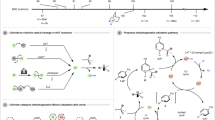
Abstract
The direct alkenylation with simple alkenes stands out as the most ideal yet challenging strategy for obtaining high-valued desaturated alkanes. Here we present a direct asymmetric dehydrogenative α-C(sp3)-H alkenylation of carbonyls based on synergistic photoredox-cobalt-chiral primary amine catalysis under visible light. The ternary catalytic system enables the direct coupling of β-keto-carbonyls and alkenes through a cooperative radical addition-dehydrogenation process involving a chiral α-imino radical and Co(II)-metalloradical intermediate. A catalytic H-transfer process involving nitrobenzene is engaged to quench in situ generated cobalt hydride species, ensuring a chemoselective alkenylation in good yields and high enantioselectivities.
Similar content being viewed by others
Introduction
The catalytic asymmetric α-alkenylation of carbonyls is a strategic C-C bond-forming transformation that grants access to chiral α-vinyl carbonyls as versatile frameworks and synthons1,2. There have been major advances along this line to enable stereoselective α-alkenylation via enolate or enamine intermediates by means of transition metal or organic catalysis3,4,5,6,7,8,9,10,11,12,13. However, most of these processes necessitate the use of pre-functionalized vinyl precursors, which require activating functional groups such as halides3,4,5,6,7, hypervalent iodinium8,9 or borate10,11,12,13 (Fig. 1, I). Simple alkenes represent the most ideal alkenylation reagents as additional activation groups can be entirely avoided and the resulting alkenylation becomes highly atom-economic and hence synthetically appealing, however, such a process remains largely unexplored14,15. In a few isolated cases, the reactions were limited to special alkenes16. To the best of our knowledge, there are few general methods to asymmetric alkenylation with alkenes, particularly in the construction of all-carbon quaternary center17,18.
Based on the continuous exploration on oxidative enamine catalysis with chiral primary amine, we reported that the corresponding secondary enamine would undergo a facile loss of proton upon single-electron oxidation to α-imino radical intermediate as a result of the enhanced N-H acidity at its radical cationic status19,20,21,22. The chiral α-imino radical catalysis have been applied to decarboxylative alknylation23 and dehydrogenative allylic alkylation reaction24. On these basis, we explored the potential of this catalysis in achieving direct asymmetric C–H alkenylation with non-functionalized alkenes25,26. In this article, we report a hydrogen-transfer strategy through α-imino radical for direct alkenylation with simple alkenes by the ternary photoredox-cobalt-chiral primary amine catalysis (Fig. 1, II). Detailed mechanistic studies have been conducted to unveil the intricate stereoscopic control mode and electron-proton-shuttle process that is indispensable in this transformation.
Results
Optimization for dehydrogenative alkenylation
We started with a model alkenylation reaction between ketoester 1a and styrene 2a. A preliminary attempt by the combination of chiral primary amine 4a (20 mol%), Co(dmgH2)2DMAPCl 5a (8 mol%) and [Ir(ppy)2dtbbpy]PF6 ([Ir], 2 mol%) gave the desired product 3a with only 20% yield, 80% ee and 10:1 E/Z (Condition A, Table 1, entry 1). Further optimization with the initial catalytic system didn’t lead to much improvement on reactivity and enantioselectivity (Table 1, entries 2–4). Interestingly, a slightly improved enantioselectivity was observed with the combination of 4,4-dimethylaminopyridine (DMAP) and a difluoroborane (BF2)- Co(II)-catalyst 5b (Table 1, entry 5, 20% yield and 86% ee). Further investigation showed that the introduction of 2-nitrotoluene (25 mol%) as H-acceptor and decreasing the reaction temperature to −10 °C were particularly effective to facilitate the alkenylation pathway. In this process, 2-nitrotoluene was fully reduced to its aniline derivative with 56% yield (in terms of 2-nitrotoluene, supplementary Fig. 1). It was found a larger ratio of 2a/1a led to higher yield of the product 3a, while slightly reduced results for both yield and stereoselectivity were observed when excess amount of 1a was engaged (Table 1, entries 7–8). The screening of amino-catalysts revealed that the morpholine-substituted 4a was the optimal one, and switching to piperidine 4b and diethylamino- 4c led to a diminishing yield and enantioselectivity (Table 1, entries 9 and 10). The use of other nitro-arenes showed comparable results (Table 1, entries 11 and 12). Under optimized conditions B, the desired alkenylation product 3a was obtained in 71% isolated yield, 93% ee, and 18:1 E/Z ratio (Table 1, entry 6, Condition B). Finally, control experiments revealed that any of the catalytic system was essential in the reaction, and no reaction were observed in their absence (Table 1, entry 13). The reaction also did not proceed in the dark without light irradiation (Table 1, entry 14), verifying its photochemical nature.
