Abstract
A mobile colistin resistance gene mcr was first reported in 2016 in China and has since been found with increasing prevalence across South-East Asia. Here we survey the presence of mcr genes in 4907 rectal swabs from mothers and neonates from three hospital sites across Nigeria; a country with limited availability or history of colistin use clinically. Forty mother and seven neonatal swabs carried mcr genes in a range of bacterial species: 46 Enterobacter spp. and single isolates of; Shigella, E. coli and Klebsiella quasipneumoniae. Ninety percent of the genes were mcr-10 (n = 45) we also found mcr-1 (n = 3) and mcr-9 (n = 1). While the prevalence during this collection (2015-2016) was low, the widespread diversity of mcr-gene type and range of bacterial species in this sentinel population sampling is concerning. It suggests that agricultural colistin use was likely encouraging sustainment of mcr-positive isolates in the community and implementation of medical colistin use will rapidly select and expand resistant isolates.
Similar content being viewed by others
Introduction
Colistin, a polymyxin antibiotic rarely used clinically until recently due to associated nephrotoxicity, is now prescribed more often due to increasing global prevalence of multidrug resistant (MDR) infections lacking alternative antibiotic options1,2. The mobile colistin resistance (mcr) gene was initially identified as the underlying cause of colistin resistance in Escherichia coli isolated from an intensive pig farm in Shanghai, China3. It was subsequently linked to the use of colistin in intensive farming as a growth promotor, and its discovery led to the colistin agricultural ban in November 20164. Unfortunately, subsequent surveillance suggests colistin use in farming remains high in South-East Asia5,6,7 and a recent report suggests colistin may be used in agriculture in some African countries8.
A review article by Anyanwu et al. published in 2021 summarises mcr prevalence in different ecosystems in Africa9 and found that unregulated use of antibiotics (including colistin) was directly linked to environmental and animal sources where bacteria carrying mcr genes had been identified. mcr positive E. coli have been detected in human, chicken, and poultry environments3, and subsequent publications have found transfer between E. coli strains and other species on a variety of plasmid backgrounds (including IncHI2, IncI2, IncX4, and IncP) via aquaculture, supply chains and slaughterhouses10. To date, 10 mcr genes (mcr-1 to mcr-10) with minor variants have been characterised11, and mcr has been reported globally12,13.
Widescale surveillance of colistin resistance became relatively common following the discovery of mcr, with a predominance of monitoring in wealthy regions across North America and Europe14,15,16,17,18,19,20, aided by the wealth of the health services. However, there is a dearth of information regarding the prevalence and spread of mcr in Africa.
mcr was first described in Africa in 2016 in a South African study evaluating the presence of mcr-1 in broiler chickens21. A literature review on PubMed (gated between 2014–2023) searching for “colistin resistance + Africa” yielded 298 results, reduced to 164 hits when “mcr + Africa was searched”. A literature review on PubMed searching for “mcr/colistin” + “Nigeria” yielded 29 hits, of which three collectively contained microbiology data19,22,23 suggesting that screening for colistin resistance, particularly from human carriage and infection is scarce. Whereas, an investigation in Southern Nigeria showed a prevalence of mcr-1 in poultry of 2.9%19.
BARNARDS is a study analysing neonatal sepsis comprising of a network of 12 clinical sites in seven low- and middle-income countries (LMICs)24,25,26,27. Mothers and their respective neonates were enrolled into the BARNARDS study between November 2015-December 2017. In addition to examining bacterial causes and risk factors of neonatal sepsis, within BARNARDS the maternal and neonatal rectal microbiota were screened for the presence of extended spectrum ß-lactamases (ESBL) and carbapenemase antibiotic resistance genes (ARG)28. Previous studies have shown that the carriage of AMR gram-negative bacteria (GNB) is not only increasing but is both a risk factor and a precursor to infection, particularly in hospitalised or immunocompromised patients29,30,31. Although our previous work in BARNARDS suggests colistin wasn’t used clinically in Nigeria during the study period, it is possible colistin was imported for use in agriculture and farming9,32.
The aim of this sub-study was firstly to retrospectively screen maternal and neonatal swabs from the three clinical sites in Nigeria (two in Abuja, and one in Kano) for the presence of mcr, and secondly to phenotypically and genotypically characterise bacterial isolates harbouring mcr gene variants.
Results
Mother/neonate characteristics and mcr carriage
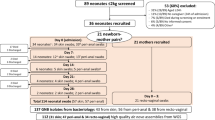
In total, 4907 rectal swabs (n = 3944 Mother Rectal [MR] and n = 963 Baby Rectal [BR]) were processed from hospitals in Kano (NK) and Abuja (NN or NW) (NK-MR n = 2140, NK-BR n = 724, NN-MR n = 909, NN-BR n = 215, NW-MR n = 895, NW-BR n = 24) (Fig. 1) and microbial growth was detected in 70% (n = 3,435) following swab enrichment for 48 h in vancomycin and colistin broth. Following screening carried out by PCR to detect mcr-1, mcr-3, mcr-8-10, 1% of samples (n = 46) were positive for at least one mcr gene (Fig. 1b). Forty-nine distinct bacterial isolates (41 MR and 8 BR) were recovered between 18th November 2015 and the 24th October 2017 (Table 1). The prevalence of mcr carriage was similar between MR and BR, at 1% (n = 39/3944) and 0.7% (n = 7/963) respectively (Fig. 1b). Forty mothers and seven neonates had mcr within their gut microbiome, however we did not find a case whereby both the mother and corresponding neonate both carried mcr. Of the three hospitals where mothers’ and neonates’ rectal microbiota were screened, NN had the highest prevalence (n = 21/1124, 1.9%), with mcr carriage in samples collected from NW at similar rates (n = 12/919, 1.3%) (Fig. 1b), compared to a lower incidence in NK (n = 13/2864, 0.5%).
a Shows a map of Nigeria highlighting the location of the three hospital sites NK Kano Nigeria: NN: National Hospital Abuja, Nigeria, NW: WUSE hospital Abuja, Nigeria, with (b) embedded pie charts showing the prevalence of mcr and the split proportion and number of which were maternal rectal samples (MR) and neonatal rectal swabs (BR). NN and NW are both in Abuja and are 8 km apart, NN to NK is 350 km. The map of Nigeria was created using ggplot2 and map_data in R.
In total, 32/39 mothers who carried mcr reported living in an urban environment (compared to rural, n = 3 and semi-rural, n = 4). Seven mothers reported previous antibiotic use including ß-lactams, macrolides, and metronidazole. Three of the seven neonatal sepsis cases (Table 1) were reported to have blood culture confirmation, and none of the Enterobacter reported from rectal carriage in this study were the causative sepsis pathogen. The three neonates with neonatal sepsis were empirically treated with either amoxicillin and ceftazidime (n = 2/3) or amoxicillin and gentamicin, (n = 1/3). Colistin was not prescribed to any mother or neonate (from available data) within this study.
Diversity of colistin resistant bacterial isolates
Of the 70% (n = 3435) of swabs producing microbial growth, 133 bacterial isolates were cultured from the colistin selective media (4 mg/L) screening. Forty-nine isolates carried an mcr gene, and short-read WGS was completed for all (Table 1, Fig. 2) with complementary long-reads generated for 16 isolates. We detected five Enterobacter spp. making up 94% (n = 46/49) of the mcr positive cohort. Twenty-six were Enterobacter kobei, 11 Enterobacter cloacae, five Enterobacter asburiae, two Enterobacter roggenkampii and two Enterobacter spp. The remaining three isolates were Shigella (n = 1), E. coli (n = 1) and K. quasipneumoniae (n = 1) (Fig. 2). mcr-10 was the most frequently detected gene variants and accounted for 92% (n = 45/49) of all rectal samples positive for mcr (Fig. 2). The remaining mcr genes detected were mcr-1 (n = 3, 6%), and mcr-9 (n = 1, 2%). mcr-8 was not detected in any of the samples, and although we detected a PCR positive hit for mcr-3, following WGS analysis this was not confirmed as mcr-3 (Supplementary Fig. 1). Eight isolates with mcr genes were recovered from seven neonates, all were Enterobacter species, and all carried mcr-10.
