Abstract
Coastal vegetated ecosystems are acknowledged for their capacity to sequester organic carbon (OC), known as blue C. Yet, blue C global accounting is incomplete, with major gaps in southern hemisphere data. It also shows a large variability suggesting that the interaction between environmental and biological drivers is important at the local scale. In southwest Atlantic salt marshes, to account for the space occupied by crab burrows, it is key to avoid overestimates. Here we found that southern southwest Atlantic salt marshes store on average 42.43 (SE = 27.56) Mg OC·ha−1 (40.74 (SE = 2.7) in belowground) and bury in average 47.62 g OC·m−2·yr−1 (ranging from 7.38 to 204.21). Accretion rates, granulometry, plant species and burrowing crabs were identified as the main factors in determining belowground OC stocks. These data lead to an updated global estimation for stocks in salt marshes of 185.89 Mg OC·ha−1 (n = 743; SE = 4.92) and a C burial rate of 199.61 g OC·m−2·yr−1 (n = 193; SE = 16.04), which are lower than previous estimates.
Similar content being viewed by others
Introduction
Over the last decade, coastal vegetated areas (e.g., salt marshes, mangroves, seagrass meadows) have been recognized for their high capacity to sequester carbon1. It is under the climate change context that the term ‘Blue carbon’ emerged to refer to the organic carbon fixed by coastal vegetation that can be stored for centuries to millennia2. Conservation and restoration of blue C ecosystems have been recognized as a need to mitigate and adapt to climate change3,4. Thus, a range of studies have documented blue C stocks around the world, in many cases as part of national C emission accounting and reduction frameworks5,6,7,8.
At a global scale, salt marsh blue C accounting is still incomplete and is heavily skewed toward studies from the northern hemisphere. Besides a recently growing availability of data from Australia9, southern hemisphere data is scarce. In particular, for South America, a recent revision10 makes evident the limited information that exists for salt marshes, with most of the estimation derived from the percentage of organic matter in surficial soils. As more data becomes available, the huge variability in C stocks and sequestration rates has become clearer, not just among different vegetation types but also within a given ecosystem. For instance, in a study of 84 Australian salt marshes, C stocks varied from 14 to 962 Mg C ha−1 in the top 1 m9 and a global analysis showed sequestration rates in salt marshes ranges from 18 to 1713 g C·m−2·year−1 11. Findings like these suggest that the interaction among environmental variables and the ecological mechanisms that determine blue C are important at the local scale and highlight that global estimates derived from indirect methods should be taken with caution.
Several environmental variables have been proposed to explain variability in blue C. Dominant species, local geomorphology, nutrient availability, hydroperiod, salinity and suspended sediment supply are among the most studied9,11,12,13,14. Previous efforts have often failed to include biological processes, even though these processes have long been recognized as important controllers of C transformation pathways. Bioturbation and herbivory, for instance, are important processes that affect C cycling with multiple antagonistic and synergistic effects15,16,17. Specifically, crab bioturbation and herbivory, through a complex network of positive, negative, direct and indirect interactions, can profoundly affect C transformation pathways in salt marshes of the southern southwest (SW) Atlantic coast15. Bioturbation and herbivory may be overlooked processes in studies of C stocks and sequestration rates in salt marshes around the world, and even other blue C ecosystems, given that coastal vegetated ecosystems are usually inhabited by burrowing organisms that consume plants and disturb the sediment18,19,20. Furthermore, through bioturbation, infaunal organisms can shape marsh geomorphology by, for instance, facilitating creek formation21 or stimulating landscape-scale accretion22.
To address the knowledge gaps, we carry out a comprehensive study of C stocks and burial rates in southern SW Atlantic salt marshes covering a latitudinal gradient from 35° to 51° S. In addition, to test the importance of selected biological and environmental variables in determining the C stocks of the region, we carried out a path analysis using SEM (structural equation modeling). Finally, we updated global estimates of C stocks and burial rates for salt marshes.
Results and discussion
Southern SW Atlantic salt marsh organic C stocks
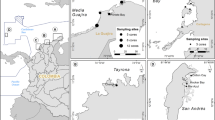
Most of the salt marshes in South America are located on the Atlantic coast, with over 95% of the surface of these Atlantic marshes located in Argentina (209,056 ha from 218,964)15,23. In this study, we sampled 11 salt marshes covering 197,577 ha, of which 145,309 ha are dominated by strictly salt marsh species (Spartina densiflora, Spartina alterniflora and Salicornia sp.) and 52,268 ha by brackish water species15. All the results shown here correspond to the area dominated by salt marsh species. These sites span along ~3000 km of coastline (35° to 51° S, Fig. 1).
a South America map60 showing sites where salt marshes occur described using black and green points. Green points indicate salt marshes in the SW Atlantic Ocean. Red circle covers the study area. b South American Atlantic coast with salt marsh sites included in this study indicated with colored stars. Inset: organic carbon (OC) stocks measured at each salt marsh by plant species and stock allocation. AG: aboveground, Sa: Spartina alterniflora, Sd: Spartina densiflora, Ssp: Salicornia sp. (Source data are provided as a Source Data file).
