Abstract
Non-alcoholic steatohepatitis (NASH) is a progressive, inflammatory liver disease with no approved pharmacological treatment. This Phase IIa, double-blind, placebo-controlled, multicentre trial (ClinicalTrials.gov: NCT03166735) investigated pharmacodynamics and safety of BI 1467335, an amine oxidase copper-containing 3 (AOC3) inhibitor, in adults with NASH from Europe and North America. Participants from 44 centres across the US, Germany, Spain, Belgium, the UK, Netherlands, Canada, France and Ireland were randomised (2:1:1:1:2; 27 July 2017 to 14 June 2019) to daily oral BI 1467335 1 mg (n = 16), 3 mg (n = 16), 6 mg (n = 17), 10 mg (n = 32) or placebo (n = 32) for 12 weeks, with follow-up to Week 16. Primary endpoint was AOC3 activity relative to baseline (%), 24 hours post-dose after 12 weeks’ treatment. Secondary biomarker endpoints included changes from baseline at Week 12 in alanine aminotransferase (ALT) and caspase-cleaved cytokeratin 18 (CK-18 caspase). Mean AOC3 activities relative to baseline at Week 12: 90.4% (placebo; n = 32), 26.5% (1 mg; n = 16), 10.4% (3 mg; n = 16), 5.0% (6 mg; n = 16), 3.3% (10 mg; n = 32). These changes indicated that BI 1467335 dose-dependently inhibited AOC3 activity; ≥3 mg doses achieved >80% inhibition ( < 20% activity) at Week 4. At Week 12 following doses of BI 1467335 ≥ 3 mg, ALT and CK-18 caspase decreased dose-dependently. All tested BI 1467335 doses were well tolerated, with no clinically relevant treatment-emergent safety signals. BI 1467335 strongly inhibited AOC3 in participants with NASH, with doses ≥3 mg dose-dependently reducing the levels of liver injury biomarkers, ALT and CK-18. This trial was registered with ClinicalTrials.gov (NCT03166735) and the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT 2016-000499-83).
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a progressive disease with associated morbidity and mortality, and is the most common cause of liver disease, with an increasing global prevalence of 25% linked to the global epidemic of obesity and type 2 diabetes1. NAFLD is an independent risk factor for chronic kidney disease2, and depression and anxiety3, An estimated 7–30% of patients with NAFLD develop non-alcoholic steatohepatitis (NASH), which is a progressive and more serious subtype characterised by lobular inflammation and liver cell injury1,4 and has a prevalence of 5% worldwide5. NASH covers a wide spectrum of disease which includes progressive fibrosis that can result in cirrhosis, and its associated complications such as liver cancer or liver failure and the need for transplantation4,6. An estimated 20% of patients with NASH will go on to develop cirrhosis and/or hepatocellular carcinoma (HCC)4,7. A recent meta-analysis reported a mortality rate over a median of 6.2 years follow-up in patients with NAFLD of approximately 18%, with around 3% of deaths being specifically related to liver disease8. The current standard of care for NAFLD/NASH includes lifestyle interventions, such as weight loss and exercise6,9, which reduce inflammation and, indirectly, fibrosis10. The investigational treatments semaglutide, lanifibranor11, and resmetirom12 are currently in Phase III trials and also aim to reduce inflammation and fibrosis. However, thus far, there are no licensed pharmacological treatments for NASH.
Persistent inflammation resulting from liver injury drives progression to fibrosis, cirrhosis and HCC13. Hepatic inflammation results from the accumulation of leukocytes recruited from the circulation, which reflects a series of steps, including interaction between leukocytes and the hepatic sinusoidal endothelium, transendothelial migration and migration within hepatic tissue toward the focus of inflammation14. Hepatic inflammation leads not only to liver-related fibrosis but also to extrahepatic comorbidities;15 therefore, given the redundancy of inflammatory pathways and the upstream causes of liver injury, the ability to reduce inflammation is central to any potential intervention for NASH13.
A key protein in promoting the recruitment of leukocytes to liver tissue is amine oxidase copper-containing 3 (AOC3), formerly known as copper-dependent semicarbazide-sensitive amine oxidase (SSAO) or vascular adhesion protein-116,17. AOC3 is constitutively expressed on human hepatic endothelium16,17 and, as a membrane-bound adhesion protein, is involved in inflammatory leukocyte recruitment and subsequent transmigration across the endothelium to sites of inflammation17,18,19,20. AOC3 is also expressed in the smooth muscle cells and endothelium of blood vessels within the human brain21. AOC3 enzymatic activity also catalyses the oxidative deamination of primary amines to produce hydrogen peroxide, ammonium and aldehyde17, which are associated with oxidative stress.
