Abstract
Floral organ shape and size in cereal crops can affect grain size and yield, so genes that regulate their development are promising breeding targets. The lemma, which protects inner floral organs, can physically constrain grain growth; while the awn, a needle-like extension of the lemma, creates photosynthate to developing grain. Although several genes and modules controlling grain size and awn/lemma growth in rice have been characterized, these processes, and the relationships between them, are not well understood for barley and wheat. Here, we demonstrate that the barley E-class gene HvMADS1 positively regulates awn length and lemma width, affecting grain size and weight. Cytological data indicates that HvMADS1 promotes awn and lemma growth by promoting cell proliferation, while multi-omics data reveals that HvMADS1 target genes are associated with cell cycle, phytohormone signaling, and developmental processes. We define two potential targets of HvMADS1 regulation, HvSHI and HvDL, whose knockout mutants mimic awn and/or lemma phenotypes of mads1 mutants. Additionally, we demonstrate that HvMADS1 interacts with APETALA2 (A-class) to synergistically activate downstream genes in awn/lemma development in barley. Notably, we find that MADS1 function remains conserved in wheat, promoting cell proliferation to increase awn length. These findings extend our understanding of MADS1 function in floral organ development and provide insights for Triticeae crop improvement strategies.
Similar content being viewed by others
Introduction
Barley (Hordeum vulgare) is one of the most important crops worldwide, mainly used for feed, food, and malt (beer and whisky) production1. Crop yield is directly related to grain size and weight2. The grain of barley, like that of most Poaceae grasses including rice (Oryza sativa), wheat (Triticum aestivum) and sorghum (Sorghum bicolor), is enclosed in spikelet hull (lemma and palea)3. In rice, the spikelet hull limits grain growth and thus affects final grain size and yield4. A series of proteins that control grain size and weight by regulating cell proliferation in the spikelet hull has been defined, including the E3 ubiquitin ligase GRAIN WIDTH AND WEIGHT 2 (GW2)5, the squamosa promoter-binding protein SPL166, the calmodulin-binding protein GW57, the G-protein γ subunit RGG18, the MKKK10–MKK4–MAPK6 cassette9, the glutaredoxin protein WIDE GRAIN 1 (WG1)10, and the ubiquitin receptor HOMOLOG OF DA1 ON RICE CHROMOSOME 3 (HDR3)11. In barley, however, the relationship between spikelet hull and grain size has not yet been determined, nor the molecular mechanism of grain size regulation defined. It is noteworthy that while the rice lemma and palea together form spikelet shell, the barley lemma entirely encloses the palea such that lemma size may be the most critical factor limiting grain size.
Another floral tissue prevalent in major cereal crops is the awn, a stiff, hair-like structure that extends from the tip of the lemma12. The awn is simple and thus often overlooked, but is evolutionarily beneficial for reproduction in wild species13 where it prevents predation by birds and animals, and aids seed dispersal and self-planting14,15,16. In rice and sorghum, awnless is considered a key domestication trait to facilitate seed harvest and storage without affecting yield13,17,18. In contrast, the barley and wheat awn has been largely preserved, although domesticated cultivars have shorter awns than their wild ancestors19, as barley and wheat awns contribute to grain filling through photosynthesis20,21,22, especially when the leaves are prematurely senescent or damaged by diseases23,24. Genes regulating awn development have been reported in different cereals. In sorghum, duplication of the ALOG transcription factor awn1 prevents awn development, leading to awn loss during domestication13,25. In rice, some genes have been artificially selected during domestication: Awn-1 (An-1) encodes a helix-loop-helix protein and positively controls cell division and the formation of awn primordia18; An-2/LONG AND BARBED AWN (LABA1) encodes a cytokinin-activating enzyme and promotes awn elongation by increasing cytokinin concentration14,26; REGULATOR OF AWN ELONGATION 2 (RAE2)/GRAIN NUMBER, GRAIN LENGTH AND AWN DEVELOPMENT 1 (GAD1)27,28 and EPIDERMAL PATTERING FACTOR-LIKE 2 (EPFL2)29 encode secreted signal proteins involved in awn development. DROOPING LEAF (DL, a YABBY gene) and OsETTIN2 (an auxin response factor gene) work together to promote awn formation30. Other reported genes include REGULATOR of AWN ELONGATION 3 encoding an E3 ubiquitin ligase31, TONGARI-BOUSHI1 encoding a YABBY protein32, CW-ZF7 encoding a CW-domain containing zinc finger protein33, and GRAIN LENGTH AND AWN 1 encoding a mitogen-activated protein kinase phosphatase34. Less is known about awn development in barley and wheat. In wheat, FRIZZY PANICLE promotes awn growth35, while a zinc finger protein expressed at the B1 locus suppresses awn development21,36. In barley, SHORT INTERNODES (SHI) controls awn elongation and pistil morphology22 and APETALA2 (AP2) regulates awn development, awn-lemma boundary and lemma identity37. Nevertheless, molecular and genetic mechanisms regulating barley and wheat awn development, as well as relationships between awn and grain yield, remain to be further elucidated.
MADS-box proteins, singly and in combinations, are the main regulators of floral organ development, as summarized in the ABCDE models of Arabidopsis and rice38. This model classifies their encoding genes into five different categories (A, B, C, D, and E) based on their homeotic functions; class E genes, encoding proteins that interact with the other four classes to regulate the development of each floral organ39, also known as SEPALLATA (SEP) genes that participate in a variety of other developmental processes, including spike architecture, flowering time, fruit ripening, and inflorescence thermo-morphogenesis40. Rice and barley contain 5 SEP genes (MADS1, MADS5, MADS34, MADS7 and MADS8), but a significant expansion has occurred in wheat38. Due to the lack of corresponding floral organ mutants and only a few known floral genes, the ABCDE model in barley and wheat has not been systematically described, and neither has the role of SEP genes in reproductive development, especially in floral organ development.
Here, we report that a SEP gene, HvMADS1, controls barley grain size and weight by regulating awn elongation and lemma transverse growth. We found that HvMADS1 regulates HvSHI and HvDL expression by directly binding to their promoters. The shi and dl mutations inhibited cell proliferation, creating shorter awns and/or narrower lemmas than wild-type (WT), mimicking the mads1 phenotype. Moreover, we found that A-class gene encoded HvAP2 interacts with HvMADS1 to promote transcriptional activity, thereby involving in awn and lemma development. We also verified that the role of MADS1 in controlling awn development is conserved in wheat. Our study provides information to broaden our understanding of barley and wheat floral organ development, and reveals a molecular and genetic role for HvMADS1 in controlling grain size in barley.
Results
HvMADS1 positively regulates awn development by promoting cell proliferation and further influences spike photosynthesis
Our previous study demonstrated that HvMADS1 in barley plays an important role in response to high temperature by maintaining a normal, unbranched inflorescence morphology40. Notably, as compared with WT, barley mads1 mutant exhibits shorter awn at ambient temperatures, indicating that HvMADS1 also regulates awn development40; however, the underlying explicit cellular and molecular mechanisms remain unclear.
We generated three knockout lines of HvMADS1 using CRISPR-Cas9 system, namely mads1-3, mads1-5 and mads1-8 in barley cv. Golden Promise (Supplementary Fig. 1a), with different alleles from those reported in previous studies40. All three homozygous mutant plants also exhibited significantly shorter awns than WT plants (Fig. 1a, d), confirming an important role of HvMADS1 in awn development as previously reported40. Cytological analyses on the central parts of the awns revealed that the width and thickness of mads1 awns were also significantly smaller than WT (Fig. 1b, d). The cell width in the transverse section of mads1 awns was slightly smaller than WT, while longitudinal cell length of mads1 was the same as WT (Fig. 1e); remarkably, cell numbers of mads1 awns in both directions were substantially reduced as compared with those in WT (Fig. 1e).
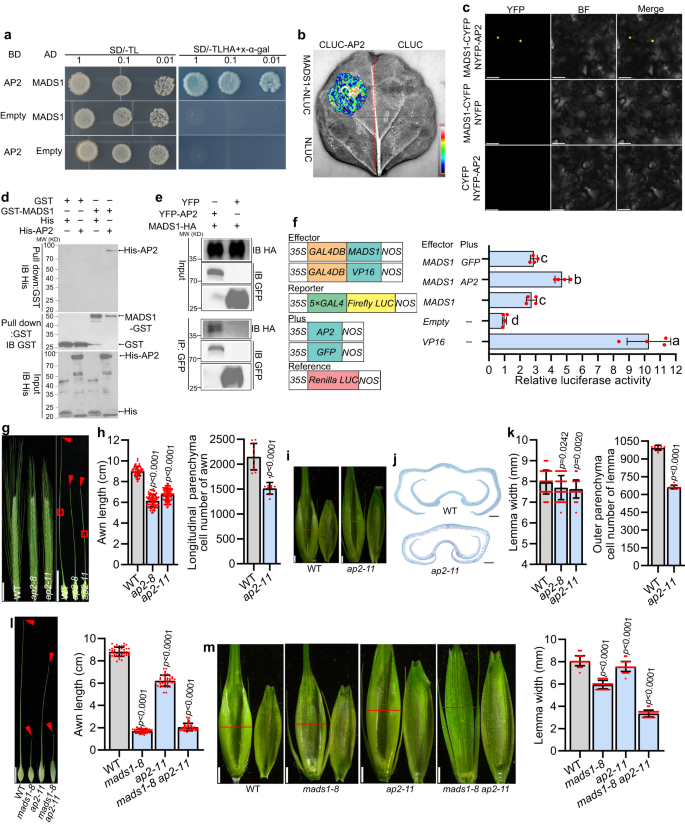
a Images of spike and awn of WT and mads1 knockout lines. Red arrowheads show awn tips; b Transverse (upper) and longitudinal (lower) sections of red boxed regions in (a). Black arrows indicate cells used for cell length measurement and cell number estimation in (e). c Photosynthetic light response curves of intact and de-awned WT and mads1 spikes (n = 3 biological replicates). PFD, photon flux density; Spike Anet, whole-spike net photosynthetic rate. d Statistic data of length, width, and thickness of WT and mads1 awns. e Statistic data of cell length and cell number in both transverse and longitudinal sections of WT and mads1 awns (n = 10 biologically independent samples). f Images of spikelet hulls of WT and mads1. Red lines indicate the position of the cross-section and location of the area used for scanning electron microscopy (SEM) in (g) and (h). g SEM images of the inner surface of WT and mads1 lemmas. h Magnified view of the red boxed lemma area in (g). i Statistic data of lemma width and inner epidermal cell width of WT and mads1 lemmas. j A cross-section of WT and mads1 spikelet hull. k Magnified images of the boxed lemma in (j). Black arrows indicate outer parenchymal cell layers. l Statistic data of the width and number of outer parenchyma cell across WT and mads1 lemmas (n = 10 biologically independent samples). Values are mean ± SD, p values obtained from two-tailed Student’s t-test; Scale bars, 2 cm (a), 100 µm (b, h), 1 mm (f), 0.8 mm (g), 500 µm (j), 50 µm (k). ch chlorenchyma tissue, le lemma, p parenchyma cells, pa palea, pi pistil, T thickness, vb vascular bundle, W width. Source data are provided as a Source Data file.