Total organic C (OC) stocks (i.e., above and belowground pooled together) ranged from 6.47 (SE = 1.5) Mg OC·ha−1 in the Salicornia sp. salt marsh in Caleta Los Loros to 111.92 (SE = 7.2) Mg OC·ha−1 in the Spartina alterniflora salt marsh in Riacho San José, averaging 42.43 (SE = 27.56) Mg OC·ha−1 across all marshes examined (Table 1, Fig. 1). OC stocks in salt marshes dominated by S. densiflora were less variable than those dominated by S. alterniflora or Salicornia sp. (Table 1, Fig. 1). In general, the greater part of the total OC stock was located belowground. The contribution of the root OC stock to the total stock could reach up to 40% in S. alterniflora and Salicornia sp. salt marshes, especially in those located in the southern sites. In contrast, the contribution of the root OC stock to the total stock was less than 10% in S. densiflora salt marshes, with the exception of Mar Chiquita and Río Negro where it was 11.6% for both of them (Table 1, Fig. 1).
The senescent aboveground biomass OC stock contributed less than 2% to the total stock in all S. alterniflora and Salicornia sp. salt marshes, while in S. densiflora salt marshes, the contribution of this stock was much higher, reaching values of 11.5, 9 and 7.7 % in Riacho San José, Río Negro and Bahía Samborombón respectively (Table 1, Fig. 1). The contribution of the green aboveground biomass OC stock was in general less than 10%, with the exceptions of the Caleta Los Loros Salicornia sp. salt marsh (11.1%) and S. densiflora salt marshes in Río Negro and Riacho San José (11.1 and 10.4% respectively; Table 1, Fig. 1).
The mean total OC stock for all the sites sampled was 42.43 (SE = 27.56) Mg OC·ha−1, with a mean belowground stock of 40.74 (SE = 2.70) Mg OC·ha−1. Altogether, the 11 salt marsh sites in this study contain 4,136,190 tons of OC, of which 3,928,493 tons were found belowground (95%, Table 2, Fig. 1).
Southern SW Atlantic salt marsh accretion and organic C burial rates
Sediment accretion rates ranged from 0.53 mm·yr−1 in the lower salt marsh of Riacho San José to 2.86 mm·yr−1 in the upper salt marsh of Bahía San Antonio. C burial rates ranged from 7.38 g OC·m−2·yr−1 in the lower part of Playas Doradas to 204.21 g OC·m−2·yr−1 in the lower part of Río Negro (Table 2). Altogether, the sites examined in this study have the potential to sequester 55,100.1 tons of OC per year.
Autochthonous and allochthonous contribution to sediment organic C stocks
C and N isotopic signatures differed among plant species, plant parts, and sites. In general, Salicornia sp. was C-depleted compared with Spartina spp. and litter was N-depleted compared with live vegetation (green leaf and roots) (Supplementary Table 1). Particulate organic matter (POM) was the main source of OC to the sediment of Bahía Samborombón, Bahía Blanca and Bahía Anegada in areas dominated by S. alterniflora, contributing 81, 77 and 93% of the OC, respectively. Similarly, POM was the main source of OC in Mar Chiquita, where S. densiflora dominated the salt marsh, contributing 80% of the OC, as well as in Bahía Blanca and Bahía Anegada in areas dominated by Salicornia sp., with a contribution of 89 and 90% of the C, respectively (Supplementary Table 2). All other sites had sediments with a mixture of OC derived from POM and plants, as well as macroalgae in the cases of Bahía San Antonio and Puerto San Julián.
According to these results, allochthonous POM is a major source of OC in southern SW Atlantic salt marshes. This finding suggests these ecosystems act as traps for allochthonous OM. Several studies have shown that the main sources for OC stocks in blue C ecosystems are allochthonous24,25, suggesting that the vegetation structure in such ecosystems can play a significant role in retaining exogenous OM. Crab burrows can act as passive traps capturing organic matter and sediments imported from adjacent systems through tides and winds26,27,28, given that we have identified crab burrows in the path analysis as a driver of belowground OC stocks, it could partly explain the mechanism that influences the principal origin of the stored OC.
Biotic and abiotic drivers of belowground organic C stocks
Path analysis of the environmental (i.e., abiotic and biotic factors) influence on belowground OC stocks showed a good fit (Fisher’s C = 125.794, P = 0.438, df = 124), and revealed that a combination of abiotic and biotic factors explained a considerable amount of the variability observed in belowground OC stocks (marginal R-squared = 0.47, conditional -i.e., including idiosyncratic differences between sites- R-squared = 0.84). The main direct drivers of belowground OC stocks were sediment accretion rates, grain size, crab burrows and dominant plant species (Fig. 2). Aboveground biomass and mean annual temperature were also retained since, although not significant (P = 0.103 and P = 0.116 respectively), their exclusion would have made the marginal R-square value fall to 0.25. Surprisingly, most significant effects were direct, with indirect effects being nonsignificant or small and often from variables that also affect belowground OC stocks directly (i.e., mean annual temperature and crab burrows through aboveground biomass). While different combinations of environmental variables affected the biotic components of the metamodel, these biotic components had a minor role in OC stocks. Only crab burrows and the dominant plant species (that also harbors differences in unmeasured abiotic variables) affected OC stocks, with aboveground biomass being marginally nonsignificant.
