Abstract
Exploring new near-room-temperature thermoelectric materials is significant for replacing current high-cost Bi2Te3. This study highlights the potential of Ag2Se for wearable thermoelectric electronics, addressing the trade-off between performance and flexibility. A record-high ZT of 1.27 at 363 K is achieved in Ag2Se-based thin films with 3.2 at.% Te doping on Se sites, realized by a new concept of doping-induced orientation engineering. We reveal that Te-doping enhances film uniformity and (00l)-orientation and in turn carrier mobility by reducing the (00l) formation energy, confirmed by solid computational and experimental evidence. The doping simultaneously widens the bandgap, resulting in improved Seebeck coefficients and high power factors, and introduces TeSe point defects to effectively reduce the lattice thermal conductivity. A protective organic-polymer-based composite layer enhances film flexibility, and a rationally designed flexible thermoelectric device achieves an output power density of 1.5 mW cm−2 for wearable power generation under a 20 K temperature difference.
Similar content being viewed by others
Introduction
With the increasing popularity of smart wearable electronic devices such as wireless headphones, smart glasses, and smartwatches, traditional battery power sources have become inadequate to meet the demand for long-term use1. There is an urgent need in the market to develop sustainable power supply technology. Among the various emerging power generation methods, flexible thermoelectric devices (F-TEDs) that can directly convert temperature differences between the human body and the external environment into electricity, have gained significant attention1. F-TEDs are easy to integrate into wearables, have no mechanical vibrations, and can provide sustainable power without the need for maintenance, making them an ideal self-powered technology2. F-TEDs are composed of pairs of p- and n-type thermoelectric materials connected in electrical series and thermal parallel3. To improve the energy conversion efficiency of F-TEDs, both p- and n-type materials require high thermoelectric performance2. This thermoelectric performance can be determined by the figure-of-merit ZT = S2σT/κ = S2σT/(κe + κl), in which S2σ is the power factor of the material, determined by the Seebeck coefficient S and electrical conductivity σ, κ represents the total thermal conductivity of the material, determined by electronic thermal conductivity κe and lattice thermal conductivity κl, and T represents the absolute temperature4. Generally, S, σ, and κe are strongly correlated with the carrier concentration n of the material, so optimizing ZT can be achieved by adjusting n through rational doping or alloying methods5. Oppositely, κl is weakly correlated with n but can be further reduced by introducing additional crystal and lattice defects to scatter heat-carrying phonons with different wavelengths. However, these defects may simultaneously scatter charge carriers, reducing the carrier mobility μ of the material and in turn, σ. Therefore, optimizing the performance of thermoelectric materials has always been a challenging research task.
As key components in F-TEDs, thermoelectric materials must simultaneously possess high performance and certain flexibility. Compared with inorganic materials, organic conducting polymers and organic/inorganic composites show relatively low ZT values although they usually have higher flexibility due to the nature of molecular chains as matrix3. Therefore, current research mostly focuses on making high-performance inorganic materials flexible6. For example, the designs of flexible inorganic thin films, aim to enhance their flexibility while maintaining their high performance6. Since F-TEDs are typically used in wearable scenarios, the materials inside the devices need to have high ZTs near room temperatures. Thus, materials meeting this criterion are generally traditional Bi2Te3-based thermoelectric thin films, known for their high thermoelectric performance near room temperatures, such as Ag-doped Bi2Te3 highly oriented thin films reported to have a ZT as high as 1.26. However, Bi2Te3 has relatively poor flexibility due to its crystal structure, and the low natural abundance of tellurium (Te) makes it relatively expensive, leading to cost inefficiency. Therefore, the development of alternative inorganic thermoelectric thin films is of paramount importance.
N-type silver selenide (Ag2Se) exhibits characteristics of an “electronic crystal and phonon liquid,” with intrinsic high σ and low κ at near-room temperatures7. Additionally, it is abundant in Earth’s crust, environmentally friendly, and represents an ideal alternative to Bi2Te38. Ag2Se possesses a stable orthorhombic structure at lower temperatures, and is considered a typical narrow-bandgap semiconductor with bandgap widths ranging from 0.02 eV to 0.22 eV7,8. Ag2Se transforms from orthorhombic phase to cubic phase above 130 °C, and most research focuses on the near-room-temperature phase of Ag2Se8. Besides, Ag2Se exhibits a certain ductility due to its crystal structure3. To enhance its practical utility, improvements in both thermoelectric performance and flexibility are required for Ag2Se-based thin films. To date, many efforts have been made to achieve these goals, such as tuning the ratio of Ag and Se9, doping with elements Cu10, S11, and Ga12, alloying with other elements (e.g., Se13 and Ag14) and compounds (e.g., CuAgSe14), and hybridizing with carbons (e.g., single-walled carbon nanotubes (SWCNTs)15) and conducting polymers (e.g., polyvinylpyrrolidone (PVP)16, polypyrrole (PPy)13, poly(3,4-ethylenedioxythiophene) (PEDOT)17 and polyvinylidene fluoride (PVDF)18). Benefiting from these novel strategies, high ZTs of ~1 have been achieved. To enhance the flexibility of the thin film, flexible substrates or supporters are usually needed, such as polyimide (PI)9,10,19,20, Nylon12,13,14,15,16,17,21,22,23,24,25, scaffold26, flexible glass18,27,28,29, polyethylene terephthalate (PET)11, and paper30. However, further enhancing its thermoelectric performance and flexibility remains an ongoing goal.
