Abstract
The coordinated metabolism of carbon and nitrogen is essential for optimal plant growth and development. Nitrate is an important molecular signal for plant adaptation to a changing environment, but how nitrate regulates plant growth under carbon deficiency conditions remains unclear. Here we show that the evolutionarily conserved energy sensor SnRK1 negatively regulates the nitrate signalling pathway. Nitrate promoted plant growth and downstream gene expression, but such effects were repressed when plants were grown under carbon deficiency conditions. Mutation of KIN10, the α-catalytic subunit of SnRK1, partially suppressed the inhibitory effects of carbon deficiency on nitrate-mediated plant growth. KIN10 phosphorylated NLP7, the master regulator of the nitrate signalling pathway, to promote its cytoplasmic localization and degradation. Furthermore, nitrate depletion induced KIN10 accumulation, whereas nitrate treatment promoted KIN10 degradation. Such KIN10-mediated NLP7 regulation allows carbon and nitrate availability to control optimal nitrate signalling and ensures the coordination of carbon and nitrogen metabolism in plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the main text and Supplementary Information. All Arabidopsis genes involved in this study can be found at TAIR (www.arabidopsis.org), with the following accession numbers: NLP7 (AT4G24020), KIN10 (AT3G01090), NIA1 (AT1G77760), NIR (AT2G15620), NRT1.1 (AT1G12110), NRT2.1 (AT1G08090), HBI1 (AT2G18300) and LBD37 (AT5G67420). Other species genes involved in this study can be found at UniProt (https://www.uniprot.org/), with the following accession numbers: A0A1B1FGW6_MARPO (https://www.uniprot.org/uniprotkb/A0A1B1FGW6/entry), A0A2K1JP51_PHYPA (https://www.uniprot.org/uniprotkb/A0A2K1JP51/entry), D8T5B1_SELML (https://www.uniprot.org/uniprotkb/D8T5B1/entry), W1PF37_AMBTC (https://www.uniprot.org/uniprotkb/W1PF37/entry), NLP3_ORYSJ (https://www.uniprot.org/uniprotkb/Q5NB82/entry), A0A3B6GRE7_WHEAT (https://www.uniprot.org/uniprotkb/A0A3B6GRE7/entry), K7VRG4_MAIZE (https://www.uniprot.org/uniprotkb/K7VRG4/entry) and A0A2Z5V8A6_LOTJA (https://www.uniprot.org/uniprotkb/A0A2Z5V8A6/entry). Extra data are available from the corresponding author upon reasonable request. All sequencing data that support the findings of this study have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and are accessible through the GEO series accession number GSE206841. Source data are provided with this paper.
References
Lawlor, D. W., Lemaire, G. & Gastal, F. Nitrogen, plant growth and crop yield. Plant Nitrogen 343–367. Edited by LEA, P.J. & Morot-Gaudry, J.F. Springer. (2001)
Vidal, E. A. et al. Nitrate in 2020: thirty years from transport to signaling networks. Plant Cell 32, 2094–2119 (2020).
Sun, C. H., Yu, J. Q. & Hu, D. G. Nitrate: a crucial signal during lateral roots development. Front. Plant Sci. 8, 485 (2017).
Fredes, I., Moreno, S., Díaz, F. P. & Gutiérrez, R. A. Nitrate signaling and the control of Arabidopsis growth and development. Curr. Opin. Plant Biol. 47, 112–118 (2019).
Liu, K. H. et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 545, 311–316 (2017).
Marchive, C. et al. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 4, 1713 (2013).
Alvarez, J. M. et al. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 11, 1157 (2020).
Hu, H. C., Wang, Y. Y. & Tsay, Y. F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 57, 264–278 (2009).
Castaings, L. et al. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J. 57, 426–435 (2009).
Konishi, M. & Yanagisawa, S. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 4, 1617 (2013).
Wang, X. et al. A transceptor–channel complex couples nitrate sensing to calcium signaling in Arabidopsis. Mol. Plant. 14, 774–786 (2021).
