Abstract
To mobilize sparingly available phosphorus (P) in the rhizosphere, many plant species secrete malate to release P sorbed onto (hydr)oxides of aluminum and iron (Fe). In the presence of Fe, malate can provoke Fe over-accumulation in the root apoplast, triggering a series of events that inhibit root growth. Here, we identified HYPERSENSITIVE TO LOW P1 (HYP1), a CYBDOM protein constituted of a DOMON and a cytochrome b561 domain, as critical to maintain cell elongation and meristem integrity under low P. We demonstrate that HYP1 mediates ascorbate-dependent trans-plasma membrane electron transport and can reduce ferric and cupric substrates in Xenopus laevis oocytes and in planta. HYP1 expression is up-regulated in response to P deficiency in the proximal zone of the root apical meristem. Disruption of HYP1 leads to increased Fe and callose accumulation in the root meristem and causes significant transcriptional changes in roots. We further demonstrate that HYP1 activity overcomes malate-induced Fe accumulation, thereby preventing Fe-dependent root growth arrest in response to low P. Collectively, our results uncover an ascorbate-dependent metalloreductase that is critical to protect root meristems of P-deficient plants from increased Fe availability and provide insights into the physiological function of the yet poorly characterized but ubiquitous CYBDOM proteins.
Similar content being viewed by others
Introduction
Phosphorus (P) is essential for undisturbed plant growth and development. However, in most soils, total amounts of P are very low or the P that is present is only poorly available to plants. The latter is because a large portion of inorganic phosphate – the P form taken up by plants – is precipitated with Ca or strongly sorbed on iron (Fe) and aluminum (Al) (hydr)oxides1. To cope with limiting P availability, plants have evolved a series of mechanisms to recycle internal P sources and to acquire P from the soil. One of these mechanisms is the release of low-molecular-weight carboxylates, such as malate, which can form complexes with Fe(III) (ferric Fe) and Al(III), thereby releasing sorbed phosphate2. This P-mining strategy likely evolved in plants growing in acidic soils with very low P concentrations3. The increased release of malate by P-deficient roots does not only increase the availability of P but also of Fe4. The impact of this interaction has been characterized in more detail in Arabidopsis thaliana. In this species, root inhibition under low P can be attenuated by removing Fe from the growth medium or by disrupting P deficiency-induced malate release5,6,7,8,9. Furthermore, it has been shown that over-accumulation of labile Fe in the apoplast of the apical region of roots triggers ectopic deposition of callose in the root apical meristem (RAM) and increased cell wall stiffening in the elongation zone5,6,10. While Fe-dependent primary root attenuation stimulates the development of a shallow and highly branched and root system11,12 that helps plants to forage P from the topsoil13, if not counteracted, excess Fe may ultimately disrupt stem-cell niche identity and RAM integrity5.
Fe uptake in most plant cells and organelles is driven by Fe(III) reduction via chemical reductants and membrane-bound metalloreductases of the ferric reductase oxidase (FRO) family14. In A. thaliana roots, Fe reduction relies on plasma membrane (PM)-bound FRO2 and the release of redox-active coumarins15,16. Interestingly, FRO2 is not expressed in the root apical zone and is repressed in response to P deficiency elsewhere17,18, whereas clear FRO3 expression in the RAM is only detected in Fe-deficient roots19. Furthermore, since the promoters of genes encoding the main coumarin biosynthesis enzymes are only active in the root hair zone16,20, it remains elusive how root tips acquire Fe.
Here, we identify and characterize a PM-bound electron transfer protein that is induced in response to P deficiency in the root apical zone of A. thaliana plants. Due to the more severe loss of meristematic integrity and more significantly inhibited cell elongation of a T-DNA insertional mutant specifically under low P conditions, we named the gene HYPERSENSTIVE TO LOW P1 (HYP1). The protein is a member of the yet poorly characterized CYBDOM family and consists of a plasma membrane-embedded cytochrome b561 domain fused to an apoplastic dopamine ß-monooxygenase N-terminal (DOMON) domain. We show that HYP1 is present in root tips, mediates ascorbate-dependent trans-PM electron transport, and can reduce ferric chelates and cupric [Cu(II)] ions. Finally, we demonstrate that HYP1-driven ferric reduction is critical to prevent malate-induced Fe overaccumulation in the apoplast and to maintain meristem integrity under low-P conditions.
Results
Identification of a CYBDOM mutant with hypersensitive root growth inhibition under low P
To identify genes required for P-dependent root growth responses, we re-analyzed a time-course root transcriptome of A. thaliana (Col-0 accession) plants grown on sufficient (625 µM) or low (100 µM) P concentrations. Previously, we used this dataset to specifically assess the expression of genes involved in P deficiency-induced root hair development21. Here, we integrated all data and performed an in-depth analysis, including an additional time point (i.e., 6 days after transfer) from the same experiment that was not considered in the previous analysis. Using log2-fold change (FC) ≥ |1.0| and FDR < 0.05 as cut-off parameters, we identified 2,327 genes that responded significantly to low P in at least one-time point (Fig. 1a; Supplementary Data 1), which were distributed into four major clusters. Among the cluster containing genes down-regulated in response to P deficiency (cluster 1, 901 genes), the gene ontology (GO) terms comprised by the cluster “iron metal homeostasis” were strongly enriched (Fig. 1b; Supplementary Data 2). The expression of major genes involved in root Fe uptake, including FRO2 and IRT1, was indeed down-regulated, as reported earlier18,22,23,24. Since we were interested in genes related with ferric Fe reduction, we first interrogated the expression of genes encoding the membrane-bound FROs and the enzymes involved with the synthesis of coumarins or ascorbate. The expression of S8H and CYP82C4, critical for the synthesis of redox-active coumarins, was strongly down-regulated under low P, while only FRO8 was consistently up-regulated among the FROs (Fig. 1c; Supplementary Data 3). However, FRO8 has been predicted to reside in mitochondrial membranes, a localization partially supported by the analysis of A. thaliana mitochondrial proteome25. Thus, our results indicated that ferric reduction by PM-bound FRO-type metalloreductases and coumarins is decreased in P-deficient roots. In line with a previous study7, the ascorbate biosynthesis gene VITAMINC2 (VTC2) and VTC4 were up-regulated in response to P deficiency, reinforcing a potential role for ascorbate under these conditions.
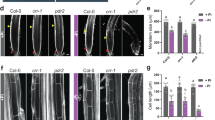
a Heat map for hierarchically clustered genes significantly regulated (|log2 FC| ≥ 1 -P versus +P, FDR < 0.05) under low P in roots (n = 3 independent root pools). b GO term enrichment network of significantly enriched terms of cluster 1 (P-values, adjusted Benjamini-Hochberg FDR). c Heat map showing differential expression (|log2 FC| ≥ 1 -P versus +P, FDR < 0.05) of selected genes. d, e Identification of a mutant with hypersensitive low P-induced primary root inhibition. Visual appearance of wild-type (Col-0) and hyp1 mutant plants (d) and primary root elongation of wild-type (Col-0), hyp1 mutant, and three independent lines expressing proHYP1::HYP1 in hyp1 (compl) 6 days after transfer (e). In a–e, seven-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (-P) with 100 µM Fe(III)-EDTA. f, g HYP1 overexpression improves root growth under low P. Appearance of plants (f) and primary root elongation of wild-type (Col-0), hyp1 mutant, and three independent transgenic lines expressing 35S::HYP1 in Col-0 (g). h, i Time-dependent changes in primary root length (h) and meristem integrity (i) after transfer to +P or -P (h) or only -P (i). j–l, Quantification of meristem cell length (j), meristem cell number (k) and mature cortical cell length (l). In f–g, ten-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (-P) with 150 µM FeCl3 and analyzed after 3 days (j–l), 6 days (f, g) or at the indicated dates after transfer (h, i). For the box plots in e and g, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum and maximum values; and dots, individual biological replicates (n = independent roots as indicated). In h, dark horizontal lines mark means and in j–l, bars represent means ± SD. Different letters indicate significant differences (one-way ANOVA followed by post-hoc Tukey’s test, P < 0.05). The exact P-values are provided as a Source Data file. In d and f, white dots indicate primary root position at the day of transfer, and arrowheads in i the boundary between meristem and transition zone. Scale bars, 1 cm (d, f) and 100 µm (i). Source data are provided as a Source Data file.
We then focused our search on other potential ferric reductases among P deficiency-induced genes. By screening for the presence of relevant protein domains, such as “ferric reductase” or “Fe/Cu oxidase”, we identified one gene, At5g35735, which was present in cluster 2 and predicted to encode a protein with a “Cytochrome b561/ferric reductase transmembrane” domain according to InterPro (www.ebi.ac.uk/interpro/). We phenotyped one available insertion line, carrying a transfer DNA (T-DNA) insertion in the sole intron of the previously uncharacterized gene At5g35735, and found that the mutant exhibited more severe primary root inhibition than the corresponding wild type (Col-0) when grown on low-P medium (Fig. 1d). Based on the conditional phenotype, we named the mutant hypersensitive to low P 1 (hyp1). Since Fe(III) complexed with malate is prone to blue light-induced photoreduction, which increases the risk of Fe(II)-driven hydroxyl radical formation in the apoplast26, we repeated the phenotypic analysis and shielded the roots from direct light exposure. With this system, only little light reached the roots, and light-induced Fe(III) reduction directly in the agar medium was almost completely prevented (Supplementary Fig. 1a–c). Since we aimed to investigate root responses induced by malate-driven Fe complexation and solubilization, we then provided Fe as the non-chelated form FeCl3. Although the amount of Fe supplied as FeCl3 was higher than that provided as Fe(III)-EDTA in standard Murashige and Skoog medium, only a small fraction was soluble (Supplementary Fig. 1d). When plants were grown under this modified cultivation setup, the severe primary root inhibition of hyp1 plants under low P supply still persisted (Fig. 1e and Supplementary Fig. 1e). We then used this cultivation setup (i.e., light-shielded roots and non-chelated Fe source) in all further experiments simulating P-limiting conditions in this study.
The expression of HYP1 in the hyp1 T-DNA insertion mutant is strongly impaired (Supplementary Fig. 2a), and the severe root inhibition of hyp1 could be fully rescued by re-introducing a construct containing the full genomic coding sequence of HYP1, including a 2.1-kb-long promoter region (Fig. 1e). Since the intron between the two exons of HYP1 is a putative rolling-circle (Helitron) transposon element (Supplementary Fig. 2b), we also expressed HYP1 complementary DNA (cDNA) under the control of the HYP1 promoter in the hyp1 mutant to rule out the possibility that the predicted transposon element was involved with the short-root phenotype. HYP1 cDNA also complemented the hypersensitive primary root inhibition of hyp1 under low P (Supplementary Fig. 2c, d). Thus, these results demonstrate that disruption of HYP1 is causal for the P deficiency-induced hypersensitive root phenotype of the isolated T-DNA line.
