Abstract
Recent research in artificial cell production holds promise for the development of delivery agents with therapeutic effects akin to real cells. To succeed in these applications, these systems need to survive the circulatory conditions. In this review we present strategies that, inspired by the endurance of red blood cells, have enhanced the viability of large, cell-like vehicles for in vivo therapeutic use, particularly focusing on giant unilamellar vesicles. Insights from red blood cells can guide modifications that could transform these platforms into advanced drug delivery vehicles, showcasing biomimicry’s potential in shaping the future of therapeutic applications.
Similar content being viewed by others
Introduction
Artificial cells are cell-like compartments that are able to reproduce at least one of the basic properties of cells: ability to compartmentalise enzymatic reactions, store and replicate information, exchange mass and energy with their environment, self-organise and regulate spatiotemporal features, sense and communicate with their environment, move, grow, reproduce and evolve1. Although the compartmentalisation property has been demonstrated in many different systems (i.e., membrane-based - such as in liposomes and polymersomes - or membrane-less - such as in emulsions, colloidosomes and coacervates)2, in order to replicate the functions of cells that occur at their surface (i.e., communication, division) the most promising approach is to build a compartment consisting of a lipid membrane hosting functional membrane proteins. Towards this end, the use of lipid vesicles consisting of a single lipid bilayer represents the most straightforward route, and, in particular, Giant Unilamellar Vesicles (GUVs) are ideal candidates since their dimensions (i.e., with diameters above 1 μm) and curvature are the most similar to eukaryotic cells3,4,5. This type of lipid vesicles has been proposed for use in a wide variety of applications, ranging from the study of cell division6, to insulin-producing artificial cells7. Despite their attractive features, these biomimetic membrane systems have become stagnant as a model system for fundamental research, without progressing into any major translational applications and virtually no clinical trials published on their potential for therapeutic use. This is in striking contrast with their smaller counterparts, Large Unilamellar Vesicles (LUVs), with diameters between 100 nm and 1 μm (commonly know as liposomes), which have been used in countless clinical applications8,9,10. Although at first glance this might appear rather surprising, given the similarities between GUVs and eukaryotic cells which routinely perform a wide set of therapeutic functions in vivo, these artificial systems are typically not stable enough to remain in circulation for long enough to provide an effective therapeutic value.
In this review we highlight the efforts made so far to produce therapeutic GUVs, taking on a biomimetic approach to shed light on what are the requirements for GUVs to become successful therapeutic artificial cells for in vivo applications. Specifically, we focus on the biomimicry of human red blood cells (RBCs), as they are a relatively simple cell model that can remain in circulation for up to 120 days11. We discuss the key aspects that provide RBCs with such exceptional biocompatibility (like size, shape, lipid composition, osmotic balance and macromolecular crowding) and we examine how these features can be mimicked within a GUV platform. Additionally, we review and compare, several RBC-mimicking carriers based on different systems, including GUVs, LUVs and modified RBCs. Finally, we list several important properties beyond advancements in RBC biomimicry that are needed to drive the nascent field of therapeutic artificial cells forward.
GUVs and RBCs: the basics
GUVs
The study and utilisation of GUVs has traditionally been constrained to obtaining fundamental insights in lipid and protein-lipid mechanics (e.g., actin-driven tubulation12, vesicle fusion13 and vesicle budding14). The scientific community, though, quickly realised that these compartments could also be proposed as cell-like environments with immense potential for research on the origins of life as well as diverse biomedical applications4,15. Just like the smaller liposomes, GUVs can be produced with well-defined materials, synthesis and purification processes. They can be further functionalised with a diversity of biomolecules, fuse with cell membranes for intracellular drug delivery and vehiculate both hydrophilic and hydrophobic molecules16,17,18. In regards to the latter, GUVs offer some inherent advantages: due to their large size they can encapsulate large quantities of drugs and also accommodate the transport of larger drug constructs19,20, which opens up the possibility of controlled release over long periods of time to maintain optimal drug levels6. GUVs can also contain multiple subcompartments, enabling the integration of complex signalling-like pathways where biochemical signals can trigger specific responses (i.e. drug release). Finally, due to their size, they can also modulate and mimic cellular processes, as shown in investigations of immune cell interactions21.
GUV production: state of the art and limitations
In order to fulfil the previous applications, it is crucial to use adequate methods to produce GUVs. And although a wide set of techniques exists for GUV generation22, these typically suffer from limitations in the structure and composition of the GUVs they can produce. In general, GUV production methods can be divided in three categories: (1) the lipid film hydration methods, (2) the inverted emulsion transfer methods, and (3) the microfluidic methods. The latter can be further subdivided into the double emulsion methods and the surfactant-mediated methods. Below, these methods are briefly introduced with a special attention highlighting some of the current limitations concerning the complex production of GUVs for biomedical applications. For a more in-depth overview on the different techniques for GUV production, the reader is invited to examine two excellent recent reviews22,23.
Lipid film hydration method
In this technique, illustrated in Fig. 1A, a dehydrated lipid film is deposited on a solid substrate, e.g., the bottom of a round bottom flask. The film is then hydrated with an aqueous solution after which the lipid film undergoes a swelling process resulting in GUV formation24. This method, though, is highly limited in the buffer and lipid conditions that can be used and often results in the production of multilamellar vesicles25. Therefore, many variants around this method have been developed, the most common incorporating an electroformation step wherein the lipid film is deposited on a conductive surface (like indium tin oxide-coated glass or platinum wires) and subjected to an AC voltage which effectively speeds up the lipid swelling into GUVs, as depicted in Fig. 1B26. Although this strategy affords a more homogeneous GUV dispersion with higher yields, it is restricted to the use of lipids with neutral net charge and buffers with low ionic strength, which hinders the possibility of working with physiological buffers and the integration of a wide set of functional proteins. Furthermore, the encapsulation efficiency of reagents inside the GUVs is rather low26. Another variation is performed by drying and hydrating the lipids on top of a polymer-based hydrogel, where the lipid swelling is enhanced by influx of hydrating solution from below. Different hydrogels have been used, including agarose, polyvinyl alcohol, and dextran polymers, among others. This method allows the use of a wider set of buffer and lipid conditions, but the obtained vesicles remain polydisperse3.
Inverted emulsion transfer method (IET)
Unlike thin film hydration methods, IET methods allow efficient encapsulation of reagents and enable a better control on the size and lamellarity of the produced GUVs27. This technique exploits the spontaneous self-assembly process of amphiphilic lipid molecules into a monolayer at the interface that results from generating a water-in-oil (w/o) emulsion. When the aqueous droplets in this emulsion are forced to move across an additional w/o interface harbouring another lipid monolayer, this second monolayer wraps around the first one forming a lipid bilayer, which results in the formation of a GUV (Fig. 1C). This method accommodates the generation of GUVs with asymmetric bilayers in which biological compounds can readily be encapsulated28. Although effective for simpler applications, this strategy is also heavily burdened by several limitations: a major issue is reproducibility, which is highly dependent on variations in the composition of the oils used and environmental conditions23,29. Furthermore, the exact lipid composition of the GUVs is difficult to tune, due to differences in adsorption kinetics of the lipid species at the w/o interface. Also, traces of oil tend to remain within the final lipid bilayer, which deteriorate its properties and curtail the function of integrated membrane proteins. Additionally, these membrane proteins can only be added afterwards by spontaneous insertion, by fusion with protein containing liposomes or they have to be produced inside the GUV via an in vitro translation system, which highly limits the repertoire of membrane proteins that can be inserted in the produced GUVs. Optimisations of this method include performing the lipid preparation in a humidity-controlled glove box and using microfluidic components5,30 or microcapillaries31.
Microfluidic methods
Similar to the previous method, in this strategy a water-in-oil-in-water (w/o/w) double emulsion is typically generated in a microfluidic chip, with each interface being stabilised by a lipid monolayer. The oil layer is then removed either by extraction of the solvent32,33,34 or through interfacial forces35, to finally deliver a GUV, as illustrated in Fig. 1D. Again, full removal of the oil from the bilayer is difficult36,37, so this method suffers from similar limitations as the IET method.
To bypass these restrictions, Weiss et al.38 developed a method that does not require any transfers through a potentially contaminating oil. In this strategy LUVs or SUVs (small unilamellar vesicles, with diameters under 100 nm) are encapsulated in w/o droplets stabilised by surfactants. By tuning the interaction strength between the surfactants and the lipids of the encapsulated vesicles, the latter can be induced to fuse at the w/o interface to form a droplet-stabilised GUV (dsGUV), which then needs to be released from the oil environment into an aqueous environment (Fig. 1E). Although the preparation of such w/o droplets does not intrinsically require a microfluidic platform (i.e., any emulsification process can be applied39), complex microfluidic techniques (e.g., picoinjection) are essential for the introduction of certain biomolecules (e.g., actin filaments) into the GUVs. Additionally, the lipid composition and buffer conditions have to be carefully chosen to efficiently release the GUVs from the droplet environment40, a procedure which, in itself, is not trivial to perform and requires the use of a demulsification reagent that may negatively affect the function of any inserted membrane proteins. Recently, these issues have been addressed by replacing the surfactant with nanoparticles41, which provides additional benefits, including a better compartmentalisation of the encapsulated biomolecules and the ability to integrate complex transmembrane proteins in the GUVs.
Table 1 provides an overview of the advantages and disadvantages of all these methods. In conclusion, in spite of a wide diversity of techniques available for GUV production, these strategies are generally ill-suited for manufacturing complex, biologically relevant GUVs featuring cell-like properties (i.e., lipid asymmetry, presence of lipid-raft-like nanodomains and complex membrane proteins and protein complexes), which is necessary to enable significant advances in the generation of artificial cells for biomedical applications. Nevertheless, to bestow advanced functionality to GUVs (i.e. targeted delivery, sensing capacity, stability, ...), many of these production techniques have been expanded or modified to accommodate the reconstitution of specific transmembrane proteins in the GUVs’ lipid bilayer, which the reader is invited to peruse in the excellent recent review from Litschel et al.22. Finally, although these functionalised GUVs have already been applied in vitro, they have only been used in vivo in exceptionally rare occasions. In the following sections these most advanced applications are reviewed in detail.
In vitro examples
In 2022, Hernandez et al.42 demonstrated the feasibility of using GUVs to target and induce apoptosis in Jurkat cells by functionalising the outer GUV surface with an antibody complex for cell-recognition and an apoptosis-inducing protein (FasL ligand), providing a first proof of concept of a cytotoxic synthetic cell.
In another work, Toparlak et al.43 developed GUVs encapsulating a responsive in vitro transcription/translation system capable of producing a neurotrophic factor in combination with a pore-forming protein enabling the release of the factor into the outside medium (see Fig. 2). The translation of these proteins was triggered in respons to the presence of lactones allowing for target specificity. Notably, the GUV solution had to be exchanged every 24 h for optimal performance.
A final, relevant example, was developed by Staufer et al.44, who created GUVs acting as artificial cellular organelles. This work encompassed the fabrication of synthetic peroxisomes with bovine catalase supporting cellular stress management, synthetic endoplasmatic reticulum acting as light-responsive calcium storage and magnetosomes encapsulating iron nanoparticles. The synthetic organelles exhibited survival up to 72 h when incubated with HaCaT and NRK cells before being degraded.