Hu, B. et al. Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 5, 401–413 (2019).
Chu, X. et al. HBI transcription factor-mediated ROS homeostasis regulates nitrate signal transduction. Plant Cell 33, 3004–3021 (2021).
Emanuelle, S., Doblin, M. S., Stapleton, D. I., Bacic, A. & Gooley, P. R. Molecular insights into the enigmatic metabolic regulator, SnRK1. Trends Plant Sci. 21, 341–353 (2016).
Baena-González, E. & Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 13, 474–482 (2008).
Sheen, J. Master regulators in plant glucose signaling networks. J. Plant Biol. 57, 67–79 (2014).
Crozet, P. et al. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front. Plant Sci. 5, 190 (2014).
Broeckx, T., Hulsmans, S. & Rolland, F. The plant energy sensor: evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 67, 6215–6252 (2016).
Emanuelle, S. et al. SnRK1 from Arabidopsis thaliana is an atypical AMPK. Plant J. 82, 183–192 (2015).
Baena-González, E., Rolland, F., Thevelein, J. M. & Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942 (2007).
Glab, N. et al. The impact of Arabidopsis thaliana SNF1-related-kinase 1 (SnRK1)-activating kinase 1 (SnAK1) and SnAK2 on SnRK1 phosphorylation status: characterization of a SnAK double mutant. Plant J. 89, 1031–1041 (2017).
Crozet, P. et al. Cross-phosphorylation between Arabidopsis thaliana sucrose nonfermenting 1-related protein kinase 1 (AtSnRK1) and its activating kinase (AtSnAK) determines their catalytic activities. J. Biol. Chem. 285, 12071–12077 (2010).
Ramon, M. et al. Default activation and nuclear translocation of the plant cellular energy sensor SnRK1 regulate metabolic stress responses and development. Plant Cell 31, 1614–1632 (2019).
Sun, D. et al. Kinase SnRK1.1 regulates nitrate channel SLAH3 engaged in nitrate-dependent alleviation of ammonium toxicity. Plant Physiol. 186, 731–749 (2021).
Jossier, M. et al. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 59, 316–328 (2009).
Sugden, C. et al. Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol. 120, 257–274 (1999).
Gómez, L. D., Gilday, A., Feil, R., Lunn, J. E. & Graham, I. A. AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J. 64, 1–13 (2010).
Zhang, Y. et al. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate1[w][OA]. Plant Physiol. 149, 1860–1871 (2009).
Zhai, Z. et al. Trehalose 6-phosphate positively regulates fatty acid synthesis by stabilizing WRINKLED1. Plant Cell 30, 2616–2627 (2018).
Wang, Y. Y., Cheng, Y. H., Chen, K. E. & Tsay, Y. F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 69, 85–122 (2018).
O’Brien, J. A. et al. Nitrate transport, sensing, and responses in plants. Mol. Plant 9, 837–856 (2016).
Li, L., Liu, K. & Sheen, J. Dynamic nutrient signaling networks in plants. Annu. Rev. Cell Dev. Biol. 37, 341–367 (2021).
Li, L. & Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 33, 116–125 (2016).
Liu, Y. et al. Diverse nitrogen signals activate convergent ROP2-TOR signaling in Arabidopsis. Dev. Cell 56, 1283–1295 (2021).
Zhai, Z., Liu, H. & Shanklin, J. Phosphorylation of WRINKLED1 by KIN10 results in its proteasomal degradation, providing a link between energy homeostasis and lipid biosynthesis. Plant Cell 29, 871–889 (2017).
Mair, A. et al. SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. eLife 4, e05828 (2015).
Carvalho, R. F. et al. The Arabidopsis SR45 splicing factor, a negative regulator of sugar signaling, modulates SNF1-related protein kinase 1 stability. Plant Cell 28, 1910–1925 (2016).
Ananieva, E. A., Gillaspy, G. E., Ely, A., Burnette, R. N. & Erickson, F. Les Interaction of the WD40 domain of a myoinositol polyphosphate 5-phosphatase with SnRK1 links inositol, sugar, and stress signaling. Plant Physiol. 148, 1868–1882 (2008).