A sunburst diagram delineating the mcr gene in the context of the sample type (MR – mother rectal, BR – baby rectal), hospital site (National Hospital Abuja NN, Wuse District Hospital Abuja NW, Murtala Mohammad Specialist Hospital Kano NK) and bacterial species. EAS Enterobacter asburiae, ECL Enterobacter cloacae, EKO Enterobacter kobei, ERO Enterobacter roggenkampii, ESP Enterobacter sp., ECO Escherichia coli, KQI Klebsiella quasipneumoniae, SHI Shigella sp. Forty-nine bacterial isolates carrying an mcr gene are represented in this sunburst diagram.
There were 17 different STs detected within the mcr positive Enterobacter isolates, with the most common being ST1, ST125 and ST691. mcr positive E. cloacae ST1 were recovered from mother and baby rectal swabs from all three Nigerian sites, but the majority were from mothers enrolled into hospitals in Abuja. Whereas E. kobei ST691 with mcr-10 were largely recovered from mothers enrolled into Kano, E. kobei ST125 with mcr-10 were all isolated from mothers enrolled in a single hospital site in Abuja (NN-MR), and all except one, were cultured from samples collected in August 2016 (n = 6).
From the mcr negative and colistin resistant cohort, we identified n = 80/83 Enterobacter species, (n = 32 E. cloacae, n = 22 E. kobei, n = 10 E. asburiae and n = 16 other Enterobacter species) and a single isolate of each Shigella sonnei, K. quasipneumoniae and Raoultella ornithinolytica. During WGS analysis there were a total of 43 previously undetected STs classified; ST1601-ST1608 (mcr positive) and ST1864-1895 (mcr negative).
Antimicrobial resistance
Minimum inhibitory concentrations (MICs) were determined for 41 mcr positive and 83 mcr negative isolates (n = 124 total) as shown in Fig. 3a, Supplementary Data 1. In addition to determining the MIC for colistin, three additional antibiotics were also selected for antimicrobial susceptibility testing: ampicillin, gentamicin and meropenem. Carbapenem resistance was reported during characterisation of clinical bacterial isolates collected during BARNARDS27 and, ampicillin and gentamicin are the empirical first line treatment options for neonatal sepsis as per the WHO guidelines. We expected colistin resistance to be high due to enrichment with colistin, and report this at 93% (n = 115/124). Likewise, ampicillin resistance was high at 94% (n = 116/124) but this was also unsurprising as most of these (95%) (n = 118/124) were intrinsically resistant Enterobacter. All Enterobacter cloacae contained blaCMH-3 and Enterobacter asburiae, hormaechei and kobei isolates all contained blaACT-2 blaACT-4, blaACT-6, or blaACT-9. Of the non-Enterobacter species, n = 5/6 were resistant to ampicillin and all six isolates carried at least one β-lactamase gene. Eleven isolates were resistant to gentamicin, ampicillin and colistin (n = 8/11 were mcr negative). Of these n = 4/11 were also resistant to meropenem, however only one isolate contained a carbapenemase ARG blaNDM-5. NW-MR576 for example carried multiple ARGs within the same plasmid including blaTEM-1, aph-(3”)-lb, tet(A) and catA1, ultimately conferring resistance to several different antibiotic classes. The majority of isolates tested (n = 97/124) were resistant to n = 2/4 antibiotics tested (Fig. 3b), with 8 isolates resistant to n = 3/4 antibiotics and 4 isolates were resistant to all antibiotics tested (Fig. 3b).
a Stacked bar graph showing the MIC profile percentages of mcr positive isolates (n = 41) and mcr negative isolates (n = 83) screened against colistin, ampicillin, gentamicin and meropenem. Blue denotes isolates sensitive to both colistin and ampicillin, gentamicin or meropenem CS XS. Yellow denotes isolates sensitive to colistin and resistant to ampicillin, gentamicin or meropenem CS XR. Orange denotes isolates resistant to colistin and sensitive to either ampicillin, gentamicin or meropenem CR XS. Pink denotes isolates resistant to both colistin and ampicillin, gentamicin or meropenem CR XR. b A stacked bar graph showing the percentage profile of isolates that were resistant to how many 0–4 of the antibiotics tested colistin and ampicillin, gentamicin or meropenem. Split by mcr positive isolates (n = 41) and mcr negative isolates (n = 83). 0 (white) shows no resistance and 4 (black) shows resistance to all 4 tested antibiotics. c A heatmap highlighting the skipped well phenomena33 in Enterobacter spp., showing that the bacteria cease growing at low concentrations (no growth depicted as yellow) of colistin but later return at higher concentrations (depicted in orange), this also indicates this is not an effect restricted to mcr positive isolates as this was observed in both mcr positive and mcr negative isolates.
mcr-1.1 conferred colistin resistance in all three isolates, two of which were from a single rectal swab (NN-MR49-1 (E. coli) and NN-MR49-2 (K. quasipneumoniae)). These isolates also contained several aminoglycoside, sulphonamide, and tetracycline ARGs (Table 2), many of which were located on the same plasmid as mcr-1.1. These two mcr-1.1 containing isolates were resistant to gentamicin and ampicillin but sensitive to meropenem. A Shigella spp. (NW-MR1609) containing mcr-1.1 in addition to chromosomally located blaCTX-M-15 and qnrS1, (Table 2) was sensitive to gentamicin and meropenem but resistant to ampicillin.
A well reported “skipped well” phenomena33 was observed in colistin MIC testing where the growth of Enterobacter was inhibited at certain concentrations, however growth was visible at higher MIC concentrations (Fig. 3c). These were exclusively observed in Enterobacter and were apparent in both mcr positive and mcr negative isolates (Fig. 3c). The single mcr-9 Enterobacter isolate remained sensitive to colistin (MIC = 0.5 mg/L), gentamicin and meropenem, the only other ARG detected were intrinsic, including fosA and blaACT-6. All Enterobacter isolates containing mcr-10, bar one, were resistant to colistin. One (NK-BR1684) of these isolates was also resistant to gentamicin and meropenem, however it did not contain any known resistance genes to aminoglycosides or carbapenems.
mcr-1 genetic context
There were two MR samples positive for mcr-1.1 with three bacterial isolates recovered (Table 1). From sample NN-MR49 both an E. coli (NN-MR49-1) ST1266, and a K. quasipneumoniae (NN-MR49-2) isolate, both carrying mcr-1.1 on large IncHI2A plasmids of 293,168 bp (E. coli) and 294,497 bp (K. quasipneumoniae) were cultured (Table 2). Comparative analysis of plasmid assemblies using Mauve alignment revealed a large degree of similarity between the plasmids (Fig. 4a). Following Blastn and PLSDB comparison, NZ_CP069683.1 (Poland) and NZ_MT929286.1 (Czech Republic) were found to be the most similar.
a A comparative analysis using Mauve of two mcr-1.1 containing IncHI2A plasmids from the same sample; E. coli (pNN-MR49-1, 293,168 bp) ST1266, and a K. quasipneumonaie (pNN-MR49-2, 294,497 bp) compared to two similar non-mcr carrying plasmids accession number NZ_CP069683.1 (from Poland) and NZ_MT929286.1 (from Czech Republic). b The genetic context of the mcr-1.1 Shigella spp. (pNW-MR1609) ST484 IncX4 plasmid relative to Klebsiella pneumoniae IncX4 plasmid (accession number CP041105) from Thailand, where the mcr−1.1 gene was the only major difference. c Despite the very different plasmid backbones for our 3 mcr-containing plasmids they all share an identical 2602 bp without evidence of IS element or transposase presence.
