Abstract
The connection between triglyceride-rich lipoproteins and cardiometabolic multimorbidity, characterized by the concurrence of at least two of type 2 diabetes, ischemic heart disease, and stroke, has not been definitively established. We aim to examine the prospective associations between serum remnant cholesterol, triglycerides, and the risks of progression from first cardiometabolic disease to multimorbidity via multistate modeling in the UK Biobank. We also evaluate the causality of these associations via Mendelian randomization using 13 biologically relevant SNPs as the genetic instruments. Here we show that elevated remnant cholesterol and triglycerides are significantly associated with gradually higher risks of cardiometabolic multimorbidity, particularly the progression of ischemic heart disease to the multimorbidity of ischemic heart disease and type 2 diabetes. These results advocate for effective management of remnant cholesterol and triglycerides as a potential strategy in mitigating the risks of cardiometabolic multimorbidity.
Similar content being viewed by others
Introduction
Blood lipids include low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, and lipoprotein(a). Despite consistent and robust evidence underscoring the deleterious effects of LDL-C on the risk of atherosclerotic cardiovascular disease, the independent relevance of triglyceride-rich lipoproteins remains uncertain. Previous studies elucidating the adverse effects of triglycerides on cardiovascular disease were hampered by the substantial inverse associations between blood levels of triglycerides and HDL-C1. Thus, hypertriglyceridemia has traditionally been considered as a biomarker or bystander for low blood HDL-C rather than an intrinsic risk factor1. Recent advances in human genetics and unanticipated outcomes from clinical trials investigating the efficacy of HDL-C-raising medication have reshaped the importance of triglycerides and HDL-C2,3,4. Elevated triglycerides were causally associated with a higher incidence of heart disease and aortic stenosis in Danish adults5,6,7. Mendelian randomization analyzes also provided genetic support indicating that elevated plasma triglycerides were superior therapeutic targets to HDL-C, despite the absence of any licensed effective triglyceride-lowering treatment beyond fibrates for heart disease prevention8,9.
Cardiometabolic diseases, including type 2 diabetes, ischemic heart disease, and stroke, remain the primary causes of premature death worldwide10. Moreover, cardiometabolic multimorbidity, characterized by the concurrence of at least two cardiometabolic diseases, has an adverse effect on the quality of life and two-fold higher risks of premature death than either of these diseases alone11. Given their role in the onset of different cardiometabolic diseases and their progression to multimorbidity, higher levels of triglyceride-rich lipoproteins have been suggested as a risk factor for multimorbidity12,13,14. Inefficient lipoprotein lipase (LPL)-mediated lipolysis and hepatic overproduction of very low-density lipoprotein (VLDL) result in accumulation of triglyceride-rich lipoprotein remnants in blood. Importantly, the remnants of triglyceride-rich lipoprotein contain about four-fold higher cholesterol per particle than LDL-C and are small enough to penetrate the endothelial barrier, which was believed to be more atherogenic than LDL-C15,16. Unlike LDL particles, cholesterol in triglyceride-rich lipoprotein remnants could be directly taken up by macrophages, facilitating the formation of foam cells and, subsequently, atherosclerotic plaque. In addition, remnant cholesterol but not LDL-C, was causally linked to low-grade inflammation17. Recent studies have reported the causal relevance of triglyceride-rich lipoproteins and their remnants for the risk of cardiovascular disease6,16,18. However, the importance of triglyceride-rich lipoproteins, namely remnant cholesterol and triglycerides, for progression from first cardiometabolic disease to multimorbidity is not fully understood. It is noteworthy that diet, like fat and cholesterol intake, and lifestyle factors might substantially influence triglyceride-rich lipoprotein metabolism19,20,21. The complex interplay between lipid species might also confound the observational relationships between triglyceride-rich lipoproteins and cardiometabolic diseases22.
In this study, we utilized multistate modeling and two-stage least squares regression-based Mendelian randomization to investigate the associations between remnant cholesterol, triglycerides and the risks of cardiometabolic multimorbidity in the UK Biobank. We found that blood remnant cholesterol and triglycerides were causally associated with higher risks of progression from first cardiometabolic disease to multimorbidity, particularly the multimorbidity of ischemic heart disease and type 2 diabetes.
Results
Baseline characteristics of study participants
Table 1 shows the baseline characteristics of participants according to serum concentrations of remnant cholesterol and triglycerides. Participants with higher serum levels of remnant cholesterol and triglycerides tended to be men and older, have higher body mass index and systolic blood pressure, have more vulnerable socioeconomic status, be current or former smokers, have a more extended smoking history, favor access alcohol intake, be physically inactive, and have less healthy diet and sleep quality (Table 1). In contrast, these covariates were more evenly distributed when stratified by the genetic risk score (GRS) quintiles (Supplementary Data 1). The average serum concentrations of remnant cholesterol and triglycerides in the entire study population were 0.70 mmol/L (median: 0.67 mmol/L; interquartile range: 0.50, 0.86 mmol/L) and 1.70 mmol/L (median: 1.44 mmol/L; interquartile range: 1.02, 2.08 mmol/L), respectively. Serum concentrations of remnant cholesterol and triglycerides were similar among participants with different fasting time (Supplementary Data 2).