In addition to conventional doping- and alloying-based compositional optimizations, designing highly orientated polycrystalline thin films is one of the effective methods to improve their overall thermoelectric performance6,31. Both theoretical and experimental results showed that (00l)-textured film can lead to a higher S than that of the (013)-textured Ag2Se-based film, contributing to an improved S2σ, which can be achieved by tuning the ratio of Ag and Se20. However, some other studies argue that films with the (00l)-preferred orientation exhibit considerably lower electrical transport performance compared to that with the (013)-preferred orientation21. This is mainly due to the fact that the σ in the (00l) orientation does not reach the same high levels as in the (013) orientation8. Therefore, there is still some controversy regarding the adjustment of film orientation to enhance thermoelectric performance. Nevertheless, it is undeniable that optimizing n and improving μ, whether it be to enhance σ or increase the S, remain focal points. As for the methods to achieve this orientation, there is currently no definitive consensus. One of the theories that can be considered is based on the Gibbs-Wulff crystal growth law32, suggesting that the introduction of micro-strain due to doping and the substitution of Se positions may result in lower free energy on the (00l) plane, leading to faster growth rates. Consequently, the controlled concentration of element doping can lead to changes in overall orientation.
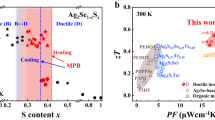
Based on this theory, in this work, we undertake theoretical calculations to confirm that Te-doping can reduce the formation energy of the (00l) plane, resulting in a strong (00l) orientation across the film to enhance its uniformity. Figure 1a, b show unit cells of Ag2Se before and after Te-doping viewed along the c- and a-axis, respectively. Based on the computational results by spin-polarized density functional theory (DFT), the (001) surface energies of pristine and Te-doped Ag2Se are calculated to be 0.004 and 0.002 eV/Å2, respectively, indicating that the introduction of Te on Se sites is beneficial for the formation of the (001) surface of Ag2Se. Other computational method based on the commercial software package Material Studio shows the same results (see Supplementary Fig. 1). Generally, the uniform thin-film structure usually results in high σ. To verify the as-calculated results, we fabricate Ag2Se thin films without and with Te doping by a vacuum thermal co-evaporation method, as illustrated by Fig. 1c, d, respectively. Here PI are used as flexible substrates to support the as-deposited thin films. The transmission electron microscopy (TEM) image in Fig. 1c indicates a typical polycrystalline feature of pristine Ag2Se thin film with anisotropic grains, while the TEM image in Fig. 1d exhibits a highly (00l)-orientated feature of Te-doped Ag2Se thin film. Figure 1e compares the S2σ and ZT values between our Te-doped Ag2Se thin film and reported works9,13,14,17,19,21,22,23,27,28,30,33,34,35, indicating that our as-achieved thermoelectric performance ranks as top values. Such high performance is derived from the Te-doping-induced suppression of n, which significantly improves the S. Also, Te-doping results in a strong (00l) orientation within the film to enhance its μ, which helps keep a high σ and in turn a high S2σ. Additionally, Te-doping-induced TeSe point defects effectively scatter phonons, effectively reducing κ. To further improve the flexibility of the as-fabricated thin film, we designed a polymer-hybrid-based coating composed of 90% poly(vinyl laurate) and 10% N-methylpyrrolidone on the thin film surface. Figure 1f compares measured increased normalized resistance ΔR/R0 of Te-doped Ag2Se thin films with and without coating as a function of bending radius. The insets show the illustration of the spin coating process (left) and the photo of testing the flexibility of the as-fabricated thin film (right). After coating the as-designed polymer-hybrid-based protect layer, the flexibility is significantly improved. Finally, we assemble an F-TED using a pair of p-type Sb2Te3 and n-type Ag2Se films, realizing an open-circuit voltage of 6 mV, an output power of 65 nW, and a power density of 1.5 mW cm−2 under a temperature difference ΔT of 20 K. The as-designed thin film and device demonstrate high potential for application in wearable power generation.
Unit cells of Ag2Se before and after Te-doping viewed along the (a) c- and (b) a-axis. Illustrations of fabricating Ag2Se thin films (c) without and (d) with Te-doping by a vacuum thermal co-evaporation method. The transmission electron microscopy (TEM) image in c indicates the polycrystalline feature of pristine Ag2Se thin film, and the TEM image in (d) indicates the highly (00l)-orientated feature of Te-doped Ag2Se thin film. e Comparison of power factor S2σ and dimensionless figure-of-merit ZT values between this work and reported works including printed Ag-Se-based thin film27, Ag2Se film on nylon membrane21, poly(3,4-ethylenedioxythiophene) (PEDOT)/Ag2Se/CuAgSe composite film17, printed β-Ag2Se33, Ag/Ag2Se composite film23, Ag2Se film on porous nylon membrane22, Ag1.8Se film9, Ag2.06Se film34, Ag2Se/Ag/CuAgSe composite film14, Ag/ Ag2Se composite film35, Ag-rich Ag2Se film30, Ag2Se/Se/polypyrrole (PPy) composite film13, Ag2Se film prepared by pulsed hybrid reactive magnetron sputtering (PHRMS)28, and microstructurally tailored β-Ag2Se thin film19 (from left to right). f Measured increased normalized resistance ΔR/R0 of Te-doped Ag2Se thin films with and without coating as a function of bending radius. The coating is composed of 90% poly(vinyl laurate) and 10% N-methylpyrrolidone. The insets show the illustration of the spin coating process (left) and the photo of testing the flexibility of the as-fabricated thin film (right).