Several lines of evidence, including its enzymatic activity and role in leukocyte recruitment, implicate AOC3 in the pathophysiology of NASH16,22. A soluble form of AOC3 (sAOC3), also found in human serum, is derived from a cleavage product of the membrane-bound protein and has amine oxidase activity. Sera from patients with chronic and inflammatory liver diseases (including alcohol-related liver disease, primary biliary cholangitis, cryptogenic cirrhosis and HCC) show elevated levels of sAOC3 (thought to be derived mainly from the liver) and AOC3 enzymatic activity23,24. Furthermore, hepatic expression of membrane-bound AOC3 and serum levels of sAOC3 are increased in patients with NAFLD, and levels of sAOC3 correlate with fibrosis stage and are predictive of progression to NASH16. Lastly, animal models suggest that AOC3 promotes progression of steatohepatitis and that AOC3 enzymatic activity is crucial for the establishment of fibrosis16. AOC3 inhibition, using anti-AOC3 antibodies, has been shown to reduce inflammatory cell recruitment to the liver in murine hepatic injury models and in vitro cultured active hepatic stellate cells from liver tissue of patients with NASH cirrhosis16. AOC3 inhibition also attenuated fibrosis in a murine NAFLD/NASH model (mice fed a methionine choline-deficient diet)16. BI 1467335 (Pharmaxis PXS-4728A) is an oral, small-molecule inhibitor of AOC325, which we hypothesised may reduce oxidative stress and hepatic inflammation in steatohepatitis, and halt or reverse the progression of fibrosis in patients with NASH16,22,25.
Herein, we report results of a randomised, placebo-controlled Phase IIa trial that assessed the safety, tolerability and pharmacodynamics of different doses of orally administered BI 1467335 during a 12-week treatment period, compared with placebo, in patients with clinical evidence of NASH.
Results
Trial patients and compliance
Between 27 July 2017 and 14 June 2019, 114 patients were enrolled and randomly assigned to one of five treatment groups. The treated population included 113 patients who received at least one dose of daily oral BI 1467335 1 mg (n = 16), 3 mg (n = 16), 6 mg (n = 17), 10 mg (n = 32) or placebo (n = 32). In total, 16 patients had at least one important protocol deviation (n = 11, treatment duration was too short; n = 5, failure to meet entry criteria; n = 4, non-compliance to trial medication; n = 2, prohibited medication use; n = 2, missing on-treatment biomarker value; n = 1, no trial medication taken) resulting in a per-protocol population of 98 patients. The safety population included 113 patients. The full analysis set, which was used for the sensitivity analysis of the primary endpoint, included 112 patients. Patient characteristics and demographics were similar between groups. There was no relevant change over time in body mass index (BMI) or any other physical parameters over the course of the trial. Almost half of the 113 patients were male (48.7%), most patients (96.5%) were white and the mean age (standard deviation [SD]) was 51.1 years (12.5) (Table 1). Baseline demographics, disease characteristics and key biomarkers are shown in Table 1.
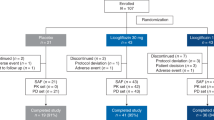
Of the 113 patients treated during the trial, 12 patients (10.6%) discontinued trial medication (all BI 1467335); one discontinuation in the 6 mg arm was due to a treatment-emergent adverse event (TEAE): moderate headache (other reasons for discontinuation are given in Fig. 1).
*Other reasons for the premature discontinuation of trial medication were the patient started prohibited concomitant medication (n = 2) and sponsor’s decision (n = 1). †Other reasons for trial discontinuation were that follow-up was not completed as planned, whether completed early or by telephone (n = 3).
Good compliance to trial medication was documented in 15 patients (94%) from the 1 mg arm and in all patients from the other arms. Patient disposition is shown in Fig. 1.
Primary outcome
BI 1467335 resulted in dose-dependent inhibition of plasma AOC3 activity, with >80% reductions from baseline seen by Week 4 with doses of ≥3 mg. Relative to baseline values, mean AOC3 activity at Week 12 (24 hours post dose) was 26.5% in the BI 1467335 1 mg dose group, (indicating 73.5% inhibition), 10.4% in the 3 mg group (indicating 89.6% inhibition), 5.0% in the 6 mg group (95.0% inhibition) and 3.3% in the 10 mg group (96.7% inhibition); mean plasma AOC3 activity in placebo recipients was 90.4%, indicating that no substantial change was observed with placebo and thus meeting the primary endpoint (Supplementary Table 1). The duration of inhibition also increased with dose: BI 1467335 treatment resulted in >80% inhibition of AOC3 for the entire 24-hour dosing interval, following the first 10 mg dose and from around Week 4 of treatment for doses of 3 mg and 6 mg, whereas for the 1 mg dose, the duration of >80% AOC3 inhibition was for approximately half a day (Fig. 2). Statistical analysis of the primary endpoint predicted that a 90% reduction of baseline AOC3 activity at Week 12 could be achieved with a daily dose of BI 1467335 3.45 mg and within a post hoc analyses an 80% reduction of baseline AOC3 activity at Week 12 could be reached with a daily dose of 1.58 mg. Plasma AOC3 concentrations fluctuated but remained similar to baseline levels throughout the trial in the placebo and across all BI 1467335 groups (Supplementary Table 2). Therefore, BI 1467335 inhibited AOC3 activity without altering the plasma concentration of AOC3.
Mean AOC3 activity relative to baseline versus time curves after once daily administration of 1 mg, 3 mg, 6 mg or 10 mg BI 1467335 or placebo for up to 85 days (whole profile days at Day 1, 28 and 84; FAS). Error bars show standard deviation from the mean. Source data are provided as a Source Data file. Dotted line indicates 20% residual AOC3 activity relative to baseline. AOC3 amine oxidase copper-containing 3, FAS full analysis set.