In vivo examples
While in vitro studies have demonstrated the therapeutic potential of GUVs, research on their application in vivo is still very limited, given their fragility.
In a first interesting study by Chen et al.45, GUVs that produced recombinant human basic fibroblast growth factor were incubated with endothelial cells and, later on, locally administered in mice through implanted matrigel plugs. These GUVs showed pro-angiogenic activity both in vitro and in vivo and were demonstrated to be non-immunogenic, albeit they were only active for 6 h.
In a second paradigmatic exampled, Krinsky et al.46 evaluated the use of GUVs excreting Pseudomonas exotoxin for the treatment of 4T1 breast cancer. First, these GUVs were incubated in vitro with 4T1 breast cancer cells, upon which 80% of the cancer cell population was killed. Next, these GUVs were tested in vivo via injection into tumours induced in the mammary fat pad of BALB/c mice, resulting in an increase of the expression of caspase 3, an apoptotic marker.
In an earlier study, Chen et al.7 produced GUVs capable of releasing insulin at high glucose concentrations as a demonstrator of artificial beta cells. GUVs were formed encapsulating glucose oxidase and peptide-shielded LUVs which encapsulated insulin. Upon the intake of glucose, this was oxidised, resulting in a decrease in pH inside the GUVs which, in turn, sterically deshielded the peptides tethered to insulin-loaded LUVs. As a result, this first set of peptides could associate with a second set of peptides, anchored to the inner surface of the GUV, inducing the fusion of the LUVs with the GUV membrane and the release of the encapsulated insulin (Fig. 2). These GUVs were dispersed in a hydrogel-forming solution which was subcutaneously injected in streptozotocin-induced type 1 diabetic mice. With this system, glucose levels could be kept normal for up to five days in the treated mice.
These compelling examples highlight both the therapeutic potential of artificial cells based on GUVs as well as the fact that the long-term in vivo survival of these systems is one of the major hurdles that needs to be overcome for their widespread application. With respect to this limitation, in the following chapters we review how RBC mimicry can be used to further improve the longevity of GUVs in in vivo applications.
RBCs
RBCs are the most abundant cell type in the human body and typically circulate for 90–120 days before they are cleared in the spleen11. The long-term stability of RBCs is a result of a complex interplay between their morphology, membrane composition and mechanical properties, which allow RBCs to squeeze through the smallest conduits of the capillary beds, enabling them to access every tissue in the human body (Fig. 3 for a visual overview). Additionally, RBCs possess a wide set of transmembrane proteins and glycoproteins which mediate their interaction with and prevent their removal by the immune system16. For these reasons, it has long been proposed that the biomimicry of RBCs can be used as a strategy to engineer drug-carrying vehicles affording long-term drug bioavailability and responsive delivery, which would greatly improve the pharmacokinetics of a wide diversity of therapeutic agents (and, particularly, large biologicals)47,48, while, at the same time, allowing to target all organs, tissues and cell types. Furthermore, RBCs interact with the immune cells in the blood, liver and spleen, which also provides a route for developing immunotherapies based on synthetic antigen-presenting cells (APCs)49. Additionally, since RBCs are cleared in the spleen by phagocytes, a biomimetic RBC variant would allow for a simple strategy to specifically target these cells47. Finally, being arguably the ‘simplest’ type of eukaryotic cells (i.e. lacking organelles and nucleus), RBCs are an ideal model to base the development of biomimetic artificial cells for therapeutic purposes11.
They have a biconcave disc shape and are extremely flexible, enabling their passage through capillaries and slits many times smaller than themselves. This flexibility is a result of their highly specialised cytoskeleton, composed of an actin-spectrin cortex (top right) that is linked to the lipid bilayer by membrane-associated protein complexes (bottom right).
RBCs as biomimetic systems
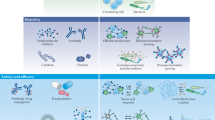
Mimicking the properties of RBCs for enhanced therapeutic vehicles has been an enduring idea through the years48. Still, most of the strategies that have been pursued to date (see “RBC-based systems” section) do not involve the manufacture of GUVs with RBC-like properties. Here, three such examples are presented which we found to be paradigmatic for their complexity and for the inclusion of more than one RBC property simultaneously (Fig. 4). More examples of RBC mimics can be found in review50, however, little in vivo applications have been reported to date.
A Polymer-RBC replica produced using RBCs as a template (reprinted with permission from ref. 51 Copyright 2020, American Chemical Society). B Coacervate coated with RBC membrane fragments (reprinted with permission from ref. 52). C Mimic produced by coating calciumhydroxide particles with first a polymer solution, then zein particles and finally RBC membranes are utilised to cover up the particles (reprinted with permission from ref. 53 Copyright 2022, American Chemical Society).
The first relevant example of RBC-like GUVs was reported by Guo et al.51, who produced an RBC mimic by first replicating the RBC shape via a silification/calcination process followed by a layer-by-layer deposition of a polymer. After this deposition, the silica template was etched away and the resulting polymeric structures were coated with an RBC membrane. These vesicles were shown to mimic the size, biconcave shape, outer membrane and oxygen-carrying capability of RBCs. What is more, they could even be loaded with drugs (doxorubicin), magnetic nanoparticles and a toxin biosensing system (based on ATP sensing). These RBC mimics were then tested ex ovo in chick embryos and in vivo in mice, whereby it was shown that both the system’s flexibility and membrane coating were essential for survival in the bloodstream. Despite the emulation of many of the RBCs properties, the elimination half-life was calculated to be 41.8 h, which is still vastly smaller than that of actual RBCs.
In an attempt to mimic the longevity and the circulatory properties of RBCs, Liu et al.52 prepared GUVs by coating dextran/dsDNA coacervate droplets with haemoglobin-containing RBC fragments. In addition, the droplets co-encapsulated glucose oxidase, which, once triggered by the presence of glucose and hydroxyurea, enabled the production of nitric oxide (NO). The RBC mimics were injected intravenously into rabbits where the produced levels of NO were capable of stimulating blood vessel vasodilation, albeit only 25% of these mimics survived for 2 h and practically all of them were cleared within 24 h.
In the last example, Hou et al.53 demonstrated a method to shape zein (a maize protein) particles in the form of RBCs which were then coated with RBC membrane fragments53. These RBC mimics were incubated with the anti-cancer drug doxorubicin and Fe2O3 nanoparticles for in vivo imaging. Upon injection in mice, these particles had a retention rate of 28% after 24 h.
These three studies highlight the enormous potential of harnessing an RBC biomimetic approach to develop a next generation of drug-delivery devices, although they also stress the intrinsic limitations that these approaches present with regards to biocompatibility. To improve on this facet, it is essential that these biomimetic carriers adequately reproduce the physicochemical properties of RBCs, properties that are outlined below. Additionally a set of different (non)-biomimetic examples are compared in Fig. 5, providing additional indication for the potential of RBC biomimicry.
Each symbol corresponds to a specific paper, the orange colour is for samples that more closely mimic RBCs. RBCM stands for RBC membrane-coated samples in contrast to NRBCM, which are the free non-RBC membrane-coated particles. While these studies are not fully comparable due to differences in materials, size and shape of the particles tested, as well as the studied species, all studies show a clear trend of prolonged retention when the vehicles integrate some of the RBC-related properties. As seen from the graph, the particles developed by Muro et al. present improved retention when produced with a discoid shape, as compared to their spherical counterparts. In the study of Merkel et al., lower percentage of cross-linker in the hydrogel, which contributed to a higher flexibility of the particles, improved retention. Merkel et al. showed that discoid-shaped hydrogel particles had an improved circulatory retention when the size of the particles resembled the size of RBCs. In Liu et al., Wang et al., & Zheng et al., an increased retention of RBC membrane-coated particles in comparison to their uncoated counterparts was demonstrated52,62,63,64,161,162,194.
Morphology and mechanics
RBCs can be regarded as the ‘contortionists’ of the mammalian cell repertoire. To be able to squeeze through narrower cavities than their own dimensions (such as blood capillaries and the spleen’s interendothelial slits), their distinctive biconcave shape is temporarily lost to adopt a diversity of particular shapes that depend on the degree of shear to which the RBCs are exposed54,55,56. This phenomenon is directly linked to their morphology (size and shape) and their membrane mechanics, which are both largely determined by their cytoskeleton.
Size and shape
Lacking a nucleus, organelles and extensive cytoplasmic structures (modifications that occur during erythropoiesis), mammalian RBCs have also evolved to adopt a biconcave shape, which is dictated by their highly specialised cytoskeleton, surface area and cellular volume57,58. With a 40% additional surface area compared to spherical cells with the same volume, RBCs are highly deformable (see “Mechanics and deformability” section>), and have optimised internal diffusion processes by eliminating the dead volume that spherical cells have at their core59. With a diameter of 7–8 μm and a height of 2.5 μm, RBCs are one of the smallest mammalian cell types. Pathological RBC morphologies present altered shapes and/or surface area-to-volume ratios (e.g. as in hereditary spherocytosis), all of which are subjected to increased retention rates in the spleen60,61. Merkel et al.62 evaluated the in vivo circulation of RBC-shaped hydrogel microparticles of several sizes in mice, demonstrating that particles with the native RBC diameter (~6.4 μm) stayed in circulation the longest (Fig. 5). Smaller particles were cleared quickly by the immune system, while larger particles became physically trapped in the liver and spleen, rapidly falling out of circulation. Similarly, this and other studies showed that microparticles with a biconcave or discoidal shape have a significantly longer circulation half-life than their spherical counterparts63,64. The exact mechanisms behind this remain unclear, but are hypothesised to be related to the altered flow mechanics, organ-specific preferences for certain particle shapes and improved flexibility in at least one dimension65,66.
The biconcave RBC shape has been replicated in many different processes and substrates. Via electrospraying, biconcave microparticles of, for example, chitosan/terephthaldehyde, pectin/oligochitosan and ethyl(hydroxyethyl)cellulose have been produced67,68,69. Other groups have succeeded in producing pectin or alginate biconcave microparticles via droplet-based microfluidic methods70,71, or using moulding processes (e.g. particle replication in non-wetting templates, PRINT) to imprint this shape in acrylate-derived hydrogel particles62,63,72. Using dextran as a shape modifier, biconcave inorganic Ca(OH)2 microcapsules were also produced which could subsequently be used as a sacrificial template for silica deposition or polyelectrolyte layer-by-layer assembly to form similarly-shaped capsules with these materials73,74,75. Biconcave microparticles of layered polyelectrolytes or silk fibroin have been produced as well, utilising as sacrificial templates calcified RBCs, biconcave poly(lactic-co-glycolic acid) or ZnO microparticles51,76,77.