An earlier phylogenetic analysis has identified HYP1 as a CYBDOM-type protein27. These proteins possess a cytochrome b561 fused to a DOMON domain, which was initially identified in dopamine β-hydroxylase, the enzyme that generates norepinephrine from dopamine28,29. Among all 11 genes encoding for DOMON-containing proteins in A. thaliana, 10 are CYBDOMs while one, AUXIN-INDUCED IN ROOT CULTURES 12 (AIR12), encodes for a protein constituted by a single DOMON associated to a glycosylphosphatidylinositol (GPI) membrane anchor27. HYP1 was the only member of the DOMON superfamily differentially regulated in response to P deficiency (Supplementary Fig. 3a, b). Nonetheless, we assessed the primary root length of T-DNA insertion mutants available for 9 DOMON-expressing genes. Besides hyp1, only T-DNA insertions disrupting the expression of At3g07390 (AIR12) and especially At3g25290 (CRR) showed significantly shorter primary root length compared to wild type, while most lines showed no or only small differences compared to wild-type plants (Supplementary Fig. 3c, d). The simultaneous disruption of HYP1 and CRR further exacerbated the primary root inhibition under low P (Supplementary Fig. 3e, f), suggesting that the activities of these two CYBDOM proteins act in an additive way to sustain root growth under low-P conditions. In the accompanying manuscript, Clúa et al.30 provide a comprehensive characterization of CRR. Next, we overexpressed HYP1 in wild-type plants using the CaMV 35S promoter. Ectopic expression of HYP1 was able to completely prevent the inhibition of primary root elongation of plants grown in low-P medium (Fig. 1f, g). Altogether, our results demonstrate that HYP1 increases the tolerance of roots to low P-induced inhibition of primary root elongation.
HYP1 is critical for the maintenance of meristem integrity and cell elongation under low P
According to our transcriptome analysis, HYP1 expression is gradually induced in roots in response to P deficiency (Supplementary Fig. 3a). A time-course analysis of primary root length after transfer to low P revealed that the primary root of hyp1 plants ceases to elongate from 2 days after transfer onwards (Fig. 1h). A closer inspection of primary root tips revealed that the loss of HYP1 decreased the meristem size, and inhibited the elongation of mature cells (Fig. 1i–l and Supplementary Fig. 4a–d). A significant decrease in mature cell length was observed already 2 days after transfer to low P, one day before the inhibition of meristem length became significant (Supplementary Fig. 4a–d). Probably as a result of the smaller meristems and shorter cells, and potentially of accelerated cell differentiation, root hairs were detected much closer to the primary root apex of hyp1 than in wild-type plants under low P (Fig. 1i). To assess stem cell integrity, we then checked the localization of WOX5 in the RAM. Under low-P conditions, proWOX5::GFP-derived fluorescence was not anymore confined to quiescent center (QC) cells but detected in several meristematic cells (Supplementary Fig. 4e), indicating the disintegration of the stem cell niche. Finally, analysis of mitotic activity with proCYCB1;1::GUS reporter revealed that loss of HYP1 activity decreased the number of cells undergoing division in the RAM in response to low P (Supplementary Fig. 4f). Taken together, these results show that the hypersensitive primary root phenotype of hyp1 plants under low P is due to loss of RAM integrity and inhibition of cell expansion in the elongation zone.
HYP1 is a plasma membrane protein expressed in different root zones
To get insights into the tissue-specific expression of HYP1, we generated proHYP1::GUS lines. In young seedlings, we detected proHYP1-driven GUS activity mainly in the two cotyledons and throughout most parts of primary and lateral roots, including the apical zone (Fig. 2a). In line with the transcriptome analysis, the intensity of GUS staining increased when plants were cultivated on low P, reinforcing that HYP1 is induced in response to P deficiency. A close-up analysis of root tips revealed that HYP1 promoter activity was prominent in root cap cells and, under low P, strongly increased in cells surrounding the stem cell niche (Fig. 2b). To assess in more detail where the HYP1 protein is located, we generated a translational fusion carrying GFP in the C-terminal part of HYP1, which was driven by the HYP1 promoter. Under sufficient P, HYP1:GFP was detected in the vasculature along the mature root hair zone, whereas in the root apex, it was confined to the root cap and mature columella cells (Fig. 2c and Supplementary Fig. 5). Low P availability increased the abundance of HYP1:GFP and, in the RAM, induced its appearance in several cell files above the stem cell niche while having no effect on HYP1:GFP-derived fluorescence in root cap cells (Fig. 2d and Supplementary Fig. 5). To verify the subcellular localization of HYP1, we used the styryl dye FM4-64 that intercalates into membranes and observed defined colocalization of HYP1:GFP with this dye (Fig. 2e). Moreover, in plasmolyzed root cells, strong HYP1:GFP-derived fluorescence was detected in the PM and in Hechtian strands (Fig. 2f). Thus, these results demonstrate that HYP1 is a PM protein present in distinct domains of the root, including the RAM.
a, b P-dependent changes in HYP1 promoter activity in whole seedlings (a) and in the indicated zones of the primary root (b). c, d HYP1:GFP localization in the apical root meristem (c) and signal quantification in root cap and in cells located above the QC (n = 10 independent roots) (d) in response to low P. Cell walls were stained with propidium iodide (PI). The arrowhead indicates the increased abundance of HYP1:GFP in the cells above the stem cell niche. In a–d, ten-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (-P) with 150 µM FeCl3. GUS staining and HYP1:GFP localization were assessed on plants grown in the indicated treatments for 3 days. e, f HYP1:GFP is localized in the plasma membrane. Localization and fluorescence intensity of GFP and the membrane stain FM4-64 (e) in the section marked with the yellow arrow, and HYP1:GFP localization in plasmolyzed cells counterstained with PI (f). The experiments were repeated two times with similar results and representative images from one experiment are shown. g, h Trans-plasma membrane current recordings (g) and calculated mean currents (h) recorded in X. laevis oocytes injected with water (H2O) or cRNA of HYP1 and exposed to 1 mM of the water-soluble electron acceptor [Fe(CN)6]3− (ferricyanide) in standard bath solution (pH 5.5) at a holding potential of −20 mV (n = 11 independent oocytes for H2O-injected; n = 18 independent oocytes for HYP1-injected). For the boxplots in d and h, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum and maximum values; and dots, individual biological replicates. In d and h, P-values according to two-sided Student’s t-test. In g, the left and right arrows indicate the addition and removal of ferricyanide, respectively. Scale bars, 1 cm (a), 100 µm (b), 50 µm (c), 2 µm (e) and 5 µm (f). Source data are provided as a Source Data file.
HYP1 mediates ascorbate-dependent trans-PM electron transport
Cytochrome b561-containing proteins have been shown to catalyze electron transport between a cytosolic electron donor and acceptors located on the noncytoplasmic side31,32. To explore the transport properties of HYP1, we performed a series of functional assays in Xenopus laevis oocytes. Employing a two-electrode voltage clamp, we detected inward currents in oocytes injected with HYP1 cRNA when ferricyanide was provided as an electron acceptor in the bathing solution (Fig. 2g, h). The currents strongly increased after raising the cytosolic concentrations of ascorbate in the oocytes (Supplementary Fig. 6a, b). The results could be fitted by a Michaelis-Menten equation revealing an apparent affinity constant of 4 mM, which is in line with the approx. 20 mM ascorbate detected in the cytosol of plant cells33. Similar to other cytochrome b561-containing proteins characterized up to date, we found that ascorbate was a specific cytosolic electron donor for HYP1 with reduced glutathione not being able to enhance HYP1-mediated currents (Supplementary Fig. 6c, d). Current amplitudes were also stimulated by increasing the concentration of the electron acceptor in the bathing solution again following patterns resembling Michaelis-Menten kinetics (Supplementary Fig. 7a–d). To rule out the possibility that the detected currents were associated with ion fluxes, we manipulated the pH and ionic composition of the bathing solutions. Replacing the standard bathing solution with a solution containing only the membrane-impermeable ions 1,3-bis[tris(hydroxymethyl)methyl-amino]propane (BTP) and 2-(N-Morpholino)ethanesulfonic acid (MES) or raising the external pH to 7.5, did not significantly alter the current amplitude detected in HYP1-injected oocytes (Supplementary Fig. 7e, f). Thus, these results demonstrate that HYP1 mediates trans-PM electron transport, an activity that is influenced by the concentration of cytosolic ascorbate and extracytoplasmic electron acceptors.
Structural features of HYP1 critical for its function in oocytes and in planta
While the crystal structure of two cytochrome b561 proteins – CYBASC1 (AtCytb561) of A. thaliana and the human duodenal cytochrome b (Dcytb) – have been determined34,35, no structural information is available for CYBDOM proteins. Therefore, we predicted HYP1 structure with AlphaFold36. Our model revealed that the cytochrome b561 domain is formed by five α-helical trans-membrane segments while the DOMON resembles a sandwiched ß-sheet structure (Fig. 3a). The presence of a secretory signal peptide in the N-terminal part and HYP1 localization in the PM (Fig. 2e, f and Supplementary Fig. 2b) suggest that HYP1’s DOMON faces the apoplast. Next, we modeled the putative binding site for ascorbate on the cytosol-facing side using Rosetta ligand docking calculations. For guiding the modeling calculations, we used the structure of ascorbate-bound cytochrome b561 (PDB ID: 4O79). The predicted binding model for ascorbate indicates that hydrogen bond interactions with the sidechains of residues arginine 307 (R307) and asparagine 311 (N311) and possibly with the backbone of proline 302 (P302) help to stabilize the binding of ascorbate (Fig. 3b). Furthermore, our structural model revealed that two conserved histidine pairs H245–H314 and H211–H278 are putative coordination sites to two b-heme groups, one at the cytoplasmic side and another at the apical side of the cytochrome b561 domain, respectively (Fig. 3c). According to our model, the Fe-to-Fe distance between the two b-heme groups is approx. 23.5 Angstrom (Å), which is comparable to the distances reported for the crystal structures of the cytochrome b561 proteins CYBASC1 (AtCytb561) and Dcytb34,35. We confirmed the critical function of the H211–H278 pair for HYP1 function by replacing these positively charged, imidazole-containing histidine residues with non-polar leucine. The concomitant H211L and H278L substitutions completely abolished HYP1-mediated electron transport in oocytes without affecting its PM localization (Fig. 3d, e and Supplementary Fig. 8c, d, g). The mutated HYP1H211L/H278L:GFP variant also lost the ability to complement the short-root phenotype of hyp1 in contrast to native HYP1:GFP (Fig. 3f).