Results and discussion
To determine the optimal Te doping concentration for achieving the best thermoelectric performance in Ag2Se thin films, we chose various ratios of elemental powders as raw materials, defining a series of nominal compositions Ag2SeTeδ (δ = 0, 0.02, 0.04, 0.06, 0.08, 0.10, and 0.12), during the thin-film preparation process. Table 1 compares nominal and actual compositions of as-fabricated Te-doped Ag2Se thin films, in which the actual compositions of the thin films were obtained from the energy dispersive spectroscopy (EDS) results. It is evident that the actual Te contents in the films do not perfectly match the nominal compositions. This discrepancy arises because during the deposition process, a portion of the Te can be evacuated, and both Se and Te can volatilize from the surface during the heat treatment process. Consequently, the final actual compositions differ somewhat from the nominal compositions. Fortunately, the actual Te contents in the films were also increased with increasing the nominal compositions of Te contents. This suggests that Te-doping can be successfully achieved, and no doping limit has been observed thus far. This indicates that under low-concentration Te-doping conditions, Te-doping has a high success rate. To better analyze the impact of Te doping on the thermoelectric performance of the films, we used the actual atomic concentration of Te as a variable, denoted as x (at.%) to assess the influence of different Te doping concentrations on thermoelectric properties.
To understand the phase information of as-fabricated Te-doped Ag2Se thin films, X-ray diffraction (XRD) was investigated. Figure 2a compares XRD patterns of Ag2Se thin films with different Te concentrations x (x = 0, 0.7, 1.3, 1.9, 2.6, 3.2, and 3.8 at.%) with 2θ ranges from 20° to 55°. All diffraction peaks perfectly match the room-temperature phase of Ag2Se, and there is no presence of impurity phases within the precision of the XRD examination. Noticeably, the pristine Ag2Se thin film without Te-doping exhibits a typical polycrystalline feature with anisotropic grains since many different diffraction peaks were observed. Oppositely, the Ag2Se thin film with Te-doping exhibits a typical highly orientated feature with isotropic grains since only (00l) peaks ((002) and (004) peaks) were observed. Figure 2b compares determined orientation factors F(00l) of the films. The calculation methods of F(00l) can be referred to the experimental details. After Te-doping, the F(00l) values were significantly enhanced to >0.9, indicating improvements in the overall orientation of the thin films. Figure 2c, d compare the pole files of Ag2Se thin films with 3.2 at.% Te along the (002) and (013) directions, respectively. The intensities belonging to the (00 l) become more significant, double-confirming the highly (00l)-oriented feature of the thin film.
a X-ray diffraction (XRD) patterns of Ag2Se thin films with different Te concentrations x (x = 0, 0.7, 1.3, 1.9, 2.6, 3.2, and 3.8 at.%) with 2θ ranges from 20° to 55°. b Determined orientation factors F(00l) of the films. Pole files of Ag2Se thin films with 3.2 at.% Te along the (c) (002) and (d) (013) directions. e Scanning electron microscopy (SEM) and corresponding electron back-scattered diffraction (EBSD) images of Ag2Se thin films with 3.2 at.% Te from the top view. The orientation information is provided for reference. f Energy dispersive spectroscopy (EDS) maps of Ag2Se thin films with 3.2 at.% Te for overlap and individual Ag, Se, and Te elements.
To understand the structural and compositional information of as-fabricated Te-doped Ag2Se thin films, scanning electron microscopy (SEM), electron back-scattered diffraction (EBSD), and EDS analysis were investigated. Figure 2e exhibits SEM and EBSD images of Ag2Se thin films with 3.2 at.% Te from the top view. The orientation information is provided for reference. As can be seen, the as-fabricated thin film with Te-doping is relatively smooth and dense without significant pores, indicating a high thin-film quality. The EBSD results indicate that the as-fabricated thin film possesses significant [001] orientation, confirming the highly oriented feature. Figure 2f exhibits EDS maps of Ag2Se thin films with 3.2 at.% Te for overlap and individual Ag, Se, and Te elements. All elements are uniformly distributed without obvious composition segregation, indicating that the doping is uniform at a microscale. The TEM results of pristine Ag2Se thin film are provided in Supplementary Fig. 2, which shows a typical polycrystalline feature with anisotropic grains, and the SEM and corresponding EDS results of Ag2Se thin films doped with different concentrations of Te (excluding 3.2 at.% Te) can be referred to Supplementary Figs. 3–7 for reference.