Observational analyzes
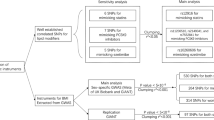
During a median follow-up of 12.5 years (4,694,404 total person-years), we identified 39,084 newly onset first cardiometabolic disease cases among 391,583 participants that were free of any cardiometabolic diseases at baseline (Supplementary Fig. 1a). Among people that experienced any cardiometabolic diseases, 3794 subsequently progressed to cardiometabolic multimorbidity. Specifically, 2370 participants further developed the co-morbidity of ischemic heart disease and type 2 diabetes, also termed IHD-T2D multimorbidity (Supplementary Fig. 1b).
We first pooled all three cardiometabolic disease together and found that elevated serum triglycerides were associated with higher risks of incident cardiometabolic disease and progression of first cardiometabolic disease to multimorbidity (Supplementary Fig. 1a). Compared with participants with serum triglycerides <0.9 mmol/L, the multivariate-adjusted hazard ratios (HRs) for progression from first cardiometabolic disease to cardiometabolic multimorbidity were 1.28 (95% CI: 1.09, 1.51), 1.29 (1.10, 1.52), 1.36 (1.16, 1.59), and 1.50 (1.29, 1.75) for those with serum triglycerides 0.9−<1.3 mmol/L, 1.3−<1.7 mmol/L, 1.7−<2.3 mmol/L, and ≥ 2.3 mmol/L, respectively (Table 2). However, when separating the cardiometabolic diseases into three distinct endpoints, it was observed that serum remnant cholesterol and triglycerides were exclusively associated with elevated risks of ischemic heart disease and type 2 diabetes, but not stroke (Supplementary Data 3).
We next tested whether serum remnant cholesterol and triglycerides involved in the trajectories from disease-free status to ischemic heart disease/type 2 diabetes and to subsequent IHD-T2D multimorbidity (Supplementary Fig. 1b). Participants with serum remnant cholesterol of ≥1.0 mmol/L had 146% (HR: 2.46; 95% CI: 2.23, 2.71; P for trend <0.001) and 63% (HR: 1.63; 95% CI: 1.52, 1.74; P for trend <0.001) higher risks of type 2 diabetes and ischemic heart disease, respectively, than those with serum remnant cholesterol of <0.4 mmol/L (Table 3). Similarly, participants with serum triglycerides of ≥ 2.3 mmol/L had 254% (HR: 3.54; 95% CI: 3.24, 3.87; P for trend <0.001) and 51% (HR: 1.51; 95% CI: 1.43, 1.59; P for trend <0.001) higher risks of type 2 diabetes and ischemic heart disease, respectively, than those with serum triglycerides of <0.4 mmol/L (Table 4). Besides, serum remnant cholesterol and triglycerides were also associated with higher risks of progression from ischemic heart disease to IHD-T2D multimorbidity. The HRs for the progression from ischemic heart disease to IHD-T2D multimorbidity were 2.14 (95% CI: 1.39, 3.31; P for trend <0.001) and 3.39 (95% CI: 2.25, 5.11; P for trend <0.001) for participants with serum remnant cholesterol of ≥1.0 mmol/L and triglycerides of ≥2.3 mmol/L, respectively (Tables 3 and 4). However, serum remnant cholesterol and triglycerides were not associated with the risks of progression from type 2 diabetes to IHD-T2D multimorbidity (Tables 3 and 4). Subgroup analyzes revealed that the associations between serum remnant cholesterol, triglycerides, and IHD-T2D multimorbidity did not differ significantly when stratified by lifestyle factors including diet, physical activity, and sleep pattern (Supplementary Data 4).
We next tested whether incorporating serum remnant cholesterol and triglycerides into the basal multivariate-adjusted regression models might increase the discrimination power for predicting cardiometabolic multimorbidity. Adding remnant cholesterol and triglycerides into the basal model substantially improves the discrimination performance with the C index increasing from 0.683 (95% CI: 0.655, 0.711) to 0.695 (95% CI: 0.668, 0.722; P for difference = 0.01) and from 0.676 (95% CI: 0.650, 0.702) to 0.700 (95% CI: 0.675, 0.725; P for difference = 2.45 × 10−6), respectively, when considering the progression from ischemic heart disease to IHD-T2D multimorbidity (Supplementary Data 5). However, incorporating remnant cholesterol and triglycerides into the basal model did not markedly influence the discrimination performance when pooling the three cardiometabolic disease together (Supplementary Data 6).
Mendelian randomization analysis
In this study, we derived a weighted genetic risks score comprising of 13 biologically relevant single nucleotide polymorphisms (SNPs), the candidate genes of which encode key enzymes and regulatory factors in triglyceride-rich lipoprotein metabolism pathway (Supplementary Data 7)5. Mendelian randomization analyzes revealed that genetically predicted remnant cholesterol and triglycerides were causally associated with higher risks of cardiometabolic multimorbidity. The causal odds ratios (ORs) for cardiometabolic multimorbidity, pooling all three cardiometabolic diseases together, and IHD-T2D multimorbidity per 1.0 mmol/L (0.98 standard deviation (SD)) increment in triglycerides were 1.21 (95% CI: 1.06, 1.39; P = 0.004) and 1.24 (95% CI: 1.06, 1.46; P = 0.007), respectively (Fig. 1). After rescaling the GRSs to represent the same magnitude (0.98 SD) of change with triglycerides, the corresponding causal ORs per 0.29 mmol/L increment in remnant cholesterol were 1.23 (95% CI: 1.07, 1.42; P = 0.005) and 1.26 (95% CI: 1.06, 1.50; P = 0.008), respectively. In consistency with the observational analysis, genetically determined blood remnant cholesterol and triglycerides were causally related to elevated risks of ischemic heart disease and type 2 diabetes but not stroke (Supplementary Fig. 2).