Secondary outcomes
At Week 12, alanine aminotransferase (ALT) and caspase-cleaved cytokeratin 18 (CK-18 [M30]) showed a significant (p < 0.05) non-flat dose–response relationship with BI 1467335 treatment (exponential, linear, logistic, quadratic and sigma Emax shapes are shown in Figs. 3 and 4, respectively). Dose–response relationships were not significant for aspartate aminotransferase (AST), alkaline phosphatase (AP), gamma-glutamyl transferase (GGT) and total cytokeratin 18 (CK-18 total [M65]) (Supplementary Fig. 2).
Error bars show MMRM-generated adjusted mean (least-square means) ALT and two-sided 90% CI estimates based on the t-distribution (detailed in Table 2) for each dose relative to baseline at Week 12. Five different candidate dose–response patterns (blue trend lines), generated using the multiple contrast test MCPMod, show the predicted shape of the dose–response relationship. The figure shows all models that had a significant test result (i.e. the null hypothesis of a flat dose-response curve was rejected with alpha 0.05, one-sided). P-values are provided thereafter for each of these models: exponential, 0.0321; linear, 0.0256; logistic, 0.0216; quadratic, 0.0254; sigEmax, 0.0212. Source data are provided as a Source Data file. ALT alanine aminotransferase, MCPMod Multiple Comparison Procedure—Modelling, MMRM mixed effects model for repeated measurements, PPS per-protocol set.
Error bars show MMRM-generated adjusted mean (least-square means) CK-18 caspase and two-sided 90% CI estimates based on the t-distribution (detailed in Table 3) for each dose relative to baseline at Week 12. Five candidate dose–response patterns (blue shapes), generated using the multiple contrast test MCPMod, show the predicted shape of the dose–response relationship. The figure shows all models that had a significant test result (i.e. the null hypothesis of a flat dose-response curve was rejected with alpha 0.05, one-sided). P-values are provided thereafter for each of these models: exponential, 0.0112; linear, 0.0093; logistic, 0.0047; quadratic, 0.0098; sigEmax, 0.0042. Source data are provided as a Source Data file. CK-18 caspase caspase-cleaved cytokeratin 18, MCPMod Multiple Comparison Procedure—Modelling, MMRM mixed effects model for repeated measurements, PPS per-protocol set.
After 12 weeks of treatment, the 3 mg, 6 mg and 10 mg doses of BI 1467335 resulted in an adjusted mean placebo-corrected relative change in ALT of −5.2% (90% CI: −21.7, 11.4), −12.0% (90% CI: −27.4, 3.3) and −15.1% (90% CI: −27.8, −2.3), respectively (Table 2). Doses of 3 mg, 6 mg and 10 mg BI 1467335 also resulted in a dose-dependent, adjusted mean placebo-corrected relative change in CK-18 caspase of −4.5% (90% CI: −35.6, 26.6), −20.8% (90% CI: −47.5, 5.8) and −23.3% (90% CI: −45.8, −0.8) (Table 3). The decrease in AST at Week 12 following treatment with 3 mg, 6 mg and 10 mg BI 1467335 showed no clear dose dependency (Supplementary Table 3). No clear change in ALT and AST was observed with the 1 mg BI 1467335 dose (although placebo-corrected values indicated increases of a similar magnitude to the decreases observed with higher doses), but an increase in CK-18 caspase was observed with the 1 mg BI 1467335 dose (confirmed by placebo-corrected values).
Changes in other biomarkers were inconsistent (GGT (Supplementary Table 4) and CK-18 total (Supplementary Table 5)) or absent (AP (Supplementary Table 6)). Placebo treatment was not associated with substantial changes in these biomarkers. The dynamic change over time relative to baseline and the concentrations over time of ALT, AST, AP, GGT, CK-18 caspase and CK-18 total are shown in Supplementary Fig. 3 and Supplementary Tables 7 to 12, respectively. At follow-up, levels of these secondary biomarkers were broadly similar in magnitude to baseline levels. A post hoc analysis performed on the pro-peptide of type lll collagen (Pro-C3) in patients with a baseline Pro-C3 value > 15 ng/mL indicated a positive but weak relationship between the change from baseline in ALT or AST and the change from baseline in Pro-C3 (Supplementary Figs. 4 and 5). This weak positive relationship seems to become more pronounced the higher the dose and the longer the treatment. However, no dose-dependent change from baseline in Pro-C3 over time was identified in the full analysis set (Supplementary Fig. 6). In general, the descriptive statistics on metabolic (fasting plasma glucose, insulin, cholesterol and triglyceride) and inflammatory markers (interferon γ and interleukins 1β, 6 and 8) and other markers and scores reflecting fibrosis status (aspartate aminotransferase to platelet ratio index [APRI], Fibrosis-4 score [Fib-4 score], enhanced liver fibrosis [ELF] score, NAFLD fibrosis score and Pro-C3) did not show relevant trends over time or differences for any BI 1467335 dose (Supplementary Tables 13–25).
