Abstract
Glutaredoxins catalyze the reduction of disulfides and are key players in redox metabolism and regulation. While important insights were gained regarding the reduction of glutathione disulfide substrates, the mechanism of non-glutathione disulfide reduction remains highly debated. Here we determined the rate constants for the individual redox reactions between PfGrx, a model glutaredoxin from Plasmodium falciparum, and redox-sensitive green fluorescent protein 2 (roGFP2), a model substrate and versatile tool for intracellular redox measurements. We show that the PfGrx-catalyzed oxidation of roGFP2 occurs via a monothiol mechanism and is up to three orders of magnitude faster when roGFP2 and PfGrx are fused. The oxidation kinetics of roGFP2-PfGrx fusion constructs reflect at physiological GSSG concentrations the glutathionylation kinetics of the glutaredoxin moiety, thus allowing intracellular structure-function analysis. Reduction of the roGFP2 disulfide occurs via a monothiol mechanism and involves a ternary complex with GSH and PfGrx. Our study provides the mechanistic basis for understanding roGFP2 redox sensing and challenges previous mechanisms for protein disulfide reduction.
Similar content being viewed by others
Introduction
Class I glutaredoxins (Grx) are glutathione-dependent thiol:disulfide oxidoreductases that reversibly convert a variety of low or high molecular weight disulfide substrates using the cysteinyl-containing tripeptide glutathione (GSH) as a reducing agent (EC 1.8.4.1–4)1,2,3,4,5,6,7,8. The disulfide substrates of glutaredoxins can be subdivided into two major groups. The first group comprises glutathione disulfide substrates (GSSR) that are reduced by one molecule of GSH, yielding a thiol (RSH) and glutathione disulfide (GSSG) as products (Fig. 1a)5,6,7,8. Model and physiological GSSR substrates are, for example, l-cysteine-glutathione disulfide (GSSCys)9,10,11,12,13,14,15, coenzyme A-glutathione disulfide3,16, as well as S-glutathionylated serum albumin9,11,17,18,19,20, sirtuin21, sulfiredoxin22, and peroxiredoxins23,24,25,26,27,28,29. The second group comprises non-glutathione disulfide substrates (RS2 or RSSR’) that are reduced by two molecules of GSH, yielding one dithiol product or two monothiol products (R(SH)2 or RSH + R’SH) and GSSG (Fig. 1a)6,7,8. Model and physiological RS2 and RSSR’ substrates are, for example, bis(2-hydroxyethyl)disulfide (HEDS)1,3,12,14,30, l-cystine1,3, redox-sensitive yellow or green fluorescent protein 2 (rxYFP or roGFP2)14,15,31,32,33,34,35, insulin36,37, collapsin response mediator protein 238,39, the transcription factor OxyR40, 3′-phosphoadenosine 5′-phosphosulfate reductase (PR)41,42, methionine sulfoxide reductase (MSR)34,43,44, and ribonucleotide reductase (RNR)2,34,45,46,47. Glutaredoxins are therefore key enzymes for redox catalysis and physiological processes such as DNA synthesis, signal transduction, and organ development2,21,34,38,40,47,48. While important mechanistic insights were gained to decipher the glutaredoxin-catalyzed reduction of glutathione disulfide substrates, there are still major gaps in our understanding of the enzyme mechanism regarding the reduction of non-glutathione disulfide substrates.
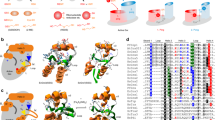
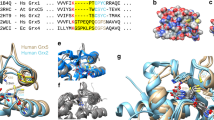
a Glutaredoxins reduce glutathione- and non-glutathione disulfide substrates using one or two molecules of GSH (shown at the top and bottom, respectively). b Glutaredoxins use a monothiol ping-pong mechanism for the reduction of glutathione disulfide substrates. The glutathione moieties of GSSR and GSH are not identical and have to interact with different protein areas of the reduced and glutathionylated enzyme. c The reduction of selected non-glutathione disulfide substrates via a dithiol mechanism requires a second active-site cysteinyl residue. d NMR-structures of the mixed disulfide between EcGrx1 and a peptide of EcRNR, EcGrx1 disulfide, glutathionylated EcGrx1, and reduced EcGrx1 in accordance with the dithiol mechanism (depicted counterclockwise). The second cysteinyl residue was removed to stabilize the mixed disulfide species. Selected residues that were previously shown to interact with glutathione or to affect catalysis in related enzymes are highlighted. e Three alternative monothiol mechanisms for the reduction of non-glutathione disulfide substrates have been suggested (see text for details). All shown reactions are reversible.
Many class I glutaredoxins are so-called dithiol enzymes because they have two cysteinyl residues in a C[P/S]Y[C/S] motif at their active site. The reversible reduction of GSSR substrates usually requires only the first active-site cysteinyl residue and is therefore catalyzed by a monothiol mechanism with ping-pong bi-bi kinetic patterns (Fig. 1b):7,8,9,10,11,12,15,17,19,30 During the oxidative half-reaction the thiolate group of the reduced glutaredoxin attacks the GSSR disulfide bond, yielding the first product (RSH) and the glutathionylated glutaredoxin Grx(SSG) as an intermediate. The Grx(SSG) intermediate is then reduced by GSH during the reductive half-reaction, yielding the reduced enzyme and GSSG. The glutathione moieties of GSSR and GSH have to interact with different protein areas as a result of the SN2 geometry of both half-reactions of the ping pong bi-bi mechanism (Fig. 1b)7,13,14,15. The 1st glutathione-interaction site, also termed glutathione-scaffold site, is rather well defined and comprises several protein areas that altogether contribute to catalysis by interacting with the glutathione moiety of GSSR7,8,13,14,15,35, therefore explaining the specificity for the γ-glutamyl moiety9,49,50. This interaction site is also occupied by the GS– ligand of iron-sulfur clusters that are bound to class II glutaredoxins7,15,33,51,52,53. The 2nd glutathione-interaction site appears to preferentially interact with the γ-glutamylcysteinyl moiety of GSH and is thought to facilitate the recruitment and activation of GSH as a preferred reducing agent7,11,13,17,50. This interaction site is less defined but comprises the glutathione moiety of Grx(SSG), parts of helix 3, and a conserved lysine/arginine residue that also contributes to the scaffold site14,15. Coupled steady-state kinetic measurements with GSSR and GSH revealed that both half-reactions usually occur with second order rate constants around 105 to 106 M–1 s–1 with the reductive half-reaction being slower for wild-type glutaredoxins11,14,15,17,19.
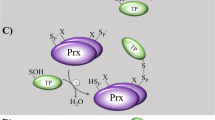
The reversible reduction of RS2 and RSSR’ substrates can be catalyzed by a monothiol and/or dithiol mechanism depending on the substrate and organism5,6,7,8. Numerous artificial and natural monothiol class I glutaredoxins efficiently reduce HEDS or redox-sensitive fluorescent proteins without a second active-site cysteinyl residue12,15,18,26,30,31,33,34,35,54,55,56. Furthermore, monothiol class I glutaredoxins can catalyze all essential glutaredoxin- and thioredoxin-dependent protein disulfide reductions in yeast, including, for example, the reduction of RNR and MSR34. A monothiol mechanism was also reported for the in vitro reduction of mammalian RNR46 and can be also employed, although with a decreased activity, by a Grx-RNR fusion protein from Facklamia ignava47. In contrast, the in vitro reduction of Escherichia coli RNR or PR was shown to require both active-site cysteinyl residues of glutaredoxin 1 (EcGrx1)41,57. The suggested dithiol mechanism for the reduction of EcRNR by EcGrx1 comprises a dithiol-disulfide exchange reaction followed by the stepwise reduction of EcGrx1(S2) and EcGrx1(SSG) by two molecules of GSH (Fig. 1c, d)57,58. According to the Cleland nomenclature the reaction should follow a uni-uni-bi-uni ping-pong mechanism8,59, although detailed kinetic studies to confirm the mechanism for EcGrx1 or the F. ignava fusion enzyme are still missing. It is plausible to postulate that the first and second GSH molecule should interact during the reductive half-reaction of the dithiol mechanism with the 1st and 2nd glutathione-interaction site, respectively. The monothiol mechanism for the reduction of non-glutathione disulfides is controversial and at least three different nonexclusive mechanisms have been discussed to date (Fig. 1e):3,9,14,15,30,34,46,57,60 (i) The thiolate of the reduced monothiol class I glutaredoxin directly attacks the disulfide substrate followed by the stepwise reduction of the mixed disulfide and Grx(SSG) by two molecules of GSH. There are two variations of the suggested mechanism depending on whether the first GSH molecule attacks the Grx(SSR) intermediate at the sulfur atom of the glutaredoxin60 or the substrate15,30. (ii) GSH nonenzymatically reacts with the disulfide substrate, yielding a GSSR substrate that is reduced as described above9,46,57. (iii) GSH is activated by the glutaredoxin and catalysis requires a ternary complex with the disulfide substrate30,34. Following the enzyme-catalyzed glutathionylation of the substrate, the deglutathionylation occurs as decribed above. Since all reactions in Fig. 1 are reversible, class I glutaredoxins can also catalyze the GSSR- or GSSG-dependent glutathionylation of thiols or the formation of (protein) disulfides3,5,35,61. Such oxidations are in direct competition with glutathione reductase and ABC transporters which usually maintain very low GSSG concentrations under physiological conditions5,7,62,63,64.
