Abstract
Quorum sensing is a mechanism of bacterial communication that controls virulence gene expression. Pseudomonas aeruginosa regulates virulence via two synthase/transcription factor receptor pairs: LasI/R and RhlI/R. LasR is considered the master transcriptional regulator of quorum sensing, as it upregulates rhlI/R. However, clinical isolates often have inactivating mutations in lasR, while maintaining Rhl-dependent signaling. We sought to understand how quorum sensing progresses in isolates with lasR mutations, specifically via activation of RhlR. We find that clinical isolates with lasR inactivating mutations often harbor concurrent mutations in rhlI. Using ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry, we discover that strains lacking lasR overproduce the RhlI-synthesized autoinducer and that RhlI variants re-calibrate autoinducer concentrations to wild-type levels, restoring virulent phenotypes. These findings provide a mechanism for the plasticity of quorum sensing progression in an acute infection niche.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa is a ubiquitous, opportunistic pathogen that causes tens of thousands of hospital-acquired infections each year1. The Centers for Disease Control and Prevention have classified P. aeruginosa infections as a serious public health threat due to the prevalence of multidrug-resistant (MDR) strains in healthcare facilities1. Moreover, the incidence of hospital-acquired MDR infections increased by 32% between 2019 and 2020 due to the emergence of the COVID-19 pandemic2. P. aeruginosa persists in healthcare niches by forming biofilms, particularly on medical equipment like catheters and ventilators, which are difficult to eradicate. Consequently, P. aeruginosa can establish infections in immunocompromised patients, such as those undergoing cancer treatment or requiring immunosuppressants, as well as patients with underlying pulmonary disorders such as cystic fibrosis (CF)3,4,5 and chronic obstructive pulmonary disorders (COPD)6,7. These infections can result in chronic pneumonia or life-threatening septic bloodstream infections, resulting in over 2000 deaths per year1.
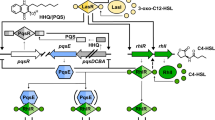
P. aeruginosa regulates biofilm formation and other virulence traits via quorum sensing (QS). QS is a mechanism of inter-bacterial communication that relies on the production and detection of signaling molecules called autoinducers (AI). In many Gram-negative bacteria, LuxI/R-type synthase/receptor pairs control QS wherein the AI molecule is secreted from the cell and diffuses across the bacterial membrane at high cell density where it binds its cognate cytosolic transcription factor receptor. The receptor-AI complex then alters gene expression to coordinate individualistic and collective behaviors. P. aeruginosa has three main hierarchical QS circuits: (1) the Las system, (2) the Rhl system, and (3) the Pqs system8,9. The Las system consists of the LuxI family AI synthase LasI, which synthesizes the acyl homoserine lactone (AHL) N-(3-oxododecanoyl)-L-homoserine lactone (3OC12HSL), and the LuxR-type receptor LasR. When bound by 3OC12HSL, LasR dimerizes and is an active transcription factor that upregulates the rhl system10,11,12, among other virulence genes. Homologous to the Las circuit, the Rhl system consists of the AI synthase/receptor pair RhlI/R. RhlI synthesizes N-butanoyl-L-homoserine lactone (C4HSL)13, which binds to and activates the transcription factor receptor RhlR. RhlR upregulates the expression of numerous virulence factors such as the genes involved in phenazine biosynthesis (with the final product being 5-methyl-1(5H)-phenazinone—pyocyanin) and rhamnolipid production. The third system, the Pseudomonas quinolone system, is upregulated by LasR but repressed by RhlR14. The pqsABCDE operon encodes enzymes responsible for the synthesis of the AI molecule 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS), which binds the receptor PqsR resulting in a positive feedback loop15,16. We have previously shown that PqsE, a metallo-β-hydrolase enzyme, physically binds to RhlR17 and enhances the affinity of RhlR for its target promoters independent of its catalytic activity18. Moreover, both C4HSL and PqsE binding are required for maximal RhlR activity19. However, recent research suggests that QS in clinical strains of P. aeruginosa does not follow the canonical model described above but signaling via RhlI-RhlR-PqsE remains intact.
The high-traffic environment of a hospital and the selective pressure of antibiotic usage drive pathogen evolution in healthcare settings. Clinical strains of P. aeruginosa often evolve lasR inactivating mutations during chronic infection20,21, leading to a re-wiring of the QS signaling network. These strains have been termed social cheaters as it is hypothesized that lasR mutations repress QS to defray the cost of synthesizing energetically expensive metabolites22. Instead, these strains achieve a fitness advantage by leeching public goods from wild-type (WT) bacteria23. Another hypothesis is that LasR- strains are better adapted to the lung environment because these mutants grow better in a chronic infection niche24. These strains maintain QS through the Rhl system despite harboring non-functional LasR variants (LasR-)23. Previous studies demonstrated that the passage of LasR- strains results in mutations in pqsR22,25,26, the receptor/transcription factor for the Pqs system. Another study showed that LasR- strains evolve mutations in mexT27,28, a regulator of the MexEF-OprN efflux system, which secretes C4HSL29. Together, these data suggest that RhlR activation converges on C4HSL in LasR- clinical strains given that (1) pqsR inactivating mutations would preclude RhlR activation by pqsE18,19 (2) mexT mutations would increase C4HSL extrusion and cell-cell signaling29 and (3) LasR- strains do not respond to 3OC12HSL24. Indeed, the levels of C4HSL are important for P. aeruginosa to establish acute infections30. In the cohort of P. aeruginosa clinical strains analyzed in this study, pqsE, rhlR, and lasI single nucleotide polymorphisms (SNPs) are infrequent and often silent. Conversely, rhlI SNPs are frequent and consistent (Supplementary Table S1), suggesting that these acute infection strains have evolved rhlI mutations in response to lasR inactivating mutations.
Here, we investigate the function of three clinically evolved, non-synonymous amino acid changes in RhlI: G62S, D83E, and P159S. We show that these clinically evolved RhlI variants restored pyocyanin production in a ∆lasR strain. We use ultra-high-performance liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry (HRMS) to directly detect and analyze the levels of AHL AI synthesized by RhlI variants. We show that RhlI variants re-calibrate the concentrations of extracellular C4HSL, resulting in increased virulence factor production in a ∆lasR background. We hypothesize that LasR- clinical strains evolve compensatory mutations in rhlI to optimize the levels of AI for RhlR activation to drive the expression of genes involved in pathogenesis.