Safety
The incidence of TEAEs was similar in the 81 patients receiving BI 1467335 (65.6–76.5%) and the 32 patients receiving placebo (62.5%) (Table 4). There was no dose-related increase in TEAEs. Most patients had TEAEs of grade ≤2. One patient receiving placebo and three patients receiving BI 1467335 had at least one grade ≥3 TEAE; all patients recovered. Two of these events were considered related to the trial medication (GGT increased in the 3 mg group and AST increased in the 10 mg group: both grade 3). The most frequently reported TEAE in >10% of the 32 patients in the placebo group was headache (12.5%). Of the 81 patients in the BI 1467335 groups, the most frequently reported TEAEs in >10% participants were nasopharyngitis (13.6%), nausea (12.3%) and headache (11.1%).
The incidence of these TEAEs did not increase with increasing doses of BI 1467335. A total of six serious TEAEs were reported in three patients (one in the placebo group [sinusitis and nasal septum deviation requiring hospitalisation], one in the 1 mg group [mild pancreatitis, not considered a serious TEAE by the investigator but categorised as such per the sponsor’s request] and one in the 3 mg group [H1N1 influenza, acute respiratory failure and chronic obstructive pulmonary disease requiring hospitalisation]); none were related to treatment and all three patients recovered. During the trial, one patient discontinued treatment with 6 mg BI 1467335 due to a moderate TEAE (headache) that was considered related to the trial medication, but the patient completed the trial.
Fewer investigator-defined drug-related TEAEs were observed in the 81 patients receiving BI 1467335 (21.0% all dose groups [1 mg, n = 16; 3 mg, n = 16; 6 mg, n = 17 and 10 mg, n = 32]) than in the 32 patients receiving placebo (25.0%). Two patients experienced liver injury events (TEAE of special interest [TEAESI]) during the trial. One patient in the placebo group had a mild AST increase (Day 140–152) that did not require treatment and a concurrent ALT increase (not considered a TEAESI) and subsequently recovered. ALT and AST levels were still elevated at follow-up. One patient in the BI 1467335 10 mg group had an AST increase (Day 15–43) of severe intensity that was not treated and was considered as possibly related to trial medication. The patient recovered. The same patient experienced an AST increase and blood bilirubin increase, starting on Day 130, both of mild intensity and not requiring treatment. ALT, AST and bilirubin levels were still elevated at follow-up. Neither patient was a Hy’s Law case since bilirubin was normal throughout treatment in the placebo case and elevation of liver enzymes was explained by Gilbert’s syndrome in the BI 1467335 10 mg case. There were no deaths in the trial.
Discussion
At Week 12 in this Phase IIa trial, BI 1467335 strongly inhibited plasma AOC3 activity in a dose-dependent manner, along with showing a reduction in markers of liver injury. There was >80% inhibition of AOC3 activity for the entire 24-hour dosing interval from Week 4 onwards of treatment for doses ≥3 mg indicating adequate target engagement. The magnitude of AOC3 inhibition was similar to that seen in previous studies in healthy volunteers [Mackie et al., manuscript in preparation]. While strong inhibition of plasma AOC3 activity was seen earlier than Week 4 with the 6 mg and 10 mg doses of BI 1467335 in healthy volunteers, this was most likely due to a difference in the timing of sample collection rather than an actual difference in these populations.
At Week 12 in this trial, the liver injury biomarkers ALT and CK-18 caspase decreased in response to treatment with 3–10 mg BI 1467335 in a dose-dependent manner, with a similar trend being observed for AST. ALT level is a marker of liver injury and elevated serum ALT indicates hepatic inflammation and liver injury in patients with various liver diseases26. CK-18 caspase is released from hepatocytes during apoptosis and is associated with inflammation and fibrosis in various chronic liver diseases, including NASH27,28,29. A positive but weak relationship was observed between the change from baseline in Pro-C3 (another marker of fibrosis) and the changes from baseline in ALT or AST. There was no clear dose effect, however this weak relationship became more pronounced with higher doses and longer treatment. No clear dose-dependent trend over time was seen for further metabolic, inflammatory and fibrosis biomarkers that, together with the increases in ALT, AST and CK-18 caspase in the BI 1467335 1 mg group is likely to be due to the presumed sub-therapeutic dose, large variability between patients, the presence of outliers and the small sample size in each group. Nevertheless, the dose-dependent reduction in the liver injury biomarkers ALT and CK-18 caspase in the absence of changes in BMI observed in the 3–10 mg BI 1467335 groups provides further support for adequate target engagement and proof-of-mechanism in patients with NASH.