NZ_CP069683.1 and the slightly smaller NZ_MT929286.1 plasmids were very similar except that they did not contain mcr-1.1 but appear as a 2602 bp insert in an identical hypothetical protein relative to both the mcr-negative plasmids. Isolates from NN-MR-49 carried near-identical mcr-1.1 plasmids in E. coli and K. quasipneumoniae, differing only by 19 SNPs and one 1335 bp insertion between pNN-MR49-1 and pNN-MR49-2. The latter took the form of an IS4321 element in the K. quasipneumoniae isolate (Fig. 4a) between the mer operon (merR) for mercury resistance and the tetracycline repressor protein (TetR) of the adjacent tet(A) gene. The IS4321 element was not found in any of the E. coli sequences (JAWPEQ00000000) but was present in three separate plasmids carried by the K. quasipneumoniae sequence (JAWPEP000000000; including the mcr-1.1 carrying plasmid) suggesting that the IS4321 transposase had been acquired from one of these other two plasmids.
Sample NW-MR1609 contained a Shigella ST484 carrying mcr-1.1 on a 33,281 bp IncX4 plasmid (pNW-MR1609), which was highly homologous to a 31,229 bp IncX4 plasmid isolated from a swine rectal swab in Thailand (accession number CP041105) that lacked the mcr-1.1 gene (Fig. 4b). Despite being found in three completely different species and two different Inc plasmid backgrounds the mcr−1.1 gene was found on an identical 2602 bp segment without any evidence of an associated IS element or transposase (Fig. 4c). Highly homologous plasmids lacking the mcr-1.1 gene were found for these plasmids and no homology or insert boundary repeat sequences (direct or indirect) could be found for the genes that were disrupted at the insertion site (Fig. 4a, b). Following Geneious (v2022.1.1) and Blastn analysis (v2.7.1) we found a similarity between pNW-MR1609 and IncX4 plasmid sequences from mcr-1.1 positive E. coli and K. pneumoniae from a Nigerian study conducted by Ngbede et al.23. Ngbede performed WGS on 15 isolates, and assembly data produced contigs ~30 Kb allowing comparison of the plasmid backbone. Furthermore, following Blastn analysis, we detected >100 hits with greater than 99 % coverage and nucleotide identity suggesting the 33 Kb IncX4 mcr-1.1 containing plasmid has been well disseminated. Analysis within PLSDB reveals that similar IncX4 plasmids have been isolated from various sectors including animals and humans from Denmark, China, Egypt, USA, and Vietnam, likewise reported in the literature34,35,36,37.
mcr-9 genetic context
Two identical copies of mcr-9.1 were detected in NW-MR803 an Enterobacter isolate most closely related to E. cloacae. One was found on the chromosome and one on a 186,925 bp IncFII/IncFIB plasmid (Fig. 5). Kieffer et al.38, showed that mcr-9.1 expression is inducible (with associated elevated colistin MIC) in the presence of colistin when mcr-9 is located upstream of the qseBC two-component regulatory system (present upstream of our plasmid mcr-9.1), but Tyson et al.39, disputed the capacity of qseBC to mediate upregulation. Only the plasmid borne mcr-9 had these adjacent genes, present on an 8251 bp mobile element flanked by IS elements IS903B and IS26 (Fig. 5). However, on the chromosome, the mcr-9.1 gene is located upstream from an IS1R locus (Fig. 5), which Jiang et al.40, have recently shown could mediate upregulation of mcr-9.2 in Genbank accession CP091482.1. Although the genomic cassette containing mcr-9.1 in NW-MR803 additionally appears to have been further modified with the adjacent insertion of an ISEsp1 element (which is also present in GenBank accession CP012999 Enterobacter sp. Isolate E20) indicating independent acquisition of these mcr-9.1 genes between plasmid and genome. Colistin MIC was found to be 2 mg/L as determined by broth microdilution, which was at the EUCAST breakpoint threshold (resistance >2 mg/L). However, a single passage in 0.5 mg/L colistin resulted in elevation of the MIC to 8 mg/L and a second passage elevated colistin MIC of 32 mg/L (Supplementary Fig. 2). However, no further elevation of MIC was observed with subsequent passages in sub-MIC colistin.
A previous publication by Tyson et al.39, had defined three separate cassettes defined by IS elements IS903B and IS1R (blue arrows). The mcr-9 gene carried on the plasmid was completely homologous to the “type A” configuration in their Fig. 3, including the qseB-like and qseC-like genes (green arrows) required for induction of colistin resistance. However, while the NN-MR-803 genomic copy of mcr-9 had similarity to Tyson et al.’s39 Fig. 3C, an additional intervening ISEsp1 insertion was observed (ISfinder identified genes shown as purple arrows). However, an identical mcr-9 configuration was found on the chromosome of GenBank accession number CP012999 Enterobacter sp. Isolate E20. Conserved cupin fold metalloprotein, WbuC is shown as a grey arrow and other open reading frames are shown as yellow arrows (including a transposase overlapping ISEsp1 from GenBank annotation). Large discrepancies between genomic and plasmid environments surrounding mcr-9 indicates independent acquisition of these from separate sources.
Mcr-10 genetic context
We analysed 12 mcr-10 positive isolates with long-read data available (Fig. 6), three contained the mcr-10 on the chromosome, nine isolates contained the mcr-10 gene on plasmids. There was one additional isolate (NK-MR1610) that contained an mcr-10-like gene on a 15,560 bp contig; however, it only had 94% identity and 15 amino acid polymorphisms indicating it could not be considered a true mcr−10 gene. Furthermore it was not preceded by tyrosine-type recombinase/integrase (GenPept accession WP_032676104.1), conserved in all other mcr-10 carrying isolates (Fig. 6). The local environment of this tyrosine-type recombinase/integrase and mcr-10 combination was only found between mis-matched IS elements that ranged in size from 5132 bp to greater than 50,000 bp (Fig. 6A–D). Three of the plasmids with mcr-10 (NK-1540, NK-MR6054, and NW-MR576) (Fig. 6C, D) and NW-MR132 with mcr-10 chromosomally located shared homologous regions between 23,881–25,447 bp with an Enterobacter roggenkampii strain 120063 plasmid (GenBank CP116250) including an operon of tellurite resistance genes ending at a ISKpn74 transposase, suggesting a common origin. Analysing the regions surrounding mcr-10 for all plasmids and chromosomal insertions using Isfinder (https://isfinder.biotoul.fr/) identified flanking transposases demarcating islands from 5903 bp to 65,813 bp containing mcr-10. However, no consistent pattern could be discerned as individual IS elements (including IS15DIV, ISKpn26, ISKpn74 and IS1B) were only found in a maximum of n = 3/12 isolates and the lack of matching IS elements indicates they would be unable to move further.
