Abstract
Fusobacterium nucleatum (F. nucleatum) promotes intestinal tumor growth and its relative abundance varies greatly among patients with CRC, suggesting the presence of unknown, individual-specific effectors in F. nucleatum-dependent carcinogenesis. Here, we identify that F. nucleatum is enriched preferentially in KRAS p.G12D mutant CRC tumor tissues and contributes to colorectal tumorigenesis in Villin-Cre/KrasG12D+/- mice. Additionally, Parabacteroides distasonis (P. distasonis) competes with F. nucleatum in the G12D mouse model and human CRC tissues with the KRAS mutation. Orally gavaged P. distasonis in mice alleviates the F. nucleatum-dependent CRC progression. F. nucleatum invades intestinal epithelial cells and binds to DHX15, a protein of RNA helicase family expressed on CRC tumor cells, mechanistically involving ERK/STAT3 signaling. Knock out of Dhx15 in Villin-Cre/KrasG12D+/- mice attenuates the CRC phenotype. These findings reveal that the oncogenic effect of F. nucleatum depends on somatic genetics and gut microbial ecology and indicate that personalized modulation of the gut microbiota may provide a more targeted strategy for CRC treatment.
Similar content being viewed by others
Introduction
Colorectal cancer is the second most common malignancy worldwide and is characterized by specific somatic mutations including lesions in oncogenes, tumor suppressor genes, and DNA repair-related genes1,2. According to The Cancer Genome Atlas (TCGA) database, APC, TP53, KRAS, and SMAD4 are the four most frequently mutated genes in patients with CRC, and their alterations often exhibit prognostic relevance3. For example, KRAS mutations are found in about half of the human CRC cases and recognized as critical determinants of therapeutic response4. In KRAS, the codon 12 of exon 2 is the most prevalent site of mutation in CRC (mainly p.G12D). As a clinical interest, there are currently no drugs that can effectively treat the KRAS p.G12D-expressing tissues5.
In addition to genetic mutations, other local factors, most notably gut microbiota, also influence CRC occurrence and progression6. Recent studies have shown that the genotoxic pathogenicity island in Escherichia coli causes a distinct mutational signature in clonal organoids7 and that a gut microbiota-generated metabolite, gallic acid, switches mutant p53 from tumor-suppressive to oncogenic8. These observations suggest that in the pathogenesis of CRC, there are cross-talks between gut microbiota and local somatic genotypes.
Cross-cohort meta-analyses have captured CRC-associated gut bacteria that were consistently identified in the cancer population spanning different continents9,10, and they include Fusobacterium nucleatum, Parvimonas micra, Gemella morbillorum, and Peptostreptococcus stomatis. Although the etiologic connection remains elusive for many of the convergent microbial signatures, considerable knowledge has been gained on the versatile roles of F. nucleatum. Studies have shown that F. nucleatum aggravates CRC development and chemoresistance via multiple routes including attachment to epithelial cells, modulation of immune microenvironment, promotion of cell cycle, activation of TLR4 signaling, and regulation of autophagy11,12,13,14. Whereas some gut microbes potentiate CRC tumor growth, others play a beneficial role. A recent mouse study involving a collection of 11 bacterial strains isolated from healthy human donors showed that the microbial intervention significantly improved the efficacy of anti-PD-1 treatment15. In addition, anti-CRC effect was also observed after introduction of single gut microbes, such as Lactobacillus acidophilus16 and Parabacteroides distasonis (P. distasonis)17. P. distasonis is defined as one of the 18 core members in the gut microbiota of human18 and thought to have important physiological functions in host19. Although these results provide the lead of substantial clinical interest, greater mechanistic insights are needed to facilitate the translation of gut microbiota-based findings.
Here, we attempt to elucidate the host and gut microbial factors crucial for F. nucleatum-dependent CRC progression. Our findings indicate that the oncogenic role of F. nucleatum is influenced by the genotype of somatic tissue, a putative host RNA helicase, and a F. nucleatum-competing gut bacterium. The results illustrate the intricacy of local effectors in modulating F. nucleatum-dependent CRC development, which may inform a more effective, personalized intervention for the disease.
Results
F. nucleatum is enriched preferentially in KRAS p.G12D-mutant CRC tissues
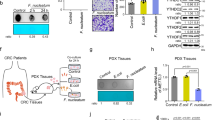
To investigate the correlation between F. nucleatum abundance and CRC somatic mutations, we performed quantitative polymerase chain reaction (qPCR) and exon sequencing on 24 pairs of tumor and matched paratumor tissues. The tumor samples were divided into wild-type (WT) tissues and mutation-bearing tissues based on exon sequencing results. Subsequent analysis revealed that F. nucleatum abundance in the KRAS mutant tissues was higher than that in the KRAS WT tissues and paratumor tissues (Fig. 1a), which was validated in an independent cohort of 239 CRC patients (CRC patients information are shown in Supplementary Table 1) (Fig. 1b). In comparison, F. nucleatum abundance exhibited no association with mutation in four tested genes namely APC, TP53, MSH2, and SMAD4.
a Exon sequencing and qPCR detection of the mutation status of CRC tissues and F. nucleatum abundance in the indicated group. The relative abundance of F. nucleatum was confirmed using abundance of F. nucleatum /quantity of pgt gene. Significant differences are indicated: one-way ANOVA with Sidak’s multiple comparison test, data are presented as the mean ± SEM. b Correlation of KRAS mutation status and F. nucleatum abundance in CRC tumor tissues. Significant differences are indicated: Chi-square test, two-sided, n = 239. c F. nucleatum (Fn) positive rates under different KRAS mutation types in tumor tissues of CRC (n = 239). d Relative abundance of F. nucleatum in the CRC patients of the indicated groups. Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, data are presented as the mean ± SEM. Source data are provided as a Source Data file.
Among patients with CRC, mutations in KRAS concentrate at codons 12, 13, and 61 of exon 2 and vary greatly in prevalence20. To examine whether F. nucleatum level correlated with particular genetic variants, we analyzed the KRAS mutation profile and F. nucleatum abundance in cohort 2 by qPCR. We found that F. nucleatum was detected in 42/83 (50.6%) tumors with a KRAS mutation in exon 2, 7/19 (36.8%) tumors with a KRAS mutation in exon 3 and 43/137 (31.4%) in KRAS WT tumors (Fig. 1c). Interestingly, the tumors with the p.G12D mutation, but not those with the adjacent p.G13D mutation, accommodated significantly higher F. nucleatum abundance than WT tumors (Fig. 1d). Collectively, our data bridge a local connection between F. nucleatum abundance and KRAS genotype.