Crozet, P. et al. SUMOylation represses SnRK1 signaling in Arabidopsis. Plant J. 85, 120–133 (2016).
Miura, K. et al. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19, 1403–1414 (2007).
Catala, R. et al. The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell 19, 2952–2966 (2007).
Miura, K. et al. Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc. Natl Acad. Sci. USA 106, 5418–5423 (2009).
Miura, K. et al. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc. Natl Acad. Sci. USA 102, 7760–7765 (2005).
Nunes, C. et al. The trehalose 6-phosphate/snRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol. 162, 1720–1732 (2013).
Sanagi, M. et al. Low nitrogen conditions accelerate flowering by modulating the phosphorylation state of FLOWERING BHLH 4 in Arabidopsis. Proc. Natl Acad. Sci. USA 118, e2022942118 (2021).
Pedrotti, L. et al. Snf1-RELATED KINASE1-controlled C/S1-bZIP signaling activates alternative mitochondrial metabolic pathways to ensure plant survival in extended darkness. Plant Cell 30, 495–509 (2018).
Nukarinen, E. et al. Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci. Rep. 6, 31697 (2016).
Cho, H. Y., Wen, T. N., Wang, Y. T. & Shih, M. C. Quantitative phosphoproteomics of protein kinase SnRK1 regulated protein phosphorylation in Arabidopsis under submergence. J. Exp. Bot. 67, 2745–2760 (2016).
Xia, X. J. et al. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 66, 2839–2856 (2015).
Saxena, I., Srikanth, S. & Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 7, 570 (2016).
Han, C. et al. KIN10 promotes stomatal development through stabilization of the SPEECHLESS transcription factor. Nat. Commun. 11, 4214 (2020).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Tian, Y. et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 9, 1063 (2018).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank H. Yu, Y. Guo and X. Zhao from the Analysis and Testing Center of SKLMT (State Key Laboratory of Microbial Technology, Shandong University) for assistance with the laser scanning confocal microscopy. This work was supported by grants from the National Natural Science Foundation of China (32070210 to M.-Y.B., 31970306 to M.F. and 31870262 to M.-Y.B.) and the Science and Technology Department of Shandong Province (2019LZGC015 to M.-Y.B. and ZR2019ZD16 to M.-Y.B.).
Author information
Authors and Affiliations
Contributions
H.W., C.H. and M.-Y.B. together designed the experiments. H.W. performed statistical analysis of plant growth, transient expression and RT–qPCR. H.W. and C.H. performed western blot and subcellular location analysis. H.W., C.H. and Z.D. performed the kinase assays and mass spectrophotometric analysis. J.-G.W., X.C., W.S., L.Y., J. C., W.H. and M.F. generated p35S:NLP7S2A-YFP/nlp7-1, p35S:NLP7S2D-YFP/nlp7-1, p35S:NLP7S2E-YFP/nlp7-1, p35S:NLP7-YFP/p35S:KIN10-Myc and p35S:NLP7-YFP/kin10 transgenic plants. H.W. performed all other experiments. Z.D. and M.F. provided the critical discussion. H.W., C.H. and M.-Y.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Shuichi Yanagisawa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 KIN10 inhibits nitrate-promoted plant growth.
a, KIN10 interacts with NLP6 in yeast. b, KIN11 interacts with NLP7 in yeast. c-f, Seedlings of Ler, p35S:KIN10-HA, Col-0, p35S:KIN10-Myc and kin10 were grown on the MGRL medium supplemented with indicated nitrate concentrations under 16 h Light/8 h Dark photoperiod for 7 days with leaf areas measurement, or for 28 days with fresh weight measurement, respectively. Scale bars represent 5 mm. Error bars indicate S.D. (n = 18). Different letters above the bars indicate statistically significant differences between the samples (One-way ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05). Asterisk between bars indicate statistically significant differences between the samples (Unpaired t test, *p < 0.05; ***p < 0.001; **** p < 0.0001). g,h, Seedlings of Ler, p35S:KIN10-HA and tps1-11 were grown on medium supplemented with different nitrogen sources under 16 h Light/8 h Dark photoperiod for 7 days. Scale bar represents 5 mm. Error bars indicate S.D. (n = 18). Different letters above the bars indicate statistically significant differences between the samples (One-way ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05).