In all occurrences the resistance gene was confined to a conserved 3378 bp region that included an adjacent tyrosine-type integrase (tyr-Int or In; brown arrows), defining the smallest unit transposon. Analysing the adjacent environment using ISfinder these unit transposons were located on inserts of various lengths bordered by a variety of IS elements (purple arrows). These could largely be classified as (A) minimal: 5132–5902 bp; B small: 14,539-17,400 bp (although NK-BR1052 was truncated by contig assembly); C intermediate: 25,862–30,548 bp (which includes a region of 20,215 bp homologous) to a Enterobacter roggenkampii plasmid (GenBank Accession number CP116250) containing a cassette of tellurite resistance genes (green arrows); and (D) complex regions of >50,000 bp, two of which include the Enterobacter roggenkampii plasmid tellurite resistance cassette, as well as genes from the tra family of conjugal elements (light blue arrows). Two of these isolates have >20,000 bp regions with high homology to a tra gene family cassette carried by an unnamed plasmid in Enterobacter sp. Z1 (Genbank accession number CP099720). No consistent pattern of boundary IS elements could be discerned to account for a larger conserved mobile element. Yellow arrows indicate open-reading frames that could not be ascribed resistance or mobility but were interspersed between other genes of interest. A full annotation of these regions is not included to highlight areas of greater interest.
Phylogenetic analysis of Enterobacter species
A core genome phylogenetic tree of 130 Enterobacter isolates obtained during this study (70 MR and 60 BR isolates) was produced to evaluate species wide diversity amongst the mcr-positive and mcr-negative colistin resistant bacteria (Fig. 7). Collectively, the most frequent species were E. kobei and E. cloacae (n = 49 and n = 44 respectively), which represented 72% of the total dataset, but nine distinct colistin resistant species of Enterobacter were present (Fig. 7). There was a large diversity with 74 distinct STs and the most common across both mcr positive and negative isolates were ST125, ST56, ST691, ST1 and ST84. A majority of BR Enterobacter were E. cloacae (Fig. 7) whereas the majority of E. kobei were from MR samples. ST125 E. kobei (n = 10) were recovered from Abuja (both in NN and NW), seven contained mcr-10 and three were negative however all were resistant to colistin (Fig. 7). SNP analysis (NN-MR773 used as the reference, single contig chromosome 4,382,297 bp) revealed that n = 6/7 mcr-10 positive isolates, collected from the same recruitment site (NN, Abuja) in August 2016 were within 10 pairwise SNPs (each to NN-MR773) suggesting clonal spread or a single acquisition point perhaps within the hospital. The single remaining mcr-10 positive ST125 E. kobei was also collected from NN in April 2016 however was >800 SNPs distant from NN-MR773. Likewise, the mcr-10 negative ST125 E. kobei from NN, were also >700 SNPs distant from mcr positive isolates. The single mcr negative ST125 E. kobei from the second hospital site in Abuja (NW) was over 1800 pairwise SNPs distant from all E. kobei isolates collected from NN.
isolates using Panaroo to generate a core genome alignment and IQtree to build the phylogeny. The colours in the circles indicate the sample type (MR, mother rectal or BR, baby rectal). The colour of the label which represents the isolate identification code (leaf) denotes the hospital location (NN, NW & NK). The date displayed is the month of the rectal swab collection. The sequence type (ST) of the isolate is denoted in the numerical text following the isolate date. The colour strip represents colistin sensitivity/resistance with red denoting resistance, yellow sensitivity, and grey indicates data not available. Followed by a heatmap of resistance genes, beginning with the presence of mcr gene, and ARGs were grouped per antibiotic class.
Two E. cloacae isolates (NN-MR1780 and NK-MR7023) sit on a separate branch distinct from the remaining (n = 42) E. cloacae. Most isolates contained blaACT/MIR as these are often intrinsic in multiple Enterobacter species, however there were four isolates of E. bugandensis that sat on a distinct branch, and a further single isolate (Fig. 7) identified as a partial match to E. cloacae, that did not contain any genetic markers conferring resistance to ß-lactams. The unidentified Enterobacter species carries mcr-9.1 and was susceptible to colistin. The remaining (n = 7) isolates susceptible to colistin (one carried mcr-10) were different species across the phylogeny.
Conjugation of Enterobacter carrying mcr-9 and mcr-10
To determine whether Enterobacter species could be acting as a reservoir genus, we performed conjugation experiments on seven distinct bacterial isolates (denoted in Table 2) with a range of plasmid Inc types including IncFIB and IncFII with plasmid sizes between 65,562 bp to 191,776 bp (denoted in Table 2). Two plasmids (pNK-BR1684 and pNN-BR1663r1; Fig. 6) contained relatively complete cassettes of transfer or tra genes that had the potential for functionality; while pNN-MR-773 and pNW-MR-576 carried truncated portions of the cassette (dbsA with either traA/traL/traE or traI/traD, respectively) that lack pilus or secretory type IV genes that would make it functional for plasmid transfer41. Further analysis of NK-BR1684 identified a 36,594 shared region of homology with the Enterobacter spp. Z1 unnamed plasmid 2 (GenBank accession number CP099720) which also contains conjugal transfer proteins traC, traD, traF, traG, traH, traI, traN, traT and traU. However, after multiple attempts of different donor recipient cell density ratios, it was not possible to conjugate any of the plasmids containing an mcr gene from Enterobacter to E. coli J53.
Discussion
To the best of our knowledge, this is the largest screening of intestinal microbiota for colistin resistance in Nigeria analysing almost 5000 samples. We found an mcr carriage of 1%, similar to our recent study which screened the same cohort of mothers and neonates for the carriage of carbapenemase ARG and reported blaNDM and blaOXA-48-like at 1.5% and 2% respectively28. Although data on colistin resistance in Africa is scarce, there has been a noticeable increase in the last three years. In 2021, Ugah and Udeani reported high phenotypic resistance to colistin in Nigerian clinical isolates42 and suggest a discord between colistin resistant bacterial infections and evidence of colistin antibiotic therapy, suggesting non-therapeutic colistin pressures are creating silent reservoirs. Furthermore, a recent study by Umair et al., 2023 indicates that there are multiple European import sources of colistin into Nigeria, likely for use in agriculture43. We detected mcr-1.1 E. coli and K. quasipneumoniae from a sample collected in November 2015, suggesting colistin resistance has been present in the Nigerian community for years and the microbiota is a likely reservoir. Mothers may be acquiring colistin resistant bacteria from the community, however neonatal acquisition may be from the community, maternal vertical transmission, or the environment. In 2018 Anyanwu et al., screened for colistin resistance in 785 cloacal swab samples from the South of Nigeria and similarly detected mcr-1.1 in E. coli and K. pneumoniae isolates further suggesting the spread of mcr in Nigeria across sectors19.
We found a microbial growth rate of 70% following enrichment in colistin broth (4 mg/mL), similar to previous studies which found broth enrichment steps can maximise mcr recovery44,45,46. While the MIC for the mcr−9 carrying isolate NN-MR803 was initially found to be below the threshold for colistin resistance (>2 mg/L), we found that a single passage in sub-MIC concentration elevated the subsequent MIC to 8 mg/L (now resistant) and a second sequential sub-MIC exposure raised the MIC to 32 mg/L (Supplementary Fig. 2). These results indicate that exposure to stepwise increases in antimicrobial concentrations in vitro may miss resistance genes that are regulated by either inducible promotors or require alteration to gene repressor mechanisms that are sensitive to gradual increases in antimicrobial concentrations. Therefore, while we did not interrogate the colistin susceptible isolates carrying mcr−10, it is possible that pre-MIC incubation with low levels of colistin may have resulted in emergence of resistant phenotypes for these isolates as well. This has been noted by other studies indicating microbiological and genomic surveillance of colistin resistance is essential to capturing all potentially resistant bacterial populations40,47,48,49,50 Whilst there are many studies utilising real-time PCR as an effective mcr screen51,52, this is costly compared to end-point PCR. An initial molecular and low-cost screen as implemented in this study can be effective and sustainable to perform in laboratories where resources and access may be restricted. It is not practical to screen all mcr genes, however mcr-1, mcr-3, mcr-8, mcr-9, and mcr-10 are often considered the most clinically important53.