To understand the micro/nanostructural and compositional information of as-fabricated Te-doped Ag2Se thin films, aberration-corrected scanning TEM (Cs-STEM) characterizations were investigated. Figure 3a shows a TEM high-angle annular dark field (HAADF) image of the Ag2Se thin film with 3.2 at.% Te from a cross-sectional view. The sample was prepared by a focused ion beam (FIB) technique. The Te-doped Ag2Se thin film exhibits a good quality with no apparent pore or noticeable impurity phase from a microscale. The thickness of the as-fabricated thin film is relatively uniform. In comparison to the undoped sample (Supplementary Fig. 2), their grain orientation is more uniform. Figure 3b shows EDS maps for overlap and individual Ag, Se, and Te elements, respectively, taken from Fig. 3a. All elements are uniformly distributed, and there is no noticeable element segregation. Figure 3c shows an EDS line scan taken from Fig. 3a. Along the thickness direction of the film, there is no variation in element distribution, further confirming that doping is exceptionally uniform. Figure 3d shows a Cs-STEM HAADF image taken from Fig. 3a. Even in the area close to the flexible substrate (PI), the as-prepared film maintains excellent crystallinity and orientation with no noticeable defects in this region. Furthermore, there is no apparent crack between the film and the substrate, indicating a strong adhesion between them. Figure 3e shows a magnified Cs-STEM HAADF image taken from Fig. 3d. The inset shows the corresponding fast Fourier transform (FFT) pattern with indexed information, indicating that the viewed direction is \(\left[\bar{2}\bar{1}0\right]\). The indexed high-resolution FFT pattern can also be referred to Supplementary Fig. 8. As can be seen, the microregion of the film exhibits excellent orientation and crystallinity. Figure 3f shows corresponding strain maps along different directions. Interestingly, the strain maps reveal a more pronounced strain along the y-direction, indicating that strains primarily affect the y-direction. This is likely attributed to Te substitutional doping. Figure 3g shows a high-resolution Cs-STEM HAADF image taken from Fig. 3e. The inset shows the crystal structure of Te-doped Ag2Se for reference. Figure 3h shows a filtered Cs-STEM HAADF image, showing the contrast difference. The arrow indicates a potential point defect of TeSe since it exhibits a higher contrast difference. Figure 3i also shows a line profile taken from Fig. 3g, confirming the potential point defect of TeSe. The EDS maps taken from such a high magnification also confirm the potential point defect of TeSe, and indicate that these point defects may be not uniformly distributed at a nanoscale (Supplementary Fig. 9) because the vacuum thermal co-evaporation method may not concisely dope Te on Se sites at an atomic level, which is understandable.
a TEM high-angle annular dark field (HAADF) image of the thin-film sample from a cross-sectional view. The sample was prepared by a focused ion beam (FIB) technique. b EDS maps for overlap and individual Ag, Se, and Te elements taken from (a). c EDS line scan taken from (a). d Spherical aberration-corrected scanning TEM (Cs-STEM) HAADF image taken from (a). e Magnified Cs-STEM HAADF image taken from (d). The inset shows the corresponding fast Fourier transform (FFT) pattern with indexed information. f Corresponding strain maps along different directions. g High-resolution Cs-STEM HAADF image taken from (e). The inset shows the crystal structure of Te-doped Ag2Se for reference. h Filtered Cs-STEM HAADF image to show the contrast difference. The arrow indicates a potential point defect of TeSe. i Line profile taken from (g) to show the potential point defect of TeSe.
In terms of the effect of Te-doping on the thermoelectric performance of Ag2Se thin films, we evaluated the thermoelectric properties of Ag2Se thin films with different Te concentrations x (x = 0, 0.7, 1.3, 1.9, 2.6, 3.2, and 3.8 at.%). Figure 4a-c show temperature-dependent σ, S, and determined S2σ. Generally, with increasing the x value, σ is decreased and S is improved, leading to an optimized S2σ of 24.8 μW cm−1 K−2 at 363 K when 3.2 at.% Te was doped into the thin film. To understand the variations of σ, S, and S2σ, Fig. 4d compares measured x-dependent n and μ at room temperature. By increasing the x value, the n suppresses but the μ enhances. The improvement in μ benefits from the more uniform structures of the thin films with highly (00l)-orientations, which help maintain high σ values even though n is decreased. To explain the decreased n, we perform first-principles DFT calculations. Figure 5a, b compare calculated band structures of Ag2Se (Ag24Se12) and Te-doped Ag2Se (Ag24Se11Te1), respectively, indicating that Ag2Se is a typical narrow-gap semiconductor, despite doping with Te or not. Interestingly, after doping Te on Se sites, the bandgap was slightly increased from 0.061 eV to 0.078 eV, explaining the improved S and reduced n. Figure 4e compares calculated x-dependent effective mass m* and deformation potential Edef at room temperature by a single parabolic band (SPB) model. With increasing the x value, the m* drops but the Edef enhances. The decrease in m* comes from the suppression of n since there is no obvious secondary phase to provide a phase boundary that can trigger the energy filtering effect36. This also confirms that the increased S does not come from the energy filtering at interfaces. In terms of the Edef, its improvement indicates that the deformation capability of the lattice becomes more difficult due to the Te doping. We also compare calculated and experimental S2σ as a function of n (Supplementary Fig. 10). The calculated data are from the SPB model. As observed, the n values are higher than those reported for Ag2Se single crystals37, and they can be progressively optimized by increasing the Te doping concentration to achieve peak S2σ in the thin film. Figure 4f shows measured temperature-dependent κ for Ag2Se thin films with different Te concentrations x (x = 0, 0.7, 1.3, 1.9, 2.6, 3.2, and 3.8 at.