Most of the commonly used oxidoreductase assays for glutaredoxins are either discontinuous and labor-intensive or unidirectional and coupled to the glutathione reductase-dependent consumption of NADPH1,2,6,8,16,17,18,30. One exception is the analysis of fluorescent protein substrates such as rxYFP or roGFP2, which allow a direct continuous detection of the reversible glutaredoxin-dependent protein disulfide reduction and formation in vitro14,15,31,32,33,34,35. Genetically encoded rxYFP or roGFP2 probes have also become extremely valuable tools for noninvasive, ratiometric real-time redox measurements in various compartments, cells, and organisms64,65,66,67,68,69,70,71. Fusion constructs between glutaredoxins or peroxidases and roGFP2 yield specific redox sensors for monitoring spatiotemporal changes of intracellular glutathione or peroxide concentrations32,61,62,69,72,73,74. Furthermore, roGFP2 fusion constructs with wild-type and mutant enzymes allow the intracellular analysis of enzyme mechanisms and structure-function relationships15,34,35,75. A current drawback of intracellular roGFP2-based mechanistic studies is, however, that the mechanisms and structure-function relationships still have to be validated in vitro. Deciphering the reversible glutaredoxin-dependent redox reactions of roGFP2 therefore not only closes a gap in our knowledge of glutaredoxin catalysis but also facilitates the intrepretation of roGFP2 responses from redox measurements and mechanistic analyses in vivo.
Here we determined the rate constants for the individual redox reactions between the two redox-sensitive cysteinyl residues of roGFP2 and the active-site cysteinyl residues of the model class I glutaredoxin PfGrx from the malaria parasite Plasmodium falciparum using stopped-flow kinetic measurements in combination with redox mobility shift assays. PfGrx shares 39% sequence identity with human Grx1, including a CPYC motif and a semiconserved GGC motif, and is well characterized in vitro and in roGFP2 assays in yeast14,26,30,35. We show (i) that PfGrx-catalyzed redox reactions outcompete uncatalyzed reactions between roGFP2 and GSH or GSSG by several orders of magnitude, (ii) that the PfGrx-catalyzed reduction and oxidation of roGFP2 occur via a mono- and not a dithiol mechanism, and (iii) that the reduction of roGFP2(S2) as a model non-glutathione disulfide substrate most likely involves a rate-limiting glutathionylation in a ternary complex with a GSH molecule that is activated at the 1st glutathione-interaction site of PfGrx. Furthermore, we confirm that the initial glutathionylation of the glutaredoxin moiety and not the glutathione transfer to the roGFP2 moiety is rate-limiting for the oxidation of fusion constructs at physiological or low micromolar GSSG concentrations, thus allowing glutaredoxin structure-function analysis in vivo.
Results
PfGrx(SSG)-dependent oxidation of reduced wild-type and monothiol roGFP2
GSSG rapidly oxidizes roGFP2 in the presence of glutaredoxins in vitro and in vivo33,34,35,61,62. To analyze the glutaredoxin-dependent oxidation kinetics for recombinant roGFP2, we first confirmed the fluorescence spectra of reduced and oxidized wild-type roGFP2 (roGFP2WT(SH)2 and roGFP2WT(S2), respectively) using a stopped-flow spectrofluorometer (Fig. 2a). Oxidation of roGFP2WT resulted in an increased fluorescence at an excitation of 400 nm and a decreased fluorescence at an excitation of 484 nm as reported previously66. To address the reactivities of the redox-sensitive cysteinyl residues, we also analyzed the recombinant monothiol roGFP2 variants roGFP2C151S and roGFP2C208S and detected small but reproducible glutathionylation-dependent changes of fluorescence at both excitation wavelengths for roGFP2C151S(SSG) and roGFP2C208S(SSG) (Fig. 2a). While glutathionylation of roGFP2C208S also caused an increased fluorescence at an excitation of 400 nm and a decreased fluorescence at an excitation of 484 nm, glutathionylation of roGFP2C151S resulted in a decreased fluorescence at both excitation wavelengths. To exclude that the small spectral changes reflected just a partial glutathionylation, we performed electrophoretic mobility shift assays (Fig. S1a). These assays confirmed that residue C208 in roGFP2C151S and residue C151 in roGFP2C208S were protected from alkylation in accordance with a complete glutathionylation under the chosen conditions.
a Fluorescence spectra of 1 µM roGFP2WT(SH)2 and roGFP2WT(S2) are shown on the left. The spectrum for roGFP2WT(S2) was recorded 2 min after the addition of 20 µM PfGrxC32S/C88S(SSG). The fluorescence spectra of 1 µM roGFP2C151S(SH) and roGFP2C208S(SH) are shown in the middle and on the right, respectively. Both monothiol roGFP2 variants were incubated with 10 mM GSSG to obtain the spectra of roGFP2C151S(SSG) and roGFP2C208S(SSG), which were recorded after the removal of GSSG and GSH. b Representative stopped-flow oxidation kinetics for the reaction between 1 µM reduced wild-type or mutant roGFP2 with 20 µM PfGrxC32S/C88S(SSG). c, d Representative stopped-flow oxidation kinetics at 484 nm for the reaction between 1 µM reduced wild-type or mutant roGFP2 with the indicated concentrations of either PfGrxC32S/C88S(SSG) or PfGrxC88S(SSG).
The spectral changes at both excitation wavelengths followed identical kinetics, which allowed us to reliably monitor the formation of roGFP2WT(S2), roGFP2C151S(SSG), or roGFP2C208S(SSG) using PfGrxC32S/C88S(SSG) as an oxidant (Fig. 2b). PfGrxC32S/C88S is the previously characterized monothiol mutant of PfGrx. C32 is the second active-site cysteinyl residue of PfGrx and C88 is the third cysteinyl residue, which is in the proximity of the active site and can also undergo redox modifications14,26,30. The PfGrxC32S/C88S(SSG)-dependent oxidation of roGFP2WT or glutathionylation of both monothiol roGFP2 variants were accelerated at increasing glutaredoxin concentrations and followed pseudo-first-order kinetics (Fig. 2c). Furthermore, the endpoint fluorescence indicated quite different redox potentials for the roGFP2 variants. While roGFP2WT was completely oxidized at even very low PfGrxC32S/C88S(SSG) concentrations, 1 µM PfGrxC32S/C88S(SSG) glutathionylated approximately 50% of 1 µM roGFP2C151S(SH) suggesting that the redox couples roGFP2C151S(SSG)/roGFP2C151S(SH) and PfGrxC32S/C88S(SSG)/PfGrxC32S/C88S(SH) have very similar redox potentials. In contrast to roGFP2C151S(SH), 50% glutathionylation of 1 µM roGFP2C208S(SH) required almost 5 µM PfGrxC32S/C88S(SSG) (Fig. 2c). The results and data interpretations of the endpoint fluorescences were independently validated by electrophoretic mobility shift assays (Fig. S1b). Pseudo-first-order kinetics were also detected using PfGrxC88S(SSG), the freshly glutathionylated dithiol variant of PfGrx26, as an oxidant (Fig. 2d). In these assays, roGFP2WT(SH)2 was again more reducing than roGFP2C151S(SH), which was again more reducing than roGFP2C208S(SH). A comparison of the endpoint fluorescences also revealed that about five times higher PfGrxC88S(SSG) than PfGrxC32S/C88S(SSG) concentrations were necessary to obtain a 50% oxidation of the roGFP2 variants (Fig. 2c, d). The results and data interpretations were again confirmed by electrophoretic mobility shift assays (Fig. S1b).
In summary, we established stopped-flow kinetic assays for the PfGrx-dependent oxidation of roGFP2WT and glutathionylation of its monothiol variants and estimated the following order of redox potentials for roGFP2C208S(SSG)/roGFP2C208S(SH) > roGFP2C151S(SSG)/roGFP2C151S(SH) ≈ PfGrxC32S/C88S(SSG)/PfGrxC32S/C88S(SH) > PfGrxC88S(SSG)/PfGrxC88S(SH) > roGFP2WT(S2) /roGFP2WT(SH)2.
Rate constants for the PfGrx-dependent oxidation of wild-type and monothiol roGFP2
Next, we fitted the pseudo-first-order glutathionylation kinetics at 484 nm from Fig. 2 and plotted the kobs values against the PfGrxC32S/C88S(SSG) or PfGrxC88S(SSG) concentration to determine the second-order rate constants for the formation of roGFP2WT(S2), roGFP2C151S(SSG), or roGFP2C208S(SSG) from the slopes of the linear fits (Fig. 3, Table 1). Both monothiol roGFP2 variants were rapidly glutathionylated with residue C208 reacting about twice as fast as residue C151 regardless whether PfGrxC32S/C88S(SSG) or PfGrxC88S(SSG) was used as the oxidant. The hightest second-order rate constant kox of 3.5 × 104 M–1 s–1 was determined for the reaction between roGFP2C151S(SH) and PfGrxC32S/C88S(SSG) (Fig. 3a–d). A similar overall rate constant of 3.0 × 104 M–1 s–1 was obtained for the PfGrxC32S/C88S(SSG)-dependent formation of roGFP2WT(S2). Thus, glutathionylation of roGFP2WT(SH)2 was most likely rate-limiting before the intramolecular disulfide bond was rapidly formed. PfGrxC32S/C88S(SSG) catalyzed the oxidation of roGFP2 via a monothiol mechanism and reacted with all roGFP2 variants 1.5 to 3 times faster than PfGrxC88S(SSG) (Fig. 3a, b, Table 1).
Secondary plots for the reaction kinetics of 1 µM reduced wild-type or monothiol roGFP2 with (a) PfGrxC32S/C88S(SSG), (b) PfGrxC88S(SSG), or (c) PfGrxC88S(S2). d Overview of the second order rate constants kox for the oxidation of the indicated roGFP2 variants by the indicated PfGrx mutants. The rate constants are also listed in Table 1. e Data interpretation of the roGFP2 oxidation kinetics. The preferred oxidation pathway is highlighted with thick arrows. All data sets were generated from at least two independent biological replicates.