Throughout the BARNARDS study we have demonstrated that Enterobacter is a clinically important genus, and important in the context of AMR. Phylogenetic analysis revealed large intraspecies diversity of colistin resistant Enterobacter suggesting a diverse reservoir of colistin resistant bacteria within the microbiota of women and neonates, with mcr genes being identified within multiple species. Enterobacter is an ESKAPE pathogen, is often antimicrobial resistant and is responsible for many nosocomial infections54. Enterobacter acquiring ESBL and carbapenemase ARG were amongst the top five non-outbreak GNB causing neonatal sepsis26, and top three within the mother/neonatal gut28. Furthermore, Liao et al., 2022 reported a high prevalence of colistin resistance and mcr-9/mcr-10 in Enterobacter over a decade in China55. More concerning however is a recent Nepalese study by Manandhar et al., 2022, where they describe a fatal neonatal sepsis outbreak caused by E. kobei carrying mcr-10 and the authors hypothesised hospital surface colonisation via the creation of biofilms56.
We were not successful in conjugating any plasmids carrying mcr-10 into E. coli J53, similar to other reports and this suggests that mcr-10 may be, at least for now, a concern largely limited within Enterobacter species. If conjugation of mcr-10 into J53 had been successful, we would have attempted to conjugate mcr-10 into wildtype strains of E. coli and K. pneumoniae, as along with Enterobacter, these species were the dominant AMR resistant isolates recovered during clinical and carriage BARNARDS samples26,28. Whilst the body of current literature primarily links mcr-10 to Enterobacter species, there are reports of plasmid mediated mcr-10 detected in other species including Cronobacter, Klebsiella, and E. coli57,58. In this study, we identified isolates containing tra genes which may assist conjugation of the mcr containing plasmid. Furthermore, all Enterobacter with additional long-read data carried a tyrosine-type recombinase/integrase upstream of the mcr-10 gene which may, contribute to the mobilisation of mcr-10 gene as suggested previously57,58 given previous evidence of an upstream xerC involvement in mediating the carbapenemase resistance gene blaIMI59. There are multiple mechanisms of colistin resistance in Enterobacter species aside from the acquisition of mcr gene suggesting that there may be undetected reservoirs of colistin resistance in the gut microbiota. Previous studies suggest that chromosomal mutations conferring resistance to colistin (pmrA/pmrB, phoQ, and mgrB genes) may be present in Enterobacter47,60 and future work to determine chromosomal mutations conferring colistin resistance are needed.
A limitation of this study was a lack of colistin susceptible isolates due to our laboratory approach. Long-read sequences were generated based on initial short-read bioinformatics analysis, therefore it was not possible to characterise the genetic context of all mcr genes. We did not collect data on the human immunodeficiency virus (HIV) status of the mother, and this, along with other conditions affecting the immune system may be drivers for AMR carriage. A further limitation was the different sample size of rectal swabs processed per site. All BR samples were processed. MR samples were processed in proportion to the total number of women enrolled into the study (NN n = 909/1902, NW n = 919/2359, and NK n = 2359/7319 samples processed). The prevalence of mcr must therefore be interpreted with caution accounting for the variation in the overall sample size per site and all findings are presented descriptively.
In conclusion, mcr carriage was higher in urban settings between 1.3–2% in Abuja compared to 0.5% in Kano. The dominant reservoir of colistin resistant species in the microbiota were Enterobacter spp., and worryingly, we detected mcr positive GNB in neonates less than a week old, including neonates presenting with sepsis. The majority of mcr genes were located on plasmids, which may, conditions permitting, disseminate wider in the intestinal microbiota and surrounding environments. The large diversity of the mcr genetic context is concerning as there are likely multiple acquisition routes suggesting that ongoing colistin resistance surveillance is vital, particularly in vulnerable population groups such as neonates. Unfortunately, due to data indicating agricultural use of colistin in Sub-Saharan Africa, these findings could likely be extrapolated across Africa and are, regrettably, higher in Asia where there is greater evidence of colistin usage agriculturally and colistin resistant infections.
Methods
Study design
A prospective cohort study was conducted through the BARNARDS network consisting of 12 clinical sites in seven countries in Africa (Ethiopia, Nigeria, Rwanda, and South Africa) and South-Asia (Bangladesh, India, and Pakistan), this work focuses exclusively on the three sites in Nigeria. Rectal samples were taken from all mothers upon recruitment and from neonates with clinical diagnosis of sepsis and upon clinical discretion from birth up to 60 days old as described in Carvalho et al., 202228. Samples were maintained at 4 °C until transfer to Cardiff University (CU) under UN3733 regulations.
Clinical variables analysed in this manuscript include admission date, enrolment date, neonatal and maternal (within last three months) antibiotic use, neonate’s date of birth, place of birth (cohort), and neonatal outcome (alive/deceased). Ethical approval was sought from each institution; NK Kano State Hospitals Management Board 8/10/1437AH 13/07/2016, NN Health Research Ethics Committee (HREC), National Hospital, Abuja NHA/EC/017/2015 27/04/2015 and NW Health Research Ethics Committee (HREC), National Hospital, Abuja NHA/EC/017/2015 27/04/2015. Consent was procured in writing in local languages by research nurses providing mothers with study information, consenting for both mother and/or neonatal enrolment. If written consent not obtainable (due to literacy barriers) and oral consent was collected from the mothers by trained researchers. Oral consent was documented by the participant signing/marking the consent form as reported in Carvalho et al., 202228.
Processing rectal swabs and bacterial isolates
We selected samples proportionally across the sampling time period from November 2015-December 2017. All BR swabs collected during BARNARDS were included for mcr screening. MR and BR were cut into 5 mL of Lysogeny Broth (LB) with vancomycin (10 mg/mL) and colistin (4 mg/mL)44 and amphotericin B (1 µg/mL) and incubated at 37 °C shaking at 180 rpm for 24 h. Swabs with no growth after 24 h were further incubated to 48 h. 1 mL of broth was transferred to a deep well MIDI 96-well plate and was centrifuged at 1530 × g for 10 min to pellet microbial growth. The supernatant was removed, and the pellet was washed with 0.2 mL 1× PBS to remove excess charcoal. The MIDI 96-well plate was further centrifuged at 1530 ×g for 1 min. The PBS was removed, and the pellet resuspended via pipette mixing in 0.1 mL of molecular grade water. The 96-well plate was centrifuged at 1530 × g for 1 min resulting in the gDNA template for PCRs. PCR conditions for mcr-1, mcr-8, mcr-9, and mcr-10 are detailed in Supplementary Table 1. Gel electrophoresis was performed with a 1.5% agarose gel for 35 min at 250 v and imaged using a G:BOX Chemi-XX6 GENESys, (Cambridge, UK). 50 μL of the overnight culture of rectal samples with a positive PCR result for any mcr variant were streak plated onto a UTI agar plate (Sigma) supplemented with vancomycin (10 mg/ml) and colistin (2 mg/ml). Each phenotypically different colony was sub-cultured, and the PCR was repeated for the gene(s) of interest. All positive bacterial isolates were identified using Microflex LT MALDI-TOF MS (Bruker Daltonik, GmbH, UK) with α-Cyano-4-hydroxycinnamic acid (HCCA) matrix (Sigma Aldrich) and preserved in TS/72 beads (Technical Service Consultants, UK) at −80 °C for gDNA extraction and a final confirmation of the mcr gene (for the positive isolates) before whole genome sequencing (WGS). A selection of bacterial isolates (selected across the sampling time and at a ratio of 2:1 negative to positive) that grew in the presence of colistin (4 mg/mL) were also preserved in TS/72 beads (Technical Service Consultants, UK) at −80 °C for gDNA extraction and WGS for comparative analysis and to look for chromosomal genes conferring resistance to colistin.