Extended Data Fig. 2 KIN10 contributes to the inhibition of carbon deficiency on the nitrate-promoted plant growth.
a-f, Carbon deficiency caused by short photoperiod, low light intensity or DCMU treatment inhibited the nitrate-promoted plant growth. Seedlings of Col-0 and kin10 were grown on the MGRL medium supplemented with different nitrogen sources under 16 h Light/8 h Dark photoperiod or 4 h light/20 h Dark photoperiod with 80 µM/m2/s light intensity for 7 days (a-b), under 20 µM/m2/s or 80 µM/m2/s light intensity in 16 h Light/8 h Dark for 14 days (c-d), or under 16 h Light/8 h Dark photoperiod with 80 µM/m2/s light intensity in the presence or absence of 5 µM DCMU treatment for 7 days (e-f). Scale bars represent 5 mm. Error bars indicate standard deviation (S.D.). (n = 30). Different letters above the bars indicate statistically significant differences between the samples (Brown-Forsythe and Welch ANOVA tests followed by Unpaired t with Welch’s correction, p < 0.05). Asterisk between the bars indicated statistically significant differences between the ratios of inhibited plant growth by low light intensity, short photoperiod or DCMU treatment in wild-type plants and kin10 mutants (Unpaired t test, ****p < 0.0001).
Extended Data Fig. 3 KIN10 and carbon deficiency regulate nitrate-responsive gene expression.
a, qRT-PCR analysis the expression of KIN10 in Ler, p35S:KIN10-HA, Col-0 and p35S:KIN10-Myc plants. Seedlings of Col-0, p35S:KIN10-Myc, Ler and p35S:KIN10-HA transgenic plants were grown on MGRL medium containing 5 mM nitrate for 7 days. Asterisk between bars indicate statistically significant differences between samples (Unpaired t test, **p < 0.01; ***p < 0.001). b-e, Quantitative RT-PCR analysis the expression of nitrate-responsive genes in wild-type, kin10 mutant and KIN10 overexpression plants. Seedlings of Col-0, p35S:KIN10-Myc and kin10 were grown on the MGRL medium containing 5 mM nitrate for 7 days, subjected to nitrate starvation for 2 days, and then treated with 5 mM KCl or 5 mM KNO3 for 1 h. Actin gene was used as an internal control. f-i, qRT-PCR analysis the expression of nitrate responsive genes in wild-type plants grown under different light intensities. Seedling of Col-0 plant were grown on medium supplemented with or without 5 mM KNO3 under 20 µM/m2/s or 80 µM/m2/s light intensity in 16 h Light/8 h Dark for 7 days. PP2A gene was used as an internal control. j-m, qRT-PCR analysis the expression of nitrate responsive gene in response to DCMU treatment. Seedling of Col-0 plant were grown on medium supplemented with/without 5 mM KNO3 and/or 5 µM DCUM under 80 µM/m2/s light intensity in 16 h Light/8 h Dark for 7 days. PP2A gene was used as an internal control. Error bars indicate SD of three biologic repeats. Different letters above the bars indicated statistically significant differences between the samples (Student t test, p < 0.05). Asterisk between the bars indicate statistically significant differences between the ratios of inhibited gene expression by low light intensity or DCUM treatment (Unpaired t test, *p < 0.05; **p < 0.01; ***p < 0.001).
