Abstract
De novo synthesis of the pyrimidine, cytidine triphosphate (CTP), is crucial for DNA/RNA metabolism and depends on the CTP synthetases, CTPS1 and −2. Partial CTPS1 deficiency in humans has previously been shown to lead to immunodeficiency, with impaired expansion of T and B cells. Here, we examine the effects of conditional and inducible inactivation of Ctps1 and/or Ctps2 on mouse embryonic development and immunity. We report that deletion of Ctps1, but not Ctps2, is embryonic-lethal. Tissue and cells with high proliferation and renewal rates, such as intestinal epithelium, erythroid and thymic lineages, activated B and T lymphocytes, and memory T cells strongly rely on CTPS1 for their maintenance and growth. However, both CTPS1 and CTPS2 are required for T cell proliferation following TCR stimulation. Deletion of Ctps1 in T cells or treatment with a CTPS1 inhibitor rescued Foxp3-deficient mice from fatal systemic autoimmunity and reduced the severity of experimental autoimmune encephalomyelitis. These findings support that CTPS1 may represent a target for immune suppression.
Similar content being viewed by others
Introduction
The cytidine nucleotide triphosphate (CTP) is a key precursor involved in the metabolism of DNA, RNA and phospholipids. CTP is in limiting concentration in cells and originates from two sources: a salvage pathway and a de novo pyrimidine synthesis pathway1. The de novo pathway depends on the CTP synthetase (CTPS) enzymatic activity, encoded by two genes: CTPS1 and CTPS2. These genes share 75% homology/identity2,3, are highly conserved in mouse (90% of homology/identity) and locate on chromosomes 1 and X respectively, both in human and mouse. CTPS1/2 catalyses the ATP-dependent amination of UTP to CTP with ammonia (NH3) transferred from hydrolysed glutamine4,5. CTPS activity is involved in cell proliferation and positively correlates with cell proliferation rates6,7,8,9,10.
We previously reported consequences of CTPS1 deficiency in humans caused by a homozygous frameshift splice mutation (c.1692-1G > C, p.T566Dfs26X) with a founder effect in populations of the Northwest of England11. The mutation leads to the skipping of exon 18 resulting in an unstable mutant protein associated with a reduction of more than 80% of total CTPS1 expression12. However, the mutation does not impact the CTP synthetase activity, and 10–20% of residual cellular CTPS activity is preserved12. All patients developed a combined immunodeficiency mainly characterised by high susceptibility to viral infections particularly Epstein Barr virus. These patients had no other clinical symptoms suggesting a critical role for CTPS1 in immune cells. Notably, T lymphocytes of patients exhibited an impaired capacity to proliferate while their effector functions like cytotoxicity and cytokine production were preserved except for IL-2 secretion. This selective effect on proliferation of activated T cells is explained by the strong up-regulation of CTPS1 expression in activated T lymphocytes upon T-cell receptor (TCR) activation. Furthermore, B cells also exhibited impaired proliferation when stimulated through the B-cell receptor (BCR). From these studies we concluded that CTPS1 could represent an attractive therapeutic target to selectively inhibit unwanted T/B cell proliferation involved in conditions such as autoimmunity diseases, T cell lymphoma/leukaemia or graft versus host disease (GvHD). Organ transplantation might also represent a good indication for CTPS1 targeting. A selective inhibitor of CTPS1 is currently being evaluated in relapsed and refractory T and B cell lymphoma patients (NCT05463263)13,14.
To acquire more knowledge on the biology of CTPS1 and CTPS2 in vivo and to better characterise their respective roles at the organism and cellular levels with a stronger focus on the immune system, several mouse models with tissue selective inactivation of Ctps1 and/or Ctps2 have been generated and studied here. We also showed that Ctps1 deletion or pharmaceutical inhibition using a selective small chemical inhibitor of CTPS1 dampened lethal poly-autoimmunity symptoms of the Scurfy mice and experimental autoimmune encephalomyelitis further supporting the proposal that targeting Ctps1 is a potential treatment of autoimmune disorders.
Results
Embryonic lethality in mouse caused by Ctps1 inactivation
To better characterise the role of CTPS1 in vivo, we first developed a mouse model mimicking the human mutation that did not result in any phenotype (Supplementary Fig. 1 and Supplementary Data information). The possible explanation for this discrepancy is that the splicing constraints for Ctps1/CTPS1 in mouse and human are different and in mouse the mutation did not disturb the splicing15. We took advantage of the KO Mouse Project consortium (KOMP, ID:89620) to obtain genetically modified Ctps1flox/flox mice allowing conditional inactivation of Ctps1 (Supplementary Fig. 2a, c). Eventually, Ctps1flox/flox animals were crossed with transgenic mouse lines expressing the cre recombinase (Cre) under the control of specific promoters (Supplementary Fig. 2d). Crossing with a CMV-Cre-transgenic mouse line16 leading to ubiquitous deletion of Ctps1 did not produce homozygous Ctps1-deficient mice (Ctps1ko/ko) at birth, while heterozygous and wild type animals were born, indicating in utero lethality when the two alleles of Ctps1 are deleted (Fig. 1a). To characterise the developmental arrest caused by Ctps1 gene inactivation, we first recovered fertilised eggs from females and cultured them for 5 days until the blastocyst stage and genotyped them by PCR. The number of Ctps1ko/ko blastocysts harvested was not significantly different from the expected Mendelian ratio, indicating that Ctps1 is not a critical requirement for blastocyst development. We next analysed embryos at different stages of development from embryonic day 6.5 (E6.5) to 9.5 (E9.5) using programmed mating (Fig. 1a–c). No homozygous Ctps1 deficient embryos were detected by day E9.5 and increasing numbers of embryos with morphological abnormalities were found between day E6.5 and E8.5. Abnormal embryos recovered at E8.5 were arrested at gastrulation stages. Supporting a role of Ctps1 at this stage, CTPS1 was found to be highly expressed in the E6.5 epiblast before gastrulation (Fig. 1c, d). However, low remaining staining was observed in Ctps1 deficient embryos probably due to tissue autofluorescence and/or antibody trapping as whole mount mouse embryos is prone to low level of background staining. Thus, our data indicate that Ctps1 is strictly required for embryo development between day E6.5 and E8.5. This is consistent with the role of cell proliferation during gastrulation as the major morphogenetic force that leads to the expansion of the ectoderm, mesoderm and endoderm layers17,18.
a Quantification of animals with a given genotype at different stages. CMV-Cre; Ctps1wt/ko males were crossed with Ctps1flox/flox females. Blastocysts were generated in vitro. Numbers in brackets indicate abnormal embryos. *** significant difference with a chi-square test, indicating embryonic lethality. b Brightfield images of Ctps1wt/flox (upper panels) and CMV-Cre; Ctps1flox/ko (lower panels) at stage E6.5, E8.5 and E9.5 (Scale bars = 100 µm). Images are representatives of 6–13 embryos analysed in three independent experiments. c Whole mount immunostaining and 3D imaging of E6.5 embryos with an anti-CTPS1 antibody (grey), which is detected in the epiblast (e) and extra-embryonic ectoderm (Xe) in controls Ctps1wt/ko embryos (Scale bars = 100 µm). d The mean pixel intensity of the CTPS1 signal from panel (c) is significantly decreased in CMV-Cre; Ctps1flox/ko mutant embryos. A t-test with Welch’s correction is used and data are presented as the mean ± SD of n = 5 control and three mutant embryos. Source data are provided in Source Data file.
Tissues with high renewal and proliferation rates are highly dependent on CTPS1
To prevent the embryonic lethality caused by Ctps1 deletion, we crossed Ctps1flox/flox mice to a Cre-ERT2 transgenic mouse line enabling inducible deletion of Ctps1 upon tamoxifen treatment (Table 1)19. As expected, in vitro tamoxifen treatment resulted in Ctps1 deletion and loss of CTPS1 expression in splenic T cells from Cre-ERT2; Ctps1flox/flox but not from Ctps1wt/flox animals (Supplementary Fig. 3a). Cre-ERT2; Ctps1wt/flox and Cre-ERT2; Ctps1flox/flox mice were either forced-fed with tamoxifen, to induce Ctps1 deletion, or with a vehicle (Figs. 2, 3). Deletion of Ctps1 by tamoxifen treatment in vivo was confirmed at protein level in T lymphocytes and by PCR (Fig. 3d and Supplementary Fig. 3b).
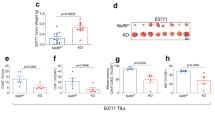
a–f Analysis of Cre-ERT2; Ctps1wt/flox and Cre-ERT2; Ctps1flox/flox animals fed with vehicle (Ctps1wt/flox and Ctps1flox/flox or tamoxifen to induce Ctps1) gene inactivation (Ctps1wt/ko and Ctps1ko/ko). a Weights and blood parameters (whole blood cells, lymphocytes, neutrophils, red blood cells, haemoglobin, and reticulocytes) of animals from day 1 of tamoxifen treatment to day 14. Values are expressed as percentage of the initial population counts at day 0. b Representative microscopy images of haematoxylin-eosin coloration of small intestine from tamoxifen treated Ctps1wt/ko and Ctps1ko/ko. Images are representatives of four animals per group. Black arrowhead highlights abnormal features (Scale bars = 100 µm). c Intestinal villi length of non-treated Ctps1wt/flox, Ctps1flox/flox, and tamoxifen treated Ctps1wt/ko and Ctps1ko/ko animals measured at day 14. d-f Analysis of Peyer patches from the intestine. Left panels of representative dot-plots from FACS analysis stained with (d) anti-B220 and anti-TCR antibodies, (e) anti-CD95 and anti-GL7 or (f) anti-CXCR5 and PD-1. Right panels of percentages of B cells and T cells (d), germinal centre (GC) B cells (e) and T follicular helper cells (Tfh) (f) from dot-plots. g–j Cre-ERT2; Ctps1wt/flox or Cre-ERT2; Ctps1flox/flox were immunised with NP-CGG at day 0 and then treated with tamoxifen at day 4 and 8 to induce Ctps1 deletion. g Experimental design. h NP-specific IgM (left), and IgG1 (right) from non-immunised (Non imm.) animals and immunised Ctps1wt/ko and Ctps1ko/ko were quantified by ELISA (arbitrary unit). i Percentages of germinal centre (GC) B cells, anti-CD95 and anti-GL7 (left) and T follicular helper cells (Tfh), anti-CXCR5 and PD-1 (right) obtained from dot-plots of Ctps1wt/ko and Ctps1ko/ko. j Representative microscopy images of haematoxylin-eosin coloration, B220 and PCNA labelling of spleen from tamoxifen treated Cre-ERT2; Ctps1wt/flox and Cre-ERT2; Ctps1flox/flox. Images are representatives of three animals per group. (Scale bars from left to right: 5 mm, 1 mm and 250 µm). Two-way ANOVA with error bar representing mean ± SEM of n = 10 (wt/flox), n = 9 (flox/flox), n = 17 (wt/ko) and n = 14 (ko/ko) animals per group (a). Unpaired t-tests two-tailed with error bar representing mean ± SD; measurement of 100 villi length (c). Data are presented as the mean ± SD of n = 8 (wt/ko) and n = 8 (ko/ko) (d). e–h Non-parametric Matt-Whitney two-tailed test were used. Data are presented as the mean ± SD of n = 8 animals per group (e, f). Data are presented as mean with ± SD of n = 6 (non-immunised); n = 5 (wt/ko) and n = 6 (ko/ko) animals per group (h) and data are presented as the mean ± SD of n = 5 (wt/ko) and n = 6 (ko/ko) (i). Source data are provided in Source Data file.