F. nucleatum exacerbates colorectal tumorigenesis in Villin-Cre/KrasG12D+/− mice
To investigate the mechanistic underpinning of the observed F. nucleatum-KRAS (p.G12D) link, we successfully generated Villin-Cre/KrasG12D+/− mice (Supplementary Fig. 1a, b). We hypothesized that F. nucleatum colonization would be increased in the colon of Villin-Cre/KrasG12D+/− mice, thus aggravating tumorigenesis. To test this, we developed an azoxymethane/dextran sulfate sodium (AOM/DSS)-induced CRC mouse model (the mice were intraperitoneally injected with 10 mg/kg AOM, followed by 5-day oral administration of 2.5% DSS starting 5 days later) in both Villin-Cre/KrasG12D+/− and Villin-Cre/KrasG12D−/− (WT) backgrounds and gavaged the mice with F. nucleatum (1 × 109 CFU) or phosphate-buffered saline (PBS) as shown in Fig. 2a. qPCR and fluorescence in situ hybridization (FISH) assays revealed that F. nucleatum was enriched in Villin-Cre/KrasG12D+/− colonic tissues compared with that in the WT tissues (Fig. 2b–d) but undetectable in the tissues of PBS-treated rodents, regardless of genetic backgrounds (Supplementary Fig. 1c). Moreover, we showed that F. nucleatum-treated Villin-Cre/KrasG12D+/− mice exhibited a pronounced augmentation in tumor formation compared with F. nucleatum-treated WT littermates and PBS-treated animals (Fig. 2e). Hematoxylin and eosin (H&E) staining revealed that F. nucleatum-treated Villin-Cre/KrasG12D+/− mice exhibited higher grades of dysplasia (Fig. 2f), tumor multiplicities and tumor loads than F. nucleatum-treated WT littermates (Fig. 2g–i). Additionally, to evaluate the interaction of F. nucleatum with the immune system in KRAS mutant mice and patients, immunofluorescence (IF) and qPCR were performed to evaluate the infiltrated immune cells and inflammatory cytokine production in colonic tissues of PBS and F. nucleatum-treated Villin-Cre/KrasG12D+/− mice. We found that F. nucleatum led to less CD3+ T cells infiltrating which is consisting with the study that F. nucleatum was inversely associated with tumor stromal CD3+ lymphocytes (Supplementary Fig. 1d, e)21. However, there were no significant differences in the number of infiltrated CD11c+ dendritic cells in colonic tissues between PBS and F. nucleatum-treated Villin-Cre/KrasG12D+/− mice (Supplementary Fig. 1d, e). The expression of Il-17a, Il-6 were also elevated after F. nucleatum treatment in Villin-Cre/KrasG12D+/− mice (Supplementary Fig. 1f). And we confirmed the results of infiltrated immune cells in tumor tissues from KRAS G12D-mutant patients by IF (Supplementary Fig. 1g, h). Together, our results suggest that F. nucleatum-mediated-colorectal tumorigenesis is aggravated in mice harboring KRAS p.G12D mutant via its increased colonization.
a Schematic diagram of the experimental design and timeline of mouse models. b qPCR analysis of F. nucleatum abundance in colonic tissues derived from Villin-Cre/KrasG12D+/− (KrasG12D) mice and WT littermates (WT) treated with AOM/DSS and F. nucleatum. Significant differences are indicated: two-tailed Student’s t-test, n = 5 (WT) and n = 4 (KrasG12D) respectively, data are presented as the mean ± SEM. c FISH detection of F. nucleatum in colonic tissues derived from Villin-Cre/KrasG12D+/− mice and WT littermates using a Cy3-conjugated F. nucleatum specific probe, the red arrows indicate F. nucleatum, n = 5, scale bar: 50 μm. d Statistical analysis of the results in (c). Significant differences are indicated: two-tailed Student’s t-test, n = 5 per group, data are presented as the mean ± SEM. e, f Representative images and H&E stainings of the colons of WT littermates and Villin-Cre/KrasG12D+/− mice treated with AOM/DSS and PBS or F. nucleatum, the red arrows indicate tumors, n = 5 per group, scale bar: 50 μm. g–i Tumor numbers, tumor loads, and size of Villin-Cre/KrasG12D+/− mice and WT littermates treated with AOM/DSS and PBS or F. nucleatum. Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 5 per group, data are presented as the mean ± SEM. Data are representative of three independent experiments. Source data are provided as a Source Data file.
P. distasonis competes with F. nucleatum in a Villin-Cre/KrasG12D+/− mouse model and human KRAS mutant CRC tissues
Given that gut microbiota is a complex ecosystem, we were curious about whether the connection between F. nucleatum and KRAS (p.G12D) involved other bacteria. We first surveyed the microbiota compositions of the colon tissues from WT and Villin-Cre/KrasG12D+/− mice and discovered that Clostridium XIVa and Ralstonia were depleted in the G12D animals (Supplementary Fig. 2a), although, no differences in the appearance, colon length and histological presentation of the colon were observed between the two groups (Supplementary Fig. 2b–d). These findings suggested that the p.G12D mutation affected the gut microbiota, which could have ramifications in F. nucleatum-related interactions. We therefore treated the mice with F. nucleatum (1 × 109 CFU) every two days for four weeks (Supplementary Fig. 2e) and subsequently detected substantial compositional differences, all depleted in Villin-Cre/KrasG12D+/− mice, between the two groups, comprising Parabacteroides, Roseburia, Akkermansia, Bacteroides, Blautia, Clostridium XI, Anaerostipes, Allobaculum, Alistipes, and Lactobacilus (Fig. 3a). Next, we used the AOM/DSS mouse model to induce CRC tumorigenesis in the two backgrounds, followed by F. nucleatum exposure (Supplementary Fig. 2f). We found that Bacteroides, Parabacteroides, Alistipes, Clostridium XI, and Intestinimonas were augmented in WT littermates compared to Villin-Cre/KrasG12D+/− mice (Fig. 3b). Among the four genera (Parabecteroides, Bacteroides, Clostridium XI, and Alistipes) exhibiting concordant alterations in both mouse experiments, Parabecteroides manifested F. nucleatum-dependent p.G12D depletion with the highest statistical significance, suggesting prominent intermicrobial antagonism.
a Heat map of differentially abundant genera between Villin-Cre/KrasG12D+/− mice (n = 3) and WT littermates (n = 6) treated with F. nucleatum (1 × 109 CFU) every two days for four weeks. b Heat map of top differentially abundant genera between Villin-Cre/KrasG12D+/− (n = 7) mice and WT littermates (n = 6) which treated by AOM/DSS and F. nucleatum. c qPCR analysis of P. distasonis and P. goldsteinii/merdae abundance in colonic tissues derived from Villin-Cre/KrasG12D+/− mice and WT littermates treated by AOM/DSS and F. nucleatum. Significant differences are indicated: two-tailed Student’s t-test, n = 4 (WT P. distasonis), n = 3 (KrasG12D P. distasonis), and n = 6 (P. goldsteinii/merdae) respectively, data are presented as the mean ± SEM. d, e Correlation analysis of F. nucleatum and P. distasonis abundance in KRAS mutant (n = 102) and KRAS WT (n = 137) CRC patients. Significant differences are indicated: Chi-square test, two-sided. Source data are provided as a Source Data file.
Parabacteroides species are diverse and P. distasonis, Parabacteroides merdae (P. merdae), and Parabacteroides goldsteinii (P. goldsteinii) have been recognized as the main probiotics conferring protection against CRC and metabolic disorders17,19,22,23. To identify which species interacts with F. nucleatum and correlates with KRAS mutation in CRC development, we examined the abundance of P. distasonis, P. merdae and P. goldsteinii in the colon of AOM/DSS-induced rodents gavaged with F. nucleatum. qPCR analysis showed that P. distasonis, but not the other two Parabacteroides species, registered greater enrichment in WT littermates when compared to Villin-Cre/KrasG12D+/− mice (Fig. 3c). Next, we showed that the inverse association between F. nucleatum and P. distasonis was found in human tissues with KRAS mutations, but absent in KRAS WT clinical samples (Fig. 3d, e). By using a logistic regression model having P. distasonis as an outcome with KRAS and F. nucleatum, and their interaction term (KRAS x F. nucleatum) as exposures, we discovered that F. nucleatum could be regarded as a protective factor for P. distasonis (OR = 0.548), and the abundance of P. distasonis is negatively correlated with F. nucleatum (p < 0.05). However, no significant difference was observed between the abundance of P. distasonis and KRAS mutation (Supplementary Table 2). Overall, our data indicate that P. distasonis may antagonize F. nucleatum during colorectal tumorigenesis in both mice and humans.