%). With increasing the x value, κ is decreased, indicating that Te-doping can help suppress the overall thermal transport of the thin film. Figure 4g compares x-dependent electronic and lattice thermal conductivities (κe and κl) at room temperature. The κe data are from the formula κe = LσT3, where L is the Lorenz number (Supplementary Fig. 11), and κl = κ – κe. Generally, with increasing the x value, both the κl and the κe decrease. The decrease in κe comes from the suppression of σ, while the dropped κl is derived from the increased intensity of TeSe point defects that effectively scatter phonons. It should be noted that when the atomic content of Te is 3.2 at.%, the κl obtained is quite low. This is partly due to the fact that thermal-conductivity measurements of thin films typically have a significant margin of error. Although methods for measuring the in-plane κ of polymer thin films have been reported38, the current technology for testing the in-plane κ of inorganic thin film is not yet fully mature39. Additionally, calculations using the SPB model also exhibit some deviation, and this deviation varies depending on the material system40. Figure 4h shows determined temperature-dependent ZT. An outstanding ZT value of 1.27 at 363 K, as well as a high ZT of 1.15 at room temperature, both rank top values among reported ones to date (Supplementary Table 1). Figure 4i also compares calculated and experimental ZT as a function of n. The calculated data are from the SPB model. As can be seen, the n value can be gradually optimized by increasing the Te doping concentration to achieve peak ZT in the thin film.
Temperature-dependent (a) electrical conductivity σ, (b) Seebeck coefficient S, and (c) determined S2σ. d Measured x-dependent carrier concentration n and mobility μ at room temperature. e Calculated x-dependent effective mass m* and deformation potential Edef at room temperature by a single parabolic band (SPB) model. Temperature-dependent (f) thermal conductivity κ. g x-dependent electronic and lattice thermal conductivities (κe and κl) at room temperature. h Temperature-dependent ZT. i Comparison of calculated and experimental ZTs as a function of n. The calculated data are from the SPB model.
Calculated band structures of (a) Ag2Se (Ag24Se12) and (b) Te-doped Ag2Se (Ag24Se11Te1). (c) Measured κl compared with calculated κl as a function of temperature. d Theoretically derived spectral κ (κs) for Ag2Se thin films with different Te concentrations x (x = 0, 0.7, 1.3, 1.9, 2.6, 3.2, and 3.8 at.%), with various phonon scatterings. Here scattering is from phonon-phonon Umklapp- and normal-process (U + N), vacancies/alloy elements (point defects, PD), grain boundaries (GB), and dislocations (DS). e Effect of point-defect scattering and (f) dependence of grain size on reducing κs as a function of Te content.
To interpret the effect of phonon scattering on κl reduction, the Callaway model is adopted. A decrease κl is mainly responsible for the reduction in κ depending on different phonon scattering phenomena. Debye Callaway model is investigated to clarify the source of reduction in κl41:
here v,θD, γ, and τT stand for sound velocity, Debye temperature, reduced phonon frequency (\(\chi\) = ħω/kBT), and total phonon relaxation time. Remarkably, strong scattering of phonon is originated by alloy element/intrinsic defects and grains that are primarily responsible for the reduction in κl for Te-doped samples. Herein, the obtained κl data curves (solid lines) exhibit inconsistency with the experimental results for Te doping concentrations lower than 3.2 at.% (Fig. 5c) at high temperatures, aligning with the trend of κl + κb (where κl is bipolar thermal conductivity) as a function of T−1 (Supplementary Fig. 15a). This inconsistency is likely due to the influence of bipolar diffusion on thermal conductivity at high temperatures (Supplementary Fig. 15b). As illustrated in Fig. 5d, phonon scattering from grain boundaries, point defects, and/or crystal defects may significantly reduce phonon transport. Simultaneously, the calculated curves/values determine the primary contribution of phonon scattering from impurity/point defects and grain boundaries (Fig. 5e, f). Details regarding the calculations and parameters used in the Debye-Callaway model for all investigated doped specimens are provided in the Supplementary Information.
After Te doping, although the structure of the Ag2Se film has been optimized in uniformity (overall orientation tends to favor (00l)), this does not necessarily mean that its flexibility has significantly improved. In fact, after doping with Te, the flexibility is only slightly improved (Supplementary Fig. 12). Additionally, the increase in Edef caused by Te doping may lead to a decrease in lattice variability, potentially resulting in increased film hardness. Therefore, to further enhance the practical value of the as-obtained high-performance thin film, significant improvements in its flexibility are required. In this work, we designed a protective layer made of organic polymer composites (90% poly (vinyl laurate) and 10% N-methylpyrrolidone), which is applied to the film surface using a spin-coating process. Figure 6a, b compare the measured ΔR/R0 of the Ag2Se thin films with 3.2 at.% Te without and with vinyl-laurate-based coatings at different bending radii and bending cycles. The inset in Fig. 6b shows a photo of the flexible thin film. For a better comparison, the vertical axis ranges of both graphs have been adjusted to be consistent. Obviously, after coating the organic polymer composites, the overall flexibility of the thin film was significantly improved. After 1000 bending cycles with a bending radius of 6.3 mm, the performance loss is only 2.5%. Such outstanding flexibility is highly competitive compared with reported works based on inorganic thermoelectric thin films (Table 2)42,43,44,45,46,47,48. Supplementary Fig. 13 also provides measured ΔR/R0 of the coated Ag2Se thin films with 3.2 at.% Te at different bending radii and heating at 95 °C by different periods for 1000 times bending, confirming the high flexibility at higher temperatures.