a–c Structural model of HYP1 predicted by AlphaFold (a), docking site of ascorbate in the cytosol-facing side of the cytochrome b561 core (b), and predicted coordination of two b-hemes on the cytochrome b561 core and one on the secreted DOMON domain (c). The distance between the Fe centers of neighboring b-hemes is indicated. d, e Trans-PM current recordings in X. laevis oocytes injected with wild-type HYP1 cRNA or with a mutated HYP1 variant carrying substitutions in the b-heme coordination sites in the apical part of the cytochrome b561 core (HYP1H211L/H278L). Current traces (d) and mean current amplitudes (e) recorded with [Fe(CN)6]3− (ferricyanide) after injection of 10 mM ascorbate in standard bath solution (pH 5.5) at a holding potential of -20 mV (n = 6 independent oocytes for HYP1; n = 8 independent oocytes for HYP1H211L/H278L). The left and right arrows indicate the addition and removal of ferricyanide, respectively. Bars represent means ± SD. P-value according to two-sided Student’s t-test. f Complementation of hyp1 with GFP translational fusions of native HYP1 or the indicated mutated variant (n = independent roots as indicated). Ten-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (-P) with 150 µM FeCl3 and primary root length analyzed after 6 days. For the box plots, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum and maximum values; and dots, individual biological replicates. Different letters indicate significant differences (one-way ANOVA followed by post-hoc Tukey’s test, P < 0.05). The exact P-values are provided as a Source Data file. g Computational docking of a b-heme molecule by a conserved methionine and histidine pair in the DOMON domain, and surface presentation showing a predicted solvent-exposed side. In a, c, and g, red spheres indicate the atom Fe at the center of a b-heme. Source data are provided as a Source Data file.
Previous studies indicated that DOMON domains can bind b-heme or sugars27,37. Based on the de novo structure model of HYP1 and compared to cellobiose dehydrogenase templates (PDBs 4QI3B, 1PL3, 4QI7), which contain a heme-binding site in a DOMON-like structure, we identified methionine 90 (M90) as a candidate for b-heme coordination. Another potential b-heme coordinating residue is H174, which is at a similar location as H176 in cellobiose dehydrogenase (PDB 4QI3) and in close spatial proximity to M90. In turn, other histidine residues in the DOMON domain, including the conserved histidine at position 54, are unlikely to participate in b-heme coordination as they are too distant from M90 or H174. According to our AlphaFold model, one edge of the b-heme putatively coordinated by the M90–H174 pair in the DOMON domain is solvent-accessible (Fig. 3g). The distance between two Fe centers of the apical heme of the cytochrome b561 domain and the heme in the DOMON is approx. 20.9 Å (Fig. 3c). Single M90L or H174L substitutions resulted in impaired PM localization of HYP1 both in oocytes and plant cells (Supplementary Fig. 8e–g).
Altogether, our results suggest the existence of a continuous electron transfer pathway across three b-hemes of HYP1, which links the cytosolic electron donor ascorbate with electron acceptors located in the apoplast.
HYP1 can mediate ferric and cupric reduction
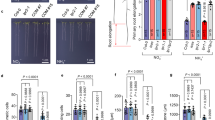
Considering the electron transport activity of HYP1, we then explored whether HYP1 is able to reduce ferric-chelates. In oocytes, HYP1-mediated currents were detected when different Fe(III) chelates, including Fe(III)-malate, were supplied in the bathing solution (Fig. 4a, b and Supplementary Fig. 9a). In order to assess the putative role of HYP1 as a ferric reductase in planta, we expressed HYP1:GFP in the fro2 mutant using the FRO2 or CaMV 35S promoter. Expression of either construct in fro2 plants significantly increased the root ferric-chelate reductase activity by approx. 1.6 times, allowing these plants to reach up to 60% of the activity detected in wild-type plants (Fig. 4c). Constitutive or Fe deficiency-induced HYP1 expression was also able to alleviate the Fe deficiency symptoms of fro2 plants by significantly increasing shoot chlorophyll concentration and shoot Fe contents (Fig. 4d, e and Supplementary Fig. 9c). These results demonstrate that HYP1 can mediate ferric-chelate reductase activity.
a, b Trans-plasma membrane currents (a) and mean amplitudes (b) elicited by 1 mM Fe(III)-malate in X. laevis oocytes injected with water (H2O) or cRNA of HYP1 (HYP1). Oocytes were pre-injected with 10 mM ascorbate and traces recorded at a holding potential of -120 mV. The left and right arrows indicate the addition and removal of Fe(III)-malate, respectively. Bars represent means ± SD (n = 6 independent oocytes). P-value according to two-sided Student’s t-test. c Ferric-chelate reductase activity of wild-type (Col-gl1), fro2, and fro2 plants expressing HYP1:GFP under the control of the FRO2 or CaMV 35S promoter. Ten-day-old seedlings were transferred to fresh medium without added Fe and assayed after 3 days. Fe(III)-EDTA was used as substrate for the ferric-chelate reductase activity assays. Bars represent means ± SD (n = 4 biological replicates constituted of 6 plants each). d, e Complementation of the Fe-deficient phenotype of fro2 by HYP1. Appearance of plants (d) and chlorophyll concentration (e) of wild-type (Col-gl1), fro2, and fro2 plants expressing HYP1:GFP under the control of the FRO2 or CaMV 35S promoter and grown for 6 days on 75 µM (control) or 25 µM Fe(III)-EDTA (low Fe). Bars represent means ± SD (n = 6 biological replicates constituted of 6 plants each). In c and d, different letters indicate statistical significance (one-way ANOVA followed by post-hoc Tukey’s test, P < 0.05). The exact P-values are provided as a Source Data file. In d, white dots indicate primary root position at the day of transfer. Scale bars, 1 cm (d). FW fresh weight. Source data are provided as a Source Data file.
Since some members of the FRO family can reduce cupric ions38,39 and 35S::HYP1 plants accumulated higher Cu concentrations in shoots when grown under low P (Supplementary Fig. 10a), we then assessed whether HYP1 can mediate cupric ion reduction. In HYP1 cRNA-injected oocytes, trans-PM currents were also detected when the Cu(II) salts CuSO4 and CuCl2 were supplied in the bathing solution (Fig. 5a, b), suggesting that Cu(II) serves as acceptor for HYP1-derived electrons. We then assessed root Cu(II) reductase activity of wild-type, hyp1, hyp1 crr and 35S::HYP1 plants. While the disruption of HYP1 alone did not significantly change the Cu(II) reduction capacity of roots, a small but significant decrease was detected in the hyp1 crr double mutant (Fig. 5c). In contrast, HYP1 overexpression increased root Cu(II) reduction activity by two-fold. Since Cu uptake relies on a reductive mechanism and excess Cu can inhibit root elongation38,40, we then phenotyped plants on elevated external Cu(II) concentrations. While the primary root of hyp1 plants was slightly longer than wild-type plants under high Cu, root growth of plants overexpressing HYP1 was strongly inhibited (Fig. 5d, e). Closer inspection of roots showed that overexpression of HYP1 decreased the length and number of cells in the meristem and the length of mature cells when plants were exposed to high Cu (Fig. 5f and Supplementary Fig. 10b). In hyp1, meristem size and cell length were less affected by high Cu and remained higher than in wild-type plants (Supplementary Fig. 10b). Altogether, our results demonstrate that HYP1 can function as Fe(III) and Cu(II) reductase and can affect the accumulation of these micronutrients in plant tissues.
a, b Trans-plasma membrane current recordings (a) and calculated mean currents (b) in X. laevis oocytes injected with water (H2O) or cRNA of HYP1 (HYP1) in response to two sources of Cu(II) (CuSO4 and CuCl2) in standard bathing solution (pH 5.5), at a holding potential of −20 mV. The left and right arrows indicate the addition and removal of the Cu substrates, respectively. Bars represent means ± SD (n = 5 independent oocytes). P values according to two-sided Student’s t-test. c Cu(II) reductase activity of wild-type (Col-0), hyp1, hyp1 crr and one transgenic line overexpressing HYP1 (35S::HYP1). Ten-day-old seedlings grown on standard half-strength MS medium were used for the assay. Bars represent means ± SD (n = biological replicates constituted of 6 plants each as indicated in the plot). P-values according to two-sided Student’s t-test. d–f HYP1 overexpression increases the sensitivity of primary roots to high Cu concentrations. Appearance of plants (a), primary root length (n = independent roots as indicated in the plot) (b) and root tip morphology (c). Ten-day-old seedlings were transferred to fresh medium containing 0.05 µM (control) or 50 µM CuSO4 and analyzed after 6 days. For the box plots, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum and maximum values; and dots, individual biological replicates. Different letters indicate significant differences (one-way ANOVA followed by post-hoc Tukey’s test, P < 0.05). The exact P-values are provided as a Source Data file. In d, white dots indicate primary root position at the day of transfer, and the arrowheads in f the boundary between meristem and transition zone. Scale bars, 1 cm (d) and 100 µm (f). FW fresh weight. Source data are provided as a Source Data file.