Causal odds ratios were calculated adjusting for age and sex. Data are presented as odd ratios with 95% confidence intervals. The dot refers to the odd ratio while the solid line refers to the 95% confidence interval. The dash line refers to odd ratio value of one as the reference. Abbreviations: CI confidence interval, IHD ischemic heart disease, OR odds ratio, T2D type 2 diabetes. Statistical significance is set at two-sided P value < 0.05.
Discussion
In this study of over 300,000 UK Biobank participants, serum remnant cholesterol and triglycerides were significantly associated with higher risks of cardiometabolic multimorbidity, particularly the risks of the progression from ischemic heart disease to IHD-T2D multimorbidity. Furthermore, Mendelian randomization analyzes conferred genetic evidence that higher levels of remnant cholesterol and triglycerides were causally related to higher risks of cardiometabolic multimorbidity, particularly in relation to IHD-T2D multimorbidity. The risks of IHD-T2D multimorbidity causally increased by 26% and 24% for each 1.0 mmol/L increment in triglycerides and 0.29 mmol/L increment in remnant cholesterol, respectively.
Previous studies have identified causal and positive associations between triglyceride-rich lipoproteins and atherosclerotic cardiovascular diseases7,23. Each 1.0 mmol/L increase in remnant cholesterol was causally associated with 2.8-fold higher risks of heart disease. Remnant cholesterol also reclassified up to 40% of incident myocardial infarction and ischemic heart disease beyond traditional cardiovascular risk factors among people free of cardiometabolic diseases6. In addition, remnant cholesterol above 1.0 mmol/L and triglycerides above 2.0 mmol/L were linked to about two-fold higher cardiovascular mortality24. Besides, remnant cholesterol was associated with risks of developing diabetes after transplantation in renal transplant recipients25. Our results parallel these previous studies showing that serum remnant cholesterol and triglycerides were causally associated with higher risks of ischemic heart disease, type 2 diabetes, and cardiometabolic multimorbidity. We further demonstrated a markedly higher risk for progression from initial ischemic heart disease to IHD-T2D multimorbidity using a multistate model. In this regard, our results provide evidence endorsing the treatment of blood triglyceride-rich lipoproteins in people with existing cardiovascular diseases to mitigate the risks of multimorbidity progression.
In the present study, using biologically plausible genetic variants as the instrumental variables, we identified causal support for associations of remnant cholesterol and triglycerides with cardiometabolic multimorbidity. Drug target Mendelian randomization studies have demonstrated prospective evidence that the triglyceride-lowering alleles in the LPL pathway, which controls triglyceride hydrolysis, may be causally associated with lower risks of coronary heart disease and type 2 diabetes independent of LDL-C26,27. The recent REDUCE-IT trial suggested that high-dose omega-3 fatty acids, which inhibit the production but enhance the clearance of triglyceride-rich VLDL, were effective in reducing ischemic events in people with hypertriglyceridemia and prescribed with statins, albeit the results of this trial have been questioned due to the adverse effects of mineral oil used for the placebo comparator on blood lipids4,28. Hence, remnant cholesterol and triglycerides were not only biological determinants but also promising therapeutic targets for the treatment of cardiometabolic diseases and prevention of progression from single disease to cardiometabolic multimorbidity.
Direct assay of triglyceride-rich lipoproteins requires labor-intensive protocols like ultracentrifugation or immunoseparation, which might hinder its implementation in large-scale cohort studies like the UK Biobank29. In contrast, in the present study, we used remnant cholesterol and triglycerides as two simple and straightforward surrogates for triglyceride-rich lipoproteins. Remnant cholesterol and triglycerides were strongly correlated but not identical. Given that there have been varied definitions for the remnants of triglyceride-rich lipoproteins, the term remnant cholesterol hereby refers to all cholesterol contents in triglyceride-rich lipoproteins, including chylomicron remnants, VLDLs, and intermediate-density lipoproteins30,31. In the present study, about 14% and 21 % of the UK Biobank participants had nonfasting serum remnant cholesterol above 1.0 mmol/L and triglycerides above 2.3 mmol/L, respectively. The distributions of remnant cholesterol and triglycerides in the UK Biobank were comparable with those in the Copenhagen General Population Study1,5. In the latter study, about 27% and 21% of participants had mild-to-moderate elevated nonfasting triglycerides and remnant cholesterol above 2.0 mmol/L and 1.0 mmol/L, respectively1,5. Random nonfasting triglycerides were, on average, 20-25% higher than the fasting values and reached the bottom after an overnight fast32. However, postprandial remnant cholesterol metabolism remains to be established, and a clinically meaningful cutoff value of remnant cholesterol is still lacking, which could facilitate translating our results into clinical practice.
