Abstract
For stable operation of ultrathin flexible transparent electrodes (uFTEs), it is critical to implement effective risk management during concurrent multi-loading operation of electrical bias and mechanical folding cycles in high-humidity environments. Despite extensive efforts in preparing solution-processed uFTEs with cost-effective and high-throughput means, achieving in-situ nano-adhesion in heterogeneous metal-oxide nanocomposites remains challenging. In this work, we observed by serendipity liquid-like behaviour of transparent metal-oxide-semiconductor zinc oxide nanoparticles (ZnONPs) onto silver nanowires (AgNWs) developed by in-situ solution processed method (iSPM). This enabled us to address the long-standing issue of vulnerability in the nanocomposite caused by the interface of dissimilar materials between AgNWs and ZnONPs, resulting in a remarkably improved multi-loading operation. Importantly, substrate-integrated uFTEs constituted of the metal-oxide nanocomposite electrode semi-embedded in the polymer matrix of greatly thin <0.5 μm thickness is successfully demonstrated with the smooth surface topography, promoted by the tri-system integration including (i) AgNW-AgNW, (ii) ZnONP-ZnONP, and (iii) AgNW-ZnONP systems. Our finding unveils the complex interfacial dynamics associated with the heterogeneous interface system between AgNWs and ZnONPs and holds great promise in understanding the in-situ nano-adhesion process and increasing the design flexibility of next generation solution-processed uFTEs.
Similar content being viewed by others
Introduction
Recent advancements in reducing device thickness in the field of ultrathin flexible transparent electrodes (uFTEs) have been favorably prevailing in state-of-art research fields and modern device innovations such as wearable optoelectronic devices, implantable bioelectronics, soft robotics, aircraft electronics, and imperceptible electronic skin since a high level of compatibility and comfortability1,2,3,4,5,6. Previously, sputtered indium tin oxide embossed electrodes have been demonstrated on target surfaces of as-prepared ultrathin flexible substrates7. However, there is an ever-increasing demand for advanced solution-processed uFTEs with fast, facile, low-cost, and high-throughput printing production8,9,10. The stringent requirements include a high figure of merit with well-balanced electrical and optical properties (i.e. <10 Ω sq.−1 of sheet resistance and >90 % of diffused transmittance) and very smooth surface morphology (i.e. <1 nm of root-mean-square (RMS)) is preferred in terms of device applications11,12,13,14,15,16,17,18. More importantly, the high operational stability under simultaneous multi-loading operations (i.e. folding cycles with a few mm radius of curvature, current bias with several hundred mA cm−2 including efficient thermal energy management, and humid environments at >85 % of relative humidity) are highly desirable, which is one of the rigorous operational situations in real applications.
Among various solution-processable conductor approaches10, substrate-integrated uFTEs constituted of nanonets of silver nanowires (AgNWs) embedded in flexible substrates have been demonstrated as the novel uFTE architectures with outstanding electrical/optical properties and mechanical flexibility by the means of fully solution-process19. However, the completely buried Ag nanonets in the insulating polymer matrix only allow narrow conductive pathways exposed to the top surface, which significantly limits the compatibility with various applications. Recently, there was a trial to extend the conductive pathways to adjacent layers by employing conductive polymers such as poly(3,4-ethylenedioxythiophene) polystyrene sulfonate in Ag nanonets for photovoltaic device applications20. However the severe instability issue increasing the resistance was observed due to the hygroscopic property of the conductive polymer in humid conditions. Meanwhile, Ag nanonets have been frequently combined with transparent metal-oxide-semiconductor nanomaterials to overcome not only the issue of the restricted conductive pathways but also avoid the vulnerability to externalities such as high humidity21,22,23,24. Nonetheless, there is still a big difficulty in achieving satisfactory performance against different operational tests. For instance, the Ag nanonets protected by atomically deposited transparent metal-oxide-semiconductors such as zinc oxide (ZnO) will break down under a continuous bias with a current density above 155 mA cm−2 due to strong Gibbs-Thomson effects in Joule heating to the Ag nanonets25. Besides, although the superior operation of ZnO-Ag nanonets under 5000-cycled bending has been reported with 3 mm of a bending radius26, there are many cracks appeared between Ag nanonets and the adjacent ZnO matrix in ZnO-Ag nanonets embedded in the polymer matrix with >20 nm of peak-to-valley (PtV) surface roughness, which will unfavorably accelerate the formation of mechanical defects under the repetitive mechanical bendings. Furthermore, there are very few studies on the high operational stability of uFTEs applicable to practical multi-loading fatigue examinations.
One of resolving the fundamental bottleneck of the instability issue is to increase adhesive interfacial regions in the nanocomposite electrode between nanonets of AgNWs and matrix of solution-processable transparent metal-oxide-semiconductor nanomaterials such as zinc oxide nanoparticles (ZnONPs) for mechanically strong interface connections as well as efficient heat-dissipating from the nanonets. In addition, ensuring strong adhesions is essential not only within the nanocomposite but also between the nanocomposite electrode and the ultrathin polymer matrix for realizing sturdy architectures of uFTEs with smooth surface topography. In the nanocomposite, there are always three different interfaces between (i) AgNWs, (ii) ZnONPs, and (iii) AgNWs and ZnONPs. Although there are various technologies for integrating individual systems of the Ag nanonets at the cross-junctions27 and cold sintering of ZnO at the grain boundaries28, far more importantly, the heterogeneous contact at the AgNW-ZnONP interface requires substantial improvement. One particular concern is the presence of organic residual wrapping on Ag nanonets prevents its direct interaction with ZnONPs29. Besides, their non-flexible geometric shapes limit the direct contact area between them30,31. As a consequence, there is a primary need to realize the integration of three different interface systems in the nanocomposite electrode of Ag nanonets and matrix of ZnONPs tightly semi-embed in an ultrathin polymer matrix for achieving milestones for the stability of robust uFTE structures.