As the mads1 awn was significantly smaller than WT, the corresponding area of chlorenchyma tissue, which contributes to spike photosynthesis and thus grain filling20, was also obviously reduced (Fig. 1b). Consequently, the whole-spike net photosynthetic rate (spike Anet) measured on intact and de-awned WT and mads1 spikes showed that the Anet of mads1 spikes was significantly lower than that of the WT, although slightly higher than that of de-awned mads1 and WT spikes at a photon flux density (PFD) ≥ 250 μmol (photons) m−2 s−1 (Fig. 1c). Most interestingly, the grain weight of de-awned WT was about 6% lower than that of the non-de-awned WT (Supplementary Fig. 1b). Collectively, these results indicated that HvMADS1 controls cell proliferation to positively regulate awn size, which directly affects spike photosynthesis in barley.
HvMADS1 controls lemma width by promoting cell proliferation
MADS1 in rice is well-known for its multiple roles in floral organ identity specification; the osmads1 null mutant displays defective inner floral organs, and an elongated leafy lemma and palea39,41. The spikelets of barley mads1 mutant contained a pair of glumes, a lemma, a palea, and three whorls of internal floral organs, including two lodicules, three stamens, and a carpel, all of which morphologically resembled WT organs (Supplementary Fig. 1c, d, e). However, unlike WT, the mads1 lemma did not completely envelop the palea (Supplementary Fig. 1c, e). Further phenotypic observations found that lemma width, but not palea width or spikelet hull length, is significantly reduced in mads1 spikelets (Fig. 1f, g, i and Supplementary Fig. 1f, g). The width of inner epidermal cell of mads1 was indistinguishable from that of WT (Fig. 1g–i), as were the outer parenchyma cell width (Fig. 1j–l). Cytological analyses revealed that it is the cell number across the lemma width that is reduced in mads1 plants (Fig. 1l). Thus, HvMADS1 also positively regulates lemma width by promoting cell proliferation.
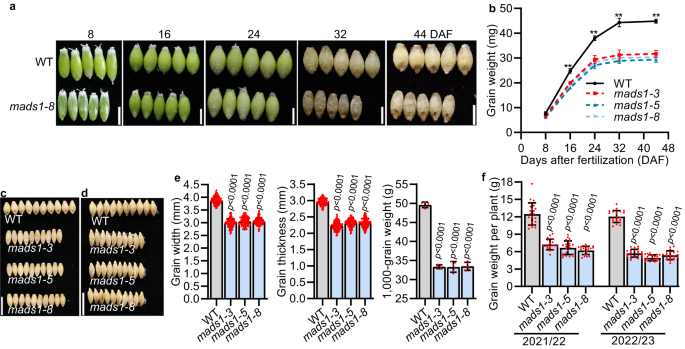
The mads1 mutant produces small grains
In barley and wheat, awns provide photosynthate for developing grains20,21. To examine whether HvMADS1 mediated awn size and lemma size ultimately affects grain size, we compared grain characteristics of WT and mads1 plants along grain development process. The obvious differences in grain size and weight were observed from 16 days after fertilization (DAF), in which the weight of mads1 grain was significantly lower than those of WT (Fig. 2a, b). Although there was a slight increase in length, significant decreases in both width and thickness of mads1 grain were observed as compared to WT (Fig. 2c–e and Supplementary Fig. 2a, b), which was consistent with observed narrower lemma in mads1 (Fig. 1f, i). To rule out the effect of carpel size on grain size, we also compared carpels size between WT and mads1 mutant, and found that the carpel size of the mutant was not significantly smaller than that of the WT (Supplementary Fig. 3a, b). In addition, to rule out the effect of endosperm on grain size we crossed mads1 (♀) with WT (♂), and found that grain size of all F2 progenies is similar to that of WT (Supplementary Fig. 3c, d). These results implied that the spikelet hull in barley is an important factor limiting grain size as it is in rice, as lemma width can directly affect the grain width and thickness (Fig. 2c–e).
Images (a) and staistic data of weight (b) of WT and mads1 grains at different developmental stage (n = 3 biological replicates). Images (c, d) and statistic data (e) of the width, thickness and 1,000-grain weight of mature WT and mads1 grains (n = 150 individual grains for grain size and n = 5 biological replicates for grain weight). f Staistic data of grain weight per plant of WT and mads1 grown in the paddy field in Shanghai for two consecutive years (2021–22 and 2022–23). Values are mean ± SD, two-tailed Student’s t-test was used to generate the p values; **p < 0.01; Scale bars, 0.5 cm (a), 1 cm (c, d). Source data are provided as a Source Data file.
The weight of mads1 grains was about 33 % lower than that of WT grain (Fig. 2e). This agronomically important result was confirmed in two-year field trials (Supplementary Fig. 2c). It is worth noting that although the spike length, spikelet number per spike and plant of mads1 were not significantly reduced compared with those of the WT (Supplementary Fig. 2d, e, f), grain numbers per spike and grain numbers per plant of mads1 were smaller than those of WT (Supplementary Fig. 2g, h). This was mainly due to much lower seed setting rate of mads1, which was 32-39% lower than that of WT (Supplementary Fig. 2i). Consequently, the overall grain yield (weight) per plant in mads1 was decreased 42–59% as compared with that of WT (Fig. 2f), suggesting that HvMADS1 is important for sustaining barley grain yield.
HvMADS1 expression promotes awn development and lemma transverse growth
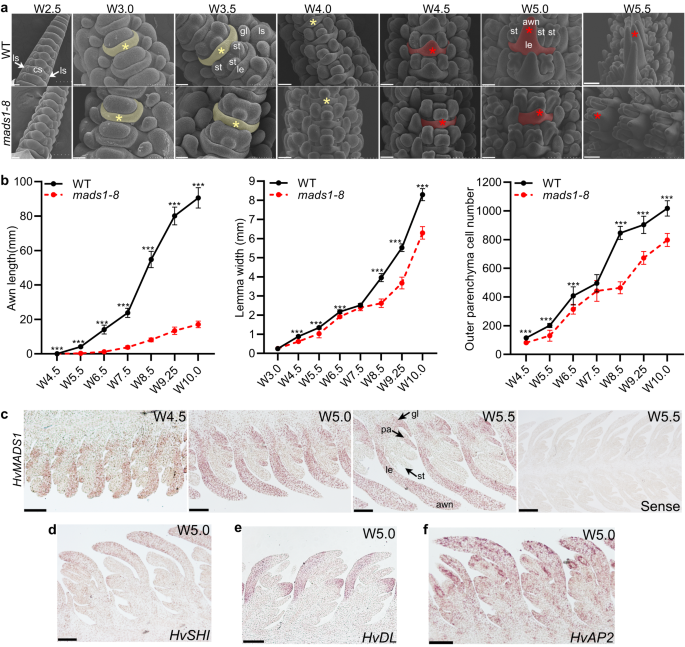
To determine the specific stages at which HvMADS1 affects awn and lemma differentiation and development, we compared WT and mads1 spikelet development using SEM at different Waddington stages (W2.5–W5.5)42, including W3.0 and W4.5 when lemma and awn primordia initiate, respectively. At stage W3.0, lemma primordia initiated normally in both WT and mads1 (Fig. 3a). During stages W4.5–W5.5, awn primordia emerged at the lemma apex similarly in both WT and mads1, which, however, elongated rapidly in WT but slowly in mads1 (Fig. 3a). Afterwards, awn length, lemma width, and the cell number of the lemma increased rapidly in WT but slower in mads1, especially after stage W7.5 (Fig. 3b).
a SEM images of WT and mads1 spikelets at different developmental stages (W2.5–W5.5). Yellow shading and star indicate lemma; red shading and star indicate lemma and awn. Scale bars, 50 µm (W3.0, W3.5), 80 µm (W2.5, W4.0, W4.5, W5.0), 160 µm (W5.5). b Dynamic changes of awn length, lemma width, and cell number across the lemma along the process of inflorescence development. The width and cell number of the lemma were obtained by analyzing lemma sections (n = 10 individual lemma samples for lemma width at stages W6.5-W10.0). c–f Results of RNA in situ hybridization of HvMADS1 (c), SHORT INTERNODES (HvSHI, d), DROOPING LEAF (HvDL, e) and APETALA2 (HvAP2, f) in longitudinal sections of developing WT inflorescences. Scale bars, 100 µm (except for W5.5 sense probe panel bar, 250 µm). (a, c–f) The representative results from three independent replicates were presented. Values are mean ± SD, asterisks indicate statistically significant differences (two-tailed Student’s t-tests; ***P < 0.001). cs central spikelet, gl glume, le lemma, ls lateral spikelet, pa palea, pi pistil, st stamen. Source data are provided as a Source Data file.