While biconcave (as well as many other shapes of) particles can be coated with a lipid bilayer, the shaping of GUVs themselves into non-spherical structures represents a much more complex challenge. Although it is possible to produce GUVs in monodisperse populations within the size range of RBCs (e.g. by utilising microfluidic architectures for step emulsification78 or droplet splitting19), the formation of GUVs with a stable biconcave shape has yet to be demonstrated. Nevertheless, GUVs have been shaped into discs, cuboids or rods with microfluidic trapping, specifically modified lipids and peptides, or by harnessing macromolecular crowding effects79,80,81,82,83. These methods for creating non-spherical GUVs, however, do not allow controlled and/or permanent shape modifications, do not reproduce the RBCs’ biconcavity or require lipid compositions that are too extrinsic from those of actual RBCs. To produce a biconcave RBC-mimicking GUV that could be deployed in vivo, permanent and reproducible shaping methods need to be developed that are compatible with relevant RBC lipid compositions and GUV production techniques. This endeavour could ideally be attained through the integration of a cytoskeleton-like structure inside a GUV, which is also how nature exerts control over cell shapes.
Mechanics and deformability
The mechanical properties of RBCs are defined by their cytoskeleton, which is composed of membrane protein complexes linked to a 2D network of nanofilaments (called the cortex) which line the inner side of the RBCs’ membrane (Fig. 3). The cortex is made up of spectrin dimer repeats ordered in a pseudo-hexagonal lattice57,84, with nodes that are centred around the transmembrane protein Band 3 which accounts for up to 25% of the RBC’s total membrane protein mass. Band 3 binds filamentous actin and many more proteins through the ‘vertical interactions’ - the ‘horizontal interactions’ being those within the cortex itself. The flexibility of this lattice ensures that RBCs can endure extreme deformations without rupturing (i.e. haemolysis), followed by a quick recovery of the original shape85. The RBCs’ deformability and overall mechanics are determined by membrane cohesion and the overall resistance of the cytosol. Dysregulation of ATP metabolism, ion transport and membrane cohesion due to pathology or senescence, can result in a change in surface area, volume and/or deformability, which leads to the clearance of RBCs from circulation in the spleen60. Besides their role in transport of oxygen and carbon dioxide, RBCs have a regulatory function which is intimately connected to their cytoskeletal mechanics. Upon their passage through capillaries, for example, release of ATP and NO is triggered by sensing of low oxygen content and by mechanotransduction pathways upon the RBCs’ deformation, as such mediating vasodilation, blood pressure and tissue oxygenation86,87.
Hence, to mimic these RBC properties, therapeutic artificial cells should ideally be supported by a cytoskeleton-like structure providing the necessary mechanical robustness for circulation through the microvasculature and to withstand osmotic stress88, while possessing an adequate deformability and the ability to recover their original shape at the same time. The importance of RBC deformability was shown by Merkel et al. and Guo et al., who demonstrated that RBC-shaped hydrogel microparticles with higher deformability displayed a longer circulation half-life compared to more rigid microparticles51,63 (Fig. 5).
Many attempts to encapsulate cytoskeleton-like structures in GUVs have been reported (Fig. 6). The most common strategies were based on synthetic hydrogels, since their production is relatively simple and their mechanical properties can be tuned in a variety of ways to match the physical properties of actual cells. Furthermore, they can be stimuli-responsive (e.g. to pH or temperature), which is useful for drug-delivery applications or mimicking physiological processes89,90,91.
Another line of research on this direction has focused on replicating the 2D architecture of cortical structures with DNA origami since its nanoscale geometries can assemble into controllable microscale structures92. Jahnke et al.93 designed DNA origami tiles that interlocked into filaments inside GUVs, resulting in the formation of a cortex-like shell that could be coupled to the membrane through DNA-cholesterol hybrids. The same group further assembled patterns of hexagonally cross-linked DNA networks inside GUVs through direct laser printing94. Kurokawa et al.95 created hexagonal DNA meshes that assembled into microspheres that, when inserted in GUVs, fused and formed a shell. Other groups created DNA skeletons with varying morphologies by varying the Mg2+ concentrations of the buffers96.
The final, most common approach to synthetically build cytoskeletal structures, has been based on the encapsulation of native cytoskeletal proteins in GUVs. More specifically, actin shells have been assembled by ion-carrier mediated Mg2+ influx97,98,99 or by co-formulating actin with actin-modulating proteins100,101,102,103,104. Merkle et al.105 reconstituted actin, spectrin and ankyrin in GUVs made from native membrane fractions, and obtained a cytoskeletal cortex that was associated to the membrane.
All these approaches, though, have produced unstructured cortical assemblies and thus far they have not been tested for their ability to support non-spherical GUVs or to enhance deformability/shape recovery in GUVs, which are all necessary features to create an RBC mimic. In addition, in order to shape GUVs in a durable and controlled way (see “Morphology and mechanics” section), the cytoskeletal structures should be anchored to the membrane, in a similar way as actin/spectrin networks are associated with Band 3 in actual RBCs. The simultaneous incorporation of cytoskeletal transmembrane proteins and cortical elements inside GUVs, however, has not frequently been reported. After all, most studies employed the IET method for the reconstitution of an actin network inside GUVs (see “GUVs” section), which is poorly compatible with the incorporation of transmembrane proteins106. Therefore, more straightforward approaches have traditionally been implemented by employing modified membrane-forming molecules, such as cholesterol-tagged cytoskeletal compounds, NTA-Ni-functionalised or biotinylated lipids and actin or by simply harnessing the electrostatic interactions between negatively-charged actin filaments and positively-charged lipids29,93,103,107.
Membrane composition
Lipids
Recent research has highlighted the importance of lipid composition in RBCs as another key determinant of their function and survival. The lipid bilayer of the RBC membrane in large part consists of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and sphingomyelin (SM)108. While these are the main components of the RBC membrane, their ratios and distributions are subject to some natural biological variability109.
Membrane asymmetry
As in many cells, the lipid composition of the inner and outer leaflet of the membrane of RBCs is different, which is referred to as membrane asymmetry. The outer leaflet is predominantly composed of PC and SM, while the inner leaflet is mainly composed of PS and PE108,110. This asymmetry is important because of the physicochemical characteristics of individual lipids, e.g. PE increases membrane fluidity and rigidity, while PS is an essential co-factor of several membrane-bound proteins (e.g. Na+/K+-ATPase, protein kinase C)111,112. The disruption of this asymmetry has important implications in various cellular processes, e.g. the presence of PS on the outer membrane leaflet of RBCs is perceived as a physical injury, which promotes blood coagulation and provides a signal for their removal by phagocytes113. Imbalances in this asymmetry, which can occur naturally, are associated with several pathologies (e.g. haemolytic anaemias)114,115,116,117,118 and RBC senescence119,120,121. It has also been shown that the RBC membranes have an intrinsic membrane potential, which is independent of the presence of ions and strictly arises from lipid asymmetry. This is important, since membrane potential governs many biological processes and might affect the manner in which an RBC-mimicking GUV would function as a drug-delivery system122.
Further evidence of the physiological relevance of this asymmetry is that it is regulated via specialised enzymes, called lipases and scramblases, which control lipid translocation in an ATP-dependent and -independent manner, respectively. These enzymes maintain the membrane asymmetry by ‘flipping’ the lipids from inner to outer membrane leaflet and vice versa116,123,124. Without the activity of these transporters the collapse of this lipid asymmetry124 would quickly lead to RBCs being removed from the bloodstream by neighbouring phagocytes116.
Due to the importance of this asymmetry in a biological system, the method to produce an RBC-mimicking GUV should allow for the formation of asymmetric lipid bilayers, with emulsion transfer or microfluidic methods being the obvious choices (Table 1). Inclusion of lipid transporters should also be considered in order to maintain the membrane asymmetry necessary for the longevity and safety of the GUVs in a circulatory system, e.g. excessive PE and PS on the outer leaflet of the drug-delivery GUVs could lead to vesicle fusion or even thrombosis119,120,121,125,126.
Membrane proteins
(Trans)membrane proteins play crucial roles in maintaining the integrity of the RBCs’ membrane as well as in providing the necessary functions to fulfil their physiological roles, e.g. signalling, transport, adhesion, and cell-cell communication127. Membrane proteins are also responsible for maintaining RBCs in circulation for long periods of time by preventing their clearance through various mechanisms such as immune evasion and cell adhesion128.
CD47 is a transmembrane protein that is expressed on the surface of many cells, including RBCs and platelets, which interact with SIRPα, a receptor expressed by macrophages, acting as a ‘do not eat me’-signal129,130. This same mechanism can be applied to prevent the GUVs from being cleared from circulation, by integration of CD47 in the GUV phospholipid bilayer. Another membrane protein that prevents the formation of membrane attack complexes and the subsequent destruction of RBCs is CD59 which binds to the C8 and C9 proteins, thus inhibiting the formation of the complex131.
Another class of membrane proteins that contribute to the longevity of RBCs are glycophorins, whose sialic acid residues act as a ‘self-marker’ for the immune system, indicating that the cells are not foreign and should not be cleared132.
An additional advantage of integrating membrane proteins in GUVs would be to provide a biomimetic strategy to achieve targeted drug delivery by harnessing specific, cell-like ligand-receptor interactions. The production and isolation of such membrane proteins, however, still presents challenges due to the need to express them in sufficient amounts in adequate recombinant systems enabling the inclusion of complex post-translational modifications and developing effective purification and stabilisation methods. Furthermore, the incorporation of these membrane proteins into GUVs, such that their function and orientation are preserved, is still a challenging task due to the intrinsic limitations of the different GUV production strategies (as mentioned in “GUVs” section).
Osmotic balance
Another important feature of RBCs that should be considered for production of effective therapeutic artificial cells is the RBCs’ ability to manage differences in chemical potential. The lipid bilayer membrane of cells (and GUVs) is a semipermeable barrier and, therefore, the latter are sensitive to osmotic imbalances between the internal and external environment88,133. The osmotic balance is directly correlated to the cellular hydration state and volume, which are properties that directly affect the RBCs’ deformability. RBCs regulate these by strictly controlling, among other parameters, intracellular Na+/K+ content, redox state, membrane surface area and haemoglobin concentration61,134. This control is mediated by transmembrane proteins like Na+/K+-ATPase, Band 3 and aquaporins135,136. In conventional GUV production protocols, osmotic imbalance is typically avoided by ensuring there is no osmolarity mismatch between the inner and outer buffers. However, to successfully deploy GUVs as RBC mimics in vivo, they should tolerate physiologically relevant levels of osmotic stress88. Stability against osmotic stress has already been established in GUVs by several strategies, including their decoration with a chitosan layer137, the inclusion of a variety of cytoskeleton structures91,95,96 or via magnetic aggregation138. Particularly, biomimetic cytoskeletal structures with larger contact area with the membrane provided superior resistance to osmotic stresses96,133. These studies highlight the importance of integrating support structures (i.e. a cytoskeleton) within GUVs to provide mechanical robustness against stress.
A second strategy towards this goal is to reconstitute transporter proteins in the GUVs’ membrane to allow them to regulate their own luminal environment. Provided ATP, K+ and Na+ are present in the adequate gradients, the reconstitution of Na+/K+-ATPase should permit such regulation136,139. Similarly, aquaporins or mechanosensitive transmembrane channels (e.g. such as the mammalian non-selective Piezo1 cation channels) that open in response to an osmosis-mediated influx of water or in response to mechanical stress140, respectively, could be incorporated in the GUV membrane. Nonetheless, the need for such advanced osmoregulation constructs is debatable, since the osmolality of blood plasma in humans usually does not vary more than 1 or 2% from the basal levels141. This is hypothetically endurable by plain GUVs, particularly when mechanically stabilised or when cholesterol is present in the lipid bilayer142,143.