The administration of up to 10 mg BI 1467335 once daily for 12 weeks did not identify any unexpected safety signals and BI 1467335 was well tolerated by the patients with NASH enrolled in this trial. Only one patient in the 6 mg BI 1467335 treatment group discontinued treatment due to a moderate TEAE (headache) and both patients who experienced a liver injury event (TEAESI) recovered and completed the trial. The safety profile of BI 1467335 in the present trial was consistent with that seen in Phase I trials in healthy volunteers (Mackie, et al. manuscript in preparation). Further development of BI 1467335 was stopped due to the risk of drug interactions of the compound with monoamine oxidase (MAO)-B in NASH patients identified in another Phase I trial30,31. Preclinical data suggest that BI 1467335 and RTU-1096 (another AOC3 inhibitor) have an IC50 against AOC3 and MAO-B in the nanomolar and micromolar range, respectively indicating a high degree of specificity for AOC332,33. Whether the MAO-B inhibition observed in humans31 is a class effect or specific to BI 1467335 is, as yet, unknown. Nevertheless, liver injury biomarkers were reduced and BI 1467335 was well tolerated in this Phase IIa trial. As such, AOC3 remains an attractive target for reducing hepatic inflammation, with the potential for subsequent beneficial effects on fibrosis in patients with NASH.
In conclusion, BI 1467335 strongly inhibited AOC3, was well tolerated at all tested doses, and showed dose-dependent reductions in serum ALT and CK-18 caspase, resulting in >10% placebo-corrected reduction from baseline for ≥6 mg doses.
Methods
Trial design and participants
All patients provided written informed consent before entering the trial and this trial was conducted in accordance with Good Clinical Practice, the ethical principles laid down in the Declaration of Helsinki and applicable regulatory requirements. The full study protocol is available as a supplementary file. Five global protocol amendments were implemented to the study protocol with Amendments 3, 4 and 5 implemented following the approval of version 3.0 of the study protocol. Details and rationale for the implemented protocol amendments are provided in the supplementary materials. The Institutional Review Boards and Independent Ethics Committees that approved each version of the study protocol are listed in Supplementary Table 26.
This multicentre, parallel-group, randomised, double-blind, placebo-controlled Phase IIa trial (ClinicalTrials.gov: NCT03166735) was conducted at 44 centres across the US, Germany, Spain, Belgium, the UK, Netherlands, Canada, France and Ireland between 27 July 2017 and 14 June 2019. Eligible patients were adults (aged 18–75 years), with either clinical evidence of NASH defined as histological evidence no more than 3 years prior to screening or clinical imaging suggestive of NASH (evidence of hepatic steatosis by magnetic resonance imaging of proton density fat fraction or ultrasound and evidence of liver fibrosis defined by stiffness >3.64 kPa with magnetic resonance elastography or >7.2 kPa with transient elastography) no more than 3 years prior to screening or within the screening phase. Patients were also required to have an ALT level >1.5 to ≤5.0 × the upper limit of normal (ULN) or historic ALT > 1.25 × ULN within 1 week to 3 months prior to screening and two consecutive ALT > 1.5 × ULN measurements at least one week apart during the screening period to be included in the trial.
Patients were excluded if they had cirrhosis; other causes of chronic liver disease; a current or recent (within 5 years) history of significant alcohol consumption (>210 g or >140 g per week in men or women, respectively); a bilirubin level >ULN; or glycosylated haemoglobin ≥9.5% or a change in body weight ≥5% in the 3 months prior to screening. A prior risk evaluation using a mechanistic static model suggested that BI 1467335 concentrations in man following 10 mg once daily could potentially inhibit MAO-B irreversibly. Due to this potential risk, concomitant medications, such as antidepressants, MAO inhibitors and serotonergic compounds, were prohibited.
Randomisation and blinding
Eligible patients were randomised using interactive response technology (IRT) 2:1:1:1:2 (block size 7) to receive placebo or BI 1467335 1 mg, 3 mg, 6 mg or 10 mg orally once daily for 12 weeks. Use of IRT provided a depersonalised patient identification code and ensured confidentiality of patient data; the randomisation list was generated using a validated system, which involved a pseudo-random number generator so that the resulting treatment was both reproducible and non-predictable. All trial participants, investigators and site staff were blinded to the assigned treatment.
Procedures
All patients received 5 film-coated tablets daily to be taken orally before breakfast, supplied as placebo or BI 1467335 in 1 mg and 5 mg dose strengths. Each treatment group received a combination of placebo and BI 1467335 tablets corresponding to the appropriate dose (e.g. the 6 mg dose group received 1 × 5 mg BI 1467335, 1 × 1 mg BI 1467335, 3 × placebo). All treatments were double blind. Assessments of sAOC3 activity and concentration, ALT, AST, AP, GGT, CK-18 caspase and CK-18 total, and other exploratory biomarkers were carried out at each study visit. Study visits took place every 2 weeks for the first 8 weeks, at Week 12 and at a follow-up visit 4 weeks after trial drug termination (Week 16). TEAEs were recorded at each study visit, at follow-up and by telephone call on Days 27 and 83. TEAESIs were liver injury events (ALT and/or AST 5–8 × baseline or >300 U/L in patients with ALT and/or AST > ULN at baseline; AST 3–8 × ULN in patients with normal AST at baseline) and trial-specific procedures for the removal of individual patients were defined in cases of increased liver enzymes (AST, ALT and total bilirubin) after randomisations34 and are shown in Fig. S1. In suspected cases of elevated liver enzymes, treatment was temporarily interrupted until other potential causes of liver injury were excluded and values were confirmed by retesting within 48 hours. If values were confirmed, trial medication remained interrupted and the patient was monitored weekly until resolution or stabilisation. Full details of interruption, restarting and stopping criteria are provided in supplementary materials.
