Abstract
With the rapid development of nuclear energy, problems with uranium supply chain and nuclear waste accumulation have motivated researchers to improve uranium separation methods. Here we show a paradigm for such goal based on the in-situ formation of π-f conjugated two-dimensional uranium-organic framework. After screening five π-conjugated organic ligands, we find that 1,3,5-triformylphloroglucinol would be the best one to construct uranium-organic framework, thus resulting in 100% uranium removal from both high and low concentration with the residual concentration far below the WHO drinking water standard (15 ppb), and 97% uranium capture from natural seawater (3.3 ppb) with a record uptake efficiency of 0.64 mg·g−1·d−1. We also find that 1,3,5-triformylphloroglucinol can overcome the ion-interference issue such as the presence of massive interference ions or a 21-ions mixed solution. Our finds confirm the superiority of our separation approach over established ones, and will provide a fundamental molecule design for separation upon metal-organic framework chemistry.
Similar content being viewed by others
Introduction
In response to the long-term energy crisis, the development of new energies is now becoming a hot topic. Nuclear energy, because of its high energy density and low carbon pollution, is thus viewed to be one of the effective alternatives1,2,3,4,5. However, the sustainable development of nuclear energy is still severely limited by the shortage and insufficient supply chain of uranium. On the other hand, the extensive use of radioactive uranium will also bring serious safety issue, such as environmental pollution and unexpectable diseases6. Thereby, it is important to carry out the research of uranium separation from used or new sources7,8,9,10,11,12,13. Spent fuel, as a typical used source, remains 93.4% unreacted uranium, which thereby can be as a major uranium source through separation, however, such separation was often blocked by the competing adsorption from a broad of metal ions, especially these physically and chemically similar f-block ions such as rare earth and other actinide ions14,15,16. Alternatively, seawater reserves abundant uranium, however, the ultralow uranium content down to 3 ~ 4 ppb and the serious vanadium ion interference still prevents the acquisition of uranium from seawater17,18,19,20,21,22,23,24,25. Therefore, significant effort should be devoted to improve uranium separation methods to meet the actual demand.
As an analogue of graphene, 2D (two-dimensional) conjugated metal-organic frameworks (MOFs), are recently receiving increasing attentions, due to its uniqueness in both structure and properties, showing important applications in supercapacitors, batteries, thermoelectric devices, chemiresistive sensors and electrocatalysts26,27,28. The design and synthesis rule of such 2D materials has been realized by the in-plane integration between π-conjugated hexa-substituted aromatic cores and late transition metal ions in a square planar coordination geometry29,30,31. Inspired by such way, we can expect the construction of similar 2D π-f conjugated MOF through a comparable in-plane integration between UO22+ ion with a planar coordination in f orbitals32,33,34 and proper π-conjugated ligands, and further make a hypothesis in uranium separation upon such MOF assembly technology. In this regard, we show herein the molecule design and uranium separation route by means of the concept of in-situ formation of π-f conjugated uranium-organic framework (UOF).
Results
2D MOF and ligand design
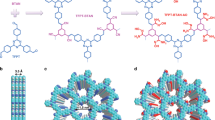
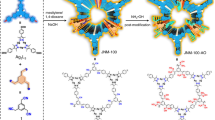
Previous research has revealed the molecular design rule for conjugated 2D MOF29,30,31. The key was the in-plane coupling between metal ions and organic ligands in a defined and periodic manner. It was found that these metal ions such as Ni2+, Co2+, and Cu2+ in the square planar coordination geometry and these hexa-substituted planar conjugated benzenes such as 1,3,5-triformylphloroglucinol (H3TFP) and 2,3,6,7,10,11-hexahydroxytriphenylene (H6HTP) meet the in-plane [3 + 2] coupling, where the organic ligands take the chelate coordination mode (model I and II, Fig. 1a) and act as three-connecting nodes, while metal ions act as two-connecting linker, finally resulting in the π-d conjugated 2D MOF (Fig. 1b)35,36. Different from these transition metal ions of Ni2+, Co2+, and Cu2+ that use d orbit for coordination, UO22+ ion, the common uranium type, affords the planar coordination feature in the f orbit and theoretically conducts more orbit to participate in coordination, generally showing the six-coordination fashion. Corresponding to this is the distinct coordination mode (model I and model II, Fig. 1c) between organic ligands and UO22+ ions and 2D MOFs generated by [3 + 2] or [3 + 3] coupling (Fig. 1d), where UO22+ ions act as three-connecting node and organic ligands act as two-connecting linker or three-connecting nodes, respectively. In this regard, we screened five comparable, planar conjugated ligands, composed of hexa-substituted H3TFP, H6HTP, and H3THQ (tetrahydroxyquinone), tetra-substituted H2HPD (2,5-dihydroxyterephthalaldehyde) and H4EAA (ellagic acid). As shown in Fig. 2, all these substrates were found to be effective in UO22+ capture from a 100 ppm UO22+ solution after contacting for 24 h, giving a hierarchy of H3TFP (100%) > H2HPD (25.6%) > H4EAA (24.8%) > H6HTP (8.3%) > H3THQ (6%), implying H3TFP being the best one. Accordingly, the next investigation is just focused on H3TFP ligand.
