Abstract
Soil organisms are affected by the presence of predatory protists. However, it remains poorly understood how predatory protists can affect plant disease incidence and how fertilization regimes can affect these interactions. Here, we characterise the rhizosphere bacteria, fungi and protists over eleven growing seasons of tomato planting under three fertilization regimes, i.e conventional, organic and bioorganic, and with different bacterial wilt disease incidence levels. We find that predatory protists are negatively associated with disease incidence, especially two ciliophoran Colpoda OTUs, and that bioorganic fertilization enhances the abundance of predatory protists. In glasshouse experiments we find that the predatory protist Colpoda influences disease incidence by directly consuming pathogens and indirectly increasing the presence of pathogen-suppressive microorganisms in the soil. Together, we demonstrate that predatory protists reduce bacterial wilt disease incidence in tomato plants via direct and indirect reductions of pathogens. Our study provides insights on the role that predatory protists play in plant disease, which could be used to design more sustainable agricultural practices.
Similar content being viewed by others
Introduction
Crop losses caused by soil-borne pathogens are becoming an ever-increasing threat to sustainable agricultural production1,2. Pathogens (e.g., Ralstonia solanacearum and Fusarium oxysporum) severely impact plant health when colonizing the plant rhizosphere3. The plant rhizosphere provides numerous ecological niches for the growth and proliferation of a variety of microorganisms4. Rhizosphere microorganisms, especially bacteria and fungi, form a tight network to improve plant health by defending against pathogens through competition for space and resources5,6. Negative impacts on rhizosphere microorganisms, such as through intense management, therefore often are reported to increase plant disease incidences and reduce plant health7,8.
Healthy plants are essential for ensuring crop productivity and food security9. Conventional agricultural management is linked to high management efforts to inhibit pests, but the intensive application of synthetic agrochemicals (e.g., chemical fertilizers and synthetic pesticides) in conventional agriculture has adverse effects on soil quality and environmental sustainability10,11. Organic farming, such as organic fertilizer application, can improve plant performance (e.g., growth and health) and minimize negative impacts of synthetic chemicals by inducing beneficial bacteria and fungi as well as their ecological interactions in soils and plant rhizospheres8,12,13. Through the promotion of plant-beneficial bacteria and fungi, organic farming is recognized as one of the most sustainable alternatives to conventional agriculture11,14.
Bacteria and fungi are also top-down controlled by predators, particularly predatory protists that are the dominant soil protists15. In turn, bacteria and fungi affect predatory protists. For example, anti-predatory compounds released by bacteria and fungi commonly inhibit protists16,17. Also species-specific interactions between protist predators and microbial prey are common, as predators specifically select for their preferred prey microorganisms15,16,18. Predatory protists are also directly affected by changes in the physicochemical surrounding (e.g., soil moisture availability and pH)19,20. Therefore, predatory protist communities are sensitive to fertilizer application, potentially even more than their microbial prey (e.g., bacteria and fungi)21,22. Predatory protists stimulate nutrient turnover (e.g., nitrogen) resulting in increased plant-availability16. Predatory protists also shift microbiome structure and functionality, often leading to enhanced soil fertility and plant yield21,22,23. While an increasing number of studies uncover the importance of protists in the complexity of disease suppression and therefore as contributors to plant health15,16, the mode of action how these predatory protists increase plant health remains unknown.
To investigate microbial mechanisms underlying fertilization-induced increases in plant health, we determined rhizosphere bacteria, fungi and predatory protists in long-term tomato fields under conventional, organic, and bioorganic fertilization regimes with different bacterial wilt disease incidences. Subsequently, we performed greenhouse experiments to validate observed direct (induced by predatory protists) and indirect (induced by microbial prey of predatory protists) effects of key predatory protistan taxa on the suppression of the major tomato bacterial wilt pathogen Ralstonia solanacearum (R. solanacearum) in the field experiment and to explore the potential bacterial wilt disease-suppressive mechanisms. We hypothesized that (1) protistan communities are more strongly affected by bioorganic fertilization than bacterial and fungal communities, (2) predatory protists explain the decrease in disease incidences and pathogen densities associated with bioorganic fertilization better than other microbial groups, (3) predatory protists directly suppress R. solanacearum through consumption and (4) indirectly improve plant health by promoting pathogen-suppressive microorganisms.
Results
Bacterial, fungal, and protistan communities and their contribution to bacterial wilt disease incidence
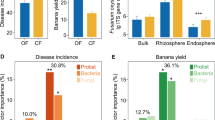
The long-term field experiment was performed with different fertilization treatments (conventional, organic, and bioorganic fertilization) that resulted in different disease incidence. Fertilization regimes had a greater impact on the diversity (one-way ANOVA: F = 7.28, p = 0.025) and community composition (PERMANOVA: R2 = 0.76, p = 0.025) of protists compared to bacteria and fungi (bacterial diversity: one-way ANOVA: F = 0.51, p = 0.625, fungal diversity: one-way ANOVA: F = 0.99, p = 0.426; bacterial community composition: PERMANOVA: R2 = 0.37, p = 0.074, fungal community composition: PERMANOVA: R2 = 0.26, p = 0.425) (Supplementary Fig. 1). The explanatory power of protistan diversity and community composition (linear model analysis: explaining 86.36% of the observed variation) for disease incidence was higher than that of bacterial and fungal diversities and community compositions (linear model analysis: bacterial diversity and community composition: explaining 10.26% of the observed variation; fungal diversity and community composition: explaining 2.61% of the observed variation, Fig. 1A). Among the selected microbial indices, protistan community composition best explained disease incidence (linear model analysis: explaining 45.36% of the observed variation, F = 299.32, p = 0.004; Fig. 1A).
The explanatory power of bacterial, fungal and protistan diversities and community compositions for disease incidence (A). Correlations between the relative abundances of predatory protists and disease incidence (B). Correlations between bacterial and fungal communities and the relative abundances of predatory protists (C). In (A), statistical significance was calculated by multiple regression using linear models. Asterisk denotes statistically significant (p < 0.05). In (B), two-sided Spearman’s correlation was performed to explore the relationship between disease incidence and the relative abundance of predatory protists. Solid lines denote statistically significant (p < 0.05). In (C), statistical significance was calculated by Mantel test. Asterisk denotes statistically significant (p < 0.05). Source data are provided as a Source Data file.
Among protistan functional groups, only the relative abundances of predatory protists were negatively correlated with disease incidence (Spearman’s correlation analysis: correlation coefficient = −0.82, p = 0.007, Fig. 1B and Supplementary Table 1). In addition, predatory protists were correlated with the bacterial community (Mantel correlation analysis: r = 0.52, p = 0.018; Fig. 1C). The relative abundances of predatory protists were highest in BF (bioorganic fertilization) (enhanced by 12.7% in BF and by 8.3% in OF (organic fertilization) compared to CF (conventional fertilization)) (one-way ANOVA with Tukey’s HSD test: F = 5.73, p = 0.041, Fig. S2). As predatory protists were linked to disease incidence and bacteria and were affected by fertilization regimes, we focused subsequent analyses on predatory protists and their links with bacteria.