Results
Identification of rhlI SNPs in clinical strains of P. aeruginosa
We analyzed whole genome sequencing data of 56 P. aeruginosa clinical isolates (Supplementary Table S1) from the Wadsworth Center, New York State Department of Health to identify SNPs in QS genes (NCBI BioProject PRJNA288601). We found that rhlR, pqsE and lasI were WT in their amino acid sequences compared to PA14, while lasR (Supplementary Table S1) and rhlI had numerous SNPs resulting in amino acid substitutions. lasR mutations in clinical strains of P. aeruginosa have been well documented. The mutations are stochastic and often result in non-functional proteins20. The rhlI SNPs we discovered were frequent and consistent, suggesting a functional role in the maintenance of these specific mutations. We chose three SNPs of interest that resulted in the following amino acid changes in RhlI: G62S, D83E, and P159S (Fig. 1a). We used AlphaFold31 to map the location of the variants on the structure of RhlI relative to the substrate binding pockets using homology modeling to the two experimentally determined LuxI synthase structures: LasI32 from P. aeruginosa and EsaI33 from Pantoea stewartii (Fig. 1b). The predicted structure aligns well with the experimentally determined structures from other systems as RhlI has a typical α-β-α fold that results in a deep cleft to allow for binding to the S-adenosylmethionine and acyl-acyl carrier protein substrates. While the structures of LasI and EsaI were not determined bound to substrate, the structure of the two binding pockets are similar to that of the GNAT family of acetyltransferases, which carry out a similar acylation reaction34. Thus, we are confident in the fold of the computationally derived structure of RhlI and the location of the variant residues therein, as it superimposes with LasI with a root mean squared deviation of approximately 2.4 Å (Supplementary Fig. S1a). The residues G62, D83, and P159 are peripheral to the core anti-parallel β-sheet fold in RhlI, indicating that they are not directly involved in binding substrate or catalysis; residues G62S and P159 flank a β-sheet predicted to be the acyl-binding site of Rhl and residue D83 is located in a loop adjacent to the active site35 (Fig. 1b). Conservation scores mapped on the PA14 RhlI structure using sequences from our cohort of 56 clinical strains revealed that these three sites were the most variable. Furthermore, the mutations of interest characterized here have been identified by other studies23, and are consistent within the cohort of isolates we analyzed, suggesting that there is a functional advantage to maintaining these particular mutations (Fig. 1c). Additionally, these sites were poorly conserved among the 150 LuxI synthase homologs sequences we examined by conservation analyses (Fig. 1d, Supplementary Fig. S1b), indicating that these residues can be variable and still maintain functionality across a diverse range of homologs.
a Dendrogram of clinical isolates based on rhlI sequences. Strains containing SNPs are colorized to highlight RhlI variants of interest: G62S is purple; D83E is blue; P159S is green. b The predicted structure of RhlI was determined using AlphaFold31. The putative acyl-chain binding site is highlighted in light green. Residues predicted to be involved in catalysis are shown in orange. The putative acyl-binding pocket and catalytic residues were assigned based on the existing experimentally solved structures of RhlI homologs LasI (PDB: 1R05)32 from P. aeruginosa and EsaI (PDB: 1KZF)33 from P. stewartii. The location of residues altered by the rhlI SNPs G62 (purple) D83 (blue), and P159 (green) are indicated and depicted in stick form. ConSurf maps depicting residue conservation in c clinical strains and d 150 LuxI synthase homologs. For both ConSurf maps, conservation was mapped on a scale of 1–9 with low conservation scores (variable residues) shown in blue and high conservation scores (conserved residues) shown in gray. e Cell-free supernatant (CFS) supplementation assay to monitor pyocyanin production in a ΔrhlI recipient strain after 8 h of growth with supernatants from WT (black), ΔrhlI (gray), RhlI G62S (purple), RhlI D83E (blue), and RhlI P159S (green) variant strains. Supplementation of WT cell-free supernatants to the ΔrhlI recipient was set to 1 in every biological replicate and mutant strains were normalized to that value. Bars represent the mean of four biological replicates. Error bars represent standard deviations of the means of biological replicates. Statistical analyses were performed using a one-way ANOVA with Dunnett’s multiple comparisons test with the ΔrhlI donor strain set as the control.
To test whether the three RhlI variants were functional AI synthases, we recombined rhlI mutants of interest into our PA14 laboratory strain. We then performed a supernatant assay in which the cell-free supernatants of strains harboring RhlI variants were supplemented to a ΔrhlI recipient strain. We used pyocyanin production as a proxy for RhlI functionality because pyocyanin is dependent on the C4HSL activation of RhlR. Thus, a ΔrhlI recipient would not produce pyocyanin unless supplemented with sufficient concentrations of C4HSL from the cell-free supernatant. Indeed, supplementation of a ΔrhlI strain with ΔrhlI strain cell-free supernatant did not result in pyocyanin production (Fig. 1e). Conversely, all three of the cell-free supernatants from strains expressing RhlI variants induced pyocyanin production in the ΔrhlI recipient strain comparable to WT levels, confirming that all three RhlI variants are functional synthases (Fig. 1e).
Strains carrying RhlI variants compensate for lasR signaling deficiency to produce pyocyanin
The frequency and consistency of rhlI SNPs in clinical P. aeruginosa isolates (Fig. 1a and Supplementary Fig. S2) led us to hypothesize that they could be compensatory mutations for non-functional lasR mutations. Indeed, these SNPs were observed in clinical isolates from other studies, suggesting that their prevalence in our cohort of isolates was not simply due to epidemiological constraints and supporting the hypothesis that rhlI SNPs are maintained in clinical populations because they confer a fitness advantage. To determine if these RhlI variants compensated for a loss-of-function lasR, we introduced each SNP at the rhlI locus in a PA14 ΔlasR background. We then performed a time course assay in phosphate-limiting media36 and monitored the production of pyocyanin. Both the growth (OD600 nm) and pyocyanin (OD695 nm) were measured at four different time points over the course of a 24-h period (Fig. 2a–d). We found that a ΔlasR strain had a pyocyanin production lag, but that the introduction of RhlI variants restored pyocyanin production in a ΔlasR background to WT levels. Furthermore, the RhlI variants did not dramatically alter the timing of pyocyanin production in a WT background, suggesting that these variants specifically aid in maintaining QS in strains lacking a functional Las system (Fig. 2c, d). All ΔlasI background (Supplementary Fig. S3A–d) strains produced pyocyanin at a similar rate as WT, and RhlI variants did not confer increased pyocyanin production in this background, indicating that there is a decoupling of the las system as it relates to its regulation of the rhl system.
a Summary graph of the pyocyanin time course experiment for WT (black), ΔlasR (magenta) and ΔrhlI (gray) strains. Measurements were taken at four time points over a 24-h period (approximately 5, 10, 18 and 24 h). Time course pyocyanin production assay for: b RhlI G62S variant data in WT background (purple) and ΔlasR backgrounds (light purple); c RhlI D83E variant data in WT (blue) and ΔlasR backgrounds (light blue); d RhlI P159S variant data in WT (green) and ΔlasR backgrounds (light green). One measurement was made per strain per time point. The experiment was performed in triplicate, resulting in the 12 total data points depicted in the graph. Non-linear regression analyses were performed for every genotype and the best fits shown; best fit lines for WT (black) and ΔlasR (magenta) control strains were plotted and copied for reference in subsequent panels for the RhlI variant strains. e Representative images of WT, ΔlasR, ΔrhlI, and ΔlasR RhlI variant swarming phenotypes.