SEM (structural equation model) analysis results carried out to explain belowground organic carbon stocks (sediment and roots) variability found in southern southwest Atlantic salt marshes. Green boxes correspond to biotic variables and yellow to abiotic. Blue arrows indicate significant positive effect and red arrows negative. Gray arrows are nonsignificant effects retained by the model. Numbers next to the arrows (estimates) are standardized path coefficients. The width of the arrows reflects the strength of the according pathway. OC organic carbon, AG aboveground, SAR sediment accretion rate, C:N ratio between carbon and nitrogen (Source data are provided as a Source Data file).
Sediment accretion rates (SAR) and grain size have been previously identified as drivers of coastal OC stocks with the general trend of larger stocks in finer sediments13,29. SAR may have counter effects on OC stock; while it has been hypothesized that higher accretion rates could help bury organic C and therefore increase the stocks, it also could have a dilution effect if the ratio between organic matter and sediments is too low26,30. Our path analysis shows that both grain size and SAR are important drivers of OC belowground stocks in southern SW Atlantic salt marshes, with a general positive effect of SAR indicating that higher rates contribute to increased stocks.
Biotic factors have received much less attention. The ecological functioning of SW Atlantic salt marshes has been well studied (e.g., ref. 31), and it has been previously suggested that burrowing crabs could have an important role in influencing OC stocks15. Several mechanisms driven by burrowing crabs affect the OC cycle in SW Atlantic salt marshes, either through herbivory or bioturbation. These mechanisms can have a positive or negative impact on the stocks, but overall, our path analysis revealed a general positive effect. Such positive effects may stem from detritus trapping in crab burrows27,29, facilitation of arbuscular mycorrhizal fungi associations, which increase plant productivity32,33, and burial of dead plant biomass and imported organic matter through sediment reworking for burrow maintenance34. However, many studies suggest a negative impact of crabs on belowground OC stocks in salt marshes. For instance, CO2 and CH4 effluxes are enhanced by the presence of crab burrows17. Burrows also promote carbon-rich fluid exchange between marsh and tidal creeks and increase oxygenation deep in the sediment, reducing the capacity for C sequestration35. In SW Atlantic salt marshes, plant consumption by crabs36,37 may further damage the leaves through increased fungal infection and lower plant productivity38,39. This may also result in large inputs of senescent biomass, which may increase aerobic decomposition in the sediment40. Furthermore, by disturbing sediment during burrow maintenance, crabs enhance erosion at the marsh edge27 and facilitate the creation of new tidal creeks21.
According to our path analysis, dominant plant species was also a driver of belowground OC stocks. This analysis provides an integrative picture highlighting the main factors that determine belowground OC stocks in southern SW Atlantic marshes. To fully understand how plant species drive OC stocks, it is necessary to consider a complex set of environmental conditions behind the dominant species identity. In particular, the plant species that dominate the SW Atlantic salt marsh present two different growth forms: Spartina species are grasses, and Salicornia sp. is a succulent. Given that the establishment of a dominant species is the result of biological interactions under specific abiotic conditions, it is important to recognize that the identity of a dominant species implies much more than a taxonomic description for explaining belowground OC stocks and probably includes an entire set of environmental conditions not fully measured here.
Updating global salt marsh blue C estimates
To compare OC stocks from this study to those reported elsewhere, we focus on belowground (roots and sediments) stocks, given that very few studies have reported aboveground stocks. OC stocks in soils have been estimated to hold an average of 99.2% of the total stock41, although the data in this study show a lower percentage (84.9%). We recalculate the global belowground C stock average pooling our results with the data compiled by Alongi42 and from recent publications (Source data are provided as a Source Data file). The updated global average C stock for salt marshes was 185.88 (SE = 4.92) Mg OC ha−1. The same procedure was used to recalculate global C accumulation rates. When we recalculate the global average accumulation rate, adding our results with the data from the publications mentioned above, we find a value of 199.61 (SE = 16.04) gOC m−2 year−1 (Source data are provided as a Source Data file).
The blue C of the Southern SW Atlantic salt marshes in the global context
Global estimations for salt marshes have varied as more data have become available, with a current average range of 162 Mg OC ha−1 1 to 317.2 Mg OC ha−1 42. However, in general, recent publications report average OC stocks below the Alongi25 estimate (e.g., 165, 62.5, 82, 38, 65, 224 and 207 Mg OC ha−1 from Australia, Florida [USA], the Arabian Gulf, China, Canada and South Africa, respectively9,43,44,45,46,47). The values found in our study, ranging from 5.74 (SE = 0.2) to 108.81 (SE = 0.71) Mg OC ha−1, are among the lowest reported. When we recalculate the global average pooling our results with the data from the publications mentioned above, we update the estimate to 185.88 Mg OC ha−1. These changes in the global average, as new information appears, highlight the high variability in marsh blue C around the world and caveats the use and extrapolation of global averages. In the case of the salt marshes from southern SW Atlantic, using a global average of 317 Mg OC ha−1 could lead to a large overestimation of the stocks. In fact, a recent publication using a literature compilation that includes different sources of data and indirect methodologies to calculate the blue C in SW Atlantic salt marshes provides an average of 270 Mg OC ha−1 10, which is far higher than the average we report here using actual data.
Sediment accretion rates for the marshes studied here range between 0.53 mm yr−1 and 2.86 mm yr−1, with a mean of 1.16 mm yr−1. These rates are substantially lower than those reported in a global review of salt marshes, which indicates a range of 2–10 mm yr−1, and a median value of 5 mm yr−1 42, suggesting lower sedimentary inputs to southern SW Atlantic marshes than marshes in other regions.