Predatory protistan and bacterial taxonomic compositions and links with bacterial wilt disease suppression
The relative abundances of seven predatory protistan OTUs negatively correlated with disease incidence, including three ciliophoran, two cercozoan, one pseudofungal and one conosan taxa (Spearman’s correlation analysis: P_OTU67: correlation coefficient = −0.90, p = 0.002; P_OTU37: correlation coefficient = −0.72, p = 0.037; P_OTU30: correlation coefficient = −0.63, p = 0.046; P_OTU39: correlation coefficient = −0.71, p = 0.032; P_OTU79: correlation coefficient = −0.75, p = 0.026; P_OTU68: correlation coefficient = −0.80, p = 0.013; P_OTU59: correlation coefficient = −0.86, p = 0.002; Fig. 2A). Of these predatory protistan OTUs, the explanatory power of ciliophoran OTUs (linear model analysis: explaining 41.64% of the observed variation) for the density of R. solanacearum was higher than that of cercozoan, pseudofungal and conosan OTUs (linear model analysis: cercozoa: explaining 13.91% of the observed variation; pseudofungi: explaining 7.98% of the observed variation; conosa: explaining 3.35% of the observed variation, Fig. 2A). In particular, the ciliophoran Colpoda P_OTU67 was the strongest predictor with respect to explaining the density of R. solanacearum (linear model analysis: explaining 19.67% of the observed variation, F = 15.53, p = 0.003, Fig. 2A). The relative abundance of Colpoda P_OTU67 was negatively correlated with the density of R. solanacearum (Spearman’s correlation analysis: correlation coefficient = −0.93, p = 2.36E-04, Supplementary Table 2). The relative abundance of Colpoda P_OTU67 was highest in BF (enhanced by 676.82% in BF and by 86.91% in OF compared to CF) (one-way ANOVA with Tukey’s HSD test: F = 63.94, p = 9.00E-05, Fig. 2A). In addition, Colpoda P_OTU67 had the greatest explanatory power for the ratio of pathogen-suppressive bacteria to pathogen-promotive bacteria (linear model analysis: explaining 38.84 % of the observed variation, F = 183.38, p = 0.047, Fig. 2B). The relative abundance of Colpoda P_OTU67 positively correlated with the ratio of pathogen-suppressive bacteria to pathogen-promotive bacteria (Spearman’s correlation analysis: correlation coefficient = 0.97, p = 2.20E-05) and this ratio negatively correlated with the density of R. solanacearum (Spearman’s correlation analysis: correlation coefficient = −0.90, p = 0.001, Fig. 2C). The relative abundance of Colpoda P_OTU67 positively correlated with the relative abundances of Pseudoxanthomonas (B_OTU128), Sphingobium (B_OTU14), Brucella (B_OTU152), Pseudomonas (B_OTU16), Luteimonas (B_OTU21), Devosia (B_OTU28), Sphingopyxis (B_OTU45), Lysobacter (B_OTU46), Streptomyces (B_OTU36) and Lentzea (B_OTU42) (Spearman’s correlation analysis: B_OTU128: correlation coefficient = 0.70, p = 0.037; B_OTU14: correlation coefficient = 0.92, p = 0.001; B_OTU152: correlation coefficient = 0.78, p = 0.014; B_OTU16: correlation coefficient = 0.83, p = 0.008; B_OTU21: correlation coefficient = 0.67, p = 0.048; B_OTU28: correlation coefficient = 0.67, p = 0.049; B_OTU45: correlation coefficient = 0.77, p = 0.021; B_OTU46: correlation coefficient = 0.74, p = 0.023; B_OTU36: correlation coefficient = 0.85, p = 0.004; B_OTU42: correlation coefficient = 0.83, p = 0.008; Fig. 2D). In turn, the relative abundances of these bacterial OTUs negatively correlated with the density of R. solanacearum (Spearman’s correlation analysis: B_OTU128: correlation coefficient = −0.67, p = 0.049; B_OTU14: correlation coefficient = −0.78, p = 0.017; B_OTU152: correlation coefficient = −0.67, p = 0.048; B_OTU16: correlation coefficient = −0.87, p = 0.005; B_OTU21: correlation coefficient = −0.67, p = 0.049; B_OTU28: correlation coefficient = −0.70, p = 0.043; B_OTU45: correlation coefficient = −0.83, p = 0.008; B_OTU46: correlation coefficient = −0.80, p = 0.014; B_OTU36: correlation coefficient = −0.68, p = 0.047; B_OTU42: correlation coefficient = −0.86, p = 0.005; Fig. 2D). In addition, the relative abundance of Colpoda P_OTU67 negatively correlated with the relative abundances of Chitinophaga (B_OTU29) and Arthrobacter (B_OTU116) (Spearman’s correlation analysis: B_OTU29: correlation coefficient = −0.78, p = 0.017; B_OTU116: correlation coefficient = −0.87, p = 0.005; Fig. 2D), which all positively correlated with the density of R. solanacearum (Spearman’s correlation analysis: B_OTU29: correlation coefficient = 0.72, p = 0.037; B_OTU116: correlation coefficient = 0.72, p = 0.037; Fig. 2D).
Heatmap illustrating the relative abundances of predatory protistan OTUs (linked with disease incidence) in all treatments and the explanatory power of these predatory protistan OTUs for the density of R. solanacearum (A). The explanatory power of predatory protistan OTUs (linked with disease incidence) for the ratio of pathogen-suppressive to pathogen-promotive bacteria (B). Correlations between the relative abundance of Colpoda P_OTU67, the ratio of pathogen-suppressive to pathogen-promotive bacteria and the density of R. solanacearum (C). Correlations between the relative abundances of predatory protistan OTUs (linked with disease incidence) and bacterial OTUs (B) and the density of R. solanacearum (D). In (A), P = predatory protist. Statistical significance was calculated by multiple regression using linear models. Asterisk denotes statistically significant (p < 0.05). The color key relates heatmap colors to the standard score (z-score). Two-sided Spearman’s correlation was performed to explore the relationship between the relative abundances of predatory protistan OTUs and disease incidence. Minus signs denote significant negative Spearman’s correlations between the relative abundances of predatory protistan OTUs and disease incidence (p < 0.05). Circles are proportional to the average relative abundances of predatory protistan OTUs across all samples. ANOVA with two-sided Tukey’s multiple comparison was used for the statistical analysis. Letters: significant differences between treatments (p < 0.05). CF conventional fertilization, OF organic fertilization, BF bioorganic fertilization. In (B), P = predatory protist. Statistical significance was calculated by multiple regression using linear models. Asterisk denotes statistically significant (p < 0.05). In (C), P = predatory protist. RA = relative abundance. Two-sided Spearman’s correlation was performed to explore the relationship between the relative abundance of Colpoda P_OTU67, the ratio of pathogen-suppressive to pathogen-promotive bacteria and the density of R. solanacearum. Solid lines denote statistically significant (p < 0.05). In (D), P = predatory protist, B = bacteria. Two-sided Spearman’s correlation was performed to explore the relationship between the relative abundances of bacterial OTUs and the density of R. solanacearum. Plus signs denote significant positive Spearman’s correlations between the relative abundances of bacterial OTUs and the density of R. solanacearum (p < 0.05). Minus signs indicate significant negative Spearman’s correlations between the relative abundances of bacterial OTUs and the density of R. solanacearum (p < 0.05). NS indicates no significant Spearman’s correlations between the relative abundances of bacterial OTUs and the density of R. solanacearum (p > 0.05). The heatmap shows significant correlations (calculated by two-sided Spearman’s correlation, p < 0.05) between the relative abundances of predatory protistan OTUs and the relative abundances of bacterial OTUs. Source data are provided as a Source Data file.
Direct effects of the key predatory protists on suppressing pathogens
Of all isolated Colpoda strains, Colpoda 2, most closely related to Colpoda inflata (Supplementary Table 3), had the most similar sequence identity (99.71%) with the key Colpoda P_OTU67 of the above field experiment (Supplementary Table 4). Co-inoculation of different Colpoda strains and R. solanacearum combinations decreased the density of R. solanacearum (Colpoda 2 + R. solanacearum: decrease of 51.08%, Colpoda 1 + R. solanacearum: decrease of 46.46%) compared to R. solanacearum inoculation in sterilized soils (one-way ANOVA with Tukey’s HSD test: F = 129.43, p = 3.46E-10; Fig. 3A). The relative change of the density of R. solanacearum in Colpoda 2 + R. solanacearum was higher than that in Colpoda 1 + R. solanacearum in sterilized soils (Student’s t test: T = −2.28, p = 0.046; Fig. 3B). As Colpoda 2 had the most similar sequence identity with the key Colpoda P_OTU67 and had the strongest directly suppressive effect on R. solanacearum, we further performed a greenhouse experiment with different concentrations of Colpoda 2 in nonsterilized soils to detect the indirect effect of the key predatory protist on promoting plant health by altering microbial communities.