We also analyzed the oxidation kinetics for roGFP2WT using the mono- and dithiol variants PfGrxC32S/C88S and PfGrxC88S following pre-treatment with the disulfide bond-inducing redox reagent 3-(dimethylcarbamoylimino)−1,1-dimethylurea (Diamide)76 (Fig. 3c, d, Table 1). Diamide-treated monothiol PfGrxC32S/C88S was intended as a negative control but had a detectable activity with roGFP2WT(SH)2 with a rate constant kox around 6.5 × 102 M–1 s–1 (Fig. 3d). The unexpected activity can be explained by the formation of variable amounts of disulfide-bridged PfGrxC32S/C88S homodimers and potential Diamide-modified monomers, summarized as PfGrxC32S/C88S(SX), as revealed by non-reducing SDS-PAGE (Fig. S1c). PfGrxC88S(S2) oxidized roGFP2WT(SH)2 only slowly with a second order rate constant of 1.5 × 102 M–1 s–1 (Fig. 3c, d). Thus, roGFP2 oxidation by PfGrx occurs preferentially via a glutathione-dependent monothiol and not a glutathione-independent dithiol mechanism (Fig. 3d, e). The slow reaction between PfGrxC88S(S2) and roGFP2WT(SH)2 was confirmed by electrophoretic mobility shift assays (Fig. S1d). No mixed disulfide intermediate was detected in accordance with a slow reaction between both proteins that was followed by a rapid intramolecular disulfide bond formation yielding roGFP2WT(S2). The instability of a mixed disulfide between roGFP2 and PfGrx was also supported by unsuccessful attempts to force and stabilize a Grx(SSR) species in titration experiments with roGFP2C151(SH) and up to 80 µM PfGrxC88S(S2) (Fig. S1e). The second order rate constant kox for the nonenzymatic reaction between GSSG and roGFP2WT(SH)2 was 0.7 M–1 s–1 (Fig. S2), clearly demonstrating that uncatalyzed glutathionylation of regular protein thiols occurs much too slowly at low physiological GSSG concentrations to compete with glutaredoxin-dependent glutathionylation63.
In summary, residues C151 and C208 of reduced roGFP2 are both susceptible to enzyme-catalyzed glutathionylation with residue C208 reacting twice as fast as residue C151. Mutation of residue C32 of PfGrx results in an up to three times faster glutathionylation of roGFP2 with a kox of 3.5 × 104 M–1 s–1. Furthermore, the PfGrx-catalyzed oxidation of roGFP2 is more than four orders of magnitude faster than the uncatalyzed reaction with GSSG and occurs two orders of magnitude faster via the glutathione-dependent monothiol mechanism than by the glutathione-independent dithiol mechanism (Fig. 3e, Table 1). Thus, roGFP2 oxidation does not efficiently occur via a Grx(SSR) intermediate and we can exclude the dithiol mechanism and monothiol mechanism (i) for roGFP2 oxidation (reverse reactions in Fig. 1c, e). Taking into account the SN2 reaction geometry, the size of roGFP2, and the accessibility of both sulfur atoms in Grx(SSG) (Fig. 1b), we favor an attack of roGFP2 at the glutathione sulfur atom as the most likely roGFP2 oxidation pathway (reverse reaction from mechanism (ii) or (iii) from Fig. 1e).
Oxidation kinetics for roGFP2WT-PfGrx fusion constructs
The in vivo oxidation kinetics from fusion constructs between roGFP2WT and a variety of glutaredoxins and mutants thereof were shown to correlate very well with the rate constants for the oxidative half-reaction from GSSCys assays in vitro, suggesting that the glutathionylation of the glutaredoxin and not the transfer of oxidation to the fused roGFP2 moiety was rate-limiting under the chosen conditions15,34,35. To test this data interpretation, we analyzed the GSSG-dependent oxidation of reduced fusion constructs between roGFP2WT and wild-type PfGrx (PfGrxWT), the dithiol mutant PfGrxC88S, or the monothiol mutant PfGrxC32S/C88S (Fig. 4, Table 1). Two separate phases were observed at an excitation of 484 nm, a rapid but small increase and a slower but more pronounced decrease of fluorescence (Fig. 4a). Similar biphasic kinetics, although a 100-fold slower, were reported for the GSSG-dependent oxidation of fusion constructs between rxYFP and a mono- or dithiol variant of ScGrx1 from yeast31. Secondary plots of the kobs values from single exponential fits of the first phase and double exponential fits of the second phase against the GSSG concentration yielded a linear and hyperbolic correlation for the first and second phase, respectively (Fig. 4b–d). The second order rate constant for the first phase depended on the PfGrx variant and was as high as 4.3 × 107 M–1 s–1 for roGFP2WT-PfGrxWT. The rate constant for the fusion construct with dithiol PfGrxC88S was about three times smaller and decreased again fivefold for monothiol PfGrxC32S/C88S (Fig. 4b–e). This trend is in contrast to the rxYFP fusion constructs with mono- and dithiol variants of ScGrx1, which were shown to react more rapidly with GSSG when the second cysteinyl residue was replaced by serine (Table 1)31. We interpret the first phase as the glutathionylation of the PfGrx moiety that became detectable for the nearby fraction of fused roGFP2 molecules (Fig. 4f). Thus, glutathionylation of roGFP2WT-PfGrxC32S/C88S by GSSG was about 2.5-times faster as the previously determined second-order rate constant for the glutathionylation of PfGrxC32S/C88S by GSSCys (Table 1)14. The faster reaction with GSSG might reflect the simultaneous interaction of the substrate with both glutathione interaction sites. An alternative interpretation for the first phase could be a rapid noncovalent interaction between PfGrx and GSSG in the vicinity of roGFP2, however, this seems less likely considering the smaller values for the structurally similar PfGrx mutants or, in particular, the rxYFP fusion constructs with ScGrx1 (Table 1). The secondary plot for the first kobs values from the second phase yielded a rate contant at GSSG saturation around 10 s–1 for all three PfGrx variants (Fig. 4e). We assign this rate constant of the second phase to the intramolecular glutathione transfer within the roGFP2WT-PfGrx fusion constructs. The strong decrease of fluorescence is then caused by the immediate formation of roGFP2WT(S2) which cannot be kinetically separated from the slower glutathionylation of the roGFP2 moiety (Fig. 4f). One explanation for the necessity to use double exponential fits for the second phase could be that a fraction of the roGFP2WT(SH)2-PfGrx(SSG) intermediates glutathionylates the roGFP2 moiety of another molecule in trans.
a Representative biphasic stopped-flow oxidation kinetics at 484 nm for the reaction between 1 µM reduced roGFP2WT-PfGrxC32S/C88S and 20 µM GSSG. Secondary plots for the kobs values from the first and second phase of the reaction between GSSG and 1 µM reduced roGFP2WT-PfGrx fusion construct for (b) PfGrxWT, (c) PfGrxC88S, or (d) PfGrxC32S/C88S. e Overview of the second order rate constants k1 for the first phase (left) and the GSSG-independent maximum rate constants k2 for the second phase (right). The rate constants are also listed in Table 1. f Data interpretation of the oxidation kinetics for the three different roGFP2 fusion constructs. The arrows in orange indicate the altered redox environment for roGFP2(SH)2 due to the glutathionylation of the PfGrx moiety. All data sets were generated from at least two independent biological replicates.
In summary, the roGFP2 oxidation kinetics of PfGrx fusion constructs reflect at up to micromolar GSSG concentrations the glutathionylation kinetics of the glutaredoxin moiety in accordance with previous intracellular structure-function analyses and data interpretations15,34,35. The GSSG-dependent oxidation of roGFP2 by fused PfGrx variants is two to three orders of magnitude faster than the oxidation by unfused PfGrx(SSG) variants and more than seven orders of magnitude faster than the uncatalyzed reaction with GSSG (Table 1). The intramolecular glutathionylation of the roGFP2 moiety only becomes rate-limiting at saturating, medium micromolar GSSG concentrations. Such high GSSG concentrations are unlikely to occur in most subcellular compartments but could be reached in the secretory pathway or during titration experiments of transgenic cell lines with an upregulated GSSG transporter35,63,64,77.
PfGrx-dependent reduction kinetics for glutathionylated monothiol roGFP2
The glutaredoxin-dependent reduction of glutathionylated monothiol roGFP2 variants was analyzed in the presence and absence of GSH (Fig. 5 and Table 1). When GSH was tested together with reduced PfGrxC88S or PfGrxC32S/C88S, the syringe of the stopped-flow spectrofluorometer also contained 0.4 U/mL glutathione reductase (GR) and 0.25 mM NADPH to ensure the removal of trace amounts of GSSG that might interfere with the redox measurements.
Representative secondary plots for the kobs values from the reaction between (a) 1 µM roGFP2C151S(SSG) or (b) 1 µM roGFP2C208S(SSG) and reduced PfGrxC88S (top) or monothiol PfGrxC32S/C88S (bottom) in the absence (left) or presence of GSH (right). c Overview of the second order rate constants kred for the PfGrx-dependent reduction of the indicated roGFP2(SSG) mutants at four different GSH concentrations. The rate constants are also listed in Table 1 and Supplementary Table S1. d Data interpretation of the reduction kinetics for both monothiol roGFP2(SSG) variants and both PfGrx mutants. The faster reduction pathways are highlighted with thick arrows. All data sets were generated from one or two independent biological replicates.
The PfGrx-dependent deglutathionylations of roGFP2C151S(SSG) and roGFP2C208S(SSG) both occurred rapidly in the absence of GSH (Fig. 5a, b). High GSH concentrations in the reaction mixtures even slightly decreased the rate constants, suggesting a competition for the 1st glutathione-interaction site (Fig. 1b). The highest second order rate constant kred of 1.4 × 106 M–1 s–1 was determined for the reaction between PfGrxC88S(SH)2 and roGFP2C208S(SSG) (Fig. 5b, c). The PfGrxC88S-catalyzed deglutathionylation of monothiol roGFP2C208S(SSG) is therefore two orders of magnitudes faster than the glutathionylation of roGFP2C208S(SH) by PfGrxC88S(SSG) (Table 1) corresponding to the strongly shifted equilibrium towards glutathionylated PfGrxC88S and reduced roGFP2C208S in the redox mobility shift assays (Fig. S1). The positions of the other equilibria from the redox mobility shift assays in Fig. S1 were also in good agreement with the more balanced ratios of the rate constants for the corresponding (de)glutathionylations from Table 1. As opposed to the reactivities of both glutathionylated PfGrx variants, reduced PfGrxC88S reacted with glutathionylated monothiol roGFP2 about six to eight times faster than PfGrxC32S/C88S (Fig. 5a–c). Thus, the presence of the thiol group of C32 in PfGrx seems to impair the glutathionylation and to facilitate the deglutathionylation of roGFP2. Furthermore, glutathionylated residue C151 of roGFP2 reacted three to four times faster than glutathionylated residue C208, which is again the opposed reactivity as observed for the oxidation kinetics of both monothiol roGFP2 variants (Table 1).