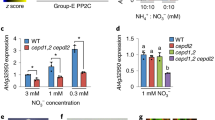
Extended Data Fig. 4 KIN10 negatively regulates NLP7-regulated gene expression.
a, Venn diagram showing the overlap between sets of genes regulated by NLP7 with or without KIN10. The Arabidopsis mesophyll protoplast of Col-0 plants were transiently expressed GFP, NLP7-GFP or Co-expressed KIN10-Myc and NLP7-GFP. b, Hierarchical cluster analysis of the expression data of 1984 genes regulated by NLP7-GFP in the presence or absence of KIN10-Myc. The numerical values for the yellow-to-blue gradient bar represent log2 of the ratio. c, Scatter plot of log2-fold change values of 1984 genes regulated by NLP7-GFP in the presence or absence of KIN10-Myc. The red line represents the trend line of the scatter plot. d, Quantitative RT-PCR analysis of the expression of genes regulated by NLP7-GFP or by KIN10-Myc and NLP7-GFP in mesophyll protoplast. PP2A gene was used as an internal control. e, Transient expression assays showed that KIN10 inhibited NLP7-induced NRT2.1 expression. The promoters of NRT2.1 and HBI1 fused to the luciferase reporter gene were co-transfected with p35S:GFP, p35S:NLP7-GFP and p35S:NLP7-GFP/p35S:KIN10-Myc into mesophyll protoplasts of wild-type plants. Error bars indicate SD of three biologic repeats. Different letters above the bars indicate statistically significant differences between the samples (Unpaired t test, p < 0.05).
Extended Data Fig. 5 KIN10-dependent phosphorylation sites on NLP7 protein.
a, Scheme of the NLP7 protein indicating KIN10 phosphorylation sites. The green dots mark in vitro KIN10 phosphorylation targets identified by mass spectrometer (this study), the purple dots refer to the previously identified CPK10/30/32 phosphorylated residue. b,c, Mass spectrometry analysis of KIN10 phosphorylation sites on NLP7.
Extended Data Fig. 6 KIN10 phosphorylates NLP7 at Ser-125 and S-306.
a, Seedlings of p35S:NLP7-YFP and p35S:NLP7-YFP/kin10 were grown on the MGRL medium containing 10 mM KNO3 under 16 h Light/ 8 h Dark photoperiod for 5 days, and then treated with 20 µM DCMU for 6 h. The immunoblots were probed with anti-GFP antibody and biotinylated Phos-tag bound with streptavidin conjugated HRP. b, The protein alignment is performed using NLPs protein sequences from green algae (Micromonas pusilla CCMP1545, A0A1B1FGW6_MARPO), moss (Physcomitrella patens, A0A2K1JP51_PHYPA), fern (Selaginella moellendorffii, D8T5B1_SELML), basal angiosperm (Amborella trichopoda, W1PF37_AMBTC), monocots (Oryza sativa, NLP3_ORYSJ; Triticum aestivum, A0A3B6GRE7_WHEAT; Zea mays, K7VRG4_MAIZE), and eudicots (Lotus japonicus, A0A2Z5V8A6_LOTJA; Arabidopsis thaliana, NLP7_ARATH). The alignment was made using the ClustalW sequence alignment program and analyzed using Vector NTI. The KIN10-mediated phosphorylation amino acid residues are indicated by red box. c, The promoter of NRT2.1 fused to the luciferase reporter gene was co-transfected with NLP7-YFP, NLP7S125A-YFP, NLP7S306A-YFP, NLP7S125AS306A-YFP (NLP7S2A-YFP) and KIN10-Myc into mesophyll protoplast of wild-type plants. The luciferase activities were normalized by Renilla luciferase as an internal control. Error bars represent S.D. (n = 3). Different letters above the bars indicate statistically significant differences between the samples (One-way ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05).