In this study, we demonstrated high operational stability of substrate-integrated uFTEs constituted of AgNW nanonets and ZnONP matrix semi-embed in colorless polyimide (cPI) substrates of ultrathin <0.5 μm thickness fabricated via fully solution-processed means. The stable operations against the simultaneous multi-loading fatigue tests were confirmed by retaining a remarkable operational current density of 8.4 MA cm−2 flowing through the individual nanowires with only 5 % variation under mechanical folding cycles with 0.5 mm radius of the curvature at 85 % of relative humidity. To achieve it, in-situ solution-processed method (iSPM) was developed for both (i) removing the capping agents from AgNWs for direct physical contact of the AgNW-AgNW system at the cross-junctions and (ii) facilitating the ZnONPs integration with flexible geometric shapes through the course of the coalescence process of ZnONP-ZnONP system. Interestingly, the provided conditions from both (i) and (ii) enable the ZnONPs to be capable of the unprecedented in-situ nano-adhesion into the cleaned surface of AgNWs via the coalescence incidents with flexible geometric shapes in AgNW-ZnONP system. The fabricated substrate-integrated uFTEs enhanced by the tri-system integration of the above-mentioned AgNW-AgNW, ZnONP-ZnONP, and AgNW-ZnONP systems showed superior electrical/optical/surface roughness properties of sheet resistance of 7.5 Ω sq.−1, the average diffused transmittance of >88 % between 400-800 nm of wavelengths when including both electrode and substrate components, and extremely smooth surface roughness with <1 nm of RMS and <5 nm of PtV. Notably, our results unveiled that the adhesive wetting process of ZnONPs to AgNWs follows the contact line friction model of hydrodynamics with viscous liquid-like behaviour. Consequently, the tri-system integration developed by the iSPM offers a general design for smooth topography for the substrate-integrated uFTEs achieved via fully solution-processed means with high operational stability of the metal-oxide nanocomposite electrodes.
Results
Solution-processed uFTEs with four phases of stability
In the metal-oxide composite electrode of AgNWs nanonets and ZnONP matrix, there are always three different types of interface systems including AgNW-AgNW, ZnONP-ZnONP, and AgNW-ZnONP, respectively, hereafter named “the tri-system”. So far, the respective key mechanisms of in-situ integration for both the AgNW-AgNW and ZnONP-ZnONP interfaces have been intensively studied32,33. However, integrating the dissimilar material interface system between AgNWs and ZnONPs is rarely examined in spite of a number of studies on these nanocomposite combinations21,22 due to the difficulty to observe their in-situ dynamics for nanoscale interactions between them. Recently, the observation of interacting dynamics between metal nanoparticles and oxide supports in the field of catalysis, which is the opposite geometric concept for this work, has been reported by strong metal-support interaction (SMSI) but the mechanism is not fully understood34,35. In particular, the non-adhesive interface between AgNWs and ZnONPs in the field of uFTEs will be vulnerable to practical dynamic operating environments such as both repetitive mechanical folding cycles and continuous electrical bias under high humid conditions, making it challenging to harness for modern devices and innovations.
Here, we develop the iSPM to achieve the tri-system integration in the metal-oxide composite electrode of AgNWs and ZnONPs for high operational stability against simultaneous multi-loading operations. As for reagents for the iSPM, a certain sodium borohydride (NaBH4) has been selected for (i) removing capping agents such as polyvinylpyrrolidone (PVP) from the surface of AgNWs, which facilitates direct contact and physical merging at the cross-junctions between the Ag nanonets (AgNW-AgNW system)36 and (ii) allowing flexible geometric shapes of ZnONPs by driving the course of the coalescence process among ZnONPs (ZnONP-ZnONP system). Interestingly, with the combination of (i) and (ii) after the iSPM, the provided conditions between AgNWs and ZnONPs enable the unprecedented adhesive motion of ZnONPs with the coalescence event to the cleansed surface of AgNWs (AgNW-ZnONP system). The NaBH4 concentration of 0.1 molar concentration (M) has been decided for the optimized iSPM since it showed a considerable reduction of sheet resistance from the original ones and the rate of the sheet resistance reduction was almost saturated after 0.1 M while there was almost no observed silver debris as a by-product (Figure S1).
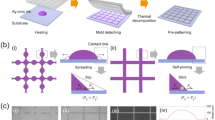
Figure 1a depicts the schematic diagram of processing the tri-system integration in the as-prepared metal-oxide nanocomposites on rigid supporting substrates (e.g. silicon wafer, glass, etc.) via the iSPM, followed by the entire fabrication progress of semi-embedding the nanocomposite electrode in cPI for the substrate-integrated uFTEs with a greatly thin thickness of <0.5 μm. The comprehensive discussions about the respective interfaces can be found in Figure S2 and Fig. 2a–f. The substrate-integrated uFTEs with different uniform thicknesses of cPI can be successfully demonstrated by the height regulator of the automatic meniscus printing equipment with an appropriate Mayer bar coating speed (Figure S3). Regarding chemicals for the polyamic acid (PAA) liquid as the precursor of cPI, 2,2′-bis(trifluoromethyl)−4,4′-diaminobiphenyl (TFDB) and 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) have been prepared due to the multi-bulky pendant trifluoromethyl groups. The crucial cPI substrate shows only a low water absorption rate of 0.2 % even after 3 days immersed in water because of the waterproofing effect of the fluorine atoms and to properly screen the thermal stability of uFTEs during the multi-loading operations, the substrate requires to have high glass transition temperatures such as the cPI (>300 °C)37. Details in the PAA synthesis have been previously reported20 and the imidization process of the PAA liquid to the cPI substrate is described in the experimental section. Figure 1b shows an optical photo of the fabricated substrate-integrated uFTEs with an extremely thin thickness of <0.5 μm floating on the surface of the water being lifted in a needle. The as-prepared nanocomposite electrodes without the cPI substrate and the cPI substrate-integrated uFTE showed an averaged diffused transmittance of >92 and >88 %, respectively. They showed negligible change in optical transparency at wavelengths between 400 nm and 900 nm with and without the iSPM (Figure S4), while the averaged reduction of 25 % in electrical sheet resistance to 7.5 ± 1 Ω sq−1 for the uFTEs was confirmed after the iSPM (Table S1). It should be noted the uFTEs with the iSPM show very good resistance uniformity at different locations of the sample (Figure S5).
a A schematic diagram exhibiting the fabrication process of the substrate-integrated uFTEs with greatly thin <0.5 μm containing the tri-system integration in the nanocomposite via the iSPM. b The photo of the uFTE (4 cm2) floating on the surface of the water getting lifted by a needle. c, Real-time monitoring of the operational current density of uFTEs according to with (blue) and without (black) the tri-system integration against the simultaneous multi-loading fatigue tests such as the initial 8.4 MA cm−2 and 10,000 cycles of repetitive folding (lasting for about 4 hours) with a folding radius of 500 μm under the humid condition of relative humidity 85 %.