Effects of different Colpoda on the density of R. solanacearum in sterilized soils (A). The relative changes of the density of R. solanacearum in co-inoculation of different Colpoda and R. solanacearum combinations (B). In (A), ANOVA with two-sided Tukey’s multiple comparison was used for the statistical analysis. Letters: significant differences between treatments (p < 0.05). Results are means ± standard deviation (n = 6 biologically independent samples). In (B), relative change = (X-control)/control, X = the copies of R. solanacearum in co-inoculation of different Colpoda and R. solanacearum combinations, control = the copies of R. solanacearum in inoculation of R. solanacearum. Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. The upper and lower whiskers represent the range of non-outlier data values. Outliers were plotted as individual points. Significant differences between different treatments are defined by two-sided Student’s t test (p < 0.05). n = 6 biologically independent samples. Source data are provided as a Source Data file.
Indirect effects of the key predatory protists on suppressing pathogens
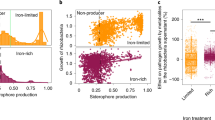
Compared with the control treatment, the different concentrations of Colpoda 2 decreased disease incidence (Colpoda 2 (102): decrease of 34.21%, Colpoda 2 (104): decrease of 55.26%, one-way ANOVA with Tukey’s HSD test: F = 31.20, p = 5.00E-06; Fig. 4A) and the density of R. solanacearum (Colpoda 2 (102): decrease of 82.04%, Colpoda 2 (104): decrease of 96.07%, one-way ANOVA with Tukey’s HSD test: F = 220.96, p = 7.45E-12; Fig. 4B) in nonsterilized soils. The density of R. solanacearum positively correlated with disease incidence (Spearman’s correlation analysis: correlation coefficient = 0.76, p = 2.68E-04; Supplementary Fig. 3). Colpoda 2 inoculation altered bacterial diversity (one-way ANOVA: F = 7.81, p = 0.005; Supplementary Figs. 4A and 4C) and community composition (PERMANOVA: R2 = 0.75, p = 0.001; Supplementary Fig. 4B), but not of fungal diversity (one-way ANOVA: F = 1.12, p = 0.354; Supplementary Fig. 4A) and community composition (PERMANOVA: R2 = 0.04, p = 0.947; Supplementary Fig. 4B). The different concentrations of Colpoda 2 altered bacterial community composition (ANOSIM: Control vs Colpoda 2(10²): R = 0.99, p = 0.002, Control vs Colpoda 2(104): R = 0.99, p = 0.003, Colpoda 2(10²) vs Colpoda 2(104): R = 0.96, p = 0.004; Fig. 4C), with bacterial Bray-Curtis distance increasing with the inoculation concentrations in comparison to control (Student’s t test: T = −13.95, p = 6.70E-22; Fig. 4D). Compared with the control treatment, Colpoda 2 inoculation treatments increased the ratio of pathogen-suppressive bacteria to pathogen-promotive bacteria (Colpoda 2 (102): increase of 120.75%, Colpoda 2 (104): increase of 400.26%, one-way ANOVA with Tukey’s HSD test: F = 224.73, p = 6.59E-12; Fig. 4E). We further examined the impact of Colpoda 2 inoculation on pathogen-suppressive and pathogen-promotive bacterial taxa. The relative abundances of 13 bacterial OTUs were negatively and fifteen positively correlated with the density of R. solanacearum (Spearman’s correlation analysis). Of these pathogen-suppressive and pathogen-promotive bacterial OTUs, Colpoda 2 (102) and Colpoda 2 (104) increased the relative abundances of seven bacterial OTUs, including Gp7, Marmoricola, Gp16, Pseudomonas, Streptomyces and Lysobacter (one-way ANOVA with Tukey’s HSD test: OTU24: F = 29.59, p = 6.00E-06; OTU211: F = 35.92, p = 2.00E-06; OTU88: F = 41.33, p = 7.90E-07; OTU78: F = 37.65, p = 1.00E-06; OTU11: F = 24.48, p = 1.90E-05; OTU41: F = 34.60, p = 2.00E-06; OTU38: F = 99.34, p = 2.23E-09; Fig. 4F), and decreased the relative abundances of thirteen bacterial OTUs, including Gp6, Pirellula, Planctopirus, Chitinophaga, Arthrobacter, Terrimicrobium, Kitasatospora, Sideroxydans, Blastocatella, Pseudoduganella and Pseudolabrys (one-way ANOVA with Tukey’s HSD test: OTU64: F = 218.59, p = 8.05E-12; OTU76: F = 59.95, p = 7.01E-08; OTU56: F = 108.87, p = 1.17E-09; OTU146: F = 34.02, p = 3.67E-07; OTU7: F = 48.07, p = 3.00E-07; OTU126: F = 124.42, p = 4.58E-10; OTU140: F = 49.81, p = 2.38E-07; OTU121: F = 143.23, p = 1.68E-10; OTU73: F = 33.75, p = 3.00E-06; OTU29: F = 43.34, p = 5.84E-07; OTU84: F = 131.22, p = 3.14E-10; OTU8: F = 154.29, p = 9.90E-11; OTU66: F = 46.60, p = 3.00E-06; Fig. 4F).
Effects of different concentrations of Colpoda 2 on disease incidence (A), density of R. solanacearum (B) and bacterial community composition (C). Bray–Curtis distances of bacterial communities between the Colpoda 2 inoculation treatments and the control treatment (D). Effects of different concentrations of Colpoda 2 on the ratio of pathogen-suppressive bacteria to pathogen-promotive bacteria (E). Effects of different concentrations of Colpoda 2 on the relative abundances of bacterial OTUs (linked with the density of R. solanacearum) (F). Control = no microbe was inoculated, Colpoda 2 (102) = Colpoda 2 was inoculated (1.0 × 102 cells g−1 dry soil), Colpoda 2 (104) = Colpoda 2 was inoculated (1.0 × 104 cells g−1 dry soil). In (A, B and E), ANOVA with two-sided Tukey’s multiple comparison was used for the statistical analysis. Letters: significant differences between treatments (p < 0.05). Results are means ± standard deviation (n = 6 biologically independent samples). In panel C, significance of bacterial community dissimilarities among different treatments are based on ANOSIM (analysis of similarities, p < 0.05). In (D), asterisks indicate significant differences as defined by two-sided Student’s t test (p < 0.001). Horizontal bars within boxes represent the median. The tops and bottoms of boxes represent 75th and 25th quartiles, respectively. The upper and lower whiskers represent the range of non-outlier data values. Outliers were plotted as individual points. n = 36 biologically independent samples. In (F), the color key relates the heatmap colors to the standard score (z-score). Two-sided Spearman’s correlation was performed to explore the relationship between the relative abundances of bacterial OTUs and the density of R. solanacearum. Plus signs denote significant positive Spearman’s correlations between the relative abundances of bacterial OTUs and the density of R. solanacearum (p < 0.05). Minus signs denote significant negative Spearman’s correlations between the relative abundances of bacterial OTUs and the density of R. solanacearum (p < 0.05). Circles are proportional to the average relative abundances of bacterial OTUs across all samples. ANOVA with two-sided Tukey’s multiple comparison was used for the statistical analysis. Letters: significant differences between treatments (p < 0.05). Source data are provided as a Source Data file.