a–e Analysis of thymocytes and splenocytes Cre-ERT2; Ctps1wt/flox and Cre-ERT2; Ctps1flox/flox animals fed with vehicle (Ctps1wt/flox and Ctps1flox/flox) or tamoxifen to induce Ctps1 gene inactivation (Ctps1wt/ko and Ctps1ko/ko). a Upper panels corresponding to representative dot-plots from FACS analysis of thymocytes for CD4 and CD8 expression. Lower panels showing total thymus cell counts and percentages of double negative CD4 and CD8 (DN), double positive CD4 and CD8 (DP), single positive CD4 (SP4) and single positive CD8 (SP8) thymocytes from FACS analyses. b Spleen cell counts (left panel) and percentages of splenic B, T, CD4, CD8 and NK cells calculated from FACS analyses. c Representative proliferation histogram profiles from FACS analysis of enriched spleen T cells labelled with cell trace violet (CTV) and activated ex vivo after tamoxifen treatment, with anti-CD3/CD28 beads plus IL-2 for 3 days. Graph bars (right panel) corresponding to proliferation indexes calculated from histogram profiles (left panels). On the right panels, same as left but 4-hydroxy tamoxifen was extemporary added to the ex vitro culture of spleen T cells (right panels). d Immunoblots for CTPS1, CTPS2 and Actin expression in lysates of non-activated or activated T cells at the day of sacrifice. Four treated Ctps1wt/ko and Ctps1ko/ko animals were analysed. Blot is the pool of two independent experiments. Molecular weights in kDa on the left. e B and T cells were sorted from total spleen of Ctps1wt/ko and Ctps1ko/ko mice. Both T and B were labelled with CTV. B-cell proliferation was induced by stimulation with LPS and IL-4 for 3 days, while T cell proliferation was induced by anti-CD3/CD28 beads plus IL-2 for 3 days. Graph bars (right panels) corresponding to proliferation indexes calculated from histogram profiles. Non-parametric Matt-Whitney two-tailed test (a–c left) and unpaired two-tailed t-tests (c right and e) were used. Data are presented as the mean ± SD of n = 9 (wt/flox), n = 8 (flox/flox), n = 12 (wt/ko) and n = 13 (ko/ko) animals per group (a), of n = 12 (wt/flox), n = 12 (flox/flox), n = 17 (wt/ko) and n = 17 (ko/ko) animals per group (b), of n = 7 per group (c-left) and of n = 4 animals per group (c right and e). Source data are provided in Source Data file.
Ctps1ko/ko presented a drastic weight loss upon tamoxifen treatment by day 6 leading to their sacrifice at day 14. In contrast to other animal groups, the Ctps1ko/ko group showed a marked drop in reticulocytes numbers associated with reduced haemoglobin, red blood cell and lymphocytes numbers, while there were no significant differences in the total number of whole blood cells and neutrophils (Fig. 2a). Considering that the Ctps1ko/ko animals were dramatically losing weight, we carefully examined the gut of Ctps1ko/ko animals. Haematoxylin-eosin coloration of gut sections revealed aberrant gut structures in Ctps1ko/ko animals. The lamina propria was detaching from the epithelium and there was an abnormal cell shedding at the top of the villi. Numerous empty crypts and vacuoles were observed in the tissue (Fig. 2b). In addition, the length of the villi was significantly reduced when compared to all other groups (Fig. 2c). These observations showed that CTPS1 is key for the proliferation and the renewal of the intestinal epithelium of the gut, which is considered as one of the highly proliferative tissues in the whole body20.
We also analysed Peyer’s patches (PP), that host numerous germinal centres (GCs)21, sites of the most intense activation and proliferation of B cells in the body22 (Fig. 2d-f). Proportions of T and B cells in PP of Ctps1ko/ko were similar to those of Ctps1wt/ko (Fig. 2d). We next examined proliferating B cells and T follicular helper cells (TFH) from the GCs23,24. While CD19+CD95+GL-7+ GC B cells were easily detected in Ctps1wt/ko, non-treated Cre-ERT2; Ctps1flox/flox or Cre-ERT2; Ctps1wt/flox animals, they were almost absent Ctps1ko/ko animals (Fig. 2e and Supplementary Fig. 3c). Likewise, CXCR5+PD-1+ TFH cells from Ctps1ko/ko animals were reduced in GCs (Fig. 2f). Thus, these observations show a key role of CTPS1 in the expansion of activated B cells and TFH in GCs. The capacity of mice to mount humoral immune responses against the T-dependent antigen 4-hydroxy-3-nitrophenyl (NP) hapten conjugated to Chicken Gamma Globulin (CGG) was then evaluated. Tamoxifen-treated Cre-ERT2; Ctps1flox/flox or Cre-ERT2; Ctps1wt/flox were immunised intraperitoneally (i.p.) with NP-CGG adsorbed on alum (Fig. 2g). At day 14 the relative serum titres of NP-specific IgM and IgG1 antibodies was measured by ELISA. A significant reduction of NP-specific IgM was observed in Ctps1ko/ko compared to Ctps1wt/ko (Fig. 2h). There was also a tendency towards a reduction NP-specific IgG1 in Ctps1ko/ko animals (although it was not statistically different). In parallel, proliferating GC B cells and T follicular helper cells (TFH) in the Peyer patches were analysed. Percentages of CD19+CD95+GL-7+ GC B cells as CXCR5+PD-1+ TFH cells were reduced after immunisation with NP-CGG in Ctps1ko/ko mice compared to Ctps1wt/ko animals (Fig. 2i). Histology analyses of spleen, after immunisation, confirmed the observations from the Peyer patches showing a reduction of proliferating B cells stained with B220 and PCNA (Fig. 2j). The capacity of mice to mount humoral immune responses against the T-dependent antigen was also tested in immunised C57BL/6 mice treated with the Stp-2 compound a small chemical inhibitor of CTPS1 (see below). A similar reduction of NP-specific IgM titres, a decreased proliferating GC B cells and T follicular helper cells (TFH) were observed (Supplementary Fig. 4a–d). Altogether these data indicate that CTPS1 is required for antibody responses.
Thymus of Ctps1ko/ko mice were smaller in size, and cellularity was reduced 10-fold compared to control (Fig. 3a). Most cells were CD4-/CD8- double negative, suggesting impaired proliferation capacity of early thymocytes. In parallel, single positive CD4 and CD8 cells tended to accumulate (Fig. 3a). In contrast, spleens of Ctps1ko/ko mice display a cellularity and composition of B, NK, dendritic cells, macrophages, and neutrophils subsets similar to those of Ctps1flox/flox or Ctps1wt/ko animals (Fig. 3b and Supplementary Fig. 3d). However, there was a slight increase of CD8+ T cells in Ctps1ko/ko mice (Fig. 3b), with no abnormalities in naive and memory CD4+ and CD8+ T cells (Supplementary Fig. 3e). Because the major immune consequence of CTPS1 deficiency in humans is the impaired capacity of activated T cells to proliferate, expansion of splenic Ctps1ko/ko T cells in response to CD3 and CD28 stimulation, was then assessed and found to be profoundly impaired which correlated with decreased CTPS1 expression (Fig. 3c, left panels and d). However, some residual CTPS1 expression was observed in Ctps1ko/ko T cells likely due to incomplete Ctps1 deletion after tamoxifen treatment. Moreover, T cells from vehicle-treated Ctps1flox/flox mice failed to proliferate when 4-hydroxy tamoxifen was extemporarily added to the culture medium (Fig. 3c, right panels). Proliferation of sorted splenic B cells from Ctps1ko/ko animals upon stimulation with LPS + IL-4 was also significantly reduced (Fig. 3e, left panel), as the proliferation of sorted T cells from Ctps1ko/ko animals upon stimulation with anti-CD3 and -CD28 (Fig. 3e, right panel).
Taken together, these data demonstrate that CTPS1 is mandatory for proliferation of cells and tissues with high proliferative and renewal potential such as thymocytes, activated lymphocytes and gut epithelial cells. These results also indicate that CTPS1 deletion even in an adult organism is not compatible with life.
An essential role of CTPS1 in haematopoiesis
Haematopoiesis, in particular erythropoiesis is a developmental process highly dependent on cell proliferation. To evaluate the role of CTPS1 in haematopoiesis, Ctps1flox/flox mice were crossed with the VAV-Cre-transgenic mouse line (Table 1)25. Mendelian distribution of the expected genotypes was found biased. Ctps1ko/ko mice were under-represented and did not survive after 6 weeks of age (Fig. 4a). Ctps1ko/ko animals were smaller in size when compared to littermate heterozygous animals (Fig. 4b and Supplementary Fig. 5a). A severe anaemia was found in these mice likely indicating an impairment of erythropoiesis (Fig. 4c). The thymus was also markedly reduced in size and cellularity. Double positive cells were reduced in Ctps1ko/ko animals while single positive CD4 and CD8 were found in higher percentages, suggesting reduced proliferation of double negative (DN) thymocytes leading to double positive (DP) stage (Fig. 4b, d). However, spleen cellularity was comparable to wild type animals or heterozygotes, but absolute numbers of B and T lymphocytes and NK cells were reduced, while macrophages and granulocytes were not affected (Fig. 4e and Supplementary Fig. 5b, c). Bone marrow (BM) cellularity was markedly reduced in Ctps1ko/ko animals. The percentage of Ter119+lin- bone marrow cells26,27 corresponding to erythrocyte progenitors was strongly decreased, while that of lin−Sca1+Kit+ (LSK+) cells which are highly enriched in haematopoietic stem and multi-potent progenitor cells26,27,28 was similar to that found in the BM of littermate heterozygous or wild type animals (Fig. 4f and Supplementary Fig. 5d). Absolute numbers of erythroid and hematopoietic progenitors were reduced as bone marrow cellularity of Ctps1ko/ko animals was low (Fig. 4f). Therefore, these data show that Ctps1 is essential for haematopoiesis of erythroid and lymphoid cells but not for myeloid cells and granulocytes.