Notwithstanding we showed a causal link between triglyceride-rich lipoproteins and type 2 diabetes risk in this study, elevated remnant cholesterol and triglycerides were unlikely to be mechanistically involved in the pathogenesis of type 2 diabetes. Seemingly, the genetic associations between remnant cholesterol, triglycerides, and type 2 diabetes could be attributed to increased likelihood of diabetes detection and diagnosis in participants with elevated triglycerides as clinicians often use elevated triglycerides as a biomarker of insulin resistance33. We also showed that baseline serum remnant cholesterol and triglycerides were associated with higher risks of progression from ischemic heart disease to IHD-T2D multimorbidity but not the progression from type 2 diabetes to IHD-T2D multimorbidity, suggesting differential roles of triglyceride-rich lipoproteins in disease progression among patients that developed heart disease or diabetes.
Mechanisms underlying the causal associations between triglyceride-rich lipoproteins and cardiometabolic multimorbidity may involve their atherogenic and pro-inflammatory properties1,15,16. Besides, most people with elevated triglycerides or remnant cholesterol are accompanied by overweight, hyperglycemia, or fatty liver15. Insulin resistance could impair hepatic clearance of VLDL, leading to overaccumulation of triglyceride-rich lipoprotein remnants34. Lipid dysmetabolism in type 2 diabetes is characterized by hypertriglyceridemia, normal or slightly higher LDL-C, and increased apolipoprotein B secretion, the main structural components of triglyceride-rich lipoproteins15. Future studies are warranted to disentangle the roles and related mechanisms of remnant cholesterol and triglycerides in the progression of cardiometabolic disease to multimorbidity.
Strengths of this study include the large sample size and comprehensive case identification using primary care, death registry, and hospital inpatient records, which allowed us to develop a multistate disease transition model with sufficient statistical power. Moreover, a combination of multistate modeling and Mendelian randomization could, to a greater extent, address the residual confounding and reverse causality, which is major methodological issues in observational studies. In addition, remnant cholesterol and triglycerides were measured from the nonfasting blood samples, which could better characterize lipid profiles over a 24 h cycle in a real-world setting5,16. Nonfasting triglycerides also had a better diagnostic performance for cardiovascular diseases than the fasting values5,32.
This study also has several limitations. First, blood samples were collected only once at baseline and thus were unable to capture long-term changes before and after the incidence of first cardiometabolic diseases. Although estimates from Mendelian randomization could indicate the life course effects of remnant cholesterol and triglycerides, repeated measurements could help to understand the impact of triglyceride-rich lipoproteins on the progression to cardiometabolic multimorbidity. Second, information concerning lipid-lowering medication use was also collected at baseline. It is unclear whether changes in the use of lipid-lowering medications after the incidence of first cardiometabolic disease could influence the risks of progression to multimorbidity via triglyceride-rich lipoproteins. Third, the potential pleiotropy of genetic instruments might lead to biased risk estimates for any Mendelian randomization analysis. In this study, we only included biologically plausible genetic variants involved in triglyceride-rich lipoproteins, minimizing the risk of pleiotropic effects. Fourth, the UK Biobank participants tended to have a better socioeconomic status than the entire UK population. However, valid findings of the exposure-outcomes associations do not necessarily require the participants to be representative of the general population at large35. Fifth, although the Harrell’s C index significantly increased after adding remnant cholesterol and triglycerides into the basal model, a risk prediction model specific for developing cardiometabolic morbidity is still lacking. Also, it remains to be studied whether remnant cholesterol and triglycerides could improve the discrimination and calibration power of the risk prediction model for progression from first cardiometabolic disease to multimorbidity. Sixth, cardiometabolic health status could influence clinical decision to prescribe lipid-lowering medication, which might a collider on the pathway from remnant cholesterol and triglycerides to cardiometabolic risk, leading to biased risk estimates36,37. However, sensitivity analysis including participants receiving lipid-lowering medication at the second-stage regression yielded similar results compared to the main analysis.
In conclusion, remnant cholesterol and triglycerides were both observationally and genetically linked to higher cardiometabolic multimorbidity risks. These findings imply that triglyceride-rich lipoproteins could serve potential risk factors and therapeutic targets for the prevention and treatment of cardiometabolic diseases, which necessitate further validation through randomized controlled trials focusing on triglyceride-rich lipoproteins.
Methods
Study population
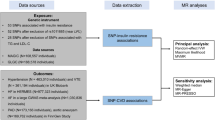
The UK Biobank has obtained ethical approval from the North West Multi-centre Research Ethics Committee as a Research Tissue Bank approval (Approval Number: 11/NW/0382, https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics). Besides, we also got approval for performing data analysis from Peking University Third Hospital Medical Science Research Ethics Committee (Approval Number: S2024080). All UK Biobank participants provided written informed consent for their participation in the study and access to their medical records. The present study was performed under Application ID 44430. The UK Biobank is a prospective study of over 500,000 UK adults aged between 37 and 73 years that were recruited between 2006 and 201038. During the baseline visit to the local assessment centers, participants were invited to complete a nurse-administrated questionnaire collecting data on demographic, socioeconomic, lifestyle, and medical history, had physical measurements recorded and provided biological samples for biochemical and genetic analyzes. In the observational analysis, participants were excluded if they had any cardiometabolic diseases (type 2 diabetes, ischemic heart disease, and stroke) at baseline, were prescribed lipid-lowering medications (n = 98,828), or had missing values for remnant cholesterol (n = 73,390) or triglycerides (n = 33,284). Therefore, 334,030 (men% = 42.5%) and 365,577 (men% = 42.1%) eligible participants were included in the observational analysis of remnant cholesterol and triglycerides, respectively (Supplementary Fig. 3a). In the Mendelian randomization analysis, participants were excluded if they were not white British (n = 59,895), did not pass the quality control for genotyping (n = 3636), or had missing values for remnant cholesterol (n = 73,390) or triglycerides (n = 33,284). To avoid collider bias, we included participants that used lipid-lowering medications in both first and second stage of Mendelian randomization analysis. Therefore, 376,712 (men% = 46.2%) and 411,930 (men% = 45.9%) eligible participants were included in the Mendelian randomization analysis of remnant cholesterol and triglycerides, respectively (Supplementary Fig. 3b).