Among these bacterial OTUs altered by Colpoda 2 inoculation (the first greenhouse experiment), the same links of Pseudomonas, Lysobacter, Streptomyces, Arthrobacter and Chitinophaga with Colpoda and R. solanacearum were observed in the above field experiment (see above Fig. 2D). Therefore, we selected these key bacterial strains (five Pseudomonas, one Lysobacter, two Streptomyces, two Arthrobacter and four Chitinophaga) and performed the second greenhouse experiment in sterilized soils to validate the potential links of these bacteria with Colpoda 2 and R. solanacearum. The relative decrease of bacterial density in Pseudomonas 1 + Colpoda 2 (decrease of 7.91%), Pseudomonas 2 + Colpoda 2 (decrease of 9.50%), Pseudomonas 3 + Colpoda 2 (decrease of 9.57%), Pseudomonas 4 + Colpoda 2 (decrease of 8.37%), Pseudomonas 5 + Colpoda 2 (decrease of 9.21%), Lysobacter 1 + Colpoda 2 (decrease of 10.59%), Streptomyces 1 + Colpoda 2 (decrease of 9.07%) and Streptomyces 2 + Colpoda 2 (decrease of 7.04%) were lower than that in Arthrobacter 1 + Colpoda 2 (decrease of 41.56%), Arthrobacter 2 + Colpoda 2 (decrease of 38.04%), Chitinophaga 1 + Colpoda 2 (decrease of 60.35%), Chitinophaga 2 + Colpoda 2 (decrease of 63.92%), Chitinophaga 3 + Colpoda 2 (decrease of 61.89%) and Chitinophaga 4 + Colpoda 2 (decrease of 61.14%) (one-way ANOVA with Tukey’s HSD test: F = 105.21, p = 1.54E-40; Fig. 5A). In the second part of the second greenhouse experiment, compared with the treatment with inoculation of R. solanacearum only, Pseudomonas 1 + R. solanacearum (decrease of 97.30%), Pseudomonas 2 + R. solanacearum (decrease of 96.92%), Pseudomonas 3 + R. solanacearum (decrease of 97.04%), Pseudomonas 4 + R. solanacearum (decrease of 97.47%), Pseudomonas 5 + R. solanacearum (decrease of 96.69%), Lysobacter 1 + R. solanacearum (decrease of 95.50%), Streptomyces 1 + R. solanacearum (decrease of 96.92%) and Streptomyces 2 + R. solanacearum (decrease of 97.71%) decreased the density of R. solanacearum and Arthrobacter 1 + R. solanacearum (increase of 93.44%), Arthrobacter 2 + R. solanacearum (increase of 139.18%), Chitinophaga 1 + R. solanacearum (increase of 58.11%), Chitinophaga 2 + R. solanacearum (increase of 52.90%), Chitinophaga 3 + R. solanacearum (increase of 73.89%) and Chitinophaga 4 + R. solanacearum (increase of 57.16%) increased the density of R. solanacearum (one-way ANOVA with Tukey’s HSD test: F = 548.13, p = 1.82E-69; Fig. 5B). The relative changes of densities of bacteria in different bacteria + Colpoda 2 treatments were negatively correlated with the relative changes of densities of R. solanacearum in different bacteria + R. solanacearum treatments (Spearman’s correlation analysis: correlation coefficient = −0.73: p = 1.99E-15; Supplementary Table 5).
Relative changes of densities of representative bacteria in different key bacteria + Colpada 2 treatments in the greenhouse experiment using sterilized soils (A). Density of R. solanacearum in treatments with inoculation of R. solanacearum and co-inoculation of representative bacteria in the greenhouse experiment using sterilized soils (B). In (A), ANOVA with two-sided Tukey’s multiple comparison was used for the statistical analysis. Letters: significant differences between treatments (p < 0.05). Relative change = (X-control)/control, X = the copies of representative bacteria in different representative bacteria + Colpoda 2 treatments, control = the copies of representative bacteria in different representative bacteria inoculation treatments. Representative bacteria are those that are affected by different concentrations of Colpoda 2 inoculation and associated with R. solanacearum density in field and greenhouse experiments (see above results). Results are means ± standard deviation (n = 6 biologically independent samples). In (B), ANOVA with two-sided Tukey’s multiple comparison was used for the statistical analysis. Letters: significant differences between treatments (p < 0.05). Relative change = (X-control)/control, X = the copies of R. solanacearum in different bacteria + R. solanacearum treatments, control = the copies of R. solanacearum in R. solanacearum inoculation treatment. Results are means ± standard deviation (n = 6 biologically independent samples). Source data are provided as a Source Data file.
Discussion
In this study, we evaluated the impacts of different fertilization regimes on disease suppression and rhizosphere microbiomes within a continuous tomato monoculture cropping system. We provide novel evidence that predatory protists stimulate plant health in response to bioorganic fertilization via direct and indirect reductions of pathogens.
We confirmed hypothesis 1 that protistan communities are more strongly affected by bioorganic fertilization than bacterial and fungal communities. This finding supports previous observations that protistan communities are more sensitive to organic fertilizer inputs than other microbial groups (e.g., bacteria and fungi) in diverse agricultural soils21,22, potentially explained by the highly diverse taxonomic, trait and functional diversity of protists that together surpasses that of other microbial groups15,24. In addition, numerous previous studies demonstrated that long-term different organic fertilization changes the soil physicochemical environment25,26. A stronger response of protistan communities to bioorganic fertilization than of bacterial and fungal communities suggests that protists are more strongly changed, potentially explained by their larger taxonomic, trait and functional diversity might make them more responsive to changes in the surrounding physicochemical environment as induced by bioorganic fertilizer inputs27,28.
We further confirmed hypothesis 2 that predatory protists explain the decrease in disease incidences and pathogen densities associated with bioorganic fertilization better than other microbial groups. The disease-suppressive function of predatory protists is related to direct and indirect interactions with the bacterial soil-borne pathogen R. solanacearum. Previous studies showed that the predominant effects of predatory protists in suppressing soil-borne diseases are determined by potential negative links between predatory protists and soil-borne pathogenic R. solanacearum already at plant establishment29. As predatory protists are the dominant, widely distributed soil protistan functional group19, our results enforce the suppression potential of the community of predatory protists present in soils. In addition, our results complement previous findings that predatory protists could increase plant health effectively through reducing fungal soil-borne pathogens (e.g., Fusarium oxysporum and Rhizoctonia solani) in other plant-pathogen systems22,30. This suggests that predatory protists have important roles in the suppression of soil-borne diseases caused by different pathogens.
We actually identified at least some key predatory protistan taxa underlying disease suppression by directly suppressing bacterial soil-borne pathogens through consumption and thereby confirmed hypothesis 3. Some ciliophoran taxa, particularly Colpoda spp., fed on R. solanacearum, which leads to a direct reduction in pathogen abundance. Colpoda is an abundant ciliate genus in soils27,31 and considered a keystone taxon in soils linked with plant growth (e.g., maize)27,32,33. Colpoda ciliates feed primarily on bacteria31. Previous studies demonstrated that predation of Colpoda ciliates can decrease the abundance of some free-living bacteria populations (e.g., Azospirillum lipoferum and Stenotrophomonas sp.), leading to changes in soil functions (e.g., nitrogen fixation)34. Thus, increases in Colpoda populations induced by bioorganic fertilization could also reduce pathogenic bacteria, which would enhance plant health. This suggests that predatory protists may be used as effective biocontrol agents to increase soil-borne diseases suppression and Colpoda may serve as a keystone genus for future biological control of diseases in diverse agricultural systems.
In addition, we confirmed hypothesis 4 that predatory protists indirectly improve plant health by promoting pathogen-suppressive microorganisms. We show that Colpoda ciliates indirectly suppressed R. solanacearum through increasing pathogen-suppressive bacteria (e.g., Pseudomonas, Lysobacter and Streptomyces), which produce antimicrobial compounds35,36. Simultaneously, Colpoda ciliates reduced pathogen-promotive bacteria (e.g., Arthrobacter and Chitinophaga) that can improve pathogen growth37. Species-specific feeding differences between pathogen-suppressive and pathogen-promotive bacteria are likely caused by highly sophisticated antagonism mechanisms of microorganisms against their predators38 and ancient co-evolutionary predator-prey relationships16. Selective predation by protists can decrease pathogen-promotive bacteria, leading to increased niche-space for growth of other microorganisms with traits conferring resistance to protists18,39. Among these are those traits that help to defend directly against protist predators and indirectly suppress plant pathogens such as through the production of general antimicrobial compounds17,22.