a Graph bars representing the percentage of animals of each genotype (Ctps1wt/wt, n = 18 Ctps1wt/ko, n = 31 and Ctps1ko/ko, n = 9) obtained from the VAV-Cre; Ctps1wt/flox breeding (n = 100). The horizontal black line represents the percentage of the expected repartition of each genotype. b Macroscopic views of VAV-Cre; Ctps1wt/flox (Ctps1wt/ko) and VAV-Cre; Ctps1flox/flox (Ctps1koko) animals and their thymus (upper right), spleen (middle right) and heart (lower right). c–e Analysis of haematological and immunological parameters at day 15-25. c Red blood cell numbers, haemoglobin, and haematocrit. d Left panels corresponding to representative dot-plots from FACS analysis of thymocytes for CD4 and CD8 expression. Right panels showing total thymus cell counts and percentages of double negative CD4 and CD8 (DN), positive CD4 and CD8 (DP), single positive CD4 (SP4) and single positive CD8 (SP8) thymocytes from FACS analyses. e Total spleen cell counts (left panel) and proportions of spleen B, T, NK cells, macrophages and granulocytes (right panels) from FACS analysis. d, e Empty red circle represent a 15-day-old animal. f Total counts of bone marrow cells. Representative dot-plots from FACS analysis of red blood cell progenitors (Ter119+) contained in the bone marrows stained with anti-Ter119 and anti-lineages antibodies. c, d–f Unpaired, two-tailed t-tests. Data are presented as the mean ± SD of n = 4 (wt/wt), n = 3 (wt/ko) and n = 4 (ko/ko) animals per group (c); of n = 4 (wt/wt), n = 4 (wt/ko) and n = 5 (ko/ko) animals per group (d–e); of n = 4 animals per group (f). Source data are provided in Source Data file.
CTPS1 is required for the proliferation of activated T cells
To go deeper into the role of CTPS1 in T lymphocytes activation, Ctps1flox/flox mice were crossed to CD4-Cre- or CD8-Cre-transgenic mouse lines (Table 1)29,30. Mendelian distribution of the different genotypes was normal. No phenotypic differences were detected in the thymus and the spleen of Ctps1ko/ko animals from breeding with CD4-Cre and CD8-Cre-Tg animals when compared to the littermate control genotypes (Fig. 5a and Supplementary Fig. 6a, d). The expression of the CD8-Cre transgene is restricted to peripheral CD8+ T cells29, while expression of the CD4-Cre transgene starts at the late DN4 stage to single positive (SP) stage leading to the deletion of Ctps1 in all T cells leaving the thymus30. As no phenotypic differences at the different stages of thymic development were detected in the thymus from CD4-Cre transgenic mice, expression of the Cre transgene could be too late to impact the DN-DP transition during which thymocytes are highly proliferating31,32. To confirm this point, we harvested thymuses from CD4-Cre-Ctps1wt/ko and CD4-Cre-Ctps1ko/ko and sorted different thymocyte populations including DN2, DN3, DN4, DP, SP4 and SP8 (Fig. 5b). PCR analysis showed that the Flox allele disappeared in DP, SP4 and SP8 populations consistent with the expression of the CD4-Cre transgene after the DN4 stage and accounting for some major phenotypic consequences in thymocytes subsets. Furthermore, no abnormalities were detected in blood, spleen cellularity and cell type composition (B cells, CD4+ and CD8+ T cells, NK, dendritic cells, macrophages and neutrophils) of CD4-Cre-Ctps1ko/ko and animals CD8-Cre-Ctps1ko/ko (Supplementary Fig. 6b, c, e). Both CD4 and CD8 enriched splenic CD4-Cre-Ctps1ko/ko T cells showed a marked reduction in proliferation upon CD3/CD28 stimulation, when compared to the proliferation of CD4-Cre-Ctps1wt/ko T cells (Fig. 5c). As expected, activated CD4-Cre-Ctps1ko/ko T cells did not express CTPS1 protein (Fig. 5d). Of note, as described for human T cells11,12, murine non-activated T cells expressed weak amounts of CTPS1 that are markedly upregulated following TCR activation, while CTPS2 expression remained relatively stable (Fig. 5d). Further analysis of T cell subsets after 3 days of activation, showed that both CD4+ and CD8+ T cells from CD4-Cre-Ctps1ko/ko presented a significant reduction of effector memory cells (CD44+CD62L-), associated with a relative increase of naive (CD44-CD62L+) T cells (Fig. 5e). CD8+ T cells, but CD4+ T cells from the spleen of CD8-Cre-Ctps1ko/ko upon activation with anti-CD3/CD28 beads, failed to proliferate leading to an effector memory CD8+ T cells impairment (Fig. 5f, g). Addition of cytidine to the culture medium during the activation by allowing CTP production through the salvage pathway rescued the proliferation of the CD8+ T cells (Fig. 5h).
a–e Analyses of Ctps1wt/flox (Ctps1wt/flox), Ctps1flox/flox (Ctps1flox/flox), CD4-Cre; Ctps1wt/flox (Ctps1wt/ko) and CD4-Cre; Ctps1flox/flox (Ctps1ko/ko). a Representative dot-plots, of two independent experiments, from FACS analyses of thymic sub-populations stained with CD4 and CD8 double negative CD4 and CD8 (DN) double positive CD4 and CD8 (DP), single positive CD4 (SP4) and single positive CD8 (SP8) cells. DN cells were further labelled with CD44 and CD25 to identify stages of DN differentiation. b RT-PCR analyses of the Ctps1 deletion in total thymus (bulk), sorted cells from DN2, DN3 and DN4 subtypes of DN cells and SP4 and SP8 cells. Corresponding amplified alleles/genotypes are indicated on the right (flox, wt and ko). c–e Analysis of splenic enriched T cells activated by anti-CD3/CD28 plus IL-2 for 3 days. c Representative proliferation dot plot profiles (left panels) from FACS analysis of T cells. Graph bars (right panels) corresponding to proliferation indexes calculated from histogram profiles on CD4+ T cells subset (upper panel), and CD8+ T cells subset (lower panel). d Immunoblots for CTPS1, CTPS2 and Actin expression in lysates of non-activated or activated T cells. Molecular weights in kDa on the left. e Proportions of T cell subsets from FACS analyses in the cultures after activation. Naive T cells (CD44-CD62L+), central memory (CD44+CD62L+), effector memory (CD44+CD62L-) are shown in both CD4+ and CD8+ T cells. Controls correspond to littermates (Ctps1wt/flox, Ctps1flox/flox and Ctps1wt/ko) of CD4-Cre; Ctps1flox/flox (Ctps1ko/ko). Blot is representative of four independent experiments. f–h Analyses of splenic enriched T cells analyses from CD8-Cre; Ctps1wt/flox (Ctps1wt/ko) and CD8-Cre; Ctps1flox/flox (Ctps1ko/ko) activated by anti-CD3/CD28 plus IL-2 for 3 days. f Representative proliferation histogram profiles from FACS analysis of enriched CD8-Cre-Ctps1ko/ko and Ctps1wt/ko splenic T CD4+ (left panels) or CD8+ (right panels) cells labelled with cell trace violet (CTV) and activated. White and red histograms correspond to Ctps1wt/ko and Ctps1ko/ko respectively. Grey histograms represent unstimulated cells. Graph bars on the right correspond to proliferation indexes calculated from histogram profiles. g Proportions of CD4+ and CD8+ T cell subsets from FACS analyses in the cultures after activation. Naive T cells (CD44-CD62L+), central memory (CD44+CD62L+), effector memory (CD44+CD62L-). Controls correspond to littermates (Ctps1wt/ko) of CD4-Cre; Ctps1flox/flox (Ctps1ko/ko). h Same as (f, right panel) except that CD8+ T cells from Ctps1wt/ko (upper panel) and Ctps1ko/ko (lower panel) were supplemented (green filled) or not (white or red filled histograms) with cytidine (200 μM). c, e–g Non-parametric Matt-Whitney two-tailed test were used. Data are presented as the mean ± SD of n = 5 (wt/flox), n = 6 (flox/flox), n = 6 (wt/ko) and n = 11 (ko/ko) animals per group (c), n = 11 per group (e), n = 12 animals per group (f) and of n = 9 animals per group (g). Source data are provided in Source Data file.
Overall, these data demonstrate the importance of CTPS1 in proliferation of activated T cells and recapitulate observations made with human CTPS1-deficient T cells11,12. However, both in humans and mice, activated CTPS1-deficient T cells retained a residual capacity to proliferate, indicating that T-cell proliferation is not fully dependent on CTPS1.
Ctps2 is not required for mice development
As CTP synthetase activity is dependent on CTPS1 and CTPS22, we next wanted to evaluate the role of CTPS2 and its putative redundancy with CTPS1. To that purpose, Ctps2flox/flox animals were obtained by using a EUCOMM construct (Supplementary Fig. 2b, c). Ctps2flox/flox animals were first crossed to CMV-Cre-transgenic mice. Viable Ctps2ko/ko animals were obtained. After birth, animals developed with no apparent abnormalities and had normal aging. Absence of the CTPS2 protein expression in Ctps2ko/ko animal was verified in lysates prepared from haematopoietic and non-haematopoietic tissues (Fig. 6a). No phenotypic difference in Ctps2ko/ko mice was detected in blood, thymus, spleen, bone marrow and gut when compared with littermate controls (Supplementary Fig. 7a–c). Proliferation of splenic Ctps2ko/ko T cells in response to CD3/CD28 was also found to be normal and comparable to that of littermate control T cells (Fig. 6b). No abnormalities in differentiation of memory T cells was noticed (Supplementary Fig. 7d). Phenotype and B cells counts were also normal in the spleen of Ctps2ko/ko animals (Supplementary Fig. 7c, e). Proliferating CD19+CD95+GL-7+ B cells in the GCs of Peyer’s patches of Ctps2ko/ko animals were found to be present in similar proportions as Ctps2wt/ko or wild-type animals (Supplementary Fig. 7e). In addition, proliferation of Ctps2ko/ko splenic B cells stimulated with LPS plus IL-4 was comparable to that of Ctps2wt/ko B cells (Fig. 6c). These data indicate that the inactivation of Ctps2 is compatible with life and has no developmental deleterious effects on development in mice. Moreover, Ctps2 is not mandatory for the proliferation of activated T and B lymphocytes.