To determine if this phenomenon was specific to pyocyanin production or general to other RhlR-dependent traits, we tested the swarming phenotypes of WT, ΔlasR, ΔrhlI, and ΔlasR strains harboring RhlI variants on semi-soft agar media (Fig. 2e). Swarming motility is controlled by RhlR via positive regulation of the rhlA promoter in a C4HSL-dependent manner37,38. Thus, we expected that RhlI variants would restore swarming in a ΔlasR strain39. Indeed, all ΔlasR strains harboring RhlI variants restored swarming motility to WT levels (Fig. 2e). These data support the hypothesis that RhlI variants are compensatory mutations and restore virulence factor production in strains lacking functional LasR.
Strains expressing RhlI variants have altered AI production
To investigate the mechanism of RhlI variant restoration of pyocyanin production in a ΔlasR background, we determined the concentrations of metabolites in the cell-free supernatant from the same time course cultures in the pyocyanin production assay described in Fig. 2 using ultra-high-performance liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry (HRMS)40,41. Absolute concentrations of C4HSL were normalized to OD600 at each time point. We found that a ΔlasR strain produced 2-fold more C4HSL than WT at later time points (Fig. 3a–d). Surprisingly, the RhlI variants attenuated C4HSL production in a ΔlasR background after the second time point, reducing the C4HSL concentrations to levels observed in the WT strain. We note that a similar profile for C6HSL was observed, albeit at lower concentrations (Supplementary Fig. S4a, b). Conversely, mass spectrometry data of a ΔrhlI strain showed increased levels of 3OC12HSL (Supplementary Fig. S4c, d), suggesting that the pool of shared precursor substrates, namely the acylated acyl carrier protein and S-adenosylmethionine (Fig. 1b) required for the AHL condensation reaction, is funneled into the opposing biosynthetic pathway when one branch of the signaling cascade is disrupted. While we observed an initial burst in C4HSL production in a ΔlasI strain at the second time point, production decreased and leveled off in subsequent time points (Supplementary Fig. S4e), unlike in a ΔlasR strain, which maintained elevated levels of C4HSL throughout the time course (Fig. 3).
Absolute concentrations of C4HSL from cell-free supernatants of strains from the time course cultures used in Fig. 2 were measured using ultra-high-performance liquid chromatography (UHPLC) coupled with a high-resolution mass spectrometer (HRMS) after a 5, b 10, c 18, and d 24 h of growth. Bars represent the mean of three biological replicates. Error bars represent standard deviations of the means of biological replicates. Statistical analyses were performed using a one-way ANOVA with Dunnett’s multiple comparisons test with the ΔlasR strain set as the control. e Pyocyanin production in a C4HSL dose-response assay of WT (black), ΔrhlI (gray), and ΔlasR (magenta) strains. Bars represent the mean of three biological replicates. Error bars represent standard deviations of the means of biological replicates.
Overall, the levels of C4HSL and C6HSL inversely correlated with the time points at which robust pyocyanin production was observed in these strains (Fig. 2a–d, Supplementary Fig. S4A,B). These results seemed counterintuitive, as higher C4HSL concentrations are often associated with the upregulation of virulence phenotypes. To confirm that additional factors in the cell-free supernatants were not obscuring our pyocyanin readings, we performed mass spectrometry analysis to detect pyocyanin, using a 5-methyl-1(5H)-phenazinone (pyocyanin) as a standard. We found that pyocyanin concentrations (Supplementary Fig. S5A–d) correlated with our absorbance readings in Fig. 2 and Supplementary Fig. S3. In total, these results showed that high concentrations of C4HSL suppressed RhlR-dependent phenotypes. This was most readily observable in the transition between the second and third time point; strains containing RhlI variants in a ΔlasR background had a decline in C4HSL concentrations and a concomitant increase in pyocyanin production. We tested if high concentrations of C4HSL could repress pyocyanin production in vivo using a dose-response assay (Fig. 3e). Pyocyanin production in a ΔrhlI and WT strain was repressed at 10 µM C4HSL, while levels remained constant in a ΔlasR strain. Therefore, we hypothesized that an optimal concentration of C4HSL is required for RhlR activation in vivo and that clinically evolved RhlI G62S, D83E, and P159S variants reduce the efficiency of the RhlI synthase to re-calibrate C4HSL levels in a ΔlasR background to appropriately activate RhlR-dependent signaling.
RhlI variants synthesize the optimal concentration of C4HSL for RhlR activation
The expression of RhlR target genes has different C4HSL dependencies42. We hypothesized that the reduced levels of C4HSL in RhlI variant strains would impact the expression of C4HSL-sensitive genes. We analyzed the expression of two genes with different C4HSL dependencies: hydrogen cyanide (hcnA) is C4HSL independent and rhamnolipid synthase (rhlA) is C4HSL dependent. We performed qRT-PCR on cell pellets from the time course experiment described in Fig. 2. For brevity, we performed these experiments with the RhlI D83E variant because all three variants altered C4HSL levels similarly in our mass spectrometry data (Fig. 3) and we expected changes in gene expression to be comparable across the variants. We found that the expression of rhlA was dynamic and heavily influenced by changes in the concentrations of C4HSL. The expression of rhlA plateaued in a ΔlasR strain and declined in a WT strain at the final time point, indicating a cessation of virulence factor production at high cell density (Fig. 4a). We note that a similar effect was observed for pyocyanin production in end-point cultures (Fig. 2). The expression of rhlA in a ΔlasR D83E strain was delayed but increased 4-fold by the final time point (Fig. 4a) and this was not due to the upregulation of either rhlI or rhlR (Fig. 4b, c). As expected, we found that hcnA expression was generally unchanged across strains (Fig. 4d). The expression of hcnA by RhlR is known to be heavily dependent on its interaction with PqsE and the modest decrease in hcnA expression is likely due to the RhlR:C4HSL-dependent repression of the pqsABCDE operon at high cell density. Taken together, these data suggested that RhlI variants synthesized optimal concentrations of AHL required for RhlR activation and subsequent virulence gene expression in vivo.