We demonstrate that bioorganic fertilization improves plant health by steering the rhizosphere protistan community, particularly stimulating predatory protists that can directly consume pathogens and indirectly suppressed pathogens by altering bacterial communities in favor of pathogen-suppressive microorganisms. Our study provides mechanistic insights on predatory protists as major agents in controlling plant health that might help in creating more sustainable agricultural practices with considering protists.
Methods
Site description, experimental design, tomato bacterial wilt disease incidence determination and rhizosphere soil collection in the field experiment
The site description, experimental design, tomato bacterial wilt disease incidence determination and rhizosphere samples collection is described in detail in40. In brief, the field experimental site is located in Hengxi town in Nanjing, Jiangsu Province, China (32°02′N,118°50′E) and the experiment was running since 2013. Three fertilization treatments were established as follows: CF (conventional fertilization), OF (organic fertilization) and BF (bioorganic fertilization). The disease incidence was calculated by counting the number of tomato plants with bacterial wilt (observations of typical wilt symptoms, including necrosis and leaf drooping) among the total number of tomato plants in each plot. The detailed fertilization scheme is shown in Supplementary Table 6. In brief, the scheme of CF (conventional fertilization) is 120 kg ha−1 nitrogen (N), 180 kg ha−1 phosphorus (P) and 120 kg ha−1 potassium (K) mineral fertilizers, the scheme of OF (organic fertilization) is 7500 kg ha−1 organic fertilizer (1.75% nitrogen (N), 0.82% phosphorus (P) and 1.42% potassium (K)) and the scheme of BF (bioorganic fertilization) is 7500 kg ha−1 bioorganic fertilizer (1.85% nitrogen (N), 0.80% phosphorus (P) and 1.46% potassium (K)). Bioorganic fertilizer was produced by inoculating 5% (v/dw) Bacillus amyloliquefaciens T-5 into the mixed compost of rapeseed meal and chicken manure (dw/dw = 1:4) compost and then fermented for 1 week. Organic fertilizer was produced using the same process as bioorganic fertilizer, but without inoculation with Bacillus. During harvest in June 2018, rhizosphere soil samples were collected and stored at −80 °C for further use. In brief, plant roots were collected and vigorously shaken in the laboratory. After that, roots were put into a triangular flask with sterile phosphate buffered saline and rhizosphere soil samples were obtained after centrifugation at 10,000 g for 10 min. Then, rhizosphere soil samples were frozen in liquid nitrogen and stored at −80 °C.
DNA extraction and quantitative PCR assay
The total genomic DNA extraction of rhizosphere soil samples is described in detail in our previous research40. In brief, we used PowerSoil Soil DNA Isolation Kits (MoBio Laboratories Inc.,USA) to extract the total genomic DNA of rhizosphere soil samples and the process of the DNA extraction follows the manufacturer’s protocol. The abundances of R. solanacearum41, Pseudomonas42, Lysobacter43, Streptomyces44, Arthrobacter45 and Chitinophaga46 were quantified using specific primers sets (R. solanacearum: forward, 5′-GAACGCCAACGGTGCGAACT-3′; reverse, 5’-GGCGGCCTTCAGGGAGGTC-3′; Pseudomonas: forward, 5′-GAGTTTGATCCTGGCTCAG-3′; reverse, 5′-GAGTTTGATCCTGGCTCAG-3′; Lysobacter: forward, 5′- GAGCCGACGTCGGATTAGCTGTT-3′; reverse, 5′-AAGGAGGTGWTCCARCC-3′; Streptomyces: forward, 5′-GAACTGAGACCGGCTTTTTGA-3′; reverse, 5′-GGTGGCGAAGGCGGA-3’; Arthrobacter: forward, 5′-GCTGGTTTGAGAGGACGACCAGC-3’; reverse, 5′-AGCCCATGACGTTTCTTTCCTGCCA-3′; Chitinophaga: forward, 5′-TTRAAGATGGSYGTGCRYC-3′; reverse, 5′-CGCTACATGACATATTCCGCT-3′) and standard curves were generated according to previously established protocols (Supplementary Table 7). Quantitative PCR assay was performed on a qTOWER Real-time PCR system of Analytik Jena (Jena, Germany). Each sample was analyzed in six replicates and the abundance of each microorganism of each sample was expressed as log10 values (target copy numbers per gram soil).
Illumina MiSeq sequencing and bioinformatic analyses
The prokaryotic 16S rRNA gene amplification and bacterial sequencing library construction is described in ref. 40. In addition, fungal ITS1 regions and the V4 region of the eukaryotic 18S rRNA gene was amplified with the primer sets ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′)/ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′)47,48 and V4_1f (5′-CCAGCASCYGCGGTAATWCC-3′)/TAReukREV3 (5′-ACTTTCGTTCTTGATYRA-3′)49, respectively. The fungal and eukaryotic sequencing libraries were constructed according to previously established protocols29,50. Paired-end sequencing of all samples was performed on an Illumina MiSeq PE 250 platform at Personal Biotechnology Co., Ltd (Shanghai, China). The raw data of the field and greenhouse experiments are available in the NCBI Sequence Read Archive (SRA) under the BioProject PRJNA957983.
The bacterial, fungal and eukaryotic raw sequences were processed and bacterial, fungal and protistan OTU tables were built with the UPARSE pipeline according to previously established protocols29,40,50. In brief, for bacteria and fungi, raw sequences were quality-filtered (sequences with a maximum expected error higher than 0.5 or a length shorter than 200 bp were deleted) and singletons were removed using USEARCH51. After that, the remaining sequences were assigned to OTUs at 97% similarity threshold, and chimeras were removed using UCHIME52. Finally, the representative sequence of each OTU was matched against the RDP bacterial 16S rRNA gene database and the UNITE fungal ITS database using the RDP classifier53,54 to obtain the bacterial and fungal OTU tables, respectively. For protists, eukaryotic raw sequences were quality-filtered (sequences with a maximum expected error higher than 0.5 or a length shorter than 350 bp were deleted) and singletons were removed using USEARCH51. After that, the remaining sequences were assigned to OTUs at 97% similarity threshold, and chimeras were removed using UCHIME52. Finally, the representative sequence of each OTU was matched against the Protist Ribosomal Reference database (PR2)55. After that, we removed OTUs assigned as Rhodophyta, Streptophyta, Metazoa, Fungi, unclassified Opisthokonta and unknown taxa sequences to obtain the protistan OTU table. Based on protistan feeding mode56,57, protistan OTUs were assigned into different functional groups, including predators, parasites, saprotrophs, plant pathogens, and phototrophs.
Isolation and identification of rhizosphere predatory protists and bacteria
Because of the observed links between Colpoda, bacterial key taxa and disease-suppression in our analyses (see Results), we isolated and identified Colpoda from the rhizosphere soil from the field experiment according to previously described protocols58 and selected key bacteria isolated in our previous study40. For Colpoda strains, the supernatant of homogenized rhizosphere soils was cultivated in each well of 96-well plates (Thermo Fisher, Massachusetts, USA) containing sterile Page’s ameba saline59 (120 mg NaCl, 4 mg MgSO4·7H2O, 4 mg CaCl2·2H2O, 142 mg Na2HPO4, and 136 mg KH2PO4 in 1 l of distilled water) buffer (containing inactivated Escherichia coli DH5α as food source of predatory protists) in the dark at 15 °C for 14 days. We performed serial dilutions for the wells to obtain single protist in 96-well plates (Thermo Fisher, Massachusetts, USA), and then selected wells containing pure protistan strains after evaluation with an inverted microscope Nikon Eclipse Ts2 (objective: Nikon CFI Achromat LWD ADL 40X, Ph1, eyepiece: Nikon TS2-W 10X) (NIKON, Tokyo, Japan). The DNA of all pure protistan strains were extracted using the TIANamp Genomic DNA Kit (TIANGEN BIOTECH, Beijing, China) following the manufacturer’s instructions. The 18S rRNA gene of protistan strains were amplified using the primers set RibA (5′-ACCTGGTTGATCCTGCCAGT-3′)/RibB (5′-TGATCCATCTGCAGGTTCACCTAC-3′) according to previously established protocols58. PCR products were sent for Sanger sequencing in Tsing Ke Biotechnology Co., Ltd. (Wuhan, China). The 18S rRNA gene sequences of pure protistan strains were obtained and blasted against the NCBI GenBank database to obtain their taxonomic information.