To confirm the correlation between HvMADS1 expression and observed phenotype, we performed gene expression analysis. Quantitative reverse transcription PCR (RT-qPCR) results revealed that HvMADS1 is relatively high expressed in developing inflorescences at stages W5.0–W9.5, the period of floral organ development including rapid growth of the lemma and awn (Supplementary Fig. 4a). RNA in situ hybridization revealed that HvMADS1 transcripts are abundant in the awn and lemma primordia at stages W4.5, W5.0, and W5.5 (Fig. 3c). Together, these results suggest that specific expression of HvMADS1 induces rapid cell division/proliferation in the lemma and awn.
HvMADS1 regulates hormone- and cell cycle–related genes
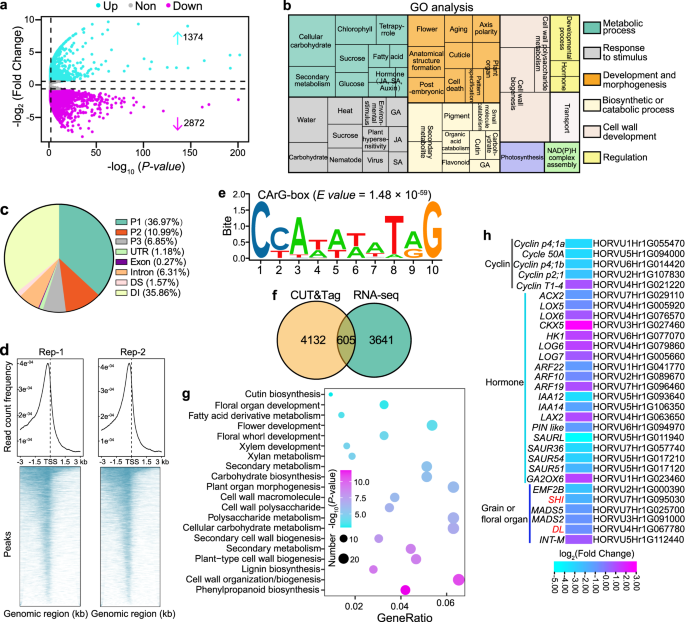
To better investigate biological processes influenced by HvMADS1 mutations, we performed transcriptomic (RNA-seq) analysis of WT and mads1 lemma and awn at stage W7.5 before the awn longitudinal growth and lemma transverse growth started to differ dramatically (Fig. 3b). Differential gene expression analysis detected 1,374 up-regulated and 2,872 down-regulated genes in mads1 relative to WT (Fig. 4a). Gene ontology (GO) term analysis revealed that differentially expressed genes (DEGs) are enriched for biological processes including flower morphogenesis, plant organ development, hormone metabolism, response processes, and cell wall development (Fig. 4b). Specifically, some of these DEGs are annotated to be involved in auxin, gibberellin, jasmonic acid, and cytokinin metabolic and signaling processes (Supplementary Fig. 5a), which is consistent with previous reports that there are many hormone-related genes downstream of MADS1, and that these four hormone families are involved in barley and/or rice spikelet development40,43,44,45. Additionally, expression of cell cycle-related genes was generally down-regulated in mads1 lemmas and awns, which was further confirmed by RT-qPCR (Supplementary Fig. 5b, c), consisting with the finding that HvMADS1 promotes cell proliferation in these tissues (Fig. 1).
a The Volcano plot of DEGs in lemma and awns between mads1 and WT. Blue and purple points represent up- and down-regulated genes, respectively; Gray points represent insignificantly changed genes. Cut-off value was set to false discovery rate (FDR) < 0.05, absolute fold change ≥ 1.5, FDR was adjusted by Benjamini–Hochberg correction. b Gene Ontology (GO) analysis of DEGs in (a). c CUT&Tag analysis of MADS1-binding regions in genomic regions of direct target genes: P1, 0–1 kb upstream of start codon; P2, 1–2 kb upstream of start codon; P3, 2–3 kb upstream of start codon; DS, 1–300 bp downstream of stop codon; DI, >300 bp downstream of stop codon. d The distribution of CUT&Tag peaks around transcriptional start sites (TSS). e Preferred CArG-box sequence for HvMADS1 binding. f The Venn diagram showing overlapped genes jointly identified by RNA-seq and CUT&Tag analyses. g GO enrichment analysis of 605 putative direct target genes identified from (f), p values were calculated by the Fisher method. h A heat-map showing expression patterns of putative HvMADS1 direct target genes in lemmas and awns between mads1 and WT.
To further unearth direct targets of HvMADS1 regulation, we performed Cleavage Under Targets and Tagmentation (CUT&Tag) assays using stage W7.5 lemma and awn from an existing HvMADS1-eGFP transgenic line40. We identified 4,737 predicted direct target genes (Supplementary Data 1); approximately half of HvMADS1 binding sites for these genes were located in the 2 kb promoter region upstream of the start codon (Fig. 4c), in agreement with the idea that HvMADS1 acts as a transcription factor. The highest frequency of binding occurred around the transcriptional start site (TSS; Fig. 4d), while one of the most enriched sequences for the in vivo HvMADS1-binding motif (E value = 1.48 × 10−59) was a cis-element CArG-box (Fig. 4e).
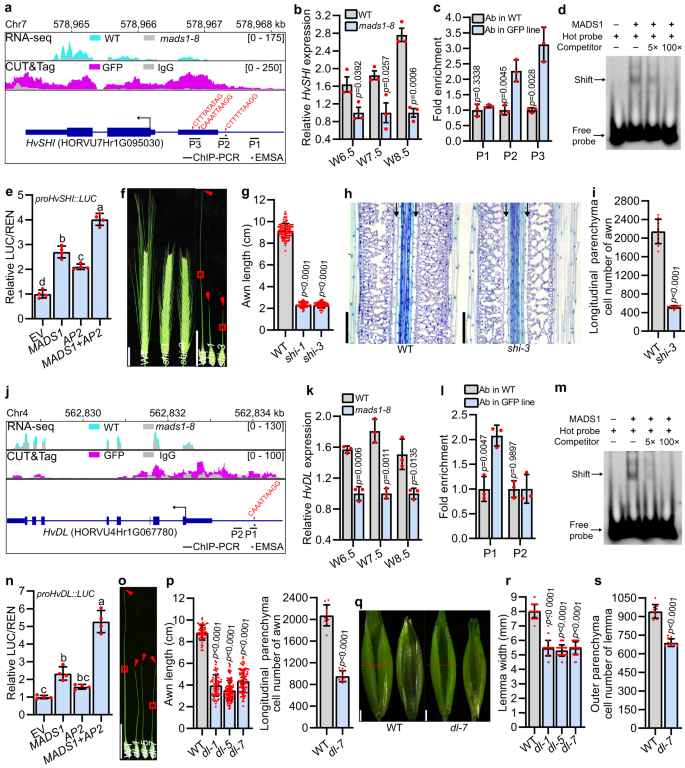
Intersection of RNA-seq and CUT&Tag data identified 605 genes as the most likely direct targets of HvMADS1 (Fig. 4f and Supplementary Data 2). Further GO analysis revealed that these targets are also significantly enriched in floral organ development, plant organ morphogenesis, and cell wall-related processes (Fig. 4g). Notably, the heatmap and genome browser view showed that HvSHI and HvDL expression is significantly suppressed in mads1 lemmas and awns (Figs. 4h, 5a, j and Supplementary Data 3). HvSHI is considered as a strong candidate for the short awn 2 (Lks2) trait involved in barley awn elongation22, whereas homologs of HvDL is involved in awn development in wheat and rice30,35. Our combined methods also revealed several other direct candidate targets of HvMADS1 that are involved in floral organ development, cell cycle, and auxin response processes (Fig. 4h and Supplementary Fig. 5d).
a, j Genome browser view of RNA-seq and CUT&Tag profiles and the schematic diagram of promoter fragments of HvSHI (a) and HvDL (j) for ChIP-PCR and EMSA assay in (c, d, l, m). Expression of HvSHI (b) and HvDL (k) in developing lemma and awn of WT and mads1. c, l Binding of HvMADS1 to the HvSHI (c) and HvDL (l) promoters in vivo as revealed by ChIP-PCR. Binding of MADS1 to probes from the HvSHI (d) and HvDL (m) promoters harboring a CArG-box (EMSA), a representative result from three replicates was presented. Transactivation activity assay of HvMADS1 and HvMADS1-HvAP2 interaction on HvSHI (e) and HvDL (n). Different letters represent significant differences (p < 0.05) determined by one-way ANOVA with Tukey’s multiple comparisons test. Images of shi (f) and dl (o) awns. Longitudinal sections of boxed regions in (o) are shown in Supplementary Fig. 7c. g Statistic data of WT and shi awns. h Longitudinal sections in the boxed regions of (f). Black arrows indicate cells used to measure cell length for cell number estimation in (i). i, Statistic data of longitudinal parenchyma cell number in WT and shi awns. p Statistic data of the length and cell number of WT and dl awns. q Images of spikelet hulls of WT and dl. Red lines indicate the position of the cross-section used in (r, s). Statistic data of the width (r) and number of the outer parenchyma cell (s) in the lemma of WT and dl. Values are mean ± SD, p values shown are from two-tailed Student’s t-test; n = 3 and 4 biological replicates for (b, c, k, l) and (e, n), respectively. Scale bars, 2 cm (f, o), 1 mm (q), 100 µm (h). Source data are provided as a Source Data file.