P. distasonis alleviates F. nucleatum-mediated CRC progression
The above findings suggested a possibility that P. distasonis could compete with F. nucleatum to mitigate CRC development. To verify this hypothesis, we administered AOM/DSS-induced Villin-Cre/KrasG12D+/− mice and WT littermates with F. nucleatum and/or P. distasonis (Supplementary Fig. 3a). To fully assess the treatment effect of P. diastonis, we administrated DSS for three cycles to ensure successful tumor development. Examination of the colon tissues showed that P. distasonis gavage alleviated F. nucleatum-promoted CRC progression in Villin-Cre/KrasG12D+/− mice (Fig. 4a, b). P. distasonis-treated mice developed dramatically fewer and smaller tumors than the F. nucleatum- or PBS-treated counterparts; moreover, concurrent exposure to F. nucleatum and P. distasonis led to diminished size and number of tumors compared to that derived from F. nucleatum-treated counterparts (Fig. 4c–e). The biotherapeutic role of P. distasonis was corroborated when we treated the clinically derived KRAS p.G12D and KRAS WT colonic organoids with F. nucleatum and/or P. distasonis (Fig. 4f, g). In addition, expression of Ki67 in the organoids was significantly decreased in both P. distasonis-treated and P. distasonis/F. nucleatum-co-treated groups, indicating that excessive multiplication of colonic epithelial cells induced by F. nucleatum was suppressed in the presence of P. distasonis (Fig. 4h and i). Collectively, our data reveal that P. distasonis could alleviate F. nucleatum-mediated progression of CRC tumors bearing the p.G12D mutation.
a Representative images of the colons of Villin-Cre/KrasG12D+/− mice treated with AOM/DSS and F. nucleatum or/and P. distasonis, n = 3 per group. b Representative H&E stainings of the colons treated in (a), n = 3 per group, scale bar: 20 μm. c–e Tumor numbers, tumor loads, and size of Villin-Cre/KrasG12D+/− mice treated in (a). Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 3 per group, data are presented as the mean ± SEM. f FISH detection of F. nucleatum (red) and P. distasonis (green) in colonic organoids derived from KRAS WT and KRAS p.G12D CRC patients treated by F. nucleatum or/and P. distasonis, scale bar: 50 μm. g Statistical analysis of the results in (f). Significant differences are indicated: two-tailed Student’s t-test, n = 5 per group, data are presented as the mean ± SEM. h Representative immunostainings of Ki67 in colonic organoids derived from KRAS WT and KRAS p.G12D CRC patients treated by F. nucleatum or/and P. distasonis, scale bar: 50 μm. i Statistical analysis of the results in (h). Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 5 per group, data are presented as the mean ± SEM. Data are representative of two independent experiments. Source data are provided as a Source Data file.
F. nucleatum invades tumor cells and binds to DHX15
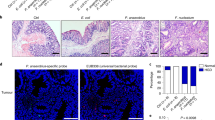
Given the extensive involvement of F. nucleatum in CRC development13,14, we assessed whether F. nucleatum can invade tumor cells. We first successfully constructed two mutant cell lines expressing KRAS p.G12D and p.G13D variants namely KRAS p.G12D and p.G13D cells. Western blot analysis confirmed that the KRAS p.G12D cells were successfully mutated (Supplementary Fig. 4a). Confocal fluorescence microscopy showed that F. nucleatum invaded more in the KRAS p.G12D cell line (on an average of 6.8/cell) than in KRAS p.G13D (on an average of 2.94/cell) or KRAS WT cell line (on an average of 0.9/cell) (Fig. 5a, b), suggesting that the observed F. nucleatum-p.G12D link involved a difference in bacterial capacity to penetrate the epithelial cells. Using cryo-focused ion beam milling and cryo-electron tomography, we visualized invasion of F. nucleatum inside the cytoplasm of infected G12D cells and confirmed that F. nucleatum contacted the nuclear membrane (Fig. 5c). Meanwhile, transmission electron microscope (TEM) experiments were performed on organoids derived from KRAS p.G12D-mutant CRC patients and found F. nucleatum invaded inside the cytoplasm of the infected organoids (Supplementary Fig. 4b).
a Representative confocal images of F. nucleatum (MOI = 100) invading to indicated tumor cells. The F. nucleatum expressed mcherry are in red and tumor cells expressed GFP are in green. Scale bar: 20 μm. b Statistical analysis of the results in (a). Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 5 per group, data are presented as the mean ± SEM. c F. nucleatum was visible inside the G12D cells and contacted the nuclear membrane by cryo-focused ion beam milling and cryo-electron tomography, data are representative of three independent experiments. d FISH detection of F. nucleatum in colonic organoids derived from indicated patients treated by F. nucleatum or KRAS p.G12D antibody, scale bar: 50 μm. e Statistical analysis of the results in (d). Significant differences are indicated: one-way ANOVA with Sidak’s multiple comparison test, n = 3 per group, data are presented as the mean ± SEM. f Western blot analysis of F. nucleatum proteins incubated by biotinylated proteins from indicated cells and detected using avidin-conjugated horseradish peroxidase. The experiment was performed in two biological replicates. g Pull-down assays were performed and validation of the FN1859-DHX15 interaction in KRAS WT, KRAS p.G12D, and KRAS p.G13D cells by western blot. The experiment was performed in three biological replicates. h The invasive level of F. nucleatum when KRAS p.G12D cells were incubated with F. nucleatum, F. nucleatum + P. distasonis, F. nucleatum + supernatant of P. distasonis. Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 5 per group, data are presented as the mean ± SEM. i Western blot analysis of DHX15 expression in Villin-Cre/KrasG12D+/− mice and WT littermates. Lanes representative of separate mice. j Statistical analysis of the results in (i). Significant differences are indicated: two-tailed Student’s t-test, n = 4 per group, data are presented as the mean ± SEM. k Western blot analysis of DHX15 expression in KRAS WT, KRAS p.G12D, and KRAS p.G13D tumor cells. The experiment was performed in three biological replicates. Source data are provided as a Source Data file.
The KRAS p.G12D peptide exhibits aberrant activation, which accompanies tumorigenesis24. To clarify whether the activation of KRAS p.G12D is linked to increased enrichment of F. nucleatum, clinically derived KRAS p.G12D or WT colonic organoids were infected with F. nucleatum, with or without incubation of KRAS p.G12D antibody. FISH assay revealed that F. nucleatum display elevated invasiveness in the KRAS p.G12D-mutant colonic organoids compared to that in the WT organoids, which was attenuated after KRAS p.G12D-specific antibody incubation (Fig. 5d, e). To investigate whether the surface proteins on KRAS p.G12D mutation that facilitated entry of F. nucleatum, we performed western blot to evaluate the expression of tight junction proteins. The reduction of tight junction proteins results in intestinal epithelial barrier dysfunction and bacterial translocation25. Results showed that KRAS p.G12D mutation cells expressed less ZO-1 and Claudin-1 proteins when compared with KRAS p.G13D mutation and KRAS WT cells (Supplementary Fig. 4c) which may facilitate the entry of F. nucleatum. These data suggested that the p.G12D mutation augments the bacterium-peptide affinity, leading to increased F. nucleatum invasion into the tumor cells.
Next, we aimed to identify the cellular component that F. nucleatum interacts with. We incubated F. nucleatum lysates with biotinylated proteins from KRAS p.G12D, KRAS p.G13D, and KRAS WT cells respectively. Subsequent western blot analysis showed that three candidate proteins from F. nucleatum might interact with KRAS p.G12D cells (Fig. 5f). The bands of interest were then submitted for mass spectrometry (MS) protein identification, which reported three proteins, FN0488 (glutamate dehydrogenase), FN1277 (aminoacyl-histidine dipeptidase), and FN1859. Among them, FN1859 was the only putative F. nucleatum surface protein according to the Uniprot database (https://www.uniprot.org).
