Abstract
The assembly of cluster-based π-stacked porous supramolecular frameworks presents daunting challenges, including the design of suitable cluster building units, control of the sufficient C-H···π interactions, trade-off between structural dynamics and stability as well as understanding the resulting collective properties. Herein, we report a cluster-based C-H···π interaction-stacked porous supramolecular framework, namely, Cu12a-π, consisting of Cu12 nanocluster as a 6-connected node, which is further propagated to a dynamic porous supramolecular frameworks via dense intralayer C-H···π interactions, yielding permanent porosity. In addition, Cu12a-π can be transformed into cluster-based nonporous adaptive crystals (Cu12b-NACs) via ligand-exchange following a dissociation-reassembly mechanism. Moreover, Cu12a-π can efficiently remove 97.2% of iodine from saturated iodine aqueous solutions with a high uptake capacity of 2.96 g·g−1. These prospective results positioned at cluster-based porous supramolecular framework and enlighten follow-up researchers to design and synthesize such materials with better performance.
Similar content being viewed by others
Introduction
Inspired by fascinating molecular pores that nature has evolved, scientists have made great progress in constructing porous materials1,2,3,4,5. Recently, a series of crystalline porous materials (CPMs) were assembled through intermolecular weak non-covalent interactions, which involve hydrogen bond6,7,8,9,10, π···π or C-H···π stacking11,12,13,14,15,16, electrostatic interactions, van der Waals forces17,18, etc. Wherein, π-stacked organic frameworks (πOFs), one of the newly emerging CPMs, have greatly broadened the boundaries of porous science, but few examples associated to such CPMs have been reported. Moreover, most of the reported πOFs are stabilized not merely by pure π···π stacking interactions, whereas other forces are also involved to a greater or lesser extent, e.g. C-H···π stacking interactions12. As a result, it is hard to figure out how much the C-H···π stacking interactions contribute the stability of CPMs. Therefore, the construction of supramolecular networks with permanent pores stabilized by C-H···π stacking interactions is meaningful to investigate the performance of such a non-covalent interaction in CPMs.
In general, the CPMs stabilized by C-H···π interactions may show the similar dynamic behavior like third-generation MOFs due to the characteristic of non-covalent interactions, and can reversibly transform their structures in response to chemical guests via physical stimulation19,20,21. The rational construction of such CPMs may require the following two main factors: 1) the robust building unit; 2) the medium strength of the bridge linker, where the frame structure can balance the stability and flexibility. However, the most reported πOFs or HOFs (hydrogen-bonded organic frameworks) are constructed by organic building units, which may limit the stability on the process of dynamically structural transformation under drastic physical stimulation22,23. In addition, the further post-transformation of such OFs must be done through the redesign of the building unit. Therefore, the development of suitable building unit to rationally construct the C-H···π interaction-stacked porous supramolecular framework (PSF) with both dynamic structure and post-transformation is an important but currently challenging subject.
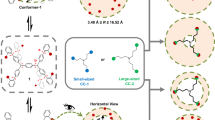
To address these challenges, we designed a three-dimensional (3D) PSF, namely Cu12a-π, based on non-covalent C-H···π interactions between cluster-based building units that are protected by 9-(prop-2-yn-1-yl)−9H-carbazole (Cbz-PrAH) and p-tert-butylthiacalix[4]arene (H4TC4A). Of these, the Cbz-PrAH contains extended aromatic ring, which is conducive to the formation of intra- and intermolecular non-covalent C-H···π interactions24; H4TC4A is a kind of macrocyclic ligand with intrinsic cavity structure25,26,27,28,29. As displayed in Fig. 1, these features of two ligands synergistically lead to construct an as-prepared PSF (Cu12a), which can transform to guest-free PSF (Cu12a-π) through a dynamical single-crystal-to-single-crystal (SC-SC) fashion by removing guest molecules under heating and vacuum conditions. The monomer Cu12 nanocluster as a zero-dimensional (0D) cluster-based building unit, can be further supramolecularly assembled into C-H···π interactions-stacked PSF. Even more impressively, the post-transformation of Cu12a-π followed a dissociation-reassembly mechanism, giving the cluster-based nonporous adaptive crystals (NACs) Cu12b-NACs with updates of both structure and properties.