Serum remnant cholesterol and triglyceride measurements
Remnant cholesterol and triglycerides are two simple measurements of triglyceride-rich lipoproteins1. Nonfasting blood samples were collected during the baseline examination39. Serum total cholesterol and triglyceride concentrations were measured using an enzymatic colorimetric method on Beckman Coulter AU5800 analyzers. Serum LDL-C concentrations were directly measured using an enzymatic protective selection method on Beckman Coulter AU5800 analyzers. Serum HDL-C concentrations were measured using the enzyme immune-inhibition analysis (Beckman Coulter AU5800). Serum remnant cholesterol was calculated as total cholesterol minus HDL-C minus LDL-C5,6,30. A circulating triglyceride above 1.7 mmol/L (150 mg/dL) is a widely accepted cutoff point for hypertriglyceridemia2. Therefore, we categorized triglycerides into five groups: <0.9 mmol/L (80 mg/dL), 0.9−<1.3 mmol/L (80− < 115 mg/dL), 1.3−<1.7 mmol/L (115 - < 150 mg/dL), 1.7−<2.3 mmol/L (150− < 204 mg/dL), and ≥ 2.3 mmol/L (≥204 mg/dL). However, there is no recommended cutoff point for elevated blood remnant cholesterol yet. We arbitrarily categorized remnant cholesterol into five groups: <0.4 mmol/L (15 mg/dL), 0.4 - <0.6 mmol/L (15− < 23 mg/dL), 0.6−<0.8 mmol/L (23 - < 31 mg/dL), 0.8−<1.0 mmol/L (31− < 39 mg/dL), and ≥ 1.0 mmol/L (≥39 mg/dL), yielding a similar pattern of participant distribution in each category between remnant cholesterol and triglycerides (Table 1).
Genetic instruments of remnant cholesterol and triglycerides
Genotyping was performed using the UK BiLEVE array and the UK Biobank Axiom array (similarity >95%)40. Mendelian randomization has three core instrumental variable assumptions: 1) the genetic instruments are strongly associated with the exposure; 2) the genetic instruments are not associated with confounding factors; 3) the genetic instruments are exclusively associated with the outcome via the exposure. To avoid pleiotropy of genetic instruments, we retrieved the 16 gain-of-function and loss-of-function single nucleotide polymorphisms in a previous study by Kaltoft et al.5. Candidate genes of these SNPs encode key enzymes, regulatory factors, and lipoproteins that have well-established effects on triglyceride-rich lipoprotein metabolism, including LPL, angiopoietin-like 3 (ANGPTL3), angiopoietin-like 4 (ANGPTL4), apolipoprotein C-III (APOC3), and apolipoprotein A-V (APOAV)7,41,42 Three rare variants (rs569107562, rs749131121, and rs267606655) were not available in the UK Biobank study. Such genetic instrument selection strategy was comparable to that used in drug target Mendelian randomization analysis, which could reduce the possibility of horizontal pleiotropy and weak instrument bias43. A total of 13 biologically relevant SNPs were included as genetic instruments for remnant cholesterol and triglycerides (Supplementary Data 7). We calculated weighted GRSs as aggregated genetic instrumentals for blood remnant cholesterol and triglycerides. The weighted GRS was calculated by summing the products of the number of lipid-raising alleles and the effect size of each SNP on blood remnant cholesterol and triglycerides (Supplementary Data 7). The weighted GRSs could explain around 2.95% and 3.01% variance of serum remnant cholesterol and triglycerides, respectively (Supplementary Data 7).
Cardiometabolic multimorbidity
The prevalent and newly on-set cardiometabolic diseases, namely type 2 diabetes, ischemic heart disease, and stroke, were identified via linkage to primary care records, hospital inpatient records, and death registry records and outcomes were coded using the International Classification of Disease, 10th revision codes: type 2 diabetes, E11; ischemic heart disease, I21−I25; stroke, I60-I64. We censored participants at the end of follow-up, at the date of the first occurrence of any cardiometabolic disease or development of multimorbidity, and at the date of loss to follow-up, whichever was first. Cardiometabolic multimorbidity was defined as the concurrence of at least two cardiometabolic diseases, like the co-existence of ischemic heart disease and type 2 diabetes (IHD-T2D multimorbidity) and the co-existence of stroke and ischemic heart disease (IHD-stroke multimorbidity). In the observational analysis, we only included participants that were free of any cardiometabolic diseases at baseline and investigated the associations between triglyceride-rich lipoproteins, newly onset cardiometabolic diseases, and subsequent progression to cardiometabolic multimorbidity (Supplementary Fig. 1). In the Mendelian analysis, we also included participants with existing cardiometabolic diseases and cardiometabolic multimorbidity at baseline.