FN1859 from F. nucleatum might interact with a cognate receptor on KRAS p.G12D cell. To capture the unknown effectors, we purified recombinant His-tagged FN1859 proteins from Escherichia coli and performed His-tag pull-down assays with WT, KRAS p.G13D, and KRAS p.G12D cell lysates. Mass spectrometry revealed the level of DHX15 was dramatically augmented in the KRAS p.G12D cell line compared with that in the WT and KRAS p.G13D cell lines (Supplementary Table 3). DHX15 is an important member of the DEAH-box RNA helicase family that is expressed in the nucleus and contributes to carcinogenesis26. To test the possible interaction between FN1859 and DHX15, pull-down assay was conducted in the three cell lines of G12D, G13D, and WT. Western blot analysis demonstrated that DHX15 exhibited specific coupling to FN1859 and that the protein-protein interaction was augmented in KRAS p.G12D cells than that in the other two cell lines (Fig. 5g). The similar results were observed in CRC samples from WT, KRAS p.G13D and KRAS p.G12D-mutant patients, shown in Supplementary Fig. 4e. To clarify whether P. distasonis involved in the interaction between F. nucleatum and DHX15, TEM was performed to assess the invasive ability of P. distasonis to tumor cells. We incubated P. distasonis with KRAS p.G12D-mutant cells and data showed that no P. distasonis was observed in the G12D cells (Supplementary Fig. 4e). Then we purified recombinant His-tagged DHX15 proteins from Escherichia coli and performed pull-down assays with P. distasonis lysates. There was no protein of P. distasonis was pulled by DHX15 (Supplementary Fig. 4f). Therefore, we supposed that P. distasonis couldn’t impact the interaction between F. nucleatum and DHX15. Furthermore, we investigated whether the invasive level of F. nucleatum could be inhibited by P. distasonis, we incubated G12D cells with F. nucleatum, F. nucleatum + P. distasonis, F. nucleatum + supernatant of P. distasonis and carried out the bacterial recovery assays. The results showed that the invasive level of F. nucleatum could be inhibited by P. distasonis, especially its supernatant implying that the metabolites of P. distasonis may involved in inhibiting F. nucleatum invasion (Fig. 5h). Finally, DHX15 expression was verified significantly increased in the colon tissues of Villin-Cre/KrasG12D+/− mice compared to WT littermates (Fig. 5i, j), and the pattern was recapitulated in the three cell lines (Fig. 5k). Together, these data reveal that DHX15 is a receptor of F. nucleatum in tumor cells and is upregulated upon the p.G12D mutation.
The ERK/STAT3 signaling mediates the expression of DHX15
The RAF/MEK/ERK and PI3K/AKT cascades are the major pathways downstream of KRAS activation and control the processes of cell growth and survival27. To better understand how DHX15 upregulation occurs in KRAS p.G12D tumors, we investigated whether these two pathways a re involved in regulating DHX15 expression upon KRAS activation. Western blot analysis showed that the level of phosphorylated ERK, but not that of AKT, was substantially upregulated in KRAS p.G12D cell line compared with the KRAS WT and KRAS p.G13D cell lines (Fig. 6a). This coincided with the increased expression of DHX15 in the cells harboring the major KRAS mutation (Fig. 6a) as well as augmented DHX15 level and p-ERK activation in the colon tissues of Villin-Cre/KrasG12D+/− mice (Fig. 6b, c). To investigate whether F. nucleatum could drive ERK activation and increase DHX15 expression, we performed experiments by co-culture F. nucleatum with KRAS WT, p.G12D and p.G13D cells and found that the level of phosphorylated ERK and DHX15 in the F. nucleatum-treated cells was the same with untreated cells (Supplementary Fig. 5a, b). To analyze the level of DHX15 in patient samples, we performed IHC on tumor tissues of patients with CRC that harbor KRAS p.G12D, p.G13D mutations, and KRAS WT allele. We found that KRAS p.G12D-bearing CRC expressed a greater level of DHX15 proteins compared with KRAS p.G13D-bearing and KRAS WT-bearing patients (Fig. 6d, e), which collectively illustrated the functional connections of the putative RNA helicase within the p.G12D-bearing tumors.
a Western blot analysis of p-ERK, p-AKT, and DHX15 expression in KRAS WT, KRAS p.G12D, and KRAS p.G13D tumor cells. The experiment was performed in three biological replicates. b Western blot analysis of p-ERK and DHX15 expression in Villin-Cre/KrasG12D+/− mice and WT littermates. Lanes representative of separate mice. c Statistical analysis of the results in (b). Significant differences are indicated: two-tailed Student’s t-test, n = 4 (KrasG12D) and n = 6 (WT) respectively, data are presented as the mean ± SEM. d Representative immunohistochemical detection of DHX15 in human CRC tissues of KRAS WT, KRAS p.G12D, and KRAS p.G13D groups, scale bar: 20 μm. e Statistical analysis of the results in (d). Significant differences are indicated: one-way ANOVA with Sidak’s multiple comparison test, n = 7 (WT), n = 10 (G12D), and n = 9 (G13D) respectively, data are presented as the mean ± SEM. f Western blotting analysis of DHX15 expression in KRAS p.G12D tumor cells after the specific inhibitor (SCH772987 for ERK and APTSTAT3-9R for STAT3) treatment. The experiment was performed in two biological replicates. g The schematic diagram shows one potential binding site of STAT3 in the putative promoter element of DHX15. h STAT3 was immunoprecipitated from indicated cells. Immunoprecipitates were assayed for the enrichment of DHX15 promoter. Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 3 per group, data are presented as the mean ± SEM. i Luciferase activity in lysates of 293T cells transfected with luciferase reporter plasmids of pGL3-basic empty vector (basic), DHX15 promoter (p-DHX15-WT) or DHX15 promoter with mutation on predicted STAT3-binding site (p-DHX15-MUT), together with STAT3 plasmid or not. Results are presented as the ratio of firefly luciferase to renilla luciferase activity, relative to that of 293T cells transfected with pGL3-basic empty vector. Significant differences are indicated: one-way ANOVA with Bonferroni’s multiple comparison test, n = 6 per group, data are presented as the mean ± SEM. Source data are provided as a Source Data file.
ERK is located in the cytoplasm and upon activation is transferred to the nucleus to regulate the activity of various transcription factors such as FOS, ELK1, MYC, and STAT1/328. To predict the putative downstream transcription factor of ERK that could directly initiate the transcription of DHX15, the bioinformatics tool JASPAR was used to identify STAT3. Correspondingly, the specific ERK inhibitor and STAT3 inhibitor were employed to examine the effect of this pathway on DHX15 expression and detected a significant downregulation at the protein level (Fig. 6f). These data indicate that the ERK-STAT3 signaling activation is required for DHX15 upregulation.
Curation of protein database identified a potential STAT3-binding site in the promoter of DHX15 (Fig. 6g). Chromatin immunoprecipitation (ChIP) assays showed that DHX15 promoter abundance was increased by 2.0-fold in KRAS p.G12D cells, 1.15-fold in KRAS WT cells, and 1.54-fold in KRAS p.G13D cells (Fig. 6h). Luciferase reporter assay verified that STAT3 bound to the promoter element of DHX15 and affected DHX15 expression (Fig. 6i). Taken together, our data demonstrate that the activation of ERK/STAT3 signaling in the downstream of KRAS is involved in DHX15 upregulation.