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs) were determined for mcr positive and mcr negative isolates purified from rectal swabs by in-house agar dilution for four antibiotics: colistin, gentamicin, ampicillin, and meropenem. E. coli ATCC 25922, E. coli NCTC 13846 (mcr-1 positive) and Pseudomonas aeruginosa ATCC 2785 were used for quality control and interpreted according to the EUCAST v12 guidelines61. Each bacterial isolate was tested in triplicate per antibiotic concentration to generate three separate data points following validation using appropriate control strains listed above from which a median value was used in Fig. 3a. Additionally, NW-MR803 (mcr-9 positive) was subjected to a three-day serial passage in a sub-MIC of colistin to determine whether resistance was inducible. MICs were determined according to EUCAST guidelines as above in Mueller-Hinton broth for colistin (0.06–128 mg/L range), comparing the original isolate to 1-day, 2-day and 3-day sub-MIC colistin induced isolates, in parallel and in triplicate. Consistent relative increases in MIC plateaued after 2 days induction (16-fold relative to original isolate).
Bacterial conjugation
Donor isolates were plated onto colistin agar (2 mg/L). J53 was used as the recipient for all experiments and was cultured onto UTI ChromoSelect agar (Sigma, UK) supplemented with 100 mg/L sodium azide (NaN3). Overnight cultures of donor and recipient isolates were prepared and diluted 1:100 in LB broth and incubated at 170 rpm at 37 °C until in log phase growth (OD 0.4–0.6 at 600 nm). The donor and recipient were mixed at ratios 1:3, 1:5 and 1:1 for each experiment and the mating cultures were incubated at 37 °C for 16–20 h. Transconjugates were selected for by plating 100 mL of suspension onto agar supplemented with NaN3 (200 mg/L) and colistin (2 mg/L). The conjugation frequency was calculated by counting CFU of donor and transconjugates, these were confirmed by PCR. A positive mcr-1 control plasmid (E. coli bacteria) was included in each experiment to calculate the conjugation frequency.
WGS and bioinformatics analysis
A colony was transferred into 1.8 mL of LB broth with colistin at 2 mg/L and incubated at 37 °C, 180 rpm for 18 h. gDNA was extracted and genomic libraries were prepared exactly as described in Carvalho et al., 202228. The same gDNA was used to generate complimentary long read sequencing data for selected isolates. SPRI beads (Mag-Bind TotalPure, Omega) were used to concentrate and purify at a 1:1 ratio with a final elution volume of 15 µL to achieve an optimal range between 40–60 ng/µL. gDNA was quantified with the dsDNA broad range assay kit using the qubit 4.0 fluorometer (Thermofisher). Genomic libraries were prepared using the Rapid Barcoding Kit (SQK-RBK004; Oxford Nanopore) with an extended incubation of 30 min following addition of RAP and sequenced on a R9.4 flow cell using a MinION (Oxford Nanopore). For all short reads we sequenced to an overall genome coverage of 30X. For all mcr isolates, additional long reads were generated to increase the hybrid genome coverage to >50–100X.
Bioinformatics was performed as described in Carvalho et al., 202228. Trimgalore (v0.6.4) was used to remove the Nextera adapter sequences and low-quality bases (--paired --phred33 -q 25 --illumina -e 0.2). Reports were generated using fastqc (v0.11.8) and collated using MultiQC (v1.12). Bacterial species were identified using Blastn nt (v2.7.1) and PathogenWatch (v.15.0.2). Genomes were screened for ARG using ABRicate (v1.0.0) with associated databases NCBI (Database accessed June 2022) and resfinder (Database accessed 2022) (98% identity/coverage cut off). Each genome was screened in the two different databases (NCBI and resfinder; both available within ABRicate) and cross checked for consistency. Previously undetected alleles, and ST profiles were submitted to BIGSbd and PubMLST for assignment. For all Enterobacter isolates, a core genome alignment was generated using Panaroo (v1.2.10), and a phylogenetic tree was constructed using IQtree (v2). Phylogenetic trees were mid-rooted, visualised, and annotated using iTOL (v6.0). Guided by ST data and the phylogenetic tree, secondary analysis was performed to determine the isolate relatedness for ST125 E. kobei. For this isolate relatedness analysis, Snippy (v4.6.0) was used with default parameters to call pairwise SNPs for ST125 E. kobei isolates using NN-MR773 as the reference. Additionally, snippy (v4.6.0) was also used to assess SNPs between complete plasmids (resulting from hybrid assemblies) and Illumina short-reads of corresponding isolates.
FAST5 reads were basecalled using Guppy (v4.5.4) within MinKnow and FASTQ reads were trimmed with Filtlong (v0.2.0) (--min_length 1000 –min_mean_q 10). Unicycler (v0.4.7)62 was used to hybrid assemble the short-read and long-read. Genomes were annotated as described above. Where hybrid assemblies produced circularisation of chromosome and/or plasmid contigs this was confirmed using Bandage (v0.9.0) and Geneious (v2022.1.1). Isolates for which Unicycler assemblies contained homopolymer-related open reading frame breaks (NW-MR803, NN-MR49-1 and NN-MR49-2) were re-sequenced on both MiSeq and MinION platforms in August 2023 for confirmatory assemblies. Confirmatory long-reads were basecalled using Guppy (v6.5.7) with an additional trimming argument (--num_extra_bases_trim 20) and short-reads were trimmed using Trim Galore (v0.6.10) using default settings. Confirmatory assemblies were performed using the Dragonflye (v1.1.1) wrapper to perform additional trimming with fastP (v0.23.4) and porechop (v0.2.4) before executing a Flye (v2.9.2) assembly with two rounds of Medaka (v 1.8.0) polishing and one round of Polypolish (v0.5.0) polishing. Plasmid contents of Dragonflye assemblies were further investigated using a specialist plasmid assembler, plassembler (v1.2.0), to confirm Flye assemblies were not missing plasmids. Multiple QC stages were reviewed in MultiQC (v1.12) using default QC collation metrics.
Genomes were downloaded from NCBI for comparative analysis and collections were screened for the presence of mcr genes using ABRicate (v1.0.0). Genomes were annotated using Prokka (v1.14.5)63, ISfinder64 (https://www-is.biotoul.fr/index.php; Database accessed May 2023) and Blastn (nt database) within Geneious. Each identified IS was downloaded into Geneious and used to annotate the genomes. All additional annotations were performed by Geneious by downloading closest homologous GenBank deposited genomes using the integral Blastn tool in Geneious to search NCBI databases. Progressive Mauve65 (Geneious plugin) was used to compare plasmid sequences and genetic context images were created from Geneious outputs in Adobe Illustrator (v26.3.1). PLSDB (https://ccb-microbe.cs.uni-saarland.de/plsdb/; Database accessed May 2023) and Blastn (nt database) were used to search for plasmids for comparative analyses. Bioinformatics analysis was performed using a high-performance computing cluster at Cardiff University (ARCCA).