Measured ΔR/R0 of the Ag2Se thin films with 3.2 at.% Te (a) without and (b) with a vinyl-laurate-based coating at different bending radii and bending cycles. The inset in (b) shows a photo of the flexible thin film. c Measured contact resistivity ρc between coated Ag2Se thin film with 3.2 at.% Te and different electrode materials. d Measured open-circuit voltages and output powers of the device as a function of loading current at different temperature differences (ΔTs). e Determined output power densities at different ΔTs. The inset shows the photo of a flexible device composed of one pair of coated Ag2Se thin film with 3.2 at.% Te as an n-type leg and coated Sb2Te3 thin film as a p-type leg. f Voltage of wearing the as-fabricated device for sitting, walking, and running as a function of time.
Based on this novel coated thin film, we designed a facile F-TED to confirm its practicality. To further enhance the output performance of the F-TED, suppressing the contact resistance ρc between the thin film and the electrodes is crucial. Therefore, we need to choose appropriate electrode materials to match the thin-film material, which has been rarely reported in the designs of flexible F-TEDs in the past2,49. Figure 6c shows measured ρc between Ag2Se thin film with 3.2 at.% Te and different electrode materials including Au, Al, Mo, and Cu. The measurement principle is provided in Supplementary Fig. 14 for reference. We selected Cu as the potential electrode material that possesses the lowest ρc. Based on this, we assembled the F-TED and evaluated its output performance. The main room-temperature thermoelectric properties of both Sb2Te3 and Ag2Se are provided in Table 3. Figure 6d compares measured open-circuit voltages and output powers of the device as a function of loading current at different ΔTs, and Fig. 6e compares determined output power densities at different ΔTs. The inset shows the photo of a flexible device composed of one pair of Ag2Se thin film with 3.2 at.% Te as an n-type leg and Sb2Te3 thin film as a p-type leg. Remarkably, a high open-circuit voltage of 6 mV, a substantial output power of 65 nW, and a competitive power density of 1.5 mW cm−2 can be simultaneously achieved at a ΔT of 20 K2,49. The comparison results are provided in Supplementary Table 3. Figure 6f compares the measured voltage of wearing the F-TED with five p-n legs for sitting, walking, and running as a function of time. The maximum voltage was obtained when wearing the device while running. This is because during running, there is better heat dissipation at the side of the device that is exposed to the air, which helps maintain a larger temperature gradient and thus allows the device to perform better.
In summary, this work reports a record-high ZT of 1.27 at 363 K in polycrystalline Ag2Se-based thin film with 3.2 at.% Te. Theoretical calculations indicate that Te-doping can reduce the formation energy of the (00l) surface, resulting in a strong (00l) orientation across the film to enhance its uniformity and in turn carrier mobility. Moreover, Te-doping slightly broadens the bandgap and results in an enhanced Seebeck coefficient and a high power factor of 24.8 μW cm−1 K−2 by optimizing the carrier concentration. Te-doping at Se sites also introduces TeSe point defects that effectively scatter phonons, reducing thermal conductivity to 0.71 W m−1 K−1. After 1000 bending cycles with a bending radius of 6.3 mm, the film shows only 2.5% performance loss by coating a protective layer made of organic-polymer-based composites on the film surface, indicating significantly enhanced overall flexibility. Finally, the assembled flexible thermoelectric device using a pair of p-type Sb2Te3 and n-type Ag2Se films achieves an open-circuit voltage of 6 mV, an output power of 65 nW, and a power density of 1.5 mW cm−2 under a temperature difference of 20 K. This work indicates Te doping can significantly improve the thermoelectric potential of Ag2Se films in the application of wearable power generation.
Methods
Thin film and device fabrication
The flexible Ag2Se thin films were fabricated on PI substrates by the vacuum thermal co-evaporation method at various temperatures. After that, a single Ag2Se-Sb2Te3 thermoelectric device was fabricated with a Cu electrode on a PI substrate. Then, an organic coating material, polyvinyl laurate, was employed to enhance the flexibility of the as-fabricated Ag2Se thin films. The experimental details can be found in the Supplementary Information.
Characterizations and thermoelectric performance evaluation
The details can be found in the Supplementary Information.
Theoretical calculation
The First-principles calculations and single parabolic (SPB) model was performed to understand the carrier transport performance. The Debye-Callaway model was investigated to clarify the source of reduction of thermal conductivity. More details can be found in the Supplementary Information.
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper.