Covariates
Age and sex were self-reported. Townsend deprivation index is an indicator of socioeconomic status, which was assessed based on local percentages of unemployment, non-car ownership, non-home ownership, and household overcrowding. A higher Townsend index suggests a more disadvantaged socioeconomic status. Body mass index was calculated as body weight divided by the square of height and expressed as kg/m2. Systolic blood pressure was measured by a digital blood pressure monitor or a manual sphygmomanometer. Smoking status was categorized as never, former, and current smokers. Pack-years of smoking were calculated as the number of cigarettes smoked per day divided by twenty, multiplied by the number of years of smoking. Alcohol intake was assessed by a food frequency questionnaire concerning beverage types and frequency. Low alcohol intake was defined as <22 units/day in men and <15 units/day in women44. Physical activity was assessed by the International Physical Activity Questionnaire short form. Adequate physical activity was defined as ≥150 min/week of moderate activity, or ≥75 min/week of vigorous activity, or ≥150 min/week of combined moderate and vigorous activity, ≥5 times/week of moderate activity, or ≥1 time week of vigorous activity45.
We derived a diet quality score as the indicator of diet quality, which is based on intakes of fruits, vegetables, processed meat, red meat, fish, whole grains, and refined grains46. Diet was assessed via a food frequency questionnaire at baseline. One point was assigned to each food item if: the average daily intake of: 1) fruits ≥ 3 servings/day; 2) vegetables ≥ 3 servings/day; 3) fish ≥ 2 servings/week; 4) processed meat ≤ 1 servings/week; 5) red meat ≤ 1.5 servings/week; 6) whole grain ≥ 3 servings/week; 7) refined grain ≤ 1.5 servings/week. The diet quality score ranged from 0 to 7 with a higher score indicating a better diet quality. A higher diet quality score indicated better adherence to a healthy dietary pattern. In addition, we also constructed a sleep quality score as an indicator of healthy sleep patterns, which is based on chronotype, sleep duration, frequency of insomnia, snoring, and daytime sleepiness46. Sleep pattern was evaluated via a touchscreen questionnaire during the baseline assessment. One point was assigned to each item if: 1) early chronotype; 2) sleep 7 to 8 h per day; 3) never or rarely had insomnia symptoms; 4) no self-reported snoring; 5) no frequent daytime sleepiness. Therefore, the sleep quality score ranged from 0 to 5 with a higher score indicating healthier sleep pattern.
Lipid-lowering medication
During the baseline assessment, the participants were invited to take a verbal interview conducted by trained staff completed at the assessment center. Triglyceride-lowering medication was identified using the following treatment/medication code: 1140861954, fenofibrate; 1140861924, bezafibrate; 1141157260, bezafibrate product; 1140861944, clofibrate; 1140862026, ciprofibrate; 1140910670 niacin; 1140861868, nicotinic acid product; 1193, omega-3/fish oil supplement. Cholesterol-lowering medication was identified using the following codes: 1141146234, atorvastatin; 1140888594, Fluvastatin; 1140888648, pravastatin; 1141192410, rosuvastatin; 1140861958, simvastatin. In addition, the participants were asked whether they regularly took cholesterol-lowering medications via a touchscreen questionnaire during the baseline assessment.
Statistical analysis
Multistate modeling
In the observational analysis, we constructed multistate models to assess the associations of blood remnant cholesterol and triglycerides with incident cardiometabolic diseases and the progression of the first cardiometabolic disease to cardiometabolic multimorbidity. The multistate model, as an extension of competing risk model, was increasingly used to study complex disease progression47. In this study, we defined three states of participants, namely free of cardiometabolic disease, incidence of first cardiometabolic disease, and development of cardiometabolic morbidity, allowing two disease transitions, namely 1) from baseline to first cardiometabolic disease and 2) from first cardiometabolic disease to multimorbidity (Supplementary Fig. 1a). For participants with the same date for first cardiometabolic disease and multimorbidity, the date of first cardiometabolic disease was calculated as the date of multimorbidity minus the median times for the transition from incident first cardiometabolic disease to multimorbidity48. In addition, we also constructed a multistate model that also included ischemic heart disease and type 2 diabetes allowing four transitions, namely 1) from baseline to incident type 2 diabetes, 2) from baseline to ischemic heart disease, 3) from type 2 diabetes to IHD-T2D multimorbidity, and 4) from ischemic heart disease to IHD-T2D multimorbidity. HRs for disease transitions across different levels of serum remnant cholesterol and triglycerides were calculated using the flexible parametric survival model, which allows time-dependent effects during disease transitions49,50. The flexible parametric survival model used restricted cubic splines to estimate hazard functions. The number of knots was selected based on Akaike’s Information Criteria and Bayesian Information Criteria. Age was used as the time scale. The proportional hazards assumption was tested using Schoenfeld residuals via transition-specific Cox regression. Age was treated as a time-varying variable. The multivariate models were adjusted for age, sex, Townsend index, BMI, systolic blood pressure, smoking status, pack-years of smoking, alcohol intake, physical activity, diet quality score, sleep quality score, and fasting time. Tests for trends were conducted by treating the median value of each lipid category as continuous variables. The discrimination performance of models with and without remnant cholesterol or triglycerides (the basal model) was compared using Harrell’s C index.