Macromolecular crowding
The interior of cells, including RBCs, is extremely crowded, with macromolecular concentrations oscillating between 50 and 400 mg/mL144,145. In an RBC, for example, the most abundant protein is haemoglobin with a concentration of 330 mg/mL, taking up 25% of the cytosolic volume145. Since other proteins are excluded from this volume, they experience a higher effective concentration which influences their folding properties, reaction rates and equilibrium constants. Compared to actual cells, the lumen of typical GUVs manufactured with the previously introduced methods is relatively devoid of macromolecules. By mimicking the inner crowded environment of RBCs in GUVs, significant stability and function gains might be achieved. It has, for example, been shown that molecular crowding results in more robust gene expression146 and cryopreservation147, and influences the assembly of a DNA origami-based cytoskeleton93.
Although potentially beneficial, molecularly crowded GUVs have been produced only in a limited number of studies, mainly due to the difficulty of creating GUVs in such conditions. Given the need for high encapsulation efficiencies, methods like the double emulsion techniques have mostly been used. But, as mentioned earlier, lipid bilayers produced with this method typically contain an oil pocket which disrupts membrane and protein function148,149. Guerzoni et al.150 developed a microfluidic chip for double emulsion production where first a macromolecular crowding agent (Ficoll PM70, bovine serum albumin or dextran) was encapsulated in a w/o droplet. In a second step, a w/o/w droplet was created which was later subjected to an osmotic pressure to further increase the concentration of the crowding agent. Finally the oil pocket was removed from the lipid bilayer by centrifugation. Although complete removal of the oil was not possible, the authors suggested this could be achieved using additional microfluidic techniques151. Despite illustrating significant steps in generating crowded GUVs, this work also highlights the limitations of current GUV production techniques to generate GUVs with biological-like features. Furthermore, the crowding agent itself is likely to have a significant impact on the final application and, therefore, the choice of such molecules needs to carefully be considered152.
Alternative RBC biomimetic systems
RBC-based systems
The use of actual RBCs modified to serve as drug-delivery systems has been demonstrated in numerous studies, with some even reaching clinical trials (Fig. 7). This approach makes use of the RBCs’ biochemical properties and consequent circulatory characteristics, which are preserved to a certain extent. Nevertheless, such systems (listed below in detail) still depend on donor-derived RBCs and are hence vulnerable to supply problems and the inherent risk of induced transfusion-associated hazards. In comparison to these, GUV-based artificial cells could be made to be safer, more universal, easier to store and would offer a larger versatility regarding encapsulation of compounds and therapeutic applications.
Drug-loaded carrier RBCs
With several procedures to load or coat RBCs with a drug, RBCs themselves can be used as delivery vehicles. As such, the RBCs’ innate protection against macrophage uptake and prolonged circulation time can directly be harnessed to improve drug pharmacokinetics without the need for engineering a biomimetic vehicle with similar properties. However, this strategy is not without its own issues. The ex vivo protocols to load the RBCs with the therapeutic agents are costly and might irreversibly damage the RBCs’ membrane, compromising their biocompatibility. Furthermore, there is a limit on the amount of drugs that can be encapsulated and the need to consider additional, complex strategies for controlling their release. Finally, the mechanical and rheological properties of the treated RBCs are usually detrimentally affected (although for unclear reasons), demonstrated by the fact that these systems display much shorter half-lives than their unmodified counterparts153 (e.g. ten days in mice for RBCs loaded with L-asparaginase154). Nonetheless, research in this field has progressed faster than with GUVs, with many RBC-based therapeutic products currently undergoing clinical trials155,156,157,158,159.
In addition to loading RBCs with drugs, these can also be deposited on the surface of RBCs. Examples include the linking of theranostics (e.g. nanoparticles) to RBCs via biotin-streptavidin or antigen-antibody binding, which can even be done on RBCs circulating inside the body153,160. This kind of ‘cellular hitchhiking’ has already been harnessed for a diversity of applications in vivo161,162,163,164.
Another strategy that utilises RBCs for novel therapeutic purposes, is by directly producing genetically modified RBCs from a diversity of progenitor sources (e.g. human hematopoietic stem cells, progenitor cells, human embryonic cells, induced pluripotent stem cells or immortalised cell lines165). Although the production of ‘cultured’ modified RBCs can bypass some of the disadvantages of donated RBCs (i.e. immunogenicity)166, this route is typically complicated by difficulties in production up-scaling, reproducibility and high manufacturing costs. Still, concepts utilising genetically modified RBCs have already been demonstrated as artificial APCs for immunotherapy applications167.
Nanoerythrosomes
Another strategy that harnesses the properties of native RBCs is embodied in the use of nanoerythrosomes (NEs), which are liposomes originating from RBCs that have been lysed to remove the original haemoglobin, and subsequently treated to form enclosed nanovesicles168,169. During the NEs’ formation process, their membrane can be modified and they can be loaded with drugs or fused with synthetic vesicles to create hybrid systems170,171. NEs are popular drug-delivery vehicles because of their increased in vivo circulation half-life and colloidal stability172, and they can extravasate into tumour tissue to release their cargo on the spot - in contrast to larger GUVs that would remain contained within the circulatory system173. Compared to GUVs, their production is relatively simple, but many of the RBC’s transmembrane proteins are lost during preparation174. Therapeutic formulations of NEs have already been applied in vivo with remarkably improved therapeutic results171,175,176.
RBC membrane-coated carriers
Another common strategy to enhance the targeting, delivery and circulation properties of micro- and nano-carriers is by coating them with fractions of RBC membranes. This bottom-up strategy has an enormous versatility: it is rather simple to implement and can be used with many different types of particles. Hence, it is considered by many as an important strategy for the future of drug delivery. An improved in vivo circulation half-life has been demonstrated with such systems177,178,179, even with RBC-shaped microparticles51. Nevertheless, and similarly to NEs, RBC-coated carriers suffer from the limitation that the extensive processing required to manufacture them results in damage of the membrane components and in changes in its composition, which detrimentally affects their stability and triggers an immune response180.
Finally, micro- and nano-carriers can also be coated with synthetic lipid formulations, resulting in a mechanically stabilised liposome-like carrier. For example, lipobeads (hydrogel particles coated with a lipid bilayer181) and coated silica microparticles have been tested in vivo with promising results182,183,184.
Alternative materials
Vesicle-like compartments can be generated with a diversity of building blocks beyond phospholipids, including particles (forming colloidosomes)185, amphiphilic block-copolymers (forming polymerosomes)186, or mixtures of polymers and lipids187. According to the properties of their building blocks and the manner in which they associate, those capsules can exhibit enhanced stability and special physicochemical properties185,188. Albeit the functionalisation with membrane proteins is not possible in these systems, colloidosomes can display highly stable encapsulation of biomolecules in a mechanically robust shell, the particles of which can easily be modified to provide additional functions185,189. Amphiphilic polymers can behave similarly to fatty acids, but their molecular weights can be tuned to be up to 20-fold higher, resulting in membranes with highly adjustable physicochemical characteristics (such as permeability, viscosity and elasticity190,191). Since polymer membranes tend to be thicker than lipid vesicles (5–50 nm compared to 3–5 nm)187,188, they are typically more stable and show lower permeability190,191, even though incorporation of functional transmembrane proteins is challenging. Moreover, an adequate selection of the polymer blocks is necessary for in vivo applications, since many of them are not sufficiently biocompatible and biodegradable.
Lipids and polymers can also be combined to form hybrid vesicles, with lipids providing the necessary biocompatibility and functionality, while the polymers bring in additional stability and functional versatility191. Their manufacture, though, is complex, given the discrepancy in dimensions and/or solubilities between lipids and polymers, often resulting in a driving force towards phase separation or even vesicle disintegration192. KaloCyte has developed a successful example of such a hybrid system, ErythroMer™, which encapsulates haemoglobin and pH-sensitive compounds to enable pH-sensitive O2-release. This artificial blood substitute can additionally be freeze-dried and has shown promising in vivo results for transfusion medicine applications193,194.
Beyond RBCs: adding more functionality
One of the advantages of developing RBC-like GUV carriers for in vivo applications is the possibility of integrating functionalities beyond those of normal RBCs. Different conventional biological functional units, like proteins and nucleic acids, can be incorporated in GUVs to provide a wide diversity of functions, such as a cell-free transcription and translation machinery195, transmembrane proteins196 and light production with luciferase197. What is more, when these biological units are exploited in non-conventional ways or when non-biological units are integrated, additional interesting functions can be integrated198. Examples of this include DNA origami nanopores199, transmembrane signal transducers built from synthetic chemicals200,201,202, magnetotactic behaviour endowed by encapsulated magnetic nanoparticles203 and photo-inducible release enabled by photopolymerisable lipids204. In the following sections, some of these extra potential functionalities are highlighted.
Protein production
During GUV production, a cell-free protein expression system can be encapsulated to produce proteins leading to a specific biological effect, thereby potentially allowing for the spatiotemporal control of the synthesis of a therapeutic agent and its controlled release188. A simple example of such a system is the production of eGFP by an E. coli cell-free expression system encapsulated in GUVs205,206. As discussed earlier, Chen et al. (2022) produced GUVs able to express recombinant human basic fibroblast growth factor for the stimulation of pro-angiogenic activity and tissue regeneration45. For an additional level of control, cell-free protein production in GUVs can also be triggered by external stimuli, e.g. with light188.
Sensing
A GUV-based RBC mimic can be potentially further modified to display additional sensing functionalities, integrating some of the complex behaviours (i.e. stimuli-responsiveness) typically displayed by actual cells. Therapeutic cell products are burdened by issues like complex genetic programmes, bio-containment and viability, while biosensing with pure cell-free expression system suffers from variable sensitivity207. By providing a robust outer shell, GUV-based artificial cells can mitigate the impact from external conditions while posing less of a biohazard, since they cannot replicate. Finally, the membrane can be modified with many different proteins acting as gates or receptors to improve the response selectivity. Examples of such sensing systems include detection of glucose levels in the bloodstream in vivo and sensing of histamine, Ca2+, bacterial acylated homoserine lactones (AHLs), hydrogen peroxide and hydrogen sulphide7,208,209,210,211.
Targeted drug release
Ideally, an effective drug-delivery vehicle should release its cargo only when required and specifically at the therapeutic site of interest. By choosing the phase transition temperature of their constituent lipids, liposomes can be optimised to release their load under mild hyperthermia212, which can be induced locally, e.g. with a laser213. Since the pH within the tumour microenvironment is typically acidic, this property was exploited to control the delivery of drugs (e.g. bleomycin) from pH-sensitive liposomes214. These liposomes contained 2-carboxycyclohexane-1- carboxylated polyglycidol modified with distearoyl phosphatidylethanolamine, a molecule that switches from a coiled to a globular conformation upon protonation of its carboxylates, leading to the release of the drug from the liposome. External signals can also serve as a cascade-triggering signal that results in drug release, for instance GUVs with connexon nanopores whose assembly was triggered by illumination215. In another example, gold nanorods were coupled to the GUV surface, inducing drug release under NIR irradiation as a result of the photothermal effect216.