The relative transcript levels of a rhlA, b rhlI, c rhIR, and d hcnA in WT (black), ΔrhlI (gray), ΔlasR (magenta), and ΔlasR RhlI D83E (blue) strains. gyrA was used as an endogenous control for qRT-PCR experiments and data was normalized to WT PA14 expression levels at the 5-h time point. Bars represent the mean of three biological replicates performed in technical duplicate. Error bars represent standard deviations of the means of biological replicates. Statistical analyses were performed using multiple paired t-tests using the two-stage step-up method between ΔlasR and ΔlasR D83E or ΔlasR D83E and WT with a p-value discovery cutoff of 0.05. Only p-values < 0.05 are shown.
We then performed qRT-PCR to determine if deletion of lasI resulted in transcription trends similar to a ΔlasR strain. Interestingly, we found that both rhlI and rhlR transcript levels increased over time in the ΔlasI strain (Supplementary Fig. S6a, b), unlike the ΔlasR strain (Fig. 4b, c). As expected, expression of hcnA was static in both the ΔlasI and ΔlasI D83E strains (Supplementary Fig. S6c), and rhlA expression increased steadily over time (Supplementary Fig. S6d), consistent with the increased levels of rhlI and rhlR. These results suggested that LasR upregulates rhlR independently of its activating ligand 3OC12HSL and supports our findings of the decoupling of the las system as it relates to regulating the rhl locus (Supplementary Fig. S3).
Phenotypic and transcriptional profiling of clinical isolates reveals a dependency on optimal C4HSL levels
To determine if the RhlI variants that restored virulence phenotypes in recombinant ΔlasR laboratory strains had the same effect on virulence factor production in clinical strains, we selected six clinical isolates from our cohort of strains (Supplementary Table S1) based on the representation of genotypic clustering (Fig. 1a). For our cohort of isolates, the genotype frequencies for lasR and rhlI are as follows: 8.5% WT lasR and WT rhlI, 30% WT lasR and mutant rhlI, 62% mutant lasR and rhlI. It should be noted that no isolate in the cohort has a mutant rhlI and WT lasR. We determined their C4HSL and pyocyanin production profiles via HRMS (Supplementary Fig. S7a). These data were largely consistent with our model, showing optimal C4HSL concentrations leading to robust pyocyanin production. Strains 680, 685, and 687 overproduced C4HSL, which led to little or no pyocyanin production in strains 680 and 687 (Supplementary Fig. S7a, b). We note that strain 685 was an outlier in our data, as it overproduced C4HSL but still synthesized high levels of pyocyanin. We address this observation below. Conversely, strain 682 produced ~5 μM C4HSL, which, in our estimation, is the optimal concentration of C4HSL production (Supplementary Fig. S7a). Indeed, strain 682 produced more pyocyanin than any other strain (Supplementary Fig. S7a, b). Strains 683 and 686 both produced between 2 and 3 μM C4HSL, which resulted in lower and more variable levels of pyocyanin compared to strain 682 (Supplementary Fig. S7a). This is likely because 683 and 686 have WT lasR with RhlI variants (Supplementary Table S1), and the levels of C4HSL are suboptimal for RhlR activation.
To follow up on these trends with additional phenotypic and transcriptional analyses, we selected specific clinical strains based on their genotype: 680 has WT lasR and rhlI and 682 and 685 have mutant lasR and rhlI (Supplementary Table S1). To determine if higher concentrations of C4HSL repressed pyocyanin production in clinical strains similar to what we observed in Fig. 3e, we performed a dose-response assay (Supplementary Fig. S7c). These experiments revealed that pyocyanin production in strains 680 and 682 can be repressed when exogenous C4HSL is added during growth. However, strain 685 produced pyocyanin in a dose-dependent manner and, at the concentrations we tested, we did not observe an inhibitory effect of C4HSL on pyocyanin production, indicating that this isolate is insensitive to the repressive effects of C4HSL. The most glaring genotypic difference between strain 685 and all other strains in the cohort is the mutation in lasR. The lasR gene in 685 has an internal, in-frame deletion that results in a 34 amino acid truncation but is otherwise intact (Δ81-115) (Supplementary Table S1). The lasR gene in strain 682 encodes a LasR variant only 80 amino acids long (Supplementary Table S1). Therefore, 685 might still be able to respond to 3OC12HSL or its LasR variant possesses a non-canonical function that we cannot account for, making its QS network responsive to higher levels of AI. This is particularly relevant within the context of the decoupling of the las system that we previously observed (Supplementary Fig. S3). We next performed qRT-PCR on clinical strains 680, 682, and 685 and found that rhlR transcript levels were relatively consistent between strains, whereas rhlA expression levels were similar to or greater than those of a ΔlasR strain (Supplementary Fig. S7d), which mirrors our results in Fig. 4a. We note that the ΔlasR parent strain is PA14, which is genotypically very different than the clinical strains. Taken together, these data show that the expression of virulence phenotypes in acute infection isolates is largely controlled by the levels of C4HSL.
LasR- strains expressing RhlI variants are more pathogenic than a LasR- in an infection model
To determine if RhlI variants were more pathogenic in an in vivo model, we performed a fast kill assay with a Galleria mellonella moth larvae infection model43. The moth larvae were infected with 106 cells of P. aeruginosa and monitored for 10 h. The death of the larvae was determined by melanization and failure to respond to physical probing. The ΔlasR strain was the least virulent and failed to kill all the larvae by the 10-h time point, while a ΔrhlI strain was the most virulent, consistent with other animal model experiments42. This suggests that decreased C4HSL signaling increases the virulence of P. aeruginosa, but overproduction of C4HSL is detrimental to the progression of pathogenesis, consistent with all other traits we monitored in this study. Indeed, ΔlasR strains harboring RhlI variants were more virulent than the ΔlasR strain and killed larvae at a rate comparable to the ΔrhlI (Fig. 5a). These results suggest that RhlI variants evolve in clinical strains of P. aeruginosa to enhance or re-establish virulence of LasR- strains during infection.
a Survival assay of G. mellonella larvae challenged with WT (black), ΔrhlI (gray), ΔlasR (magenta), ΔlasR G62S (purple), ΔlasR RhlI D83E (blue), ΔlasR P159S (green) over the course of a 10-h infection. The dotted black line represents a PBS blank injection. TPI time post-infection in hours. Experiments were performed in biological triplicate with 5 larvae per strain. All strains for each survival assay replicates were performed at the same time and plotted on separate graphs for clarity. Error bars represent the standard error of the mean. Statistical analyses of the larval survival curves for ΔlasR compared to ΔlasR RhlI variant strains were performed using the Gehan-Breslow-Wilcoxon test. b Model for RhlI variant restoration of virulence factor expression by modulation of C4HSL (green) and 3OC12HSL (pink) levels in clinical strains of P. aeruginosa. ΔlasR strains synthesize inhibitory levels of C4HSL early, resulting in the delayed production of virulence factors like pyocyanin (blue triangles). RhlI variants (i.e., D83E) produce less C4HSL, restoring pyocyanin production in a ΔlasR background. The figure was made with BioRender.