Knock out of Dhx15 in Villin-Cre/Kras G12D+/− mice attenuates the CRC phenotype
To verify the regulatory effect of DHX15 in colorectal tumorigenesis, we knocked out DHX15 in Villin-Cre/KrasG12D+/− mice by generating Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice (Supplementary Fig. 6a). Then we developed an AOM/DSS-induced CRC mouse model and gavaged F. nucleatum in both Villin-Cre/KrasG12D+/− and Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice (Fig. 7a). We verified that F. nucleatum-treated Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice exhibited a relieve in tumor formation compared with F. nucleatum-treated Villin-Cre/KrasG12D+/− mice (Fig. 7b). H&E staining confirmed that F. nucleatum-treated Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice showed lower grades of dysplasia (Fig. 7c), tumor multiplicities and tumor loads than F. nucleatum-treated Villin-Cre/KrasG12D+/− mice (Fig. 7d–f). To investigate whether immune factors involved in the pro-tumorigenic activities of DHX15-F. nucleatum interaction, IF, and qPCR were performed to evaluate the infiltrated immune cells and inflammatory cytokine production in colonic tissues of F. nucleatum-treated Villin-Cre/KrasG12D+/− and Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice. We found that there were no significant differences in the number of infiltrated CD3+ T cells and CD11c+ dendritic cells between the two groups (Fig. 7g and 7h). However, the expression of Il-6, Il-17a were elevated after F. nucleatum treatment in Villin-Cre/KrasG12D+/− mice when compared to Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice (Fig. 7i) suggested that the inflammatory cytokines might contribute to the pro-tumorigenic activities of F. nucleatum. Overall, we verified that the F. nucleatum-mediated-colorectal tumorigenesis is attenuated in mice activating Kras p.G12D and lacking DHX15.
a Schematic diagram of the experimental design and timeline of mouse models. b, c Representative images and H&E stainings of the colons of Villin-Cre/KrasG12D+/− mice and Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice treated with AOM/DSS and F. nucleatum, the red arrows indicate tumors, n = 5 per group, scale bar: 50 μm. d–f Tumor numbers, tumor loads, and size of Villin-Cre/KrasG12D+/− mice and Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice treated with AOM/DSS and F. nucleatum. Significant differences are indicated: two-tailed Student’s t-test, n = 5 per group,data are presented as the mean ± SEM. g Representative immunofluorescence detection of CD3 and CD11c positive cells in Villin-Cre/KrasG12D+/− mice and Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice treated with AOM/DSS and F. nucleatum, scale bar: 50 μm. h Statistical analysis of the results in (g). Significant differences are indicated: two-tailed Student’s t-test, n = 5 per group, data are presented as the mean ± SEM. i qPCR analysis of Il-17a, Il-6 mRNA expression Villin-Cre/KrasG12D+/− mice and Villin-Cre/KrasG12D+/−/Dhx15fl/fl mice treated with AOM/DSS and F. nucleatum. Significant differences are indicated: two-tailed Student’s t-test, n = 4 (KrasG12D) and n = 5 (KrasG12D/Dhx15−/−) respectively, data are presented as the mean ± SEM. Data are representative of two independent experiments. Source data are provided as a Source Data file.
Discussion
Colorectal cancer is characterized by gut microbiota abnormalities and specific somatic mutations. Recent studies revealed that high F. nucleatum abundance in the tumor tissues was significantly associated with BRAF mutations and CpG island methylator phenotype (CIMP)-positive CRC patients in univariate analyses and the amount of F. nucleatum was also associated with microsatellite instable (MSI)-high tumors independent of CIMP and BRAF mutation status, supporting the notion that gut microbiota has links to the intratumor genetics and epigenetics of CRC29,30,31. Our study presents a greater complexity of niche elements converged in the F. nucleatum-dependent CRC tumorigenesis, as the functional links comprise a somatic mutation, a putative host RNA helicase, a signaling pathway crucial for multiple malignancies, and a F. nucleatum-antagonizing bacterium. The findings indicate that a comprehensive approach is needed to disentangle the connection between the gut microbiota shift and CRC development.
Considerable effort has been devoted to understand and manage KRAS mutant-mediated therapeutic resistance. The biophysical differences between individual KRAS mutant alleles including the p.G12D, p.G12V and p.G13D variants are sufficient to generate a range of sensitivities to EGFR inhibitor32. Previous evidence has shown that in both early-onset and later-onset tumors, KRAS mutation prevalence was higher in the cecum compared with that in the other subsites33. Among CRCs in all colorectal sites, cecal cancers show the highest KRAS mutation frequency34. Therefore, cecal cancers appeared to represent a unique subtype, characterized by a high frequency of KRAS mutation35. Interestingly, F. nucleatum is most abundant in cecal cancers36 which support our findings in this study that patients harboring KRAS p.G12D mutation are correlated with an elevated F. nucleatum presence. In fact, the relationship between F. nucleatumn and KRAS mutations is not conclusive based on the previous studies. Kosuk Mima et al. have observed 1069 CRC samples and found that the amount of F. nucleatum was not associated with KRAS mutation30. Ivan Borozan et al. investigated 1,994 CRC cases and concluded that mutation status of APC, PIK3CA, KRAS, BRAF, ERBB2, and SMAD4 were not associated with F. nucleatum prevalence31. In the two studies above, the genomic DNA of F nucleatum was extracted from FFPE tissue sections of CRC. However, in another research, KRAS mutation was found to be more frequently observed in CRC samples infected with F. nucleatum and the genomic DNA of F nucleatum was extracted from 43 fresh-frozen CRC tissue samples and the matched adjacent normal tissues37. Consistent with this, we evaluated 24 fresh-frozen CRC tissue samples and the matched adjacent normal tissues and 239 fresh-frozen CRC tumor tissues and verified that F. nucleatum abundance was associated with KRAS mutation. The potential differences in different researches maybe due to the tissue status (fresh frozen vs. FFPE). What’s more, here we identified that KRAS genotype affects the activation of downstream ERK signaling pathway and KRAS p.G12D mutation-induced activation of ERK signaling is stronger than KRAS p.G13D and KRAS WT which is similar with a previous report that BRAF V600E could induce stronger ERK activity than KRAS p.G12V mutation38.
The activation of ERK signaling leads to expression of DHX15 which is a member of the DEAH-box RNA helicase family and is required for virus-induced innate immune gene expression39,40. Studies have reported that DHX15 contributes to carcinogenesis in several malignancies including prostate cancer, non-small cell lung cancer, and hepatocellular carcinoma26,41,42. However, a functional tie with disease-related gut microbes remains poorly characterized, although it has recently been reported that DHX15 deficient mice are susceptible to infection by enteric bacteria Citrobacter rodentium and that the protein plays an important role in the antimicrobial response in colitis43. Here we show that the elevated expression of DHX15 is pertinent to KRAS p.G12D mutation and DHX15 as a specific receptor in the nuclei of tumor cells interact with F. nucleatum to mediated-colorectal tumorigenesis.
P. distasonis negatively correlates with several adverse health conditions including inflammatory bowel disease, multiple sclerosis, and obesity19,44, which coincides with its protective role against intestinal epithelial damage and colonic tumorigenesis in mice45. In this study, we show a competition between P. distasonis and F. nucleatum that is more pronounced in p.G12D-bearing mice and patients than their counterparts with the WT allele. These findings collectively raise the possibility of developing the bacterium as probiotics or auxiliary therapeutics for colorectal cancer, although more in-depth exploration is needed. Recently, combination of KRAS inhibitor AMG510 and anti-PD-1 monotherapy delayed tumor growth, with complete regression in nine of ten tumors and generated most attention46. Whether the combination of microbiota such as P. distasonis and anti-PD-1 monotherapy could represent a potentially trans-formative therapy for patients for whom effective treatments are lacking also deserves further investigation.
Taken together, we propose a pathogenic model of CRC dependent on somatic genotype. In KRAS p.G12D mutation CRC tissues, F. nucleatum invades more and the ERK signaling is activated and subsequently induces DHX15 expression. The interaction of FN1859-DHX15 potentiates colorectal tumorigenesis and could be alleviated by P. distasonis. Our findings may provide a basis for personalized therapy for the subset of treatment-recalcitrant CRC patients carrying the KRAS p.G12D mutation.
Methods
Mice
Villin-Cre/KrasG12D+/− mice and Villin-Cre/KrasG12D−/− littermates constructed in C57BL/6J background were purchased from The Shanghai Model Organisms Center. Mice were bred and maintained under specific pathogen-free (SPF) conditions. Age- and sex-matched mice at 6-8 weeks of age and mixed mice were randomly used for all experiments. According to the guidelines (GB/T 35892-2018), the ethics committee specified that the maximal tumor burden is no more than 10% of the body weight of animals and the average diameter is less than 20 mm. During the experiment, the tumor sizes of the mice complied with the regulations. All animal protocols were reviewed and approved by the Ethics Review Committee for Animal Experimentation at Shanghai Tenth People’s Hospital.