a π-d in-plane integration in the manner of model I and II. b View of the π-d conjugated 2D MOFs by means of [3 + 2] coupling with π-conjugated organic ligand as three-connected node and metal ions in the square planar coordination geometry as two-connected linker. c π-f in-plane integration in the manner of model I and II. d View of the π-f conjugated 2D MOFs by means of [3 + 2] or [3 + 3] coupling with UO22+ ion as three-connected node and π-conjugated organic ligand as two-connected linker or three-connected node.
To clarify the difference in UO22+ capture for these organic ligands, we then carried out the calculation on the binding energy (ΔG) of these organic ligands with UO22+ ion by density functional theory (DFT) method. A planar six-coordination model of one UO22+ ion coordinated by three these organic ligands was used to carried out DFT calculation. The optimized coordination structures of them were shown in Supplementary Fig. 1. The binding energy (ΔG) gives a hierarchy of H4THQ (−0.29 eV)>H6HTP (−0.69 eV)>H4EAA (−1.02 eV)>H2HPD (−2.99 eV)>H3TFP (−3.91 eV). Generally, negative binding energy (ΔG) suggests the reaction thermodynamically spontaneous, while this also obeys a rule, viz. the more negative, and the stronger the binding. Thus, the negative ΔG values mean that all these organic ligands can capture UO22+ ion through coordination, which is consistent with the experimental results, while the smallest and biggest ΔG value in, respectively, H3TFP and H4THQ means the strongest and weakest binding and consequently the biggest and smallest UO22+ uptake, which is also in good agreement with the experimental results. Moreover, the hierarchy in binding energy (ΔG) is also in accord with the hierarchy in the UO22+ uptake, confirming the adsorption of UO22+ ions by these organic ligand obeying the defined planar coordination principle. In addition, seen from the optimized coordination structures of them, it is found that the chelate coordination from the combination of one aldehyde oxygen and one hydroxyl oxygen (such as H4TFP and H2HPD) is more beneficial for strengthening U-O coordination and planar coordination configuration over the chelate coordination from two hydroxyl oxygens (such as H4EAA, H6HTP, H4THQ), due to steric hindrance effect. And this could be the key to determine the UO22+ uptake performance. Moreover, the bigger conjugated organic molecule is beneficial for strengthening planar coordination configuration and consequently enhancing UO22+ uptake, e.g., H6HTP and H4EAA vs. H4THQ.
Adsorption kinetics
Fast adsorption process is usually the way we want. We then tested the adsorption kinetics of H3TFP ligand from a 50 ppm U(VI) solution with pH = 3 through using 10 mg adsorbent (Fig. 3a). Notably, the adsorption equilibrium was finished within 15 min with 100% removal efficiency, suggesting ultrafast adsorption kinetics, due to the thermodynamicly spontaneous chemical reaction through coordination assembly between UO22+ ions and H3TFP ligand. After extending the contact time to 24 h and 48 h, we found that the residual uranium concentration was decreased down to ultralow level of 0.18 ppb and 0.15 ppb, respectively, far lower than the World Health Organization (WHO) standard (15 ppb) for uranium content in drinking water. In light of this data, the distribution coefficient, Kd, was calculated up to 9.6 × 107 mL/g (a Kd value exceeding 1.0 × 105 mL/g is usually considered as excellent adsorbent), implying strong affinity between adsorbents and uranium resulted from the strong U-O coordination interactions. This value ranks the top level among all established uranium adsorbents, including POP1-AO (1.1 × 106 mL/g)37, MIGPAF-13 (2.0 × 106 mL/g)38, SMON-PAO (3.7 × 105 mL/g)39, and PIDO/NF (2.8 × 105 mL/g)7. This exceptional uranium removal ability was further attested for a low concentration of U(VI) solution (1 ppm), giving a residual uranium concentration of 2.3 ppb after 5 min and 0.13 ppb after 48 h (Fig. 3b), also far exceeding the WHO standard. Big Kd value up to 2.1 × 106 mL/g was also observed.
a Adsorption kinetics from 50 ppm U solution upon H3TFP adsorbent. b Adsorption kinetics from 1 ppm U solution upon H3TFP adsorbent. c Adsorption capacity of H3TFP adsorbent. Highlight in blue represents the theoretical adsorption capacity. d A comparison in U uptake capacity between reported U adsorbents and our case. e Influence of interfering ions on U adsorption. f Selective U capture from a 21-ions mixture solution. The error bars indicate the standard deviation (n = 3).