HvMADS1 could regulate awn and lemma development through direct regulation of potential targets HvSHI and HvDL
Expression analysis revealed that both HvSHI and HvDL are expressed in the developing inflorescence of WT (Supplementary Figs. 6a, 7a), and that their expression levels are significantly downregulated in mads1 lemma and awn at stages W6.5, W7.5, and W8.5 (Fig. 5b, k). In situ hybridization confirmed the specific expression of HvSHI in awn (Fig. 3d and Supplementary Fig. 4b) and HvDL in both lemma and awn (Fig. 3e and Supplementary Fig. 4c), suggesting that these two genes likely function in awn and/or lemma development. ChIP-qPCR assays further corroborated that HvMADS1 directly binds in vivo to HvSHI and HvDL promoter fragments containing a CArG-box motif (Fig. 5c, l), which was subsequently verified in vitro by electrophoretic mobility shift assay (EMSA; Fig. 5d, m). Dual-luciferase (LUC) assays in barley protoplasts showed that HvMADS1 can transiently activate the expression of HvSHI and HvDL (Fig. 5e, n), confirming that HvMADS1 can directly bind to promoters of HvSHI and HvDL to activate their transcription.
To confirm whether HvSHI and HvDL affect barley awn and lemma development, we created HvSHI mutants (named shi-1 and shi-3; Supplementary Fig. 6b) and HvDL mutants (dl-1, dl-5 and dl-7; Supplementary Fig. 7b) via CRISPR/Cas9-mediated gene editing. As in mads1 mutants, awns of the shi and dl mutants were significantly shorter than that of WT, primarily due to a reduction in cell number rather than in cell size (Fig. 5f–i, o, p, and Supplementary Fig. 6c, 7c, d). Correspondingly, the 1,000-grain weight of shi mutant was significantly smaller than that of WT (Supplementary Fig. 6d).
Notably, our observations showed that the dl mutant exhibits a significantly narrower lemma (Fig. 5q, r) but similar spikelet hull length and palea width, as compared with WT (Supplementary Fig. 7e, f). As dl mutants were unable to set seeds due to defective pistils with ectopic carpel-like organs (Supplementary Fig. 7g), the grain size of dl mutants could not be obtained. Microscopic observation of lemma cross-sections further revealed significantly reduced cell number in dl, which mimicked the defect in mads1 (Fig. 5s and Supplementary Fig. 7h). SEM results confirmed that, similar to mads1, it is not the initiation of dl lemma but the growth of shi and dl awns is slowed down as compared to that in WT (Supplementary Fig. 8). Correspondingly, expression levels of a number of cell cycle genes, whose expression was significantly decreased in mads1, were also remarkably down-regulated in shi and dl mutants (Supplementary Fig. 6e, 7i). These results implied that the direct regulation of potential targets HvSHI and HvDL by HvMADS1 could contribute to HvMADS1-dependent regulation of awn and/or lemma development through controlling cell proliferation.
Interaction of HvMADS1 with HvAP2
SEP proteins, to which MADS1 belongs, often form higher-order complexes with other ABCDE proteins to regulate floral organ development46,47. Our yeast two-hybrid (Y2H) assay confirmed above mentioned idea and found that HvMADS1 interacts with other A-, B-, C-, D-, E-class, and AGL6 subfamily homeotic proteins except HvMADS4 and HvMADS14 (Supplementary Fig. 9). Most interestingly, HvMADS1 interacted with HvAP2 in yeast cells (Fig. 6a). Considering the fact that HvAP2 was reported to regulate awn development37, we speculate that HvMADS1 and HvAP2 might function together in barley awn development. The interaction of the HvAP2 with HvMADS1 was subsequently confirmed by split firefly luciferase complementation (SFLC) assays and bimolecular florescence complementation (BiFC) assays in tobacco epidermal cells (Fig. 6b, c), and further validated by in vitro pull down and in vivo Co-immunoprecipitation (Co-IP) assays (Fig. 6d, e). Further investigations showed that the intervening (I) and keratin-like (K) domain of HvMADS1 are required for physical interaction with HvAP2 (Supplementary Fig. 10a), and that HvAP2 interacts with HvMADS1 likely through its C- and N-terminal domain (Supplementary Fig. 10b). A transient transcriptional activity assay demonstrated that the interaction of HvMADS1 with HvAP2 significantly enhances transcriptional activity of HvMADS1 (Fig. 6f). Additional RT-qPCR and in situ hybridization revealed that HvAP2 is ubiquitously expressed in developing floral organs, including in the awn and lemma during inflorescence development (Fig. 3f, Supplementary Figs. 4d and 11a), which overlapped with that of HvMADS1.
a–e Interaction between MADS1 and HvAP2. Yeast two-hybrid assay (a): AD, GAL4 activation domain; BD, GAL4 DNA-binding domain; SD/-TL and SD/-TLHA, synthetic defined medium without Trp and Leu or without Trp, Leu, His and Ade, respectively. Split firefly luciferase complementation (SFLC) assay in tobacco leaves (b): Scale bar represents luminescence intensity. Bimolecular florescence complementation (BiFC) assay in tobacco epidermal cells (c). In vitro pull-down assay (d): GST-MADS1 was incubated with His-AP2, pulled down using anti-GST beads, and detected by anti-His immunoblotting. IB, Immunoblot. In vivo Co-immunoprecipitation (Co-IP) assay in tobacco leaves (e): GFP beads were used for immunoprecipitation (IP). f Effects of HvMADS1–HvAP2 interaction on HvMADS1-induced transcriptional activation (n = 4 biological replicates); Different letters indicate significant differences according to one-way ANOVA with Tukey’s multiple comparisons test (p < 0.05). Images (g) and statistic data of length, and cell number (h) of WT and ap2 awns. Longitudinal sections of boxed regions in (g) are shown in Supplementary Fig. 11c. i Images of WT and ap2 spikelet hulls. Red lines indicate the position of the cross-section used in (j, k). j Cross-sections of WT and ap2 spikelet hulls. k Statistic data of the width and outer parenchyma cell number of WT and ap2 lemmas. Statistic data of awn length (l), images and statistic data of lemma width (m) of WT, mads1-8, ap2-11 and mads1-8 ap2-11 double mutant. Values are mean ± SD, p values shown are from two-tailed Student’s t-test; For (a, b, c, d, e), a representative result is shown from three independent replicates. Scale bars, 50 μm (c), 2 cm (g), 1 cm (l), 1 mm (i, m), and 250 µm (j). Source data are provided as a Source Data file.
To further explore the functions of HvAP2, we generated ap2 knockout mutants (ap2-8 and ap2-11; Supplementary Fig. 11b). Phenotypic analysis confirmed that HvAP2 also promotes awn growth mainly by affecting cell number (Fig. 6g, h, and Supplementary Fig. 8, 11c, d), and that the ap2 lemma is also slightly narrower due to the reduced cell number (Fig. 6i–k). The width and thickness of ap2 grain were significantly narrower than WT (Supplementary Fig. 11g, h). The observed phenotypes of awn length and grain size of ap2 mutants are consistent with previous reports37. To further test the genetic relationship between MADS1 and AP2 with respect to awn length and lemma width, we created mads1-8 ap2-11 double mutant by crossing mads1-8 with ap2-11. Phenotypic similarities of the degree of awn length shortening between mads1-8 and mads1-8 ap2-11 (Fig. 6l) indicated that HvMADS1 is genetically epistatic to HvAP2 in the control of awn growth. The lemma width of double mutant was significantly reduced, much narrower than that of mads1-8, ap2-11 and WT (Fig. 6m). Taken together, these findings indicate that HvMADS1 and HvAP2 synergistically regulate the awn and lemma development. Expression analysis found that expression levels of HvSHI and HvDL, as well as several cell cycle genes that are downregulated in mads1, are also significantly reduced in ap2 mutants (Supplementary Fig. 11i). Dual-LUC assays demonstrated that HvSHI and HvDL promoter activation is much significantly enhanced when HvAP2 and HvMADS1 are co-transformed than HvMADS1 alone (Fig. 5e, n). Thus, HvMADS1 and HvAP2 may form a complex and regulate the common target genes HvSHI and HvDL to control awn size and/or lemma transverse growth.