Substrate Scope
As shown in Fig. 2, styrenes bearing various para-substituents on the aryl ring such as alkyl (3c), phenyl (3d), alkoxyl (3e), phenoxyl (3f) and halogen (3h‒3j) were well tolerated in the reactions to afford the E-selective alkenylation products with yields ranging from 30% to 74% and high levels of enantioselectivities (Fig. 2, entries 2‒11). Interestingly, the configuration of alkenes was mostly of Z-form when a fluoro-substituted photoredox catalyst [Ir]-dF was used, and similar results were also observed for other substrates (Fig. 2, entries 1‒4). The substrate with long linear alkoxyl group also worked well, providing the corresponding product 3k with 42% yield, 90% ee, and 3:1 E/Z ratio. Moreover, meta-, ortho- as well as multi-substituted styrenes reacted smoothly with satisfying results (Fig. 2, entries 13‒22). Generally, the reaction favors electron-donating styrenes (3e‒3g, 3q, 3r, and 3w) and slightly decreased yields were observed for styrenes bearing electron-withdrawing group (3i, 3j, and 3n).
aReaction conditions: 1 (0.1 mmol), 2 (0.5 mmol), 4a (20 mol%), [Ir] (2 mol%), 5b (8 mol%), 2-nitrotoluene (25 mol%), DMAP (8 mol%), 0.3 mL of MeCN, deaerated and irradiated for 48 h by 30 W blue LED under −10 °C. Yield with isolated product. E/Z ratio was determined by 1H NMR analysis. ee was determined by HPLC analysis. b[Ir]-dF instead of [Ir]. cReaction under room temperature. d1.0 Equivalent of alkenes and 2.0 equivalent of 1a were added. e2.5 Equivalent of alkenes was added.
It should be noted that ortho- group has little effect on reactivity as 2,4,6-trimethylstyrene gave the corresponding product 3v with 40% yield, 86% ee, and 6:1 E/Z ratio (Fig. 2, entry 23). Thiophene-substituted alkenes worked well in the reaction with single E-alkene stereoisomer 3w (Fig. 2, entry 24). Unfortunately, internal β-methyl styrene, cyclohexane, and terminal substrates such as allylbenzene and 1,1-diphenyl ethylene did not work under the present conditions (Fig. 2, entries 41‒44).
The applicability of the β-ketocarbonyls was next investigated. Different esters could be incorporated to furnish the corresponding alkenylation products with good yields and enantioselectivities (Fig. 2, entries 25 and 26). Dihydrofuranone, cyclohexanone or acyclic ketoesters could also be applied, showing unfortunately low reactivity (Fig. 2, entries 27‒29). The reactions worked well with β-ketoamides to give the corresponding single E-alkenylation products 3ac‒3af and the five-membered cyclopentanones showed higher activity and stereoselectivity than their six-membered counterparts (Fig. 2, entries 30‒33), likely due to the more propensity of five-membered rings to form exocyclic double bond, a preferred geometry for the key radical intermediate (Fig. 1).
The current catalytic protocol could be extended to late-stage functionalization of structurally complex substrates bearing natural products and pharmaceuticals. Firstly, celestolide 3ag and tonalid 3ah derived alkenes showed excellent enantioselectivities and E/Z ratio (Fig. 2, entries 34 and 36). Furthermore, similar results were also observed for substrates bearing pharmaceutically active ibuprofen 3aj and camphanic acid group 3aj (Fig. 2, entries 36 and 37). Of further significance is the observation that the protocol enables late-stage functionalization of L-tyrosine and L-phenylalanine derivatives (3ak, 3al) in good yields and high levels of stereoselectivity (Fig. 2, entries 38 and 39). In addition, diacetone-fructose derived β-ketoester also worked smoothly to furnish the corresponding product 3am albeit with relatively low activity (Fig. 2, entry 40). Finally, a gram-scale reaction (10 mmol) of β-ketoester 1a and styrene was performed to probe the practicability, and comparable results were obtained in the presence of a reduced catalyst loading (Fig. 2, entry 2).