Discussion
QS in clinical strains of P. aeruginosa does not occur as it has been canonically described in laboratory PA14 or PAO1 strains. Frequent inactivating mutations in the QS master transcriptional regulator lasR have been reported, and these strains tend to evolve compensatory mutations in other QS-related genes to restore group behavior signaling. Here, we identified SNPs in the rhlI gene of clinical isolates that altered the function of RhlI to compensate for the loss of LasR function. Specifically, we showed that RhlI variants synthesize lower levels of C4HSL and that the reduction of C4HSL concentrations in a ΔlasR background results in the restoration of virulence expression both in vitro and in vivo. Meta-bromo-thiolactone (mBTL) is a small molecule agonist of RhlR that partially inhibits its regulatory function at high concentrations44. Similarly, we anticipate that the loss of lasR funnels the pool of acyl homoserine lactone precursor molecules into the C4HSL biosynthetic pathway, resulting in C4HSL levels that repress RhlR instead of activating it. While LasR- clinical isolates of P. aeruginosa possess a fitness advantage over LasR+ strains, either by social cheating22 or enhanced fitness in a lung niche22, these strains are rendered less virulent. We purport that LasR- clinical strains evolve secondary mutations in rhlI to enhance virulence (Fig. 5b). The rhlI mutations characterized in this study (D83E, G62S, and P159S) have been identified by other studies, suggesting that the evolution of these mutations is not an epidemiological constraint of our cohort of isolates. Therefore, the results of this study are applicable to widely circulating modern clinical strains of P. aeruginosa.
While it was unexpected that the lasI and lasR deletions had different effects on rhlR expression, it is not a novel observation, as other studies have shown that ΔlasR and ΔlasI strains have different phenotypes and pathogenicity45,46. However, the discrepancy in the progression of C4HSL production over time in ΔlasR and ΔlasI backgrounds might explain why loss-of-function mutations in lasI are not readily observed in clinical strains. While we are unsure of the mechanism of the decoupling of lasI and lasR behaviors, it does support our findings from Fig. 2 and Supplementary Fig. S3a, which showed that the time-course dependency of pyocyanin production in ΔlasR and ΔlasI strains were different. Furthermore, it supports our conclusions that the concentration of C4HSL is the main driver of signaling in these backgrounds. This is also evidenced by increased rhlR transcription in a ΔlasI strain but not in a ΔlasR background (Supplementary Fig. S6b), which suggests that unliganded LasR can act as a transcription factor. It was recently reported that 3OC12HSL accumulates in LasR- strains grown on a solid surface47, suggesting that 3OC12HSL has an alternative role in QS other than activating LasR.
We note that RhlI variant strains produce similar levels of C4HSL in both LasR+ and LasR- backgrounds and that those levels are comparable to a WT strain. If RhlI variants were catalytically deficient, it would be expected that a C4HSL deficiency would also be observed in a LasR+ strain. The RhlI variants studied here are not located in the predicted catalytic site of RhlI48. It is possible that the rhlI mutations identified in this study simply reduce the affinity of RhlI for acyl-chain-carrying substrates, thereby reducing enzyme efficiency. In a WT background, LasI competes with RhlI for shared precursors. Mass spectrometry data of 3OC12HSL levels showed that a ΔrhlI strain produced high levels of 3OC12HSL, and vice versa (Supplementary Fig. S4c), suggesting that the ratio of 3OC12HSL:C4HSL is important for the activation of RhlR. We expect that in a LasR- strain, where lasI is not upregulated and no 3OC12HSL is produced, rhlI mutants evolve to reduce the binding affinity of RhlI to re-calibrate the levels of C4HSL synthesized in a LasR- background. This would be beneficial in a LasR- background in which the pool of shared acyl homoserine lactone precursors overwhelm the C4HSL biosynthetic pathway.
Our data revealed that the ratio of C4HSL concentration to cell density, rather than C4HSL levels alone, dictates the timing of virulence factor production in clinical strains. We hypothesize that the loss of LasR inadvertently prevents the activation of RhlR by increasing C4HSL concentrations to an inhibitory level reducing virulence. High concentrations of C4HSL at low cell density inhibit RhlR, whereas attenuated C4HSL levels are appropriate for the activation of cytosolically available RhlR. It should also be noted that it is still unclear how the rhl system is upregulated in clinical LasR- strains, and this is an avenue of research that we are pursuing. Furthermore, we hypothesize that SNPs in rhlI evolved in clinical strains of P. aeruginosa to compensate for the loss of lasR by restoring the balance of C4HSL synthesis, resulting in optimal concentrations for RhlR activation and delayed but robust virulence factor expression (Fig. 5b). This could be particularly important for the adaptability of LasR- strains21, and could mediate reversion from a chronic phenotype to cause new infections, as LasR- strains are deficient in establishing acute infections30,45,46.
Methods
The research conducted in this study complies with all regulations set forth by the Wadsworth Center Institutional Review Board.
Cell-free supernatant supplementation assay
Donor and recipient strains were cultured from freezer stocks in 3 mL LB overnight at 37 °C with shaking. High-cell density recipient cultures were back-diluted 1:10 (500 µL) in 4 mL FDS- media (2% glycerol, 10 g/L DL-alanine, 50 µM iron (III) citrate, 0.1 M Na2SO4, 20 mM MgCl2, 500 µM K2HPO4)49. High-density donor cultures were pelleted, and 500 µL cell-free supernatant was supplemented to the back-diluted recipient culture. Cultures were incubated for 5 h at 37 °C with shaking. Growth was measured at OD600 (1:10 dilution) and pyocyanin at OD695. Pyocyanin production was calculated using the equation below.
Pyocyanin time course assay
Strains were cultured from freezer stocks in 3 mL LB overnight at 37 °C with shaking. High-cell density cultures (OD600 ~ 2.0) were then back-diluted 1:10 in 5 mL FDS- media and incubated for 24 h. Time points were collected at 5, 10, 18, and 24 h. 1 mL of culture was collected at each time point and growth was measured by diluting the culture 1:10 in 1 mL FDS- media and reading the absorbance at OD600. The remainder of the 1 mL sample was pelleted, and the absorbance of cell-free supernatant was measured at OD695 to quantify pyocyanin. Cell pellets and 100 µL of cell-free supernatant were frozen for RNA extraction and mass spectrometry, respectively.