Mendelian randomization
We used one-sample Mendelian randomization to study the causal associations of remnant cholesterol and triglycerides with incident cardiometabolic disease and multimorbidity. The Causal ORs were calculated via the two-stage least squares method51. For comparing remnant cholesterol with triglycerides, the GRSs were rescaled to 1.0 mmol/L increment in triglycerides (standard deviation (SD): 1.02 mmol/L; 0.98 SD) and 0.29 mmol/L increment in remnant cholesterol (SD: 0.30 mmol/L; 0.98 SD). Because genotype is determined at conception and is less susceptible to confounding, the model only adjusted for age and sex. In the sensitivity analysis, we included participants receiving lipid-medications in the second-stage regression in Mendelian randomization to test whether use of lipid-lowering medication might be a collider on the pathway from remnant cholesterol and triglycerides to cardiometabolic multimorbidity. The endpoints of interest in the Mendelian randomization analysis were cardiometabolic multimorbidity.
Statistical analysis was performed in Stata/MP version 17.0 and R version 4.2.2 with significance set at two-sided P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This research was conducted using the UK Biobank Resource (https://www.ukbiobank.ac.uk/) under Application ID 44430. Data from UK Biobank is accessible to eligible researchers via applying to www.ukbiobank.ac.uk. Data supporting the findings of this study are available in the article and its Supplementary information. Source data are provided with this paper.
Code availability
Multistate model was developed using the stmerlin stata command: https://github.com/RedDoorAnalytics/stmerlin. Forest plots were developed using the R package: https://cran.r-project.org/web/packages/forestploter. Analytical codes have been deposited at Zenodo (https://doi.org/10.5281/zenodo.10560862)52.
References
Nordestgaard, B. G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circulation Res. 118, 547–563 (2016).
Reiner, Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat. Rev. Cardiol. 14, 401–411 (2017).
Pirillo, A., Casula, M., Olmastroni, E., Norata, G. D. & Catapano, A. L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 18, 689–700 (2021).
Ridker, P. M. et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin-1β, interleukin-6, c-reactive protein, oxidized low-density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein-associated phospholipase a2: a reduce-it biomarker substudy. Circulation 146, 372–379 (2022).
Kaltoft, M., Langsted, A. & Nordestgaard, B. G. Triglycerides and remnant cholesterol associated with risk of aortic valve stenosis: Mendelian randomization in the Copenhagen General Population Study. Eur. heart J. 41, 2288–2299 (2020).
Doi, T., Langsted, A. & Nordestgaard, B. G. Elevated remnant cholesterol reclassifies risk of ischemic heart disease and myocardial infarction. J. Am. Coll. Cardiol. 79, 2383–2397 (2022).
Varbo, A. et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 61, 427–436 (2013).
Holmes, M. V. et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. heart J. 36, 539–550 (2015).
Gordillo-Marañón, M. et al. Validation of lipid-related therapeutic targets for coronary heart disease prevention using human genetics. Nat. Commun. 12, 6120 (2021).
Kivimäki, M. et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2, e277–e285 (2017).
Di Angelantonio, E. et al. Association of cardiometabolic multimorbidity with mortality. Jama 314, 52–60 (2015).
Borén, J., Taskinen, M.-R., Björnson, E. & Packard, C. J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 19, 577–592 (2022).
Huh, J. H. et al. Remnant cholesterol and the risk of cardiovascular disease in type 2 diabetes: a nationwide longitudinal cohort study. Cardiovasc Diabetol. 21, 228 (2022).
Hu, X. et al. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc Diabetol. 21, 117 (2022).
Chait, A. et al. Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes 69, 508–516 (2020).
Ginsberg, H. N. et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. heart J. 42, 4791–4806 (2021).
Varbo, A., Benn, M., Tybjærg-Hansen, A. & Nordestgaard, B. G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 128, 1298–1309 (2013).
Castañer, O. et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J. Am. Coll. Cardiol. 76, 2712–2724 (2020).
Lai, C. Q. et al. The impact of alcoholic drinks and dietary factors on epigenetic markers associated with triglyceride levels. Front Genet 14, 1117778 (2023).
Oelrich, B., Dewell, A. & Gardner, C. D. Effect of fish oil supplementation on serum triglycerides, LDL cholesterol and LDL subfractions in hypertriglyceridemic adults. Nutr., Metab., cardiovascular Dis.: NMCD 23, 350–357 (2013).
Vergara, M. et al. Associations of Changes in Blood Lipid Concentrations with Changes in Dietary Cholesterol Intake in the Context of a Healthy Low-Carbohydrate Weight Loss Diet: A Secondary Analysis of the DIETFITS Trial. Nutrients 13, 1935 (2021).
Ramos-Cáceres, M. et al. Triglyceride Metabolism Modifies Lipoprotein(a) Plasma Concentration. J. Clin. Endocrinol. Metab. 107, e3594–e3602 (2022).