Interestingly, 1,1 di-substituent alkenes could be also applied to this asymmetric dehydrogenative transformation. Unexpectedly, thermodynamically-stable allyl alkylation product 3an was obtained with satisfactory result from 1-methylene-dihydroindene 2an, an inactive substrate by our previous strategy (Fig. 3, 3an)11,24. When there is no 2-nitrotoluene, only the isomerization product 2an’ is obtained with a yield of 95%, and no desired allylic adduct was isolated. Similar allylic alkylation were also obtained with five, six, and seven-membered cyclic methylenes with liner and cyclic β-ketoester (Fig. 3, 3ao‒3as).
Mechanistic studies
Acid-base effect on the catalytic system
In asymmetric dehydrogenative allylic alkylation reaction, we noticed that balancing basicity between tertiary amino moiety of aminocatalyst and axial ligand of cobaloxime was critical for the transformation24. In the current reaction system with BF2-Co(II) 5b, the catalytic amount of base additive was also found to be critical for both reactivity and stereoselectivity, and there was virtually no reactivity in its absence. A survey of different organic base revealed a clear trend between basicity (pKaH) and catalytic performance (Fig. 4, III). DMAP with a pKaH = 7.9 gave the best results in terms of both yield and enantioselectivity. Strong basic amines such as DABCO (pKaH 9.1), guanidine (pKaH 13.2), DBU (pKaH 13.9), and DBN (pKaH 15.3) demonstrated poor activity but maintaining selectivity. Similar behaviors were also observed with less basic amines such as N-methyl imidazole (pKaH 6.4) and pyridine (pKaH 3.4)27,28. From Fig. 4, it is also clear that basicity seems only affect activity but not the stereoselectivity, suggesting the base-mediated proton shuttle may facilitate the conversion, but do not directly participate in the stereodetermining step. In this regard, the loading of DMAP also has a dramatic effect on the catalysis, both decreasing and increasing the loading led to a reduction of activity (Fig. 4, I). Furthermore, the basicity of tertiary amine moiety also significantly influences this reaction. Piperidine and diethylamine, with stronger basicity than morpholine, markedly diminish both reactivity and enantioselectivity, highlighting a delicate acid-base balance in the reaction (Fig. 4, II, and III).
I Effect of DMAP loading. II Effect of tertiary amine moiety on chiral amine. III Effect of base additive. IV Proposed proton shuttle. aDMAP (8 mol%) as base additive. b4a was engaged as catalyst, the pKa values were recorded from the iBonD databank or determined through DFT calculation in DMSO solution.
A proton-shuttle network involving the key intermediate (e.g. 6a-I) and external bases can be invoked to account for the observed acid-base effect (Fig. 4, IV). DMAP (pKaH = 7.9) with moderate basicity would facilitate the photo-induced electron transfer by proton abstraction to form the most active radical intermediate 6a-I as a mono-protonated species (pKa = 9.9). A stronger base may further deprotonate to form neutral radical species 6a-III, which is less reactive in radical addition. The reactivity bias toward electron-rich styrene is in line with this scenario. In addition, the morpholine (pKaH = 9.2) side chain as in 4a also favors an internal proton shift toward 6a-I (pKa = 9.9) over 6a-II. On the other hand, piperidine (pKaH = 10.5) and diethylamine (pKaH = 10.9) side chains (as in 4b and 4c, respectively) would favor the equilibration to 6a-II, explaining the observed poor activity of these two catalysts.