Swarming motility assay
Swarming assays were performed as described previously39. Briefly, cultures of P. aeruginosa PA14 and the mutants were grown overnight in LB. 2 µL of the stationary phase cultures were spotted onto swarming agar medium (1 mM MgSO4, 8.6 mM NaCl, 1 mM CaCl2, 20 mM NH4Cl, 22 mM KH2PO4, 12 mM Na2SO4, 0.2% glucose, 0.5% casamino acids, 0.5% agar). The plates were incubated overnight at 37 °C and imaged after 24 h.
P. aeruginosa strain construction
P. aeruginosa clinical strains were obtained from the New York State Department of Health Bacteriology Laboratory at the Wadsworth Center. Standard cloning and molecular biology techniques were used to generate plasmids for E. coli and P. aeruginosa conjugation experiments. Briefly, mutagenesis was performed using a pEXG2 suicide vector containing the coding sequence for the rhlI gene plus 500 bp flanking DNA. Conjugative competent E. coli SM10 λpir strains harboring pEXG2-rhlI mutant plasmids were conjugated with PA14 WT, ΔlasR and ΔlasI laboratory strains. All strains and plasmids used in this study are shown in Supplementary Table S2. Primers are shown in Supplementary Table S3.
Whole genome assembly and phylogenetic analyses
Paired-end reads were downloaded from the National Center for Biotechnology Information BioProject database (NCBI accession number: PRJNA288601). Reads were trimmed and assessed for quality using Trim Galore! v0.6.750,51. Trimmed reads were mapped to the P. aeruginosa UCBPP-PA14 reference assembly (NCBI accession number: NC_008463.1) using the BWA-MEM52 algorithm within SAMtools v1.1.053. Variant calling and filtering were performed by BCFtools mpileup v1.1.0.254. Consensus FASTA sequences were generated using BCFtools consensus V1.1.0.2. To identify RhlI SNPs and sequence relatedness, consensus sequences were annotated using Prokka v1.1455, using default parameters. RhlI-annotated protein sequence alignments were generated using MUSCLE v3.8.155156. Columns with >20% gaps were trimmed and filtered by similarity with trimAl (v1.4.1; option --gt 0.80 and –st 0.001)57. The maximum-likelihood tree was generated from RhlI protein sequence alignments (n = 56) with IQ-TREE v1.6.1258. Model selection was performed using an automatic substitution model based on the Bayesian information criteria (BIC) score, where the HKY+F+I model was chosen. The tree was visualized and annotated with Interactive Tree Of Life (iTOL v. 6.7.1)59. To identify RhlI variants among the isolates, we used the Pseudomonas Genome DB60. We utilized the NCBI BLAST61,62 search function to compare rhlI isolate sequences to the P. aeruginosa UCBPP-PA14 reference assembly (NCBI accession number: NC_008463.1). Mismatches were identified using the pairwise output format.
To contextualize RhlI sequence similarity among a diverse range of bacterial species, we obtained protein sequences of RhlI orthologs from the OrthoDB v1163 online database. Protein sequences were filtered for those which were between 185-215 amino acids. The remaining sequences were aligned using MUSCLE v3.8.1551 and trimmed with trimAl v1.4.1. The maximum-likelihood tree was generated from orthologous RhlI protein sequence alignments (n = 264) with IQ-TREE v1.6.12. Model selection was performed using an automatic substitution model based on the Bayesian information criteria (BIC) score, where the LG+F+R7 model was chosen. The tree was visualized and annotated with Interactive Tree Of Life (iTOL) v6.7.1. Custom code and all commands issued in this study can be found at https://github.com/calebmallery/RhlI_manuscript_methods.
High-resolution mass spectrometry analysis
An ultra-high-performance liquid chromatography (UHPLC) coupled with a high-resolution mass spectrometer (HRMS) method was used for the cell-free supernatant analysis during the time-course study. A high-throughput and direct sample preparation method was developed to make it possible to prepare 100 samples in 2 h, enabling rapid quantitation of the time course study samples. Briefly, 50 µL filtered supernatants were directly extracted by 200 µL methanol and vortexed for 15 s. The precipitated proteins were separated from the extraction solvent using centrifugation (2 min, 8000–10,000×g), the supernatant was mixed with 0.1% formic acid in water in an HPLC vial with a 1:1 ratio, and then directly used for UHPLC-HRMS analysis. For UHPLC-HRMS high-throughput analysis, the instrumental system contained a Vanquish UHPLC coupled with a high-resolution QE-Orbitrap mass spectrometer (ThermoFisher Scientific) operating in the positive electrospray-ionization (ESI) mode with a heated ion source (HESI). Analyte separation was achieved using a C18 bonded beads (Hypersil GOLD column, 50 × 2.1 mm, 1.9 μm particle size, ThermoFisher) as the stationary phase and solvent A and B as mobile phases. For gradient elution from the UHPLC, mobile phase A was 0.1% v/v formic acid with 5 mM ammonium formate in water, and mobile phase B was 0.1% v/v formic acid with 5 mM ammonium formate in methanol. The flow rate was 0.450 mL/min, and the column oven was maintained at 45 °C. The binary gradient was initially at 5.0% mobile phase B and increased to 100% mobile phase B over 3.5 min. After a hold at 100% mobile phase B for 1 min, the mobile phase composition was returned to 5% mobile phase B in 0.1 min and maintained for another 1 min to equilibrate the column. The sample injection volume was 2 µl. An external calibration curve was used for quantifying the analytes with quality control samples to ensure the system stability was qualified for each batch. For quantification, a 5 ppm accurate mass window was used for peak integration. Data were acquired using Xcalibur 4.1 software and processed with TraceFinder 5.0 software (both from ThermoFisher).
RNA extraction and qRT-PCR
RNA extraction and subsequent qRT-PCR experiments on cell pellets from time course assays were performed as described previously18. Briefly, pellets were thawed and resuspended in 600 µL TRI Reagent (Sigma) and transferred to a 2 mL screw-cap tube containing 100 µL zirconia beads. Cells were disrupted with a bead beater and 100 µL chloroform was added. Samples were vigorously shaken by hand, incubated at RT for 2 min, and then pelleted at maximum speed for 15 min at 4 °C. The aqueous layer was aspirated and transferred to a clean 1.5 mL microcentrifuge tube. Isopropanol was added to precipitate the RNA. Samples were washed once with 70% ethanol, dried for 2 min, and resuspended in 100 µL nuclease-free water. Resuspended samples were DNase treated using the TURBO DNase kit according to the manufacturer’s instructions (ThermoFisher). DNA libraries were prepared with the Superscript IV kit (ThermoFisher) using 500 ng of DNase-treated RNA. SYBR 2x Master Mix (ThermoFisher) was used to perform qRT-PCR on 2 µL of cDNA (diluted 1:5) in a total reaction volume of 20 µL.