Adsorption capacity
Large adsorption capacity is also one of the goals pursued by adsorbent. Next, we investigated the adsorption capacity upon H3TFP adsorbent from a uranium solution with concentration of 10–1000 ppm. Interstingly, this adsorbent enabled 100% removal for all these uranium solutions (Fig. 3c), which is never observed in the literature. The experimental uranium uptake capacity was as high as 1.0 g/g. If taking the formation of UOF through [3 + 3] coupling into account, the theoretical adsorption capacity is estimated as big as 1.6 g/g, which surpasses all established uranium adsorbents (Fig. 3d, Supplementary Table 1)40, including these benchmark adsorbents such as MIL-101-DAMN (0.60 g/g)41, Cu-BTC (0.61 g/g)42, TFPPy-BDOH (0.98 g/g)43, and POP3-AO (1.07 g/g)37. This also confirmed the advantage of our method over established approaches.
pH effect
It was found that pH value showed significant effect on the uranium uptake performance (Supplementary Fig. 2). The optimal performance was found under pH = 3 and 5, whereas both increasing acidity and alkalinity would lead to sharp decrease in the uranium uptake, for example, 100% uranium removal under pH = 3 and 5 vs. 12.6% uranium removal under pH = 1 or 21.8% uranium removal under pH = 9. High acidity leading to a sharp decrease in uranium uptake is mainly due to the protonation that will significantly affect the coordination of H3TFP hydroxyl groups, and high alkalinity resulting in a sharp decrease in uranium uptake is mainly due to the solution of H3TFP under such condition that will significantly affect the UOF formation.
Selectivity towards UO2 2+
As we know, divalent copper and trivalent iron ion were found to construct 2D MOF with H3TFP ligands35, while the presence of trivalent 4f ions, or tetravalent thorium ion23, or vanadium ion were often found to give significant effect on UO22+ capture37,38. Thereafter, it is important to research the uranium adsorption performance in the presence of these interfering ions. Then, we carried out the uranium adsorption experiments form a 10 ppm uranium solution under the presence of massive other interfering ions with U/M (M = Cu2+, Fe3+, Nd3+, Th4+, and VO3−) ratio from 1:10 to 1:100 (Fig. 1e). Notably, no decrease in the uranium uptake was observed in the presence of other interfering ions, even expanding the U/M ratio to 1:100, completely excluding the influence of interfering ions. Such selectivity towards UO22+ ion over other ions is highly rare in the literature44,45. Furthermore, we carried out a selectivity test from a 21-ions mixture solution, including in Na+, K+, Cs+, Mg2+, Ca2+, Sr2+, Zn2+, Cd2+, Pd2+, Mn2+, Cu2+, Co2+, Ni2+, Cr3+, Fe3+, La3+, Gd3+, Nd3+, Th4+, UO22+, and VO3- (Fig. 1f). It was found that UO22+ was 100% captured, whereas other ions just gave less than 10% removal efficiency, suggesting highly selective capture of UO22+ ion over other 20 ions.
The selectivity of UO22+ over Fe3+, Nd3+, Th4+, and VO3− can be easy to understand, since UO22+ is planarly coordinated and can effectively construct the π-f conjugated 2D uranium-organic framework, whereas other ions such as Fe3+, Nd3+, Th4+, and VO3− are spherical coordinated and can not construct corresponding π-d/f conjugated 2D metal-organic framework. However, Cu2+ ions own the planar coordination feature, and can form the π-d conjugated 2D metal-organic framework, which will theoretically result in considerably competitive adsorption with UO22+ ion. Thereby, to understand the UO22+/Cu2+ selectivity in this work, DFT calculation on binding energy was carried out (Supplementary Fig. 3). The negative binding energy (ΔG) of −1.16 eV implies the adsorption potential of H3TFP ligand towards Cu2+ ions. But the binding towards Cu2+ ion is far weaker than that towards UO22+ ion, as evidenced by the ΔG value (−1.16 eV) in Cu2+ ion that is far bigger than that (−3.91 eV) in UO22+ ion; thus H3TFP ligand can enable selective UO22+ capture over Cu2+ ion.