Hydrogen transfer with cobalt
[CoIII]-H species are known to undergo reversible addition-elimination with alkenes24,29. Under hydrogen-evolving conditions in the absence of H-acceptor, the reaction afforded mainly alkene-dimerization byproduct b1 (42% yield) (Fig. 5, I), derived from [CoIII]-H mediated radical process. The desired alkenylation adduct 3a was isolated in a minor 20% yield and 80% ee. During further optimization, the use of BF2 Co(II)-catalyst 5b in the presence of nitrotoluene was identified to effectively suppress the hydroalkylation by-pathway, leading exclusively to the desired alkenylation reaction (Supplementary Fig. 1 vs Fig. 2). Previously, we found oxime-Co(III) catalyst 5a was able to promote the deuteration of styrene through [CoIII]-H mediated hydroalkylation-dehydrogenation process29. In contrast, 5b showed virtually no activity in the H/D exchange reaction (Fig. 5, II). On the other hand, both 5a and 5b showed comparable activity in the photo-reduction of 2-nitrotoluene30,31,32. These observations suggest that [CoIII]-H derived from 5b can preferentially react with polar nitro- moiety instead of alkene, explaining the observed chemoselectivity.
Photoredox cycle and stereocontrol model
A series of stoichiometric experiments with preformed enamine 6a were tested under the dual photoredox and cobalt catalytic conditions. The alkenylation reaction proceeds to give the desired product 3a with excellent enantioselectivity, verifying the enamine catalysis nature (Fig. 6, I). The addition of water to the stochiometric reaction led to a minor yet noticeable drop of the enantioselectivity. Similar detrimental polar effects were observed during the process of optimization of solvents, with protic solvents showing rather poor reactivity and enantioselectivity (Supplementary Table 8). Stepwise multivariant linear free energy correlation (LFER) analysis revealed an excellent correlation between enantioselectivity and the solvent acidity scale, Catalán’s SA (Fig. 6, II)33,34. The observed water additive effect and solvent LFER analysis suggest a critical ionic interaction in the stereocontrolling step. Furthermore, Stern-Volmer fluorescence quenching experiments revealed that the excited state [IrIII]* was only quenched effectively by cobalt 5a or 5b, a clear indication of a reductive quenching mechanism, supporting a SET sequence involving cobalt-iridium-enamine (Fig. 6, III).
Proposed cycle
On these bases, a catalytic cycle was proposed as shown in Fig. 7. The combination of [Ir], DMAP, and cobalt 5b provides an efficient photoredox oxidative system, leading to the generation of α-imino radical and [CoIII]-H species through a sequence of electron and proton transfer process. A radical-radical complex A, an ion pair consisting of the imino radical 6a-I and Co(II) that is sensitive to polar media, was proposed to dictate the stereoselectivity. Subsequent cooperative radical addition to alkene through transition state TS-I results in the formation of the critical C-C bond (Fig. 7). A following photo-mediated dehydrogenation leads to the alkenylation product and another [CoIII]-H species. Subsequent hydrolysis would regenerate aminocatalyst 4a and complete the catalytic cycle. The reduction process between 2-nitrotoluene and the two molecules of [CoIII]-H species complete the cobalt catalysis, and its effectiveness is critical to override the undesired hydroalkylation process.
Discussion
We have developed an efficient catalytic system to achieve the direct oxidation of secondary enamine intermediate which can be applied to enantioselective α-C(sp3)-H functionalization of carbonyls with alkenes by combining photoredox-cobalt-chiral primary amine catalysis under visible light irradiation. This synergistic system leads to the formation of alkenylation adducts with excellent stereoselectivity through a cooperative radical addition process that involves a chiral α-imino radical and Co(II)-metalloradical. A series of mechanistic studies revealed an elaborate electron and proton transfer process that was involved. The successful development of asymmetric alkenylation is also attributed to the presence of hydrogen acceptor to quench the in-situ generated cobalt hydride, thus improving chemoselectivity. We believe the current strategy would find broad applications in elusive asymmetric radical transformations.
Methods
General procedure for dehydrogenative alkenylation
In an oven-dried 5 mL pyrex tube equipped with a magnetic stir bar, β-ketocarbonyls 1 (0.1 mmol), [Ir(ppy)2dtbbpy]PF6 (1.86 mg, 2 mol%), Co(dmgBF2)2 ·2H2O (3.36 mg, 8 mol%), chiral primary amine 4a (6.72 mg, 20 mol%), DMAP (0.98 mg, 8 mol%), alkene 2 (0.5 mmol) and MeCN (0.3 mL) were added. The mixture was equipped with a rubber septum and bubbled with argon gas. The sample was then irradiated by a 30 W blue LED under −10 °C condition for 48 h. After that, the reaction mixture was directly loaded onto silica gel column and eluted with ethyl acetate/hexane to obtain the alkenylation product.