The AgNW-AgNW interface. a, b The TEM images illustrating the respective Ag-PVP-Ag and Ag-Ag junctions regarding before and after the iSPM, respectively. c, d The ZnONP-ZnONP interface. TEM images of the respective ZnONPs before and after the iSPM. The respective insets are the corresponding diffraction patterns. The AgNW-ZnONP interface. e The as-prepared nanocomposite demonstrating the non-physical connection between AgNWs and ZnONPs in the presence of the PVP layer in the middle. f The TEM image magnifying near the interfacial region between AgNWs and ZnONPs. The representative FFT images were extracted from the corresponding (i), (ii), and (iii) boxes, showing an example of the well-matched lattice fringe between AgNWs and ZnONPs. There are many well-matched lattice fringe alignments that can be frequently found. The surface topography of the substrate-integrated uFTEs. g and h The SEM images of uFTEs according to with and without the tri-system integration, respectively. The respective insets are the corresponding magnified SEM images, illustrating ZnONP-poor (purple) and -rich (blue) surfaces, respectively. i and j, The AFM height profiles at the top-surface of the substrate-integrated uFTEs according to with and without the tri-system integration, respectively. The respective yellow lines are for measuring the corresponding average height profiles.
We will express the exceptional operations of the robust substrate-integrated uFTEs with the tri-system integration in terms of four phases of mechanical-electrical-thermal-moisture stability. Figure 1c exhibits real-time monitoring for the operational current density against the simultaneous multi-loading operations of 10,000 times folding cycles with 500 μm of a folding radius at 85 % relative humidity condition. The current density of 8.4 MA cm−2 has been set as the initial current density flowing through the individual nanowires with an averaged diameter of 30 nm38, which surpasses the highest reported current densities at about 5 and 5.3 MA cm−2 for the previously AgNW-ZnO and AgNW-titanium dioxide (TiO2) based electrodes, respectively, so far according to our literature reviews25,39. All the tested uFTEs were prepared on the supporting cPI with a thickness of 10 μm as a carrier film for the dynamic fatigue tests. From the results, the unstable current density of the optimized uFTEs without the tri-system integration was confirmed with a gradual decrease and they eventually exhibited breakdown at the folding cycle of 4-5 K times. In contrast, the robust uFTEs with the tri-system integration show remarkable improvement with an insignificant current density variation of less than 5 % under the same operation. Apparently, the improvement could be partially ascribed to the respective ZnONP-ZnONP and AgNW-AgNW interface integrations of the tri-system. The most difficult part of the technology is to optimize AgNW-ZnONP interfacical interaction in order to complete the tri-system integration. First of all, to clarify the reason for the mechanical degradation regarding the non-adhesive AgNW-ZnONP interface, it is proven that many torn surfaces caused by ruptures along the weak interfaces between AgNWs and the adjacent ZnONP matrix were generated at the folded area with a radius of 250 μm (Figure S6b). On the other hand, the robust uFTEs with the tri-system integration can preserve the firm surface in the same mechanical folding condition (Figure S6c). On top of that, in conjunction with applying the mechanical folding with a folding radius of 500 μm, the escalated maximum temperatures (T) at the most folded edge of the uFTEs with different current densities were measured by an infrared thermometer (Figure S6d). Generally, the results show that the detected maximum T values from the uFTEs with the tri-system integration are always reduced at the same current density as compared to the ones without the tri-system integration. For instance, the T of 116 °C at 8.4 MA cm−2 was measured from the robust uFTEs with the tri-system integration but the ones without the tri-system integration reached the higher T of 174 °C at the same current density. The difference between the two measured temperatures implies the critical information that even though Ag nanonets are combined with ZnONPs matrix with the average 5 nm diameters of ZnONPs filling very fine gaps, there is still an inefficient heat-dissipating between AgNWs and ZnONPs at the interface unless direct adhesive binding is involved (Figure S7), which is consistent with the phenomenon of the discontinuous aggregation of titanium oxide nanoparticles to AgNWs with weak attachments23. To provide different detailed results into the degradation, the uFTEs were tested under the continuous current bias without mechanical stress/strain as shown in Figure S8. Moreover, the high joule heating of Ag nanonets exposed to the high humidity due to the detachment from the ZnONP matrix will further accelerate the degradation of AgNWs by the corrosive reactions40. Eventually, our results show that the reason for the broken uFTEs without the tri-system integration after the multi-loading fatigue test is caused by the complex mechanical-electrical-thermal-moisture effects on the physically weak AgNW-ZnONP interface system, resulting in fatal damages on the most folded area (Figure S9). A trend of sequential quantum drops in operational current density was the direct evidence of the most serious annihilation when the absence of the tri-system integration (Figure S10), meaning that groups of AgNW networks in the nanocomposite electrode were broken down sequentially due to the respective regional hotspot propagations under electrical stress41. In addition, we investigated the thermal stress on both uFTEs and cPI by characterizing the resistance changes of the uFTEs and the SEM images for surface morphology evolution of cPI substrates with elevated temperatures, respectively (Figure S11 and S12). It was found that the iSPM-modified uFTEs showed less resistance change with temperature particularly when the temperature was higher than 150 °C as compared to the unmodified ones. Yet, there is a concern that after the PVP removals from the AgNW networks, the air exposure could potentially be harmful. To confirm it, the iSPM-treated uFTEs with of PVP removal were placed in an ambient environment for 35 days. Interestingly, the uFTEs with of PVP removal showed stable resistance which could be ascribed to both the passivation by the ZnONPs matrix and fluorinated cPI despite the PVP removals (Figure S13a). Consequently, the <0.5 μm-thick cPI substrate-integrated uFTEs have been successfully demonstrated and achieved the sturdy composite electrode of AgNWs and ZnONPs with the in-situ tri-system integration for realizing the mechanical-electrical-thermal-moisture (i.e., four phases) stability for the robust and long stability in promising solution-processed uFTEs.
Extremely morphological smooth uFTEs with the tri-system integration
We first exhibit the discernible evidence of the tri-system integration before and after the iSPM on the respective AgNWs, ZnONPs, and composite of AgNWs and ZnONPs, by investigating the transmission electron microscopy (TEM) images (Fig. 2a–f). Please note that the respective samplings of Fig. 2a–f were made by transferring to the copper grid substrates of TEM from the corresponding samples on glass substrates.
