by RSC and SWI/SNF. Mol. Cell. Biol. 35, 4083–4092 (2015).
Mashtalir, N. et al. Chromatin landscape signals differentially dictate the activities of mSWI/SNF family complexes. Science 373, 306–315 (2021).
Nakayama, R. T. et al. SMARCB1 is required for widespread BAF complex–mediated activation of enhancers and bivalent promoters. Nat. Genet. 49, 1613–1623 (2017).
Mittal, P. & Roberts, C. W. M. The SWI/SNF complex in cancer—biology, biomarkers and therapy. Nat. Rev. Clin. Oncol. 17, 435–448 (2020).
Zhu, B., Zhang, W., Zhang, T., Liu, B. & Jiang, J. Genome-wide prediction and validation of intergenic enhancers in Arabidopsis using open chromatin signatures. Plant Cell 27, 2415–2426 (2015).
Zeng, L. et al. Mechanism and regulation of acetylated histone binding by the tandem PHD finger of DPF3b. Nature 466, 258–262 (2010).
Ren, X. et al. Histone benzoylation serves as an epigenetic mark for DPF and YEATS family proteins. Nucleic Acids Res. 49, 114–126 (2021).
Local, A. et al. Identification of H3K4me1-associated proteins at mammalian enhancers. Nat. Genet. 50, 73–82 (2018).
Yan, W. et al. Dynamic control of enhancer activity drives stage-specific gene expression during flower morphogenesis. Nat. Commun. 10, 1705 (2019).
Zaret, K. S. & Mango, S. E. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr. Opin. Genet. Dev. 37, 76–81 (2016).
Pajoro, A. et al. Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol. 15, R41 (2014).
Jin, R. et al. LEAFY is a pioneer transcription factor and licenses cell reprogramming to floral fate. Nat. Commun. 12, 626 (2021).
Efroni, I. et al. Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev. Cell 24, 438–445 (2013).
Wu, M.-F. et al. SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proc. Natl Acad. Sci. USA 109, 3576–3581 (2012).
Zhao, M. et al. Arabidopsis BREVIPEDICELLUS interacts with the SWI2/SNF2 chromatin remodeling ATPase BRAHMA to regulate KNAT2 and KNAT6 expression in control of inflorescence architecture. PLoS Genet. 11, e1005125 (2015).
Zhang, D., Li, Y., Zhang, X., Zha, P. & Lin, R. The SWI2/SNF2 chromatin-remodeling ATPase BRAHMA regulates chlorophyll biosynthesis in Arabidopsis. Mol. Plant 10, 155–167 (2017).
Zhang, W., Zhang, T., Wu, Y. & Jiang, J. Genome-wide identification of regulatory DNA elements and protein-binding footprints using signatures of open chromatin in Arabidopsis. Plant Cell 24, 2719–2731 (2012).
Maher, K. A. et al. Profiling of accessible chromatin regions across multiple plant species and cell types reveals common gene regulatory principles and new control modules. Plant Cell 30, 15–36 (2018).
Wang, F.-X. et al. Chromatin accessibility dynamics and a hierarchical transcriptional regulatory network structure for plant somatic embryogenesis. Dev. Cell 54, 742–757. e8 (2020).
Farmer, A., Thibivilliers, S., Ryu, K. H., Schiefelbein, J. & Libault, M. Single-nucleus RNA and ATAC sequencing reveals the impact of chromatin accessibility on gene expression in Arabidopsis roots at the single-cell level. Mol. Plant 14, 372–383 (2021).
Wang, Z.-P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 1–12 (2015).
Nishimura, A., Aichi, I. & Matsuoka, M. A protocol for Agrobacterium-mediated transformation in rice. Nat. Protoc. 1, 2796 (2006).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Kolde, R. Pheatmap: pretty heatmaps (R package version 1, 2012).
Warnes, G. R. et al. Gplots: various R programming tools for plotting data (R package version 2, 2014).