References
Liu, R., Wang, Z. L., Fukuda, K. & Someya, T. Flexible self-charging power sources. Nat. Rev. Mater. 7, 870–886 (2022).
Zhang, L., Shi, X.-L., Yang, Y.-L. & Chen, Z.-G. Flexible thermoelectric materials and devices: From materials to applications. Mater. Today 46, 62–108 (2021).
Yang, Q. et al. Flexible thermoelectrics based on ductile semiconductors. Science 377, 854–858 (2022).
Xiao, Y. & Zhao, L.-D. Seeking new, highly effective thermoelectrics. Science 367, 1196 (2020).
Jiang, B. et al. High-entropy-stabilized chalcogenides with high thermoelectric performance. Science 371, 830–834 (2021).
Zheng, Z.-H. et al. Harvesting waste heat with flexible Bi2Te3 thermoelectric thin film. Nat. Sustain. 6, 180–191 (2023).
Wei, T.-R. et al. Ag2Q-Based (Q = S, Se, Te) silver chalcogenide thermoelectric materials.Adv. Mater 35, 2110236 (2023).
Zhu, M., Shi, X.-L., Wu, H., Liu, Q. & Chen, Z.-G. Advances in Ag2S-based thermoelectrics for wearable electronics: Progress and perspective. Chem. Eng. J. 475, 146194 (2023).
Hou, S. et al. High performance wearable thermoelectric generators using Ag2Se films with large carrier mobility. Nano Energy 87, 106223 (2021).
Hou, S. et al. High-performance, thin-film thermoelectric generator with self-healing ability for body-heat harvesting. Cell Rep. Phys. Sci. 3, 101146 (2022).
Xin, C. et al. Solution-processed flexible n-Type S-doped Ag2Se thermoelectric generators for near-ambient-temperature energy harvest. Mater. Today Energy 33, 101266 (2023).
Lu, Y., Liu, Y., Li, Y. & Cai, K. The influence of Ga doping on preparation and thermoelectric properties of flexible Ag2Se films. Compos. Commun. 27, 100895 (2021).
Li, Y. et al. Exceptionally high power factor Ag2Se/Se/Polypyrrole composite films for flexible thermoelectric generators. Adv. Funct. Mater. 32, 2106902 (2022).
Lu, Y. et al. Ultrahigh power factor and flexible silver selenide-based composite film for thermoelectric devices. Energy Environ. Sci. 13, 1240–1249 (2020).
Hu, Q.-X. et al. SWCNTs/Ag2Se film with superior bending resistance and enhanced thermoelectric performance via in situ compositing. Chem. Eng. J. 457, 141024 (2023).
Jiang, C. et al. Ultrahigh performance polyvinylpyrrolidone/Ag2Se composite thermoelectric film for flexible energy harvesting. Nano Energy 80, 105488 (2021).
Lu, Y. et al. Ultrahigh performance PEDOT/Ag2Se/CuAgSe composite film for wearable thermoelectric power generators. Mater. Today Phys. 14, 100223 (2020).
Park, D., Ju, H. & Kim, J. Enhanced thermoelectric properties of flexible N-type Ag2Se nanowire/polyvinylidene fluoride composite films synthesized via solution mixing. J. Ind. Eng. Chem. 93, 333–338 (2021).
Lei, Y. et al. Microstructurally tailored thin β-Ag2Se films towards commercial flexible thermoelectrics. Adv. Mater. 34, 2104786 (2021).
Zheng, Z.-H. et al. Significantly (00l)-textured Ag2Se thin films with excellent thermoelectric performance for flexible power applications. J. Mater. Chem. A 10, 21603–21610 (2022).
Ding, Y. et al. High performance n-Type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 10, 841 (2019).
Jiang, C. et al. Ultrahigh performance of n-Type Ag2Se films for flexible thermoelectric power generators. ACS Appl. Mater. Interfaces 12, 9646–9655 (2020).
Gao, Q. et al. High power factor Ag/Ag2Se composite films for flexible thermoelectric generators. ACS Appl. Mater. Interfaces 13, 14327–14333 (2021).
Wang, Z. et al. High performance Ag2Se/Ag/PEDOT composite films for wearable thermoelectric power generators. Mater. Today Phys. 21, 100553 (2021).
Geng, J. et al. High power factor n-Type Ag2Se/SWCNTs hybrid film for flexible thermoelectric generator. J. Phys. D. Appl. Phys. 54, 434004 (2021).
Mallick, M. M., Franke, L., Rösch, A. G. & Lemmer, U. Shape-versatile 3D thermoelectric generators by additive manufacturing. ACS Energy Lett. 6, 85–91 (2021).
Mallick, M. M. et al. High-performance Ag–Se-Based n-Type printed thermoelectric materials for high power density folded generators. ACS Appl. Mater. Interfaces 12, 19655–19663 (2020).
Perez-Taborda, J. A., Caballero-Calero, O., Vera-Londono, L., Briones, F. & Martin-Gonzalez, M. High thermoelectric zT in n-Type silver selenide films at room temperature. Adv. Energy Mater. 8, 1702024 (2018).