HYP1 prevents Fe overaccumulation in root meristems under low P
The ferric-chelate reduction capacity of HYP1 prompted us to investigate the role of Fe in HYP1-mediated root growth in response to P deficiency. Omitting Fe from the growth medium fully rescued the primary root growth of hyp1 plants (Fig. 6a, b). Next, we monitored Fe distribution in the apical zone of primary roots with Perls/DAB. Under sufficient P, Fe was more strongly distributed in the meristematic zone and especially near the stem cell niche, with Perls/DAB-dependent staining being comparable in wild-type and hyp1 roots (Fig. 6c, d). This pool of stainable Fe likely reflects Fe precipitated as Fe-phosphate in the apoplast, as shown previously5. Under low P, Fe accumulation remained more concentrated around and within the stem cell niche but was strongly intensified in hyp1 roots (Fig. 6c, d). Interestingly, Perls/DAB-stainable apoplastic Fe was decreased when HYP1 was overexpressed, almost completely disappearing under low P. By staining callose with aniline blue, we found that the increased Fe accumulation in hyp1 meristems was accompanied by increased callose deposition, especially in the stem cell niche and around cortical and endodermal cells in the meristem (Fig. 6e, f). Furthermore, we detected decreased mobility of the transcription factor SHORT ROOT (SHR) from the stele into quiescent center cells (Supplementary Fig. 11), suggesting that cell-to-cell mobility of proteins between these cells was impaired in hyp1 roots.
a, b Appearance of plants (a) and primary root length of wild-type (Col-0), hyp1 mutant and one HYP1-overexpressing line (n = independent roots as indicated) (b). Ten-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (−P) with 150 µM FeCl3 (+Fe) or without added Fe (−Fe) and analyzed after 6 days. c−f Fe accumulation and distribution (c) and callose deposition (e) in the apical region of primary roots of wild-type (Col-0), hyp1 mutant and one HYP1-overexpressing line. Quantification of Perls/DAB-stained Fe (n = 8 independent roots) (d) and aniline blue-stained callose (n = independent roots as indicated) (f). Ten-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (−P) with 150 µM FeCl3 and roots stained after 3 days. g Longitudinal sections of Perls/DAB-stained root tips 2 days after transfer from +P to -P medium. The experiment was repeated two times with similar results and representative images from one experiment are shown. h Heat map for hierarchically clustered genes significantly regulated in response to low P (|log2 FC| ≥ 1 −P versus +P, FDR < 0.05) in root tips of wild type (Col-0), hyp1 mutant and one HYP1-overexpressing line (35S::HYP1) (n = 3 independent root pools). Ten-day-old seedlings were transferred to a fresh medium containing 625 µM P (+P) or 5 µM P (−P) with 150 µM FeCl3 for 3 days. i UpSet plots showing the number of genes exclusively or commonly up- or down-regulated. j Heat map showing differential expression (|log2 FC| ≥ 1 -P versus +P, FDR < 0.05) of genes related to Fe reduction, uptake or storage. For the box plots in b, d, and f, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum, and maximum values; and dots, individual biological replicates. Different letters indicate significant differences (one-way ANOVA followed by post-hoc Tukey’s test). The exact P-values are provided as a Source Data file. Scale bars, 1 cm (a), 100 µm (c), 50 µm (e), 20 µm (g). In a, white dots indicate the primary root position on the day of transfer. Source data are provided as a Source Data file.
To better determine where Fe was accumulating in the root meristems of low-P plants, we prepared longitudinal cross-sections of Perls/DAB-stained meristems. In root tips of wild-type plants, Fe was mainly deposited around stem cells, cortical cells and in some cells of the columella and root cap, while in meristems of 35S::HYP1 plants stainable Fe was strongly diminished with only little deposits being detected around the QC (Fig. 6g). In hyp1 meristems, Perls/DAB staining was more intense and was additionally detected around endodermal cells and in the stele, thus largely overlapping with the pattern of callose deposition (Fig. 6e, g). The stained Fe pools appeared mainly apoplastic, as the staining was strongly concentrated around cell boundaries and was highly reminiscent of apoplastic Fe localization reported previously5. Thus, these results suggested that HYP1 prevents aberrant Fe accumulation and callose deposition in the meristem of roots exposed to low-P medium.
HYP1-dependent transcriptional responses in roots
To gain more insights into the putative role of HYP1 in Fe homeostasis and global transcriptional responses under low P, we performed a whole transcriptome sequencing (RNA-seq) analysis in root tips. Clear differences were detected when comparing the expression pattern of genes differentially expressed (|log2 FC| ≥ 1.0, FDR < 0.05) in response to low P in wild type, hyp1 and 35S::HYP1 (Fig. 6h; Supplementary Data 4). In wild-type root tips, 1113 genes were differentially expressed after 3 days of growth under low P compared to sufficient P (Fig. 6i; Supplementary Data 4). Disruption of HYP1 resulted in the largest number of differentially expressed genes (DEGs) with 829 genes being differentially expressed exclusively in hyp1 roots (322 up-regulated and 507 down-regulated). In contrast, a comparatively small number of genes (169 in total) was significantly altered in root tips of low P-grown 35S::HYP1 plants, most of them being up-regulated. The majority of DEGs in 35S::HYP1 overlapped with genes significantly responding to low P also in wild type and hyp1 (Fig. 6i; Supplementary Data 4). According to GO analysis, this subset of DEGs was significantly enriched with functional groups related to phosphate starvation responses, phosphate transport and phosphate homeostasis (Fig. 6i and Supplementary Fig. 12a), suggesting that primary P starvation responses were not significantly disturbed by HYP1 overexpression or disruption. This result also indicated that HYP1 overexpression restricted the transcriptional responses mainly to P starvation and less to secondary effects typically derived from the interaction with Fe. In fact, the expression of many Fe-related genes, including IRT1, FRO2, FRO8, S8H, CYP82C4, and FER1, which were strongly responsive to low P in wild-type and hyp1 roots, were only mildly or not differentially regulated in 35S::HYP1 (Fig. 6j; Supplementary Data 5). Among genes that failed to respond to low P in hyp1 roots (i.e., up-regulated in wild type but not in hyp1) we detected GO terms related to cell wall composition or organization, while genes related to heme binding, Fe-S cluster binding, and cell-cell junction organization were specifically down-regulated in roots of hyp1 in response to low P (Supplementary Fig. 12b, c). Together, these results reinforce a putative involvement of HYP1 in maintaining Fe acquisition under low-P conditions and highlight that processes related to P deficiency responses in the apoplast are significantly affected when HYP1 activity is lacking.
HYP1 counteracts increased Fe availability resulting from low P-induced malate release
Considering the Fe-dependent root phenotype of the hyp1 mutant, the HYP1-dependent ferric reductase activity in roots and the weak Perls/DAB staining in root tips of 35S::HYP1 plants, we hypothesized that HYP1 function is required to counteract the increased Fe solubility resulting from P deficiency-induced malate release. To test this possibility, we generated a hyp1 almt1 double mutant. In the absence of ALMT1, disruption of HYP1 did neither increase primary root sensitivity to low P nor Perls/DAB-stainable Fe pools in root tips (Fig. 7a–c and Supplementary Fig. 13a). Loss of HYP1 in the lpr1 lpr2 mutant background also had no effect on primary root growth or Fe staining. Thus, these results suggest that HYP1 activity becomes critical only when ALMT1-mediated malate export and LOW PHOSPHATE ROOT (LPR)-dependent apoplastic Fe(II) oxidation are active.
a–c HYP1 function under low P is critical only in the presence of ALMT1 and LPR1 and LPR2. Appearance of plants (a), primary root length after 6 days (n = independent roots as indicated) (b) and Perls/DAB staining of Fe after 3 days (c). d Root ferric-chelate reductase activity assayed with freshly prepared Fe(III)-malate complexes as substrate (n = 6 biological replicates consisting of 6 plants each). The assay was performed after transferring plants for 3 days to medium containing 625 µM P (+P) or 5 µM P (-P) with 150 µM FeCl3. Bars represent means ± SD. e–i Cell type-specific complementation of hyp1 with GFP translational fusions of HYP1 under the control of the indicated promoters. Primary root length (e), confocal images of propidium iodide-stained mature root zones and RAMs (f) and quantification of meristem cell length (g), meristem cell number (h), and mature cortical cell length (i) (n = independent roots as indicated). Ten-day-old seedlings were transferred to fresh medium containing 625 µM P (+P) or 5 µM P (-P) with 150 µM FeCl3 and analyzed after 3 days. For the box plots in b, e, and g–i, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum and maximum values; and dots, individual biological replicates. In b, d, e, and g–i, letters indicate significant differences (one-way ANOVA followed by post-hoc Tukey’s test, P < 0.05). The exact P-values are provided as a Source Data file. In a, dots indicate primary root position at the day of transfer. Scale bars, 1 cm (a), 100 µm (c, j) and 50 µm (f). Source data are provided as a Source Data file.
As malate becomes a predominant Fe(III)-chelator in P-starved roots6,7, we next assessed HYP1-dependent ferric reduction of freshly prepared Fe(III)-malate complexes. Probably as a result of the strong down-regulation of FRO2 in mature root zones, the capacity of roots of P-deficient wild-type plants to reduce Fe(III) decreased by approx. 50% (Fig. 7d). An even stronger decrease was detected for the hyp1 crr double mutant, while HYP1 overexpression maintained the root ferric-chelate reductase activity at the same level as under sufficient P. Thus, these results demonstrate that overexpression of HYP1 can overcome ALMT1-induced Fe solubilization by restoring, together with CRR, ferric reduction capacity in P-deficient roots.
Locally defined HYP1 activity in the RAM sustains root growth under low P
Since HYP1 is not only expressed in the RAM, we next investigated which expression domain was most critical for HYP1-mediated tolerance to low P by expressing HYP1:GFP under the control of different promoters. Recapitulating HYP1 expression in root cap cells, in mature endodermal and vascular cells and in cells above the QC in the proximal part of the RAM with a 2151-bp HYP1 promoter sequence fully restored primary root elongation, mature cell length and meristem size and integrity (Fig. 7e–i). Using the promoter of BEARSKIN1 (BRN1) to confine HYP1 expression in root cap cells did neither significantly improve root growth nor prevent loss of RAM integrity (Fig. 7e). Expression of HYP1 in root cap cells did also not significantly change the RAM size and the length of mature cells (Fig. 7g–i). Expression of HYP1 in mature endodermal cells with the promoter of SCHENGEN1 (SGN1) was able to significantly increase the elongation of mature cells and partially restore primary root length but had limited effect in restoring RAM integrity and size (Fig. 7e–i). However, full recovery of primary root growth, mature cell length and RAM size and integrity was achieved when we used the promoter of LPR1 to drive HYP1 expression in the stem cell niche and in proximal endodermal and cortical cells in the RAM, and in endodermal cells in differentiated root zones (Fig. 7e–i). Thus, these results suggest that HYP1 activity in a confined zone in the proximal zone of the RAM is required to prevent RAM disintegration, while primary root elongation further relies on HYP1 expression in mature endodermal cells.