Recycle use and liquid-liquid extraction
More interestingly, H3TFP adsorbent can be conveniently recovered in the form of precipitate through using 3 M HNO3 as eluent, while the adsorbed uranium on adsorbent can be 100% desorbed into solution in the form of UO2(NO3)2, possibly due to a disassembly of UOF with the breakage of all U-O coordination bonds. Such complete solid-liquid separation (Fig. 4) facilitates our following recycle use to an ideal form, as evidenced by observation of no decrease in both uranium adsorption and adsorbent recovery from a 100 ppm uranium solution after repeating adsorption-desorption cycles (Fig. 4) for 11 times (Supplementary Fig. 4).
Interestingly, although H3TFP is insoluble in water, however it gave good solubility in many organic solvents, involved in dichloromethane (DCM), toluene (PhMe), ortho-dichlorobenzene (ODCB), p-xylene (PX), m-xylene (MX), ortho-xylene (OX), and trimethylbenzene (TB). Thus, we further developed the liquid-extraction route (Fig. 4) and the results were shown in Supplementary Fig. 5. Notably, such liquid-extraction route was found to be also effective for uranium capture. Especially, H3TFP in DCM and ODCB was found to give 100% removal for a 50 ppm uranium solution within 15 min. This result is comparable with that observed in the solid-extraction route. We also extended the contacting time up to 48 h for H3TFP in DCM, and found low residual concentration of uranium (1.24 ppb), which is also far below the WHO standard of drinking water.
In both solid- and liquid-extraction routes, a key step in the desorption process is the solid-liquid separation between UO2(NO3)2 solution and H3TFP precipitation regeneration from desorption. Thus, it is important to reveal the solubility of H3TFP in water. As shown in Supplementary Fig. 6, it was clear that H3TFP is completely soluble in ODCM, but insoluble in water. This can be further attested by UV-visiblespectral test (Supplementary Fig. 7), where H3TFP in ODCM gave a strong adsorption peak at 320 nm, while no obvious adsorption was observed for H3TFP in water.
Extraction of uranium from seawater
To confirm the practical application of our uranium separation method, we further explored uranium capture performance from natural seawater. It was found that the use of 10 mg H3TFP adsorbent can reduce a 10 L natural seawater (3.3 ppb U) to 0.1 ppb after contacting for 5 days, showing uranium uptake as high as 3.2 mg/g. If considering the time cost, the uranium uptake efficiency is 0.64 mg·g−1·d−1. Such value far exceeds the top adsorbents (Supplementary Table 2) such as PPH-OP (0.36 mg·g−1·d−1)46, AP-PIM-1 (0.32 mg·g−1·d−1)47,48, MIGPAF-13 (0.28 mg·g−1·d−1)38, and POP1-AO (0.15 mg·g−1·d−1)37. In addition, we also conducted the uranium extraction under a longer contacting time from a 20 L natural seawater (3.3 ppb U) using 10 mg H3TFP adsorbent. Impressively, 97% uranium can be captured after 10 days, giving uranium uptake as high as 6.4 mg/g, while extending contacting time up to 14 days did not increase uranium uptake. A comparison in uranium uptake between established materials and our case is shown in Supplementary Table 3, which clearly suggests our case with the location of top level in the field of uranium extraction from seawater. Moreover, we should also consider the economy of uranium extraction from seawater. In generally, the manufacturing cost of adsorbents dominates the total cost for uranium extraction from seawater. For our case, the cost of preparing H3TFP adsorbent is as low as 1.3 $/g, confirming its economic feasibility. Such value is far below the current benchmark adsorbents such as COF 4 P (4.7 $/g)14 and COF-4 (2.7 $/g)15.
Discussion
Uranium capture mechanism
As mentioned above, the uranium capture upon H3TFP adsorbent majorly obeys the rule of in-situ formation of π-f conjugated 2D UOF, where the key is the assembly between π-conjugated H3TFP ligands and f ions of UO22+ in the defined and periodic manner. The use of f orbit to participate in bonding for UO22+ ions suggests the formation of considerably more ionic compounds than transition metals, meaning that UO22+ ions are more likely to form crystalline MOFs than transition metals;26,47 this can reasonably explain the selectivity of uranium over transition metal ions such as Cu2+ and Fe3+, even though these metal ions can also form π-d conjugated 2D MOFs. The f orbit in UO22+ ion shows the in-plane coordination feature, meeting the in-plane assembly, whereas other f ions such as rare-earth (trivalent state) and thorium (tetravalent state) ions afford sphere coordination feature49,50, which fully excludes the in-plane assembly; this can reasonably explain the selectivity of uranium over Nd3+ and Th4+ ions. Similarly, vanadium ions also own the sphere coordination feature, thus excluding the in-plane assembly and finally leading to the big selectivity of UO22+ over VO3− ion.