As for the as-prepared AgNWs prior to the iSPM, the cross-junction shows the distinct junction of the Ag-PVP-Ag constitution (Fig. 2a). On the other hand, the one after the iSPM exhibits the direct Ag-Ag junction at the cross-junction due to the absence of the capping agents (Fig. 2b), which stands for better current flows through the Ag nanonets with the extended electrical pathways. A number of the better conductive cross-junctions can be found with direct contacts and physical mergings between the Ag nanonets (Figure S2d). Regarding the ZnONP morphology after the iSPM (Fig. 2d), it is discovered that ZnONPs can lead to physical merges among the ZnONPs, as compared to the ones with the non-interactions between many separate ZnONPs before the iSPM (Fig. 2c). The inserted diffraction pattern of Fig. 2d extracted from the corresponding TEM image explicates the particular contribution of the iSPM on crystallinities of the merged ZnONPs cannot be found as compared to the ones before the iSPM (inset of Fig. 2c)42. The impact on the chemical composition was negligible confirmed with no particular chemical composition change before and after the iSPM by X-ray photoelectron spectroscopy (XPS) measurements (Figure S14). The observed change in the ZnONPs geometries can also support the higher densification degree of the ZnONP matrix as an increasing number of the iSPM (Figure S15). The densely packed ZnONP matrix resulted from the merging ZnONPs after the iSPM can provide uFTEs with more high heat transfer efficiency due to the enlarged contact areas between ZnONPs (Figure S2e) rather than the loosely packed matrix with air (Figure S2b)43.
In the case of the composite of AgNWs and ZnONPs before the iSPM, there is the obvious presence of the insulating organic PVP ligand layer sandwiched between AgNWs and ZnONPs, causing the generation of the resistive Ag-PVP-ZnO connections (Fig. 2e). The PVP barrier physically blocked the contact between AgNWs and ZnONPs, leading to weak adhesions along the AgNWs at the interface (Figure S16a). Very differently, there are various atomic arrangement cases of many ZnONPs continuously adherent to the surfaces of an AgNW in the absence of the blocking layer after the iSPM (Fig. 2f). Many well-matched lattice fringe alignments can be easily found between (i) Ag (111) and ZnO (002), (ii) Ag (200) and ZnO (101), etc. Additionally, a number of the adhesive interfaces were shown (Figure S16b), verifying that the iSPM can create attractive adhesive interactions between the cleaned surface of AgNWs and the flexible geometric shapes of ZnONPs. These evolutions can also contribute to understanding the more closely packed nanocomposite electrode after the iSPM (Figure S2f) evolved from the case with many protruding AgNWs before the iSPM (Figure S2c). In fact, a large amount of additional ZnONPs could provide the AgNW networks with a well-coverage. However, unless there is a strong attractive adhesion of ZnONPs to AgNWs, the temporary cover by the additional ZnONPs could easily detach from AgNWs whenever applied to dynamic operations. The adhesive interface between AgNWs and ZnONPs can not only explain the mechanically robust nano-adhesion but also the efficient heat-dissipating mechanism from AgNWs to the directly contacted ZnONPs for the use of uFTEs. The representative fast Fourier transform (FFT) images are extracted from the corresponding (i), (ii), and (iii) dotted boxes that indicate lattice structures of Ag (200), ZnO (002), and both of them inclusive, respectively (right of Fig. 2f). Based on the referred from the experimental TEM images, an atomistic pattern of the Ag (111) and ZnO (0001) surface is presented to provide the direct support of chemically seamless integrations between these two constitutional materials with the minimal lattice mismatch (Figure S17a)44. The XPS results can well explain the replacement of PVP with ZnONP in the direct bonding with Ag (Figure S17b)45.
The smooth morphology is well-known as one of the most important requirements in the compatibility with multi-layered electronics. The tri-system integration via the iSPM significantly contributed to semi-embedding the smooth surface roughness of the nanocomposite electrode in the cPI matrix (Fig. 2h) as compared to the comparison sample without the tri-system integration (Fig. 2g). The optical microscopic images in dark and phase modes for surface topography of the substrate-integrated uFTEs are provided for comprehensive evaluation (Figure S18). The comparison surface exhibited very rough topography with over 4 nm of RMS and 20 nm of PtV roughness, respectively, (Fig. 2i). The rough surface could be formed between complex situations including the rigid supporting substrate, nanocomposite electrode, and cPI matrix, respectively. For instance, the disconnected ZnONP matrix and weak interaction with Ag nanonets can locally tear apart the nanocomposite electrode. In addition, the PAA liquid precursor can be able to permeate into the loosely packed gaps between AgNWs and ZnONPs within the nanocomposite electrode without the iSPM, which hinders the densification of the nanocomposite electrode (Figure S2c). The rough surface can be frequently observed due to the weak binding to both cPI and AgNWs in terms of ripping the ZnONP matrix away from the cPI substrate and protruding AgNWs to the top ZnONP-poor surface (Fig. 2g). A similar phenomenon can consistently be observed in the previously reported work with no tri-system integration26. In contrast, the uFTEs with the tri-system integration showed an extremely smooth surface topography with <1 nm of RMS and <5 nm of PtV roughness (Fig. 2j). This can be explained by both the tight-bound AgNW-ZnONP composite electrode to the cPI matrix and the strongly adhesive nanocomposite being completely delaminated with ZnONP-rich surface (Fig. 2h). To establish the pragmatic viability of the developed uFTEs, the demonstration in real optoelectronic devices such as organic photovoltaics and its comprehensive study are provided in the Supporting Information (Figure S19). As a consequence, the unprecedentedly strong adhesion between AgNWs and ZnONPs has been experimentally demonstrated. The successfully semi-embedded nanocomposite electrode in the cPI matrix showed the extremely smooth surface roughness promoted by the tri-system integration, which played a decisive role in real optoelectronic devices.