Jindal, S., Singh, S., Saini, G. S. S. & Tripathi, S. K. Optimization of thermoelectric power factor of (013)-oriented Ag2Se films via thermal annealing. Mater. Res. Bull. 145, 111525 (2022).
Gao, J. et al. Thermoelectric flexible silver selenide films: compositional and length optimization. iScience 23, 100753 (2020).
Lu, Y. et al. Staggered-layer-boosted flexible Bi2Te3 films with high thermoelectric performance. Nat. Nano 18, 1281–1288 (2023).
Karato, S.-I. An introduction to the rheology of Solid Earth. Vol. 463. 2008: Cambridge University Press Cambridge, UK.
Mallick, M. M. et al. New frontier in printed thermoelectrics: formation of β-Ag2Se through thermally stimulated dissociative adsorption leads to high ZT. J. Mater. Chem. A 8, 16366–16375 (2020).
Zheng, Z.-H. et al. Achieving ultrahigh power factor in n-type Ag2Se thin films by carrier engineering. Mater. Today Energy 24, 100933 (2022).
Li, X. et al. Exceptional power factor of flexible Ag/Ag2Se thermoelectric composite films. Chem. Eng. J. 434, 134739 (2022).
Almufarij, S. R. et al. Enhanced the thermoelectric power factor of n-type Bi2Te3 thin film via energy filtering effect. Inorg. Chem. Commun. 157, 111439 (2023).
Lin, S. et al. Revealing the promising near-room-temperature thermoelectric performance in Ag2Se single crystals. J. Materiomics 9, 754–761 (2023).
Weathers, A. et al. Significant electronic thermal transport in the conducting polymer Poly(3,4-ethylenedioxythiophene). Adv. Mater. 27, 2101–2106 (2015).
Li, D. et al. Ce-filled Ni1.5Co2.5Sb12 skutterudite thin films with record-high figure of merit and device performance. Adv. Energy Mater. 13, 2301525 (2023).
Zhu, J. et al. Restructured single parabolic band model for quick analysis in thermoelectricity. NPJ Comput. Mater. 7, 116 (2021).
Jabar, B. et al. Enhanced power factor and thermoelectric performance for n-type Bi2Te2.7Se0.3 based composites incorporated with 3D topological insulator nanoinclusions. Nano Energy 80, 105512 (2021).
Shang, H. et al. High-performance Ag-Modified Bi0.5Sb1.5Te3 films for the flexible thermoelectric generator. ACS Appl. Mater. Interfaces 12, 7358–7365 (2020).
Kong, D., Zhu, W., Guo, Z. & Deng, Y. High-performance flexible Bi2Te3 films based wearable thermoelectric generator for energy harvesting. Energy 175, 292–299 (2019).
Cao, Z., Tudor, M. J., Torah, R. N. & Beeby, S. P. Screen printable flexible BiTe–SbTe-based composite thermoelectric materials on textiles for wearable applications. IEEE T. Electron Dev. 63, 4024–4030 (2016).
Li, Y. et al. A flexible and infrared-transparent Bi2Te3-carbon nanotube thermoelectric hybrid for both active and passive cooling. ACS Appl. Electron. Mater. 2, 3008–3016 (2020).
Shang, H. et al. Bi0.5Sb1.5Te3-based films for flexible thermoelectric devices. J. Mater. Chem. A 8, 4552–4561 (2020).
Jin, Q. et al. Cellulose fiber-based hierarchical porous bismuth telluride for high-performance flexible and tailorable thermoelectrics. ACS Appl. Mater. Interfaces 10, 1743–1751 (2018).
Varghese, T. et al. Flexible thermoelectric devices of ultrahigh power factor by scalable printing and interface engineering. Adv. Funct. Mater. 30, 1905796 (2020).
Cao, T., Shi, X.-L. & Chen, Z.-G. Advances in the design and assembly of flexible thermoelectric device. Prog. Mater. Sci. 131, 101003 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 62274112), Guangdong Basic and Applied Basic Research Foundation (2022A1515010929), and Science and Technology plan project of Shenzhen (JCYJ20220531103601003). ZGC thanks the financial support from the Australian Research Council, and QUT Capacity Building Professor Program, and acknowledges the National Computational Infrastructure, supported by the Australian government, for providing computational resources and service. The authors are thankful for the assistance on STEM-HAADF observation received from the Electron Microscope Center of Shenzhen University. This work was enabled by the use of the Central Analytical Research Facility hosted by the Institute for Future Environments at QUT.
Author information
Authors and Affiliations
Contributions
D.Y. and X.-L.S. contributed equally to this work. Z.-G.C. and Z.H.Z. supervised the project and conceived the idea. D.Y. and X.-L.S. designed the experiments and wrote the manuscript. D.Y. carried out the synthesis of materials. M.L., X.-L.S., W.L., H.M. and X.Z. undertook the theoretical work. M.N., A.M., S.C., Y.C., L.F., G.-X.L., P.F. undertook the thermoelectric performance evaluation. All the authors discussed the results and commented on the manuscript. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Supree Pinitsoontorn, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, D., Shi, XL., Li, M. et al. Flexible power generators by Ag2Se thin films with record-high thermoelectric performance. Nat Commun 15, 923 (2024). https://doi.org/10.1038/s41467-024-45092-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45092-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.