Mannose-modified liposomes were created to specifically target APCs for antigen delivery217. GUVs were produced from different types of special lipids which can be functionalised with different proteins: biotinylated lipids can be coupled to biotinylated proteins via streptavidin, NTA-tagged lipids can interact with His-tagged proteins and DOPE lipids can react with hydroxysuccimide-functionalised molecules. Via these methods, Staufer et al.19 coupled anti-CD3 antibodies and RGD-ligands to GUVs, inducing their specific interaction with a specific population of cells.
Imaging
In addition to satisfy their intended biomedical application, the ability to assess the biodistribution of biomimetic carriers can be achieved by incorporating different types of contrast/signalling agents inside the GUVs. Magnetic nanoparticles, for example, were incorporated in biomimetic carriers to allow for their visualisation via MRI, which offered the additional benefit of enabling their magnetic manipulation51,203. Alternatively, fluorescently labelled particles can be incorporated for imaging purposes218.
Storage
Finally, another important consideration for the therapeutic applicability of GUV-based artificial cells, is their shelf life, which is highly dependent on their production and storage conditions. With the ability to fully engineer these systems (it is, for example, possible to add lyoprotectant during the GUV production) it might be possible to store GUVs for longer periods of time than actual RBCs. The latter can only be stored for 42 days, as defined by the FDA, since they undergo a cascade of biochemical changes that adversely affect their function and properties219,220.
One of the most attractive approaches for long-term storage of therapeutics is lyophilisation/freeze-drying, which, for LUVs, has been shown to be effective for up to six months221,222. The use of lyoprotectants during lyophilisation allows bacteria and yeast cells to regain their function upon rehydration. Mammalian cells, in general, do not survive this process, but some functions still remain (e.g. freeze-dried platelets retain some clotting properties)223,224. For RBCs, the recovery rate has been shown to be less than 25% for pre-transfusion alloantibody screening225. These results might be improved by addition of lyoprotectants inside the cells, but this is rather difficult and the loading process might damage the cells. With GUV-based artificial cells, though, this should be less of an issue since lyoprotectants can directly be co-encapsulated during GUV production223.
Another option is the storing the samples in a frozen state. In the presence of glycerol, RBCs can be stored frozen for up to 10 years226,227. However, due to the high costs involved in the storage and removal of the glycerol and a limited shelf life after thawing, this approach has found limited practical use227. Furthermore, storage of extracellular vesicles and LUVs by cryopreservation has been demonstrated228,229,230, which provides a route for exploring the long-term storage of GUV-based artificial cells, which has not been done to date.
Conclusion
In this review, the potential of GUV-based artificial cells for in vivo therapeutic use has been critically assessed. Over recent years, great progress has been made in the field of artificial cells, where GUVs featuring a wide variety of functionalities have been developed. However, only a limited number of in vivo applications with these systems has been demonstrated, and no studies have yet reported their viability to circulate in the bloodstream. Amidst numerous strategies that have been explored to enhance the circulation half-life of drug-delivery systems (which include a diversity of surface modifications and variations in vehicle morphology and mechanical properties65), biomimicry of RBCs represents a promising strategy that can help create a robust, durable and versatile vehicle, especially for GUVs. To this end, replication of the main features that provide RBCs with such properties via biomimicry offers the largest potential. These have been identified as size and shape, cytoskeleton, deformability, membrane composition, transmembrane proteins, osmotic balance and macromolecular crowding. Although some of these aspects can be reproduced to a satisfactory extent, the combination of several of them is still challenging and some of them (i.e. biconcavity) remain to be demonstrated. This is due to inherent limitations in the currently available techniques for GUV production, which can only accommodate the recreation of certain of these specific features separately. Therefore, for the future production of therapeutic artificial cells, it is necessary to continue to develop new GUV-manufacturing techniques enabling the creation of lipid-asymmetric vesicles that incorporate a wide set of transmembrane proteins assembled into nanodomains. At the same time these techniques would still need to allow the encapsulation of active compounds like haemoglobin (for artificial red blood cells) as well as the reconstruction of a cytoskeletal architecture providing mechanical stability and deformability, all in a molecularly crowded environment. Finally, these strategies should also be developed taking into account production scalability and cost.
Compared to RBC-based systems, the biggest advantages of GUV-based artificial cells as a delivery vehicle would be their engineerability and manufacturability: RBC-like GUVs built from the bottom up, could be manufactured with fully controlled compositions and functionalities. In contrast with liposomes, the GUVs’ size would enable the encapsulation of complex systems, such as large biological constructs and drug quantities.
A very basic, yet relevant example of a potential application of these GUV-based systems would be the ability to generate an artificial RBC mimic. In such system the presence of a biological membrane containing transmembrane proteins could provide more functions than the mere encapsulation of haemoglobin, such as the on-demand export of ATP and NO regulation, which are currently lacking in many RBC mimics. In more developed, complex systems, advanced functionalities could be integrated to transcend the normal function of an RBC. Examples of functions that could be integrated are: protein production (e.g. a drug), environmental sensing, stimuli-responsiveness and inclusion of lyoprotectants for improved cryopreservation. Potential applications are not limited to an advanced RBC mimic: artificial cell-like systems could be imagined that take over other types of functions like those of T-Cells for cancer treatment, for maintaining blood-sugar levels and advanced biosensors for monitoring drug levels.
Overall, the generation of in vivo-circulating, multifunctional GUV-based artificial cells has the potential to revolutionise the field of drug delivery, by providing a cell-like platform with cell-like properties, but more research is required to achieve these goals.
References
Yewdall, N. A., Mason, A. F. & van Hest, J. C. M. The hallmarks of living systems: towards creating artificial cells. Interface Focus 8, 20180023 (2018).
Spoelstra, W. K., Deshpande, S. & Dekker, C. Tailoring the appearance: what will synthetic cells look like? Curr. Opin. Biotechnol. 51, 47–56 (2018).
Dimova, R., Stano, P., Marques, C. M. & Walde, P. Preparation methods for giant unilamellar vesicles. In The Giant Vesicle Book (eds Dimova, R. & Marques, C. M.) Ch. 1, 3–20 (CRC Press, Boca Raton, FL, 2019).
Lasic, D. D. Giant Vesicles: A Historical Introduction. In Perspectives in Supramolecular Chemistry: Giant Vesicles, Vol. 6 (eds Pier Luigi Luisi & Peter Walde) Ch. 2, 11–24 (John Wiley & Sons, Baffins Lane, UK, 1999).
Litschel, T. et al. Reconstitution of contractile actomyosin rings in vesicles. Nat. Commun. 12, 2254 (2021).
Adepu, S. & Ramakrishna, S. Controlled drug delivery systems: current status and future directions. Molecules 26, 5905 (2021).
Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 14, 86–93 (2018).
Gregoriadis, G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 13, 527–537 (1995).
Akbarzadeh, A. et al. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 8, 102 (2013).
Feng, Z., Gu, Y., Yuan, M., Xiao, R. & Fei, Z. Clinical trials of liposomes in children’s anticancer therapy: a comprehensive analysis of trials registered on ClinicalTrials.gov. Int. J. Nanomed. 17, 1843–1850 (2022).
Vincy, A. et al. Recent progress in red blood cells-derived particles as novel bioinspired drug delivery systems: challenges and strategies for clinical translation. Front. Chem. 10, 905256 (2022).
Liu, A. P. et al. Membrane-induced bundling of actin filaments. Nat. Phys. 4, 789–793 (2008).
Heuvingh, J., Pincet, F. & Cribier, S. Hemifusion and fusion of giant vesicles induced by reduction of inter-membrane distance. Eur. Phys. J. E 14, 269–276 (2004).
Wick, R., Walde, P. & Luisi, P. L. Light microscopic investigations of the autocatalytic self-reproduction of giant vesicles. J. Am. Chem. Soc. 117, 1435–1436 (1995).
Lussier, F., Staufer, O., Platzman, I. & Spatz, J. P. Can bottom-up synthetic biology generate advanced drug-delivery systems? Trends Biotechnol. 39, 445–459 (2021).
Glassman, P. M. et al. Red blood cells: the metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv. Drug Deliv. Rev. 178, 113992 (2021).
Emir Diltemiz, S. et al. Use of artificial cells as drug carriers. Mater. Chem. Front. 5, 6672–6692 (2021).
Lee, Y. & Thompson, D. H. Stimuli-responsive liposomes for drug delivery. WIREs Nanomed. Nanobiotechnol. 9, e1450 (2017).
Staufer, O. et al. Microfluidic production and characterization of biofunctionalized giant unilamellar vesicles for targeted intracellular cargo delivery. Biomaterials 264, 120203 (2021).
Saito, A. C., Ogura, T., Fujiwara, K., Murata, S. & Nomura, S.-iM. Introducing micrometer-sized artificial objects into live cells: a method for cell-giant unilamellar vesicle electrofusion. PLoS ONE 9, e106853 (2014).
Jenkins, E. et al. Reconstitution of immune cell interactions in free-standing membranes. J. Cell Sci. 132, jcs219709 (2018).
Litschel, T. & Schwille, P. Protein reconstitution inside giant unilamellar vesicles. Annu. Rev. Biophys. 50, 525–548 (2021).
Walde, P., Cosentino, K., Engel, H. & Stano, P. Giant vesicles: preparations and applications. Chembiochem 11, 848–865 (2010).
Reeves, J. P. & Dowben, R. M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 73, 49–60 (1969).
Akashi, K., Miyata, H., Itoh, H. & Kinosita, K. J. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 71, 3242–3250 (1996).
Angelova, M. I. & Dimitrov, D. S. Liposome electroformation. Faraday Discuss. Chem. Soc. 81, 303–311 (1986).
Pautot, S., Frisken, B. J. & Weitz, D. A. Production of unilamellar vesicles using an inverted emulsion. Langmuir 19, 2870–2879 (2003).
Pautot, S., Frisken, B. J. & Weitz, D. A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA 100, 10718–10721 (2003).
Tsai, F.-C., Stuhrmann, B. & Koenderink, G. H. Encapsulation of active cytoskeletal protein networks in cell-sized liposomes. Langmuir 27, 10061–10071 (2011).
Blosser, M. C., Horst, B. G. & Keller, S. L. cDICE method produces giant lipid vesicles under physiological conditions of charged lipids and ionic solutions. Soft Matter 12, 7364–7371 (2016).
Venero, O. M., Sato, W., Heili, J. M., Deich, C. & Adamala, K. P. Liposome preparation by 3D-printed microcapillary-based apparatus. In Cell-Free Gene Expression, Vol. 2433 (eds Karim, A. & Jewett, M.) 227–235 (Humana, New York, NY, USA, 2022).
Petit, J., Polenz, I., Baret, J.-C., Herminghaus, S. & Bäumchen, O. Vesicles-on-a-chip: a universal microfluidic platform for the assembly of liposomes and polymersomes. Eur. Phys. J. E 39, 59 (2016).
Soga, H. et al. In vitro membrane protein synthesis inside cell-sized vesicles reveals the dependence of membrane protein integration on vesicle volume. ACS Synth. Biol. 3, 372–379 (2014).