To confirm the formation of π-f conjugated 2D UOF during the uranium adsorption upon H3FTP adsorbent, we carried out a series of characterizations on the uranium-loaded samples (namely UOF-TFP), including in IR (infrared spectrum), XPS (X-ray photoelectron spectroscopy), PXRD (powder X-ray diffraction), TG (thermogravimetric analysis), N2 adsorption, SEM-EDS (scanning electron microscope plus energy-dispersive X-ray spectroscopy), and TEM (transmission electron microscope). IR spectra disclosed new peak at 922 cm−1, belonging to the antisymmetric vibration of uranyl ions, which, relative to UO2(NO3)2·6H2O with peak at 960 cm−1 (Supplementary Fig. 8)44,45, shows big red shift, implying strong coordination interactions between H3TFP adsorbent and uranyl ions. And coordination with the participation of both aldehyde and phenolic groups of H3TFP adsorbent can be further read out from IR peaks showing big shift or almost disappearance for aldehyde and phenolic groups, relative to free H3TFP molecule. The success in uranium capture upon H3TFP adsorbent can be further reflected in XPS spectrum, showing typical UO22+ peaks at 381.8 eV and 392.7 eV for U4f7/2 and U4f5/2, respectively (Supplementary Fig. 9). The values are significantly lower than that of UO2(NO3)2·6H2O (382.5 eV and 393.4 eV)46,47, also confirming the strong coordination interactions between H3TFP ligands and UO22+ ions. The presence of two satellite peaks at higher binding energy, relative to U4f7/2 and U4f5/2, confirms the U(VI) oxidation state in UOF-TFP.
The single crystal of UOF-TFP can not be obtained, blocking us to access its exact structure. But, we can simulate its structure from powder X-ray diffraction (PXRD) plus pawley refinements44,45. PXRD pattern showed high crystallinity of UOF-TFP. In light of the coordination feature of both UO22+ ions and H3TFP ligands, we then proposed a 2D MOF model, and then Pawley refinements were carried out on the experimental PXRD (Fig. 5a). It was found that the refined PXRD pattern was in good agreement with the experimental counterpart, as evidenced by the small difference and reasonable Rp = 2.65% and Rwp = 3.78% values. The layered nets in UOF-TFP showed a staggered orientation (AB stacking). The structure of UOF-TFP was shown in Fig. 5b. UO22+ ions afforded the planar six-coordination by three TFP3− ligands using three phenolic oxygen and three aldehyde oxygen atoms. The TFP3- ligands afforded a chelate coordination mode through using adjacent one phenolic oxygen and one aldehyde oxygen atom to fix one UO22+ ion. The U-O bond length of 2.40–2.70 Å is in the normal range33,34. The π-f conjugated 2D net was built in a [3 + 3] way that each TFP3− ligand connects to three UO22+ ions, while each UO22+ ion also connects to three TFP3− ligands, resulting in an overall binodal hcb net. Moreover, such AB stacking led to small void among layers (Supplementary Fig. 10), occupied by water molecules. TG test disclosed the loss of solvent water molecules before 230 °C, and decomposition of UOF-TFP occurred after 310 °C (Supplementary Fig. 11). In addition, the micoporosity was confirmed by N2 adsorption at 77 K (Supplementary Fig. 12).
More impressively, such π-f conjugated 2D net can be intuitively read out by STEM-HADDF test. First, SEM disclosed the nanorod morphology of UOF-TFP (Fig. 5c). EDS element analysis clearly disclosed the presence of uranium element, and the U/C atom ratio is well consistent with the structural data, confirming the accuracy of our structural simulation. Furthermore, such nanorod morphology can be also clearly read out from TEM images. Impressively, UO22+ ions and its location can be clearly read out from STEM-HADDF images (Fig. 5d), where the UO22+ ions are approximately arranged in a hcb fashion with a distance of ca. 0.9 nm between two adjacent U ions, which is similar to that observed in the simulated structure data with a distance of ca. 0.81 nm.