Extended Data Fig. 7 Phosphorylation mimic versions of NLP7 failed to rescue the growth defective phenotypes of nlp7-1.
a, The growth phenotype of wild type, nlp7-1 and transgenic plants expressing different versions of NLP7 grown in soil for 3 weeks under long-day condition. Scale bar represents 1 cm. b, RT-PCR showed the expression levels of NLP7 and mutation forms of NLP7 in wild type, nlp7-1 and different transgenic plants. PP2A represented the equal cDNA loading. c, Immunoblot analysis showed the protein levels of NLP7 and mutation forms of NLP7 in wild type, nlp7-1 and different transgenic plants. Actin was used as protein loading control. d, The promoting effects of nitrate on the expression of HBI1 were reduced in the NLP7S2D/nlp7-1 and NLP7S2E/nlp7-1 transgenic plants. Seedlings of wild-type plants and indicated mutants were grown on the MGRL medium containing 5 mM KNO3 for 5 days, transferred to nitrogen-free medium for 2 days, and then treated with 10 mM KNO3 or 10 mM KCl for 3 h. PP2A was used as an internal control. Error bars indicate SD of three biologic repeats. Different letters above the bars indicated statistically significant differences between the samples (Unpaired t test, p < 0.05). e, Transient assays showed the expression of HBI1 was induced by NLP7-YFP and NLP7S2A-YFP, but not by NLP7S2D-YFP and NLP7S2E-YFP. The promoter of HBI1 fused to the luciferase reporter gene was co-transfected with NLP7-YFP, NLP7S2A-YFP, NLP7S2D-YFP and NLP7S2E-YFP into mesophyll protoplast of wild-type plants. The luciferase activities were normalized by Renilla luciferase as an internal control. Error bars represent S.D. (n = 3). Different letters above the bars indicate statistically significant differences between the samples (Brown-Forsythe and Welch ANOVA tests followed by Unpaired t with Welch’s correction, p < 0.05).
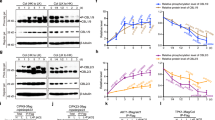
Extended Data Fig. 8 Overexpression KIN10 decreased the NLP7 protein stability.
a, Immunoblot analysis of the protein levels of pNLP7:NLP7-YFP in Col-0 and p35S:KIN10-Myc backgrounds. Seedlings of pNLP7:NLP7-YFP and pNLP7:NLP7-YFP/p35S:KIN10-Myc were grown on the medium containing 5 mM KNO3 for 6 days. b, qRT-PCR analysis of NLP7 expression in pNLP7:NLP7-YFP and pNLP7:NLP7-YFP/p35S:KIN10-Myc plants. Seedlings of pNLP7:NLP7-YFP and pNLP7:NLP7-YFP/p35S:KIN10-Myc were grown on the medium containing 5 mM KNO3 for 6 days. PP2A gene was used as an internal control. Error bars indicate SD of three biologic repeats. ns above bars indicates that there was no statistically significant differences between the samples (Welch’s t test, p < 0.05). c-f. Immunoblot analysis of the protein levels of NLP7-Myc in Col-0, kin10 and p35S:KIN10-Myc backgrounds with or without CHX treatment. Seedlings of p35S:NLP7-Myc, p35S:NLP7-Myc/kin10 and p35S:NLP7-Myc/p35S:KIN10-Myc were grown on ½ MS liquid medium for 7 days, then treated with 50 µM cycloheximide (CHX) for different time. The band intensities of Myc and actin were quantified by Image J software. Error bars represent S.D. (n = 3). Different letters above the dots indicate statistically significant differences between the samples (Unpaired t test, p < 0.05).
Extended Data Fig. 9 Carbon deficiency promotes the cytoplasmic localization of NLP7.