Unveiling the viscous liquid-like behaviour of ZnONPs into AgNWs
Observing the merging dynamics of dissimilar nanomaterials at the heterogeneous interface plays an important role in understanding the formation of complex interface systems46,47,48. In the present section, via the iSPM, we discuss the finding of both the in-situ coalescence dynamics among solid-state ZnONPs and the in-situ wetting dynamics into the surface of AgNWs, which will be potentially useful as a general guidance for durable design in various combinations between metal nanowires and transparent metal-oxide-semiconductor nanoparticles. We noticed that the observations of the in-situ dynamics were allowed by the sampling of TEM with natural drying after the iSPM directly on the copper grid substrate but without annealing. The iSPM could be able to provide ZnONPs with the releasing energy from the deep potential-energy well by substrates in TEM49. The coalescence process may be accelerated by the electron beam irradiation in TEM but the limited influence of the irradiation is discussed in Supporting Information.
Figure 3a exhibits the in-situ evolution as the coalescence process between a pair of ZnONPs from the initial stage that the pair is apart from each other (Supplementary Movie 1). The time at 0 s has been set at a necking event between the collided pair after the initial stage. Notedly, the time set for the necking event might not be precise due to the recording frame rate limit. It can be clearly seen that the iSPM enables the in-situ coalescence dynamics of the pair of ZnONPs to proceed toward the energetically minimized atomic rearrangement and ended up with a certain round-shaped geometric. They evolved to a bigger size with about 4 nm of diameter than that of the original ZnONP individuals of the initial stage with a diameter of about 3 nm, respectively, in which the evolution preserves the total surface area within error ranges of 5%. Nonetheless, during the evolution, we noted that the crystallinity of one of the ZnONP pair at the initial stage before the collision was (002) and the crystallographic orientation of the merged ZnONP at 50 s also maintained as (002) as well. This phenomenon is similar to the recently reported liquid-like behaviour of metal nanoparticles discussing that the diffusion of atoms on free surfaces quickly reconstructed the original geometry of metal crystal structures50, however, our finding here is in relatively more rigid ZnONPs. The in-situ evolution as the coalescence process was not just only observed in a single pair but also in multiple ZnONPs at the same time (Figure S20). Please note that the as-prepared ZnONPs before the iSPM retained stably rigid shapes and could not observe the dynamics (Figure S21).
a Sequential TEM images of in-situ dynamics of the representative of two ZnONP pair. After the initial stage that the two ZnONPs are away from each other, and the necking has been set as the standard to start counting at 0 s. The evolving boundary is marked with yellow color. b In-situ sequential TEM images of a ZnONP with 5 nm of R. The time was started to measure until 110 s from t0 (e.g. t110 = t0 + 110 s), in which the wetting dynamics were captured from the almost beginning. The t0 was set on the coalescence event of the ZnONP to the AgNW. The evolving boundary of the ZnONP with R of 7 nm is marked with red color. c, d The respective TEM images of ZnONPs being wetted on the surface of AgNWs measured after 70 s for the estimated 6 nm of R and 90 s for the estimated 7 nm of R from t0, respectively, in which t0 is the standard to start counting t (e.g. t70 = t0 + 70 s and t90 = t0 + 90 s). The measurement started to count from the middle of the wetting process of ZnONPs on the surface of AgNWs. The evolving boundary is marked with green and blue for 5 and 6 of R in the respective ZnONPs. e The plot of r/R ~ (σt/Rμt) interpreting that spreading dynamics of different R of ZnONPs for 5 (red), 6 (green), and 7 (blue) nm follows a viscous liquid-like behaviour tha the temporal evolution of t r/R is proportional to ~t1/2.
As for the adhesive wetting dynamics between AgNWs and ZnONPs, the existing random coalescence approach of ZnONPs can reach out to the adjacent cleaned surface of AgNWs to initiate the in-situ wetting dynamics for forming the adhesive AgNW-ZnONP interface. Figure 3b shows that a ZnONP with a radius (R) of 5 nm as a representing case collides with the surface of an AgNW at a certain moment, followed by gradually spreading out on the surface. To offer temporal TEM images, time was started to measure from the arbitrary contact event t0 between the AgNW and the ZnONP (i.e. t110 = t0 + 110 s in the unit of second). Impressively, the ZnONP showed a liquid-like behaviour, in which the spreading process until t110 can be almost spread out without discrete/abrupt morphological change (Supplementary Movie 2). The same mechanism was found with the other ZnONPs with 6 and 7 nm of the respective estimated R and depicted in Fig. 3c and d, respectively, in which time for the respective temporal TEM images were counted from the middle of the in-situ wetting dynamics.
The liquid-like wetting process of the viscous ZnONPs enabled by the iSPM on the clean surface of AgNWs is demonstrated by the relation of r/R ~ (σt/Rμf) as illustrated in Fig. 3e, where r is the spreading radius, σ is the surface energy and μf is the friction coefficient51. In this analysis, μf is chosen as 0.1 as an intrinsic material property of the surface and σ varies inversely as the size of ZnONP decreases52,53. Coherently, the collapsed lines of Fig. 3e are matched as previously proposed by de Gennes, which is regarded as a viscous liquid-like behaviour that r/R should be proportional to ~t1/254. Notedly, it is different from the ideal liquid as described by Tanner’s law with ~t1/1055. Consequently, the adhesive solid-state ZnONPs acting like a viscous liquid can intensively get wetted into the cleaned surface of AgNWs.
Discussion
The robust operational stability of the entirely solution-processed substrate-integrated uFTEs with greatly thin <0.5 μm has been demonstrated with the tri-system integration within the composite electrode of Ag nanonets and ZnONP matrix. The remarkable mechanical-electrical-thermal-moisture stability is featured by small current density fluctuation of less than 5 % against the simultaneous multi-loading fatigue test (10,000 cyclic mechanical folding with folding radius of 0.5 mm, continuous electrical bias of 8.4 MA cm−2, and relative humid condition of 85 %). This robustness is attributed to the iSPM approach for achieving the unique tri-system integration of (i) AgNW-AgNW, (ii) ZnONP-ZnONP, and (iii) AgNW-ZnONP at the same time. The tri-system integration via the iSPM enabled by (i) in-situ removals of organic residuals from AgNWs, (ii) flexible geometric shapes of ZnONPs under the course of in-situ coalescence process between themselves, and (iii) the liquid-like behaviour of solid-state ZnONPs in-situ wetting onto AgNWs. Meanwhile, the nanocomposite electrode was firmly semi-embedded in the plastic matrix to form the substrate-integrated uFTEs via fully solution-processed means. As a good uFTE candidate harnessing solution-process, the excellent electrical/optical properties when including both electrode and substrate components (7.5 Ω sq.−1 of sheet resistance and >88 % of diffused transmittance in the region of wavelengths 400-900 nm) and extremely smooth surface topography (within <1 nm of RMS and <5 nm of PtV roughness) have been demonstrated. The flexible OSCs on the developed uFTEs with and without the tri-system integration were fabricated and the maximum PCE of 13.66 % from the one without the iSPM has been substantially improved to that of 16.51 % for ones on the iSPM-modified uFTEs. Consequently, the strategic design for the lost-cost, solution-processed, and high-throughput uFTEs not only offers a comprehensive understanding of the formation mechanisms of the tri-system integration but also advances the integration of hybrid combinations of nano-materials for employing uFTEs in real device applications.