Funakoshi, K., Suzuki, H. & Takeuchi, S. Formation of giant lipid vesiclelike compartments from a planar lipid membrane by a pulsed jet flow. J. Am. Chem. Soc. 129, 12608–12609 (2007).
Deshpande, S., Caspi, Y., Meijering, A. E. C. & Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 7, 10447 (2016).
Campillo, C. et al. Unexpected membrane dynamics unveiled by membrane nanotube extrusion. Biophys. J. 104, 1248–1256 (2013).
Moga, A., Yandrapalli, N., Dimova, R. & Robinson, T. Optimization of the inverted emulsion method for high-yield production of biomimetic giant unilamellar vesicles. ChemBioChem 20, 2674–2682 (2019).
Weiss, M. et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 17, 89–96 (2018).
Göpfrich, K. et al. One-pot assembly of complex giant unilamellar vesicle-based synthetic cells. ACS Synth. Biol. 8, 937–947 (2019).
Haller, B. et al. Charge-controlled microfluidic formation of lipid-based single- and multicompartment systems. Lab Chip 18, 2665–2674 (2018).
Waeterschoot, J. et al. Formation of giant unilamellar vesicles assisted by fluorinated nanoparticles. Adv. Sci. n/a, 2302461 (2023).
Hernandez Bücher, J. E. et al. Bottom-up assembly of target-specific cytotoxic synthetic cells. Biomaterials 285, 121522 (2022).
Toparlak, Ö. D. et al. Artificial cells drive neural differentiation. Sci. Adv. 6, eabb4920 (2020).
Staufer, O., Schröter, M., Platzman, I. & Spatz, J. P. Bottom-up assembly of functional intracellular synthetic organelles by droplet-based microfluidics. Small 16, 1906424 (2020).
Chen, G. et al. Implanted synthetic cells trigger tissue angiogenesis through de novo production of recombinant growth factors. Proc. Natl Acad. Sci. USA 119, e2207525119 (2022).
Krinsky, N. et al. Synthetic cells synthesize therapeutic proteins inside tumors. Adv. Healthc. Mater. 7, 1701163 (2018).
Glassman, P. M. et al. Vascular drug delivery using carrier red blood cells: focus on RBC surface loading and pharmacokinetics. Pharmaceutics 12, 440 (2020).
Hu, C.-M. J., Fang, R. H. & Zhang, L. Erythrocyte-inspired delivery systems. Adv. Healthc. Mater. 1, 537–547 (2012).
Wauters, A. C. et al. Artificial antigen-presenting cell topology dictates T cell activation. ACS Nano 16, 15072–15085 (2022).
Luo, Z. et al. Erythrocyte inspired functional materials for biomedical applications. Adv. Sci. 10, 2206150 (2023).
Guo, J. et al. Biomimetic rebuilding of multifunctional red blood cells: modular design using functional components. ACS Nano 14, 7847–7859 (2020).
Liu, S. et al. Enzyme-mediated nitric oxide production in vasoactive erythrocyte membrane-enclosed coacervate protocells. Nat. Chem. 12, 1165–1173 (2020).
Hou, K. et al. A multifunctional magnetic red blood cell-mimetic micromotor for drug delivery and image-guided therapy. ACS Appl. Mater. Interfaces 14, 3825–3837 (2022).
Skalak, R. & Brånemark, P. I. Deformation of red blood cells in capillaries. Science 164, 717–719 (1969).
Li, H., Liu, Z. L., Lu, L., Buffet, P. & Karniadakis, G. E. How the spleen reshapes and retains young and old red blood cells: a computational investigation. PLoS Comput. Biol. 17, e1009516 (2021).
Mauer, J. et al. Flow-induced transitions of red blood cell shapes under shear. Phys. Rev. Lett. 121, 118103 (2018).
Nigra, A. D., Casale, C. H. & Santander, V. S. Human erythrocytes: cytoskeleton and its origin. Cell. Mol. Life Sci. 77, 1681–1694 (2020).
Fischer, T. M. Shape memory of human red blood cells. Biophys. J. 86, 3304–3313 (2004).
Mohandas, N. & Gallagher, P. G. Red cell membrane: past, present, and future. Blood 112, 3939–3948 (2008).
Pivkin, I. V. et al. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl Acad. Sci. USA 113, 7804–7809 (2016).
Diez-Silva, M., Dao, M., Han, J., Lim, C.-T. & Suresh, S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. 35, 382–388 (2010).
Merkel, T. J. et al. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J. Control. Release 162, 37–44 (2012).
Merkel, T. J. et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl Acad. Sci. USA 108, 586–591 (2011).
Muro, S. et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 16, 1450–1458 (2008).
Wei, Y., Quan, L., Zhou, C. & Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 13, 1495–1512 (2018).
Zhu, X., Vo, C., Taylor, M. & Smith, B. R. Non-spherical micro- and nanoparticles in nanomedicine. Mater. Horiz. 6, 1094–1121 (2019).
Ju, X. et al. Red-blood-cell-shaped chitosan microparticles prepared by electrospraying. Particuology 30, 151–157 (2017).
Crouse, J. Z. et al. Development of a microscale red blood cell-shaped pectin-oligochitosan hydrogel system using an electrospray-vibration method: preparation and characterization. J. Appl. Biomater. Funct. Mater. 13, 326–331 (2015).
Hayashi, K., Hayashi, H., Yamada, S., Sakamoto, W. & Yogo, T. Cellulose-based molecularly imprinted red-blood-cell-like microparticles for the selective capture of cortisol. Carbohydr. Polym. 193, 173–178 (2018).
Marquis, M., Davy, J., Fang, A. & Renard, D. Microfluidics-assisted diffusion self-assembly: toward the control of the shape and size of pectin hydrogel microparticles. Biomacromolecules 15, 1568–1578 (2014).
Mazutis, L., Vasiliauskas, R. & Weitz, D. A. Microfluidic production of alginate hydrogel particles for antibody encapsulation and release. Macromol. Biosci. 15, 1641–1646 (2015).
Chen, K. et al. Low modulus biomimetic microgel particles with high loading of hemoglobin. Biomacromolecules 13, 2748–2759 (2012).
Yu, C. et al. Construction of biconcave hemoglobin-based microcapsules and electrochemical evaluation for its ability of oxygen carry. Sens. Actuators B Chem. 256, 217–225 (2018).
She, S. et al. Fabrication of biconcave discoidal silica capsules and their uptake behavior by smooth muscle cells. J. Colloid Interface Sci. 426, 124–130 (2014).
She, S., Li, Q., Shan, B., Tong, W. & Gao, C. Fabrication of red-blood-cell-like polyelectrolyte microcapsules and their deformation and recovery behavior through a microcapillary. Adv. Mater. 25, 5814–5818 (2013).
Doshi, N., Zahr, A. S., Bhaskar, S., Lahann, J. & Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl Acad. Sci. USA 106, 21495–21499 (2009).
Cao, S. et al. Shape-dependent biodistribution of biocompatible silk microcapsules. ACS Appl. Mater. Interfaces 11, 5499–5508 (2019).
George, J. E., Manna, R., Roy, S., Kumari, S. & Paul, D. Pump-free and high-throughput generation of monodisperse hydrogel beads by microfluidic step emulsification for dLAMP-on-a-chip. Preprint at bioRxiv https://doi.org/10.1101/2023.03.12.532292 (2023).
Yamashita, Y., Masum, S. M., Tanaka, T. & Yamazaki, M. Shape changes of giant unilamellar vesicles of phosphatidylcholine induced by a de novo designed peptide interacting with their membrane interface. Langmuir 18, 9638–9641 (2002).
Fanalista, F. et al. Shape and size control of artificial cells for bottom-up biology. ACS Nano 13, 5439–5450 (2019).
Neuhaus, F. et al. Vesicle origami: cuboid phospholipid vesicles formed by template-free self-assembly. Angew. Chem. Int. Ed. 56, 6515–6518 (2017).
Bhattacharya, A. et al. Single-chain β-D-glycopyranosylamides of unsaturated fatty acids: self-assembly properties and applications to artificial cell development. J. Phys. Chem. B 123, 3711–3720 (2019).
Garenne, D., Libchaber, A. & Noireaux, V. Membrane molecular crowding enhances MreB polymerization to shape synthetic cells from spheres to rods. Proc. Natl Acad. Sci. USA 117, 1902–1909 (2020).
Pan, L., Yan, R., Li, W. & Xu, K. Super-resolution microscopy reveals the native ultrastructure of the erythrocyte cytoskeleton. Cell Rep. 22, 1151–1158 (2018).
Teliska, L. H. & Rasband, M. N. Spectrins. Curr. Biol. 31, R504–R506 (2021).
Abudara, V., Retamal, M. A., Del Rio, R. & Orellana, J. A. Synaptic functions of hemichannels and pannexons: a double-edged sword. Front. Mol. Neurosci. 11, 435 (2018).
Ulker, P., Gunduz, F., Meiselman, H. J. & Baskurt, O. K. Nitric oxide generated by red blood cells following exposure to shear stress dilates isolated small mesenteric arteries under hypoxic conditions. Clin. Hemorheol. Microcirc. 54, 357–369 (2013).
Zong, W., Li, Q., Zhang, X. & Han, X. Deformation of giant unilamellar vesicles under osmotic stress. Colloids Surf B Biointerfaces 172, 459–463 (2018).
Allen, M. E., Hindley, J. W., Baxani, D. K., Ces, O. & Elani, Y. Hydrogels as functional components in artificial cell systems. Nat. Revi. Chem. 6, 562–578 (2022).
Jesorka, A., Markström, M., Karlsson, M. & Orwar, O. Controlled hydrogel formation in the internal compartment of giant unilamellar vesicles. J. Phys. Chem. B 109, 14759–14763 (2005).
Li, D.-Y., Zhou, Z.-H., Yu, Y.-L. & Deng, N.-N. Microfluidic construction of cytoskeleton-like hydrogel matrix for stabilizing artificial cells. Chem. Eng. Sci. 264, 118186 (2022).
Dey, S. et al. DNA origami. Nat. Rev. Methods Primers 1, 13 (2021).
Jahnke, K., Huth, V., Mersdorf, U., Liu, N. & Göpfrich, K. Bottom-up assembly of synthetic cells with a DNA cytoskeleton. ACS Nano 16, 7233–7241 (2022).
Walther, T., Jahnke, K., Abele, T. & Göpfrich, K. Printing and erasing of DNA based photoresists inside synthetic cells. Adv. Funct. Mater. 32, 2200762 (2022).
Kurokawa, C. et al. DNA cytoskeleton for stabilizing artificial cells. Proc. Natl Acad. Sci. USA 114, 7228–7233 (2017).
Arulkumaran, N., Singer, M., Howorka, S. & Burns, J. R. Creating complex protocells and prototissues using simple DNA building blocks. Nat. Commun. 14, 1314 (2023).
Häckl, W., Bärmann, M. & Sackmann, E. Shape changes of self-assembled actin bilayer composite membranes. Phys. Rev. Lett. 80, 1786–1789 (1998).
Limozin, L., Roth, A. & Sackmann, E. Microviscoelastic moduli of biomimetic cell envelopes. Phys. Rev. Lett. 95, 178101 (2005).