Gendrel, A.-V., Lippman, Z., Martienssen, R. & Colot, V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2, 213–218 (2005).
Zhang, Y. et al. Model-based analysis of ChIP–seq (MACS). Genome Biol. 9, R137 (2008).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Rossinnes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2011).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 16, 284–287 (2012).
Liu, W. et al. IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics 31, 3359–3361 (2015).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32025003) and by the National Key Research and Development Program of China (2016YFA0500801) from the Chinese Ministry of Science and Technology. We thank the Research Platform of Protein Science at Institute of Biophysics, Chinese Academy of Sciences, for providing the technical assistance on the ITC assay.
Author information
Authors and Affiliations
Contributions
J.G. (Beijing Normal University), G.C., Y.-X.Z., Z.-C.Z., Z.-Z.L., J.G. (National Institute of Biological Sciences), L.L. and S.C. performed the experiments. J.G. (Beijing Normal University), Y.-Q.L., Y.-N.S., D.-Y.Y. and X.-W.C. performed the bioinformatics analyses. J.G. (Beijing Normal University) and X.-J.H. designed the experiments. J.G. (Beijing Normal University) and X.-J.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Sebastian Sacharowski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
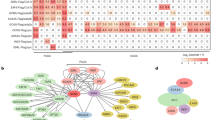
Extended Data Fig. 1 Phylogenetic analysis of SWI/SNF chromatin remodelers in different plant species and identification of SWI/SNF complexes in Oryza sativa.
a, A neighbor-joining tree was generated using the full-length protein sequences of BRM, SYD, MINU1, MINU2, and their orthologues in different plant species. The human SWI/SNF chromatin remodeler BRG1 was introduced as the outgroup. Bootstrap values: % invariant branches during resampling. b, Heatmap visualization of proteins co-purified with GFP-tagged OsBCL7, OsDEC, OsCHB704, OsLFR and OsBSH as determined by AP-MS. The matched queries of a prey protein are normalized by the average matched queries of the total subunits purified by a bait protein. c, Schematic representations of BAS, SAS and MAS complexes in Oryza sativa. Shared subunits are shown in grey. Specific subunits of BAS, SAS and MAS complexes are shown in green, blue and red, respectively.
Extended Data Fig. 2 Interactions between subunits of BAS, SAS, and MAS complexes as determined by Y2H assays.
a, Interactions of BAS complex subunits. The truncated versions of BRM used in Y2H assays. b, Interactions of SAS complex subunits. The truncated SYD versions SYD-ΔC and SYD-C used in Y2H assays are shown on the top panel. c, Interaction of MAS complex subunits. The interactions detected by Y2H assays in this study are highlighted in blue, and the previously published interactions are labelled by red frames. Blank boxes indicate that no interaction was detected.
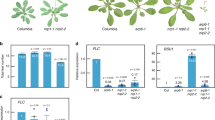
Extended Data Fig. 3 Developmental phenotypes of SWI/SNF mutants.
a, Schematic representations of mutations in SYS1, SYS2 and SYS3 mediated by CRISPR/Cas9. The translation start sites are marked by +1. The untranslated regions (UTR) and exon regions are indicated with blank and black boxes, respectively. Introns are indicated with fold lines. The mutations validated by Sanger sequencing are illustrated by red boxes. WT: wild type. Scale bar: 100 bp. b, Morphological phenotypes of cotyledons and rosette leaves taken from bolting plants of SWI/SNF mutants and wild type. c, Morphological phenotypes of 5-week-old plants of SWI/SNF mutants and wild type. d, Measurement of the first cauline branching angles in indicated genotypes. P values determined by two-tailed Student’s t-test or Welch’s t-test indicate the difference between the mutants and the wild-type control. n = 23. e, The ratio of abnormal cauline branches of which the growth positions are not at the axils of cauline leaves. n = 15. P values determined by two-tailed Mann Whitney U test indicate the difference between the mutants and the wild-type control. f, Petal number in the indicated genotypes. The average petal number of five flowers was calculated for each plant. The petal numbers of 20 plants were determined for each genotype (except for swp73b, n = 14). P values determined by two-tailed Mann Whitney U test indicate the difference between the mutants and the wild-type control. g, The average length of all the siliques from the main stem was determined for each plant. n = 15. P values determined by two-tailed Student’s t-test or Welch’s t-test indicate the difference between the mutants and the wild-type control. Data are presented as mean values + /- SD in d-g.