Greenhouse experiments description
The first greenhouse experiment was performed to detect the effects of Colpoda strains on R. solanacearum suppression in the rhizosphere and contained two parts. The first part was performed to detect direct effects of Colpoda strains on R. solanacearum suppression using sterilized soil. The second part was performed with different inoculation amounts of Colpoda 2 to validate concentration effects of this predatory protist on suppressing R. solanacearum by altering microbial communities in nonsterilized soils. The second greenhouse experiment was performed to validate the potential interactions of key bacterial taxa (found from the second part of the first greenhouse experiment and the field experiment) with Colpoda and R. solanacearum using sterilized soils. Detailed processes and inoculation treatments of the first and second greenhouse experiments are shown as follows.
The first part of the first greenhouse experiment was performed in sterilized soils to detect direct effects of Colpoda strains on Ralstonia solanacearum suppression. Treatments in the first part of the first greenhouse experiment as follows: (1) R. solanacearum, Ralstonia solanacearum QLRs-1115 (1.0 × 104 cells g−1 dry soil) was inoculated, (2) Colpoda 2 + R. solanacearum, Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 104 cells g−1 dry soil) were inoculated, 3) Colpoda 1 + R. solanacearum, Colpoda strain 1 (1.0 × 102 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 104 cells g−1 dry soil) were inoculated. Ralstonia solanacearum was inoculated two days after seedling transplantation. For co-inoculation treatments of different Colpoda strains and Ralstonia solanacearum combinations, Ralstonia solanacearum was inoculated 2 days after seedling transplanting and Colpoda strains were inoculated 2 weeks after inoculation of Ralstonia solanacearum. Rhizosphere samples were collected 3 weeks after the inoculation of protists.
The second part of the first greenhouse experiment was performed with different inoculation concentrations of Colpoda 2 in nonsterilized soils to validate indirect effects of this predatory protist on pathogen suppression by altering microbial communities. Treatments in the second part of the first greenhouse experiment as follows: (1) Control, no microbe was inoculated, (2) Colpoda 2 (102), Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) was inoculated, (3) Colpoda 2 (104), Colpoda strain 2 (1.0 × 104 cells g−1 dry soil) was inoculated. Rhizosphere samples were collected four weeks after the inoculation of protists.
The first part of the second greenhouse experiment was performed in sterilized soils to validate the potential interactions of key bacterial taxa (found from the field experiment and the second part of the first greenhouse experiment) with Colpoda. Treatments in the first part of the second greenhouse experiment as follows: (1) Pseudomonas 1: Pseudomonas strain 1 (1.0 × 104 cells g−1 dry soil) was inoculated, (2) Pseudomonas 1+ Colpoda 2: Pseudomonas strain 1 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, (3) Pseudomonas 2: Pseudomonas strain 2 (1.0 × 104 cells g−1 dry soil) was inoculated, (4) Pseudomonas 2+ Colpoda 2: Pseudomonas strain 2 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, (5) Pseudomonas 3: Pseudomonas strain 3 (1.0 × 104 cells g−1 dry soil) was inoculated, 6) Pseudomonas 3+ Colpoda 2: Pseudomonas strain 3 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, (7) Pseudomonas 4: Pseudomonas strain 4 (1.0 × 104 cells g−1 dry soil) was inoculated, (8) Pseudomonas 4+ Colpoda 2: Pseudomonas strain 4 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 9) Pseudomonas 5: Pseudomonas strain 5 (1.0 × 104 cells g−1 dry soil) was inoculated, 10) Pseudomonas 5+ Colpoda 2: Pseudomonas strain 5 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 11) Lysobacter 1: Lysobacter strain 1 (1.0 × 104 cells g−1 dry soil) was inoculated, 12) Lysobacter 1+ Colpoda 2: Lysobacter strain 1 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 13) Streptomyces 1: Streptomyces strain 1 (1.0 × 104 cells g−1 dry soil) was inoculated, 14) Streptomyces 1+ Colpoda 2: Streptomyces strain 1 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 15) Streptomyces 2: Streptomyces strain 2 (1.0 × 104 cells g−1 dry soil) was inoculated, 16) Streptomyces 2+ Colpoda 2: Streptomyces strain 2 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 17) Arthrobacter 1: Arthrobacter strain 1 (1.0 × 104 cells g−1 dry soil) was inoculated, 18) Arthrobacter 1+ Colpoda 2: Arthrobacter strain 1 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 19) Arthrobacter 2: Arthrobacter strain 2 (1.0 × 104 cells g−1 dry soil) was inoculated, 20) Arthrobacter 2+ Colpoda 2: Arthrobacter strain 2 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 21) Chitinophaga 1: Chitinophaga strain 1 (1.0 × 104 cells g−1 dry soil) was inoculated, 22) Chitinophaga 1+ Colpoda 2: Chitinophaga strain 1 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 23) Chitinophaga 2: Chitinophaga strain 2 (1.0 × 104 cells g−1 dry soil) was inoculated, 24) Chitinophaga 2+ Colpoda 2: Chitinophaga strain 2 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 25) Chitinophaga 3: Chitinophaga strain 3 (1.0 × 104 cells g−1 dry soil) was inoculated, 26) Chitinophaga 3+ Colpoda 2: Chitinophaga strain 3 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) were inoculated, 27) Chitinophaga 4: Chitinophaga strain 4 (1.0 × 104 cells g-1 dry soil) was inoculated, 28) Chitinophaga 4+ Colpoda 2: Chitinophaga strain 4 (1.0 × 104 cells g−1 dry soil) and Colpoda strain 2 (1.0 × 102 cells g−1 dry soil) in were inoculated. Different bacteria were inoculated two days after seedling transplanting. For co-inoculation treatments of different Colpoda strains and bacteria combinations, different bacteria were inoculated two days after seedling transplanting and Colpoda strains were inoculated two weeks after inoculation of bacteria. Rhizosphere samples were collected three weeks after the inoculation of protists.
The the second part of second greenhouse experiment was performed in sterilized soils to validate the potential interactions of key bacterial taxa (found from the field experiment and the second part of the first greenhouse experiment) with Ralstonia solanacearum. Treatments in the second part of the second greenhouse experiment as follows: 1) R. solanacearum: Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) was inoculated, 2) Pseudomonas 1 + R. solanacearum: Pseudomonas strain 1 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 3) Pseudomonas 2 + R.solanacearum: Pseudomonas strain 2 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) was inoculated, 4) Pseudomonas 3 + R. solanacearum: Pseudomonas strain 3 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 5) Pseudomonas 4 + R. solanacearum: Pseudomonas strain 4 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 6) Pseudomonas 5 + R. solanacearum: Pseudomonas strain 5 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 7) Lysobacter 1 + R. solanacearum: Lysobacter strain 1 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 8) Streptomyces 1 + R. solanacearum: Streptomyces strain 1 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 9) Streptomyces 2 + R. solanacearum: Streptomyces strain 2 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 10) Arthrobacter 1 + R. solanacearum: Arthrobacter strain 1 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 11) Arthrobacter 2 + R. solanacearum: Arthrobacter strain 2 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 12) Chitinophaga 1 + R. solanacearum: Chitinophaga strain 1 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 13) Chitinophaga 2 + R. solanacearum: Chitinophaga strain 2 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 14) Chitinophaga 3 + R. solanacearum: Chitinophaga strain 3 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated, 15) Chitinophaga 4 + R. solanacearum: Chitinophaga strain 4 (1.0 × 105 cells g−1 dry soil) and Ralstonia solanacearum QLRs-1115 (1.0 × 105 cells g−1 dry soil) were inoculated. Two days after seedling transplanting, different bacteria were inoculated and Ralstonia solanacearum was inoculated 2 weeks after inoculation of different bacteria. Rhizosphere samples were collected 3 weeks after the inoculation of Ralstonia solanacearum.