Statistics & reproducibility
No statistical method was used to predetermine sample size. No data were excluded from the analyses, the experiments were not randomised, and the investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All short-read sequences generated were submitted to the European Nucleotide Archive (ENA) and given the project number PRJEB44720 (Supplementary Data 2). All hybrid assembled genomes (from short-read and long-read) were uploaded to NCBI and given the project number PRJNA860154. All raw MIC data is available within the Supplementary Data 1 file. Isolate availability will be considered by the authors under the remit of a mutually accepted material transfer agreement.
Code availability
All bioinformatics software used has been extensively mentioned in the methods section. All default parameters used unless specifically detailed in the methods section and therefore no bespoke code was generated during this study.
References
Editorial. When the drugs don’t work. Nat. Microbiol. 1, 16003 (2016).
Paterson, D. L. & Harris, P. N. A. Colistin resistance: a major breach in our last line of defence. Lancet Infect. Dis. 16, 132–133 (2016).
Liu, Y. Y. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168 (2016).
Walsh, T. & Wu, Y. China bans colistin as a feed additive for animals. Lancet Infect. Dis. 16, 1102–1103 (2016).
Nguyen, N. T. et al. Use of colistin and other critical antimicrobials on pig and chicken farms in southern Vietnam and its association with resistance in commensal Escherichia coli bacteria. Appl. Environ. Microbiol. 82, 3727–3735 (2016).
Wongsuvan, G., Wuthiekanun, V., Hinjoy, S., Day, N. P. J. & Limmathurotsakul, D. Antibiotic use in poultry: a survey of eight farms in Thailand. Bull. World Health Organ. 96, 94–100 (2018).
Mohsin, M. et al. Excessive use of medically important antimicrobials in food animals in Pakistan: a five-year surveillance survey. Glob. Health Action. 12, 1697541 (2019).
Mankhomwa, J. et al. A qualitative study of antibiotic use practices in intensive small-scale farming in urban and peri-urban blantyre, malawi: implications for antimicrobial resistance. Front. Vet. Sci. 9, 876513 (2022).
Anyanwu, M. U., Okpala, C. O. R., Chah, K. F. & Shoyinka, V. S. Prevalence and traits of mobile colistin resistance gene harbouring isolates from different ecosystems in Africa. BioMed. Res. Int. 2021, 1–20 (2021).
Shen, Y. et al. Integrated aquaculture contributes to the transfer of mcr-1 between animals and humans via the aquaculture supply chain. Environ. Int. 130, 104708 (2019).
Hussein, N. H., AL-Kadmy, I. M. S., Taha, B. M. & Hussein, J. D. Mobilized colistin resistance (mcr) genes from 1 to 10: a comprehensive review. Mol. Biol. Rep. 48, 2897–2907 (2021).
Wang, R. et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 9, 1179 (2018).
Nang, S. C., Li, J. & Velkov, T. The rise and spread of mcr plasmid-mediated polymyxin resistance. Crit. Rev. Microbiol. 45, 131–161 (2019).
Sia, C. M. et al. The characterization of mobile colistin resistance (mcr) genes among 33,000 Salmonella enterica genomes from routine public health surveillance in England. Microb. Genom. 6, e000331 (2020).
Mendes, A. C. et al. mcr-1 in carbapenemase-producing Klebsiella pneumoniae with hospitalized patients, Portugal, 2016-2017. Emerg. Infect. Dis. 24, 762–766 (2018).
Mendes Oliveira, V. R., Paiva, M. C. & Lima, W. G. Plasmid-mediated colistin resistance in Latin America and Caribbean: a systematic review. Travel Med. Infect. Dis. 31, 101459 (2019).
Timmermans, M., Wattiau, P., Denis, O. & Boland, C. Colistin resistance genes mcr-1 to mcr-5, including a case of triple occurrence (mcr-1, −3 and −5), in Escherichia coli isolates from faeces of healthy pigs, cattle and poultry in Belgium, 2012–2016. Int. J. Antimicrob. Agents 57, 106350 (2021).
Wise, M. G., Estabrook, M. A., Sahm, D. F., Stone, G. G. & Kazmierczak, K. M. Prevalence of mcr-type genes among colistin resistant Enterobacteriaceae collected in 2014-2016 as part of the INFORM global surveillance program. PLoS One. 13, e0195281 (2018).
Anyanwu, M. U. et al. Isolation and characterisation of colistin-resistant Enterobacterales from chickens in Southeast. Niger. J. Glob. Antimicrob. Resist. 26, 93–100 (2021).
Monte, D. F. et al. Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 genes in South America. Antimicrob. Agents Chemother. 61, e02718–16 (2017).
Perreten, V., Strauss, C., Collaud, A. & Gerber, D. Colistin resistance gene mcr-1 in avian-pathogenic Escherichia coli in South. Afr. J. Antimicrob. Chemother. 60, 4414–4415 (2016).
Olaitan, A. O. et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int. J. Antimicrob. Agents 44, 500–507 (2014).
Ngbede, E. O. et al. Identification of mobile colistin resistance genes (mcr-1.1, mcr-5 and mcr-8.1) in Enterobacteriaceae and Alcaligenes faecalis of human and animal origin, Nigeria. Int. J. Antimicrob. Agents 56, 106108 (2020).
Sands, K. et al. Characterisation of Staphylococci species from neonatal blood cultures in low- and middle-income countries. BMC Infect. Dis. 22, 593 (2022).
Milton, R. et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob. Health 10, e661–e672 (2022).
Sands, K. et al. Characterization of antimicrobial-resistant Gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat. Microbiol. 6, 512–523 (2021).
Thomson, K. M. et al. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS). Lancet Infect. Dis. 21, 1677–1688 (2021).
Carvalho, M. J. et al. Antibiotic resistance genes in the gut microbiota of mothers and linked neonates with or without sepsis from low- and middle-income countries. Nat. Microbiol. 7, 1337–1347 (2022).
Arzilli, G. et al. Screening for antimicrobial-resistant Gram-negative bacteria in hospitalised patients, and risk of progression from colonisation to infection: Systematic review. J. Infect. 84, 119–130 (2022).
Bezabih, Y. M. et al. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J. Antimicrob. Chemother. 76, 22–29 (2021).
Tseng, W. P., Chen, Y. C., Chen, S. Y. & Chang, S. C. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant Gram-negative bacteria colonization or infection. Antimicrob. Resist. Infect. Control 7, 83–93 (2018).
Anyanwu, M. U. et al. Is Africa ready for mobile colistin resistance threat? Infect. Ecol. Epidemiol. 11, 1962781 (2021).
Ezadi, F., Ardebili, A. & Mirnejad, R. Antimicrobial susceptibility testing for polymyxins: challenges, issues, and recommendations. J. Clin. Microbiol. 57, e01390–18 (2019).
Sun, J. et al. Genetic analysis of the IncX4 plasmids: implications for a unique pattern in the mcr-1 acquisition. Sci. Rep. 7, 424 (2017).
Soliman, A. M. et al. Complete genome sequences of two Escherichia coli clinical isolates from Egypt carrying mcr-1 on IncP and IncX4 plasmids. Front. Microbiol. 13, 989045 (2022).
Dantas Palmeira, J. et al. Worldwide disseminated Incx4 plasmid carrying mcr-1 arrives to wild mammal in Portugal. Microbiol. Spectr. 10, e01245–22 (2022).
Habib, I. et al. First report from supermarket chicken meat and genomic characterization of colistin resistance mediated by mcr-1.1 in ESBL-producing, multidrug-resistant Salmonella minnesota. Int. J. Food Microbiol. 379, 109835 (2022).
Kieffer, N. et al. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob. Agents Chemother. 63, e00965–19 (2019).