Outcomes
The primary endpoint was plasma AOC3 activity relative to baseline measured as a percentage, 24 hours post dose after 12 weeks of treatment. AOC3 activity was measured via a quasi-quantitative, one-step fluorometric activity assay (Amplex® Red Monoamine Oxidase Assay Kit). In this assay, hydrogen peroxide, produced during oxidation of benzylamine by AOC3, was used as a proxy for quantification of AOC3 activity. Hydrogen peroxide oxidised Amplex Red to its fluorescent analogue, resorufin, allowing for colorimetric analysis of AOC3 activity. The number and percentage of patients with drug-related adverse events (AEs) was a secondary endpoint in this trial. Safety and tolerability were further assessed based on the general occurrence of TEAEs, safety laboratory parameters, physical examination, vital sign measurements and a 12-lead electrocardiogram. The intensity of AEs was classified and recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Secondary biomarker endpoints were relative changes from baseline in ALT, AST, AP, GGT, CK-18 caspase, and CK-18 total at Week 12. Further biomarker endpoints included markers of metabolism (e.g. fasting plasma glucose, insulin and lipids), inflammation (e.g. interleukins 1β, 6 and 8, and interferon γ) and markers and scores reflecting fibrosis status (e.g. APRI, Fib-4 score, ELF score, NAFLD fibrosis score and Pro-C3).
Sample size calculation
The sample size calculation was based on the primary endpoint, as well as the ALT change from baseline; the latter being the biomarker with the least favourable ratio of anticipated effect size to variance. A sample size of 108 patients with an allocation ratio of 2:1:1:1:2 for placebo, BI 1467335 1 mg, 3 mg, 6 mg or 10 mg, with an assumed 10% discontinuation rate and assumed maximum change of 30%, was anticipated to provide sufficient precision for the primary endpoint evaluation as well as an 84.8% probability of detecting a 20% relative change from baseline in ALT at Week 12. This calculation was based on 1000 simulations using Multiple Comparison Procedure—Modelling (MCPMod)35, assuming a baseline ALT of 80 U/L with an SD of 40, a placebo effect of −10 U/L and a treatment effect of −34 U/L at Week 12, with the null hypothesis of no dose relationship rejected at one-sided alpha 0.05.
Missing data
Missing AOC3 activity data were subject to imputation of missing values. Prior to imputation, the pattern of missing data was explored using a blinded dataset. If the pattern was monotone, a regression model was used for the imputation. Otherwise, a Markov chain Monte Carlo step was applied to create monotone data in multiple datasets prior to using a regression method. A decreasing 3-parameter curve model was then applied. For the secondary biomarkers, the pattern of missing values was assumed to be random, and directly handled within the mixed effects model for repeated measurements (MMRM) based on the likelihood method. If one of the three electrocardiogram cardiac cycles was missing, the arithmetic mean for this single electrocardiogram was computed using the available cardiac cycles. Imputation was not performed for safety endpoints, further exploratory biomarker and pharmacogenomic endpoints or other variables. Only patients without missing baseline data and with at least one non-missing post-baseline, on-treatment biomarker measurement for any primary, secondary or further biomarker endpoint were included in the full analysis set and the per-protocol set.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC, USA). Primary and secondary non-safety endpoints were evaluated using the per-protocol population of all randomised patients, excluding patients with no baseline and/or on-treatment value and important protocol deviations leading to exclusion. Safety analyses used all randomised patients who received trial treatment. The dose–response relationship of the primary endpoint was analysed using a nonlinear regression model (decreasing Emax curve) applied to the AOC3 activity at Week 12. The fitted regression model was used to derive the smallest dose where the mean plasma AOC3 activity curve dropped below 10%. For the secondary biomarker endpoints, the MMRM was used to generate adjusted mean and 90% CI estimates for the treatment effects at Week 12. These estimates, together with the corresponding covariance matrix, were used to analyse the dose–response relationship by examining the fit of eight shapes modelled using MCPMod36,37, allowing for simultaneous evaluation of different potential dose–response patterns, while protecting the overall probability of type I error (one-sided alpha of 0.05). A test for non-flat dose–response relationship was first performed; if a relationship could be shown, all significant models from a set of candidate models were selected and fitted to the data as a second step.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all authors who are not working for Boehringer Ingelheim access to relevant clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript and this request will be evaluated within 3 months. The data access criteria can be found on the Boehringer Ingelheim’s member page: https://vivli.org/ourmember/boehringer-ingelheim/. Researchers should use the https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/ link to request access to study data. The research proposal should provide the scientific rationale of the planned analysis and explain the potential public interest, and will be reviewed by Boehringer Ingelheim and Pharmaxis, as well as an external independent review panel of experts. In addition to anonymised clinical study data, upon request, Boehringer Ingelheim provides the redacted study protocol, statistical analysis plan, annotated case report forms, clinical study reports and data specifications, as appropriate. The Study Protocol and Statistical Analysis Plan are available as Supplementary Notes 1 and 2 in the Supplementary Information file. The remaining data are available within the Article, Supplementary Information. Controlled access to patient level clinical study data is an essential element of data protection requirements. Further information can be found at https://www.mystudywindow.com/msw/datasharing. Source data are provided with this paper.