a, b, KIN11 promotes NLP7 protein cytoplasmic localization in protoplasts. The Arabidopsis mesophyll protoplasts were transfected with NLP7-YFP and/or KIN11-Myc and Histone3−RFP. c,d, The nuclear localization of NLP7-YFP was inhibited by KIN10, but such inhibiting effects of KIN10 were reduced in NLP7S2A-YFP. The Arabidopsis mesophyll protoplasts were transfected with NLP7S2A-YFP or NLP7-YFP and/or KIN10-Myc and Histone3−RFP. e,f, DCMU treatment promotes the cytoplasmic localization of NLP7 in the tobacco leaves. The tobacco leaves that were scooped out from soil and grown on the nitrate free medium for 2 days were transformed with the Agrobacterium containing p35S:NLP7-YFP construct. After 24 h, these plants were treated with 10 mM KNO3 and/or 50 μM DCMU for 24 h. g-j, Carbon deficiency promotes the cytoplasmic localization of NLP7. Seedlings of p35S:NLP7-YFP/nlp7-1 and p35S:NLP7S2A-YFP/nlp7-1 were grown on the 10 mM KNO3 medium for 6 days under 16 h Light/8 h Dark photoperiod with 20 µM/m2/s or 80 µM/m2/s light or under 16 h Light/8 h Dark photoperiod with 80 µM/m2/s light treated with/without 50 µM DCMU for 24 h. The box plots showed the ratio of nuclear to cytoplasmic signals of NLP7-YFP or mutant form of NLP7-YFP. Scale bars represent 20 μm. Error bars represent S.D. (n = 80). Asterisk between bars indicate statistically significant differences between the samples (Welch’s t test, ****p < 0.0001). Different letters above the bars indicate statistically significant differences between the samples. (Brown-Forsythe and Welch ANOVA tests followed by Unpaired t with Welch’s correction, p < 0.05).
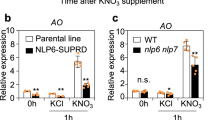
Extended Data Fig. 10 Nitrate regulates SnRK1 activity and NLP7 subcellular localization.
a, Nitrate depletion induced the expression of SnRK1 target genes DIN1 and TPS8. Seedlings of Col-0 were grown on the MGRL medium containing 10 mM KNO3 for 5 days, and transferred to medium containing 10 mM KNO3 (Mock) or nitrogen-free medium (N depletion) for 24 h. b, Nitrate resupply reduced the expression of SnRK1 target genes DIN1 and TPS8. Seedlings of Col-0 were grown on the MGRL medium containing 10 mM KNO3 for 5 days, then transferred to nitrogen-free medium (Mock) for 24 h and resupplied with 10 mM KNO3 for 12 h (N resupply). Actin gene was used as an internal control. Error bars indicate SD of three biologic repeats. Asterisk above the bars indicate statistically significant differences between the samples (Unpaired t test, *p < 0.05; **p < 0.01, ***p < 0.001). c, Seedlings of p35S:KIN10-Myc were grown on the MGRL medium containing 10 mM KNO3 for 5 days, transferred to nitrogen-free medium for 2 days, then treated with 10 mM NH4+ for indicated time. Total KIN10 proteins were probed with anti-Myc and phosphorylation KIN10 were probed with anti-P-AMPK antibodies. d, The leaves of tobacco plants that were scooped out from soil and grown on the nitrate free medium were transformed with the Agrobacterium containing p35S:NLP7-YFP and p35S:NLP7S2A-YFP construct. After 36 h, these plants were treated with 10 mM KNO3 or 10 mM KCl for 3 h. The box plots showed the ratio of nuclear to cytoplasmic signals of NLP7-YFP and mutant versions of NLP7-YFP. Scale bars represent 20 μm. Error bars represent S.D. (n = 80). Different letters above the bars indicated statistically significant differences between the samples. (Brown-Forsythe and Welch ANOVA tests followed by Unpaired t with Welch’s correction, p < 0.05).
Supplementary information
Supplementary Table
Table 1. Nitrate-regulated genes in wild-type plants; Table 2. Nitrate-regulated genes in KIN10-Ox plants; Table 3. NLP7-regulated genes in protoplasts; Table 4. NLP7andKIN10 co-regulated genes in protoplasts; Table 5. Oligo used in this study and ANOVA.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots and gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unmodified gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blots and gels.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data and unprocessed western blots and gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and gels.
Source Data Extended Data Fig. 8
Unprocessed western blots and gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Extended Data Fig. 10
Unprocessed western blots and gels.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Han, C., Wang, JG. et al. Regulatory functions of cellular energy sensor SnRK1 for nitrate signalling through NLP7 repression. Nat. Plants 8, 1094–1107 (2022). https://doi.org/10.1038/s41477-022-01236-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01236-5