Extended Data Fig. 4 Comparison of the effects of SWI/SNF mutations on development and gene expression.
a, Fresh weight of 23-day-old plants of SWI/SNF mutants and wild type. The fresh weight of 15 plants were determined for each genotype. P values determined by two-tailed Student’s t-test or Welch’s t-test indicate the difference between the mutants and the wild-type control. Data are presented as mean values + /- SD. b, Scatter plot with a fitted regression line and a 95% confidence interval band showing the correlation between the plant fresh weight and the differentially expressed genes in SWI/SNF mutants. R represents the Pearson correlation coefficient. P value were determined by two-tailed t-test. c-f, Determination of the correlation of development-related differentially expressed genes between different SWI/SNF mutants. Heatmaps show the Pearson Correlation Coefficient of brm, brd1/2/13, brip1/2, syd, swi3d, sys1/2/3, minu1/2, pms2a/2b based on the expression changes of leaf development-related genes (c), flower development-related genes (d), flowering time-related genes (e), and embryo development-related genes (f).
Extended Data Fig. 5 Comparison of BAS-, SAS- and MAS-occupied TSS-flanking regions and genes as determined by ChIP-seq.
a, Profile plots showing the enrichment of the SWI/SNF components at the genes bound by each component. b, Boxplots showing the enrichment of SWI/SNF components at the TSS-flanking region of their binding genes. The enrichment of SWI/SNF components was calculated at 200-bp bins of the TSS-flanking region. Sample size of each box plot: BRD1 (n = 10928), SWI3C (n = 11110), SYD (n = 7904), SWI3D (n = 10492), SYS1 (n = 8929), MINU2 (n = 10835), PMS2B (n = 13467). In box plots, center lines and box edges are medians and the interquartile range (IQR), respectively. Whiskers extend within 1.5 times the IQR. c, Venn diagrams showing the overlap of genes occupied by indicated SWI/SNF components. The overlapping significance is shown by P values as determined by hypergeometric test (one-tailed). RF (representation factor) represents the number of overlapping genes divided by the expected number of overlapping genes drawn from two independent groups.
Extended Data Fig. 6 Determination of the correlation between the enrichments of BAS, SAS and MAS components at chromatin.
a, b, Heatmaps showing the enrichment of indicated SWI/SNF components at all the SWI/SNF components-occupied genes. The genes are subjected to sorting by the maximum enrichment value of each region of SYD (a) and MINU2 (b) at these genes. c, d, Enrichment of indicated SWI/SNF components at SYD (c) and MINU2 (d) enrichment deciles of the total regions (n = 18552) occupied by SWI/SNF components. In box plots of c and d, center lines and box edges are medians and the interquartile range (IQR), respectively. Whiskers extend within 1.5 times the IQR.
Extended Data Fig. 7 The histone modification and expression levels of genes occupied by BAS, SAS and MAS components.