References
Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology 158, 1851–1864 (2020).
Kaps, L. et al. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United European Gastroenterol. J. 8, 942–948 (2020).
Labenz, C. et al. Nonalcoholic fatty liver disease increases the risk of anxiety and depression. Hepatol. Commun. 4, 1293–1301 (2020).
Sheka, A. C. et al. Nonalcoholic steatohepatitis: a review. JAMA 323, 1175–1183 (2020).
Paik, J. M., Golabi, P., Younossi, Y., Mishra, A. & Younossi, Z. M. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD. Hepatology 72, 1605–1616 (2020).
European Association for the Study of the Liver, European Association for the Study of Diabetes & European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Povsic, M., Oliver, L., Jiandani, N. R., Perry, R. & Bottomley, J. A structured literature review of interventions used in the management of nonalcoholic steatohepatitis (NASH). Pharmacol. Res. Perspect. 7, e00485 (2019).
Taylor, R. S. et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 158, 1611–1625.e1612 (2020).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, P367–P378 (2015).
Newsome, P. N. et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 384, 1113–1124 (2021).
Francque, S. M. et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med 385, 1547–1558 (2021).
Schuppan, D. & Kim, Y. O. Evolving therapies for liver fibrosis. J. Clin. Investig. 123, 1887–1901 (2013).
Lalor, P. F., Shields, P., Grant, A. & Adams, D. H. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 80, 52–64 (2002).
Gehrke, N. & Schattenberg, J. M. Metabolic inflammation-A role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 158, 1929–1947 (2020).
Weston, C. J. et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Investig. 125, 501–520 (2015).
Salmi, M. & Jalkanen, S. Vascular adhesion protein-1: A cell surface amine oxidase in translation. Antioxid. Redox Signal. 30, 314–332 (2019).
Noda, K. et al. Vascular adhesion protein-1 regulates leukocyte transmigration rate in the retina during diabetes. Exp. Eye Res. 89, 774–781 (2009).
Almulki, L. et al. Localization of vascular adhesion protein-1 (VAP-1) in the human eye. Exp. Eye Res. 90, 26–32 (2010).
Salmi, M., Kalimo, K. & Jalkanen, S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J. Exp. Med. 178, 2255–2260 (1993).
Hernandez-Guillamon, M. et al. VAP-1/SSAO plasma activity and brain expression in human hemorrhagic stroke. Cerebrovasc. Dis. 33, 55–63 (2012).
Torok, N. J. Vascular adhesion protein 1 in nonalcoholic steatohepatitis: A novel biomarker? Hepatology 62, 1313–1315 (2015).
Kurkijärvi, R. et al. Circulating form of human vascular adhesion protein-1 (VAP-1): Increased serum levels in inflammatory liver diseases. J. Immunol. 161, 1549–1557 (1998).
Kurkijärvi, R. et al. Circulating soluble vascular adhesion protein 1 accounts for the increased serum monoamine oxidase activity in chronic liver disease. Gastroenterology 119, 1096–1103 (2000).
Jarnicki, A. G. et al. The inhibitor of semicarbazide-sensitive amine oxidase, PXS-4728A, ameliorates key features of chronic obstructive pulmonary disease in a mouse model. Br. J. Pharmacol. 173, 3161–3175 (2016).
Kim, W. R., Flamm, S. L., Di Bisceglie, A. M. & Bodenheimer, H. C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 47, 1363–1370 (2008).
Li, J., Verhaar, A. P., Pan, Q., de Knegt, R. J. & Peppelenbosch, M. P. Serum levels of caspase-cleaved cytokeratin 18 (CK18-Asp396) predict severity of liver disease in chronic hepatitis B. Clin. Exp. Gastroenterol. 10, 203–209 (2017).
Cusi, K. et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 60, 167–174 (2014).
Jazwinski, A. B. et al. Elevated serum CK18 levels in chronic hepatitis C patients are associated with advanced fibrosis but not steatosis. J. Viral. Hepat. 19, 278–282 (2012).
Boehringer Ingelheim. Boehringer Ingelheim discontinues development of BI 1467335 for NASH, https://www.boehringer-ingelheim.us/press-release/boehringer-ingelheim-discontinues-development-bi-1467335-nash (accessed 10 August 2022).
ClinicalTrials.gov. NCT03927209. A phase I, open-label, positron emission tomography study in healthy male subjects to explore the inhibition of monoamine oxidase B in the brain after multiple oral doses of BI 1467335 (non-randomized, open-label, parallel-group study), https://clinicaltrials.gov/ct2/show/NCT03927209 (accessed 10 August 2022).
Matsuda, T. et al. Vascular adhesion protein-1 blockade suppresses ocular inflammation after retinal laser photocoagulation in mice. Investig. Ophthalmol. Vis. Sci. 58, 3254–3261 (2017).
Schilter, H. C. et al. Effects of an anti-inflammatory VAP-1/SSAO inhibitor, PXS-4728A, on pulmonary neutrophil migration. Respir. Res. 16, 42 (2015).