a–c Analyses of T and B lymphocytes of CMV-Cre; Ctps2flox/flox (Ctps2ko/ko) and control littermate (Ctps2wt/flox, Ctps2flox/flox and Ctps2wt/ko) animals. a, b Analysis of splenic enriched T cells activated by anti-CD3/CD28 plus IL-2 for 3 days. a, c Analysis splenic purified B cells activated with LPS plus IL-4 for 3 days. a Immunoblots for CTPS1, CTPS2 and Actin expression in lysates of activated T cells (left panel), activated B cells (central panel) and fibroblasts recovered from ear skin (right panel). Molecular weights in kDa on the left. Blots represent two pooled, independent experiments. b Representative proliferation dot plot profiles (left panels) from FACS analysis of enriched T cells labelled with cell trace violet (CTV) and stained for CD25, as an activation marker. Graph bars (right panels) corresponding to proliferation indexes calculated from histogram profiles. c Representative proliferation dot plot profiles (left panels) from FACS analysis of B cells labelled with cell trace violet (CTV) and stained for IA/IE, as an activation marker. Graph bars (right panels) corresponding to proliferation indexes calculated from histogram profiles. d–f Analysis of from CD4-Cre; Ctps1wt/floxxCtps2wt/flox (Ctps1wt/ko, Ctps2wt/ko) empty black circle, CD4-Cre; Ctps1flox/floxxCtps2wt/flox (Ctps1ko/ko, Ctps2wt/ko) filled red circle, CD4-Cre; Ctps1wt/floxxCtps2flox/flox (Ctps1wt/ko, Ctps2ko/ko) filled blue circle and CD4-Cre; Ctps1flox/floxxCtps2flox/flox (Ctps1ko/ko, Ctps2ko/ko) filled purple circle. d Spleen cell counts (left panel) and percentages of splenic B, T, CD4+, CD8+ and NK cells calculated from FACS analyses. e Immunoblots for CTPS1, CTPS2 and Actin protein expression in lysates from non-activated or activated splenic T cells, sorted and non-sorted on CD3+. Molecular weights in kDa on the left. Blot is representative of two independent experiments. f Representative proliferation dot plot profiles (right panels) from FACS analysis of CD4+ (upper panels) and CD8+ (lower panels) T cells labelled with cell trace violet (CTV) and stained for CD25 as an activation marker. Graph bars (right panels) corresponding to proliferation indexes calculated from histogram profiles. Non-parametric Matt-Whitney two-tailed test (b) and Unpaired t-tests two-tailed (d, f) were used. Data are presented as the mean ± SD of n = 5 animals per group (b), of n = 8 (controls), n = 6 (Ctps1ko), n = 9 (Ctps2ko) and n = 6 (DKO) animals per group (d) and of n = 7 (controls), n = 5 (Ctps1ko), n = 7 (Ctps2ko) and n = 5 (DKO) animals per group (f). Source data are provided in Source Data file.
A non-redundant role of CTPS2 in proliferation of activated CTPS1-deficient T cells
However, CTPS2 could partially compensate for the absence of CTPS1 in T lymphocytes explaining the residual proliferation detected in Ctps1ko/ko T cells from CD4-Cre; Ctps1flox/flox mice breeding. T cells, in which both Ctps1 and Ctps2 were inactivated, were obtained to test this hypothesis. To do so, CD4-Cre; Ctps1flox/flox were crossed to Ctps2flox/flox. Like in Ctps1ko/ko animals from CD4-Cre; Ctps1flox/flox breeding, cellularity and cell population distribution of blood, thymus and spleen were normal in double CTPS1ko/ko-CTPS2ko/ko with the noticeable exception of T lymphocyte counts that were significantly diminished (Fig. 6d and Supplementary Fig. 7f–h). Absence of CTPS1 and/or CTPS2 proteins was checked by western blot. Both CTPS1 and CTPS2 proteins were absent in lysates of enriched activated T cells from double Ctps1ko/ko-Ctps2ko/ko animals. CTPS1 was detectable but not CTPS2 in lysates of activated T cells from Ctps2ko/ko animals, while CTPS2 but not CTPS1 was present in lysates of Ctps1ko/ko activated T cells (Fig. 6e). The deletion of both enzymes had a drastic effect on proliferation of activated CD4+ and CD8+ T lymphocytes resulting in an almost complete absence of proliferation (Fig. 6f), while T lymphocytes from Ctps1ko/ko-Ctps2wt/ko animals displayed a reduced proliferation, similar to that observed in CD4-Cre; Ctps1flox/flox T cells. Remarkably some Ctps1ko/ko-Ctps2ko/ko CD8+ T lymphocytes exhibited a residual proliferation that might depend of CTP salvage pathway. Taken together, these results indicate that, although Ctps1 has an essential role in T cell proliferation that Ctps2 cannot compensate, the lack of both Ctps1 and Ctps2 further reduces T-cell proliferation, implying that CTPS2 has an additional role to CTPS1.
Both genetic deletion and pharmaceutical inhibition of Ctps1 rescue Scurfy mice from lethal autoimmunity
We next tested whether deletion of Ctps1 could dampen pathological T-cell responses involved in autoimmunity and in inflammatory diseases. We took advantage of the Scurfy (Sf) mouse model that mimics the IPEX syndrome (immune dysregulation, poly-endocrinopathy, enteropathy, X-linked) responsible in humans for a severe poly-autoimmunity and inflammatory disorder, caused by hemizygous loss-of-function mutations in the X-linked gene FOXP3/FoxP333,34. FOXP3/FoxP3 gene is critical for the development of CD4+ regulatory T cells (Treg)35,36 which are key to immune tolerance37. FOXP3-deficient mice and human males exhibit T cell-dependent inflammation and autoimmunity linked to exacerbated T cell activation and responses among other findings leading to premature death by day 25–35 in mice38,39,40 (Fig. 7a). As previously reported38,39,40, Scurfy (FoxP3Sf/Y) mice are smaller than their littermate controls and have scaly skin and ears and squinted eyes. Histological evaluation indeed demonstrated thickening of ears and tissue liver disruption with severe inflammation consisting of massive cellular infiltration38,39,40 (Fig. 7b–d).
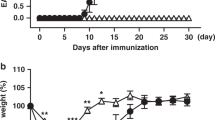
a-i Analyses of CD4-Cre; Ctps1wt/floxxFoxP3wt/Y (Ctps1wt/ko-FoxP3wt/Y) CD4-Cre; Ctps1wt/floxxFoxP3sf/Y (Ctps1wt/ko-FoxP3sf/Y) and CD4-Cre; Ctps1flox/floxxFoxP3sf/Y (Ctps1ko/ko-FoxP3sf/Y). a Kaplan Meier survival curves. b Animal weights measured at different days as indicated. c Macroscopic views of Ctps1wt/ko-FoxP3wt/Y, Ctps1wt/ko-FoxP3sf/Y and Ctps1ko/ko-FoxP3sf/Y animals and organs. Tail, thymus, and spleen at day 21 (top), day 44 (middle) and day 66 (bottom). Ears also shown at day 44. d Representative microscopy images of haematoxylin-eosin coloration of ear and liver at day 21. Infiltration highlighted with yellow arrowheads. Images are representatives of four animals per group. (Scale bars 250 µm). e Representative proliferation dot plot profiles (right panels) from FACS analysis of splenic T cells from animals from day 25 labelled with cell trace violet (CTV), stained for CD25 as an activation marker and activated by anti-CD3/CD28 plus IL-2 for 3 days. Graphs (right panel) corresponding to proliferation indexes calculated from histogram profiles. f Dot-plots from FACS analysis of FoxP3 intracellular staining on splenic T cells from the same animals as in (e). Numbers in the gates correspond to proportions or graph bars of CD4+CD25+FoxP3+. Gates defined based on staining with control Ig isotypes. g–i Analyses of haematological and immunological parameters. g Red blood cells, haemoglobin levels, whole blood cells numbers and proportions of lymphocytes, monocytes and neutrophils in blood, analysed from day 18–50. h Graph bars showing total thymus cell counts (left panel) at different periods of time as indicated. Proportions (right panels) from FACS analysis of double positive CD4 and CD8 (DP), single positive CD4 (SP4) and single positive CD8 (SP8) thymocytes. i Graph bars showing total spleen cell counts (left panel) and proportions of splenic B, T, NK cells, macrophages and neutrophils (right panels) from FACS analysis. A Log-rank (Mantel-Cox) statistical test for curve comparison was used: n = 11 (control), n = 12 (scurfy) and n = 11 (rescue) (a). Data are from n = 9 (control), n = 10 (scurfy) and n = 6 (rescue) animals per group (b). Unpaired t-tests two-tailed were used (e, g–i). Data are presented as the mean ± SD of n = 3 (control), n = 3 (scurfy) and n = 6 (rescue) (e), of n = 15 (control), n = 10 (scurfy) and n = 8 (rescue) (g), of n = 17 (control), n = 5 (scurfy) and n = 9 (rescue) (h), and of n = 18 (control), n = 6 (scurfy) and n = 11 (rescue) (i). Source data are provided in Source Data file.
Selective deletion of Ctps1 in T cells of Scurfy mice (afterward designated as Scurfy-Ctps1ko/ko) was obtained by crossing CD4-Cre; Ctps1flox/flox mice to Scurfy (FoxP3Sf/Y). In Scurfy-Ctps1ko/ko, both CD8+ and CD4+ T cells had, as expected, a reduced capacity to expand in response to CD3/CD28 stimulation (Fig. 7e, Supplementary Fig. 8a). Similarly, to Scurfy mice, Scurfy-Ctps1ko/ko animals exhibit a deficiency in Treg cells (CD4+, CD25+, FoxP3+) (Fig. 7f), indicating that Ctps1 deletion has no effect on Treg differentiation. Importantly, the phenotype of Scurfy mice was rescued in Scurfy-Ctps1ko/ko (Fig. 7a–d). Scurfy-Ctps1ko/ko survived at least up to 80 days and did not present scaly tails and ears while histological analyses did not reveal inflammation and tissue disruption at day 44 (Fig. 7d). Nevertheless, the weight of rescued mice never reached that of control animals (Fig. 7b). Blood parameters were reduced in Scurfy animals at day 21, while Scurfy-Ctps1ko/ko animals had values comparable to controls, except for monocyte and lymphocyte proportions that were intermediate (Fig. 7g). In the thymus, cellularity and distribution of DP, SP4 and SP8 were profoundly perturbed in Scurfy animals and were restored to normal in Scurfy-Ctps1ko/ko animals (Fig. 7h), although by day 44, rescued mice developed enlarged spleen while thymuses had a reduced cellularity (Fig. 7c, h). The spleen cellularity was slightly increased in Scurfy-Ctps1ko/ko animals, percentages of B and NK cells stayed low, while values of total T cells returned to the normal (Fig. 7i and Supplementary Fig. 8b). However, accumulation of effector memory CD4+ and CD8+ T cells seen in the spleen of Scurfy mice was significantly reduced in Scurfy-Ctps1ko/ko animals, but although not reaching proportions of control mice (Supplementary Fig. 8c). These data thus demonstrate that inactivation of Ctps1 in T lymphocytes prevents the autoimmune and inflammatory disease in Scurfy mice allowing their survival.