a, The H3ac, H3K4me3, and H3K27me3 levels at regions occupied by indicated BAS, SAS, and MAS components in the wild-type plants. The sample size of each boxplot: BRD1 (n = 12973), SWI3C (n = 13851), SYD (n = 9687), SWI3D (n = 13099), SYS1 (n = 11875), MINU2 (n = 11729), PMS2B (n = 15207), random (n = 10000). P values for Kruskal-Wallis tests indicate the differences of the histone modification levels within a group (marked by black lines). Lowercase letters above the boxplots show the results of Dunnett’s test, with statistically similar clusters grouped by the same letter. b, The expression levels of genes occupied by indicated BAS, SAS, and MAS components in the wild-type plants. The sample size of each boxplot: BRD1 (n = 12973), SWI3C (n = 13851), SYD (n = 9687), SWI3D (n = 13099), SYS1 (n = 11875), MINU2 (n = 11729), PMS2B (n = 15207), random (n = 10000). P values for Kruskal-Wallis tests indicate the expression differences within a group (marked by black lines). Lowercase letters above the boxplots show the results of Dunnett’s test, with statistically similar clusters grouped by the same letter. c, Heatmap showing the enrichment of H3ac and H3K4me3 at all the SWI/SNF components-occupied genes. The genes are subjected to sorting by the maximum enrichment value of BRD1 (left), SYD (middle), and MINU2 (right)-occupied regions. d, Enrichment of H3ac, H3K4me3, and H3K27me3 at BRD1, SYD and MINU2 enrichment deciles of total regions (n = 18552) occupied by SWI/SNF components. In box plots of a, b and d, center lines and box edges are medians and the interquartile range (IQR), respectively. Whiskers extend within 1.5 times the IQR.
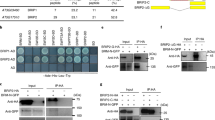
Extended Data Fig. 8 The bromodomains of BRD1, BRD2 and BRD13 bind to acetylated histones and the second PHD domain of PMS2B binds to methylated H3 at lysine 4.
a, Sequence alignment of the bromodomains of HsBRD9, AtBRD1, AtBRD2, AtBRD13, HsBRM, ScSTH1, and AtBRM. The BRD1 bromodomain conserved residues Y207, N251 and Y258 marked with asterisks were subjected to point mutation. b, Coomassie blue staining of the wild-type BRD1, BRD2, and BRD13 bromodomains and of the mutated BRD1 bromodomain (BRD1-M) tagged by HIS. The fusion proteins were purified from E. coli. The experiments were repeated for at least two times with similar results. c,d, ITC binding assays measuring the binding affinity of the bromodomains of BRD1,BRD2, BRD13 and BRD1-M for acetylated and unmodified H3 peptides (c) and acetylated and unmodified H4 peptides (d). e, Schematic representations indicate the domain architecture of PMS1A and PMS2B and the truncated versions of PMS1A and PMS2B purified from E. coli. f, A phylogenetic tree of the PHD domains in PMS1A, PMS1B, PMS2A, PMS2B, and in the known PHD-containing H3K4me3 readers ARID5, SHL and EBS. The RING fingers of PMS2A and PMS2B that are closely related to the conserved PHD finger were included for analysis. g, Coomassie blue staining of the wild-type PMS1A and PMS2B PHD domains and of the mutated PMS2B PHD domain tagged by GST. The proteins were expressed and purified from E. coli. The experiments were repeated for at least two times with similar results. h, Determination of the binding of wild-type PMS1A and PMS2B PHD domains to methylated and unmethylated histone peptides by in vitro binding assays. i, Sequence alignment of the second PHD fingers of PMS2A and PMS2B (PMS2A-PHD2 and PMS2B-PHD2) and the PHD fingers of ARID5, SHL and EBS. Three conserved aromatic residues (Y148, Y155, and W170) in the PMS2B-PHD2 marked with asterisks were mutated to alanine in the mutated version of PMS2B (PMS2B-PHD-M). The experiment was repeated for two times with similar results. j, The effect of the PMS2B-PHD2 mutation on the binding ability of PMS2B for methylated H3 at lysine 4 as determined by in vitro binding assays. The experiment was repeated for two times with similar results.