Galleria mellonella infection experiments
P. aeruginosa strains were cultured from freezer stocks in 3 mL LB overnight at 37 °C with shaking. To prepare larvae inoculant, LB cultures were back-diluted 1:10 in FDS- media and incubated at 37 °C overnight, then normalized to 106 CFU in PBS. For fast kill assays, late instar larvae (Carolina Biological Supply Company) of uniform size and color were selected for infection. Larvae were injected between the third and fourth proleg with 20 µl of inoculum or PBS control using a BD Micro-Fine IV U-100 Insulin Needle and monitored for 10 h. Death was determined by melanization and failure to respond to physical probing.
Statistics and reproducibility
All statistical analyses were performed using GraphPad Prism 9.5.1. All experiments were performed in technical duplicate with at least three independent biological replicates performed on different days. Sample sizes were chosen based on previous studies that conducted pyocyanin and C4HSL measurements. No statistical method was used to predetermine the sample size. No data were excluded from the analyses. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The P. aeruginosa UCBPP-PA14 reference assembly has a National Center for Biotechnology Information accession number of NC_008463.1. Paired-end reads from clinical strains of P. aeruginosa were downloaded from the National Center for Biotechnology Information BioProject database NCBI accession number: PRJNA288601. Individual accession numbers for each sequenced isolate can be found in Supplementary Table S1. Source data are provided with this paper.
Code availability
Custom code and all commands issued in this study can be found at https://github.com/calebmallery/RhlI_manuscript_methods.
References
Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019. https://doi.org/10.15620/cdc:82532 (2019).
Centers for Disease Control and Prevention. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. https://doi.org/10.15620/CDC:117915 (2022).
Fricks-Lima, J. et al. Differences in biofilm formation and antimicrobial resistance of Pseudomonas aeruginosa isolated from airways of mechanically ventilated patients and cystic fibrosis patients. Int. J. Antimicrob. Agents 37, 309 (2011).
Yoon, S. S. et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3, 593–603 (2002).
Bhagirath, A. Y. et al. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 16, 1–22 (2016).
Montero, M., Domínguez, M., Orozco-Levi, M., Salvadó, M. & Knobel, H. Mortality of COPD patients infected with multi-resistant Pseudomonas aeruginosa: a case and control study. Infection 37, 16–19 (2009).
Murphy, T. F. et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177, 853–860 (2012).
Lee, J. & Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6, 26–41 (2015).
Kostylev, M. et al. Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc. Natl Acad. Sci. USA 116, 7027–7032 (2019).
Pesci, E. C., Pearson, J. P., Seed, P. C. & Iglewski, B. H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179, 3127–3132 (1997).
de Kievit, T. R., Kakai, Y., Register, J. K., Pesci, E. C. & Iglewski, B. H. Role of the Pseudomonas aeruginosa las and rhl quorum-sensing systems in rhlI regulation. FEMS Microbiol. Lett. 212, 101–106 (2002).
Schuster, M., Urbanowski, M. L. & Greenberg, E. P. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl Acad. Sci. USA 101, 15833–15839 (2004).
Parsek, M. R., Val, D. L., Hanzelka, B. L., Cronan, J. E. & Greenberg, E. P. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl Acad. Sci. USA 96, 4360–4365 (1999).
McGrath, S., Wade, D. S. & Pesci, E. C. Dueling quorum sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol. Lett. 230, 27–34 (2004).
Déziel, E. et al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 55, 998–1014 (2005).
Diggle, S. P. et al. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 50, 29–43 (2003).
Taylor, I. R. et al. Inhibitor mimetic mutations in the Pseudomonas aeruginosa PqsE enzyme reveal a protein-protein interaction with the quorum-sensing receptor RhlR that is vital for virulence factor production. ACS Chem. Biol. 16, 740–752 (2021).
Simanek, K. A. et al. The PqsE-RhlR interaction regulates RhlR DNA binding to control virulence factor production in Pseudomonas aeruginosa. Microbiol. Spectr. 10, e0210821 (2022).
Feathers, J. R., Richael, E. K., Simanek, K. A., Fromme, J. C. & Paczkowski, J. E. Structure of the RhlR-PqsE complex from Pseudomonas aeruginosa reveals mechanistic insights into quorum-sensing gene regulation. Structure 30, 1626–1636.e4 (2022).
Feltner, J. B. et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 7, e01513-16 (2016).
Zhao, K. et al. Evolution of lasR mutants in polymorphic Pseudomonas aeruginosa populations facilitates chronic infection of the lung. Nat. Commun. 14, 1–14 (2023).
Chen, R., Déziel, E., Groleau, M. C., Schaefer, A. L. & Greenberg, E. P. Social cheating in a Pseudomonas aeruginosa quorum-sensing variant. Proc. Natl Acad. Sci. USA 116, 7021–7026 (2019).
Wang, Y. et al. Characterization of lasR-deficient clinical isolates of Pseudomonas aeruginosa. Sci. Rep. 8, 1–10 (2018).
Harrison, F., Muruli, A., Higgins, S. & Diggle, S. P. Development of an ex vivo porcine lung model for studying growth virulence, and signaling of Pseudomonas aeruginosa. Infect. Immun. 82, 3312–3323 (2014).
Smalley, N. E. et al. Evolution of the quorum sensing regulon in cooperating populations of Pseudomonas aeruginosa. mBio 13, e0016122 (2022).
Jayakumar, P., Figueiredo, A. R. T. & Kümmerli, R. Evolution of quorum sensing in Pseudomonas aeruginosa can occur via loss of function and regulon modulation. mSystems 7, e0035422 (2022).
Juarez, P., Broutin, I., Bordi, C., Plésiat, P. & Llanes, C. Constitutive activation of MexT by amino acid substitutions results in MexEF-OprN overproduction in clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 62, e02445-17 (2018).
Oshri, R. D., Zrihen, K. S., Shner, I., Omer Bendori, S. & Eldar, A. Selection for increased quorum-sensing cooperation in Pseudomonas aeruginosa through the shut-down of a drug resistance pump. ISME J. 12, 2458–2469 (2018).
Maseda, H. et al. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48, 1320 (2004).