Data availability
All data are available from the corresponding author upon request. Supplementary Information is available and includes general information, substrate and reagent synthesis, optimization details, general experimental procedures, and compound characterization, determination of the absolute configuration, mechanistic studies, HPLC, NMR spectra, and DFT calculations. Source data are present. Source data are provided with this paper.
References
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H Bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Le Bras, J. & Muzart, J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 111, 1170–1214 (2011).
Kučera, R. et al. Enantioselective total synthesis of (−)-himalensine a via a palladium and 4-hydroxyproline co-catalyzed desymmetrization of vinyl-bromide-tethered cyclohexanones. J. Am. Chem. Soc. 145, 5422–5430 (2023).
Lou, S. & Fu, G. C. Enantioselective alkenylation via nickel-catalyzed cross-coupling with organozirconium reagents. J. Am. Chem. Soc. 132, 5010–5011 (2010).
Taylor, A. M., Altman, R. A. & Buchwald, S. L. Palladium-catalyzed enantioselective α-arylation and α-vinylation of oxindoles facilitated by an axially chiral P-stereogenic ligand. J. Am. Chem. Soc. 131, 9900–9901 (2009).
Dai, X., Strotman, N. A. & Fu, G. C. Catalytic asymmetric hiyama cross-couplings of racemic α-bromo esters. J. Am. Chem. Soc. 130, 3302–3303 (2008).
Chieffi, A., Kamikawa, K., Åhman, J., Fox, J. M. & Buchwald, S. L. Catalytic asymmetric vinylation of ketone enolates. Org. Lett. 3, 1897–1900 (2001).
Guo, J. et al. Nickel(II)-catalyzed enantioselective α-vinylation of β-keto amides/esters with hypervalent iodine salts. Org. Lett. 18, 5540–5543 (2016).
Skucas, E. & MacMillan, D. W. C. Enantioselective α-vinylation of aldehydes via the synergistic combination of copper and amine catalysis. J. Am. Chem. Soc. 134, 9090–9093 (2012).
Xiong, P., Hemming, M., Ivlev, S. I. & Meggers, E. Electrochemical enantioselective nucleophilic α-C(sp3)–H alkenylation of 2-acyl imidazoles. J. Am. Chem. Soc. 144, 6964–6971 (2022).
Stevens, J. M. & MacMillan, D. W. C. Enantioselective α-alkenylation of aldehydes with boronic acids via the synergistic combination of copper(II) and amine catalysis. J. Am. Chem. Soc. 135, 11756–11759 (2013).
Kim, H. & MacMillan, D. W. C. Enantioselective organo-SOMO catalysis: the α-vinylation of aldehydes. J. Am. Chem. Soc. 130, 398–399 (2008).
Li, L., Li, Y., Fu, N., Zhang, L. & Luo, S. Catalytic asymmetric electrochemical α-arylation of cyclic β-ketocarbonyls with anodic benzyne intermediates. Angew. Chem. Int. Ed. 59, 14347–14351 (2020).
Lu, M.-Z. et al. Recent advances in alkenyl sp2 C–H and C–F bond functionalizations: scope, mechanism, and applications. Chem. Rev. 122, 17479–17646 (2022).
Tang, S., Liu, K., Liu, C. & Lei, A. Olefinic C–H functionalization through radical alkenylation. Chem. Soc. Rev. 44, 1070–1082 (2015).
Liang, K., Zhang, Q. & Guo, C. Nickel-catalyzed switchable asymmetric electrochemical functionalization of alkenes. Sci. Adv. 8, eadd7134 (2022).
Corey, E. J. & Guzman-Perez, A. The catalytic enantioselective construction of molecules with quaternary carbon stereocenters. Angew. Chem. Int. Ed. 37, 388–401 (1998).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Zhu, L. et al. Catalytic asymmetric oxidative enamine transformations. ACS Catal. 8, 5466–5484 (2018).