Soils of the greenhouse experiments were collected from the fields treated with conventional fertilization. All soils were taken back to the laboratory and air-dried in the shade. After that, soils were passed through a 2-mm sieve to ensure soil homogenization in subsequent greenhouse experiments. Soils were sterilizated by Co75 γ-ray irradiation (65 kGy) at Xiyue Technology Co., Ltd (Nanjing, China). Each of the six replicates contained 12 sterilized tomato seedlings (cultivar “Hong ai sheng”) and each seedling was planted in a black polypropylene pot containing 300 g dry soil. All pots were cultivated in a greenhouse (daytime: 16 h and average 30 °C, night: 8 h and average 25 °C) at the Nanjing Agriculture University. Pots were watered with deionized water every 2 days and with ½ strength Hoagland nutrient solution (Hopebio, Qingdao, China) every 10 days. The pots were weekly randomized throughout the experiments. Rhizosphere soil samples were collected at the end of each greenhouse experiment according to the method as described above. DNA extractions, quantitative PCR assays and Illumina MiSeq sequencing of rhizosphere soil samples, bioinformatic analyses of data and disease incidence determination were according to methods described above.
Statistical analyses
We used nonparametric Shannon indexes (calculated by MOTHUR)60,61 to estimate bacterial, fungal and protistan α-diversities. We used principal coordinate analysis (PCoA) based on the Bray–Curtis distance to compare the differences of bacterial, fungal and protistan community compositions at the OTU level in R (R version 4.0.1). We used permutational multivariate analysis of variance (PERMANOVA)62 to assess the effects of different fertilization regimes on the community compositions of rhizosphere microorganisms by the adonis function with 999 permutations using R package “vegan”63 (R version 4.0.1). We used one-way ANOVA to assess effects of different fertilization regimes on the α-diversities of rhizosphere microorganisms using SPSS v20.0 (SPSS Inc. USA). ANOSIM (analysis of similarities, calculated by MOTHUR)61,64 was performed to investigate significant differences of bacterial community composition between different treatments in the greenhouse experiments. We selected bacterial, fungal and protistan α-diversities (Shannon index) and community compositions (PCoA1) as main microbial predictors to calculate the significance of effects of these predictors on disease incidence using multiple regression by linear models in R (R version 4.0.1). The explanatory power and significance of each microbial predictor was assessed using R package “relaimpo”65 (R version 4.0.1). We used Spearman’s correlation coefficient to evaluate correlations between abundant predatory protistan OTUs (average relative abundance >0.1%) and disease incidence, key predatory protistan OTUs (linked with disease incidence) and abundant bacterial OTUs (average relative abundance >0.1%) in the field experiment, and abundant bacterial OTUs (average relative abundance >0.1%) and the density of R. solanacearum in the greenhouse experiments, respectively. The correlation coefficients and p values of Spearman’s correlations were calculated through the “corr.test” function using R package “psych”66 (R version 4.0.1). We used heatmaps to show Spearman’s correlations between key predatory protistan OTUs (linked with disease incidence) and abundant bacterial OTUs (average relative abundance >0.1%) using R package “pheatmap”67 (R version 4.0.1). Mantel test was conducted to evaluate the correlations between rhizosphere bacterial and fungal communities and the relative abundances of rhizosphere predatory protists using R package “vegan”63 (R version 4.0.1). One-way ANOVA with Tukey’s HSD test was used for multiple comparisons and student’s t-test was performed to compare significances of the difference between distinct treatments in SPSS v22.0 (SPSS Inc. USA). For pathogen-suppressive microorganisms and pathogen-promotive microorganisms, putative pathogen-suppressive microorganisms were defined as those with relative abundances that negatively correlated with the density of R. solanacearum (Spearman’s correlation: p < 0.05), and putative pathogen-promotive microorganisms were defined as those with relative abundances that positively correlated with the density of R. solanacearum (Spearman’s correlation: p < 0.05). The formula for the ratio of pathogen-suppressive microorganisms to pathogen-promotive microorganisms was SUM-PPS/SUM-PPP, where SUM-PPS is the sum of the relative abundances of putative pathogen-suppressive microorganisms and SUM-PPP is the sum of the relative abundances of putative pathogen-promotive microorganisms.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data used in this study are available in the NCBI Sequence Read Archive (SRA) under accession code BioProject PRJNA957983. Source data are provided with this paper.
Code availability
All codes used in this study are available on GitHub (https://github.com/SaiGuo92/Code-for-paper1) and Zendo68.
References
Teng, P. S., Shane, W. W. & MacKenzie, D. R. Crop losses due to plant pathogens. Crit. Rev. Plant Sci. 2, 21–47 (1984).
Savary, S., Ficke, A., Aubertot, J.-N. & Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 4, 519–537 (2012).
Mendes, R., Garbeva, P. & Raaijmakers, J. M. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37, 634–663 (2013).
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T. & Singh, B. K. Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621 (2020).
Richardson, A. E., Barea, J.-M., McNeill, A. M. & Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321, 305–339 (2009).
Semenov, M. V., Krasnov, G. S., Semenov, V. M. & van Bruggen, A. Mineral and organic fertilizers distinctly affect fungal communities in the crop rhizosphere. J. Fungi 8, 251 (2022).
Berendsen, R. L., Pieterse, C. M. J. & Bakker, P. A. H. M. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486 (2012).
Liu, H. et al. Continuous application of different organic additives can suppress tomato disease by inducing the healthy rhizospheric microbiota through alterations to the bulk soil microflora. Plant Soil 423, 229–240 (2018).
Flood, J. The importance of plant health to food security. Food Secur. 2, 215–231 (2010).
Tilman, D. et al. Forecasting agriculturally driven global environmental change. Science 292, 281–284 (2001).
Scotti, R., Bonanomi, G., Scelza, R., Zoina, A. & Rao, M. A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 15, 333–352 (2015).
Maeder, P. et al. Soil fertility and biodiversity in organic farming. Science 296, 1694–1697 (2002).
Schmidt, J. E., Kent, A. D., Brisson, V. L. & Gaudin, A. C. M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 7, 146 (2019).
Reganold, J. P. & Wachter, J. M. Organic agriculture in the twenty-first century. Nat. Plants 2, 1–8 (2016).
Geisen, S. et al. Soil protists: a fertile frontier in soil biology research. FEMS Microbiol. Rev. 42, 293–323 (2018).
Gao, Z., Karlsson, I., Geisen, S., Kowalchuk, G. & Jousset, A. Protists: puppet masters of the rhizosphere microbiome. Trends Plant Sci. 24, 165–176 (2019).
Jousset, A. & Bonkowski, M. The model predator Acanthamoeba castellanii induces the production of 2, 4, DAPG by the biocontrol strain Pseudomonas fluorescens Q2-87. Soil Biol. Biochem. 42, 1647–1649 (2010).
Jousset, A. et al. Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J. 3, 666–674 (2009).
Oliverio, A. M. et al. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 6, eaax8787 (2020).
Hu, Z. et al. Precipitation changes, warming, and N input differentially affect microbial predators in an alpine meadow: evidence from soil phagotrophic protists. Soil Biol. Biochem. 165, 108521 (2022).