In summary, we established an assay for the direct and continuous monitoring of a glutaredoxin-dependent deglutathionylation reaction using roGFP2(SSG) monothiol variants as model substrates. The residue-specific reactivities and kred values for C151 and C208 in roGFP2(SSG) as well as active-site residue C29 in reduced dithiol PfGrxC88S and monothiol PfGrxC32S/C88S in Fig. 5 correlate inversely with the reactivities and kox values for the same residues of the reverse reactions in Fig. 3. The thiol group of C32 in PfGrx therefore facilitates the C29-dependent deglutathionylation of roGFP2(SSG) without forming an intramolecular disulfide bond. The kred value of 1.4 × 106 M–1 s–1 for PfGrxC88S(SH)2 is about two orders of magnitude higher than the highest kox value for PfGrxC32S/C88S(SSG) in accordance with a physiological relevance of glutaredoxins as deglutathionylating enzymes.
PfGrx-dependent reduction kinetics for roGFP2WT(S2)
To address the previously suggested alternative models for the glutaredoxin-dependent reduction of non-glutathione protein disulfides (Fig. 1c, e)6,34,60, we conducted a detailed analysis of the reaction between reduced PfGrx variants and roGFP2WT(S2) (Figs. 6 and 7, Table 1). The reduction of roGFP2WT(S2) required both PfGrx and GSH (Fig. 6) as opposed to the reduction of the glutathionylated monothiol roGFP2 variants (Fig. 5). The GSH-containing syringe of the stopped-flow spectrofluorometer was supplemented again with 0.4 U/mL GR and 0.25 mM NADPH to remove trace amounts of GSSG and to prevent the thermodynamically favored reverse reaction32. The nonenzymatic initial reaction between 1 µM roGFP2WT(S2) and physiological millimolar GSH concentrations was too slow to be detectable and to be of any relevance in the presence of glutaredoxins in accordance with previous analyses32. Thus, we can exclude a direct uncatalyzed reduction of roGFP2WT(S2) by GSH (i.e. mechanism (ii) from Fig. 1e). Neither reduced monothiol PfGrxC32S/C88S nor dithiol PfGrxC88S reacted with roGFP2WT(S2) in the absence of GSH, and the reduction velocities depended on both the PfGrx and GSH concentration (Fig. 6a–d). A rapid direct reaction between reduced PfGrx and roGFP2WT(S2) in accordance with the dithiol mechanism from Fig. 1c or the monothiol mechanism (i) from Fig. 1e therefore also seems unlikely. Adequate fits of the biphasic kinetic traces for the reduction of roGFP2WT(S2) from Fig. 6a required a double exponential equation. Secondary plots for the rate constants of the rapid first reaction phase at variable PfGrx concentrations deviated from linearity and showed a hyperbolic correlation at millimolar GSH concentrations (Fig. 6b, c). Apparent kred values were estimated from linear fits at low PfGrx concentrations. The reactions with PfGrxC88S were faster than with PfGrxC32S/C88S and the highest kredapp value of 3.4 × 103 M–1 s–1 was detected for PfGrxC88S at 5 mM GSH (Fig. 6d, Table 1). The addition of the competitive inhibitor S-methyl glutathione (GSMe) significantly decreased the kredapp value (Fig. 6d). GSMe is usually a much weaker inhibitor in the HEDS assay or in assays with GSSR substrates11,14,17, suggesting that the competition between GSH and GSMe in the roGFP2 measurements occurred at the 1st and not the 2nd glutathione interaction site. No reaction was observed when l-cysteine was used instead of GSH in accordance with a specific substrate recognition (Fig. S3). Furthermore, alanine mutations of the established glutathione-scaffold site residue D90 or of crucial residue K26 in monothiol PfGrxC32S/C88S slowed down the reaction and resulted in linear secondary diagrams for the first rate constant (Fig. 6e). The kredapp values for the D90 and K26 mutants at 5 mM GSH were 78 and 5.6% of the kredapp value for PfGrxC32S/C88S as a control. These percentages are almost identical to the percentages for the second-order rate constants of the same mutants with GSSCys as a substrate14, indicating that GSH also occupies the 1st glutathione-interaction site of PfGrx for the reduction of roGFP2WT(S2). Based on the assumption that a ternary complex is formed between PfGrx, GSH, and roGFP2WT(S2), we estimated true kred values from tertiary plots at variable GSH concentrations of 7.3 × 105 M–2 s–1 and 1.3 × 105 M–2 s–1 for PfGrxC88S and PfGrxC32S/C88S, respectively (Fig. 6f).
a Representative biphasic stopped-flow reduction kinetics at 484 nm for the reaction between 1 µM roGFP2WT(S2) and reduced dithiol PfGrxC88S (left) or monothiol PfGrxC32S/C88S (right) in the presence of 5 mM GSH. b, c Representative secondary plots of the kobs values for the first phase with reduced PfGrxC88S or PfGrxC32S/C88S at 1 mM (left) or 5 mM GSH (right). d Overview of the apparent second-order rate constants kredapp for the first phase of the PfGrx-dependent reduction of roGFP2(S2) at different GSH concentrations (left) or with 1 mM GSH in the presence or absence of the competitive inhibitor GSMe (right). No activity was detected for 0 mM GSH. The rate constants are also listed in Table 1 and Supplementary Table S1. e Secondary plots for the first phase of the reduction kinetics of roGFP2(S2) at 5 mM GSH using either a D90A (left) or K26A (right) mutant of reduced monothiol PfGrxC32S/C88S. f Tertiary plots of the GSH-dependent kredapp values for reduced PfGrxC88S (left) or PfGrxC32S/C88S (right). True kred values were estimated by linear regression and are listed in Table 1. All data sets were generated from at least two independent biological replicates.
Simulation of the biphasic kinetic data for the reaction between 1 μM roGFP2WT(S2) and 1, 2.5, or 5 mM GSH in the presence of 1–40 µM reduced PfGrxC88S. Best fits (dashed lines) were obtained for a reaction within a ternary complex regardless whether (a) roGFP2(S2) or (b) GSH was first bound to PfGrx. Rounded rate constants from Table 1 in black were chosen as input parameters to calculate the missing parameters in blue. The relative fluorescence intensity of each roGFP2 redox species was taken from Fig. 2.
Finally, we simulated the PfGrxC88S-dependent reduction kinetics for roGFP2(S2) using the three alternative mechanisms from Fig. 1e and the rate constants from Table 1 (Fig. 7). We excluded the possibility that the first reaction phase reflects the deglutathionylation of roGFP2WT since this would necessitate an earlier drastic increase of the fluorescence due to the opening of the disulfide bond in roGFP2WT(S2). Simulations for alternative monothiol mechanisms (i) and (ii) from Fig. 1e did not reveal appropriate fits and were excluded based on incorrect fluorescence amplitudes and deviating, substrate concentration-dependent rate constants for the data sets at 1, 2.5, and 5 mM GSH. In contrast, good fits with concentration-independent rate constants were obtained for the monothiol mechanism (iii) from Fig. 1e, regardless whether the formation of the ternary complex was initiated by GSH or roGFP2WT(S2) (Fig. 7). We therefore suggest a mechanism that can be described according to the Cleland nomenclature as a combination of a random bi-uni mechanism and a ping-pong bi-bi (or uni-uni-uni-uni) mechanism in which the single product of the rate-limiting random mechanism becomes the first substrate of the ping-pong mechanism (Fig. 8a, see also discussion). The small second-order rate constant for the simulated reaction between GSH and the complex of reduced PfGrxC88S and roGFP2WT(S2) might reflect a poor accessibility of the 1st glutathione interaction site in this complex (Fig. 7a), whereas the simulated reaction for roGFP2(S2) was by two orders of magnitude faster when GSH was already bound to PfGrxC88S (Fig. 7b). Whether the roGFP2WT(SSG) intermediate is released presumably depends on its size and the geometry of the first and second transition state as outlined below (Fig. 8a, b).
a Cleland scheme of the deduced monothiol reaction mechanism for the PfGrx-catalyzed redox reactions of roGFP2. The reaction with roGFP2(S2) as a model non-glutathione disulfide substrate begins with a rate-limiting random bi-uni mechanism and is shown from left to right. The subsequent deglutathionylation of the roGFP2(SSG) intermediate follows a ping-pong mechanism. The reverse reaction with GSSG (or GSSR) and roGFP2(SH)2 as model protein with surface-exposed cysteinyl residues is shown from right to left and will probably deviate by a nonenzymatic shortcut yielding roGFP2(S2) from roGFP2(SSG). See text for details. b Schematic representations of the three predicted transition states for the reactions from panel a. c Potential effects of the size and reaction geometry on the formation and orientation of the first transition state from (a). d Schematic representations of the four predicted transition states for the suggested reduction of HEDS according to monothiol mechanism (i). e Schematic representations for the putative reduction of Grx(SSR) intermediates according to alternative monothiol mechanism (i) are shown on the left and in the middle. If the Grx(SSR) species cannot be attacked by GSH, a second or moderately conserved third cysteinyl residue in glutaredoxins could prevent the accumulation of trapped Grx(SSR) species according to the cysteine resolving model shown on the right.