OC sequestration rates in the marshes were also comparatively low, varying between 7.38 gOC·m−2·yr−1 in the lower marsh of Playas Doradas and 204.21 gOC·m−2·yr−1 in the lower marsh of Río Negro with a mean of 47.62 gOC·m−2·yr−1. Estimates of global mean OC sequestration rates in marshes vary between 212 (SE = 18) gOC m−2 year−1 as reported by Alongi42 (minimum of 9 and maximum of 1713 g C m−2 year−1) and 244.7 gOC m−2 yr−1 as reported by Ouyang & Lee11 (summarizing data from 143 sites). The summary of OC sequestration rates provided by Alongi42 is derived from 168 sites in Europe, North America, Asia, and Australia, but notably with only 8 sites in Australia and no South American or African sites included. Alongi42 suggested that insufficient data existed from East Asia, the Arctic and Australia to obtain sound global estimates, and we further note that data is largely missing from the southern hemisphere.
Filling geographical gaps and adding understanding behind blue C in salt marshes
Within the climate change context, there is a need to undertake carbon accounting at a national level and identify the main sources and sinks of carbon. The identification of ecosystems and areas that provide high rates of OC sequestration is essential for the development of effective measures and policies for climate change mitigation. Likewise, understanding the factors that regulate OC stocks and sequestration rates is capital for the adoption of strategies that can maximize blue C reservoirs and, thus, adaptation to climate change. This study provides a comprehensive characterization of OC stocks and sequestration rates for southern SW Atlantic salt marshes. It also highlights the importance of incorporating both environmental (i.e., abiotic) and biological (i.e., biotic) variables for an understanding of blue C storage.
Findings from this study also show the substantial global variation in blue C stocks and burial rates, as well as the large data gaps in the southern hemisphere, particularly in South America and Africa. With the inclusion of our data, it seems that overestimations in global blue C stocks and burial rates are likely to have been made. Indirect calculations derived from equations that do not incorporate local factors can be a large source of bias. For instance, in the case of the SW Atlantic salt marsh to account for the space occupied by crab burrows is key to avoid overestimates. Thus, greater effort should be made toward the assessment of blue C stocks and burial rates in understudied regions. Furthermore, reporting on aboveground biomass, which appears to not be accounted for in many studies, should be included. Our results show that in some areas, this can be a significant proportion of the total C stocks (10%).
Methods
Study sites
SW Atlantic salt marshes are dominated by three plant species: Spartina alterniflora, Spartina densiflora and Salicornia sp. Depending on the area, one, two, or all the dominant species can be found. The southern limit of Spartina species is 43°S, below that, Salicornia sp. dominates. Where both Spartina species coexist, S. densiflora occupies the upper intertidal area while S. alterniflora dominates in the lower area, where it is subjected to daily tidal inundation. The mean tidal range among the studied sites varied from 0.78 to 8.07 m (micro- to macro-tidal). The dominance and presence of one or another species is mostly explained by the freshwater input48. Areas receiving a higher freshwater contribution are dominated by S. alterniflora, while those that are more saline are dominated by Salicornia sp.
A conspicuous characteristic of these salt marshes and the adjacent bare intertidal habitats is that they are densely inhabited by the burrowing crab Neohelice (=Chasmagnathus) granulata. Crabs’ burrow density can reach over 120 burrows per m2 and they can be up to 1 m depth42. This crab species can remove up to 2.4 kg of sediment per day per m2 49. The impacts of this on ecological functions in SW Atlantic salt marshes is either by herbivory, consuming large amounts of plant biomass, or via bioturbation through the construction and maintenance of their burrows34.
Most of the salt marshes in South America are located on the Atlantic coast, with over 95% of the recorded area of the SW Atlantic salt marshes located in Argentina (209,056 ha from 218,964)14,22. In this study, we sampled 11 salt marshes covering 132,796 ha spanning along ~3000 km of coastline (Fig. 1, Table 1).
Samples collection and processing
Samples were collected in January 2016, during the southern hemisphere summer period and processed following the Blue C Manual recommendations50, as detailed below. Sediment and vegetation samples were collected at each of the eleven salt marshes. When more than one of the dominant plant species was present in a site, sampling was undertaken in each plant community. The sampling consisted of 3 to 5 units (25 × 25 cm quadrats) per site and dominant plant species. Above and belowground biomass and OC (organic carbon) stocks, crab burrow diameter and abundance, surficial (2 cm depth) sediment for C and N stable isotope signatures and OC stocks belowground up to 1 m depth were measured at each sampling unit.
To measure aboveground plant biomass, all the biomass above the sediment within the sampling unit was collected and transported to the lab, where the material was separated into green and senescent. Samples were dried at 60 °C and weighed. A subsample of each sample was separated to determine C and N content and for stable isotope analysis. Samples of root biomass were taken using a Russian corer down to 50 cm deep and 9.05 cm2 area and transported to the lab, where they were sieved. All roots were separated, dried in an oven at 60 °C and weighted. Subsamples were also processed for C and N content and stable isotopes.