Hence, we assumed that targeting CTPS1 with a specific molecule inhibiting its function can suppress the lethal autoimmune symptoms of Scurfy mice. A series of small molecules that selectively inhibit human and mouse CTPS1 activity was generated41. In contrast to 3-Deazuridine42, an uridine/UTP analogue, that inhibits both CTPS1 and CTPS2, the Stp-2 compound showed efficient reduction of in vitro T cell proliferation and had the highest selectivity towards murine Ctps1 among other compounds tested (Supplementary Fig. 8d, e). Stp-2 was injected subcutaneously to Scurfy animals from day 10 and every 2 days (Fig. 8a–g). The dose of 30 mg/kg for the Stp-2 compound was chosen based on its solubility and potency. As controls, Scurfy males injected with a vehicle died between day 15 to 30. In contrast, a significant number (50%) of Scurfy males treated with Stp-2, survived beyond 50 days (Fig. 8a), had normal mobility and behaviour and did not present severe phenotypical symptoms like scaly tail, and ear and squinted eyes (Fig. 8b). Histological images of ears showed no sign of inflammation or thickening while liver images showed reduced sign of cellular infiltration (Fig. 8d). Nevertheless, their weight did not increase as the one of their treated littermate heterozygous treated sisters (Fig. 8c).
a–d Analyses of FoxP3sf/Y vehicle-treated animals (black) or with an inhibitor of Ctps1 (green) with drug-treated littermate female FoxP3wt/sf animals as controls (white). a Kaplan Meier survival curves. b Weights of animals from day 10 and every two-three day along the life of animals of vehicle-treated littermate females FoxP3wt/sf and males FoxP3sf/Y treated with the inhibitor of Ctps1. c Macroscopic view of FoxP3sf/Y animals at day 21 and FoxP3sf/Y treated with the Ctps1 inhibitor at day 21, 29 and 43. Thymus, and spleen are shown at day 43. d Representative microscopy images of haematoxylin-eosin coloration of ear and liver at day 52. Images are representatives of three animals per group. (Scale bars 500 µm). e–g Analyses of haematological and immunological parameters. e Red blood cells, haemoglobin levels, haematocrit percentage, whole blood cells numbers and proportions of lymphocytes, monocytes and neutrophils in blood. f Graph bars showing total thymus cell counts (left panel) and proportions (right panels) from FACS analysis of double positive CD4 and CD8 (DP); single positive CD4 (SP4) and single positive CD8 (SP8) thymocytes. g Graph bars showing total spleen cell counts (left panel) and proportions of splenic B, T, CD4+, CD8+, NK cells, macrophages and neutrophils cells (right panels) from FACS analysis. A Log-rank (Mantel-Cox) statistical test for curve comparison was used: n = 8 (vehicle-treated), n = 17 (drug-treated) (a). Data are from n = 10 (female, drug-treated), n = 26 (male, drug-treated) animals per group (c). Unpaired two-tailed t tests were used (e, f–g). Data are presented as the mean ± SD of n = 11 (control), n = 3 (vehicle-treated), n = 12 (drug-treated) (e), of n = 3 (control) and n = 6 (rescue) (f–g). Source data are provided in Source Data file.
Along the treatment of Scurfy animals with the compound Stp-2, red blood cell counts and haematocrit returned to normal ranges, while haemoglobin was still slightly reduced as compared to treated littermate heterozygous females (Fig. 8e). The percentage of blood lymphocytes remained low in treated Scurfy mice compared to treated females. Blood monocyte and neutrophil proportions remained as high as in non-treated Scurfy males (Fig. 8e). Some treated males were sacrificed at day 52–58 as their littermate sisters. Of note, one treated male was kept until 125 days. Heterogeneity in the thymus cellularity was observed among treated males. Indeed, in two mice, thymus cell numbers and thymus subpopulations returned to normal (Fig. 8f), while in four others, these numbers were close to those of non-treated Scurfy mice (before they died) (Fig. 7h). Spleen abnormalities in cell population distribution remained similar to that of non-treated Scurfy mice (Fig. 8g and Fig. 7i). The results show that targeting Ctps1 with a specific selective small chemical inhibitor improved the lifespan of Scurfy animals and several blood parameters returned to normal levels although lymphocyte populations did not recover in contrast to Scurfy-Ctps1ko/ko.
Pharmaceutical inhibition of Ctps1 limits experimental autoimmune encephalomyelitis
The Stp-2 compound was further tested in a more common model of autoimmunity, the induced-experimental autoimmune encephalomyelitis (EAE). EAE is a T-cell-mediated autoimmune disease characterised by lymphocyte infiltration in the central nervous system (CNS) associated with local inflammation, resulting in primary demyelination of axonal tracks, associated impaired axonal conduction in the CNS, and progressive hind-limb paralysis43. C57BL/6 mice were immunised with MOG peptide to induce EAE and then treated with the Stp-2 compound or vehicle every two days post immunisation (Supplementary Fig. 4a). The treatment with Stp-2 allowed a robust reduction of the clinical score along the disease (Fig. 9a). The beneficial effect was stable over time and sustained until day 30. We also measured the general ambulatory ability of CTPS1 inhibitor and vehicle-treated EAE-induced animals compared to non-immunised animals using an open field maze. Confirming the clinical score evaluation, EAE-induced mice treated with Stp-2 showed a good mobility compared to non-immunised mice, while vehicle-treated EAE animals tended to stay at the peripheral of the open field unable to travel a long distance (Fig. 9b). Mice were sacrificed and their spinal cords were analysed for cellular infiltration and demyelination which are a hallmark of EAE. While vehicle-treated EAE animals presented with extensive lymphocyte infiltration associated with demyelination, Stp2-treated animals were comparable to non-immunised animals lacking signs of infiltration with preserved myelinated areas (Fig. 9c, d). Thus, our data demonstrate that treatment targeting Ctps1 improves EAE clinical signs and controls inflammation and demyelination.
a–d EAE was induced by the immunisation of 12-wk-old C57BL/6 mice with MOG peptide. a Clinical score for Stp-2-drug (green) and vehicle (black) treated animals. b Representative traces of locomotor activity recorded in an open field cage for 10 min in control mice with no EAE and EAE-induced mouse treated with vehicle (Vt) and in Stp-2 compound (Dt) treated mouse. Quantification of the total distances travelled for 10 min. c, d Assessment of cellular infiltration by staining with haematoxylin/eosin and luxol blue counterstained with cresyl violet. Representative haematoxylin-eosin staining (c) and Luxol fast blue/cresyl violet counter-staining (d) of spinal cord sections of non-EAE and EAE-induced mouse treated with vehicle (Vt) and in Stp-2 compound (Dt) treated mouse. Images are representatives of four animals per group. (Scale bars 500 µm and 100 µm). Two-way ANOVA. Data are presented as mean ± SEM of n = 10 (vehicle-treated) and n = 13 (drug-treated) animals per group (a). One way ANOVA with Tukey’s multiple comparisons test n = 3 animals per group. Source data are provided in Source Data file.
Discussion
Herein, we show that Ctps1 but not Ctps2 is essential for life in mice. In humans, CTPS1 deficiency causes combined immunodeficiency and has no developmental effect. However, the mutation causing CTPS1 deficiency in human is hypomorphic and preserved around 10–15% of the CTPS1 expression and activity12. This residual CTPS1 activity is in all likelihood sufficient for human normal development further highlighting the key role of CTPS1 in immunity and in particular in lymphocytes. Inactivation of Ctps2 has no effect on mice development indicating that Ctps1 and Ctps2 have quite distinct roles. We recently showed that CTPS2 exhibits a lower CTP synthetase activity than CTPS1 that might explain the lesser importance of CTPS2 contribution in cell proliferation10. Our results indicate that Ctps1 is not only mandatory for early development but also for later steps and during all life. Indeed, inducible inactivation of Ctps1 in normally developed mice results in rapid health deterioration associated with a rapid weight loss requiring the sacrifice of the animals.
Overall, it seems that the degree of reliance on Ctps1 in a given tissue (or a cell type/population) positively correlates with its proliferative rate. As such renewal of gut epithelium, production of red blood cells and thymopoiesis are associated with the highest proliferation rate in the organism including T-cell expansion upon antigenic stimulation (see below). Thymic development has been previously shown to be also dependent on the CTP salvage pathway44,45. However, our study demonstrates the CTP de novo synthesis pathway is critical for thymic development. Interestingly, in contrast to lymphocytes, myeloid cells appear to be less or not dependent on CTPS activity for their development and maintenance. Thus, in myeloid cells CTP synthesis may be achieved by the salvage pathway rather than by de novo synthesis.
As we previously reported in humans, we observed in the different mouse models that T-cell proliferation upon TCR/antigenic stimulation is highly dependent on CTPS1, as well as GC B cells are. CTPS1 expression in splenic T cells is rapidly up regulated in response to TCR engagement. Interestingly, residual proliferation of activated Ctps1-deficient T cells is dependent on CTPS2. We also noticed that few CD8+ T cells in absence of both Ctps1 and Ctps2 were able to proliferate, while no CD4+ T expanded. This could be explained by the different metabolic constraints between CD8+ and CD4+ T lymphocytes46. The CTP salvage pathway could be more active in CD8+ T cells than in CD4+ T cells.
Inborn errors of purine and pyrimidine metabolism are a diverse group of disorders that present with a wide range of phenotypes including mental retardation, autism, growth retardation, renal stones, and immunodeficiency disorders47. Deficiencies in adenosine deaminase (ADA)48, a housekeeping enzyme of purine metabolism encoded by the ADA gene or in the purine nucleoside phosphorylase (PNP) deficiency49, are characterised by recurrent infections, neurologic symptoms, and autoimmune disorders. Both genetic deficiencies lead to a blockade of the purine pathway leading to the intracellular accumulation of deoxynucleosides and deoxynucleotides, which are poisonous for both dividing and non-dividing lymphocytes. Some disorders of the pyrimidine metabolism are also associated with a marked susceptibility to infections such as orotic aciduria (caused by bi-allelic mutations in UMPS), and pyrimidine nucleotide depletion syndrome47. However, to date CTPS1-deficiency is the only genetic defect impairing the pyrimidine pathway with a clinical phenotype restricted to immunodeficiency likely explained by the hypomorphic nature of the CTPS1 mutation12.
We provide evidence that inhibiting CTPS1 in T cells significantly attenuates the poly-autoimmune and inflammatory disease of Scurfy mice. Unsurprisingly, inactivation of Ctps1 appears more efficient than the treatment with the Stp-2 compound. Treatment with Stp-2 allows mice to survive with reduced phenotypic characteristics of the disease. We also showed that the Step-2 compound efficiently reduces the severity of the disease in EAE, a more common model of autoimmunity and inflammation. Therefore, our results provide preliminary evidence to utilise highly selective CTPS1 inhibitors to treat human autoimmune and/or inflammatory conditions that are driven by pathological T-cell responses. Importantly, the study of these different mouse models also informs on possible adverse effects of treatments with CTPS1 inhibitors that might include anaemia, intestine injury, and lymphopenia. However, we did not notice that treatment with Stp-2 had such effects in heterozygous littermate FoxP3wt/sf females.