In summary, reduction of roGFP2WT(S2) as a model non-glutathione protein disulfide substrate requires the presence of GSH and PfGrx. The kinetic data can be best explained by a ternary complex in which PfGrx recruits and activates GSH at the 1st glutathione-interaction site to facilitate the rate-limiting glutathionylation of roGFP2WT(S2) and its subsequent deglutathionylation in accordance with monothiol mechanism (iii) from Fig. 1e (Fig. 8a, b).
Discussion
The enzymatic formation and reduction of disulfides in physiological systems clearly differs from uncatalyzed redox equilibrations in vitro5,7,63,78,79. Since fundamental biochemical pathways evolved in the absence of molecular oxygen, most intracellular disulfides are rather transient and most cysteinyl residues are kept in a reduced state with the help of the thioredoxin or the glutathione/glutaredoxin system7,63,77. While in vitro equilibrations between redox couples such as roGFP2 and GSH/GSSG require minutes or even hours to reach a thermodynamic endpoint (ΔG = 0), enzyme-catalyzed thiol-disulfide exchange reactions usually occur very rapidly and either maintain or reach a steady state (ΔG ≠ 0)7,31,32,60,63,67,78,79. Here we determined the mechanisms and rate constants for the glutathione/glutaredoxin-dependent redox reactions of the widely used redox probe roGFP2 and addressed the question of how mono- and dithiol glutaredoxins catalyze the reduction of roGFP2(S2) as a model non-glutathione protein disulfide substrate. We showed that the PfGrx-dependent oxidation and reduction of roGFP2 both efficiently occur via a monothiol mechanism (Fig. 8a). The dithiol mechanism was too slow to compete with the monothiol mechanism in the presence of GSH or GSSG and was only detected for the oxidation of roGFP2 using diamide-treated PfGrx without GSH. The latter result confirms the previous study on the ScGrx1-catalyzed oxidation of rxYFP, although the removal of the second active-site cysteinyl residue had opposite effects on the reactivity of PfGrx and ScGrx1 (Table 1)31. Furthermore, we established a stopped-flow kinetic assay for the direct continuous detection of the glutaredoxin-dependent deglutathionylation of monothiol roGFP2 variants. The rate constants from our deglutathionylation assays between 6.4 × 104 and 1.4 × 106 M–1 s–1 are very similar to the rate constants for PfGrx or plant and mammalian glutaredoxins from discontinuous or coupled enzymatic assays with other high and low molecular weight GSSR substrates (Table 1)9,11,14,19, thus confirming our assay as an alternative reliable method for the direct analysis of enzyme-catalyzed deglutathionylations. Although residues C32 and C88 of PfGrx are redox-inactive during the monothiol mechanism, they both facilitate the deglutathionylation of GSSR substrates. Analogous residues in other glutaredoxins were also shown to increase11,20,31,56 or to decrease18,55,56,57 their activity. While the second-order rate constants of PfGrxC88S and PfGrxC32S/C88S for GSSG are one order of magnitude higher than for bulky roGFP2(SSG), the rate constant for the glutathionylation of PfGrxC32S/C88S by GSSG is twice that with GSSCys (Table 1)14. Thus, not only the size but also the charge and shape of the glutathionylated substrate are relevant with GS– in GSSG being the optimal leaving group in accordance with a 2nd glutathione-interaction site (Fig. 1b).
What can we learn about the mechanism for the reduction of roGFP2(S2)? Despite the thermodynamically highly favorable reverse reaction, PfGrxC88S(S2) reacted very slowly with roGFP2WT(SH)2 most likely due to the instability and high energy of the Grx(SSR) intermediate. Thus, it is unlikely that the formation of a Grx(SSR) intermediate is a preferred reaction pathway for the reduction of roGFP2(S2). Our kinetic data indeed support an enzymatic reduction of roGFP2(S2) that requires a ternary complex between the glutaredoxin, GSH, and the non-glutathione disulfide substrate (Fig. 8a, b). The presence of the second active-site cysteinyl residue in PfGrx improves the reactivity of the ternary complex but does not form an intramolecular disulfide bond in contrast to a dithiol mechanism. Glutathionylated roGFP2 is subsequently reduced via the monothiol ping-pong mechanism for GSSR substrates. The enzyme-catalyzed reverse reaction between GSSG or GSSR and reduced roGFP2 releases roGFP2(SSG) which can instantaneously react via a nonenzymatic shortcut yielding GSH and roGFP2(S2) (Fig. 8a). Thus, the enzymatic reduction of roGFP2(S2) by GSH follows monothiol mechanism (iii) and requires a glutaredoxin to form roGFP2(SSG). On the basis of microscopic reversibility of enzymatic reactions, the enzyme-catalyzed oxidation of roGFP2(SH)2 by GSSG or GSSR follows the reverse reaction of mechanism (iii). However, the nonenzymatic formation of roGFP(S2) from roGFP2(SSG) also proceeds extremely rapidly and competes with the final enzyme-catalyzed reaction step with implications for redox measurements as outlined below. The reduction of roGFP2(S2) is therefore a good example for an enzyme using an alternative reaction pathway to overcome a kinetic challenge, and we hypothesize that other non-glutathione disulfide substrates with reactive proximal cysteinyl residues and rather negative redox potentials also require a ternary complex for their reduction. The reaction mechanism for roGFP2(S2) as a model non-glutathione disulfide substrate resembles the first part of the GSH-dependent reduction of the sulfenic acid of human peroxiredoxin 6 that is catalyzed by glutathione transferase P1-1 (which shares several structural features with glutaredoxins)7,80,81,82. The first GSH molecule is activated at the 1st glutathione-interaction site of the glutaredoxin. This step might involve acid-base catalysis by the active-site cysteinyl thiolate (which could explain an effect of the second cysteinyl residue), and/or the conserved lysine residue, yielding GS– in analogy to glutathione transferases (Fig. 8b)7. Please note that the previously designated glutathione-activator site for the monothiol mechanism with GSSR substrates corresponds to the 2nd and not the 1st glutathione-interaction site (Fig. 1b)7,8,13,14,15. Since we have now shown that the 1st glutathione-interaction site can serve not only as a scaffold site for GSSR substrates but also as an activator site for GSH for the reduction of non-glutathione disulfide substrates, we would like to suggest to preferably use the terms 1st and 2nd glutathione-interaction site (e.g., in combination with the function as a scaffold or activator) to avoid ambiguities in the future. The 1st glutathione-interaction site should be frequently (although transiently) occupied by GSH at millimolar concentrations, whereas diverse non-glutathione disulfide substrates are far less abundant in vivo and should encounter the active site less frequently. Hence, although future kinetic studies might confirm a random mechanism for the rate-limiting first part of monothiol mechanism (iii) in vitro, GSH is probably the first and the non-glutathione disulfide the second substrate under physiological conditions in vivo (Fig. 8a). Since GSH interacts with the 1st glutathione-interaction site, glutathionylated products of the random bi-uni mechanism might already adopt a suitable orientation as classical GSSR substrates for the subsequent deglutathionylation (Fig. 8b, c) to avoid a futile cycle due to the nonenzymatic shortcut (Fig. 8a). Whether the deglutathionylation requires a release or reorientation of the GSSR intermediate should depend on whether the four sulfur atoms of the disulfide substrate, GSH, and the active-site cysteinyl residue can be arranged like beads on string. While such an arrangement could be achieved by small disulfides such as HEDS, bulky protein substrates might cause significant deviations from this reaction geometry (Fig. 8b, c). We therefore assume that the reaction sequence for the reduction of roGFP2(S2) requires at least a reorientation of the GSSR intermediate and that this may also be the case for other non-glutathione protein disulfide substrates. Whether this hypothesis is correct or whether accessible disulfides (e.g., within an extended loop or a flexible N- or C-terminus) circumvent such steric limitations remains to be analyzed.