Guo, S. et al. Protists as main indicators and determinants of plant performance. Microbiome 9, 64 (2021).
Guo, S. et al. Trophic interactions between predatory protists and pathogen-suppressive bacteria impact plant health. ISME J. 16, 1932–1943 (2022).
Chandarana, K. A. & Amaresan, N. Soil protists: an untapped microbial resource of agriculture and environmental importance. Pedosphere 32, 184–197 (2022).
Geisen, S. et al. Soil protistology rebooted: 30 fundamental questions to start with. Soil Biol. Biochem. 111, 94–103 (2017).
Liu, J. et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404, 115287 (2021).
Puzniak, O., Hrynchyshyn, N., Datsko, T., Andruszczak, S. & Hulko, B. Consequences of the long-term fertilization system use on physical and microbiological soil status in the western polissia of Ukraine. Agriculture 12, 1955 (2022).
Foissner, W. Protozoa as bioindicators in agroecosystems, with emphasis on farming practices, biocides, and biodiversity. Agric. Ecosyst. Environ. 62, 93–103 (1997).
Payne, R. J. Seven reasons why protists make useful bioindicators. Acta Protozool. 52, 105–113 (2013).
Xiong, W. et al. Rhizosphere protists are key determinants of plant health. Microbiome 8, 27 (2020).
Long, J. J. et al. Interactions of free-living amoebae with the rice fungal pathogen, Rhizoctonia solani. BMC Res. Notes 12, 746 (2019).
Foissner, W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Eur. J. Protistol. 34, 195–235 (1998).
Bamforth, S. S. Protozoa: recyclers and indicators of agroecosystem quality. In Fauna in Soil Ecosystems (CRC Press, 1997).
Zhang, W., Lin, Q., Li, G. & Zhao, X. The ciliate protozoan Colpoda cucullus can improve maize growth by transporting soil phosphates. J. Integr. Agric. 21, 855–861 (2022).
Martínez-Reyes, C. M., Rodríguez-Zaragoza, S., Cabirol, N., Alarcón, A. & Mendoza-López, M. R. Effect of predation by Colpoda sp. in nitrogen fixation rate of two free-living bacteria. Microb. Ecol. 83, 1026–1035 (2022).
Li, S. et al. An antibiotic complex from Lysobacter enzymogenes strain C3: antimicrobial activity and role in plant disease control. Phytopathology 98, 695–701 (2008).
Tyc, Olaf, Song, C., Dickschat, J. S., Vos, M. & Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 25, 280–292 (2017).
Li, M. et al. Indirect reduction of Ralstonia solanacearum via pathogen helper inhibition. ISME J. 16, 868–875 (2022).
Jousset, A. Ecological and evolutive implications of bacterial defences against predators. Environ. Microbiol. 14, 1830–1843 (2012).
Bauer, M. A., Kainz, K., Carmona-Gutierrez, D. & Madeo, F. Microbial wars: competition in ecological niches and within the microbiome. Microb. Cell 5, 215 (2018).
Deng, X. et al. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities. N. Phytol. 235, 1558–1574 (2022).
Schönfeld, J., Heuer, H., van Elsas, J. D. & Smalla, K. Specific and sensitive detection of Ralstonia solanacearum in soil on the basis of PCR amplification of fliC fragments. Appl. Environ. Microbiol. 69, 7248–7256 (2003).
Tao, C. et al. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 8, 137 (2020).
Hu, Y. Detection Methods for the Genus Lysobacter and the Species Lysobacter Enzymogenes. (University of Nebraska - Lincoln, 2010).
Li, C., Tian, Q., Rahman, M. K. U. & Wu, F. Effect of anti-fungal compound phytosphingosine in wheat root exudates on the rhizosphere soil microbial community of watermelon. Plant Soil 456, 223–240 (2020).
Ruiz, O. N. et al. Metagenomic characterization reveals complex association of soil hydrocarbon-degrading bacteria. Int. Biodeterior. Biodegrad. 157, 105161 (2021).
Hayes, A. C., Liss, S. N. & Allen, D. G. Growth kinetics of Hyphomicrobium and Thiobacillus spp. in mixed cultures degrading dimethyl sulfide and methanol. Appl. Environ. Microbiol. 76, 5423–5431 (2010).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols (eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J.) 315–322 (Academic Press, 1990).
Gardes, M. & Bruns, T. D. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118 (1993).
Bass, D. et al. Coprophilic amoebae and flagellates, including Guttulinopsis, Rosculus and Helkesimastix, characterise a divergent and diverse rhizarian radiation and contribute to a large diversity of faecal-associated protists. Environ. Microbiol. 18, 1604–1619 (2016).
Xiong, W. et al. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 107, 198–207 (2017).
Edgar, R. Usearch. (2010).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264 (2019).
Guillou, L. et al. The Protist Ribosomal Reference database (PR2): a catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 41, D597–D604 (2013).
Dumack, K., Fiore‐Donno, A. M., Bass, D. & Bonkowski, M. Making sense of environmental sequencing data: ecologically important functional traits of the protistan groups Cercozoa and Endomyxa (Rhizaria). Mol. Ecol. Resour. 20, 398–403 (2020).
Xiong, W. et al. A global overview of the trophic structure within microbiomes across ecosystems. Environ. Int. 151, 106438 (2021).
Gao, Z., Jousset, A., Kowalchuk, G. A. & Geisen, S. Five groups in the genus Allovahlkampfia and the description of the new species Vahlkampfia bulbosis n.sp. Protist 173, 125870 (2022).
Thomas, V., Herrera-Rimann, K., Blanc, D. S. & Greub, G. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72, 2428–2438 (2006).
Chao, A. & Shen, T.-J. Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 10, 429–443 (2003).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Anderson, M. J. Permutational multivariate analysis of variance (PERMANOVA). In: Wiley StatsRef: Statistics Reference Online 1–15 (American Cancer Society, 2017).
Oksanen, J. et al. Package ‘vegan’. R. Package 2, 1–295 (2013).
Clarke, K. R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18, 117–143 (1993).
Grömping, U. Relative importance for linear regression in R: the package relaimpo. J. Stat. Softw. 17, 1–27 (2006).
Revelle, W. & Revelle, M. W. Package ‘psych’. R. Package 337, 338 (2015).
Kolde, R. & Kolde, M. R. Package ‘pheatmap’. R Package 1, 790 (2018).
Guo, S. et al. Code-for-paper1. Zenodo. https://doi.org/10.5281/zenodo.10489177 (2024).
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (KYT2023001 (to R.L.), QTPY2023003 (to R.L.), XUEKEN2023031 (to H.L.) and XUEKEN2023039 (to Q.S.)), the National Natural Science Foundation of China (42277294 (to R.L.), 42307171 (to S.G.) and 42377294 (to X.D.)), the Postdoctoral Science Foundation of China (2023M731724) (to S.G.), the Excellent Postdoctoral Program of Jiangsu Province, China (2023ZB706) (to S.G.), the Guidance Foundation, the Hainan Institute of Nanjing Agricultural University (NAUSY-MS10) (to R.L.),the Achievement Transformation Fund project of Hainan Research Institute of Nanjing Agricultural University (NAUSY-CG-ZD-01) (to R.L.) and the Natural Science Foundation of Jiangsu Province, China (BK20210390) (to C.T.).
Author information
Authors and Affiliations
Contributions
S.G., R.L., Q.S., G.A.K. and S.G. developed the ideas and designed the experimental plans. S.G., Z.J., Z.Y., X.Y., X.D., C.T. and H.L. performed the experiments. S.G., W.X., R.L. and S.G. analyzed the data. S.G., R.L., Q.S., G.A.K. and S.G. participated in the completion of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yang Bai, Hang-Wei Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, S., Jiao, Z., Yan, Z. et al. Predatory protists reduce bacteria wilt disease incidence in tomato plants. Nat Commun 15, 829 (2024). https://doi.org/10.1038/s41467-024-45150-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45150-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.