Most conventional immunomodulatory agents act by inhibiting activation or reducing proliferation of lymphocytes, notably by targeting purine and pyrimidine biosynthesis. Each of these drugs has its own mechanism of action and is used to manage a variety of autoimmune conditions such as rheumatoid arthritis, psoriasis, systemic lupus erythematosus, systemic sclerosis or organ transplantation. For example, methotrexate inhibit several enzymes responsible for nucleotide synthesis including dihydrofolate reductase, thymidylate synthase, aminoimidazole caboxamide ribonucleotide transformylase (AICART) and, amido-phosphoribosyltransferase50. Azathioprine has an antagonist effect on purine metabolism leading to broad inhibition of DNA, RNA, and protein synthesis51. Another drug commonly used is the mycophenolate, which is an inhibitor of the inosine-5’-monophosphate dehydrogenase, which result in depletion of guanosine nucleotide preferentially in T and B lymphocytes thus inhibiting their proliferation52. Teriflunomide selectively and reversibly inhibits dihydro-orotate dehydrogenase, a key mitochondrial enzyme in the de novo pyrimidine synthesis pathway, leading to a reduction in proliferation of activated T and B lymphocytes without causing cell death53,54. Although these drugs, which interfere with nucleotide synthesis, have been shown to be effective, they have more than one mechanism of action and the precise way in which they exert their effects is often unknown. Moreover, their anti-proliferative and cytotoxic effects are in most cases not specific to the immune system explaining their toxicity and side-effects. In our case, we hypothesised based on our acknowledge of CTPS1, that targeting CTPS1 (with selective inhibitors) would be more specific and could lead to less adverse side-effects. Indeed, we widely characterised the role of CTPS1 in proliferation in activated T cells and activated T cells represent one of the highest CTPS1 expressing tissue11,12. One possible limitation of the use of CTPS1 inhibitors is to impair the immune response to viral infections including EBV reactivation (as it is observed in CTPS1-deficient patients). This unwanted side effect could be resolved by an adjusted dosage of CTPS1 inhibitor (as it is done for other immunosuppressive drugs).
In conclusion, our study by the establishment and the analyses of these different Ctps1 deficient mouse models provide important findings on the respective roles of CTPS1 and CTPS2 notably in haematopoiesis and T lymphocyte activation. We also provide several lines of evidence that CTPS1 represents a promising target for the treatment of autoimmune and inflammatory conditions.
Methods
Mouse ethic authorisation
All mouse experiments were performed in accordance with European Union (EU) Directive 2010/63/EU. Animal procedures were approved by the animal committee of the University of Paris Descartes (Paris, France) and the Ministry of Higher Education, Research and Innovation (APAFIS#32465). Animal were housed in individual ventilated cages of group of 5 animals maximum with enrichment, under specific and opportunistic pathogen-free (SOPF) conditions. Animals were fed standard chow diet ad libitum, and kept under ambient temperature (21–22 °C) and 50–60% humidity, with 12–12 h on-off light cycle. Both male and female are used in this study unless specified for specific experiments.
Mice
To obtain genetically modified mice carrier the same mutation as human, mouse mutated allele was generated through CRISPR/Cas9 mediated HR in C57BL/6J mouse zygotes using CRISPR ribonucleoprotein (RNP) complex direct delivery in pronuclei by electroporation. Briefly a guide targeting intron-exon 18 was engineered by CRISPR/Cas9 technology using CRISPOR web site and the matrix, to mimic the human mutation, was designed (Supplementary Table 1). Recombinant Cas9, tracrRNA, crRNA and ssODN were purchased from Integrated DNA technologies. 200 ng/uL tracrRNA:crRNA duplex and 2 µM Cas9 protein were mixed and allowed to form an active rRNP complex for 10 min at room temperature, to which 200 ng/μL ssODN were added in Opti-MEM buffer (31985062, ThermoFisher Scientific). Four weeks-old female mice (Janvier Labs, France) were super-ovulated and mated with C57BL/6J male mice. The next day, zygotes were collected and batches of 50 zygotes were aligned between the electrodes, and repeated pulses of electroporation were delivered by the NEPA21 electroporator, allowing the RNPs/ssODN to enter the zygotes. Surviving zygotes were placed in KSOM media (MR-106-D, Merck Millipore) and cultured overnight at 37 °C and 5%CO2 to reach two-cell stage. Two-cell embryos were transferred into pseudo-pregnant females using standard surgical procedures. F0 founders were genotyped and presence of the mutation was confirmed by sequencing. Founders were backcrossed with C57BL/6J mice to remove potential off-targets and to segregate the mutated allele.
Ctps1 and Ctps2 ES cells, purchase from KOMP (ID:89620) and EUCOMM (ID:114201) respectively, were injected into blastocysts to generate founder. Chimeric animals were obtained and bred for germ line transmission. Founders were further backcrossed to C57Bl/6J wild type mice (purchased from Janvier Labs; #000664). These animals were further bred to transgenic mice expressing the flipase transgene (Tg-FLP)55 to delete the KOMP cassette and generate animals with flox alleles (Ctps1flox/flox). Homozygous Ctps1flox/flox animals were then crossed to specific CRE transgenic animals. The following CRE transgenic mouse were used: Tg Flipase JAX stock #012930, CMV-Cre JAX stock #006054, Vav-icre JAX stock #008610, CD8-Cre JAX stock #008766, CD4-Cre JAX stock #017336, Cre-ERT2 JAX stock #008463. The Scurfy mice line, stock #004088 was maintained by backcrossing on a B6.129S7-Rag1tm1Mom/J stock #002216 background, allowing the generation of FoxP3Sf/Sf animals. Rag1−/− female mice do not develop disease. Crossing of these female mice with wild type C57BL/6J mice resulted in litters in which all the males are FoxP3Sf/Y. Thus Rag1−/+ males develop the Scurfy phenotype, while heterozygous females do not56. All mice were euthanized using cervical dislocation. Age- and sex-matched mice between 6 and 12 weeks of age were used in the experiments.
Treatment of Scurfy mice with Stp-2
Stp-2 is derived compound of Cp27741 with stronger selectivity against murine CTPS1/CTPS2 (IC50 against mCTPS1 of 15 nM and 586 nM against mCTPS2). A 3 mg/ml solution of Stp-2 compound was prepared in 10% Benzyl alcohol and 90% Castor oil, maximum solubility of Stp-2 being 6 mg/mL. Litters from Scurfy breeding were sub-cutaneously injected in the back with 50 μl of Stp-2 (30 mg/kg) starting day ten and every 2 days along the life of the animal.
Induction of the Cre- ER T2 transgene
Tamoxifen for in vivo Cre induction or 4-hydroxy tamoxifen (4-OHT) for in vitro experiments, were purchase from Sigma-Aldrich. Tamoxifen was prepared in corn oil to a final concentration of 10 mg/mL. 100 μL of tamoxifen was given by oral gavage using a flexible feeding tube (04 × 040) from ECIMED at day 1 and day 4 of the experiment. 4-OHT was added to the in vitro cell culture at 300 nM.
Blastocysts
Four to six weeks-old female mice from the Ctps1flox/flox line were super-ovulated by intraperitoneal injection of 5 IU PMSG (SYNCRO-PART@ PSMG 600UI, Ceva), followed by 5 IU of hCG (Chorulon 1500 IU, Intervet) at an interval of 46–48 h, and mated with a CMV-Cre+; Ctps1wt/ko male. The next day, zygotes were collected from the female oviducts and exposed to hyaluronidase (H3884, Sigma-Aldrich) to remove the cumulus cells and then placed in KSOM medium (MR-106-D, Merck) and cultured for 3 days into a CO2 incubator (5% CO2, 37 °C), to reach the blastocyst stage. Blastocysts were recovered in individual tubes and DNA was prepared in 20 μL of DNA lysis buffer. 2 μL were used for the genotyping by Polymerase chain reaction (PCR) amplification.
Embryo analysis
Two proestrus or oestrus females were mated with one male overnight, plugs were checked in the morning. Females were sacrificed and embryos at E6.5, E8.5 and E9.5 were collected and dissected. Whole embryos were then lysed in DNA lysis buffer and genotyped by PCR. E6.5 embryos were fixed in 4% PFA and stained with anti-CTPS1 antibody. Immunofluorescence on whole mount E6.5 embryos was performed using CUBIC clearing as described57. Samples were incubated overnight in the lipid-removing Reagent-1, then 48 h with anti-CTPS1 primary antibody (1/150, ab133743) in TNB blocking buffer (PerkinElmer-FP1020) containing 0.5% triton, and overnight with a Donkey anti-Rabbit Alexa Fluor 488 conjugated secondary antibody (1/500, Invitrogen-A21206) in PBS with 0.01% triton. Samples were finally incubated overnight in Reagent-2 for adjustment of the refractive index and mounted in 0.4% agarose in Reagent-2. Multi-channel 16-bit images were acquired with a Z.1 lightsheet microscope (Zeiss). 3D data was visualised using Imaris software, and CTPS1 mean signal intensity was measured in every slide of each stack using FIJI. The mean value of all measurements per embryo is plotted.
Genotyping
DNA was extracted form ear punch biopsies using the REDExtract-N-Amp™ Tissue PCR kit from Sigma. Polymerase chain reaction (PCR) amplification was then performed with specific primers and analysed on agarose gel after electrophoresis. The list of primers is provided in Supplementary Table 1. For Scurfy mice, the FoxP3 mutation was validated by Sanger sequencing using the BigDye™ Terminator v3.1 Cycle Sequencing Kit (Life Technologies) and a 3500xL Genetic Analyzer (Applied Biosystems) according to the manufacturer’s instructions. All collected sequences were analysed using 4peaks software (Version 1.8; A. Griekspoor and T. Groothuis, http://nucleobytes.com/index.php/4peaks). For genotyping of E6.5 mouse embryos, the ectoplacental cone or the whole embryo was lysed. Yolk sac tissue was used for the genotyping of E8.5 and E9.5 embryos. Different stages of thymic T cells differentiation including DN2, DN3, DN4, DP, SP4 and SP8 from were sorted on a FACS Aria. 5000 cells were recovered and directly lysed in DNA lysis buffer and genotyped by PCR.