Lutter, E. I., Purighalla, S., Duong, J. & Storey, D. G. Lethality and cooperation of Pseudomonas aeruginosa quorum-sensing mutants in Drosophila melanogaster infection models. Microbiology 158, 2125–2132 (2012).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Gould, A. et al. Structure of the Pseudomonas aeruginosa acyl-homoserinelactone synthase LasI. Mol. Microbiol. 53, 1135–1146 (2004).
Watson, W. T., Minogue, T. D., Val, D. L., Von Bodman, S. B. & Churchill, M. E. A. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell 9, 685–694 (2002).
Churchill, M. E. A. & Chen, L. Structural basis of acyl-homoserine lactone-dependent signaling. Chem. Rev. 111, 68–85 (2011).
Shin, D. et al. N-Acyl homoserine lactone analog modulators of the Pseudomonas aeruginosa Rhll quorum sensing signal synthase. ACS Chem. Biol. 14, 2305–2314 (2019).
Long, J., Zaborina, O., Holbrook, C., Zaborin, A. & Alverdy, J. Depletion of intestinal phosphate following surgical injury activates the virulence of P. aeruginosa causing lethal gut-derived sepsis. Surgery 144, 189 (2008).
Medina, G., Juárez, K., Valderrama, B. & Soberón-Chávez, G. Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J. Bacteriol. 185, 5976 (2003).
Keegan, N. R. et al. Promoter selectivity of the RhlR quorum-sensing transcription factor receptor in Pseudomonas aeruginosa is coordinated by distinct and overlapping dependencies on C4-homoserine lactone and PqsE. Preprint at bioRxiv https://doi.org/10.1101/2023.08.07.552226 (2023).
Paczkowski, J. E. et al. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 292, 4064 (2017).
Mellbye, B. L., Spieck, E., Bottomley, P. J. & Sayavedra-Soto, L. A. Acyl-homoserine lactone production in nitrifying bacteria of the genera Nitrosospira, Nitrobacter, and Nitrospira identified via a survey of putative quorum-sensing genes. Appl. Environ. Microbiol. 83, 1540–1557 (2017).
Girard, L. et al. Characterization of N-acyl homoserine lactones in Vibrio tasmaniensis LGP32 by a biosensor-based UHPLC-HRMS/MS method. Sensors 17, 906 (2017).
Mukherjee, S., Moustafa, D., Smith, C. D., Goldberg, J. B. & Bassler, B. L. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 13, e1006504 (2017).
Tsai, C. J. Y., Loh, J. M. S. & Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7, 214 (2016).
O’Loughlin, C. T. et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl Acad. Sci. USA 110, 17981–17986 (2013).
Rumbaugh, K. P. et al. Quorum sensing and the social evolution of bacterial virulence. Curr. Biol. 19, 341–345 (2009).
Rumbaugh, K. P., Griswold, J. A., Iglewski, B. H. & Hamood, A. N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 67, 5854–5862 (1999).
Pereira, T. de O., Groleau, M.-C. & Déziel, E. Surface growth of Pseudomonas aeruginosa reveals a regulatory effect of 3- oxo-C12-homoserine lactone in absence of its cognate receptor, LasR. mBio 14, e0092223 (2023).
Zhang, B. et al. Directed evolution of RhlI to generate new and increased quorum sensing signal molecule catalytic activities. Enzyme Microb. Technol. 134, 109475 (2020).
Soto-Aceves, M. P., Cocotl-Yañez, M., Servín-González, L. & Soberón-Chávez, G. The Rhl quorum-sensing system is at the top of the regulatory hierarchy under phosphate-limiting conditions in Pseudomonas aeruginosa PAO1. J. Bacteriol. 203, 475–495 (2021).
Felix Krueger, Frankie James, Phil Ewels, Ebrahim Afyounian, Michael Weinstein, Benjamin Schuster-Boeckler, Gert Hulselmans, & sclamons. (2023). FelixKrueger/TrimGalore: v0.6.10. Zenodo. https://doi.org/10.5281/zenodo.7598955.
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://arxiv.org/abs/1303.3997 (2013).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078 (2009).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, 1–4 (2021).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Nguyen, L. T., Schmidt, H. A., Von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Winsor, G. L. et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 44, D646–D653 (2016).
Johnson, M. et al. NCBI BLAST: a better web interface. Nucleic Acids Res. 36, W5–W9 (2008).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Kuznetsov, D. et al. OrthoDB v11: annotation of orthologs in the widest sampling of organismal diversity. Nucleic Acids Res. 51, D445–D451 (2023).
Acknowledgements
This work was supported by National Institutes of Health training grant T32GM132066 to C.P.M. and S.S., NIH grant R01GM14436101, New York Community Trust Foundation grant P19-000454, Cystic Fibrosis Foundation grant PACZKO21G0, and American Lung Association Innovation Award INALA2023 to J.E.P. The authors thank Dr. Kimberlee Musser, Clinical Director of the Wadsworth Center, New York State Department of Health, and the staff in the Bacteriology Group at the Wadsworth Center, for access to clinical isolates of P. aeruginosa and their genome sequences. The Wadsworth Center serves as the Northeast AR Lab Network regional laboratory for antimicrobial resistance surveillance. The authors also thank the research laboratories in the Division of Genetics at the Wadsworth Center for helpful discussions on the research and for resource sharing. This work was made possible with the help of the dedicated staff scientists at the Advanced Genomics Technologies Center and Media & Tissue Core facilities at the Wadsworth Center.
Author information
Authors and Affiliations
Contributions
K.A.S. and J.E.P. conceived the study and designed experiments. K.A.S. performed the experiments shown in Figs. 1e, 2a–d, 3a–d, 4a–d, Supplementary Figs. S3a–d, S4a–e, S5a–d, S6a–d, and S7a–d. M.L.S. performed the experiment shown in Fig. 5. C.P.M. provided bioinformatic analyses displayed in Fig. 1a, Supplementary Table S1, Supplementary Figs. S1a and S2. S.S. performed the experiment shown in Fig. 2e. L.L. provided technical assistance and analyzed all mass spectrometry experiments shown in Fig. 3, Supplementary Figs. S4 and S5. J.E.P. performed structural analyses of the RhlI synthase and homologs. K.A.S., M.L.S., C.P.M., L.L., and J.E.P. wrote the manuscript. J.E.P. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simanek, K.A., Schumacher, M.L., Mallery, C.P. et al. Quorum-sensing synthase mutations re-calibrate autoinducer concentrations in clinical isolates of Pseudomonas aeruginosa to enhance pathogenesis. Nat Commun 14, 7986 (2023). https://doi.org/10.1038/s41467-023-43702-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43702-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.