Next, we monitored the impact of HYP1 on the fate of apoplastic Fe by assessing Perls/DAB-stainable Fe pools in the RAM after transferring plants from a Fe-containing to a Fe-depleted medium. When phosphate was present in the Fe-depleted medium, Fe pools were not significantly altered within 1 day after transfer and were comparable in wild type and hyp1 (Fig. 8a, b). Under low P, Perls/DAB-stainable Fe pools were significantly depleted in the RAM of wild-type plants while remaining largely unchanged in hyp1 plants. We hypothesized that the HYP1-dependent depletion of stainable Fe pools could be associated with HYP1-driven reduction of soluble Fe(III)-malate complexes formed under P-limiting conditions. Indeed, Fe(III)-malate reduction capacity of excised root tips (approx. 3 mm-long) from P-deficient hyp1 plants was significantly lower compared to wild-type and HYP1-overexpressing plants (Supplementary Fig. 13b). We also visualized Fe(II) in the primary root with the turn-on fluorescent probe RhoNox-141,42. In order to compare RhoNox-1 signals in similar RAM areas, the staining was performed 1 day after transfer, when the size and integrity of the RAM of low P-exposed hyp1 plants are not yet significantly altered (Fig. 1i and Supplementary Fig. 4a–d). In line with the ferric-chelate reductase activity detected in excised root tips, RhoNox-1-derived fluorescence decreased in response to low P and was significantly lower in the RAM of hyp1 and much higher in the RAM of 35S::HYP1 compared to wild-type plants (Supplementary Fig. 13c–e). Together, these results suggested that, under low P, HYP1 promotes the depletion of apoplastic Fe pools by reducing Fe(III) solubilized by malate.
a, b HYP1 expression in the meristem depletes apoplastic ferric Fe pools under low P. Perls/DAB staining of Fe (a) and quantification of signals (n = independent roots as indicated) (b) in the RAM of the indicated plants after preculture with 625µM P in a Fe-containing medium (+P+Fe) and after 1 day of growth on Fe-depleted medium containing 625 µM P (+P-Fe) or 5 µM P (-P-Fe). For the box plots in b, horizontal line, median; edges of boxes, 25th (bottom) and 75th (top) percentiles; whiskers, minimum and maximum values; and dots, individual biological replicates. Letters indicate significant differences (one-way ANOVA followed by post-hoc Tukey’s test, P < 0.05). The exact P-values and source data are provided as a Source Data file. c Hypothetical model for the function of HYP1 in root adaptation to low-P conditions. Increased ALMT1-mediated export of malate in response to P deficiency releases phosphate (Pi) from precipitated Fe-phosphate complexes while increasing the apoplastic concentration of soluble ferric-malate complexes. Ferric reduction by HYP1 facilitates the uptake of excess Fe by a yet unknown root tip-expressed Fe(II) transporter. Depletion of soluble Fe can prevent its participation in apoplastic redox cycling reactions potentially involving Fe(III) reduction by blue light or apoplastically-located chemical reductants and Fe(II) re-oxidation by the cell-wall-localized multicopper ferroxidases LPR1 and LPR2. Uncontrolled apoplastic redox cycling induces ROS-dependent aberrant callose deposition in the root apical meristem, causing meristem exhaustion. A putative interplay of HYP1 and LPRs remains elusive. ROS reactive oxygen species, PHTs phosphate transporters, Asc ascorbate, MDHA monodehydroascorbate.
Discussion
Ferric Fe reduction is key to several processes that maintain Fe homeostasis, including Fe uptake, allocation, and storage. Up to date, all characterized ferric-chelate reductases in plants belong to the flavo-cytochromes of the FRO family. Interestingly, plants also encode for proteins containing a cytochrome b561 domain, which is common in several ferric reductases active in animal cells, such as the human duodenal cytochrome b (Dcytb) involved with dietary nonheme Fe uptake35,43. In plants, the cytochrome b561 domain can occur as a single unit, as in diheme b-containing cytochrome b561 proteins (CYBASCs), or associated with one or more DOMON domains, as in CYBDOMs31. Our data uncovered a mechanism of ferric/cupric reduction in plants involving a CYBDOM protein. Although ferric reductase activity had been previously recorded for cytochrome b561-containing proteins in oocytes32,44, the participation of such proteins in ferric reduction in plants had not yet been determined. By expressing HYP1 in the fro2 mutant, we show that HYP1 can mediate ferric-chelate reduction in planta and improve growth under low-Fe conditions (Fig. 4c–e). Our results also demonstrate that HYP1 can mediate trans-PM electron transfer to cupric substrates in oocytes and that root cupric reductase activity is significantly increased when HYP1 is overexpressed (Fig. 5a–c). Other plant CYBDOMs likely also possess such activity, as a significant decrease in Cu(II) reduction was only detected in the hyp1 crr double mutant (Fig. 5c). Whereas the relevance of HYP1-mediated cupric reduction is still unclear, we demonstrate that the ability of HYP1 to reduce ferric chelates has a significant impact on root growth under low-P conditions.
Plants contain a larger number of CYBDOM genes than other organisms27,45, suggesting that the association of DOMONs with the redox-active cytochrome b561 plays an important role in plants. Our AlphaFold-supported modelling indicated that the DOMON domain of HYP1 can coordinate one b-heme group (Fig. 3a, c), in line with previous electron paramagnetic resonance analysis of the DOMON-only protein AUXIN-INDUCED IN ROOT CULTURES 12 (AIR12; ref. 27). Thus, our results suggest that the three b-hemes of HYP1 form an electron conduit connecting cytosolic ascorbate with electron acceptors located in the apoplast, such as ferric and cupric substrates. Unfortunately, disruption of the predicted b-heme coordination site in the DOMON impaired HYP1 localization in the PM (Supplementary Fig. 8), preventing us to directly investigate its requirement for trans-PM electron transport in oocytes and for HYP1 function in planta. Regarding the electron donor, instead of NADPH, the common electron donor of FRO-type metalloreductases15, cytochrome b561-containing proteins oxidize cytosolic ascorbate to reduce electron acceptors on the opposite side of the membrane. Our results indeed demonstrate that ascorbate serves as a cytosolic electron donor for HYP1 (Supplementary Fig. 6). The upregulation of genes encoding ascorbate biosynthesis genes in response to P deficiency (ref. 7; Fig. 1c) suggests that the electron donor for CYBDOM-mediated trans-PM electron transport is increased in P-deficient root cells. Thus, ascorbate may indirectly drive ferric-chelate reduction at the PM besides functioning as a chemical reductant of apoplastic ferric pools as proposed previously7. However, determining whether the levels and redox state of apoplastic ascorbate in the root tips are altered depending on P availability or HYP1 activity still remains a challenge.
The accumulation of soluble Fe(III)-malate complexes in the apoplast of root tips has been proposed as a key determinant of the inhibition of primary root growth under low-P conditions6,7. Once mobilized, Fe is more prone to engage in apoplastic redox cycling reactions, producing ROS and triggering aberrant callose deposition5. Primary root length inhibition in relation with excessive accumulation of Fe in the apoplast has also been recorded in ammonium-stressed plants46,47. In the phloem of roots, ammonium-induced Fe overaccumulation induces massive callose deposition which results in decreased sucrose delivery to sustain root growth46. Under low P, Fe pools likely become more reactive as less phosphate is available to chemically interact with Fe while malate increases the pool of mobile Fe. Based on our findings, we hypothesize that HYP1-mediated ferric reduction prevents that excessive amounts of malate-mobilized Fe accumulate in the apoplast (Fig. 8c). Our cell type-specific complementation experiments indicated that HYP1 activity in the proximal RAM is critical to maintain meristem integrity and root growth (Fig. 7e–i). It is within this zone that HYP1 expression and protein accumulation is most strongly induced in response to low P and where increased callose deposition is detected in the RAM of hyp1 plants (Figs. 2b, c and 6e). In mature root zones, expression of HYP1 in inner-root tissue (including the endodermis) can restore cell elongation but has limited ability to prevent RAM disintegration.
Our results further suggest that, in the context of P deficiency, the function of HYP1 appears to be less critical to support whole-plant Fe nutrition but rather to maintain Fe homeostasis in root tips. The identification of another CYBDOM (CRR) critical for maintaining root growth under low P in the accompanying study of Clúa et al.30 reinforce the importance of these proteins in this process. Interestingly, the main strategy-I mechanism of Fe uptake formed by FRO2 and the Fe(II) transporter IRON-REGULATED TRANSPORTER1 (IRT1) is not present in the root meristem and elongation zone17,48. Although future studies with Fe tracers will be required to quantify the Fe uptake capacity of cells within these two zones, we hypothesize that HYP1 may represent a component of a root tip-active Fe acquisition mechanism induced under low-P conditions (Fig. 8c). The up-regulation of HYP1 in P-deficient roots indicates that the proposed root tip-active Fe acquisition mechanism is under a differential regulation compared to FRO2 and IRT1 in the root hair zone. One possibility to explain this difference is that cellular processes in the root apex are more sensitive to Fe-driven radical formation in the apoplast5,47,49. Thus, Fe-dependent modulation of root growth may attenuate root growth on low P-containing soil patches and increase exploration of P in the topsoil. In the root apex, LPR1 is expressed in the stem cell niche and early cortical and endodermal cells49, thus partially overlapping with HYP1 under low-P conditions. Since the activity of the multicopper oxidase LPR1 maintains higher Fe levels in the apoplast5, this potentially increases the risk that apoplastic Fe engages in redox cycling and ROS formation. In this context, HYP1-mediated ferric reductase activity may either supply or, if the formed Fe(II) is immediately taken up, remove the substrate for LPR1. Our results favor the second scenario, as HYP1 activity is only necessary when LPRs are active (Fig. 7a–c). The ferric reduction by HYP1 could help plants to fine tune root growth in low-P soils by altering the pool of apoplastic Fe that is necessary to attenuate root growth while simultaneously protecting RAM integrity against P deficiency-induced Fe toxicity.
Beyond P deficiency and outside the RAM, HYP1, and other CYBDOMs might help to fine-tune other Fe-dependent redox processes in concert with LPR1 or LPR250,51. Furthermore, some CYBDOMs have been found to show strong coregulation with auxin-regulated peroxidases, laccases, and GDSL-motif-containing lipases expressed in root endodermal cells52. Thus, it will be interesting to investigate whether the ferric and cupric reduction capacities of plant CYBDOMs can potentially modulate cell wall formation and composition and to determine whether these proteins can reduce other apoplastic electron acceptors. By establishing the phenotypic changes evoked upon altered HYP1 expression at the organismal level, our study sheds light on the physiological roles of these poorly characterized proteins.
Methods
Plant materials
If not noted otherwise, the Arabidopsis thaliana accession Columbia-0 (Col-0) was used as wild type in this study. The following T-DNA tagged and transgenic lines were used in this study: SALK_115548 (hyp1), SALK_009629 (almt1), SALK_016297 and SALK_091930 (lpr1 lpr2), SALK_202530 (crr), SALK_013527 (At4g12980), SALK_006551 (At3g59070), SALK_092983 (At4g17280), SALK_204800 (At5g47530), SALK_110375 (At3g07570), SALK_111270 (At3g61750), SALK_089061 (At5g54830), SALK_205490 (air12), SALK_012348 (At2g04850), proSHR::SHR:GFP53, proCYCB1;1::GUS54, proWOX5::GFP55. All A. thaliana T-DNA insertion lines were obtained from the Nottingham Arabidopsis Stock Centre, except the lpr1 lrp2 double mutant which was kindly provided by Prof. Dr. Steffen Abel. The lines almt1 hyp1, hyp1 crr and lpr1 lpr2 hyp1 were generated by crossing the single almt1 and crr or double lpr1 lrp2 mutants with hyp1. Homozygous double or triple mutant plants were identified by PCR using the primers listed in Supplementary Table 1. The A. thaliana accession Columbia glabra (Col-gl1) was used as wild type for the fro2 mutant.