Perrier, D. L. et al. Response of an actin network in vesicles under electric pulses. Sci. Rep. 9, 8151 (2019).
Chen, S., Sun, Z. G. & Murrell, M. P. In vitro reconstitution of the Actin Cytoskeleton Inside Giant Unilamellar Vesicles. JOVE (186), 64026 (2022).
Baldauf, L. et al. Biomimetic actin cortices shape cell-sized lipid vesicles. Preprint at bioRxiv https://doi.org/10.1101/2023.01.15.524117 (2023).
Baldauf, L., Frey, F., Perez, M. A., Idema, T. & Koenderink, G. H. Branched actin cortices reconstituted in vesicles sense membrane curvature. Biophys. J. 122, 2311–2324 (2023).
Guevorkian, K., Manzi, J., Pontani, L.-L., Brochard-Wyart, F. & Sykes, C. Mechanics of biomimetic liposomes encapsulating an actin shell. Biophys. J. 109, 2471–2479 (2015).
Wubshet, N. H., Wu, B., Veerapaneni, S. & Liu, A. P. Differential regulation of GUV mechanics via actin network architectures. Biophys. J. 122, 1–14 (2022).
Merkle, D., Kahya, N. & Schwille, P. Reconstitution and anchoring of cytoskeleton inside giant unilamellar vesicles. ChemBioChem 9, 2673–2681 (2008).
Jørgensen, I. L., Kemmer, G. C. & Pomorski, T. G. Membrane protein reconstitution into giant unilamellar vesicles: a review on current techniques. Eur. Biophys. J. 46, 103–119 (2016).
Nishigami, Y., Ito, H., Sonobe, S. & Ichikawa, M. Non-periodic oscillatory deformation of an actomyosin microdroplet encapsulated within a lipid interface. Sci. Rep. 6, 18964 (2016).
Virtanen, J. A., Cheng, K. H. & Somerharju, P. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc. Natl Acad. Sci. USA 95, 4964–4969 (1998).
Chakrabarti, R. S. et al. Variability of cholesterol accessibility in human red blood cells measured using a bacterial cholesterol-binding toxin. eLife 6, e23355 (2017).
Van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids : where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Dawaliby, R. et al. Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. J. Biol. Chem. 291, 3658–3667 (2016).
Zwaal, R. F. A., Comfurius, P. & Bevers, E. M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 62, 971–988 (2005).
Savill, J., Dransfield, I., Gregory, C. & Haslett, C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975 (2002).
Bevers, E. M., Comfurius, P. & Zwaal, R. F. A. Changes in membrane phospholipid distribution during platelet activation. Biochim. Biophys. Acta Biomembr. 736, 57–66 (1983).
Engeland, M. V., Ramaekers, F. C. S., Schutte, B. & Reutehgsperger, C. P. M. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. J. Int. Soc. Anal. Cytol. 139, 131–139 (1996).
Fadeel, B. & Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44, 264–277 (2010).
Vermes, I., Haanen, C. & Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 243, 167–190 (2000).
Tse, W. T. & Lux, S. E. Red blood cell membrane disorders. Br. Jo. Haematol. 104, 2–13 (1999).
Berezina, T. L. et al. Influence of storage on red blood cell rheological properties. J. Surg. Res. 102, 6–12 (2002).
Fadok, V. A., De Cathelineau, A., Daleke, D. L., Henson, P. M. & Bratton, D. L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276, 1071–1077 (2001).
Martínez-Vieyra, I. et al. Alterations to plasma membrane lipid contents affect the biophysical properties of erythrocytes from individuals with hypertension. Biochim. Biophys. Acta Biomembr. 1861, 182996 (2019).
Gurtovenko, A. A. & Vattulainen, I. Lipid transmembrane asymmetry and intrinsic membrane potential: two sides of the same coin. J. Am. Chem. Soc. 129, 5358–5359 (2007).
Zwaal, R. F. A. & Schroit, A. J. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89, 1121–1132 (1997).
Daleke, D. L. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr. Opin. Hematol. 15, 191–195 (2008).
Witasp, E., Kagan, V. & Fadeel, B. Programmed cell clearance: molecular mechanisms and role in autoimmune disease, chronic inflammation, and anti-cancer immune responses. Curr. Immunol. Rev. 4, 53–69 (2008).
Chen, W. et al. Comparison of erythrocyte membrane lipid profiles between NAFLD patients with or without hyperlipidemia. Int. J. Endocrinol. 2020, 9501826 (2020).
Cournia, Z. et al. Membrane protein structure, function, and dynamics: a perspective from experiments and theory. J. Membr. Biol. 248, 611–640 (2015).
Lew, V. L. & Tiffert, T. On the mechanism of human red blood cell longevity: roles of calcium, the sodium pump, PIEZO1, and gardos channels. Front. Physiol. 8, 977 (2017).
Brittain, J. E., Mlinar, K. J., Anderson, C. S., Orringer, E. P. & Parise, L. V. Activation of sickle red blood cell adhesion via integrin-associated protein/CD47-induced signal transduction. J. Clin. Invest. 107, 1555–1562 (2001).
Burger, P., Hilarius-Stokman, P., De Korte, D., Van Den Berg, T. K. & Van Bruggen, R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119, 5512–5521 (2012).
Ninomiya, H. & Sims, P. J. The human complement regulatory protein CD59 binds to the α-chain of C8 and to the ’b’ domain of C9. J. Biol. Chem. 267, 13675–13680 (1992).
Chasis, J. A. & Mohandas, N. Red blood cell glycophorins. Blood 80, 1869–1879 (1992).
Ho, J. C., Rangamani, P., Liedberg, B. & Parikh, A. N. Mixing water, transducing energy, and shaping membranes: autonomously self-regulating giant vesicles. Langmuir 32, 2151–2163 (2016).
Glogowska, E. & Gallagher, P. G. Disorders of erythrocyte volume homeostasis. Int. J. Lab. Hematol. 37, 85–91 (2015).
Mathai, J. C. et al. Functional analysis of aquaporin-1 deficient red cells. J. Biol. Chem. 271, 1309–1313 (1996).
Kaplan, J. H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 (2002).
Quemeneur, F., Rammal, A., Rinaudo, M. & Pépin-Donat, B. Large and giant vesicles “decorated" with chitosan: effects of pH, salt or glucose stress, and surface adhesion. Biomacromolecules 8, 2512–2519 (2007).
Li, Q., Li, S., Zhang, X., Xu, W. & Han, X. Programmed magnetic manipulation of vesicles into spatially coded prototissue architectures arrays. Nat. Commun. 11, 232 (2020).
Bhatia, T. et al. Spatial distribution and activity of Na + /K + -ATPase in lipid bilayer membranes with phase boundaries. Biochim. Biophys. Acta Biomembr. 1858, 1390–1399 (2016).
Cahalan, S. M. et al. Piezo1 links mechanical forces to red blood cell volume. eLife 4, e07370 (2015).
Verbalis, J. G. Disorders of body water homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 17, 471–503 (2003).
Knop, J.-M. et al. Life in multi-extreme environments: brines, osmotic and hydrostatic pressure - a physicochemical view. Chem. Rev. 123, 73–104 (2023).
Liu, X., Stenhammar, J., Wennerström, H. & Sparr, E. Vesicles balance osmotic stress with bending energy that can be released to form daughter vesicles. J. Phys. Chem. Lett. 13, 498–507 (2022).
Chapanian, R. et al. Enhancement of biological reactions on cell surfaces via macromolecular crowding. Nat. Commun. 5, 4683 (2014).
Shou, K. et al. Effect of red blood cell shape changes on haemoglobin interactions and dynamics: a neutron scattering study. R. Soc. Open Sci. 7, 201507 (2020).
Ding, Y., Contreras-Llano, L. E., Morris, E., Mao, M. & Tan, C. Minimizing context dependency of gene networks using artificial cells. ACS Appl. Mater. Interfaces 10, 30137–30146 (2018).
Gore, M., Narvekar, A., Bhagwat, A., Jain, R. & Dandekar, P. Macromolecular cryoprotectants for the preservation of mammalian cell culture: Lessons from crowding, overview and perspectives. J. Mater. Chem. B 10, 143–169 (2022).
Deng, N.-N. et al. Macromolecularly crowded protocells from reversibly shrinking monodisperse liposomes. J. Am. Chem. Soc. 140, 7399–7402 (2018).
Vibhute, M. A. et al. Transcription and translation in cytomimetic protocells perform most efficiently at distinct macromolecular crowding conditions. ACS Synth. Biol. 9, 2797–2807 (2020).
Guerzoni, L. P. et al. High macromolecular crowding in liposomes from microfluidics. Adv. Sci. 9, 2201169 (2022).
Tivony, R., Fletcher, M., Al Nahas, K. & Keyser, U. F. A microfluidic platform for sequential assembly and separation of synthetic cell models. ACS Synth. Biol. 10, 3105–3116 (2021).
Fujiwara, K. & Yanagisawa, M. Generation of giant unilamellar liposomes containing biomacromolecules at physiological intracellular concentrations using hypertonic conditions. ACS Synth. Biol. 3, 870–874 (2014).
Zarrin, A., Foroozesh, M. & Hamidi, M. Carrier erythrocytes: recent advances, present status, current trends and future horizons. Expert Opin. Drug Deliv. 11, 433–447 (2014).
Kravtzoff, R., Ropars, C., Laguerre, M., Muh, J. P. & Chassaigne, M. Erythrocytes as carriers for L-asparaginase. methodological and mouse in-vivo studies. J. Pharm. Pharmacol. 42, 473–476 (1990).
Chessa, L. Current and future therapeutic strategies to treat ataxia telangiectasia. Expert Opin. Orphan Drugs 2, 877–887 (2014).
Leuzzi, V. et al. Positive effect of erythrocyte-delivered dexamethasone in ataxia-telangiectasia. Neurol. Neuroimmunol. Neuroinflamm. 2, e98 (2015).
Hunault-Berger, M. et al. A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study. Am. J. Hematol. 90, 811–818 (2015).
Bachet, J.-B. et al. Asparagine synthetase expression and phase I study with l-asparaginase encapsulated in red blood cells in patients with pancreatic adenocarcinoma. Pancreas 44, 1141–1147 (2015).
A study of safety, tolerability, pharmacodynamics, and pharmacokinetics of KAN-101 in people with celiac disease https://www.clinicaltrials.gov/ct2/show/NCT05574010 (2022).
Sahoo, K. et al. Nanoparticle attachment to erythrocyte via the glycophorin A targeted ERY1 ligand enhances binding without impacting cellular function. Pharm. Res. 33, 1191–1203 (2016).
Wang, Y. et al. Red blood cell-hitchhiking chitosan nanoparticles for prolonged blood circulation time of vitamin K1. Int. J. Pharm. 592, 120084 (2021).
Zheng, J. et al. Red blood cell-hitchhiking mediated pulmonary delivery of ivermectin: effects of nanoparticle properties. Int. J. Pharm. 619, 121719 (2022).
Zhao, Z. et al. Screening of Zwitterionic liposomes with red blood cell hitchhiking and tumor cell active transporting capability for efficient tumor entrance. Adv. Funct. Mater. 33, 2214369 (2023).