In addition, to gain deep insight into the local coordination sphere of the uranium species, U K-edge X-ray absorption near-edge structure (XANES, Supplementary Fig. 13) and extended X-ray absorption fine structure (EXAFS, Supplementary Fig. 14) spectroscopy were performed. UO2(NO3)2·6H2O was used as the reference standard. It was found that UOF-TFP rendered comparable XANES and EXAFS spectra with that observed in UO2(NO3)2·6H2O, confirming their similarity in the valence state and coordination surrounding of uranium. As we know, uranium in UO2(NO3)2·6H2O is hexavalent in the form of UO22+, and UO22+ in UO2(NO3)2·6H2O takes planar six coordination with two NO3- ions and two coordination water molecules, where each NO3− ion displays a chelate coordination mode with two NO3− oxygen atoms to fix one UO22+ ion. Accordingly, we can deduce UO22+ ions with the planar six coordination in UOF-TFP (Supplementary Table 4). High-quality fits of the EXAFS data for UOF-TFP (Fig. 6a, b) strongly suggest an eight coordination of uranium, where two U-O coordinations with bond length of 1.85 ± 0.01 Å are assigned to U = O bond of UO22+ ion, other six U-O coordinations with longer bond length are assigned to the planar U-O coordination from TFP3- ligand. This also reveals two types of U-O bond with equal numbers in the bond length of 2.42 ± 0.02 Å and 2.51 ± 0.01 Å, respectively. We then comparied the results from EXAFS data with the results from structural simulation, and found that the U = O bond of UO22+ ion form EXAFS data is well consistent with the results from structural simulation (1.84 Å), while the bond length of 2.42 ± 0.02 Å and 2.51 ± 0.01 Å from EXAFS data is close to U-Ohydroxyl bond (2.40 Å, hydroxyl oxygen of TFP3− as coordination atom) and U-Oaldehyde bond (2.70 Å, aldehyde oxygen of TFP3− as coordination atom) from structural simulation. Thereby, the structure of UOF-TFP can be once again confirmed by EXAFS results.
In summary, we have demonstrated a proof-of-concept of in-situ formation of π-f conjugated 2D UOF for targeting at highly efficient and selective uranium separation. Taking both coordination and self-assembly chemistry into account, through using the simple but powerful organic ligand of H3TFP molecule, we can easily appoint the self-assembly between π-conjugated H3TFP ligands and in-plane coordinated f UO22+ ions in a defined and periodic manner, leading to the rapid and massive formation of UOF. Such feature finally offered an ideal uranium separation process, as evidenced by its superior performance, including in recorded theoretical uptake capacity, ultrafast adsorption kinetics, large distribution coefficient, big selectivity, and long-term repeatability. This work not only outlines a concept of how to use MOF chemistry to solve practical problems, but also points out a promising direction in the field of uranium separation.
Methods
Materials and measurements
H3TFP (99%), H2HPD (99%) and H6HTP (99%) were purchased from Jilin Chinese Academy of Sciences - Yanshen Technology Co., Ltd. H4THQ (99%), H4EAA (99%), UO2(NO3)2·6H2O (99%), and these organic solvents (99%) were purchased from Aladdin Biochemical Technology Co., Ltd. These were used as received without further purification. X-ray powder diffraction were collected by a Bruker AXSD8 Discover powder diffractometer at 40 kV, 40 mA for Cu Kλ (λ = 1.5406 Å). The simulated powder patterns were calculated by Mercury 1.4. Infrared Spectra (IR) were measured by a Bruker VERTEX70 spectrometer in the 700–3600 cm−1 region. The gas adsorption isotherms were collected on a Belsorp-max. Ultrahigh-purity-grade (>99.999%) N2 gases were used during the adsorption measurement. The analyses of concentrations of U ions in the solution was carried out by ThermoFisher iCap7600 ICP-OES or iCap RQplus ICP-MS instruments. X-ray photoelectron spectra (XPS) were collected by Thermo Scientific ESCALAB 250 Xi spectrometer. Scanning electron microscopy (SEM) images were recorded on a Hitachi SU 8100 Scanning Electron Microscope. Transmission Electron Microscope (TEM) was recorded on a Talos F200x from Thermo Fisher Scientific. UV-vis spectroscopy were recorded at room temperature on a SHIMADZU UV-2700 spectrophotometer.
Data availability
The authors declare that all the data supporting the findings of this study are available within the article (and Supplementary Information Files), or available from the corresponding author on request. All the data generated in this study have been deposited in the Figshare database under [https://doi.org/10.6084/m9.figshare.24085956]. Source data are provided with this paper.
References
Hoffert, M. I. et al. Energy implications of future stabilization of atmospheric CO2 content. Nature 395, 881–884 (1998).