What could be the reasons for possible alternative glutaredoxin mechanisms for the reduction of non-glutathione disulfide substrates? While the dithiol mechanism for EcGrx1 and EcRNR or EcPR can be explained by specific protein-protein interactions (Fig. 1c, d)42,58, a rather unspecific GSH-dependent monothiol mechanism might be better suited for the reduction of a variety of physiological non-glutathione disulfide substrates to maintain metabolites and proteins in a reduced state (Fig. 1e)34. Several candidate non-glutathione disulfide substrates for the dithiol mechanism have been identified using monothiol glutaredoxin mutants as bait proteins for the trapping of Grx(SSR) species (Fig. 1c)83,84,85. Non-glutathione disulfide substrates that employ monothiol mechanism (iii) should not be trapped as Grx(SSR) intermediates, which might explain the rather small number of verified non-glutathione disulfide substrates of glutaredoxins to date. A rate-limiting step involving a ternary complex according to Fig. 8a could also explain the previously observed sequential kinetic patterns for the small non-glutathione substrate HEDS (Fig. 8c)3,7,12,14,15,30. A nonexclusive alternative explanation for the kinetic patterns with HEDS (and the decreased enzyme activity at high GSH concentrations) could be a Grx(SSCH2CH2OH) intermediate that is attacked by GSH according to monothiol mechanism (i) from Fig. 1e, yielding a GSSR product that has to change its inhibitory orientation before it can be reduced at the 1st glutathione-interaction site (Fig. 8d)3,14,15,30. Monothiol mechanism (i) and (iii) remain to be analyzed in more detail for HEDS and small non-glutathione disulfides. Mechanism (ii) from Fig. 1e has also been suggested (and is still commonly propagated) for HEDS as a non-glutathione substrate9,57. However, the kinetic patterns do not support a ping-pong mechanism and the nonenzymatic first reaction with a second order rate constant of 0.6 M–1 s–1 is too slow to explain the rapid turnover3,12,14,15,30. Many other nonenzymatic glutathionylations according to mechanism (ii) probably also have such small rate constants63,86. The reduction of human RNR, which was suggested to follow mechanism (ii)46, therefore needs to be analyzed and monothiol mechanism (iii) should be also considered. A dithiol mechanism according to Fig. 1c and the monothiol mechanism (i) from Fig. 1e were suggested for slow thermodynamic thiol-disulfide equilibrations between different glutaredoxins, GSH, and the metal-binding domains of the proteins HMA4 and Atox160. The suggested dithiol mechanism was based on experiments at a single substrate concentration that required more than 8 h for redox equilibration in the presence of 0.5 µM glutaredoxin with or without 1 µM GSH; conditions that are neither physiological nor exemplary for rapid glutaredoxin catalysis. Furthermore, the relevance of a Grx(SSR) intermediate and an attack by GSH at the glutaredoxin sulfur atom according to monothiol mechanism (i) were postulated for the reduction of the non-glutathione disulfide bond of the HMA4 domain60. The slow protein reduction was analyzed at a single substrate concentration in the presence of 0.1 µM glutaredoxin and 0.8 mM GSH. Neither the accessibility of the Grx(SSR) sulfur atoms (Fig. 8e) nor the presence or concentration of the mixed disulfide were addressed. In contrast, a GSSR intermediate was detected and its decreased steady-state concentration at higher glutaredoxin concentrations was suggested to support monothiol mechanism (i)60. However, this result is expected considering the very high activity of glutaredoxins as deglutathionylating enzymes and a decreased GSSR concentration neither excludes mechanism (ii) or (iii) from Fig. 1e. We therefore suggest for future studies on monothiol mechanism (i) to take the bulkiness of the putative Grx(SSR) intermediate, an SN2 reaction geometry, and a possible accumulation of Grx(SSR) as an artifact into account (Fig. 8e). For example, if the glutaredoxin sulfur atom in Grx(SSR) species was accessible for a productive reaction with GSH, one would not expect to trap such species by monothiol glutaredoxin mutants83,84,85. We have previously encountered a potentially similar mechanistic misdirection regarding the relevance of a mixed disulfide between PfGrx and the peroxiredoxin PfAOP. While electrophoretic mobility shift assays and mass spectrometry data both suggested a potential role of this mixed disulfide for catalysis26, kinetic assays later revealed the irrelevance for the catalytic cycle and thus confirmed that the Grx(SSR) species was an artifact29. The reduction of roGFP2(S2) according to monothiol mechanism (iii) and previous experiments in yeast also raise the question whether the dithiol mechanism is crucial for the reduction of many protein substrates34. At this point it cannot be ruled out that some of the proteins that were captured with monothiol glutaredoxin mutants as Grx(SSR) species might have just accumulated as unspecific dead-end products. Thus, the second and a moderately conserved third cysteinyl residue (e.g. C32 and C88 in PfGrx) could not only form an intramolecular disulfide during catalysis or affect the reactivity of the active-site thiolate but also function as a resolving cysteine for trapped Grx(SSR) species (Fig. 8e)8,13,26,34. As outlined above, studies on non-glutathione disulfide substrates unfortunately regularly lack more detailed kinetic analyses, first, to confirm the efficient turnover of the substrate candidates and, second, to validate the dithiol mechanism. For example, control experiments with millimolar GSH and a monothiol glutaredoxin mutant to exclude a monothiol mechanism often appear to be missing40,41,87. In summary, monothiol mechanism (iii), as exemplified for roGFP2(S2) in Fig. 8a, b, provides a plausible alternative mechanism for the reduction of non-glutathione disulfide substrates that could complement the established dithiol mechanism for EcGrx1 and EcRNR. Whether this mechanism is commonly employed for a variety of substrates and whether monothiol mechanisms (i) and (ii) are really relevant for the reduction of other non-glutathione disulfide substrates remains to be analyzed.
What are the implications of our study regarding noninvasive redox measurments? Our roGFP2 measurements confirm (i) that dynamic roGFP2 sensing (or the physiological glutathionylation/deglutathionylation of regular surface-exposed cysteinyl residues) requires a glutaredoxin activity, and (ii) that glutathionylated proteins or metabolites should be very transient redox species when GSH and glutaredoxins are present (unless they are stabilized by a kinetic uncoupling mechanism)5,32,34,35,61,62,63,64. The very low rate constants for direct, glutathione-independent dithiol-disulfide exchange reactions between glutaredoxins and roGFP2 or other protein(S2)/protein(SH)2 couples (despite favorable thermodynamics) are a prerequisite and great advantage for reliable redox measurements in vivo. If Grx(SSR) intermediates and mixed disulfides with roGFP2 were more stable and were readily formed, glutaredoxins would sense and transfer a plethora of other redox states to roGFP2 probes. Regarding intracellular roGFP2-based structure-function analysis of glutaredoxins, we now showed that the GSSG-dependent oxidation of the fused glutaredoxin moiety (and not the glutathionylation of the roGFP2 moiety) is rate-limiting unless saturating GSSG concentrations are present. Such high micromolar GSSG concentrations are unlikely to occur in most subcellular compartments but could be reached in the secretory pathway or during titration experiments of transgenic cell lines with an upregulated GSSG transporter35,63,64,77. This explains why intracellular oxidation kinetics for roGFP2 fusion constructs with glutaredoxin mutants showed excellent correlation with the second order rate constants of the oxidative half-reaction from in vitro experiments15,26,34,35. No saturation behavior for GSSG was previously detected for the fusion construct between rxYFP and ScGrx131, which makes sense considering that PfGrx and many other glutaredoxins are at least two orders of magnitude more active than ScGrx1 (Table 1)14,56. One anomaly from previous measurements was the high intracellular steady-state oxidation of roGFP2 for fusion contructs with ScGrx7, in particular, when a WP-motif or a loop from class II glutaredoxins was introduced. In principle, all reactions in Fig. 8a are reversible. However, the negative redox potential of roGFP2, the instantaneous nonenzymatic formation of roGFP2(S2) from roGFP2(SSG), and the very slow nonenzymatic reaction of roGFP2(S2) with GSH make the nonenzymatic shortcut from Fig. 8a practically irreversible and independent of the fused glutaredoxin. Furthermore, the reduction of roGFP2(S2) is the slowest of the enzymatic reaction steps in Fig. 8a (Table 1). We therefore suggest that the intracellular steady-state oxidation of roGFP2 predominantly reflects the ability of the fused glutaredoxin to form a productive ternary complex with GSH and roGFP2(S2). This hypothesis can now be tested in a follow-up study.
Methods
Materials
Diamide, dithiothreitol (DTT), ethylenediaminetetraacetic acid (EDTA), GSH, GSSG, and yeast GR were from Sigma-Aldrich, l-cysteine was from Carl Roth, isopropyl-β-d-1-thiogalactopyranoside was from Serva, methyl ether poly(ethylene glycol)24 maleimide (mmPEG) was from Iris Biotech, and NADPH was from Gerbu. PCR primers were purchased from Metabion. All restriction enzymes, Phusion DNA polymerase, and T4 DNA ligase were from New England Biolabs (NEB).
Cloning and site-directed mutagenesis
All plasmids and primers for cloning and site-directed mutagenesis are listed as separate Supplementary Data. Fusion constructs ROGFP2WT-PFGRXWT, ROGFP2WT-PFGRXC88S, and ROGFP2WT-PFGRXC32S were PCR-amplified with Phusion DNA polymerase using the corresponding p416TEF plasmids as a template35 and subcloned into the KpnI and AvrII restriction sites of pET45b in strain XL1-Blue. Point mutations in ROGFP2WT-PFGRXC32S/C88S, ROGFP2C151S or ROGFP2C208S were introduced by PCR with Pfu polymerase using ROGFP2WT-PFGRXC32S/pET45b or ROGFP2/pET28a as a template. The template DNA was then digested with DpnI and the plasmids were cloned in strain XL1-Blue. Correct mutations and sequences were confirmed for all constructs by sequencing both strands.
Heterologous expression and sample preparation
Recombinant C-terminally LEH6-tagged wild-type and mutant roGFP2, N-terminally MRGSH6GS-tagged PfGrx variants, and N-terminally MAH6VGT-tagged roGFP2WT-PfGrxWT, roGFP2WT-PfGrxC88S, and roGFP2WT-PfGrxC32S/C88S were produced in E. coli strain BL21 (DE3) or strain XL1-Blue and purified by Ni-NTA affinity chromatography as described previously14,26,35,88. Similar yields and purities were obtained when the recombinant proteins were produced for 4 h at 30 °C in E. coli strain SHuffle T7 express (NEB) following induction with 0.5 mM isopropyl-β-d-1-thiogalactopyranoside at an OD of 0.5. Cultures were subsequently cooled in an ice water bath for 15 min and centrifuged (4.000g, 15 min, 4 °C). Cell pellets were resupended in ice-cold buffer 1 containing 20 mM imidazole, 50 mM NaxHyPO4, 300 mM NaCl, pH 8.0 at 4 °C and stirred on ice with DNase I and 10 mg/mL lysozyme for 1 h before sonication. The lysates were centrifuged (10.000g, 30 min, 4 °C) and the supernatants were loaded on equilibrated Ni-NTA agarose columns (Qiagen). The columns were washed with 15 column volumes of buffer 1 followed by the elution with 200 mM imidazole, 50 mM NaxHyPO4, 300 mM NaCl, pH 8.0 at 4 °C. The purity of all proteins was confirmed by analytical SDS-PAGE and the concentrations were determined spectrophotometrically at 280 nm.