Flow cytometry
Cell suspensions were prepared from thymii and spleen by mechanical disruption on cell strainers 70 μm and resuspended on ice in FACS buffer (PBS containing 2% FCS). Prior to staining, all samples were blocked with TruStain FcXtm PLUS (anti-mouse CD16/32, clone S17011E) from BioLegend in FACS buffer for 15 min on ice. Surface staining was done on ice for 30 min. Surface staining was performed with directly conjugated antibodies (BD Pharmingen, BioLegend and eBioScience) according to standard techniques and analysed on LSR-Fortessa X20 flow cytometer (Becton Dickinson). The following mouse antibodies were conjugated to fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), phycoerythrin-cyanin5 (PE-Cy5), phycoerythrin-cyanin7 (PE-Cy7), Peridinin-chlorophyll-cyanin5.5 (PerCP-Cy5.5), allophycocyanin (APC), allophycocyanin-Cyanin7 (APC-Cy7), alexa-700, Brilliant Violet 421 (BV421), Brilliant Violet 510 (BV510), Brilliant Violet 605 (BV605), Brilliant Violet 650 (BV650), Brilliant Violet 711 (BV711), Brilliant Violet 785 (BV785): anti-TCR (clone H57-597), anti-CD4 (clone RMA4-5), anti-CD8 (clone 53-6.7), anti-CD11b (clone M1/70), anti-CD11c (clone N418), anti-CD19 (clone 6D5), anti-B220 (clone RA3-6B2), anti-CD24 (clone M1/69), anti-CD25 (clone PC61), anti-CD27 (clone LG3A10), anti-CD44 (clone IM7), anti-CD62L (clone MEL-14), anti-CD95 (clone DX2), anti-CD117 (clone 2B8), anti-GR1 (clone RB6-8C5), anti-F4/80 (clone BM8), anti-Ter119 (clone Ter119), anti-Sca1 (clone D7), anti-NKP46 (clone 29A1.4), anti-NK1.1 (clone PK136), anti-IA/IE (clone M5/114.15.2), anti-GL-7 (clone GL-7), anti-CXCR5 (clone L138D7), anti-PD-1 (clone 29 F.1A12), anti-IgM (clone RMM-1), anti-IgD (clone 11-26c2a).
For intracellular FoxP3 staining, samples were blocked with TruStain FcXtm PLUS, surfaced labelled then, isotype control-APC (Rat IgG2a, eBR2a) or anti-FoxP3-APC (FJK-16S, eBioScience) antibodies were used with the staining buffer set and protocol from eBioscience. The dilution of antibodies is provided in Supplementary Table 2.
The gating strategies used in all figures and extended data figures are shown in Supplementary Fig. 9.
T and B-cell proliferation assays
For in vitro T cell activation, T cells were enriched using the Dynabeads Untouched mouse T cells kit from Invitrogen, according to manufacturer. Enriched T cells were further incubated with Cell Trace Violet (CTV) (Biolegend) according to manufacturer and cultured in DMEM+Glutamax supplemented with 10% FCS, penicillin and streptomycin, non-essential amino acids, HEPES and sodium pyruvate and beta mercapto-ethanol (all from Gibco). CTV-labelled enriched T cells were activated with Dynabeads mouse T-activator (anti-CD3/anti-CD28), according to manufacturer plus IL-2 (1000 U/mL) in 48 wells plate at a concentration of 1million/mL for 3 days. 3-deazauridine (3-DU) (Sigma–Aldrich) was used as a non-selective inhibitor and Stp-1 and Stp-2 as specific murine-Ctps1 inhibitors. 200 µM of cytidine (Sigma–Aldrich) was in some experiments added to the culture to complement proliferation. Sorted B cells, using CD19 beads from Miltenyi. Sorted B cells were incubated with CTV and cultured in DMEM+Glutamax supplemented with 10% FCS, penicillin and streptomycin, non-essential amino acids, HEPES and sodium pyruvate and beta mercapto-ethanol (all from Gibco). CTV-labelled B cells were activated with LPS (25 μg/mL; Sigma) plus mIL-4 (25 ng/mL; Peprotec) in 48 wells plate at a concentration of 1million/mL for 3 days. T and B cells proliferation was assessed via CTV dilution after surface staining and analysed by cytometry on LSR-Fortessa X20 flow cytometer (Becton Dickinson), with DIVA software. Data from FACS were processed using FlowJo2 (version 10.7.1, Becton Dickinson & Compagny).
Biochemical analysis
Lysate from activated and non-activated spleen T cells, activated spleen B cells or fibroblasts were separated on 8% SDS-PAGE gel. After transfer to nitrocellulose membranes, the following primary antibodies were used: rabbit monoclonal anti-CTPS1 EPR8086 (Abcam ab133743) or rabbit polyclonal anti-CTPS2 (C-ter) (Abcam ab190462) (dilution 1/1000) and mouse monoclonal anti-actin (Santa-Cruz-4778) (dilution 1/5000). Membranes were then washed and incubated with anti-mouse (7076S) or anti-rabbit HRP (7074S) conjugated antibodies (dilution 1/10000) from Cell Signalling Technology and Pierce ECL western blotting substrate was used for detection. Uncropped blots are supplied in the Source Data File.
Histology
Liver and ear were dissected out and fixed in 4% PFA in PBS over-night at 4 °C. The intestine was dissected out, faecal matter was gently removed by using a syringe filled with PBS. The intestine was rolled on itself and placed in a cassette and in fixative solution (4% PFA in PBS) for over-night at 4 °C. The next day organs were washed with cold PBS and left in cold PBS over-night. Two days later, organs were transferred in a 30% sucrose solution for 3 h at 4 °C. Finally, tissues were transferred in cryomolds, quick froze in OCT and kept at −80 °C.
Sections were prepared with an ultramicrotome (RM 2235, Leica). Serial 10-µm sections were cut and collected on glass slides. The sections were stained with haematoxylin and eosin (H&E) after drying and photographed with Nanozoomer 2.0 HT microscopy (Hamamatsu).
Drug testing
Knock-out Jurkat cells for CTPS1 have been previously reported7. KO-Jurkat cells were transduced with a lentiviral vector (pLVX-EF1a-IRES-mCherry (Clontech) with either human-CTPS1, human-CTPS2, murine-Ctps1 or murine-Ctps2. Transfected cells were seeded at a density of 150000 cells/ml in 96-well plates and treated with the indicated compounds diluted in complete culture medium. 3-deazauridine (3-DU) (Sigma–Aldrich) was used as a non-selective inhibitor and Stp-2 as a specific murine-Ctps1 inhibitor. At the 24 and 48 h, 10 μL of celltiter-blue (CellTiter-Blue® Cell Viability Assay, Promega) were added to the culture medium and the cells further incubated at 37 °C for up to 4 h. Absorbance at 560/595 nm was measured and analysed according to the manufacturer’s instructions using a Tecan Infinite 200 Pro-plate reader (Tecan Life Sciences).
NP-CGG immunisation
Immunisations were realised in two animal groups. The first group was Cre-ERT2; Ctps1wt/flox and Cre-ERT2; Ctps1flox/flox animals in which Ctps1 deletion was achieved by oral gavage with tamoxifen at day 4 and 8 (Fig. 2g). In the second group, wild type C57BL/6 animals were treated every two days with sub-cutaneous injection in the back with 50 μl of Stp-2 compound (green) or vehicle (black), specifically inhibiting Ctps1 expression (Supplementary Fig. 4a). Mice were immunised intraperitoneally with 100 μg of NP-CGG (4-hydroxy-3-nitrophenylacetyl-Chicken Gamma Globulin, Biosearch Technologies) dissolved in PBS and adsorbed on Alhydrogel® aluminium hydroxyde following manufacturer’s instructions (Invivogen). Mice were then sacrificed at day 14. Serum was separated after blood clotting by two serial centrifugations for detection of specific IgM and IgG1. Serum from non-immunised C57BL/6 animal was also recovered. Briefly F96 MaxiSorp immunoplates (Nunc) were coated during 1 h at 37 °C with 50 μL per well of a 10 μg.ml−1 solution of goat anti-mouse Ig (#1010-01, SouthernBiotech), or of NP-BSA capture antigen (NP23-BSA, Biosearch Technologies) in coating buffer (carbonate-bicarbonate, pH 9.6). Plates were then saturated overnight at 4 °C with 100 μl PBS-1% BSA per well and, incubated for 1 h at RT with 50 μL per well of serum samples serially diluted in PBS. Two duplicate dilution series were performed for each sample. After a last incubation with 100 μl of HRP-conjugated goat anti-mouse IgG1 and IgM (SouthernBiotech) diluted 2 000 times in PBS-1% BSA-0.05% Tween 20, each well was incubated 5–10 min at RT with 50 μl of KPL SureBlue TMB Microwell Peroxidase Substrate (SeraCare) and the reaction was stopped by addition of a 0.6 N H2SO4 solution. Optical density was measured at 450 nm, background signal measured at 620 nm was subtracted for calculation. For NP-specific Ig titres, a reference pool of six sera taken from C57BL/6 control mice 14 days after immunisation with NP-CGG was used as an internal control on every plate to calculate relative Ig titres. Germinal centres from Peyer patches were analysed by cytofluorimetry as already described (see mat et med in the section Flow cytometry). Spleen were harvested, fixed in 4% PFA and embedded in paraffin. Slides were deparaffinized with Neo-clear (Sigma) and rehydrated. Serial sections (5 μm) were used for haematoxylin-eosin staining (mounted with PERTEX) and immunochemistry. For immune-histochemical assessment, sections were labelled with the following antibodies: biotin conjugated anti-B220 (Invitrogen) and anti-PCNA (Abcam) and a Dako Envision Kit (Dako North America, Inc) was used to reveal the staining. Slides were mounted in Aquatex medium (Merck) and images were taken with Nanozoomer 2.0 HT microscopy (Hamamatsu).
EAE induction
EAE was induced by subcutaneous immunisation of 12-wk-old C57BL/6 mice at two sites with 100 μg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) (Polypeptide Group) emulsified in CFA (Sigma) and supplemented with 6 mg/ml Mycobacterium tuberculosis (strain H37RA) (BD). Pertussis toxin (Sigma) (300 ng) was injected intraperitoneal at day 0 and day 2 post-immunization. Clinical score evaluation of symptoms was performed daily according to the standard EAE grading scale. The Stp-2 compound, or vehicle was injected sub-cutaneous every two days starting the day of EAE induction. Disease severity was scored daily on a 5 points scale: 0.5: tip tail is limp; 1: limp tail; 1.5: limp tail and hind leg inhibition; 2: limp tail and weakness of hind legs; 2.5: limp tail and dragging of hind legs; 3: limp tail and complete paralysis of hind legs; 3.5: limp tail and complete paralysis of hind legs plus unable to right itself; 4: complete hind and partial front leg paralysis; 4.5: complete hind and partial front leg paralysis no more movement around; 5: mouse found dead. The general activity of mice was evaluated at day 20 by open field task measurement and recording for 10 min. At day 30 after EAE induction, animals were sacrificed, and lumbar spinal cord sections were harvested fixed in 4% PFA and embedded in paraffin. Deparaffined serial sections (5 μm) were used for eosin-haematoxylin. Adjacent sections were stained with 0.1% Luxol® Fast Blue (Sigma) at 55 °C for 2 h. Sections were dehydrated in 70% alcohol for 15 s and further differentiated in lithium carbonate solution (0.05%) finally counterstained with 0.1% cresyl violet (Sigma) for 5 min. Then, sections were washed, dehydrated in alcohol series, cleared in toluene, mounted in Eukitt medium (Sigma), dried over- night, and photographed with Nanozoomer 2.0 HT microscopy (Hamamatsu).