Human subjects
The primary tumor tissues and the adjacent normal tissues were obtained from patients with colorectal adenocarcinoma who underwent a surgical resection. 254 fresh tumor tissues and 24 adjacent normal tissues were collected at the time of surgical resection and were immediately frozen in liquid nitrogen and stored at −80 °C. Additionally, formalin-fixed, paraffin-embedded specimens (one from each case) were collected from Department of Pathology of Shanghai Tenth People’s Hospital Affiliated to Tongji University. According to the TNM staging system, tumors were classified independently by two pathologists. The clinic pathological parameters included age, sex, tumor size, tumor location, pathological grade, and tumor size. All individuals provided informed consent and the ethics approval also covered the “Establishment of patient-derived organoids”. The study was performed in accordance with the Declaration of Helsinki Principles and approved by the Research Ethics Board of Shanghai Tenth People’s Hospital, Tongji University School of Medicine (SHDSYY-2019-2751).
16S rRNA sequencing analysis
Colon tissues from patients or mice were collected. The total DNA was then extracted with the QIAamp DNA Mini kit (QIAGEN). The 16S rRNA high-throughput sequencing was performed by Realbio Genomics Institute (Shanghai, China) using the Illumina MiSeq. Variable regions V3-V4 on 16S rRNA genes of bacteria were amplified with forward primer F341 5′-ACTCCTACGGGRSGCAGCAG-3′ and reverse primer R806 5′-GGACTACVVGGGTATCTAATC-3′. The raw data were then subjected to a quality control procedure using UPARSE. The qualified reads were clustered to generate operational taxonomic units (OTUs) at the 97% similarity level using Usearch. Principal components analysis (PCA), heatmap analysis, Bray-Curtis similarity cluster, and species abundance analysis were performed.
Targeted gene sequencing
Five genes were screened for the detection of mutations in patients with CRC by sequencing using the Illumina NovaSeq 6000. To identify the mutations in these genes, we designed PCR primers using the primerXL pipeline. Three hundred and eighty oligonucleotide pairs were produced and encompassed all of the CDSs and most of the untranslated regions of the 5 genes. The amplification reactions were conducted using an AB 2720 Thermal Cycler (Life Technologies Corporation) with the following cycling conditions: 95 °C for 2 min; 11 cycles of 94 °C for 20 s, 63 °C per cycle for 40 s, 72 °C for 1 min; 24 cycles of 94 °C for 20 s, 65 °C for 30 s, 72 °C for 1 min and 72 °C for 2 min. The PCR products were used generate a library for further detection, and the DNA-adapter-ligated and -indexed fragments from ten libraries were then pooled and hybridized. After hybridization of the sequencing primer, base incorporation was performed using the Illumina NovaSeq 6000 in a single lane following the manufacturer’s standard cluster generation and sequencing protocols for 250 cycles of sequencing per read to generate paired-end reads, including 250 bp at each end and 8 bp of the index tag. The primer sequences are provided in Source Data file.
Animal experiments
Following the single dose of AOM (10 mg/kg, intraperitoneal) injection, mice were treated 2.5% DSS (molecular weight 36,000–50,000, MP biomedicals) in drinking water for 5 successive days (To investigate whether F. nucleatum exacerbates colorectal tumorigenesis in Villin-Cre/KrasG12D+/− mice in early time, mice were administrated DSS for one cycle. To assess the treatment effect of P. diastonis and the role of DHX15 respectively, mice were administrated DSS for three cycles to ensure successful tumor development.) and then gavaged with 1 × 109 CFU of F. nucleatum and/or P. distasonis every 2 days for 4 weeks. The animals in the control group were gavaged by the same volume of PBS. Then mice were sacrificed to harvest colon tissues for analysis.
Bacterial recovery assay
1 × 105 cells were grown in a 24-well plate and co-cultured with bacteria for 1 h (MOI = 100) under anaerobic conditions. After co-culture, Ampicillin (200 mg/ml) and Gentamicin (200 mg/ml) were used to eliminate extracellular bacteria for 1 h, and medium was removed and cells were washed with PBS three times. To lyses the cells, 100 μl of H2O was added for 20 min, followed by the addition of 900 μl of Wilkins–Chalgren anaerobe broth to homogenize the cells. The invaded F. nucleatum colonies were recovered on Wilkins–Chalgren anaerobe agar plate under anaerobic conditions; the number of colonies was counted.
FISH
Frozen colon tissues were fixed in Carnoy’s solution overnight and embedded in paraffin; 5 μm thick sections were hybridized in the hybridization buffer (0.9 M NaCl, 20 mM Tris/HCl, pH 7.3, 0.01% SDS). Stringency was used with the form amide concentration from 0 to 30% (v/v). Pre-warmed hybridization buffer (20 ml) was mixed with approximately 5 pmol of the oligonucleotide probe and carefully applied to the tissue sections. After incubation for 5 h in a dark humid chamber at 46 °C, each of the slides were rinsed with sterile double-distilled water, air-dried in the dark, and mounted with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific). The probe sequences used to detect F. nucleatum are listed as followed: 5′-CGCAATACAGAGTTGAGCCCTGC-3′47.
DNA extraction and qPCR
Mucosal tissues were digested in PBS containing an enzymatic cocktail of mutanolysin (250 U/ml) and lysozyme (1 mg/ml) (Sigma-Aldrich) at 37 °C for 1 h, total genomic DNA was extracted with the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s instructions. qPCR was performed to detect the F. nucleatum or P. distasonis level by using 40 ng genomic DNA in 20 μl universal SYBR Green PCR Master Mix (Roche) in a ViiA 7 Real-Time PCR System (Applied Biosystems). F. nucleatum or P. distasonis quantitation was measured relative to the pgt gene. The primers used are listed as followed: F. nucleatum forward: CAACCATTACTTTAACTCTACCATGTTCA, F. nucleatum reverse: GTTGACTTTACAGAAGGAGATTATGTAAAAATC, P. distasonis forward: CCACGCAGTAAACGATGA, P. distasonis reverse: 5′-CTTAACGCTTTCGCTGTG-3′, prostaglandin transporter (pgt) forward: 5′-ATCCCCAAAGCACCTGGTTT-3′, pgt reverse: 5′-AGAGGCCAAGATAGTCCTGGTAA-3′.
Cell culture and stable cell lines construction
The human colon cancer cell line HT-29 was obtained from Stem Cell Bank, Chinese Academy of Sciences, and was maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, #SH30243.01) supplemented with 10% FBS (Gibco, #10270-106), 1% penicillin/streptomycin (Beyotime, #C0222). All cells were cultured at 37 °C supplied with 5% CO2. The cell lines with stable overexpression of KRAS p.G12D, p.G13D, or control cells were generated by infection HT-29 cells with KRAS G12D-sgRNA-Cas-EGFP, KRAS G13D-sgRNA-Cas-EGFP and negative scramble control-EGFP lentiviral plasmids which were purchased from GeneChem (Shanghai, China) according to the manufacturer’s instructions.