Stopped-flow kinetic measurements
For all stopped-flow measurements, freshly purified proteins were reduced with 5 mM DTT on ice for 30 min. Excess DTT and imidazole were removed on a PD-10 desalting column (Merck), and the reduced proteins were eluted with 3.5 mL ice-cold assay buffer containing 100 mM Tris/HCl, 1 mM EDTA, pH 8.0. Protein concentrations were again determined spectrophotometrically at 280 nm. Glutathionylated roGFP2C151S, roGFP2C208S, PfGrxC88S, and PfGrxC32S/C88S were produced by incubation of the reduced enzymes with 5 mM GSSG for 60 min on ice. Oxidized roGFP2WT, PfGrxC88S, and PfGrxC32S/C88S were produced by incubation of the reduced enzymes with 5 mM Diamide for 60 min on ice. The glutathionylated/oxidized enzymes were again purified via Ni-NTA affinitiy chromatograpghy followed by desalting on a PD-10 column as described above. Stopped-flow measurements were performed at 25 °C in a thermostatted SX-20 spectrofluorometer (Applied Photophysics). The change of fluorescence was measured for up to 600 seconds after mixing (total emission at an excitation wavelength of 400 or 484 nm with a slit width of 2 mm). The oxidation of roGFP2 was investigated by mixing 2 µM of reduced roGFP2 in syringe 1 with variable concentrations of glutathionylated or diamide-oxidized PfGrx in syringe 2. For the reduction of roGFP2, 2 µM diamide oxidized roGFP2WT or 2 µM glutathionylated roGFP2C151S or roGFP2C208S in syringe 1 were mixed with variable concentrations of reduced PfGrx and GSH with 0.4 U/mL GR and 0.25 mM NADPH in syringe 2. The oxidation of the fusion-constructs was followed by mixing 2 µM reduced protein in syringe 1 with variable concentrations of GSSG in syringe 2. The traces of three consecutive measurements were averaged and fitted by single exponential or double exponential regression using the Pro-Data SX software (Applied Photophysics). Rate constants kobs were plotted against the substrate concentration in SigmaPlot 13.0 to obtain second order rate constants from the slopes of the linear fits or first-order rate constants from hyperbolic fits. The Pro-KIV 1.01 software (Applied Photophysics) was used to perform kinetic simulations of the mechanistic models using the determined rate constants from Table 1 based on the indicated reaction schemes.
Redox mobility shift assays
For the redox mobility shift assays, proteins were purified, reduced and oxidized as described above. The glutathionylation or oxidation of roGFP2 was investigated by mixing reduced roGFP2C151S or roGFP2C208S with mmPEG in the presence of 5 mM GSSG or Diamide. To monitor the oxidation of roGFP2WT by the intramolecular disulfide of PfGrxC88S, 1 µM reduced roGFP2WT was mixed with 2 µM diamide-oxidized PfGrxC88S at room temperature. After different time points 160 µM mmPEG was added and the solution was incubated on ice for 15 min. Proteins were precipitated by adding four volumes of ice-cold acetone and subsequent centrifugation (21.000g, 20 min, 4 °C). The supernatant was discarded and samples were resuspended in 5 × Laemmli buffer. To monitor the transfer of the glutathione moiety from PfGrx to roGFP2, 5 µM reduced roGFP2WT, roGFP2C151S or roGFP2C208S were mixed with variable concentrations of glutathionylated PfGrxC88S or PfGrxC32S/C88S at room temperature for 1 h. The reaction was quenched by the addition of 5 × Laemmli and 400 µM mmPEG and incubation for 15 min on ice. Samples in Laemmli buffer were boiled at 95 °C for five min and applied to a 15% SDS gel, separated by SDS-PAGE, and stained with coomassie. Uncropped and unprocessed scans of Fig. S1 are provided in the Source Data file.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting the findings of this study are provided in the Manuscript and its Supplementary Information/Source Data files. The data regarding electrophoretic mobility shift assays, nonenzymatic reaction between roGFP2WT(SH)2 and GSSG, thiol-specificity of the PfGrx-catalyzed reduction of roGFP2WT(S2), and rate constants at variable GSH concentrations are provided in the Supplementary Information. Uncropped and unprocessed scans of Supplementary Fig. 1 are provided in the Source Data file. A list of primers and plasmids are provided as separate Supplementary Data 1. The structures referenced in Fig. 1d are available at the following PDB accession codes: 1EGO, 1GRX, 1QFN and 1EGR. Source data are provided with this paper.
References
Nagai, S. & Black, S. A thiol-disulfide transhydrogenase from yeast. J. Biol. Chem. 243, 1942–1947 (1968).
Holmgren, A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc. Natl Acad. Sci. USA 73, 2275–2279 (1976).
Mieyal, J. J., Starke, D. W., Gravina, S. A. & Hocevar, B. A. Thioltransferase in human red blood cells: kinetics and equilibrium. Biochemistry 30, 8883–8891 (1991).
Couturier, J., Jacquot, J. P. & Rouhier, N. Evolution and diversity of glutaredoxins in photosynthetic organisms. Cell Mol. Life Sci. 66, 2539–2557 (2009).
Gallogly, M. M., Starke, D. W. & Mieyal, J. J. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid. Redox Signal 11, 1059–1081 (2009).
Lillig, C. H. & Berndt, C. Glutaredoxins in thiol/disulfide exchange. Antioxid. Redox Signal 18, 1654–1665 (2013).
Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 1830, 3217–3266 (2013).
Liedgens, L. & Deponte, M. in Glutathione (ed. Flohe, L.) 251–261 (CRC Press, 2019).
Gravina, S. A. & Mieyal, J. J. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry 32, 3368–3376 (1993).
Yang, Y. et al. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry 37, 17145–17156 (1998).
Gallogly, M. M., Starke, D. W., Leonberg, A. K., Ospina, S. M. & Mieyal, J. J. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles. Biochemistry 47, 11144–11157 (2008).
Mesecke, N., Mittler, S., Eckers, E., Herrmann, J. M. & Deponte, M. Two novel monothiol glutaredoxins from Saccharomyces cerevisiae provide further insight into iron-sulfur cluster binding, oligomerization, and enzymatic activity of glutaredoxins. Biochemistry 47, 1452–1463 (2008).
Eckers, E., Bien, M., Stroobant, V., Herrmann, J. M. & Deponte, M. Biochemical characterization of dithiol glutaredoxin 8 from Saccharomyces cerevisiae: the catalytic redox mechanism redux. Biochemistry 48, 1410–1423 (2009).
Begas, P., Liedgens, L., Moseler, A., Meyer, A. J. & Deponte, M. Glutaredoxin catalysis requires two distinct glutathione interaction sites. Nat. Commun. 8, 14835 (2017).
Liedgens, L. et al. Quantitative assessment of the determinant structural differences between redox-active and inactive glutaredoxins. Nat. Commun. 11, 1725 (2020).
Chang, S. H. & Wilken, D. R. Participation of the unsymmetrical disulfide of coenzyme A and glutathione in an enzymatic sulfhydryl-disulfide interchange. I. Partial purification and properties of the bovine kidney enzyme. J. Biol. Chem. 241, 4251–4260 (1966).
Srinivasan, U., Mieyal, P. A. & Mieyal, J. J. pH profiles indicative of rate-limiting nucleophilic displacement in thioltransferase catalysis. Biochemistry 36, 3199–3206 (1997).
Johansson, C., Lillig, C. H. & Holmgren, A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J. Biol. Chem. 279, 7537–7543 (2004).
Zaffagnini, M. et al. Glutaredoxin s12: unique properties for redox signaling. Antioxid. Redox Signal 16, 17–32 (2012).
Corteselli, E. M. et al. Structural and functional fine mapping of cysteines in mammalian glutaredoxin reveal their differential oxidation susceptibility. Nat. Commun. 14, 4550 (2023).
Brautigam, L. et al. Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1. Proc. Natl Acad. Sci. USA 110, 20057–20062 (2013).
Boukhenouna, S. et al. Evidence that glutathione and the glutathione system efficiently recycle 1-cys sulfiredoxin in vivo. Antioxid. Redox Signal 22, 731–743 (2015).
Rouhier, N. et al. Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor. Plant Physiol. 127, 1299–1309 (2001).
Pauwels, F., Vergauwen, B., Vanrobaeys, F., Devreese, B. & Van Beeumen, J. J. Purification and characterization of a chimeric enzyme from Haemophilus influenzae Rd that exhibits glutathione-dependent peroxidase activity. J. Biol. Chem. 278, 16658–16666 (2003).
Hanschmann, E. M. et al. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-Cys peroxiredoxin Prx3. J. Biol. Chem. 285, 40699–40705 (2010).
Djuika, C. F. et al. Plasmodium falciparum antioxidant protein as a model enzyme for a special class of glutaredoxin/glutathione-dependent peroxiredoxins. Biochim. Biophys. Acta 1830, 4073–4090 (2013).
Peskin, A. V. et al. Glutathionylation of the active site cysteines of peroxiredoxin 2 and recycling by glutaredoxin. J. Biol. Chem. 291, 3053–3062 (2016).
Schumann, R., Lang, L. & Deponte, M. Characterization of the glutathione-dependent reduction of the peroxiredoxin 5 homolog PfAOP from Plasmodium falciparum. Protein Sci. 31, e4290 (2022).
Lang, L. et al. Substrate promiscuity and hyperoxidation susceptibility as potential driving forces for the co-evolution of Prx5-Type and Prx6-Type 1-Cys peroxiredoxin mechanisms. ACS Catalysis 13, 3627–3643 (2023).
Begas, P., Staudacher, V. & Deponte, M. Systematic re-evaluation of the bis(2-hydroxyethyl)disulfide (HEDS) assay reveals an alternative mechanism and activity of glutaredoxins. Chem. Sci. 6, 3788–3796 (2015).
Bjornberg, O., Ostergaard, H. & Winther, J. R. Mechanistic insight provided by glutaredoxin within a fusion to redox-sensitive yellow fluorescent protein. Biochemistry 45, 2362–2371 (2006).
Meyer, A. J. et al. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 52, 973–986 (2007).
Trnka, D. et al. Molecular basis for the distinct functions of redox-active and FeS-transfering glutaredoxins. Nat. Commun. 11, 3445 (2020).