Extended Data Fig. 9 Different effects of BAS, SAS and MAS mutations on chromatin accessibility.
a, The number of differential accessible regions (DARs) in indicated SWI/SNF mutants. DARs with increased accessibility (ATAC-up) were represented in red and DARs with decreased accessibility (ATAC-down) were represented in blue. b, The percentage of BAS/SAS/MAS-bound and unbound regions among the regions with decreased accessibility (ATAC-down regions) in corresponding BAS/SAS/MAS mutants. c, The number of genes with increased accessibility and genes with decreased accessibility in indicated SWI/SNF mutants. Genes with increased accessibility (ATAC-up) were represented in red and genes with decreased accessibility (ATAC-down) were represented in blue. d, Boxplots showing H3K27me3 levels at the regions bound by each complex and the regions bound by each complex with decreased (ATAC-down) or maintained (ATAC-maintain) accessibility. The sample size of each box plot: BAS-bound (n = 12392), ATAC-down (n = 1470), ATAC-maintain (n = 10922), SAS-bound (n = 11398), ATAC-down (n = 2195), ATAC-maintain (n = 9203), MAS-bound (n = 11129), ATAC-down (n = 1637), ATAC-maintain (n = 9492), random (n = 10000). In box plots, center lines and box edges are medians and the interquartile range (IQR), respectively. Whiskers extend within 1.5 times the IQR. P values determined by two-tailed Mann Whitney U test indicate the difference between indicated samples.
Extended Data Fig. 10 Different effects of BAS, SAS and MAS mutations on chromatin accessibility.
a, Heatmap depicting the effects of BAS, SAS, and MAS enzyme mutations on chromatin accessibility at genes whose chromatin accessibility is specifically reduced in brm, syd, and minu1/2. WT: wild type. b, Boxplots showing the chromatin accessibility levels in the brm, syd and minu1/2 mutants and the wild type at the 400~1500 bp upstream region of TSS and at the −400~200-bp TSS-flanking region. Those genes with reduced chromatin accessibility specifically in brm, syd, and minu1/2 mutants were independently analyzed. In box plots, center lines and box edges are medians and the interquartile range (IQR), respectively. Whiskers extend within 1.5 times the IQR. P values determined by two-tailed Wilcoxon signed rank test indicate the difference between the mutants and the wild-type control. WT: wild type. P values of reduced and increased signals in indicated mutants are shown in blue and red, respectively.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Supplementary Tables 1–3.
Supplementary Data 1
Full list of Arabidopsis proteins identified by affinity purification followed by mass spectrometry analysis.
Supplementary Data 2
Full list of rice proteins identified by affinity purification followed by mass spectrometry analysis.
Supplementary Data 3
DEGs identifed by RNA-seq.
Supplementary Data 4
Expression of development-related genes in SWI/SNF mutants.
Supplementary Data 5
Peaks identified by ChIP–seq.
Supplementary Data 6
DARs identified by ATAC–seq.
Supplementary Data 7
GO analysis of genes with decreased accessibilily in SWI/SNF mutants identified by ATAC–seq.
Supplementary Data 8
Motif analysis of regions with decreased accessibility in SWI/SNF mutants identified by ATAC–seq.
Supplementary Data 9
Primers used in the study.
Source data
Source Data Extended Data Fig. 8
Unprocessed gels and western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Cai, G., Li, YQ. et al. Comprehensive characterization of three classes of Arabidopsis SWI/SNF chromatin remodelling complexes. Nat. Plants 8, 1423–1439 (2022). https://doi.org/10.1038/s41477-022-01282-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01282-z
This article is cited by
-
BCL7A and BCL7B potentiate SWI/SNF-complex-mediated chromatin accessibility to regulate gene expression and vegetative phase transition in plants
Nature Communications (2024)
-
Molecular basis of chromatin remodelling by DDM1 involved in plant DNA methylation
Nature Plants (2024)
-
Mapping nucleosome-resolution chromatin organization and enhancer-promoter loops in plants using Micro-C-XL
Nature Communications (2024)
-
BRIP1 and BRIP2 maintain root meristem by affecting auxin-mediated regulation
Planta (2024)