Establishment of patient-derived organoids (PDOs)
Biopsies from CRC patients were collected in 5 ml PBS containing penicillin/streptomycin on ice. Following washing and mincing tissues into around 1–2 mm3, samples were digested with 10 ml of cell dissociation reagent (Stem Cell, #07174) on ice on a rocking platform for 30 min. Dissociated cells were passed through 100 μm cell strainer, and then pelleted and suspended in ice-cold PBS. Centrifuge cells for 300 g, 5 min, and then resuspend cells in growth factor reduced (GFR) matrigel (Corning, #356231), and seed cells on 48-well cell culture plate (Corning, #3548). Following solidified in 37 °C and 5% CO2 incubator for 30 min, 300 μl of human IntestiCult™ Organoid Growth Medium (Stem Cell, #06010) which were additionally added 10 μM of Y27632 (Stem Cell, #72304) for the primary culture and were overlaid in the well coated with matrigel. As for the passaging of PDOs, organoids were harvested with ice-cold PBS and pipetted with mechanical force through 1 ml pipette (160 times per well). Dissociated PDOs were pipetted and washed with ice-cold PBS. Resuspend the dissociated cells in GFR matrigel and re-seeded on 48-well flat bottom cell culture plate. PDOs were frozen in FBS containing 10% DMSO for biobanking. The information of the patients from whom the organoids derived is provided in Supplementary Table 1.
Far-western assay
The far-western assay was performed as the reference48. To prepare the biotinylated cell proteins, cell proteins were extracted with PBS containing 1% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature, then labeled with 1 mM EZ-Link Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific) for 2 h at 4 °C. Whole F. nucleatum proteins were extracted with PBS containing 1% Triton X-100, separated by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was blocked with 5% BSA for 1 h and incubated with biotinylated cell proteins overnight at 4 °C. Biotin-labeled proteins were detected using avidin-conjugated horseradish peroxidase (HRP). The corresponding bands in SDS-PAGE were excised for identification by mass spectrometry.
His pull-down assay
The recombinant His-FN1859 or His-DHX15 was produced in Escherichia coli strains. To perform the His pull-down assay, His-FN1859 or His-DHX15 was incubated with Ni-NTA Magnetic Beads, followed by adding cell or bacteria proteins indicated overnight at 4 °C. The beads were then washed and boiled with SDS-PAGE gel loading buffer. Eluted proteins were separated by SDS-PAGE and analyzed by mass spectrometry.
Mass spectrometry
Scoop out the area where the protein is located in SDS-PAGE and cut it into small pieces about 1 mm3. Add double steaming water to soak the glue block, shake for 10 minutes, absorb the lotion. The glue block was immersed in 50% ACN/100 mM NH4HCO3 (pH 8.0) solution, shook for 10 min, and the lotion was absorbed. The process was repeated three times. Add 100% ACN-impregnated glue block, shake for 10 min, absorb the lotion, and then drain the glue block in a vacuum draining machine. 10 mM DTT/50 mM NH4HCO3 (pH 8.0) solution was added to the glue block, incubated at 56 °C for 1 h for reduction reaction, and then the leaching solution was removed. After that, 55 mM iodoacetamide/50 mM NH4HCO3 (pH 8.0) solution was added, and the solution was incubated at room temperature and dark place for 30 min. Add 100% ACN, shake for 20 min, absorb the infusion, drain the glue block. Appropriate amount of trypsin was added to the glue blocks, and 50 mM NH4HCO3 solution was added to completely cover the glue blocks, and the glue blocks were incubated at 37 °C overnight for enzyme digestion. Then 60% ACN/5% formic acid was added, ultrasonic shock was performed for 10 min, and the supernatant was absorbed into the new centrifuge tube after centrifugation. After the extraction process is repeated twice, the extracted liquid is combined and drained in the centrifugal concentrator. The peptide was desalted using C18 column and frozen at −20 °C for machine detection.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed using the SimpleCHIP enzymatic chromatin immunoprecipitation kit (Cell Signaling Technology, #9002) according to the manufacturer’s protocol with minor modifications. The genomic DNA recovered from the ChIP assays was amplified with primers specific to the STAT3-binding elements of the DHX15 promoter region. The specificity of the primer set was verified by analyzing the dissociation curve of each gene-specific PCR product and listed in Supplementary Table 4.
Luciferase reporter assays
PGL3-basic vector (Promega, #E1751) was used to clone the promoter of DHX15. Site-specific mutant was generated by PCR. 293T cells were provided from the cell bank of Chinese Academy of Sciences and seeded in a 96-well plate with a density of 0.8 × 105/well 1 day before transfection. Wells was transfected with a mixture of 100 ng pGL3 luciferase vector, DHX15 WT plasmid or DHX15 mutant plasmid or/and 100 ng STAT3 plasmid and 25 ng pRL-TK renilla vector using Lipofectamine 3000 Transfection Reagent (Invitrogen, #L3000-15). Twelve hours post-transfection, luciferase activity was measured on a microplate reader (Berthold, TriStar LB941) by using the Dual-Luciferase Reporter Assay System (Promega, #2920). The ratio of firefly luciferase to renilla luciferase was calculated for each well.
RNA extraction, reverse transcription, and qPCR
Total RNA was extracted from colon tissues from mice using the TRIzol reagent (Invitrogen, #15596-026), and NanoDrop spectrophotometer (ND-1000) was used for RNA quality control. cDNA was synthesized using PrimeScriptTM RT Master Mix (TaKaRa, #RR036A). qPCR was carried out with the TB Green Premix Ex TaqTM II (TaKaRa, #RR820A) in a ViiA 7 Real-Time PCR System (Applied Biosystems). The relative expression of target genes was confirmed using quantity of target gene/quantity of β-actin. Primer sequences are listed in Supplementary Table 4.
Western blotting
Mouse colons or cultured cells were lysed in radio immunoprecipitation assay buffer supplemented with protease and phosphatase inhibitor cocktail (Thermo Scientific, #78440). Antibody used are listed in Supplementary Table 5. The signal was detected with ECL Western Blotting Substrate (Thermo Scientific, #34095) and Amersham Imager 600 (GE Healthcare). Images have been cropped for presentation.
Immunohistochemistry and immunofluorescence
The human or mouse colons were fixed in formalin and embedded in paraffin. Sections (6 μm) were stained with hematoxylin and eosin (H&E). For immunohistochemistry, Ki67 or DHX15 expression was evaluated in colon sections using rabbit anti-Ki67 Ab (1:100 dilution, Cell Signaling Technology, #12202) or rabbit anti-DHX15 Ab (1:100 dilution, Proteintech, #12265-1-AP), following the manufacturer’s instructions. For immunofluorescence, CD3 or CD11c expression was evaluated in colon sections using rat anti-CD3 Ab (1:100 dilution, Abcam, #ab11089) or mouse anti-CD11c Ab (1:100 dilution, Abcam, #ab254183), following the manufacturer’s instructions. For observation F. nucleatum invasion, cells were seeded in 35 mm plates and exposed to F. nucleatum with a MOI of 100 under anaerobic conditions. Then the cells were washed for three times and imaged immediately or fixed in 4% formaldehyde containing 0.1% Triton X-100 at room temperature for 15 min. DAPI was used to visualize the nuclei. Images were obtained by Olympus BX51 microscope or Zeiss LSM900 confocal microscope.
Bacterial strains and culture conditions
F. nucleatum (25586) was purchased from ATCC and P. distasonis was isolated from CRC patients and has a 99.72% identity with the 16S rRNA gene sequence of Parabacteroides distasonis strain ATCC 8503 (NCBI accession number NR_074376.1) (Supplementary Table 6). Identification of cultured F. nucleatum and P. distosonis by Nanopore Sequencing is shown in Supplementary Table 7. The strains were maintained in bottled Thioglycollate anaerobe broth (Sanyao, #15611) or Columbia agar in an anaerobic chamber (5% CO2, 2% H2, and 93% N2) at 37 °C.
Transmission electron microscope
Cells were harvested after trypsin digestion, and fixed with 2.5% glutaraldehyde solution. PDOs were dissociated by TrypLETM Express (Gibco, 12604-013), and fixed in 2.5% glutaraldehyde solution. TEM (Hitachi) was operated at a voltage of 80 kV and equipped for morphological observation after sample preparation.