DeCanio, S. J. & Fremstad, A. Economic feasibility of the path to zero net carbon emissions. Energy Policy 39, 1144–1153 (2011).
Nuclear key to a clean energy future: IEA World Energy Outlook (2016). https://www.iea.org/reports/world-energy-outlook-2016.
Nifenecker, H. Future electricity production methods. Part 1: Nuclear energy. Rep. Prog. Phys. 74, 022801 (2011).
Sovacool, B. K. Valuing the greenhouse gas emissions from nuclear power: a critical survey. Energy Policy 36, 2940–2953 (2008).
Craft, E. et al. Depleted and natural uranium: chemistry and toxicological effects. J. Toxicol. Environ. Health B Crit. Rev. 7, 297–317 (2004).
Wang, D. et al. Significantly enhanced uranium extraction from seawater with mass produced fully amidoximated nanofiber adsorbent. Adv. Energy Mater. 8, 1802607 (2018).
Sun, Q. et al. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv. Mater. 30, 1705479 (2018).
Sun, Q. et al. Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste. Nat. Commun. 9, 1644 (2018).
Wang, X. X. et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci. China Chem. 62, 933–967 (2019).
Yuan, Y. et al. Molecularly imprinted porous aromatic frameworks and their composite components for selective extraction of uranium ions. Adv. Mater. 30, 1706507 (2018).
Li, J. et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 47, 2322–2356 (2018).
Zheng, T. et al. Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system. Nat. Commun. 8, 15369 (2017).
Yang, H. et al. Tuning local charge distribution in multicomponent covalent organic frameworks for dramatically enhanced photocatalytic uranium extraction. Angew. Chem. Int. Ed. 62, e202303129 (2023).
Chen, Z. S. et al. Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance. Nat. Commun. 14, 1106 (2023).
Zhang, H. L. et al. Three mechanisms in one material: uranium capture by a polyoxometalate-organic framework through combined complexation, chemical reduction, and photocatalytic reduction. Angew. Chem. Int Ed. 58, 16110–16114 (2019).
Wang, Z. Y. et al. Constructing an ion pathway for uranium extraction from seawater. Chem 6, 1683–1691 (2020).
Feng, L. J. et al. In situ synthesis of uranyl-imprinted nanocage for selective uranium recovery from seawater. Angew. Chem. Int. Ed. 61, 82–86 (2022).
Cui, W. R. et al. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew. Chem. Int. Ed. 59, 17684–17690 (2020).
Yuan, Y. et al. A molecular coordination template strategy for designing selective porous aromatic framework materials for uranyl capture. ACS Cent. Sci. 5, 1432–1439 (2019).
Yuan, Y. & Zhu, G. S. Porous aromatic frameworks as a platform for multifunctional applications. ACS Cent. Sci. 5, 409–418 (2019).
Kalaj, M. et al. MOF-polymer hybrid materials: from simple composites to tailored architectures. Chem. Rev. 120, 8267–8302 (2020).
Yuan, Y. H. et al. A Bio-inspired nano-pocket spatial structure for targeting uranyl capture. Angew. Chem. Int. Ed. 59, 4262–4268 (2020).
Chen, Z. et al. N, P, and S codoped graphene-like carbon nanosheets for ultrafast uranium (VI) capture with high capacity. Adv. Sci. 5, 1800235 (2018).
Yu, Q. H. et al. A universally applicable strategy for construction of anti-biofouling adsorbents for enhanced uranium recovery from seawater. Adv. Sci. 6, 1900002 (2019).
Skorupskii, G. et al. Efficient and tunable one-dimensional charge transport in layered lanthanide metal-organic frameworks. Nat. Chem. 12, 131–136 (2020).
Sheberla, D. et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 16, 220–224 (2016).
Feng, D. W. et al. Robust and conductive two-dimensional metal-organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 3, 30–36 (2018).
Dou, J. H. et al. Signature of metallic behavior in the metal-organic frameworks M3(hexaiminobenzene)2 (M = Ni, Cu). J. Am. Chem. Soc. 139, 13608–13611 (2017).
Sheng, S. C. et al. A one-dimensional conductive metal-organic framework with extended π-d conjugated nanoribbon layers. Nat. Commun. 13, 7599 (2022).
Xing, G. L. et al. Conjugated nonplanar copper-catecholate conductive metal-organic frameworks via contorted hexabenzocoronene ligands for electrical conduction. J. Am. Chem. Soc. 145, 8979–8987 (2023).
Lv, K., Fichter, S., Gu, M., März, J. & Schmidt, M. An updated status and trends in actinide metal-organic frameworks (An-MOFs): From synthesis to application. Coor. Chem. Rev. 446, 214011 (2021).