Plant growth conditions
Seeds were surface sterilized by 70% ethanol with 0.05% (v/v) Triton X-100, and pre-cultured on half-strength Murashige and Skoog salt mixture (½ MS) solid medium containing 0.5% sucrose, 1 mM MES (pH 5.5), and 1% Difco agar. Seeds were stratified for 2 days in the dark at 4 °C. Afterwards, Petri dishes were transferred to a growth chamber vertically positioned under a 22 °C/18 °C and 10-h/14-h (light/dark) regime at light intensity of 120 μmol photons m−2 s−1. For the microarray profiling shown in Fig. 1a–c, growth conditions inducing primary root inhibition at intermediate low-P conditions were used11. First, seedlings were precultured as described above for 7 days. Then, seedlings were transferred to fresh ½ MS solid medium containing either 625 µM KH2PO4 (+P) or 100 µM KH2PO4 + 525 µM KCl (-P) with Fe supplied as 75 µM Fe(III)-EDTA. Whole roots were harvested 1, 2, 4, and 6 days after transfer to treatments. Roots of 50 plants were pooled together to constitute one biological replicate. The same growth conditions were also used in the initial phenotyping experiment shown in Fig. 1d. In all other experiments, roots were shielded from direct light exposure by placing Petri dish plates inside cardboard boxes and intercalating each Petri dish with 3-cm thick black foam that extended from the bottom of the box, as shown in Supplementary Fig. 1a, b. In these experiments, 10-day-old seedlings instead of seven-day-old were transferred from the pre-culture to square Petri dishes (12 x 12 cm) containing modified half-strength MS medium with 0.5% sucrose, 2.5 mM MES (pH 5.5), 1% Difco agar, 625 μM KH2PO4 (+P) or 5 μM KH2PO4 + 620 µM KCl (-P), with or without 150 μM FeCl3 (+Fe or -Fe, respectively), which was supplied as the sole Fe source after the autoclaved agar was cooled down. In experiments with complemented fro2 plants, different Fe conditions were obtained by transferring 10-d-old seedlings to a modified half-strength MS medium with 0.5% sucrose, 1 mM MES (pH 5.5), 1% Difco agar, 75 μM Fe(III)-EDTA (+Fe, control), 20 μM Fe(III)-EDTA (low Fe), no added Fe (-Fe), as indicated in the legends of the corresponding figures. To phenotype plants under Cu excess, 10-day-old plants cultivated as indicated above were transferred to fresh solid half-strength MS medium containing 0.05 µM (control) or 50 µM CuSO4. Phenotyping under low Fe and high Cu was also performed using the root-shaded setup described above.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from homogenized root samples using the NucleoSpin RNA Mini Kit (Machery-Nagel), followed by on-column DNase treatment (QIAGEN), according to the manufacturers’ protocols. cDNA was synthesized from 0.5-1 µg RNA by reverse transcription using the RevertAid First Strand cDNA synthesis Kit (Thermo Fisher Scientific) and oligo(dT) primer. A ten- or twenty-times diluted cDNA sample was then used for quantitative real-time (RT) PCR analysis with the CFX384 Touch Real-Time PCR Detection System (Bio-Rad Laboratories) and the iQ SYBR Green Supermix (Bio-Rad Laboratories), using the primers listed in Supplementary Table 2. Amplification cycle protocols were as follows: 2 min at 95 °C; 40 cycles of 6 s at 95 °C and 30 s at 60 °C. Melting curves were verified at the end of 40 cycles for confirmation of primer specificities. All reactions were repeated in two technical and three biological replicates. Average Cq values were normalized by ∆∆CT formula against UBIQUITIN 2 (AT2G36170) expression, as indicated in the legend of the corresponding figures.
Transcript profiling and GO term enrichment analysis
For the identification of Fe-related genes responsive to P deficiency, we performed a microarray study. The expression of selected root hair-related genes from the microarray presented in this study was already reported previously21. Here, we present a more detailed analysis of the dataset, including one additional time-point from the same experiment. In brief, RNA was extracted from whole roots using a RNeasy plant mini kit (Qiagen) with on-column DNase treatment according to the manufacturer’s instructions. cDNA was prepared using random hexamer primers and SuperScript II reverse transcriptase (Life Technologies) according to the manufacturer’s protocol. RNA amplification, labeling and hybridization to Agilent microarrays (Arabidopsis V4, 021169, Gene Expression Microarray) were conducted following the manufacturer’s protocol (Agilent Technologies). The full dataset was deposited in the NCBI Gene Expression Omnibus under accession GSE217790. Analysis of microarray data was performed with the R package limma56. Raw feature intensities were background corrected using “normexp,” and “quantile” normalization method was used to normalize between arrays. Differential expression was calculated by fitting a linear model to log2-transformed data by an empirical Bayes method57. To extract genes with significant expression differences, the cutoff |log2 FC| ≥ 1 and the Benjamini–Hochberg false discovery rate (FDR) < 0.05 was applied. Significant differentially expressed genes (DEGs) over four time points between -P and +P samples were visualized in a heat map (|log2 FC | ≥ 1, FDR < 0.05) generated with the hierarchical clustering (HCL) algorithm by using Pearson distance and average linkage clustering.
For RNA-seq analysis, total RNA was extracted as described above. Library construction and sequencing were performed at Novogene (UK) Company Limited. Briefly, RNA integrity and quantitation were determined with the Agilent 2100 Bioanalyzer. Total mRNA was enriched with oligo(dT) beads and randomly fragmented with fragmentation buffer. A stranded-specific library was then prepared for each sample and sequenced with a NovaSeq 6000 PE150 platform, yielding about 44 million high-quality reads per sample. After the removal of adapters, reads containing ploy-N and low-quality reads, the clean reads were aligned to the Arabidopsis genome (TAIR10) using HISAT258 with the default parameters. The reads mapped on exons were counted using featureCounts59. Significant DEGs were identified with |log2FC| ≥ 1 and FDR < 0.05 using DESeq260. The full dataset was deposited in the European Nucleotide Archive (ENA) under project ID PRJEB65916. To visualize intersections of DEGs in the three different genotypes in UpSet plots the R package Complex UpSet61 was applied. Heat maps were drawn using the R package Complex Heatmap62 by using Euclidean distance and cetroid linkage clustering. Gene ontology (GO) enrichment analyses were performed with clusterProfiler63. Statistical significance of GO terms was declared when the P-value of Benjamini–Hochberg FDR for multiple testing correction was < 0.05. To reduce redundant GO terms and keep one representative term, the method simplify was applied with a similarity cutoff > 0.7. Dot plot representations of significant GO terms were obtained using the R package enrichPlot64.
Plasmid construction and generation of transgenic lines
All constructs for plant expression were generated by GreenGate modular cloning65. The 2151-bp HYP1 promoter sequence and the 2019-bp of HYP1 open reading frame (without stop codon) were amplified from genomic DNA of Col-0 (CS60000) and the 1212-bp long HYP1 coding sequence (without stop codon) amplified from complementary DNA (cDNA). To drive HYP1 expression in specific cell types, the 2008-bp promoter fragment of BRN1 (At1g33280), 2086-bp of SGN1 (At1g61590), 2309-bp of LPR1 (At1g23010) and 1941-bp of FRO2 (At1g01580) were amplified from genomic DNA of Col-0 (CS60000). Amplification was performed using the Phusion High-Fidelity DNA Polymerase (New England Biolabs) using primers listed in Supplementary Table 3. Promoters, open reading frames and coding sequences were cloned into the GreenGate entry modules pGGA000 and pGGC000, respectively. Correct integration of the fragments into entry modules was verified by restriction digestion reactions and the cloned sequences were verified by sequencing. To generate transcriptional reporters and translational GFP fusions, individual entry modules were assembled either into the GreenGate binary vector pGGZ001, including a phosphinothricin or hygromycin resistance cassette for antibiotic selection in plants, or into pFASTR A-G for selection of transgenic plants based on the presence of red fluorescence in seeds. Mutated HYP1M90L, HYP1H174L and HYP1H211L/H278L variants were generated by site-directed mutagenesis using the double-primer method66 and the primers listed in Supplementary Table 2. The final binary vectors were transformed into the Agrobacterium tumefaciens strain GV3101 (containing the helper plasmid pSOUP if transformed with pGGZ001) and finally transferred to A. thaliana (Col-0; CS60000) plants via the flower dip method67. Representative transgenic lines for each construct were selected from 6-8 independent transformants and used for analysis. If not indicated otherwise in the figures, 35S::HYP1 line 5d was used as the representative HYP1-overexpressing line in Col-0 background. For oocyte expression, the cDNA sequence of native or mutated HYP1 variants was amplified with primers containing XmaI (forward) or XbaI (reverse) restriction sites and cloned into pGEMHE32.
Root phenotypical analysis
To determine primary root lengths, roots were scanned using an Epson Expression 12000XL scanner (Seiko Epson) with a resolution of 300 dots per inch after they were clearly separated from one another on the agar plate. Primary root length was quantified with ImageJ software. Measurements of meristem cell length and mature cortical cell length were performed with ZEN 3.4 Blue Edition imaging software (Carl Zeiss Microscopy).
Oocyte expression and two-electrode voltage clamp recordings
Oocytes were surgically removed from adult female Xenopus laevis frogs treated with collagenase (1 mg mL−1) for 30 min at room temperature under constant shaking, and washed twice with standard bath solution composed of 96 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES (pH 7.5). Isolated oocytes were injected with HYP1 cRNA at 40 ng per oocyte (or with an equivalent volume of nuclease-free water) using a Drummond “Nanoject” microinjector. Oocytes were incubated at 18 °C in standard solution (supplemented with 0.1 mg mL−1 gentamycin) for at least 24 h before voltage-clamp experiments.
Whole-cell membrane currents were measured at room temperature using a home-made two-electrode voltage-clamp amplifier and 0.2–0.4 MΩ glass electrodes with a tip diameter of a few micrometers, filled with 3 M KCl. By convention, negative currents are inward currents mediated by the efflux of anions/electrons (or influx of cations). Membrane currents elicited by exposure to extracellular electron acceptors were generally recorded at a holding potential of −20 mV. In some assays, as indicated in the text and figure legends, the standard bath solution was modified by setting the pH to 5.5 by substituting 10 mM HEPES with an equimolar concentration of MES, or by replacing NaCl, KCl, CaCl2 and MgCl2 with 200 mM MES and 20 mM BTP at pH 5.5 (BTP-MES solution). In experiments with a negative holding voltage of −120 mV, 1 mM lanthanum chloride was supplied to the bath solution, in order to inhibit endogenous background currents activated at these negative voltages. A gravity-driven perfusion system was used for continuous superfusion of oocytes during voltage-clamp recordings and for switching between different bath solutions. Ferric compounds were prepared as stock solutions and diluted appropriately in the bath solution.