To determine belowground OC stocks, up to 1 m depth samples were collected using a Russian corer. Sample depths varied between sites according to sediment hardness, reaching 1 m or hard rock were the limits. Considering this natural soil limit, we do not extrapolate the stock up to 1 m depth so as not to overestimate; rather we considered the limit for C accumulation. Each sample was sliced from top to bottom at 5, 10 and then every 10 cm up to a maximum of 1 m. All the slices were dried in at 60 °C and weighed. Subsamples of each dried slice (~2gr) were weighed, incinerated at 450 °C for 8 h and reweighed. All sediment samples (incinerated and non-incinerated) were sent to the Dauphin Island Sea Laboratory (Alabama, USA) for determination of total C and N. Incinerated samples are considered to have only inorganic C while non-incinerated have both organic and inorganic (loss on ignition (LOI) technique). Therefore, organic C content was determined as the difference between the non-incinerated and incinerated.
To determine the contribution of allochthonous and autochthonous organic matter sources to the sediment OC stocks, C and N stable isotopes from potential sources and surficial sediment were used. To determine stable N and C isotopes in surficial sediment, we took 2 cm depth samples using a 2 cm diameter plastic tube. Samples were dried at 60 °C and then divided into two subsamples. One half was acidified to eliminate carbonates and to measure stable isotopes of C, and the other half was not acidified and used to determine stable isotopes of N. To determine C and N stable isotopes from autochthonous sources, vegetation (green and senescent leaf and roots) subsamples were ground to a fine powder and stored in Eppendorf vials. Sediment and vegetation samples were sent to the Stable Isotope Geosciences Facility, Texas A&M University (Texas, USA) for C and N stable isotope determination. Stable isotope signatures of allochthonous sources (i.e., particulate organic matter (POM) and macroalgae) were obtained from the literature (Supplementary Information Table. 3). When information from a specific site was not found, we used averages of regional data. Most of the sites have two main potential sources (salt marsh plants and POM), with the exception of Bahía San Antonio and Puerto San Julián, where macroalgal beds were also observed and reported in the literature.
To determine crab burrow abundances, all burrows present in the sampling units were counted after harvesting aboveground biomass and before sampling sediment and belowground biomass. In addition, the diameter of five burrows at each sampling unit was measured with a caliper.
A separate series of two cores were also collected from each field site to undertake a geochronological assessment of the sediment to determine accretion and OC burial rates. In the field, a walkover survey was conducted to identify areas with representative stratigraphy in the lower and upper marsh areas. Sections of the sites with minimal bioturbation were surveyed by inserting a 75 mm diameter 50 cm long core into the sediment, making sure to limit subsurface compaction (<10%51). Cores were sealed and packed top and bottom for transport to the lab to limit disturbance of the sediment stratigraphy52. In the lab, cores were removed and assessed for compaction (no compaction or disturbance recorded), and then core extremities were cleaned laterally to prevent downcore mixing and contamination and then cut into 1 cm subsamples, weighed, dried at 40 °C to constant weight and the reweighed, to assess water loss (used in the dating equations, 7). Subsamples were placed in calibrated dimension vials and analyzed in an ultra-low background HP-Ge gamma spectrometer with a mean count time of 500,000 s. Radionuclides used were 210Pb, 214Pb (as a proxy for background 210Pb, as per ref. 50) and 137Cs for independent verification53. Gamma spectroscopy is a well-established technique to assess rates of sediment accretion and carbon sequestration in coastal wetland soils5,7,51,53,54,55. To evaluate sediment accretion rates, the robust 210Pb Constant Flux:Constant Sedimentation (CF:CS) method was used and compared with the 137Cs impulse dating method, based on deposition from the global peak around 1963 in the southern hemisphere52,56. CF:CS was used to determine accretion rates for all sites but Punta Loyola given 210Pb in excess was not separated from 214Pb (Supplementary Fig. 1). In this case 137Cs was used, showing a peak at 45 mm depth. In addition, the samples from Mar Chiquita were so bioturbated that dating was not possible. Carbon sequestration rates were calculated using the rates of sediment accretion and density as per Greiner et al.5.
Following loss on ignition, minerogenic samples were analyzed for granulometry. Prior to analysis, samples were carefully disaggregated using a pestle and mortar, then mixed with sodium hexametaphosphate (to prevent flocculation), and placed on a shaker for 30 min to further disaggregate the sediments. Three runs were undertaken using a Malvern Mastersizer 2000 laser particle size analyzer with an ultrasonic prior to each run to obtain an average grain size value for each sample52. Samples were classified using the Wentworth scale.
OC stocks calculation
To determine OC stocks in the aboveground and root biomass, the OC content determined in each vegetation compartment (green, senescent and roots) was multiplied by their respective biomasses measured at each site. Total belowground OC stocks were estimated by adding the OC determined along each sediment profile and expressed as mega grams (tons) per hectare (MgOC·ha). These measurement units are widely used in blue C studies, facilitating comparison. Empty space occupied by crab burrows was considered in the calculation to not overestimate OC stocks. To undertake this, the average number of burrows and diameters measured per site were used to calculate the sediment volume without C.
Statistical analysis
The relative contribution of allochthonous (POM and macroalgae) and autochthonous (salt marsh plants) sources to the OC stocks was assessed using Stable Isotope Bayesian mixing models (MixSIAR: Stock et al. 2018, R package version 3.6). The models were run using the δ13C and δ15N signatures of the sediment and the 3 potential organic matter sources. The contribution of salt marsh plants was analyzed using the pooled isotopic data from green and senescent leaves and roots. The mean and standard deviations of isotopic signatures for the three sources were obtained from the literature, and concentration dependence was not incorporated into the models. Results of the mixing models are given as a probabilistic contribution (%) of each source to the sedimentary organic matter pool (mixtures).