The function of bread wheat MADS1 in regulating awn size
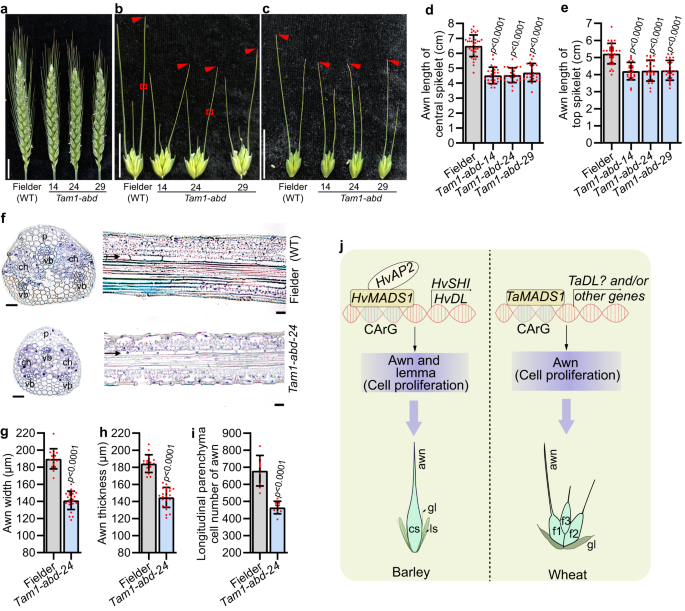
There are three wheat homologs of HvMADS1, namely TaMADS1-A (TraesCS4A01G058900), TaMADS1-B (TraesCS4B01G245700), and TaMADS1-D (TraesCS4D01G243700; Supplementary Fig. 12a). To investigate whether TaMADS1 is involved in wheat awn and lemma development, we generated a triple recessive mutant of TaMADS1 (Tam1-abd) in hexaploid bread wheat cultivar Fielder by CRISPR-Cas9 (Supplementary Fig. 12b). Interestingly, the awns of both central and top spikelets in Tam1-abd were significantly smaller compared to those of WT (Fig. 7a–e, g, h). Further histological experiments demonstrated that the number of longitudinal parenchyma cell of the Tam1-abd awn was reduced by 31.8 % compared to that of WT, while the cell length was not significantly changed (Fig. 7f, i and Supplementary Fig. 12c). Thus, consistent with barley results, reduction in cell number due to the Tam1-abd mutation likely resulted in shorter awns in wheat mutant. Notably, Tam1-abd did not show any phenotypes in lemma and other floral organs, due likely to the functional redundancy since TaMADS1 has duplicated in wheat (Supplementary Fig. 12a).
a Images of Fielder (WT) and three independent Tam1-abd mutant spikes at heading day. b, c Images of WT and Tam1-abd awns from the central (b) and top (c) spikelets. Statistic data of awn lengths from the central (d) and top (e) spikelets. f Sections from the boxed regions of (b) showing the size and number of cells in WT and Tam1-abd awns. g–i Statistic data of width (g), thickness (h), and longitudinal parenchyma cell number (i) of WT and Tam1-abd awn. j A proposed model for MADS1-mediated awn and lemma development. In barley, HvMADS1 and HvAP2 form a complex that jointly activates potential downstream genes HvSHI and HvDL, further influencing cell proliferation and ultimately controlling the elongation of the awn and lemma transverse growth. In wheat, TaMADS1 can also control awn elongation by influencing cell proliferation. Values are mean ± SD, p values obtained from two-tailed Student’s t-test; Scale bars, 2 cm (a, b, c), and 25 µm (f). ch chlorenchyma tissue, f1–3 flower 1–3, cs central spikelet, gl glume, p parenchyma cells, ls lateral spikelet, vb vascular bundle. Source data are provided as a Source Data file.
Discussion
Grain size is both a critical yield trait and a complex developmental trait regulated by many factors, including the photosynthetic rate of source tissue and spikelet hull size4,17. Research on regulators of barley grain size has lagged significantly behind that of rice due to the lack of mutants, artificial populations for genetic analysis, and natural populations with high-quality sequencing data. With the help of CRISPR-Cas9 genome editing technology, we have functionally characterized that HvMADS1 controls barley grain size and weight by regulating awn length that influences spike photosynthesis and lemma width that affects spikelet hull size. We have further shown that HvMADS1 directly regulates downstream target genes, and can physically interact with other proteins to do so; its molecular network in barley has been proposed based on our multiple level experimental evidence (Fig. 7j). Notably, the function of MADS1 in controlling awn length is conserved in wheat. Since TaDL has also been reported to be involved in wheat awn development35, further studies on the regulatory relationship between TaMADS1 and TaDL will help to establish the wheat molecular network for awn development.
Divergence in biological function of genes can arise through sub- or neo-functionalization. We show here that SEP genes may undergo functional divergence among rice, barley, and wheat. In rice, LOFSEP genes (OsMADS1, OsMADS5 and OsMADS34) are predominantly involved in inflorescence branching, spikelet and floral development48; while SEP3 clade genes (OsMADS7 and OsMADS8) regulate inner floral organ development49. Unlike OsMADS1, which is responsible for floral organ identity specification in rice, HvMADS1 seemed to only regulate awn length and lemma width without changing floral organ properties in barley. This finding is consistent with our previous work that single or double mutants of barley LOFSEP genes (HvMADS1, HvMADS5 and HvMADS34) have no obvious phenotypic changes in floral organs, spikelet and inflorescence branching under normal temperature40. There are four SEP genes in Arabidopsis, including AtSEP3 and the LOFSEP clade (AtSEP1, AtSEP2, and AtSEP4); any single mutant shows no or weak phenotypes. However, in the quadruple atsep1/2/3/4 mutant, all floral organs are transformed into leaf-like structures50, and only quadruple mutant of AtSEP4 and three MADS-box genes of other classes (SOC1/SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1, SVP/SHORT VEGETATIVE PHASE and AGL24/AGAMOUS-LIKE 24), namely, atsep4/soc1/svp/agl24, is defective in inflorescence branching51. The LOFSEP genes have undergone significant duplication in wheat; MADS1 and MADS5 are sisters to 10 and 7 wheat proteins, respectively52. Thus, we speculate that SEP genes have diverged through sub-functionalization in barley and wheat, and that some functions of barley and wheat SEP genes remain unidentified due to redundancy with SEP genes or with other classes of MADS-box genes. The mads1/5/34 mutant, mads1/5/34/7/8 mutant, higher order mutants of HvSEP genes with other barley MADS-box genes, and the equivalent mutants in wheat, will need to be created to further unravel the molecular regulatory network of SEP genes in Triticeae crops.
A combined RNA-seq and CUT&Tag approach identified multiple direct target genes of HvMADS1, including genes involved in the synthesis and signaling of hormones, such as cytokinin, gibberellins, jasmonates, and auxin, which is consistent with previous ChIP-seq data that OsMADS1 can bind to auxin-related genes44. In rice, auxin response factors OsETT230 and OsARF653, and the cytokinin synthesis protein OsAn-214,26, control awn length and/or grain size; future studies in barley could reveal whether these hormone-related genes regulate awn size and spikelet hull growth or not. Interestingly, the mads1 mutation affected expression of multiple cell-cycle genes, and whether HvMADS1 can directly bind to promoters of these genes to regulate awn and lemma development requires further investigations.
HvSHI, a downstream target gene of HvMADS1, affects awn growth through regulating cell proliferation, which is in accord with the HvMADS1 mechanism; both mutants also have a similar degree of awn shortening. Although the awn extends from the lemma tip, it is currently believed that the awn is a modified leaf, while the lemma may be modified sepal or novel organ with bract and sepal features36,54. Since HvSHI is specifically expressed in the awn primordia, it is reasonable that it affects only awn development but not lemma development, which also supports the idea that awn and lemma development are independent processes. The fact that the 1000-grain weight of shi grains was significantly lighter than those of WT with a 6.1% reduction (Supplementary Fig. 6d) indicate that this difference is due to the diminished photosynthetic capacity of the shorter awn, which is consistent with the results of artificial de-awning that reduces WT grain weight (Supplementary Fig. 1b). In rice, the regulatory mechanisms of grain size have been elaborated in detail5,55, and several recent reviews have also summarized these regulatory pathways in detail4,56. Generally, cereal grain size is mainly determined by maternal spikelet hull, pericarp, overall pistil (including carpel), and endosperm5,7,8,9,56,57,58,59. In this study, we also demonstrated that changes in mads1 grain weight is highly associated with changes in lemma width (Figs. 1i, 2e). We also ruled out the carpel and endosperm as the cause of the smaller mads1 grains (Supplementary Fig. 3a–d). Thus, the 33% reduction in grain weight of mads1 grain (Fig. 2e) is thus mainly due to the narrower lemma width; nevertheless, currently we cannot completely rule out the effect of pericarp. It is worth noting that plot sizes used in our field trials (around 2 m2/line) were much smaller than those typically used by breeders in breeding selections. In addition, our field experiments were carried out in the same region for two consecutive years without setting up of different plot replications for the same line. Therefore, larger-scale field trials with plot replications in different regions are needed for subsequent field yield analyses.
The lemma and palea together protect the inner floral organ to ensure its stable growth and seed setting, and help to define the final grain size. The reduced seed setting rate of mads1 plants in field trials (Supplementary Fig. 2) may be due to the inability of the narrowed lemma to enclose the palea, thus resulting in florets opening at the fertilization stage. Although ongoing research has revealed several genetic factors that control lemma development in rice60,61,62, for example, OsDL regulates vascular patterning of the lemma63, but its mechanism of regulating lemma development is not clearly elucidated. In barley, even less is known about factors affecting lemma development. Here, we have shown that HvDL, the other downstream target gene of HvMADS1, affects lemma width; the lemma width of dl mutant is significantly reduced (Fig. 5r), mimicking the mads1 phenotype. Interestingly, HvDL also controls carpel properties (Supplementary Fig. 7g) and the number of lemma vascular bundles (Supplementary Fig. 7h), suggesting that there may be other proteins or pathways that control HvDL expression to regulate floral organ properties. Further investigations into upstream and downstream regulatory networks of the HvMADS1–DL module may help to identify the molecular regulators of floral organ development, including lemmas, in barley. Combining a series of biochemical experiments with the phenotypes of single mutants, we inferred that the binding of HvMADS1 to HvSHI and HvDL is critical for HvMADS1 in controlling awn length and lemma width. The future work should focus on the genetic analysis of double mutants (mads1 shi and mads1 dl), mads1 mutants overexpressing HvDL and/or HvSHI, and transgenic plants with site-directed mutated motifs of HvDL/HvSHI that bound by HvMADS1, to further strengthen the understanding of the biological significance of the HvMADS1-HvSHI/HvDL module.