Varbo, A. & Nordestgaard, B. G. Remnant cholesterol and risk of ischemic stroke in 112,512 individuals from the general population. Ann. Neurol. 85, 550–559 (2019).
Wadström, B. N., Pedersen, K. M., Wulff, A. B. & Nordestgaard, B. G. Elevated remnant cholesterol, plasma triglycerides, and cardiovascular and non-cardiovascular mortality. Eur. Heart J. 44, 1432–1445 (2023).
Szili-Torok, T. et al. Remnant lipoprotein cholesterol is associated with incident new onset diabetes after transplantation (NODAT) in renal transplant recipients: results of the TransplantLines Biobank and cohort Studies. Cardiovasc Diabetol. 21, 41 (2022).
Lotta, L. A. et al. Association of genetically enhanced lipoprotein lipase-mediated lipolysis and low-density lipoprotein cholesterol-lowering alleles with risk of coronary disease and Type 2 Diabetes. JAMA Cardiol. 3, 957–966 (2018).
Ference, B. A. et al. Association of triglyceride-lowering lpl variants and ldl-c-lowering ldlr variants with risk of coronary heart disease. Jama 321, 364–373 (2019).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med 380, 11–22 (2019).
Duran, E. K. & Pradhan, A. D. Triglyceride-rich lipoprotein remnants and cardiovascular disease. Clin. Chem. 67, 183–196 (2021).
Varbo, A. & Nordestgaard, B. G. Directly measured vs. calculated remnant cholesterol identifies additional overlooked individuals in the general population at higher risk of myocardial infarction. Eur. heart J. 42, 4833–4843 (2021).
Stürzebecher, P. E., Katzmann, J. L. & Laufs, U. What is ‘remnant cholesterol’? Eur. Heart J. 44, 1446–1448 (2023).
Langsted, A., Freiberg, J. J. & Nordestgaard, B. G. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 118, 2047–2056 (2008).
De Silva, N. M. et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes 60, 1008–1018 (2011).
Gordts, P. L. et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Investig. 126, 2855–2866 (2016).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of uk biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Coscia, C. et al. Avoiding collider bias in Mendelian randomization when performing stratified analyses. Eur. J. Epidemiol. 37, 671–682 (2022).
Holmberg, M. J. & Andersen, L. W. Collider Bias. Jama 327, 1282–1283 (2022).
Littlejohns, T. J., Sudlow, C., Allen, N. E. & Collins, R. UK Biobank: opportunities for cardiovascular research. Eur. Heart J. 40, 1158–1166 (2019).
Elliott, P. & Peakman, T. C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 37, 234–244 (2008).
Bycroft, C. et al. Genome-wide genetic data on ~500,000 UK Biobank participants. Preprint at bioRxiv, 166298 (2017).
Jørgensen, A. B., Frikke-Schmidt, R., Nordestgaard, B. G. & Tybjærg-Hansen, A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med 371, 32–41 (2014).
Dewey, F. E. et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med 374, 1123–1133 (2016).
Harrison, S. C. et al. Genetic association of lipids and lipid drug targets with abdominal aortic aneurysm: a meta-analysis. JAMA Cardiol. 3, 26–33 (2018).
Innes, H. et al. Characterizing the risk interplay between alcohol intake and body mass index on cirrhosis morbidity. Hepatol. (Baltim., Md.) 75, 369–378 (2022).
Lloyd-Jones, D. M. et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121, 586–613 (2010).
Zhao, Y. et al. Associations of polysocial risk score, lifestyle and genetic factors with incident type 2 diabetes: a prospective cohort study. Diabetologia 65, 2056–2065 (2022).
Han, Y. et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur. Heart J. 42, 3374–3384 (2021).
Wu, Y. et al. Ambient air pollution associated with incidence and dynamic progression of type 2 diabetes: a trajectory analysis of a population-based cohort. BMC Med 20, 375 (2022).
Shang, Y., Nasr, P., Widman, L. & Hagström, H. Risk of cardiovascular disease and loss in life expectancy in NAFLD. Hepatol. (Baltim., Md.) 76, 1495–1505 (2022).
Crowther, M. J. & Lambert, P. C. Parametric multistate survival models: Flexible modelling allowing transition-specific distributions with application to estimating clinically useful measures of effect differences. Stat. Med 36, 4719–4742 (2017).
Palmer, T. M. et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am. J. Epidemiol. 173, 1392–1403 (2011).
Zhao, Y. Elevated blood remnant cholesterol and triglycerides are causally related to the risks of cardiometabolic multimorbidity. Zenodo. https://doi.org/10.5281/zenodo.10560862. (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers: 82204026 (receiver: Y.Z.) and 82173499 (receiver: T.H.)).
Author information
Authors and Affiliations
Contributions
Y.Z. and T.H. conceptualized this study. Y.Z., Z.Z., Y.L., W.X., Z.S., N.H., W.W. and X.D. carried out the data analysis. Y.Z. carried out data visualization and drafted the manuscript. T.H. and R.C. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Zhuang, Z., Li, Y. et al. Elevated blood remnant cholesterol and triglycerides are causally related to the risks of cardiometabolic multimorbidity. Nat Commun 15, 2451 (2024). https://doi.org/10.1038/s41467-024-46686-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46686-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.