Given that belowground OC stocks likely depend on many interrelated biotic and abiotic variables14, we evaluated their relative importance as well as their direct and indirect pathways. Briefly, we used path analysis (a form of structural equation modeling without latent variables) to evaluate the direct and indirect roles of key abiotic and biotic factors on belowground OC stocks. Abiotic variables include grain size, tidal amplitude, mean annual temperature, freshwater inputs, mean annual precipitation, sediment accretion rates and sediment layer depth. Biotic variables include plant litter, above and belowground biomass and quality (C:N ratio), crab densities, and dominant plant species. We described the theoretical background behind each potential link in Supplementary Table 4.
Most abiotic variables were determined from external data sources at the site level. We obtained temperature and precipitation data using WorldClim (version 2; http://www.worldclim.org/)57 through bioclimatic variables 1 (mean annual temperature, °C) and 12 (mean annual precipitation, mm). Mean tidal amplitude (m) was obtained from the Argentinean National Hydrographic Service (http://www.hidro.gov.ar/), using the information from the nearest site to our sampling locations. Freshwater input was coded as a binary variable, which indicates whether the site is associated with freshwater input from rivers (1) or not (0), partly determined following Isacch et al.48. All other variables were obtained in situ and at the plot level (except grain size and sediment accretion rates for which we took one measurement per dominant species with a maximum of two species per site). Grain size was estimated as the inverse of the sorting coefficient (thus showing larger values for poorly sorted sediments, typical of sediments with larger mean grain size). Plant biomass and C:N ratio (senescent and green aboveground tissues and roots) were determined as explained above and expressed as g m−2 and unitless, respectively. We also estimated the area occupied by burrows by measuring the diameter of up to five burrows per plot, calculating the average surface of those burrows, and multiplying this average surface by the number of burrows per square meter (i.e., m2 burrows per m2 sediment; equivalent to the proportional surface area occupied by crab burrows; unitless). Sediment layer depth (cm) was estimated as the maximum depth we could bury the Russian corer to obtain sediment samples. Finally, belowground OC stock (g OC m−2) was obtained by multiplying OC density by sediment surface without burrows (see above), including OC from sediment and roots. (Source data are provided as a Source Data file).
For our path analysis, we performed a three-step procedure: (1) we first specified the metamodel carefully checking that all potential relationships were supported by scientific evidence, (2) then we simplified the individual models of each dependent variable following Deguines et al.58, and (3) finally we performed a step-wise deletion and addition of variables that improved overall model fit and that were originally considered in the metamodel. All individual models from the metamodel included site as a random factor and thus were performed using linear mixed-effects models (lme function from the nike package for R) to evaluate the significance of the hypothesized pathways explaining each dependent variable. To ensure normality of model residuals, we transformed sediment OC stock, belowground biomass and the three C:N ratios (senescent and green aboveground tissue and roots) using log10, and the surface of crab burrows, litter and aboveground biomass using square root. For each model, we graphically assessed the variance of the residuals for signs of heterogeneity. When heterogeneity was suspected (senescent tissue C:N), we refit the model with different variance structures and selected the structure that yielded the best results according to Akaike’s information criterion56. We simplified each model by removing terms lacking support (P > 0.05) in this preliminary analysis. We performed marginal F-tests with univariate analysis of deviance57 to investigate the effects of explanatory variables in each model (always coincident with Akaike’s information criterion)56. Following these preliminary results, we conducted piecewise structural equation modeling to join the multiple models into a single SEM. In this implementation, individual models are constructed separately for each predicted variable. The mixed-effects models were then combined and tested with the d-separation test for goodness of fit58 and path coefficients extracted from models that fit, as well as potential paths that were missing from the model. We implemented the model in R using the package piecewiseSEM59. We only deleted paths that were not significant and whose deletion improved model fit. In addition, we only added paths that were suggested by Shipley’s test and that were originally considered in our metamodel.
Data availability
Source data are provided with this paper. Organic carbon stocks, plant biomasses and crab burrow abundances and diameters data generated in this study, as well as all the data compiled for the SEM analysis and to update global estimates of OC stock and burial rate have been deposited in the Figshare database under the code: https://doi.org/10.6084/m9.figshare.24260338.
References
Duarte, C. M. et al. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 3, 961–968 (2013).
Nellemann, C. et al. Blue Carbon. A Rapid Response Assessment. United Nations Environment Programme. GRID-Arendal. http://www.grida.no/ (2009).
Masson-Delmotte, V. et al. Global Warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, 93–174 (Cambridge University Press, 2018).
Cooley, S. et al. Oceans and coastal ecosystems and their services. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (eds Pörtner, H.-O. et al.) 379–550 (Cambridge University Press, 2022).
Greiner, J. T. et al. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS ONE 8, e72469 (2013).
Lima, M. et al. Environmental drivers of carbon stocks in temperate seagrass meadows. Hydrobiologia 847, 1773–1792 (2020).
Ward, R. Carbon sequestration and storage in Norwegian Arctic coastal wetlands: impacts of climate change. Sci. Total Environ. 748, 141343 (2020).