Previous studies have shown that HvAP2 has pleiotropic roles in floral organ and grain development, regulating lemma identity and length as well as grain length and width37. In this study, we measured the width of the entire unfolded lemma rather than the curled lemma as did in other studies37. We found that HvAP2 also regulates lemma transverse growth since the cell number in the transverse section of ap2 lemma was significantly reduced, lemma width of ap2 mads1 double mutant was narrower than those of single mutants (Fig. 6k, m). A previous study assumed that HvAP2 could control floral development by modulating its targeting genes including HvMADS137. Our genetic and biochemical analyses results in this study verified that HvAP2 interacts with HvMADS1, forming a complex, at least in part, to participate in a common pathway promoting awn development and lemma transverse growth (Fig. 6). Interestingly, Arabidopsis AP2 is a repressor, which interacts with co-repressors TOPLESS to regulate downstream floral homeotic genes to control floral organ development64. More studies are needed to clarify the working model for HvAP2, and to answer following questions: whether HvAP2 can also act as a repressor? Is there a co-repressor similar to TOPLESS in barley? How do the two interact and thus participate in barley floral organ development?
Although grain size is a key trait for crop breeding, genes affecting grain size in an agriculturally meaningful way are still a scarce resource. In rice, a C-terminal mutation in MADS1 forms a semidominant allele that promotes longitudinal cell proliferation in the spikelet hull, and introduction of this allele into several cultivars is favorable for both grain length and yield improvement55,65. Our results here suggest that MADS1 can regulate other aspects of grain development to affect final grain size, so genome-edited superior alleles of MADS1 will be a key resource in the future for high-yield barley breeding.
Methods
Plant materials and growth conditions
Barley (Hordeum vulgare cv. Golden Promise) was planted either in a greenhouse with a 16 h photoperiod at 18 °C/15 °C (day/night) or in a field in Minhang (31° 04′ N, 121° 45′ E; Shanghai, China) under natural conditions during November 2021–June 2022 and November 2022–June 2023. For field experiments, each WT and mads1 mutant barley line was planted in ten rows with 0.20 m between rows, the length of each row is about 1 meter and 15 seeds were planted per row. These barley plants were grown under normal conditions and followed local agricultural practices for field management, avoiding floods, droughts, pests and diseases. 300 kg compound fertilizer per hectare (Nitrogen: Phosphorus: Potassium = 15%: 15%: 15%,) and 250 kg urea fertilizer per hectare were applied at the sowing and jointing stages, respectively. In the June of the following year, plants were randomly sampled to measure spike length, spikelet number per spike/plant, grain number per spike/plant, grain weight per plant, seed setting and thousand-grain weight. Grain weight and size were measured in grains that were treated in a 37 °C oven for one week. Wheat (Triticum aestivum cv. Fielder) was grown in a greenhouse with a photoperiod of 16 h at 20 °C/16 °C (day/night). For BiFC and SFLC assays, tobacco (Nicotiana benthamiana) was planted in a greenhouse at 23 °C with a 16 h/8 h (day/night) period.
Knockout mutants of HvMADS1, HvSHI, HvDL, and HvAP2 were generated by CRISPR-Cas9 technology. For each gene, two targets were chosen, one single guide RNA (sgRNA) was driven by the OsU6a, and the other by the OsU6b; the tandem sgRNA cassette was cloned into pYLCRISPR–Cas9Pubi–H66. Agrobacterium tumefaciens AGL1–mediated transformation of immature barley embryos67 and selection of mutant lines were performed. A Cas9-sgRNA expression vector for TaMADS1 knockout was constructed and introduced into Agrobacterium EHA105 for transformation of immature wheat embryos68. Homozygous progeny plants (≥T2 generation) were used in the analyses. Primers used for constructs are listed in Supplementary Data 4.
Morphological and histological analysis
Barley grains and spikelets were collected from the central parts of the main spike of at least ten individual plants per genotype for phenotyping analysis. For wheat, at least twenty-four spikelets from main spike were collected from the central and top parts of at least eight individual plants for each genotype for phenotypic observation. Barley spike photosynthetic gas exchange parameters were measured as described69 with minor modifications. Briefly, the entire awn on all spikelets of uniform main spikes of healthy WT plants at heading were cut off using sharp blades, and the whole-spike net photosynthetic rate was measured three days after de-awning to rule out the effect of wounding. The grain weight of both WT and de-awned WT was measured at harvest time. For SEM analysis, fresh awn, spikelet, and inflorescence were fixed in fresh FAA (3.5% formalin, 5% acetic acid and 50% ethanol) for 24 h. After being dehydrated in a graded ethanol series, samples were dried and coated with gold using Leica EM SCD050 sputtering device, then photographed with a scanning electron microscope (Hitachi, S-3400N). For paraffin sectioning, after being dehydrated and infiltrated by Histo-Clear II and ethanol, samples were transferred into paraffin, sectioned at 8 µm thickness, stained with toluidine blue, and observed under the light microscope (Nikon, ECLIPSE 80i). ImageJ software was used to calculate cell length, cell width and cell number, and at least 9 individual samples were used for such analyses. For the length and number of awn cells, the awn was longitudinally cut at the middle part of the awn, cell length per awn across the cut entire awn region was measured, and the resulting value was divided by the awn length to yield cell numbers per awn. For the size and number of lemma cells, lemma was cut transversely at the middle part of the lemma, and cell length and cell numbers were directly measured and counted, respectively.
In situ mRNA hybridization
Fresh developing inflorescences were cut and immediately fixed in FAA solution for 6 h, then dehydrated, infiltrated, embedded, and sectioned as described above for paraffin sectioning. RNA probes (sense and anti-sense) were labelled using the SP6/T7 DIG RNA labeling kit (Roche) according to manufacturer’s instructions, and listed in Supplementary Data 4. Pre-treatment of sections, hybridization, and immunological detection were performed70.
RT-qPCR and RNA-seq analysis
Lemma and awn from spikelets in the central part of stage W7.5 spike from WT and mads1 mutants were collected for RNA-seq and RT-qPCR. In addition, whole shoot (7 days after germination), developing inflorescences at W2.0–W9.5, mature lemma, awn, palea, and developing grains from 0–35 days after fertilization (DAF) were collected from WT and mads1 plants for spatiotemporal expression analysis of HvMADS1, HvSHI, HvDL and HvAP2 expression. All tissues collected contain three biological repeats, each is a pooled tissue from 15-30 individual plants. Total RNA was isolated using TRIzol Universal Reagent (Tiangen) and cDNA synthesized using the Tiangen FastQuant RT Kit according to manufacturer’s instructions. Quantitative real-time PCR (RT-qPCR) assays were performed using QuantiNova SYBR Green PCR Kit (Qiagen). Expression of HvActin7 was used as the internal control71 and primers used for RT-qPCR are all listed in Supplementary Data 4.
For transcriptome analysis (RNA-seq), library construction and sequencing were performed by BGI (Wuhan, China) using an Illumina 2500 platform. After screening and trimming, clean reads were mapped to the barley reference genome, and transcript levels calculated as fragments per kilobase million (FPKM) using RSEM (v1.3.3). Differentially expressed genes (DEGs) were selected using R package DESeq2 (v.1.30.0) with a criterion of |fold change| ≥ 1.5 and false discovery rate <0.05. DEGs were analyzed for enriched pathways by Gene Ontology (GO) using AgriGO272. REVIGO was used to summarize and visualize the GO term results73.
Cut&Tag and ChIP-qPCR
Cut&Tag treatments were performed as described74 with minor modifications. Nuclei were extracted from W7.5 lemma and awn of pro::HvMADS1–eGFP transgenic plants (created in our previous work40), each with two biological replicates. Primary antibodies were anti-GFP (ab290, Abcam) and IgG (as control; 12-370, Sigma–Aldrich), and the guinea pig anti-Rabbit IgG (PAB9407, Abnova) was used as secondary antibody. The tagmentation assay was performed after use of Tn5 transposase according to the manufacturer’s instructions (Vazyme, S603-01). Libraries were prepared using the TruePrep Index Kit V2 for Illumina (Vazyme, TD202) and NEBNext Hi-Fi mix (NEB, M0541S), purified with AMPure beads (Beckman, A63881), and sequenced at Personal Biotechnology (Shanghai) with an Illumina Nova. Cut&Tag data processing and analysis were performed based on the existing pipeline74,75.
The ChIP-qPCR assay was performed as reported with minor modifications76. Approximately 2 g of W7.5 inflorescences from WT and pro::HvMADS1–eGFP plants were cross-linked with 1% (v/v) formaldehyde and ground in liquid nitrogen. The chromatin was sonicated, then the DNA fragments were incubated with GFP-Trap Magnetic Agarose (ChromoTek, Munich). Immunoprecipitated DNA was analyzed by quantitative PCR using HvSHI and HvDL primers (Supplementary Data 4). Samples prior to precipitation were used as input controls, and the final result was a comparison of the enrichment levels of target genes between WT and GFP lines.
Electrophoretic mobility shift assay (EMSA)
The complete MADS1 coding sequence (CDS) was fused to 6×His and cloned into the pGADT7 vector for in vitro protein synthesis using the TNT T7/SP6 Wheat Germ Protein Expression System (Promega). Briefly, the recombinant MADS1–6×His protein was incubated with fluorescein amidite (FAM)-labeled probe and competition probe in binding buffer for 30 min at 25 °C. Samples were loaded onto a 6% native polyacrylamide gel at 4 °C, and the FAM signals were detected by the Cy2 channel of a ChemiDoc MP imaging system (BioRad). All probe sequences are listed in Supplementary Data 4.