Guo, J. et al. Modular assembly of red blood cell superstructures from metal-organic framework nanoparticle-based building blocks. Adv. Funct. Mater. 31, 2005935 (2021).
Ferenz, K., Karaman, O. & Shah, S. B. Artificial red blood cells. In Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood (eds Denizli, A., Nguyen, T. A., Rajan, M., Alam, M. F. & Rahman Blood Transfusion, and Artificial Blood, K. B. T. N. f. H.) 397–427 (Elsevier, 2022).
Douay, L. Why industrial production of red blood cells from stem cells is essential for tomorrow’s blood transfusion. Regener. Med. 13, 627–632 (2018).
Zhang, X. et al. Engineered red blood cells as an off-the-shelf allogeneic anti-tumor therapeutic. Nat. Commun. 12, 2637 (2021).
Deák, R. et al. Physicochemical characterization of artificial nanoerythrosomes derived from erythrocyte ghost membranes. Colloids Surf. B Biointerfaces 135, 225–234 (2015).
Le, Q.-V., Lee, J., Lee, H., Shim, G. & Oh, Y.-K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B 11, 2096–2113 (2021).
Ma, W. et al. Biomimetic nanoerythrosome coated aptamer-DNA tetrahedron/maytansine conjugates: pH responsive and targeted cytotoxicity for HER2 positive breast cancer. Adv. Mater. 34, 2109609 (2022).
Han, X. et al. Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 5, 6870–6893 (2019).
Kuo, Y.-C. et al. Colloidal properties of nanoerythrosomes derived from bovine red blood cells. Langmuir 32, 171–179 (2016).
Mehta, S., Dumoga, S., Malhotra, S. & Singh, N. Comparative analysis of PEG-liposomes and RBCs-derived nanovesicles for anti-tumor therapy. Colloids Surf. B Biointerfaces 218, 112785 (2022).
Bóta, A. et al. Lipid nanoparticles with erythrocyte cell-membrane proteins. J. Mol. Liquids 369, 120791 (2023).
Agnihotri, J. & Jain, N. K. Biodegradable long circulating cellular carrier for antimalarial drug pyrimethamine. Artif. Cells Nanomed. Biotechnol. 41, 309–314 (2013).
Gupta, N., Patel, B., Nahar, K. & Ahsan, F. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur. J. Pharm. Biopharm. 88, 1046–1055 (2014).
Wang, P. et al. Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf. B Biointerfaces 189, 110842 (2020).
Li, M. et al. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater. Sci. 8, 1802–1814 (2020).
Lee, J. Y. et al. Red blood cell membrane bioengineered Zr-89 labelled hollow mesoporous silica nanosphere for overcoming phagocytosis. Sci. Rep. 9, 7419 (2019).
Malhotra, S., Dumoga, S. & Singh, N. Red blood cells membrane derived nanoparticles: applications and key challenges in their clinical translation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14, 1–26 (2022).
Kazakov, S. Lipobeads. In Liposomes (ed. Catala, A.) Ch. 3, 49–93 (InTech, London, UK, 2017).
Ulker, D., Ozyurt, R., Erkasap, N. & Butun, V. Magnetic targeting of 5-fluorouracil-loaded liposome-nanogels for in vivo breast cancer therapy and the cytotoxic effects on liver and kidney. AAPS PharmSciTech 23, 289 (2022).
Olden, B. R. et al. Cell templated silica microparticles with supported lipid bilayers as artificial antigen presenting cells for T cell activation. Adv. Healthc. Mater. 8, 1801188 (2019).
Ashley, C. E. et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 10, 389–397 (2011).
Huo, C., Li, M., Huang, X., Yang, H. & Mann, S. Membrane engineering of colloidosome microcompartments using partially hydrophobic mesoporous silica nanoparticles. Langmuir 30, 15047–15052 (2014).
Antonietti, M. & Förster, S. Vesicles and liposomes: a self assembly principle beyond lipids. Adv. Mater. 15, 1323–1333 (2003).
Jiang, W. et al. Artificial cells: past, present and future. ACS Nano 16, 15705–15733 (2022).
Jayaraman, P. et al. Cell-free optogenetic gene expression system. ACS Synth. Biol. 7, 986–994 (2018).
Wei, M., Lin, Y. & Qiao, Y. Engineered colloidosomes as biomimetic cellular models. Giant 13, 100143 (2023).
Chang, T. M. S. Therapeutic applications of polymeric artificial cells. Nat. Rev. Drug Discov. 4, 221–235 (2005).
Lu, Y., Allegri, G. & Huskens, J. Vesicle-based artificial cells: materials, construction methods and applications. Mater. Horiz. 9, 892–907 (2022).
Dao, T. P. T. et al. Phase separation and nanodomain formation in hybrid polymer/lipid vesicles. ACS Macro Lett. 4, 182–186 (2015).
Mittal, N. et al. Erythromer (EM), a nanoscale bio-synthetic artificial red cell. In Blood Substitutes and Oxygen Biotherapeutics (eds Liu, H., Kaye, A. D. & Jahr, J. S.) Ch. 24, 253–265 (Springer, Cham, 2022).
Pan, D. et al. Erythromer (EM), a nanoscale bio-synthetic artificial red cell: proof of concept and in vivo efficacy results. Blood 128, 1027 (2016).
Nguyen, H. T., Lee, S. & Shin, K. Controlled metabolic cascades for protein synthesis in an artificial cell. Biochem. Soc. Trans. 49, 2143–2151 (2021).
Komiya, M. et al. Advances in artificial cell membrane systems as a platform for reconstituting ion channels. Chem. Rec. 20, 730–742 (2020).
Chakraborty, T. & Wegner, S. V. Cell to cell signaling through light in artificial cell communities: glowing predator lures prey. ACS Nano 15, 9434–9444 (2021).
Hindley, J. W., Law, R. V. & Ces, O. Membrane functionalization in artificial cell engineering. SN Appl. Sci. 2, 593 (2020).
Langecker, M. et al. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 338, 932–936 (2012).
Langton, M. J., Keymeulen, F., Ciaccia, M., Williams, N. H. & Hunter, C. A. Controlled membrane translocation provides a mechanism for signal transduction and amplification. Nat. Chem. 9, 426–430 (2017).
Langton, M. J., Scriven, L. M., Williams, N. H. & Hunter, C. A. Triggered release from lipid bilayer vesicles by an artificial transmembrane signal transduction system. J. Am. Chem. Soc. 139, 15768–15773 (2017).
Søgaard, A. B. et al. Transmembrane signaling by a synthetic receptor in artificial cells. Nat. Commun. 14, 1646 (2023).
Mikhaylov, G. et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 6, 594–602 (2011).
Yavlovich, A. et al. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. J. Therm. Anal. Calorim. 98, 97–104 (2009).
Nishimura, K. et al. Cell-free protein synthesis inside giant unilamellar vesicles analyzed by flow cytometry. Langmuir 28, 8426–8432 (2012).
Noireaux, V. & Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. USA 101, 17669–17674 (2004).
Boyd, M. A. & Kamat, N. P. Designing artificial cells towards a new generation of biosensors. Trends Biotechnol. 39, 927–939 (2021).
Dwidar, M. et al. Programmable artificial cells using histamine-responsive synthetic riboswitch. J. Am. Chem. Soc. 141, 11103–11114 (2019).
Majumder, S. et al. Cell-sized mechanosensitive and biosensing compartment programmed with DNA. Chem. Commun. 53, 7349–7352 (2017).
Lentini, R. et al. Two-way chemical communication between artificial and natural cells. ACS Cent. Sci. 3, 117–123 (2017).
Erguven, H., Wang, L., Gutierrez, B. & Izgu, E. C. Biomimetic Vesicles with Designer Phospholipids Can Sense Environmental Redox Cues. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2023-p0nwc (2023).
Yatvin, M. B., Weinstein, J. N., Dennis, W. H. & Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202, 1290–1293 (1978).
Mackanos, M. A. et al. Laser-induced disruption of systemically administered liposomes for targeted drug delivery. J. Biomed. Opt. 14, 44009 (2009).
Yuba, E. et al. Bleomycin-loaded pH-sensitive polymer-lipid-incorporated liposomes for cancer chemotherapy. Polymers 10, 74 (2018).
Sihorwala, A. Z., Lin, A. J., Stachowiak, J. C. & Belardi, B. Light-activated assembly of connexon nanopores in synthetic cells. J. Am. Chem. Soc. 145, 3561–3568 (2023).
Yuan, Z., Das, S., Lazenby, R. A., White, R. J. & Park, Y. C. Repetitive drug releases from light-activatable micron-sized liposomes. Colloids Surf. A Physicochem. Eng. Asp. 625, 126778 (2021).
Wang, C. et al. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine 32, 5475–5483 (2014).
Belwal, V. K. & Singh, K. P. Nanosilica-supported liposome (protocells) as a drug vehicle for cancer therapy. Int. J. Nanomed. 13, 125–127 (2018).
Raphael, J. L. The role of policy in red blood cell storage and transfusion in children. Pediatr. Res. 82, 894–896 (2017).
Sparrow, R. L. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus. 8, s26–30 (2010).
Cliff, R. O. et al. Liposome encapsulated hemoglobin: long-term storage stability and in vivo characterization. Biomater. Artif. Cells Immob. Biotechnol. 20, 619–626 (1992).
Stark, B., Pabst, G. & Prassl, R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: effects of cryoprotectants on structure. Eur. J. Pharm. Sci. 41, 546–555 (2010).
Zhang, M. et al. Freeze-drying of mammalian cells using trehalose: preservation of DNA integrity. Sci. Rep. 7, 6198 (2017).
Sum, R. et al. Wound-healing properties of trehalose-stabilized freeze-dried outdated platelets. Transfusion 47, 672–679 (2007).
Alves, D., Sparrow, R. & Garnier, G. Rapidly freeze-dried human red blood cells for pre-transfusion alloantibody testing reagents. J. Biomed. Mater. Res. Part B Appl. Biomater. 109, 1689–1697 (2021).
Chaudhari, C. Frozen red blood cells in transfusion. Med. J. Armed Forces India 65, 55–58 (2009).
Henkelman, S., Noorman, F., Badloe, J. F. & Lagerberg, J. W. M. Utilization and quality of cryopreserved red blood cells in transfusion medicine. Vox Sang. 108, 103–112 (2015).
Jeyaram, A. & Jay, S. M. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 20, 1 (2018).
Susa, F., Bucca, G., Limongi, T., Cauda, V. & Pisano, R. Enhancing the preservation of liposomes: The role of cryoprotectants, lipid formulations and freezing approaches. Cryobiology 98, 46–56 (2021).
Bosch, S. et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 6, 36162 (2016).
Zhang, W. et al. Biomimetic erythrocytes engineered drug delivery for cancer therapy. Chem. Eng. J. 433, 133498 (2022).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: formulation of the concept and structure: J.W.; figure generation: J.W. and S.L.; writing and editing of the manuscript: J.W., S.L., W.G. and X.C.; J.W., S.L., W.G. and X.C. reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waeterschoot, J., Gosselé, W., Lemež, Š. et al. Artificial cells for in vivo biomedical applications through red blood cell biomimicry. Nat Commun 15, 2504 (2024). https://doi.org/10.1038/s41467-024-46732-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46732-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.