Wu, J. et al. Opportunities for blue carbon strategies in China. Ocean Coast. Manag. 194, 105241 (2020).
Macreadie, P. et al. Carbon sequestration by Australian tidal marshes. Sci. Rep. 7, 44071 (2017).
Hatje, V. et al. Vegetated coastal ecosystems in the Southwest Atlantic Ocean are an unexplored opportunity for climate change mitigation. Commun. Earth Environ. 4, 160 (2023).
Ouyang, X. & Lee, S. Y. Update estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences 11, 5057–5071 (2014).
Kennedy, H. et al. Species traits and geomorphic setting as drivers of global soil carbon stocks in seagrass meadows. Glob. Biogeochem. Cycles 36, GB007481 (2022).
Kelleway, J. J. et al. Sedimentary factors are key predictors of carbon storage in SE Australian salt marshes. Ecosystems 19, 865–880 (2016).
Kelleway, J. J. et al. Sediment and carbon deposition vary among vegetation assemblages in a coastal salt marsh. Biogeosciences 14, 3763–3779 (2017).
Martinetto, P. et al. Crab bioturbation and herbivory may account for variability in carbon sequestration and stocks in south west Atlantic salt marshes. Front. Mar. Sci. 3, 122 (2016).
Mueller, P. et al. Top-down control of carbon sequestration: grazing affects microbial structure and function in salt marsh soils. Ecol. Appl. 27, 1435–1450 (2017).
Agusto, L. E. et al. Fiddling with the blue carbon: fiddler crab burrows enhance CO2 and CH4 efflux in saltmarsh. Ecol. Indic. 144, 109538 (2022).
Coverdale, T. G. et al. Belowground herbivory increase vulnerability of New England salt marshes to die-off. Ecology 93, 2085–2094 (2012).
Wolfrath, B. Burrowing of the fiddler crab Uca-tangeri in the Ria Formosa in Portugal and its influence on sediment structure. Mar. Ecol. Prog. Ser. 85, 237–243 (1992).
Wang, J. Q. et al. Bioturbation of burrowing crabs promotes sediment turnover and carbon and nitrogen movements in an estuarine salt marsh. Ecosystems 13, 586–599 (2010).
Escapa, M. et al. Direct and indirect effects of burrowing crab Chasmagnathus granulatus activities on erosion of southwest Atlantic Sarcocornia-dominated marshes. Limnol. Oceanogr. 52, 2340–2349 (2007).
Crotty, S. M. et al. Faunal engineering stimulates landscape-scale accretion in southeastern US salt marshes. Nat. Commun. 14, 881 (2023).
Mcowen, C. et al. A global map of saltmarshes. Biodivers. Data J. 5, e11764 (2017).
Santos, R. et al. Superficial sedimentary stocks and sources of carbon and nitrogen in coastal vegetated assemblages along a flow gradient. Sci. Rep. 9, 610 (2019).
Oreska, M. P. J. et al. Non‐seagrass carbon contributions to seagrass sediment blue carbon. Limnol. Oceanogr. 63, S3–S18 (2018).
Gorham, C. et al. Heterogeneous tidal marsh soil organic carbon accumulation among and within temperate estuaries in Australia. Sci. Total Environ. 787, 147482 (2021).
Escapa, M. et al. Sediment dynamics modulated by burrowing crab activities in contrasting SW Atlantic intertidal habitats. Estuar. Coast. Shelf Sci. 80, 365–373 (2008).
Gorham, C. et al. Soil carbon stocks vary across geomorphic setting in Australian temperate tidal marsh. Ecosystems 24, 319–334 (2021).
Botto, F. et al. Ecological importance of passive deposition of organic matter into burrows of the SW Atlantic crab Chasmagnathus granulatus. Mar. Ecol. Prog. Ser. 312, 201–210 (2006).
Lovelock, C. E. et al. Contemporary rates of carbon sequestration though vertical accretion of sediments in mangrove forests and saltmarshes of South East Queensland, Australia. Estuar. Coasts 37, 763–771 (2014).
Alberti, J. et al. Can a single species challenge paradigms of salt marsh functioning? Estuar. Coasts 38, 1178–1188 (2015).
Daleo, P. et al. Ecosystem engineers activate mycorrhizal mutualism in salt marshes. Ecol. Lett. 10, 902–908 (2007).
Daleo, P. et al. Mycorrhizal fungi determine salt-marsh plant zonation depending on nutrient supply. J. Ecol. 96, 431–437 (2008).
Montemayor, D. I. et al. Effect of dominant Spartina species on salt marsh detritus production in SW Atlantic estuaries. J. Sea Res. 66, 104–110 (2011).
Guimond, J. A. et al. A physical-biogeochemical mechanism for negative feedback between marsh crabs and carbon storage. Environ. Res. Lett. 15, 034024 (2019).
Costa, C. S. B. et al. Plant zonation in irregularly flooded salt marshes: relative importance of stress tolerance and biological interactions. J. Ecol. 91, 951–965 (2003).
Alberti, J. et al. Local and geographic variation in grazing intensity by herbivorous crabs in SW Atlantic salt marshes. Mar. Ecol. Prog. Ser. 349, 235–243 (2007).
Daleo, P. et al. Grazer facilitation of fungal infection and the control of plant growth in SW Atlantic salt marshes. J. Ecol. 97, 781–787 (2009).