Statistics and reproducibility
Each experiment was performed at least two biological replicates. The data were analyzed with Graphpad Prism 5 and Spss 22.0. Acquired data were presented as mean values, and error bars represent the SEM. Student’s t-test was used when two conditions were compared, and one-way ANOVA with Bonferroni post-test or Sidakʼs post-test was used for multiple comparisons. The correlations between F. nucleatum, P. distasonis abundance and KRAS mutation status were analyzed using Chi-square. The probability values of <0.05 were considered statistically significant. Exact p-value was provided.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data in this study have been deposited in the GSA with accession number CRA013275, CRA013274, CRA013276, CRA013455, HRA006025. Dataset HRA006025 is available under restricted access for human genetics data privacy concerns; access can be obtained by the DAC (Data Access Committees) of the GSA-human database. The approximate response time for accession requests is about 3 days. Once access has been approved, the data will be available for 3 months. The user can also contact the corresponding author directly. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD048684, PXD048686. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided as a Source Data file. Source data are provided with this paper.
References
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Yu, J. et al. Novel recurrently mutated genes and a prognostic mutation signature in colorectal cancer. Gut 64, 636–645 (2015).
Vaughn, C. P., Zobell, S. D., Furtado, L. V., Baker, C. L. & Samowitz, W. S. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 50, 307–312 (2011).
Knickelbein, K. & Zhang, L. Mutant KRAS as a critical determinant of the therapeutic response of colorectal cancer. Genes Dis. 2, 4–12 (2015).
Lotfollahzadeh, S., Recio-Boiles, A. & Cagir, B. Colon cancer. In: StatPearls [Internet]. NBK470380 [bookaccession], http://www.ncbi.nlm.nih.gov/pubmed/29262132 (StatPearls Publishing, Treasure Island (FL), 2024).
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature 580, 269–273 (2020).
Kadosh, E. et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 586, 133–138 (2020).
Gao, R. et al. Integrated analysis of colorectal cancer reveals cross-cohort gut microbial signatures and associated serum metabolites. Gastroenterology 163, 1024–1037.e9 (2022).
Liu, N. N. et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 7, 238–250 (2022).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Yang, Y. et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology 152, 851–866.e24 (2017).
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563.e16 (2017).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Chen, C. C. et al. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 107, 1623–1634 (2012).
Koh, G. Y. et al. Parabacteroides distasonis attenuates toll-like receptor 4 signaling and Akt activation and blocks colon tumor formation in high-fat diet-fed azoxymethane-treated mice. Int J. Cancer 143, 1797–1805 (2018).
Falony, G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564 (2016).
Wang, K. et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5 (2019).
Pajkos, G., Kiss, I., Sandor, J., Ember, I. & Kishazi, P. The prognostic value of the presence of mutations at the codons 12, 13, 61 of K-ras oncogene in colorectal cancer. Anticancer Res. 20, 1695–1701 (2000).
Borowsky, J. et al. Association of Fusobacterium nucleatum with specific T-cell subsets in the colorectal carcinoma microenvironment. Clin. Cancer Res. 27, 2816–2826 (2021).
Chu, W. et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil. Steril. 113, 1286–1298.e4 (2020).
Wu, T. R. et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 68, 248–262 (2019).
Boutin, A. T. et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 31, 370–382 (2017).
Drolia, R., Tenguria, S., Durkes, A. C., Turner, J. R. & Bhunia, A. K. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe 23, 470–484.e7 (2018).
Xie, C., Liao, H., Zhang, C. & Zhang, S. Overexpression and clinical relevance of the RNA helicase DHX15 in hepatocellular carcinoma. Hum. Pathol. 84, 213–220 (2019).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3, 11–22 (2003).
Guo, Y. J. et al. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med 19, 1997–2007 (2020).
de Carvalho, A. C. et al. Microbiota profile and impact of Fusobacterium nucleatum in colorectal cancer patients of Barretos Cancer Hospital. Front Oncol. 9, 813 (2019).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016).
Borozan, I. et al. Molecular and pathology features of colorectal tumors and patient outcomes are associated with Fusobacterium nucleatum and Its subspecies animalis. Cancer Epidemiol. Biomark. Prev. 31, 210–220 (2022).
McFall, T. et al. A systems mechanism for KRAS mutant allele-specific responses to targeted therapy. Sci. Signal 12, eaaw8288 (2019).
Ugai, T. et al. Molecular characteristics of early-onset colorectal cancer according to detailed anatomical locations: comparison with later-onset cases. Am. J. Gastroenterol. 118, 712–726 (2023).
Rosty, C. et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod. Pathol. 26, 825–834 (2013).
Yamauchi, M. et al. Colorectal cancer: a tale of two sides or a continuum? Gut 61, 794–797 (2012).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin. Transl. Gastroenterol. 7, e200 (2016).
Proenca, M. A. et al. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World J. Gastroenterol. 24, 5351–5365 (2018).
Brandt, R. et al. Cell type-dependent differential activation of ERK by oncogenic KRAS in colon cancer and intestinal epithelium. Nat. Commun. 10, 2919 (2019).
Fraile, J. M. et al. Loss of the deubiquitinase USP36 destabilizes the RNA helicase DHX33 and causes preimplantation lethality in mice. J. Biol. Chem. 293, 2183–2194 (2018).
Pattabhi, S., Knoll, M. L., Gale, M. Jr. & Loo, Y. M. DHX15 is a coreceptor for RLR signaling that promotes antiviral defense against RNA virus infection. J. Interferon Cytokine Res. 39, 331–346 (2019).
Yao, G. et al. Long non-coding RNA JHDM1D-AS1 interacts with DHX15 protein to enhance non-small-cell lung cancer growth and metastasis. Mol. Ther. Nucleic Acids 18, 831–840 (2019).
Jing, Y. et al. DHX15 promotes prostate cancer progression by stimulating Siah2-mediated ubiquitination of androgen receptor. Oncogene 37, 638–650 (2018).
Wang, Y. et al. The RNA helicase Dhx15 mediates Wnt-induced antimicrobial protein expression in Paneth cells. Proc. Natl Acad. Sci. USA 118, e2017432118 (2021).
Cekanaviciute, E. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl Acad. Sci. USA 114, 10713–10718 (2017).
Koh, G. Y., Kane, A. V., Wu, X. & Crott, J. W. Parabacteroides distasonis attenuates tumorigenesis, modulates inflammatory markers and promotes intestinal barrier integrity in azoxymethane-treated A/J mice. Carcinogenesis 41, 909–917 (2020).
Canon, J. et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Gao, Y. et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target Ther. 6, 398 (2021).
Long, X. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol 4, 2319–2330 (2019).
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (81972221, 82072634, 81801564, 81902422); Shanghai Sailing Program (18YF1419400); Clinical Research Plan of SHDC (SHDC2020CR2069B); Jiangsu Natural Science Foundation (BK20231245); Program of Jiangsu Commission of Health (No. M2020024). We are very grateful for the kind offer of Dhx15-floxed mice from Prof. Shu Zhu (Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230027, China), and the help for using cryo-focused ion beam milling and cryo-electron tomography from Prof. Quan Wang and Xuebo Yang (Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai, 201210, China).
Author information
Authors and Affiliations
Contributions
H. Zhu, Q. Wei, and H. Qin designed the research. H. Zhu, M. Li, and H. Yang conducted most of the experiments. M. Li helped with organoids culture. D. Bi and H. Liu offered help in data analysis. F. Song helped in RNA interference. C. Kong and Y. Zhang helped with mouse model construction. Y. Gao and Y. Zhu helped with the experimental details. R. Xie and J. Zheng contributed to specimen preparation. X. Yan and Q. Xu helped with data discussion. H. Zhu wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Federica Facciotti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Li, M., Bi, D. et al. Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15. Nat Commun 15, 1688 (2024). https://doi.org/10.1038/s41467-024-45572-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45572-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.