Dual-luciferase (dual-LUC) and transcriptional activity assays in barley protoplasts
The preparation and transformation of barley protoplasts77 were performed. For dual-LUC assays, the HvSHI and HvDL promoters were amplified and cloned into pGreenII-0800-LUC to drive luciferase (LUC) expression as reporters. Effectors were created by ligating HvMADS1 and HvAP2 CDS into the pGreenII-0000 construct. Reporter and effector vectors were co-transformed into barley protoplasts via polyethylene glycol-mediated transformation78, and cultured overnight at 22 °C in the dark. LUC activity was measured using a Dual-Luciferase Reporter Assay System (Promega) with Renilla luciferase (REN) as an internal control.
For the transcriptional activity assay, 5×GAL4-LUC and 35S::REN were used as the reporter and the internal control, respectively. 35S::HvAP2 or 35S::GFP were used as plus vectors. Full-length CDS of MADS1 and VP16 (constitutive transcriptional activator) were fused to GAL4BD to generate effector vectors. Protoplast transformation and LUC/REN measurement followed the same protocols as for dual-LUC assays.
Yeast two-hybrid assays
Yeast two-hybrid assays were performed following manufacturer’s instructions (Clontech). The full-length or truncated CDS of HvMADS1 and of HvAP2 were amplified and cloned into inserted into pGADT7 and pGBKT7, respectively. Different combinations of these vectors were co-transformed into yeast strain AH109 and selected on SD-Leu/-Trp or SD-His/-Ade/-Leu/-Trp for 2–3 days.
Split firefly luciferase complementation (SFLC) and bimolecular fluorescence complementation (BiFC) assays
For SFLC assays, HvMADS1 and HvAP2 CDS was cloned into the pCAMBIA1300-nLUC and pCAMBIA1300-cLUC vectors79, respectively. For BiFC assays, HvMADS1 and HvAP2 CDS was ligated into pXY104-cYFP and pXY106-nYFP80, respectively. Recombinant constructs were transformed into A. tumefaciens GV3101 and different combinations of Agrobacterium were co-infiltrated into tobacco leaves. Subsequently, the plants were grown in the dark for 48 h, and the activated luciferase reconstitution signals were captured with Tanon 5200 imaging system after injecting 1 mM luciferin to leaf regions. The YFP fluorescence signal was observed under a Leica SP5 confocal laser-scanning microscope.
In vitro pull-down assay
The pull-down assay was performed as described in the Glutathione Affinity Handbook (Qiagen, 3rd Edition). The HvMADS1 and HvAP2 CDS was cloned into pGEX4T1 and pET-32a to produce GST-MADS1 and His-AP2, respectively. The GST-MADS1 and His-AP2 recombinant proteins were expressed in Escherichia coli BL21 (DE3), and purified with Glutathione HiCap Matrix resin (Qiagen) and Ni-NTA Agarose (Qiagen), respectively. GST-MADS1 and GST were incubated with glutathione beads, respectively, and then His-AP2 or His added to the reaction, followed by incubation overnight at 4 °C. Beads were washed and the eluted proteins were separated by 10% SDS-PAGE, and finally immunoblotted and probed with anti-GST and anti-His antibody, respectively.
In vivo co-immunoprecipitation (Co-IP) assay
Co-IP assays were performed as described81 with minor modifications The 35S::MADS1-HA and 35S::YFP-AP2 plasmids in Agrobacterium GV3101 were transiently co-expressed into tobacco leaves as described above. The empty-vector 35S::YFP was used as a negative control. Proteins were isolated with extraction buffer containing 10 mM Tris–HCl at pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 0.2 % TrixtonX-100, 0.2 % NP-40, 1 mM DTT, 2% (v/v) PVP40, 0.1% (v/v) Tween-20, and 1×protease inhibitor cocktail (Roche). A small portion of the protein extract was saved as input and the rest incubated with GFP beads (GFP-Trap® Agarose, Chromo Tek) for 4 h at 4 °C. Beads were then denatured in protein loading buffer, and subjected to SDS-PAGE. Anti-GFP (G1544, Sigma) and anti-HA (M20003, Abmart) antibodies were used to detect protein interaction signals.
Phylogenetic analyses
Homologous proteins in other species, including wheat, Brachypodium, sorghum, and rice, were obtained using the HvMADS1 (HORVU4Hr1G067680) protein sequence to query against the EnsemblPlants database (http://plants.ensembl.org). The amino acid alignment of these MADS-box proteins was imported into MEGA5 using the neighbor-joining method to generate a phylogenetic tree with bootstrap values of 1000 replicates.
Statistics & Reproducibility
Statistical analyses of all bar graphs were performed using GraphPad Prism (Version 8.0.2) (https://www.graphpad.com/scientific-software/prism/) or Microsoft Excel (2016). Values were represented as mean ± SD, p values are made by two-tailed Student’s t-test or one-way ANOVA with Tukey’s multiple comparisons test. The biological replicates of experiments presented in this study are indicated in the respective figure legends. No data were excluded from the analyses and no statistical method was used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The transcriptomic data and CUT&Tag data generated in this study have been deposited in the National Center for Biotechnology Information under accession code GSE228410 and PRJNA1012547, respectively. All data supporting the conclusions of this study are presented within the paper and its Supplementary Information files. Source data are provided with this paper.
References
FAO. World food situation. Food and Agriculture Organization of the United Nations (accessed 27 April 2022). https://www.fao.org/home/en.
Sakuma, S. & Schnurbusch, T. Of floral fortune: tinkering with the grain yield potential of cereal crops. N. Phytol. 225, 1873–1882 (2020).
Yuan, Z. et al. Molecular and genetic pathways for optimizing spikelet development and grain yield. aBIOTECH 1, 276–292 (2020).
Li, N. et al. Molecular networks of seed size control in plants. Annu. Rev. Plant Biol. 70, 435–463 (2019).
Song, X.-J. et al. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 39, 623–630 (2007).
Wang, S. et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954 (2012).
Liu, J. et al. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 3, 17043 (2017).
Tao, Y. et al. RGG1, involved in the cytokinin regulatory pathway, controls grain size in rice. Rice 13, 76 (2020).
Xu, R. et al. Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol. Plant 11, 860–873 (2018).
Hao, J. et al. The GW2-WG1-OsbZIP47 pathway controls grain size and weight in rice. Mol. Plant 14, 1266–1280 (2021).
Gao, Q. et al. The ubiquitin-interacting motif-type ubiquitin receptor HDR3 interacts with and stabilizes the histone acetyltransferase GW6a to control the grain size in rice. Plant Cell 33, 3331–3347 (2021).
Ntakirutimana, F. & Xie, W. Morphological and genetic mechanisms underlying awn development in monocotyledonous grasses. Genes 10, 573 (2019).
Zhou, L. et al. Gene duplication drove the loss of awn in sorghum. Mol. Plant 14, 1831–1845 (2021).
Hua, L. et al. LABA1, a domestication gene associated with long, barbed awns in wild rice. Plant Cell 27, 1875–1888 (2015).
Elbaum, R. et al. The role of wheat awns in the seed dispersal unit. Science 316, 884–886 (2007).
Jin, J. et al. Two awn development-related peptides, GAD1 and OsEPFL2, promote seed dispersal and germination in rice. Mol. Plant 16, 485–488 (2022).
AuBuchon-Elder, T. et al. Sterile spikelets contribute to yield in Sorghum and related grasses. Plant Cell 32, 3500–3518 (2020).
Luo, J. et al. An-1 encodes a basic helix-loop-helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell 25, 3360–3376 (2013).
Haas, M. et al. Domestication and crop evolution of wheat and barley: Genes, genomics, and future directions. J. Integr. Plant Biol. 61, 204–225 (2019).
Maydup, M. L. et al. The contribution of the awns of bread wheat (Triticum aestivum L.) to grain filling: Responses to water deficit and the effects of awns on ear temperature and hydraulic conductance. Field Crops Res. 167, 102–111 (2014).
DeWitt, N. et al. Sequence-based mapping identifies a candidate transcription repressor underlying awn suppression at the B1 locus in wheat. N. Phytol. 225, 326–339 (2020).
Yuo, T. et al. A SHORT INTERNODES (SHI) family transcription factor gene regulates awn elongation and pistil morphology in barley. J. Exp. Bot. 63, 5223–5232 (2012).
Rebetzke, G. J. et al. Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. J. Exp. Bot. 67, 2573–2586 (2016).
Tambussi, E. A. et al. The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. Crit. Rev. Plant Sci. 26, 1–16 (2007).
Takanashi, H. et al. DOMINANT AWN INHIBITOR encodes the ALOG protein originating from gene duplication and inhibits awn elongation by suppressing cell proliferation and elongation in Sorghum. Plant Cell Physiol. 63, 901–918 (2022).
Gu, B. et al. An-2 encodes a cytokinin synthesis enzyme that regulates awn length and grain production in rice. Mol. Plant 8, 1635–1650 (2015).
Bessho-Uehara, K. et al. Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proc. Natl Acad. Sci. USA 113, 8969–8974 (2016).
Jin, J. et al. GAD1 encodes a secreted peptide that regulates grain number, grain length, and awn development in rice domestication. Plant Cell 28, 2453–2463 (2016).
Xiong, L. et al. Small EPIDERMAL PATTERNING FACTOR-LIKE2 peptides regulate awn development in rice. Plant Physiol. 190, 516–531 (2022).
Toriba, T. & Hirano, H.-Y. The DROOPING LEAF and OsETTIN2 genes promote awn development in rice. Plant J. 77, 616–626 (2014).