For ascorbate injection experiments, ascorbate stock solutions (100 mM) were freshly prepared and adjusted to pH 7.0 with KOH. To raise the cytosolic ascorbate (Asccyt) concentration to 10 mM, oocytes were removed from the voltage-clamp setup after the initial recording series and injected with ascorbate stock solution using the Drummond “Nanoject” microinjector. A first injection of 50 nL stock solution and a second injection of 100 nL resulted in an increase of the initial cytosolic ascorbate (Asccyt) of 10 and 30 mM, respectively, considering a volume of about 500 nL for a mean oocyte diameter of 1 mm. After the injection, the oocyte volume transiently increased but recovered to its original size within minutes. Injected oocytes were allowed to recover for 30 min at room temperature before voltage-clamp measurements were resumed.
GUS histochemical assay
GUS activity of plants expressing proHYP1::GUS and proCYCB1;1::GUS was assessed by incubating root samples in a solution containing 20 mg mL−1 5-bromo-4 chloro-3-indolyl-β-D-glucuronic acid (X-gluc) in 100 mM sodium phosphate, 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6 and 0.1% (v/v) Triton X-100 at 37 °C in darkness. Samples were then mounted on a clearing solution (chloral hydrate : water : glycerol = 8:3:1) and imaged using a Axio Imager 2 light microscope (Carl-Zeiss).
Callose staining
Callose deposition was assessed by staining roots with aniline blue as described previously5. Briefly, roots were incubated for 1.5 hours in a solution containing 0.1% (w/v) aniline blue (AppliChem) in 100 mM Na-phosphate buffer (pH 7.2). Stained roots were mounted on water and directly visualized with a laser-scanning confocal microscope.
Confocal microscopy analyses
For protein localization studies, roots of transgenic lines expressing GFP were excised, stained with propidium iodide (10 µg mL−1) for 10 minutes, and mounted in water. GFP- and propidium iodide-dependent fluorescence were acquired with a confocal laser-scanning microscope (LSM 780; Zeiss, Germany) equipped with a 20X/0.8 M27 objective. GFP was excited with a 488 nm Argon laser and the emitted light was detected at 505–535 nm while propidium iodide was excited at 561 nm and detected at 600–700 nm. For co-localization of GFP with PM, root segments were stained with 4 µM FM6-64 (Invitrogen) in 50 mM Na-phosphate buffer (pH 7.2) for 15 min at 4 °C, and mounted in 50 mM Na-phosphate buffer (pH 7.2). FM4-64 was excited at 488 nm and detected at 640 nm. Aniline blue-stained callose was excited at 405 nm and emission detected at 415–480 nm. Fe(II)-RhoNox-1 complex was excited at 514 nm and emission detected at 531–703 nm.
Confocal microscopy analyses of X. laevis oocytes expressing HYP1:EGFP, HYP1H211L/H278L:EGFP, and HYP1M90L:EGFP were performed using a Leica SP2 microscope equipped with a 40x oil-immersion objective. FM4-64, diluted at a final concentration of 15 µM, was used to stain the PM. Both EGFP and FM4-64 were excited at 488 nm, and emissions were monitored at 505 to 512 nm and 645 to 655 nm, respectively. Confocal images were obtained by scanning the laser scan line axially (z scan) along a 10-µm section (0.5-µm steps) at the bottom of the oocyte.
Fe staining
The staining of Fe by Perls/DAB was based on a procedure described previously68, with modifications. In our assays, roots were rinsed one time with ultrapure water for 1 min, and then incubated for 5 min in a freshly prepared Perls staining solution [0.5% (v/v) HCl and 0.5% (w/v) K-ferrocyanide]. Roots were shortly rinsed in water to remove agar adhering to roots, while the more diluted Perls staining solution compared to the original protocol was necessary to prevent meristem disintegration by otherwise to high HCl concentration. Subsequently, roots were incubated in a methanol solution containing 10 mM NaN3 and 0.3% (v/v) H2O2 for 1 h in the dark at room temperature. After washing three times with 100 mM phosphate buffer (pH 7.4) for 1 min each, roots were finally incubated for 5 min in an intensification solution containing 0.025% (w/v) DAB and 0.005% (v/v) H2O2 in 100 mM phosphate buffer (pH 7.4). Samples were then mounted on a clearing solution (chloral hydrate : water : glycerol = 8:3:1) and immediately imaged using a Axio Imager 2 light microscope (Carl Zeiss Microscopy, Germany). For Fe localization in root sections, roots stained with Perls/DAB as described above were subjected to a series of aldehyde fixation, dehydration, and resin embedding as described in Supplementary Table 4. Semi-thin sections of 2.5 μm thickness were cut using a Leica UCT microtome (Leica Microsystems, Germany), and mounted on slides in rapid mounting medium Entelan (Sigma-Aldrich, Germany). Sections were recorded with a 40X objective at fixed exposure time using a Zeiss Axio Imager M2 (Carl Zeiss Microscopy, Germany).
For detection of Fe(II) in the apical meristem, root tips were excised and incubated for 10 min in a freshly prepared buffer solution (2 mM CaSO4 and 500 mM MES pH 5.5 adjusted with KOH) containing 2.5 µM RhoNox-1 fluorescent dye41. Roots were rinsed and mounted with the same buffer solution without the probe and immediately visualized with a confocal laser-scanning microscope.
Ferric-chelate and cupric reductase assays
For ferric-chelate reductase activity, the reaction solution consisted of 0.2 mM CaSO4, 5 mM MES pH 5.5 (KOH), 0.2 mM FerroZine [3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′-disulfonic acid]. The ferric substrates in these assays were either 0.1 mM Fe(III)-EDTA, when HYP1 complementation of fro2 was tested, or freshly prepared 0.1 mM FeCl3 : L-(−)-Malic acid complexes (1:2), when ferric-chelate reductase was assayed in response to P deficiency. Cupric reductase activity was assessed as described previously38. The reaction solution was composed by 0.2 mM CuSO4, 0.6 mM Na3citrate, and 0.4 mM BCDS (Sigma-Aldrich) in ultrapure water. The absorbance of formed Fe(II)-FerroZine or Cu(I)-BCDS complexes was determined at 562 nm or 483 nm, respectively, using a NanoDrop 2000 (NanoDrop). Extinction coefficients were 28.6 mM−1 cm−1 for the Fe(II)-FerroZine complex and 12.25 mM−1 cm−1 for the Cu(I)-BCDS complex.
Shoot chlorophyll analysis
Whole shoot samples were weighted and incubated for 1–2 d at 4 °C in N,N’-dimethylformamide (Roth). The absorbance of the extracts was measured at 647 nm and 664 nm using a NanoDrop 2000 (NanoDrop) and the total chlorophyll concentration determined with the equations described in ref. 69.
Elemental analysis
Whole root or shoot samples were dried at 65 °C and weighted into polytetrafluoroethylene tubes. Plant material was digested with concentrated HNO3 (67-69%; Bernd Kraft) and pressurized in a high-performance microwave reactor (UltraCLAVE IV, MLS GmbH). Digested samples were diluted with de-ionized water (Milli-Q Reference A+, Merck Millipore). Element analysis was carried out by high-resolution inductively coupled plasma mass spectrometry (HR-ICP-MS) (ELEMENT 2, Thermo Fisher Scientific, Germany).
Structure modeling
The structure of HYP1 was predicted using AlphaFold36. In addition, a homology model of HYP1 was developed before the release of AlphaFold, using the comparative modeling protocol available in the Rosetta software70. The following template structures were considered in comparative modeling: the cytochrome domain of cellobiose dehydrogenase enzymes from Phanerodontia chrysosporium (PDB: 1D7C), Myriococcum thermophilum (PDB: 4QI3) and Neurospora crassa (4QI7), and the cytochrome domain of Pyranose dehydrogenase from Coprinopsis cinerea (6JT6) as templates for the DOMON domain of HYP1, as well as the A. thaliana cytochrome b561 (PDB: 4O6Y, 4O79, 4O7G) and human duodenal cytochrome b structures (PDB: 5ZLE, 5ZLG) as templates for the cytochrome b domain of HYP1. The AlphaFold- and Rosetta-predicted HYP1 structure models were in close agreement with each other (Cα atom RMSDs of 1.5 Å and 2.3 Å over the cytochrome b and DOMON domains, respectively). For modeling the interactions with b-heme and ascorbate, the AlphaFold-generated model of HYP1 was used because of its estimated higher accuracy.
Three b-heme molecules were placed in the HYP1 structure model at positions inferred from the template structures listed above. The positions of the b-heme molecules were refined using the FastRelax protocol in the Rosetta software. Distance restraints between the Fe atom in the b-heme molecule and the coordinating histidine residues were applied in this step: restraints were set up between H245 and H314 and one b-heme in the cytochrome b561 domain, between H211 and H278 and the other b-heme in the cytochrome b561 domain, and between H174 and the b-heme in the DOMON domain. The final step was an unrestrained minimization of the whole structure in the Amber14:EHT force field using the MOE software (v. 2022.02 Chemical Computing Group ULC, Montreal, Canada).
Ligand docking
The molecular binding mode of ascorbate (PubChemID: 54679076) was modeled using ligand docking calculations with the Rosetta software. Ascorbate was docked into HYP1’s cytoplasmic facing pocket, which was identified by structural comparison with the ascorbate-bound structure of cytochrome b561 (PDB ID: 4O79). The structure analysis indicated that residues K77, K81, and R150 in cytochrome b561 coordinate ascorbate through hydrogen bonds. The equivalent residues in HYP1 are K234, R307 and R299. The centroid of these three residues was used as a starting point to place the ascorbate molecule in the HYP1 pocket prior to the docking calculations.
Statistics and reproducibility
To analyze the significant differences among multiple groups, one-way ANOVA followed by post hoc Tukey’s test at P < 0.05 was applied. Significant differences between two groups were assessed by a two-sided Student’s t-test. Statistical tests were performed using SigmaPlot 11.0 software and plots prepared with GraphPad Prism software v.9.3.1 (https://www.graphpad.com/). All experiments were conducted at least two times with similar results and data from a single representative experiment are presented.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw microarray data generated in this study have been deposited and available in the NCBI Gene Expression Omnibus (GEO) under accession GSE217790, while the raw RNA sequencing data have been deposited and available in the European Nucleotide Archive (ENA) under project ID PRJEB65916. All data generated during this study are included in this published article (and its Supplementary Information File). Source data are provided as a Source Data file. Source data are provided with this paper.