Zhang, L., Fu, N. & Luo, S. Pushing the limits of aminocatalysis: enantioselective transformations of α-branched β-ketocarbonyls and vinyl ketones by chiral primary amines. Acc. Chem. Res. 48, 986–997 (2015).
Li, Y., Wang, D., Zhang, L. & Luo, S. Redox property of enamines. J. Org. Chem. 84, 12071–12090 (2019).
Li, Y., Zhang, L. & Luo, S. Bond energies of enamines. ACS Omega 7, 6354–6374 (2022).
Wang, D., Zhang, L. & Luo, S. Enantioselective decarboxylative α-alkynylation of β-ketocarbonyls via a catalytic α-imino radical intermediate. Org. Lett. 19, 4924–4927 (2017).
Jia, Z., Zhang, L. & Luo, S. Asymmetric C–H dehydrogenative allylic alkylation by ternary photoredox-cobalt-chiral primary amine catalysis under visible light. J. Am. Chem. Soc. 144, 10705–10710 (2022).
Capacci, A. G., Malinowski, J. T., McAlpine, N. J., Kuhne, J. & MacMil-lan, D. W. C. Direct, enantioselective α-alkylation of aldehydes using simple olefins. Nat. Chem. 9, 1073–1077 (2017).
Xu, G.-Q., Wang, W. D. & Xu, P.-F. Photocatalyzed enantioselective functionalization of C(sp3)–H bonds. J. Am. Chem. Soc. 146, 1209–1223 (2024).
Yang, J.-D., Xue, X.-S., Ji, P., Li, X. & Cheng, J.-P. Internet Bond-energy Databank (pKa and BDE): iBonD Home Page. First accessed date: 2024-01-10. Version number: iBonD 2.0 Version. http://ibond.nankai.edu.cn.
Yang, Q. et al. Holistic prediction of pKa in diverse solvents based on machine learning approach intermediates. Angew. Chem. Int. Ed. 19, 19282–19291 (2020).
Jia, Z. & Luo, S. Visible light promoted direct deuteration of alkenes via Co(III)–H mediated H/D exchange. CCS Chem. 5, 1069–1076 (2023).
Meng, S.-L. et al. Cobaloxime: selective nitrite reduction catalysts for tandem ammonia synthesis. Energy Environ. Sci. 16, 1590–1596 (2023).
Yang, Q. et al. Visible-light-promoted asymmetric cross-dehydrogenative coupling of tertiary amines to ketones by synergistic multiple catalysis. Angew. Chem. Int. Ed. 56, 3694–3698 (2017).
Zhong, J.-J. et al. A cascade cross-coupling and in situ hydrogenation reaction by visible light catalysis. Adv. Synth. Catal. 356, 2846–2852 (2014).
Minisci, F. et al. Polar effects in free-radical reactions. Solvent and isotope effects and effects of base catalysis on the regio- and chemoselectivity of the substitution of protonated heteroaromatic bases by nucleophilic carbon-centered radicals. J. Org. Chem. 52, 730–736 (1987).
Han, Y., Yang, K., Zhang, L., Luo, S. & Cheng, J.-P. Understanding how charge-charge interaction affects the stereochemistry of enamine fluorination by chiral primary amine catalysis. Sci. China Chem. 66, 2828–2835 (2023).
Acknowledgements
We thank the National Key R&D Program of China (2023YFA1506401, S.L. and L.Z.), the National Fundamental Resource Investigation Program of China (2018FY201200, S.L.), the Natural Science Foundation of China (22031006 and 22393891, to S.L.), and Haihe Laboratory of Sustainable Chemical Transformations for financial support (S.L. and L.Z.).
Author information
Authors and Affiliations
Contributions
S.L. conceived and directed the project.; Z.J. optimized the reaction conditions, examined the substrate scope, and studied the mechanism with the help of L.C.; L.Z. performed the computational studies. S.L. and Z.J. wrote the manuscript, with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, Z., Cheng, L., Zhang, L. et al. Asymmetric C–H Dehydrogenative Alkenylation via a Photo-induced Chiral α‑Imino Radical Intermediate. Nat Commun 15, 4044 (2024). https://doi.org/10.1038/s41467-024-48350-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48350-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.