Methods
Materials and chemicals
Ethanol (AnalaR NORMAPUR(R) ACS, ≥99.8%, VWR Chemicals) was used in this work. AgNWs dispersed in ethanol were bought from ACS Material with a diameter of 30 nm and length of 100-300 μm. ZnONPs used in this work were synthesized according to the previous work42. The concentration of 3 mg mL−1 of AgNWs and 15 mg mL−1 of ZnONPs dispersed in ethanol, respectively, were used for the nanocomposite electrodes. As for the preparation of the iSPM, NaBH4 powder (ReagentPlus(R), 99%, Sigma-Aldrich) was dissolved for 0.1 M of an optimized molar concentration in a mixture of deionized water and ethanol with an 8:2 ratio. The fluorinated cPI used in this work was synthesized according to the previous work20. As for the (poly(amic acid) (PAA)) as the viscous precursor for achieving cPI, 2,2’-bis(trifluoromethyl)−4,4’-diaminobiphenyl (TFDB) and 4,4’-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) with multi-bulky pendant trifluoromethyl groups were bought from J&K Scientific LLC. The PAA of the 6FDA:TFDB (1:1) co-polymer structure in a solvent of Dimethylacetamide (DMA) (SuperDry, J&K Scientific LLC). BHT was prepared by mildly stirring for 6 hours.
Fabrication of the entirely solution-processed uFTEs
Step 1: Preparation of the as-prepared AgNWs-ZnONPs electrodes.
Rigid supporting substrates of 4 cm2 with smooth surface roughness (e.g. silicon wafer, glass, etc.) were cleaned using deionized water, acetone, and ethanol. The cleaned substrates were then treated with ultraviolet-ozone (UVO) for 15 minutes. The AgNWs networks were then formed on the treated substrates using Mayer rod coating or spin-coating. On top of the as-prepared AgNW networks, the ZnONPs was deposited by the same means.
Step 2: The iSPM on the as-prepared AgNWs-ZnONPs electrodes.
The as-prepared AgNWs-ZnONPs samples were treated by dipping them in the iSPM for 30 s. After that, the samples were washed with ethanol and deionized water. This iSPM process was repeated three times to get the final nanocomposite electrodes with the tri-system integration.
Step 3: Formation of cPI on iSPM-treated AgNW-ZnONP samples.
On the iSPM-treated AgNW-ZnONP samples, the PAA precursor solution of the cPI was coated to cover the whole surface by Mayer bar coating. Right after the PAA coating process, the samples will undergo the imidization processing by annealing with a gradual increase of temperature to 200 °C to form cPI. After the imidization processing temperatures, the samples were cooled down to room temperature.
Step 4: Peeling-off process.
After cooling down to room temperature, the substrate-integrated AgNW-ZnONP sample in the cPI were submerged in deionized water and then kept for the natural delamination for the formation of the electrode integrated with cPI substrate.
Fabrication of the flexible organic solar cell devices
Two different pairs of donor and acceptor materials of P3HT:PC61BM and D18-Cl:N3 as organic active layers were purchased from Solarmer Materials Inc. 1,2-dichlorobenzene (DCB) anhydrous and chloroform (CF) ACS used for active layer solvents were purchased from Sigma-Aldrich. The solvent 1-butanol for ZnONP solution as an electron transporting layer was bought from J&K Scientific LLC. Polydimethylsiloxane (PDMS) coated on a glass substrate was used as an adhesive film so that the peeled uFTEs were stuck to glass/PDMS as rigid supporting adhesive substrates for the flexible OSC device fabrication. All of the OSC devices with two different bulk heterojunction active layers were inverted devices with structures of cathode / ZnONPs as electron transport layer / active layers / evaporated MoO3 as hole transport layer / evaporated Ag as anode. The ITO-coated glass substrates of the reference OSCs were cleaned using sequential ultrasonication in deionized water, acetone, and ethanol for 15 minutes, respectively. Glass/ITO was treated by the UVO for 15 minutes. The ZnONPs solution with a concentration of 15 mg mL−1 in 1-butanol as electron-transporting layers were deposited on both the uFTEs/PDMS/glass and ITO/glass by spin-coating at 3000 rpm for 40 s, followed by drying at 80 °C for 5 minutes. Two different kinds of organic bulk heterojunction active layers with P3HT:PC61BM (1:1, 20 mg mL−1 in DCB) and D18-Cl:N3 (1:1.2, 12 mg mL−1 in CF) were prepared. After transferring samples into the nitrogen-filled glovebox, these two organic active layers were spin-coated on the ZnONPs layers, followed by post-treatment of 1 h slow growth and then annealing at 130 °C for 10 minutes for the P3HT:PC61BM film (static dispense at 670 rpm for 40 s, 30 uL), and annealing at 100 °C for 10 minutes for the D18-Cl:N3 film (dynamic dispense at 2500 rpm for 40 s, 17 uL). The devices were then finished by thermal evaporation of 7 nm MoO3 and 100 nm Ag under a vacuum of 2 × 10−6 Torr in the high-vacuum deposition system with a device area of 0.08 cm2.