Bessho-Uehara, K. et al. Regulator of Awn Elongation 3, an E3 ubiquitin ligase, is responsible for loss of awns during African rice domestication. Proc. Natl Acad. Sci. USA 120, e2207105120 (2023).
Tanaka, W. et al. The YABBY gene TONGARI-BOUSHI1 is involved in lateral organ development and maintenance of meristem organization in the rice spikelet. Plant Cell 24, 80–95 (2012).
Zhang, Z. et al. Functional characterization of rice CW-domain containing zinc finger proteins involved in histone recognition. Plant Sci. 263, 168–176 (2017).
Wang, T. et al. GRAIN LENGTH AND AWN 1 negatively regulates grain size in rice. J. Integr. Plant Biol. 61, 1036–1042 (2019).
Du, D. et al. FRIZZY PANICLE defines a regulatory hub for simultaneously controlling spikelet formation and awn elongation in bread wheat. N. Phytol. 231, 814–833 (2021).
Huang, D. et al. Dominant inhibition of awn development by a putative zinc-finger transcriptional repressor expressed at the B1 locus in wheat. N. Phytol. 225, 340–355 (2020).
Shoesmith, J. R. et al. APETALA2 functions as a temporal factor together with BLADE-ON-PETIOLE2 and MADS29 to control flower and grain development in barley. Development 148, dev194894 (2021).
Callens, C. et al. Dissecting the role of MADS-box genes in monocot floral development and diversity. J. Exp. Bot. 69, 2435–2459 (2018).
Wu, D. et al. Loss of LOFSEP transcription factor function converts spikelet to leaf-like structures in rice. Plant Physiol. 176, 1646–1664 (2017).
Li, G. et al. MADS1 maintains barley spike morphology at high ambient temperatures. Nat. Plants 7, 1093–1107 (2021).
Hu, Y. et al. Interactions of OsMADS1 with floral homeotic genes in rice flower development. Mol. Plant 8, 1366–1384 (2015).
Waddington, S. R. et al. A quantitative scale of spike initial and pistil development in barley and wheat. Ann. Bot. 51, 119–130 (1983).
Youssef, H. M. et al. VRS2 regulates hormone-mediated inflorescence patterning in barley. Nat. Genet. 49, 157–161 (2017).
Khanday, I. et al. Rice LHS1/OsMADS1 controls floret meristem specification by coordinated regulation of transcription factors and hormone signaling pathways. Plant Physiol. 161, 1970–1983 (2013).
Cai, Q. et al. Jasmonic acid regulates spikelet development in rice. Nat. Commun. 5, 3476 (2014).
Immink, R. G. H. et al. SEPALLATA3: the ‘glue’ for MADS box transcription factor complex formation. Genome Biol. 10, R24 (2009).
Kong, X. et al. The wheat AGL6-like MADS-box gene is a master regulator for floral organ identity and a target for spikelet meristem development manipulation. Plant Biotechnol. J. 20, 75–88 (2022).
Zhu, W. et al. Rice SEPALLATA genes OsMADS5 and OsMADS34 cooperate to limit inflorescence branching by repressing the TERMINAL FLOWER1-like gene RCN4. N. Phytol. 233, 1682–1700 (2022).
Cui, R. et al. Functional conservation and diversification of class E floral homeotic genes in rice (Oryza sativa). Plant J. 61, 767–781 (2010).
Ditta, G. et al. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 14, 1935–1940 (2004).
Liu, C. et al. A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Dev. Cell 24, 612–622 (2013).
Schilling, S. et al. Genome-wide analysis of MIKC-type MADS-box genes in wheat: pervasive duplications, functional conservation and putative neofunctionalization. N. Phytol. 225, 511–529 (2020).
Qiao, J. et al. A novel miR167a-OsARF6-OsAUX3 module regulates grain length and weight in rice. Mol. Plant 14, 1683–1698 (2021).
Schrager-Lavelle, A. et al. Grass flowers: an untapped resource for floral evo-devo. J. Syst. Evol. 55, 525–541 (2017).
Liu, Q. et al. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 9, 852 (2018).
Ren, D. et al. Molecular bases of rice grain size and quality for optimized productivity. Sci. Bull. 68, 314–350 (2023).
Liu, H. et al. TaDA1, a conserved negative regulator of kernel size, has an additive effect with TaGW2 in common wheat (Triticum aestivum L.). Plant Biotechnol. J. 18, 1330–1342 (2020).
Yang, X. et al. Ovule cell wall composition is a maternal determinant of grain size in barley. N. Phytol. 237, 2136–2147 (2023).
Xie, Q. et al. Carpel size, grain filling, and morphology determine individual grain weight in wheat. J. Exp. Bot. 66, 6715–6730 (2015).
Si, F. et al. Control of OsARF3a by OsKANADI1 contributes to lemma development in rice. Plant J. 110, 1717–1730 (2022).
Song, X. et al. Rice RNA-dependent RNA polymerase 6 acts in small RNA biogenesis and spikelet development. Plant J. 71, 378–389 (2012).
Ren, D. et al. The pleiotropic ABNORMAL FLOWER AND DWARF1 affects plant height, floral development and grain yield in rice. J. Integr. Plant Biol. 58, 529–539 (2016).
Li, H. et al. Rice MADS6 interacts with the floral homeotic genes SUPERWOMAN1, MADS3, MADS58, MADS13, and DROOPING LEAF in specifying floral organ identities and meristem fate. Plant Cell 23, 2536–2552 (2011).
Krogan, N. T., Hogan, K. & Long, J. A. APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Development 139, 4180–4190 (2012).
Wang, C. et al. Dissecting a heterotic gene through GradedPool-Seq mapping informs a rice-improvement strategy. Nat. Commun. 10, 2982 (2019).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Harwood, W. A. et al. Barley transformation using Agrobacterium-mediated techniques. in Transgenic Wheat, Barley and Oats: Production and Characterization Protocols (eds. Jones, H. D. & Shewry, P. R.) 137–147 (Humana Press, Totowa, NJ, 2009).
Zhang, S. et al. Targeted mutagenesis using the Agrobacterium tumefaciens-mediated CRISPR-Cas9 system in common wheat. BMC Plant Biol. 18, 302 (2018).
Chang, T.-G. et al. An in situ approach to characterizing photosynthetic gas exchange of rice panicle. Plant Methods 16, 92 (2020).
Dreni, L. et al. The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J. 52, 690–699 (2007).
Liu, H. et al. Transcriptome profiling reveals phase-specific gene expression in the developing barley inflorescence. Crop J. 8, 71–86 (2020).
Tian, T. et al. AgriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45, W122–W129 (2017).
Supek, F. et al. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6, e21800 (2011).
Zhao, L. et al. Dynamic chromatin regulatory programs during embryogenesis of hexaploid wheat. Genome Biol. 24, 7 (2023).
Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019).
Bowler, C. et al. Chromatin techniques for plant cells. Plant J. 39, 776–789 (2004).
Saur, I. M. L. et al. A cell death assay in barley and wheat protoplasts for identification and validation of matching pathogen AVR effector and plant NLR immune receptors. Plant Methods 15, 118 (2019).
Shan, Q. et al. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 9, 2395–2410 (2014).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146, 323–324 (2007).
Mao, Z. et al. Photoexcited CRY1 and phyB interact directly with ARF6 and ARF8 to regulate their DNA-binding activity and auxin-induced hypocotyl elongation in Arabidopsis. N. Phytol. 225, 848–865 (2020).
Zhang, P. et al. THERMOSENSITIVE BARREN PANICLE (TAP) is required for rice panicle and spikelet development at high ambient temperature. N. Phytol. 237, 855–869 (2023).
Acknowledgements
The authors would like to dedicate this paper to Prof. Dabing Zhang whose background work and ideas were the basis of this project and who tragically passed away in June 2023. The authors would also like to thank Dr. Xuelei Lin and Prof. Jun Xiao (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for their technical assistance with the CUT&Tag assays. We thank Dr. Natalie Betts for editing this manuscript, Prof. Genying Li (Crop Research Institute, Shandong Academy of Agricultural Sciences) for creating the wheat mads1 mutant lines, Prof. Chengdao Li (Murdoch University) for performing the analysis of natural variation in HvMADS1, Prof. Yaoguang Liu (South China Agricultural University) for providing CRISPR-Cas9 vectors, and Mr. Ting Luo (Shanghai Jiao Tong University) for helping with barley cultivation. This research was funded by grants from the Australian Research Council (DP210100956 and DP230102476 to D.Z.); the Australian Research Council Training Centre for Accelerated Future Crop Development (IC210100047 to D.Z.); the Australian Research Council Linkage (LP210301062 to D.Z.); the National Natural Science Foundation of China (32130006 and 31970803 to D.Z.); the Yazhou Bay Seed Laboratory Project (B21HJ8104 to D.Z.); the Innovative Research Team (Ministry of Education); and the 111 Project (B14016 to D.Z.).
Author information
Authors and Affiliations
Contributions
D.Z. conceived the project and supervised the study. D.Z., Y.Z., G.L. and J.X.S designed experiments. Y.Z. and C.S. performed most of the experiments and analyzed data. J.S. performed bioinformatics analyses. Y.Y. helped to evaluate some phenotypic data. L.Y. created the barley shi and ap2 mutant lines. Q.S. conducted spike photosynthesis analysis. C.S. and Y.Z. performed CUT&Tag assays. Y.Z., C.S., and D.Z. wrote the manuscript with comments from all authors. J.X.S revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sarah McKim, Nils Stein and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Shen, C., Li, G. et al. MADS1-regulated lemma and awn development benefits barley yield. Nat Commun 15, 301 (2024). https://doi.org/10.1038/s41467-023-44457-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-44457-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.