Statistics
All graphs and statistical analyses were performed with GraphPad Prism (version 9.4.1; GraphPad Software, Inc, San Diego, CA). Statistical tests included: t-test with Welch’s correction, two-way ANOVA, unpaired t-tests, non-parametric Matt-Whitney two-tailed test, log-rank (Mantel-Cox) statistical test for curve comparison and one-way ANOVA with Tukey’s multiple-comparison test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information, Source Data file or from the corresponding author upon request (claire.soudais@inserm.fr). Source data are provided with this paper.
References
Evans, D. R. & Guy, H. I. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J. Biol. Chem. 279, 33035–33038 (2004).
Kassel, K. M., Au da, R., Higgins, M. J., Hines, M. & Graves, L. M. Regulation of human cytidine triphosphate synthetase 2 by phosphorylation. J. Biol. Chem. 285, 33727–33736 (2010).
van Kuilenburg, A. B., Meinsma, R., Vreken, P., Waterham, H. R. & van Gennip, A. H. Identification of a cDNA encoding an isoform of human CTP synthetase. Biochim. Biophys. Acta 1492, 548–552 (2000).
Lieberman, I. Enzymatic amination of uridine triphosphate to cytidine triphosphate. J. Biol. Chem. 222, 765–775 (1956).
Long, C. W. & Pardee, A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J. Biol. Chem. 242, 4715–4721 (1967).
Ellims, P. H., Gan, T. E. & Medley, G. Cytidine triphosphate synthetase activity in lymphoproliferative disorders. Cancer Res. 43, 1432–1435 (1983).
Kizaki, H., Williams, J. C., Morris, H. P. & Weber, G. Increased cytidine 5’-triphosphate synthetase activity in rat and human tumors. Cancer Res. 40, 3921–3927 (1980).
Williams, J. C., Kizaki, H., Weber, G., Morris, H. P. & Increased, C. T. P. synthetase activity in cancer cells. Nature 271, 71–73 (1978).
Lin, Y. et al. CTPS1 promotes malignant progression of triple-negative breast cancer with transcriptional activation by YBX1. J. Transl. Med. 20, 17 (2022).
Minet, N. et al. Differential roles of CTP synthetases CTPS1 and CTPS2 in cell proliferation. Life Sci. Alliance 6, e202302066 (2023).
Martin, E. et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 510, 288–292 (2014).
Martin, E. et al. Impaired lymphocyte function and differentiation in CTPS1-deficient patients result from a hypomorphic homozygous mutation. JCI Insight 5, e133880 (2020).
Asnagli, H. et al. CTP synthase 1 is a novel therapeutic target in lymphoma. Hemasphere 7, e864 (2023).
Pfeiffer, C. et al. CTPS1 is a novel therapeutic target in multiple myeloma which synergizes with inhibition of CHEK1, ATR or WEE1. Leukemia 38:181–192 (2023).
Kol, G., Lev-Maor, G. & Ast, G. Human-mouse comparative analysis reveals that branch-site plasticity contributes to splicing regulation. Hum. Mol. Genet. 14, 1559–1568 (2005).
Schwenk, F., Baron, U. & Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081 (1995).
Tam, P. P. & Behringer, R. R. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 68, 3–25 (1997).
Power, M. A. & Tam, P. P. Onset of gastrulation, morphogenesis and somitogenesis in mouse embryos displaying compensatory growth. Anat. Embryol. 187, 493–504 (1993).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Rath, E. & Haller, D. Intestinal epithelial cell metabolism at the interface of microbial dysbiosis and tissue injury. Mucosal. Immunol. 15, 595–604 (2022).
Fagarasan, S., Kawamoto, S., Kanagawa, O. & Suzuki, K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 28, 243–273 (2010).
Reboldi, A. & Cyster, J. G. Peyer’s patches: organizing B-cell responses at the intestinal frontier. Immunol. Rev. 271, 230–245 (2016).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Naito, Y. et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol. Cell Biol. 27, 3008–3022 (2007).
Ogilvy, S. et al. Transcriptional regulation of vav, a gene expressed throughout the hematopoietic compartment. Blood 91, 419–430 (1998).
Koulnis, M. et al. Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J. Vis. Exp. (54):2809 (2011).
Seita, J. & Weissman, I. L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 640–653 (2010).
Okada, S. et al. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 78, 1706–1712 (1991).
Maekawa, Y. et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat. Immunol. 9, 1140–1147 (2008).
Sawada, S., Scarborough, J. D., Killeen, N. & Littman, D. R. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell 77, 917–929 (1994).
Egerton, M., Scollay, R. & Shortman, K. Kinetics of mature T-cell development in the thymus. Proc. Natl Acad. Sci. USA 87, 2579–2582 (1990).
Robert, P. A., Kunze-Schumacher, H., Greiff, V. & Krueger, A. Modeling the dynamics of T-cell development in the thymus. Entropy 23, 437 (2021).
Wildin, R. S. et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27, 18–20 (2001).
Bennett, C. L. et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27, 20–21 (2001).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Clark, L. B. et al. Cellular and molecular characterization of the scurfy mouse mutant. J. Immunol. 162, 2546–2554 (1999).
Godfrey, V. L., Wilkinson, J. E. & Russell, L. B. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138, 1379–1387 (1991).
Sharma, R., Sung, S. S., Fu, S. M. & Ju, S. T. Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice. J. Biomed. Sci. 16, 20 (2009).
Novak, A. et al. Discovery and optimization of potent and orally available CTP synthetase inhibitors for use in treatment of diseases driven by aberrant immune cell proliferation. J. Med. Chem. 65, 16640–16650 (2022).
McPartland, R. P., Wang, M. C., Bloch, A. & Weinfeld, H. Cytidine 5’-triphosphate synthetase as a target for inhibition by the antitumor agent 3-deazauridine. Cancer Res. 34, 3107–3111 (1974).
Constantinescu, C. S., Farooqi, N., O’Brien, K. & Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164, 1079–1106 (2011).
Austin, W. R. et al. Nucleoside salvage pathway kinases regulate hematopoiesis by linking nucleotide metabolism with replication stress. J. Exp. Med. 209, 2215–2228 (2012).
Toy, G. et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc. Natl Acad. Sci. USA 107, 5551–5556 (2010).
Seder, R. A. & Ahmed, R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4, 835–842 (2003).
Nyhan, W. L. Disorders of purine and pyrimidine metabolism. Mol. Genet. Metab. 86, 25–33 (2005).
Hirschhorn, R. Overview of biochemical abnormalities and molecular genetics of adenosine deaminase deficiency. Pediatr. Res. 33, S35–41 (1993).
Markert, M. L. Purine nucleoside phosphorylase deficiency. Immunodefic. Rev. 3, 45–81 (1991).
Funk, R. S., van Haandel, L., Becker, M. L. & Leeder, J. S. Low-dose methotrexate results in the selective accumulation of aminoimidazole carboxamide ribotide in an erythroblastoid cell line. J. Pharmacol. Exp. Ther. 347, 154–163 (2013).
Kelley, W. N., Rosenbloom, F. M. & Seegmiller, J. E. The effects of azathioprine (imuran) on purine synthesis in clinical disorders of purine metabolism. J. Clin. Invest. 46, 1518–1529 (1967).
Allison, A. C. Mechanisms of action of mycophenolate mofetil. Lupus 14, s2–8 (2005).
Bar-Or, A., Pachner, A., Menguy-Vacheron, F., Kaplan, J. & Wiendl, H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 74, 659–674 (2014).
Scherer, S. et al. Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development. Nat. Immunol. 24, 501–515 (2023).
Raymond, C. S., Soriano, P. & High-efficiency, F. L. P. and PhiC31 site-specific recombination in mammalian cells. PLoS One 2, e162 (2007).
Delville, M. et al. A combination of cyclophosphamide and interleukin-2 allows CD4+ T cells converted to Tregs to control scurfy syndrome. Blood 137, 2326–2336 (2021).
Le Garrec, J. F. et al. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. Elife 6, e28951 (2017).
Acknowledgements
We thank Geoffrey de Ribains, Tim Bourne and Pascal Georges for their support and discussions over the years. We also acknowledge Virginie Puchois, Corinne Lebreton, Marie Chérier and Julien Diana for their technical and experimental advice. We would like to particularly thank Francina Langa-Vives head of the Mouse Genetics Engineering Center at the Institut Pasteur. We also thank the members of the Latour lab for discussions. This work was supported by grants from Ligue Contre le Cancer—Equipe Labellisée (France) (to S.L.), Inserm (France), the Agence Nationale de Recherche (ANR, France): ANR-18-CE15-0025-01 (to S.L.), ANR-10-IAHU- 01 (Imagine Institute), ANR-21-CE15-0018-01 (to E.M.) and the European Research Council: Proof of Concept ERC-2015-PoC_Master/ERC-2015-PoC_680465_SAFEIMMUNOSUPPRESS (to A.F., S.L.), INCA PL-BIO2020 (to S.L.). N.M. is a fellowship recipient of the Association Nationale de la Recherche Technologique (ANRT) under agreement with the industrial partner Step-Pharma. P.P.G. is recipient of a Pasteur-Roux-Cantarini fellowship from the Institut Pasteur. S.M.M. is a senior scientist at the INSERM. S.L. is a senior scientist at the Centre National de la Recherche Scientifique (CNRS, France).
Author information
Authors and Affiliations
Contributions
Conceptualisation: C.S., S.L.; Methodology: C.S., S.S., E.M.; Investigation: C.S., R.S., C.B., N.M., S.M., C.G., C.L.S., P.D., C.C., P.P.G., S.S., S.M.M. and E.M.; Resources: H.A., A.P., F.E.S.; Project administration: C.S.; Funding acquisition: A.F., E.M., S.L.; Supervision: S.L.; Visualisation: C.S.; Writing—original draft: C.S., S.L.; Writing—review & editing: C.S., N.M., A.P., F.E.S., S.M.M., A.F., E.M., S.L.
Corresponding authors
Ethics declarations
Competing interests
H.A. was an employee of Step-Pharma SAS. A.P. is a current employee of Step Pharma SAS. N.M. was a fellowship recipient of the Association Nationale de la Recherche Technologique (France) under agreement with Step-Pharma. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Christopher Goodnow, Barbara Zimmermann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soudais, C., Schaus, R., Bachelet, C. et al. Inactivation of cytidine triphosphate synthase 1 prevents fatal auto-immunity in mice. Nat Commun 15, 1982 (2024). https://doi.org/10.1038/s41467-024-45805-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45805-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.