Tyson, G. H. et al. The mcr-9 gene of Salmonella and Escherichia coli is not associated with colistin resistance in the United States. Antimicrob. Agents Chemother. 64, e00573–20 (2020).
Jiang, S. et al. Molecular antibiotic resistance mechanisms and co-transmission of the mcr-9 and metallo-β-lactamase genes in carbapenem-resistant Enterobacter cloacae complex. Front. Microbiol. 13, 1032833 (2022).
Shen, Z., Tang, C. M. & Liu, G.-Y. Towards a better understanding of antimicrobial resistance dissemination: what can be learnt from studying model conjugative plasmids? Mil. Med. Res. 9, 1–3 (2022).
Ugah, U. & Udeani, T. High prevalence of phenotypic resistance to colistin, tigecycline and netilmicin in a region with no history of colistin administration in Nigeria. Clin. Lab. 67, 1–12 (2021).
Umair, M. et al. International manufacturing and trade in colistin, its implications in colistin resistance and One Health global policies: a microbiological, economic, and anthropological study. Lancet Microbe 4, e264–e276 (2023).
Zurfluh, K. et al. Screening for fecal carriage of MCR-producing Enterobacteriaceae in healthy humans and primary care patients. Antimicrob. Resist. Infect. Control. 6, 28 (2017).
Germ, J. et al. Surveillance cultures for detection of rectal and lower respiratory tract carriage of colistin-resistant Gram-negative bacilli in intensive care unit patients: comparison of direct plating and pre-enrichment step. J. Med. Microbiol. 68, 1269–1278 (2019).
Sun, Q. et al. Alkaline Peptone water-based enrichment method for mcr-3 from acute diarrheic outpatient gut samples. Front. Med. 5, 99 (2018).
Mills, J. P. et al. Risk factors for and mechanisms of CO-listin resistance among Enterobacterales: getting at the CORE of the issue. Open Forum Infect. Dis. 8, ofab145 (2021).
Vendrik, K. E. W. et al. A prospective matched case-control study on the genomic epidemiology of colistin-resistant Enterobacterales from Dutch patients. Commun. Med. 2, 55 (2022).
Bastidas-Caldes, C. et al. Worldwide prevalence of mcr-mediated colistin-cesistance Escherichia coli in isolates of clinical samples, healthy humans, and livestock—A systematic review and meta-analysis. Pathogens 11, 659 (2022).
Wu, L. et al. Occurrence and characteristics of mcrs among Gram-negative bacteria causing bloodstream infections of infant inpatients between 2006 and 2019 in China. Microbiol. Spectr. 10, e0193821 (2022).
Mentasti, M. et al. Rapid detection and differentiation of mobile colistin resistance (mcr-1 to mcr-10) genes by real-time PCR and melt-curve analysis. J. Hosp. Infect. 110, 148–155 (2021).
Hu, S. et al. Novel Quadruplex PCR for detecting and genotyping mobile colistin resistance genes in human samples. Diagn. Microbiol. Infect. Dis. 101, 115419 (2021).
Hu, S., Lv, Z., Wang, Y., Shen, J. & Ke, Y. Rapid detection of human origin colistin-resistance genes mcr-1, mcr-3, mcr-8, mcr-10 in clinical fecal samples. Arch. Microbiol. 203, 4405–4417 (2021).
Mulani, M. S., Kamble, E. E., Kumkar, S. N., Tawre, M. S. & Pardesi, K. R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front. Microbiol. 10, 539 (2019).
Liao, W. et al. High prevalence of colistin resistance and mcr-9/10 genes in Enterobacter spp. in a tertiary hospital over a decade. Int. J. Antimicrob. Agents 59, 106573 (2022).
Manandhar, S. et al. A fatal outbreak of neonatal sepsis caused by mcr-10-carrying Enterobacter kobei in a tertiary care hospital in Nepal. J. Hosp. Infect. 125, 60–66 (2022).
Yang, J. et al. Potential mobilization of mcr-10 by an integrative mobile element via site-specific recombination in Cronobacter sakazakii. Antimicrob. Agents Chemother. 65, e01717–e01720 (2021).
Wang, C. et al. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 9, 508–516 (2020).
Octavia, S. et al. Genomic study of blaIMI-positive Enterobacter cloacae complex in Singapore over a five-year study period. Antimicrob. Agents Chemother. 64, e00510–e00520 (2020).
Doijad, S. P. et al. Resolving colistin resistance and heteroresistance in Enterobacter species. Nat. Commun. 14, 140 (2023).
EUCAST. EUCAST V12: The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0, 2022 (2022).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, 1–22 (2017).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Siguier, P. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36 (2006).
Darling, A. C. E., Mau, B., Blattner, F. R. & Perna, N. T. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14, 1394–1403 (2004).
Acknowledgements
We would firstly like to acknowledge all the mothers’ and neonates that enrolled into the BARNARDS study, and all hospital and research staff at each of the participating clinical sites. BARNARDS was funded by two awards $4.28 M (OPP1119772) and $849 K (OP1191522) from the Bill and Melinda Gates Foundation at 500 Fifth Avenue North-Seattle, WA 98109. We would like to thank the team at Bill and Melinda Gates Foundation namely Padmini Srikantiah, Rasa Izadnegahdar, Keith Klugman and Sara Vernam. The funders had no role in the design of the study, data collection, analysis, interpretation of data or manuscript preparation. We thank and acknowledge the hard work of the team at Liofilchem®, Roseto, Italy, specifically F. Brocco and M. Collett. We would like to acknowledge Specialist Antimicrobial Chemotherapy Unit (SACU) at Public Health Wales for support with bacterial isolate identification using MALDI-TOF MS. We would like to thank Mei Li for support with the bacterial conjugation protocol. We would like to thank Jordan Mathias and Jawaria Aziz for support in re-sequencing NN-MR803, NN-MR49-1 and NN-MR49-2. We would also like to acknowledge Wales Gene Park and ARCCA for their continued bioinformatics support. Bioinformatics analysis was undertaken using the supercomputing facilities at Cardiff University operated by Advanced Research Computing at Cardiff (ARCCA) on behalf of the Cardiff Supercomputing Facility and the HPC Wales and Supercomputing Wales (SCW) projects. We acknowledge the support of the latter, which is part-funded by the European Regional Development Fund (ERDF) via the Welsh Government. The Centre for Trials Research is funded by Health and Care Research Wales and Cancer Research UK. We thank the team of curators for the databases hosted on PubMLST https://pubmlst.org/databases/.
Author information
Authors and Affiliations
Contributions
E.A.R.P. and K.S. designed the study and wrote the manuscript. K.S., E.A.R.P. and M.B. performed the literature search. E.A.R.P., K.S., C.F., I.B. and E.J. performed the microbiology experiments. K.S., E.A.R.P. and C.F. analysed the data. E.A.R.P. and K.S. performed the whole genome sequencing experiments. K.S., I.B. and O.B.S. performed the bioinformatics analysis. K.I., F.M., S.U., C.A., L.A., E.C., A.H.Y., A.A., D.M., S.G., J.S. and A.S.M. collected all clinical data and samples from the three Nigerian hospitals enrolled into BARNARDS. R.M. provided the epidemiological data. M.J.C., R.M., T.R.W. and K.I. contributed to the BARNARDS study design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Portal, E.A.R., Sands, K., Farley, C. et al. Characterisation of colistin resistance in Gram-negative microbiota of pregnant women and neonates in Nigeria. Nat Commun 15, 2302 (2024). https://doi.org/10.1038/s41467-024-45673-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45673-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.