References
Shen, J. et al. Phosphorus dynamics: from soil to plant. Plant Physiol. 156, 997–1005 (2011).
Ryan, P., Delhaize, E. & Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 527–560 (2001).
Lambers, H. et al. Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil 476, 133–160 (2022).
Lopez-Hernandez, D., Flores, D., Siegert, G. & Rodriguez, J. V. The effect of some organic anions on phosphate removal from acid and calcareous soils. Soil Sci. 128, 1483820 (1979).
Muller, J. et al. Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Dev. Cell 33, 216–230 (2015).
Balzergue, C. et al. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat. Commun. 8, 15300 (2017).
Mora-Macias, J. et al. Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc. Natl Acad. Sci. USA 114, E3563–E3572 (2017).
Ward, J. T., Lahner, B., Yakubova, E., Salt, D. E. & Raghothama, K. G. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 147, 1181–1191 (2008).
Svistoonoff, S. et al. Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Genet. 39, 792–796 (2007).
Gutierrez-Alanis, D. et al. Phosphate starvation-dependent iron mobilization induces cle14 expression to trigger root meristem differentiation through clv2/pepr2 signaling. Dev. Cell 41, 555–570.e553 (2017).
Gruber, B. D., Giehl, R. F. H., Friedel, S. & von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 163, 161–179 (2013).
Perez-Torres, C. A. et al. Phosphate availability alters lateral root development in arabidopsis by modulating auxin sensitivity via a mechanism involving the tir1 auxin receptor. Plant Cell 20, 3258–3272 (2008).
Lynch, J. P. & Brown, K. M. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant Soil 237, 225–237 (2001).
Jain, A., Wilson, G. T. & Connolly, E. L. The diverse roles of FRO family metalloreductases in iron and copper homeostasis. Front Plant Sci. 5, 100 (2014).
Robinson, N. J., Procter, C. M., Connolly, E. L. & Guerinot, M. L. A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697 (1999).
Rajniak, J. et al. Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat. Chem. Biol. 14, 442–450 (2018).
Connolly, E. L., Campbell, N. H., Grotz, N., Prichard, C. L. & Guerinot, M. L. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 133, 1102–1110 (2003).
Li, W. & Lan, P. Genome-wide analysis of overlapping genes regulated by iron deficiency and phosphate starvation reveals new interactions in Arabidopsis roots. BMC Res. notes 8, 555 (2015).
Mukherjee, I., Campbell, N. H., Ash, J. S. & Connolly, E. L. Expression profiling of the Arabidopsis ferric chelate reductase (FRO) gene family reveals differential regulation by iron and copper. Planta 223, 1178–1190 (2006).
Schmid, N. B. et al. Feruloyl-CoA 6’-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 164, 160–172 (2014).
Bhosale, R. et al. A mechanistic framework for auxin dependent Arabidopsis root hair elongation to low external phosphate. Nat. Commun. 9, 1409 (2018).
Misson, J. et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl Acad. Sci. USA 102, 11934–11939 (2005).
Thibaud, M. C. et al. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J. 64, 775–789 (2010).
Hoehenwarter, W. et al. Comparative expression profiling reveals a role of the root apoplast in local phosphate response. Bmc Plant Biol. 16, 106 (2016).
Heazlewood, J. L. et al. Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell 16, 241–256 (2004).
Zheng, Z., Wang, Z., Wang, X. Y. & Liu, D. Blue light-triggered chemical reactions underlie phosphate deficiency-induced inhibition of root elongation of arabidopsis seedlings grown in petri dishes. Mol. Plant 12, 1515–1523 (2019).
Preger, V. et al. Auxin-responsive genes air12 code for a new family of plasma membrane b-type cytochromes specific to flowering plants. Plant Physiol. 150, 606–620 (2009).
Levin, E. Y., Levenberg, B. & Kaufman, S. The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine. J. Biol. Chem. 235, 2080–2086 (1960).
Aravind, L. DOMON: an ancient extracellular domain in dopamine beta-monooxygenase and other proteins. Trends Biochem. Sci. 26, 524–526 (2001).
Clúa, J. et al. A CYBDOM protein impacts iron homeostasis and primary root growth under phosphate deficiency in Arabidopsis. Nat. Commun. https://doi.org/10.1038/s41467-023-43911-x (2024).
Asard, H., Barbaro, R., Trost, P. & Berczi, A. Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid. Redox Signal 19, 1026–1035 (2013).
Picco, C. et al. Direct recording of trans-plasma membrane electron currents mediated by a member of the cytochrome b561 family of soybean. Plant Physiol. 169, 986–995 (2015).
Zechmann, B. Compartment-specific importance of ascorbate during environmental stress in plants. Antioxid. Redox Sign 29, 1488–1501 (2018).
Lu, P. et al. Structure and mechanism of a eukaryotic transmembrane ascorbate-dependent oxidoreductase. Proc. Natl Acad. Sci. USA 111, 1813–1818 (2014).
Ganasen, M. et al. Structural basis for promotion of duodenal iron absorption by enteric ferric reductase with ascorbate. Commun. Biol. 1, 120 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Iyer, L. M., Anantharaman, V. & Aravind, L. The DOMON domains are involved in heme and sugar recognition. Bioinformatics 23, 2660–2664 (2007).
Bernal, M. et al. Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24, 738–761 (2012).
Yi, Y. & Guerinot, M. L. Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J. 10, 835–844 (1996).
Lequeux, H., Hermans, C., Lutts, S. & Verbruggen, N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Bioch 48, 673–682 (2010).
Hirayama, T., Okuda, K. & Nagasawa, H. A highly selective turn-on fluorescent probe for iron(ii) to visualize labile iron in living cells. Chem. Sci. 4, 1250–1256 (2013).
Platre, M. P. et al. The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants. Nat. Commun. 13, 4445 (2022).
McKie, A. T. et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291, 1755–1759 (2001).
Berczi, A., Su, D. & Asard, H. An Arabidopsis cytochrome b561 with trans-membrane ferrireductase capability. Febs Lett. 581, 1505–1508 (2007).
Tsubaki, M., Takeuchi, F. & Nakanishi, N. Cytochrome b561 protein family: Expanding roles and versatile transmembrane electron transfer abilities as predicted by a new classification system and protein sequence motif analyses. Biochimica et. Biophysica Acta (BBA) - Proteins Proteom. 1753, 174–190 (2005).
Liu, X. X. et al. Phloem iron remodels root development in response to ammonium as the major nitrogen source. Nat. Commun. 13, 561 (2022).
Liu, Y. et al. PDX1.1-dependent biosynthesis of vitamin B6 protects roots from ammonium-induced oxidative stress. Mol. Plant 15, 820–839 (2022).
Vert, G. et al. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233 (2002).
Naumann, C. et al. Bacterial-type ferroxidase tunes iron-dependent phosphate sensing during Arabidopsis root development. Curr. Biol. 32, 2189–2205.e2186 (2022).
Zhu, Q. Y. et al. The ferroxidases are critical for Fe(II) oxidation in xylem to ensure a healthy Fe allocation in Arabidopsis thaliana. Front Plant Sci. 13, 958984 (2022).
Xu, Z. R. et al. The ferroxidases LPR1 and LPR2 control iron translocation in the xylem of Arabidopsis plants. Mol. Plant 15, 1962–1975 (2022).
Ursache, R. et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 7, 353–364 (2021).
Nakajima, K., Sena, G., Nawy, T. & Benfey, P. N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311 (2001).
Ferreira, P. C. G. et al. Developmental expression of the arabidopsis cyclin gene Cyc1at. Plant Cell 6, 1763–1774 (1994).
Blilou, I. et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 (2005).
Ritchie M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3 (2004).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Lex, A., Gehlenborg, N., Strobelt, H., Vuillemot, R. & Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Computer Graph. 20, 1983–1992 (2014).
Gu, Z. Complex heatmap visualization. iMeta 1, e43 (2022).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Yu G. enrichplot: visualization of functional enrichment result. In: R package version 1.20.1 (ed^(eds) (2023).
Lampropoulos, A. et al. GreenGate - a novel, versatile, and efficient cloning system for plant transgenesis. Plos One 8, e83043 (2013).
Zheng, L., Baumann, U. & Reymond, J.-L. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32, e115–e115 (2004).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Roschzttardtz, H., Conejero, G., Curie, C. & Mari, S. Identification of the endodermal vacuole as the iron storage compartment in the arabidopsis embryo. Plant Physiol. 151, 1329–1338 (2009).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents - verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochimica Et. Biophysica Acta 975, 384–394 (1989).
Leman, J. K. et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat. Methods 17, 665–680 (2020).
Acknowledgements
We thank Dr. Benjamin D. Gruber for discussions, Jacqueline Fuge, Annett Bieber, and Elis Fraust for excellent technical assistance and Dr. Yudelsy A. Tandron Moya for conducting HR-ICP-MS analyses (Leibniz Institute of Plant Genetics and Crop Plant Research). We thank Prof. Dr. Paolo Trost (University of Bologna Alma Mater) for critically reading the manuscript. We are grateful to Prof. Dr. Steffen Abel (Leibniz Institute of Plant Biochemistry) for sharing the proSHR::SHR:GFP reporter line and the lpr1 lpr2 double mutant. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) grant WI1728/24-1 to N.v.W. and “Progetti di Ricerca di Interesse Nazionale” grant 20222CS2B3 to A. C. Costs for open access publication were partially funded by the DFG, grant 491250510.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.F.H.G. and N.v.W. conceived, designed, and coordinated the project. R.A.M., C.P., A.G., J.S.S., M.M., and R.F.H.G. performed all experimental work. A.H. analyzed transcriptome data. F.E. and G.K. performed the structural modeling. A.C. supervised the two-electrode voltage clamp experiments. G.K, C.P., A.C., N.v.W., and R.F.H.G. analyzed data; R.F.H.G. wrote the manuscript with inputs of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dai-Yin Chao, Ute Krämer and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maniero, R.A., Picco, C., Hartmann, A. et al. Ferric reduction by a CYBDOM protein counteracts increased iron availability in root meristems induced by phosphorus deficiency. Nat Commun 15, 422 (2024). https://doi.org/10.1038/s41467-023-43912-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43912-w
This article is cited by
-
A CYBDOM protein impacts iron homeostasis and primary root growth under phosphate deficiency in Arabidopsis
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.