Freitas, R. F. et al. Grazing scar characteristics impact degree of fungal facilitation in Spartina alterniflora leaves in a South American salt marsh. Braz. Arch. Biol. Technol. 58, 103–108 (2015).
Fanjul, E. et al. Effect of crab bioturbation on organic matter processing in South West Atlantic intertidal sediments. J. Sea Res. 94, 194–212 (2014).
Kennedy, H. et al. Coastal wetlands. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands (eds Hiraishi, T. et al.) Ch. 4 (IPCC, 2014).
Alongi, D. M. Salt marshes. Blue Carbon. Springer Briefs in Climate Studies (Springer, 2018).
Radabaugh, K. R. et al. Coastal blue carbon assessment of mangroves, salt marshes, and salt barrens in Tampa Bay, Florida, USA. Estuar. Coasts 41, 1496–1510 (2018).
Cusack, M. et al. Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ. Res. Lett. 13, 074007 (2018).
Yuan, Y. et al. Distribution of organic carbon storage in different salt-marsh plant communities: a case study at the Yangtze Estuary. Estuar. Coast. Shelf Sci. 243, 106900 (2020).
Gailis, M. et al. Quantifying blue carbon for the largest salt marsh in southern British Columbia: implications for regional coastal management. Coast. Eng. J. 63, 275–309 (2021).
Human, L. R. D. et al. Blue carbon and nutrient stocks in salt marsh and seagrass from urban African estuary. Sci. Total Environ. 842, 156955 (2022).
Isacch, J. P. et al. Distribution of salt marsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. J. Biogeogr. 33, 888–900 (2006).
Iribarne, O. et al. Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Mar. Ecol. Prog. Ser. 155, 137–145 (1997).
Howard, J. et al. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows. Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature (International Union for Conservation of Nature, 2014).
Ward, R. et al. Recent rates of sedimentation on irregularly flooded Boreal Baltic coastal wetlands: responses to recent changes in sea level. Geomorphology 217, 61–72 (2014).
Ward, R. Sedimentary response of Arctic coastal wetlands to sea level rise. Geomorphology 370, 107400 (2020).
Teasdale, P. et al. Recent estuarine sedimentation rates from shallow inter-tidal environments in western Scotland: implications for future sea-level trends and coastal wetland development. Quat. Sci. Rev. 30, 109–129 (2011).
Callaway, J. et al. Sediment accretion rates from four coastal wetlands along the Gulf. Mex. J. Coast. Res. 13, 181–191 (1997).
Arias-Ortiz, A. et al. Reviews and syntheses: Pb-derived sediment and carbon accumulation rates in vegetated coastal ecosystems—setting the record straight. Biogeosciences 15, 6791–6818 (2018).
Foucher, A. et al. A worldwide meta-analysis (1977–2020) of sediment core dating using fallout radionuclides including 137Cs and 210Pbxs. Earth Syst. Sci. Data 13, 4951–4966 (2021).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Deguines, N. et al. Precipitation alters interactions in a grassland ecological community. J. Anim. Ecol. 86, 262–272 (2017).
Zuur, A. F. et al. Mixed Effect Models and Extensions in Ecology with R (Springer, 2009).
Becker, R. A. & Wilks, A. R. Draw Geographical Maps. https://cran.r-project.org/web/packages/maps/index.html (2022).
Acknowledgements
We thank L. Linn from Dauphin Island Sea Laboratory, E. B. Roark from the Stable Isotope Geoscience Facility (TAMU) for laboratory analysis services and D. Navarro from IIMyC for technical and field assistance. This study was funded by grants from CONICET, FONDECyT and UNMdP (Argentina) to P.M. and O.I., NOAA (Office of Sea Grant, U.S. Dept. of Commerce, under Mississippi Alabama Sea Grant Consortium) and Mississippi State University Extension Service to E.S. Dr. N. Marbà was supported by OBAMA-NEXT, “Observing and mapping marine ecosystems - Next generation tools” project funded by the European Union under the Horizon Europe programme (grant Agreement: 101081642). The present research was carried out within the framework of the activities of the Spanish Government through the “Maria de Maeztu Centre of Excellence” accreditation to IMEDEA (CSIC-UIB) (CEX2021-001198). Dr. Raymond Ward was supported in this study by the Rising Stars Award, within the University of Brighton and by P190251PKKK to support the project “The impact of global environmental changes on coastal ecosystem services”.
Author information
Authors and Affiliations
Contributions
P.M., J.A., J.C., O.I., N.M., D.M., E.S. and R.W. conceptualized the project; P.M., J.A., J.C., N.M., D.M., E.S. and R.W. carried out the fieldwork and processed the samples; J.A. and D.M. carried out the SEM analysis; M.E.B. carried out the mixing model analysis (MixSIAR); R.W. analyzed the SAR (sediment accretion rates) and the CBR (carbon burial rates); P.M. and J.A. made the figures; P.M. lead the project and wrote the manuscript which was reviewed, edited and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Micheli Duarte de Paula Costa, Janine Adams and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinetto, P., Alberti, J., Becherucci, M.E. et al. The blue carbon of southern southwest Atlantic salt marshes and their biotic and abiotic drivers. Nat Commun 14, 8500 (2023). https://doi.org/10.1038/s41467-023-44196-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-44196-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.