Mei, S. et al. Assembling a heterobimetallic actinide metal-organic framework by a reaction-induced preorganization strategy. Angew. Chem. Int. Ed. 62, e202306360 (2023).
Hanna, S. L. et al. Discovery of spontaneous de-interpenetration through charged point-point repulsions. Chem 8, 225–242 (2022).
Zhang, Z. et al. Ultrafast interfacial self-assembly toward supramolecular metal-organic films for water desalination. Adv. Sci. 9, 2201624 (2022).
Xie, L. S., Skorupskii, G. & Dincă, M. Electrically conductive metal-organic frameworks. Chem. Rev. 120, 8536–8580 (2020).
Song, Y. P. et al. Nanospace decoration with uranyl-specific “hooks” for selective uranium extraction from seawater with ultrahigh enrichment index. ACS Cent. Sci. 7, 1650 (2021).
Wang, Z. Y. et al. Constructing uranyl-specific nanofluidic channels for unipolar ionic transport to realize ultrafast uranium extraction. J. Am. Chem. Soc. 143, 14523–14529 (2021).
Yuan, Y. H. et al. Charge balanced anti-adhesive polyacrylamidoxime hydrogel membrane for enhancing uranium extraction from seawater. Adv. Funct. Mater. 29, 1805380 (2019).
Mei, D. C., Liu, L. J. & Yan, B. Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coor. Chem. Rev. 475, 214917 (2023).
Zhang, J. C. et al. Diaminomaleonitrile functionalized double-shelled hollow MIL-101 (Cr) for selective removal of uranium from simulated seawater. Chem. Eng. J. 368, 951–958 (2019).
Liu, R., Zhang, W., Chen, Y. T. & Wang, Y. S. Uranium (VI) adsorption by copper and copper/iron bimetallic central MOFs. Colloid Surf. A. 587, 124334 (2020).
Niu, C. P. et al. A conveniently synthesized redox-active fluorescent covalent organic framework for selective detection and adsorption of uranium. J. Hazard. Mater. 425, 127951 (2022).
Xiong, X. H. et al. Ammoniating covalent organic framework (COF) for high-performance and selective extraction of toxic and radioactive uranium ions. Adv. Sci. 6, 1900547 (2019).
Xu, Y., Yu, Z. W., Zhang, Q. Y. & Luo, F. Sulfonic-pendent vinylene-linked covalent organic frameworks enabling benchmark potential in advanced energy. Adv. Sci. 10, 2300408 (2023).
Yuan, Y. H. et al. Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat. Sustain. 4, 708–714 (2021).
Yang, L. S. et al. Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat. Sustain. 5, 71–80 (2022).
Wang, Y. L. et al. Umbellate distortions of the uranyl coordination environment result in a stable and porous polycatenated framework that can effectively remove cesium from aqueous solutions. J. Am. Chem. Soc. 137, 6144–6147 (2015).
Zhang, H. L. et al. Ultrafiltration separation of Am(VI)-polyoxometalate from lanthanides. Nature 616, 482–487 (2023).
Wang, Y. X. et al. Emergence of uranium as a distinct metal center for building intrinsic X-ray scintillators. Angew. Chem. Int. Ed. 57, 7883–7887 (2018).
Acknowledgements
This work was supported financially by the National Natural Science Foundations of China (22376024, 21966002, 22227809) (F.L. and L.Z.), the Youth leading talent project of FuZhou (NO. 2020ED64) (F.L.), the Jiangxi project (DHSQT22021007) (F.L.), Shanghai Science and Technology Innovation Action Plan (22142200300) (J.W.) and the Photon Science Center for Carbon Neutrality. The authors would also like to thank Wenqian Liu from Shiyanjia Lab (www.Shiyanjia.com) for the XRD, SEM, and STEM analysis.
Author information
Authors and Affiliations
Contributions
F.L. conceived the experiments, writing, and editing. Q.Z. carried out the major experiments. Q.Z. carried out PXRD simulation. Z.H., F.G., and X.X. carried out part of the experiments. L.Z., J.Z., and J.W. carried out EXAFS and XANES tests and discussion. L.G. carried out DFT calculation. All authors contributed to the discussion, and gave approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shengqian Ma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q.Y., Zhang, L.J., Zhu, J.Q. et al. Ultra-selective uranium separation by in-situ formation of π-f conjugated 2D uranium-organic framework. Nat Commun 15, 453 (2024). https://doi.org/10.1038/s41467-023-44663-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-44663-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.