Chalasani, N. & Regev, A. Drug-induced liver injury in patients with preexisting chronic liver disease in drug development: How to identify and manage? Gastroenterology 151, 1046–1051 (2016).
Bornkamp, B., Pinheiro, J. & Bretz, F. MCPMod: An R package for the design and analysis of dose-finding studies. J. Stat. Softw. 29, 1–23 (2009).
Pinheiro, J., Bornkamp, B. & Bretz, F. Design and analysis of dose-finding studies combining multiple comparisons and modeling procedures. J Biopharm Stat 16, 639–656 (2006).
Pinheiro, J., Bornkamp, B., Glimm, E. & Bretz, F. Model-based dose finding under model uncertainty using general parametric models. Stat. Med. 33, 1646–1661 (2014).
Acknowledgements
The study was supported and funded by Boehringer Ingelheim. Boehringer Ingelheim was involved in the study design, and collection and analysis of data, and was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations. The authors retained responsibility for the final decision to submit the paper for publication. Medical writing support was provided by Tom Priddle, DPhil, and Anna Wydra, MSc, of OPEN Health Communications (London, UK) and funded by Boehringer Ingelheim. PNN was supported by the National Institute of Health Research (NIHR) Birmingham Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Agreements between Boehringer Ingelheim and the authors included the confidentiality of the study data. We thank all the patients and study investigators who participated in the clinical study described here.
Funding
Supported/funded by Boehringer Ingelheim.
Author information
Authors and Affiliations
Consortia
Contributions
All authors were involved in the conceptual design of this manuscript, drafting and development, and agreement to publish. In addition: P.N.N., A.J.S., J.E., J.L., A.M., V.R. were involved in study design; J.E. was responsible for trial initiation and maintenance; C.S. was involved in development and validation of the assay for measurement of AOC3 activity; A.J.S., J.M.S., P.N.N., G.N., V.R., E.L. were the study investigators; E.L. was involved in study consultation; J.M.S, P.N.N., V.R., E.L. were involved in patient enrolment; P.N.N, J.L., A.M., C.S. were involved in data analysis; E.L. was involved in data review; and J.M.S., P.N.N., J.E., J.L., A.M., V.R., E.L. were involved in data interpretation.
Corresponding author
Ethics declarations
Competing interests
P.N.N. reports, on behalf of the University of Birmingham, grant/research support from Pharmaxis, Boehringer Ingelheim, Echosens and Novo Nordisk, and consulting fees from BMS, Boehringer Ingelheim, Gilead Sciences, Novo Nordisk, Pfizer and Poxel. A.J.S. is President of Sanyal Biotechnology and has stock options in Genfit, Akarna Therapeutics, Tiziana Life Sciences, Indalo, Durect, Inversago and Galmed Pharmaceuticals. He has served as a consultant to AstraZeneca, Nitto Denko, Conatus, Nimbus, Salix, Tobira, Takeda, Janssen, Gilead Sciences, Terns, Bird Rock Bio, Merck, Valeant, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, HemoShear, Larimar Therapeutics, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept Pharmaceuticals, Echosens, Immuron, Galectin, Fractyl Health, Synlogic, Afimune, Chemomab, Zydus, Nordic Bioscience, Albireo, ProSciento and Surrozen. His institution has received grant support from Gilead Sciences, Salix, Tobira, Bristol Myers Squibb, Shire, Intercept Pharmaceuticals, Merck, AstraZeneca, Mallinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate. G.N. reports speaker’s bureau fees from Intercept Pharmaceuticals, grant support from Echosens, consultancy fees from Auxilium. J.M.S reports consultancy fees from Boehringer Ingelheim, Bristol Myers Squibb, Genfit, Gilead Sciences, Intercept Pharmaceuticals, Madrigal Pharmaceuticals, Novartis, Novo Nordisk, Nordic Bioscience, Pfizer, Roche, Sanofi, Siemens Healthineers, research funding from Gilead Sciences, Boehringer Ingelheim, Siemens Healthineers, and speakers bureau fees from the Falk Foundation. V.R. reports consultancy fees from Boehringer Ingelheim. E.L. reports research/grant support from 89bio, Allergan, Akero Therapeutics, AstraZeneca, Axcella Health, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Durect, Eli Lilly and Company, Elobix, Enanta Pharmaceuticals, Enyo, Galmed Pharmaceuticals, Genfit, Gilead Sciences, Hanmi Pharmaceuticals, Intercept Pharmaceuticals, Laboratory for Advanced Medicine, Madrigal Pharmaceuticals, Merck, Metacrine, Novartis, Novo Nordisk, Octeta Therapeutics, Poxel, Roche Pharmaceuticals, Viking and Zydus. J.E., J.L., A.M. and C.S are employed by Boehringer Ingelheim.
Peer review
Peer review information
: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Newsome, P.N., Sanyal, A.J., Neff, G. et al. A randomised Phase IIa trial of amine oxidase copper-containing 3 (AOC3) inhibitor BI 1467335 in adults with non-alcoholic steatohepatitis. Nat Commun 14, 7151 (2023). https://doi.org/10.1038/s41467-023-42398-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42398-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.