Characterization and measurements
The surface topography of the nanocomposites and substrate-integrated uFTEs was examined by SEM (Hitachi S-4800), TEM (Philips Tecnai G2 20 S-TWIN and Thermo Scientific Talos F200X G2), Optical microscope (Ti-2E, Nikon), and tapping mode AFM (NT-MDT NTEGRA), respectively. Temperature elevated in the uFTEs under electrical bias was measured by an IR thermometer of Fluke. XPS spectra was measured in the ultrahigh vacuum environment using Physical Electronics PHI 5802 with a monochromatic Al Kα X-ray source. The work functions of the ZnONPs films were measured by a SKP5050 scanning KPM (KP Technology Ltd.). The UPS was characterized by using a He discharged lamp (He I 21.22 eV, Kratos Axis Ultra DLD). The sheet resistance of the uFTEs was directly measured from a four-point probe approach. The diffused transmission spectra were obtained from a UV/vis. spectrophotometer of Shimadzu. The current density-voltage (J-V) characteristics of all the fabricated OSC devices were measured under a shadow mask with an opening area of 0.05 cm2 using Enlitech AM 1.5 G solar simulator with a light intensity of 100 mW cm−2 calibrated with a standard silicon reference cell and the data was collected using a Keithley 2635 sourcemeter. As for the Mayer bar equipment, the laboratory-type high-precision printing and coating machine with the built-in Mayer bar (from Peking University Yangtze Delta Institute of Optoelectronics) was used for the AgNW, ZnONP, and PAA coatings. The experimental adjustments have been carried out to both coating speed with 10-50 mm s−1 and height distance of less than 100 μm between the bar and the substrates depending on the target thickness and the viscosity of different materials/solutions. Ultraviolet-ozone (UVO) cleaner with a wavelength of 253.7 nm (Jelight Company, Inc.). As for the folding task, we have used home-made folding machines with linear motion guides motorized with stepper motors. Regarding the software, we used the Arduino home-made program to adjust the linear distance with the minimum value of 1 mm. The detailed procedure of the folding task can be divided by 5 steps. Step (i): The uFTE samples were loaded to the sample holders with one end of the electrode fixed on the non-moving ground and the other end to the linear movement ground. Step (ii): For the measurement of the electrical properties, the uFTE samples were connected using the 3 M electrical contact clips. Step (iii): The initial distance between the two ends of the electrode was set in the program to make the fixed uFTE sample straightened out. Step (iv): The moving distance and the number of forward/backward cycles were set in the program for running the folding test. Step (v): The uFTE samples were un-loaded after the folding test. A recorded video of the mechanical folding test of the OSC device is provided (Supplementary Movie 3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available in the main text or the supplementary materials. Source data generated in this study are provided in the Source Data file. Source data are provided with this paper.
References
White, S. M. et al. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photon. 7, 811–816 (2013).
Kim, D. et al. Epidermal electronics. Science 333, 838–843 (2011).
Someya, T. & Amagai, M. Toward a new generation of smart skins. Nat. Biotechnol. 37, 382–388 (2019).
Rich, S. I., Wood, R. J. & Majidi, C. Untethered soft robotics. Nat. Electron. 1, 1020112 (2018).
Kaltenbrunner, M. et al. Flexible high power-per-weight perovskite solar cells with chromium oxide-metal contacts for improved stability in air. Nat. Mater. 14, 1032–1039 (2015).
Kaltenbrunner, M. et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 499, 458–463 (2013).
Park, S. et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 561, 516–521 (2018).
Kumar, A. & Zhou, C. The race to replace Tin-Doped Indium oxide: which material will win? ACS Nano 4, 11–14 (2010).
Pasquarelli, R. M., Ginley, D. S. & O’Hayre, R. Solution processing of transparent conductors: from flask to film. Chem. Soc. Rev. 40, 5406–5441 (2011).
Meng, L. et al. Solution-processed flexible transparent electrodes for printable electronics. ACS Nano 17, 4180–4192 (2023).
Gordon, R. G. Criteria for choosing transparent conductors. MRS Bull. 25, 52–57 (2000).
Ellmer, K. Past achievements & future challenges in the development of optically transparent electrodes. Nat. Photon. 6, 809–817 (2012).
Lee, H. J. et al. Haze-suppressed transparent electrodes using IZO/Ag/IZO Nanomesh for highly flexible and efficient blue organic light-emitting diodes. Adv. Opt. Mater. 9, 2002010 (2021).
Takemoto, A. et al. Fully transparent, ultrathin flexible organic electrochemical transistors with additive integration for bioelectronic applications. Adv. Sci. 10, 2204746 (2023).
Kim, N. et al. Work-function-tunable metal-oxide mesh electrode and novel soluble bipolar host for high-performance solution-processed flexible TADF-OLED. Nano Energy 105, 108028 (2023).
Xu, Y. et al. Recent progress of electrode materials for flexible Perovskite solar cells. Nanomicro Lett. 14, 117 (2022).
Kang, D. Y. et al. Dopant-tunable ultrathin transparent conductive oxides for efficient energy conversion devices. Nanomicro Lett. 13, 211 (2021).
Yang, Y. et al. High-performance organic light-emitting diodes using ITO anodes grown on plastic by room-temperature ion-assisted deposition. Adv. Mater. 16, 21–324 (2004).
Xu, F. & Zhu, Y. Highly conductive and stretchable silver nanowire conductors. Adv. Mater. 24, 5117–5122 (2012).
Kim, J. et al. High performance flexible transparent electrode via one-step multifunctional treatment for ag nanonetwork composites semi-embedded in low-temperature-processed substrate for highly performed organic photovoltaics. Adv. Energy Mater. 10, 1903919 (2020).
Ghosh, D. S., Chen, T. L., Mkhitaryan, V., Formica, N. & Pruneri, V. Solution processed metallic nanowire based transparent electrode capped with a multifunctional layer. Appl. Phys. Lett. 102, 221111 (2013).
Kim, A., Won, Y., Woo, K., Kim, C. & Moon, J. Highly transparent low resistance ZnO/Ag nanowire/ZnO composite electrode for thin film solar cells. ACS Nano 7, 1081–1091 (2013).
Zhu, R. et al. Fused silver nanowires with metal oxide nanoparticles and organic polymers for highly transparent conductors. ACS Nano 5, 9877–9882 (2011).
Zilberberg, K. et al. Highly robust indium-free transparent conductive electrodes based on composites of silver nanowires and conductive metal oxides. Adv. Funct. Mater. 24, 1671–1678 (2014).
Chen, D. et al. Mitigation of electrical failure of silver nanowires under current flow and the application for long lifetime organic light-emitting diodes. Adv. Electron. Mater. 2, 1600167 (2016).
Chen, D. et al. Thermally stable silver nanowire–polyimide transparent electrode based on atomic layer deposition of zinc oxide on silver nanowires. Adv. Funct. Mater. 25, 7512–7520 (2015).