Zimmermann, J. et al. One cysteine is enough: a monothiol Grx can functionally replace all cytosolic Trx and dithiol Grx. Redox Biol. 36, 101598 (2020).
Zimmermann, J., Oestreicher, J., Geissel, F., Deponte, M. & Morgan, B. An intracellular assay for activity screening and characterization of glutathione-dependent oxidoreductases. Free Radic. Biol. Med. 172, 340–349 (2021).
Katzen, H. M., Tietze, F. & Stetten, D. Jr Further studies on the properties of hepatic glutathione-insulin transhydrogenase. J. Biol. Chem. 238, 1006–1011 (1963).
Rahlfs, S., Fischer, M. & Becker, K. Plasmodium falciparum possesses a classical glutaredoxin and a second, glutaredoxin-like protein with a PICOT homology domain. J. Biol. Chem. 276, 37133–37140 (2001).
Brautigam, L. et al. Vertebrate-specific glutaredoxin is essential for brain development. Proc. Natl Acad. Sci. USA 108, 20532–20537 (2011).
Gellert, M. et al. Identification of a dithiol-disulfide switch in collapsin response mediator protein 2 (CRMP2) that is toggled in a model of neuronal differentiation. J. Biol. Chem. 288, 35117–35125 (2013).
Zheng, M., Aslund, F. & Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721 (1998).
Lillig, C. H. et al. New thioredoxins and glutaredoxins as electron donors of 3’-phosphoadenylylsulfate reductase. J. Biol. Chem. 274, 7695–7698 (1999).
Berndt, C., Schwenn, J. D. & Lillig, C. H. The specificity of thioredoxins and glutaredoxins is determined by electrostatic and geometric complementarity. Chem. Sci. 6, 7049–7058 (2015).
Tarrago, L. et al. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases B by glutaredoxins and thioredoxins. J. Biol. Chem. 284, 18963–18971 (2009).
Kim, H. Y. Glutaredoxin serves as a reductant for methionine sulfoxide reductases with or without resolving cysteine. Acta Biochim. Biophys. Sin. (Shanghai) 44, 623 (2012).
Holmgren, A. Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J. Biol. Chem. 254, 3664–3671 (1979).
Zahedi Avval, F. & Holmgren, A. Molecular mechanisms of thioredoxin and glutaredoxin as hydrogen donors for Mammalian s phase ribonucleotide reductase. J. Biol. Chem. 284, 8233–8240 (2009).
Rozman Grinberg, I. et al. A glutaredoxin domain fused to the radical-generating subunit of ribonucleotide reductase (RNR) functions as an efficient RNR reductant. J. Biol. Chem. 293, 15889–15900 (2018).
Berndt, C., Poschmann, G., Stuhler, K., Holmgren, A. & Brautigam, L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2, 673–678 (2014).
Peltoniemi, M. J., Karala, A. R., Jurvansuu, J. K., Kinnula, V. L. & Ruddock, L. W. Insights into deglutathionylation reactions. Different intermediates in the glutaredoxin and protein disulfide isomerase catalyzed reactions are defined by the gamma-linkage present in glutathione. J. Biol. Chem. 281, 33107–33114 (2006).
Saaranen, M. J., Salo, K. E., Latva-Ranta, M. K., Kinnula, V. L. & Ruddock, L. W. The C-terminal active site cysteine of Escherichia coli glutaredoxin 1 determines the glutathione specificity of the second step of peptide deglutathionylation. Antioxid. Redox Signal 11, 1819–1828 (2009).
Banci, L. et al. [2Fe-2S] cluster transfer in iron-sulfur protein biogenesis. Proc. Natl. Acad. Sci. USA 111, 6203–6208 (2014).
Iwema, T. et al. Structural basis for delivery of the intact [Fe2S2] cluster by monothiol glutaredoxin. Biochemistry 48, 6041–6043 (2009).
Johansson, C. et al. The crystal structure of human GLRX5: iron-sulfur cluster co-ordination, tetrameric assembly and monomer activity. Biochem. J. 433, 303–311 (2011).
Izquierdo, A., Casas, C., Muhlenhoff, U., Lillig, C. H. & Herrero, E. Saccharomyces cerevisiae Grx6 and Grx7 are monothiol glutaredoxins associated with the early secretory pathway. Eukaryot. Cell 7, 1415–1426 (2008).
Rouhier, N., Gelhaye, E. & Jacquot, J. P. Exploring the active site of plant glutaredoxin by site-directed mutagenesis. FEBS Lett. 511, 145–149 (2002).
Discola, K. F. et al. Structural aspects of the distinct biochemical properties of glutaredoxin 1 and glutaredoxin 2 from Saccharomyces cerevisiae. J. Mol. Biol. 385, 889–901 (2009).
Bushweller, J. H., Aslund, F., Wuthrich, K. & Holmgren, A. Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14––S) and its mixed disulfide with glutathione. Biochemistry 31, 9288–9293 (1992).
Berardi, M. J. & Bushweller, J. H. Binding specificity and mechanistic insight into glutaredoxin-catalyzed protein disulfide reduction. J. Mol. Biol. 292, 151–161 (1999).
Cleland, W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim. Biophys. Acta 67, 104–137 (1963).
Ukuwela, A. A., Bush, A. I., Wedd, A. G. & Xiao, Z. Glutaredoxins employ parallel monothiol-dithiol mechanisms to catalyze thiol-disulfide exchanges with protein disulfides. Chem. Sci. 9, 1173–1183 (2018).
Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 (2008).
Morgan, B. et al. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat. Chem. Biol. 9, 119–125 (2013).
Deponte, M. The incomplete glutathione puzzle: just guessing at numbers and figures? Antioxid. Redox Signal 27, 1130–1161 (2017).
Oestreicher, J. & Morgan, B. Glutathione: subcellular distribution and membrane transport (1). Biochem. Cell Biol. 97, 270–289 (2019).
Dooley, C. T. et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279, 22284–22293 (2004).
Hanson, G. T. et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279, 13044–13053 (2004).
Ostergaard, H., Tachibana, C. & Winther, J. R. Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 166, 337–345 (2004).
Albrecht, S. C., Barata, A. G., Grosshans, J., Teleman, A. A. & Dick, T. P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829 (2011).
Schwarzlander, M., Dick, T. P., Meyer, A. J. & Morgan, B. Dissecting redox biology using fluorescent protein sensors. Antioxid. Redox Signal 24, 680–712 (2016).
Fujikawa, Y. et al. Mouse redox histology using genetically encoded probes. Sci. Signal 9, rs1 (2016).
Haag, M., Kehrer, J., Sanchez, C. P., Deponte, M. & Lanzer, M. Physiological jump in erythrocyte redox potential during Plasmodium falciparum development occurs independent of the sickle cell trait. Redox Biol. 58, 102536 (2022).
Morgan, B., Sobotta, M. C. & Dick, T. P. Measuring E(GSH) and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 51, 1943–1951 (2011).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Calabrese, G. et al. Hyperoxidation of mitochondrial peroxiredoxin limits H2 O2 -induced cell death in yeast. EMBO J. 38, e101552 (2019).
Staudacher, V. et al. Redox-sensitive GFP fusions for monitoring the catalytic mechanism and inactivation of peroxiredoxins in living cells. Redox Biol. 14, 549–556 (2018).
Kosower, N. S. & Kosower, E. M. Diamide: an oxidant probe for thiols. Methods Enzymol. 251, 123–133 (1995).
Deponte, M. in Redox Chemistry and Biology of Thiols (ed. Alvarez, B.) 241–275 (ScienceDirect, 2022).
Flohe, L. The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta 1830, 3139–3142 (2013).
Deponte, M. & Lillig, C. H. Enzymatic control of cysteinyl thiol switches in proteins. Biol. Chem. 396, 401–413 (2015).
Manevich, Y., Feinstein, S. I. & Fisher, A. B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl Acad. Sci. USA 101, 3780–3785 (2004).
Ralat, L. A., Misquitta, S. A., Manevich, Y., Fisher, A. B. & Colman, R. F. Characterization of the complex of glutathione S-transferase pi and 1-cysteine peroxiredoxin. Arch. Biochem. Biophys. 474, 109–118 (2008).
Ralat, L. A., Manevich, Y., Fisher, A. B. & Colman, R. F. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry 45, 360–372 (2006).
Rouhier, N. et al. Identification of plant glutaredoxin targets. Antioxid. Redox Signal 7, 919–929 (2005).
Sturm, N. et al. Identification of proteins targeted by the thioredoxin superfamily in Plasmodium falciparum. PLoS Pathog. 5, e1000383 (2009).
Schutte, L. D. et al. Identification of potential protein dithiol-disulfide substrates of mammalian Grx2. Biochim. Biophys. Acta 1830, 4999–5005 (2013).
Nagy, P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid. Redox Signal 18, 1623–1641 (2013).
Lopez-Maury, L., Sanchez-Riego, A. M., Reyes, J. C. & Florencio, F. J. The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. strain PCC 6803. J. Bacteriol. 191, 3534–3543 (2009).
Urscher, M., More, S. S., Alisch, R., Vince, R. & Deponte, M. Tight-binding inhibitors efficiently inactivate both reaction centers of monomeric Plasmodium falciparum glyoxalase 1. FEBS J. 279, 2568–2578 (2012).
Acknowledgements
This work was funded by the DFG grant DE 1431/20-1 to M.D. and, in part, by DFG RTG 2737 (STRESSistance). We thank Marisa Jakobs for the help with initial redox mobility shift assays and Robin Schumann for cloning wild-type and mutant ROGFP2.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.G. and L.L. performed the stopped-flow kinetic experiments and analyzed the data. B.H. performed the redox mobility shift assays. M.D. and B.M. conceptualized the study. M.D. supervised the study and wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kathrin Ulrich and John Mieyal for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geissel, F., Lang, L., Husemann, B. et al. Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction. Nat Commun 15, 1733 (2024). https://doi.org/10.1038/s41467-024-45808-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45808-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.