Park, J. H. et al. Flash-induced self-limited plasmonic welding of silver nanowire network for transparent flexible energy harvester. Adv. Mater. 29, 1603473 (2016).
Guo, J. et al. Cold sintered ceramic nanocomposites of 2D MXene and zinc oxide. Adv. Mater. 30, 1801846 (2018).
Yang, C. et al. Silver nanowires: from scalable synthesis to recyclable foldable electronics. Adv. Mater. 23, 3052–3056 (2011).
Boles, M. A., Ling, D., Hyeon, T. & Talapin, D. V. The surface science of nanocrystals. Nat. Mater. 15, 141–153 (2016).
Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009).
Garnett, E. C. et al. Self-limited plasmonic welding of silver nanowire junctions. Nat. Mater. 11, 241–249 (2012).
Liu, L. et al. Connecting energetics to dynamics in particle growth by oriented attachment using real-time observations. Nat. Commun. 11, 1045 (2020).
Wang, H. et al. Strong metal–support interactions on gold nanoparticle catalysts achieved through Le Chatelier’s principle. Nat. Catal. 4, 418–424 (2021).
Frey, H., Beck, A., Huang, X., van Bokhoven, J. A. & Willinger, M. G. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science 376, 982–987 (2022).
Ge, Y. et al. Direct room temperature welding and chemical protection of silver nanowire thin films for high performance transparent conductors. J. Am. Chem. Soc. 140, 193–199 (2018).
Matsuura, T., Hasuda, Y., Hishi, S. & Yamada, N. Polyimide derived from 2,2’-bis(trifluoromethyl)−4,4’-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2’-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 24, 5001–5005 (1991).
Resende, J. et al. Time of failure of metallic nanowire networks under coupled electrical and thermal stress: implications for transparent electrodes lifetime. ACS Appl. Nano Mater. 5, 2102–2112 (2022).
Khan, A. et al. Understanding the mechanisms leading to failure in metallic nanowire-based transparent heaters, and solution for stability enhancement. Nanotechnol 28, 055709 (2017).
Patil, J. J. et al. Failing forward: stability of transparent electrodes based on metal nanowire networks. Adv. Mater. 33, 2004356 (2021).
Thomas, S. et al. Electrical mapping of silver nanowire networks: a versatile tool for imaging network homogeneity and degradation dynamics during failure. ACS Nano 12, 4648–4659 (2018).
Beek, W. J. E., Wienk, M. M., Kemerink, M., Yang, X. & Janssen, R. A. J. Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells. J. Phys. Chem. B 109, 9505–9516 (2005).
Swartz, E. T. Thermal boundary resistance. Rev. Mod. Phys. 61, 605–668 (1989).
Lin, Z. & Bristowe, P. D. Microscopic characteristics of the Ag(111)/ZnO(0001) interface present in optical coatings. Phys. Rev. B 75, 205423 (2007).
Moulder, J. F., Stickle, W. F., Sobol, P. E. & Bomben, K. D. Handbook of X-Ray Photoelectron Spectroscopy. (Perkin-Elmer Corporation, Physical Electronics Division, Eden Prairie, Minnesota, 1992).
Grouchko, M. et al. Merging of metal nanoparticles driven by selective wettability of silver nanostructures. Nat. Commun. 5, 2994 (2014).
Zhao, G. et al. Stable ultrathin partially oxidized copper film electrode for highly efficient flexible solar cells. Nat. Commun. 6, 8830 (2015).
Aabdin, Z. et al. Bonding pathways of gold nanocrystals in solution. Nano Lett. 14, 6639 (2014).
Ajayan, P. M. & Marks, L. D. Experimental evidence for quasimelting in small particles. Phys. Rev. Lett. 63, 279–282 (1989).
Sun, J. et al. Liquid-like pseudoelasticity of sub-10-nm crystalline silver particles. Nat. Mater. 13, 1007–1012 (2014).
Carlson, A., Bellani, G. & Amberg, G. Universality in dynamic wetting dominated by contact-line friction. Phys. Rev. E 85, 045302(R) (2012).
Chai, Z., Lu, X. & He, D. Friction mechanism of zinc oxide films prepared by atomic layer deposition. RSC Adv. 5, 55411–55418 (2015).
Koster, R. S., Fang, C. M., Dijkstra, M., van Blaaderen, A. & van Huis, M. A. Stabilization of rock salt ZnO nanocrystals by low-energy surfaces and mg additions: a first-principles study. J. Phys. Chem. C. 119, 5648–5656 (2015).
de Gennes, P. G. Wetting: statics and dynamics. Rev. Mod. Phys. 57, 827–863 (1985).
Tanner, L. H. The spreading of silicone oil drops on horizontal surfaces. J. Phys. D. 12, 1473 (1979).
Acknowledgements
The work was supported by University Grant Council of the University of Hong Kong Grants (Grant# 202011159254 and 202111159113), General Research Fund (Grant# 17201819, 17211220, 17200021, and 17200823) and Collaborative Research Fund (Grant no. C7035-20G) from the Research Grants Council (RGC), as well as Innovation and Technology Fund from Innovation and Technology Commission (Grant No. ITS/277121FP and MRP/040/21X) of Hong Kong Special Administrative Region, China. The authors acknowledge Guanding Mei and Rosita Lau for help in technical drawing and Kevin Tsia and Kelvin Lee for the measurements of optical microscopes.
Author information
Authors and Affiliations
Contributions
K.J.J., C.W.C.H. and S.H. conceived the idea. K.J.J. conducted the fabrication of ultrathin flexible transparent electrodes and flexible organic solar cell devices and performed the characterization of liquid-like behaviour between silver nanowires and zinc oxide nanoparticles. S.K. and Z.J. performed the stability measurement of the multi-loading fatigue tests on the uFTEs. H.X., S.A. and Z.H. performed measurement of SEM, XPS, UPS, and AFM measurement and analysed results. C.W.C.H. coordinated the work. K.J.J. finished the original draft of the manuscript and all authors discussed the results and revised and approved on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.J., Shuji, K., Zheng, J. et al. Tri-system integration in metal-oxide nanocomposites via in-situ solution-processed method for ultrathin flexible transparent electrodes. Nat Commun 15, 2070 (2024). https://doi.org/10.1038/s41467-024-46243-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46243-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.