Results
Synthesis and structural characterization
To implement the synthesis of the as-prepared PSF, Cu12a, we selected copper(I) precursor, Cbz-PrACu (Please see experimental details in the Supplementary Methods) to react with H4TC4A via solvothermal method, giving the red rhombic crystals of Cu12a in a quite high yield of 91% (Supplementary Fig. 1). The process involved the depolymerization of the starting Cbz-PrACu precursor upon heating. Concomitantly, the H4TC4A was deprotonated, resulting in the formation of 12-nuclei copper(I) nanoclusters protected by Cbz-PrA‒ and TC4A4‒ ligands. The infrared (IR) spectroscopy confirmed the existence of Cbz-PrA‒ in Cu12a. Besides, elements of Cu12a were confirmed by energy-dispersive x-ray (EDX) spectroscopy. More synthetic details and basic characterizations are listed in the Supplementary Figures and Tables (Supplementary Fig 2–4 and Supplementary Tables 1, 2).
Single-crystal X-ray diffraction (SCXRD) analyses revealed that the monomer of Cu12a crystallizes in a monoclinic unit cell under the P21/c space group with the asymmetric unit containing a half of nanocluster (Supplementary Fig. 5 and Supplementary Table 1), and its formula is determined as [Cu12(Cbz-PrA)4(TC4A)2]·(CH3COOCH2CH3). The structural anatomy of the monomer was shown in Fig. 2. The metal core can be viewed as an approximate cube-like pattern composed of three rectangular Cu4 units rotated by 45° in sequence, the equatorial copper atoms are bulged from the centres of four vertical faces of the cube about 0.446 Å. The Cu12 kernel exhibits a C4 symmetry with a C4 axis passing through the centres of two horizontal planes, and is consolidated by short Cu···Cu contacts (average distance of 2.76 Å), which are shorter than the sum of Bondi’s van der Waals radii of two Cu atoms (2.80 Å)30, implying an appreciable cuprophilic interactions. Furthermore, the types of capping ligands in the monomer of Cu12a are somewhat reminiscent of the Cu13a and Cu13b, where Cbz-PrA− and TC4A4− capped on the periphery of the copper kernel in our previous work31.
The difference is that four Cbz-PrA− ligands like nails hold only one coordination mode (μ4-η1: η1: η2: η2, Supplementary Fig. 6a, b), which are evenly distributed on the equator of the kernel, and their carbazole rings are oriented to two directions (up and bottom). The Cu-C bond lengths are in the range of 1.927 ~ 2.241 Å. Apart from the Cbz-PrA− ligands, the poles of the kernel are clutched by two TC4A4− ligands. The phenolic hydroxyl and bridging sulfur donors of the TC4A4− are bound to four copper atoms, showing the same coordination mode, μ4-κo2:κo2:κo2:κo2:κs1:κs1:κs1:κs1 (Supplementary Fig. 6c). The Cu-O and Cu-S bond lengths related to TC4A4− fall in the ranges of 1.946 ~ 2.390 Å. The four Cbz-PrA− and two TC4A4− ligands define the basic configuration of the Cu12 cluster, and have substantial impacts on the crystal stability and adsorption behavior (vide infra).
Crystalline supramolecular assembly
Recently, the framework adaptability to guest uptake and stimuli-responsiveness are two intriguing features in flexible CPMs that are receiving increasing attentions32,33,34,35,36. Inspired by this, the guest EA molecules in Cu12a may endow it with similar behavior. Thus, the crystalline sample of Cu12a has been heated at 338 K upon vacuum for two days. Surprisingly, the activated sample of Cu12a, termed Cu12a-π, can still be analyzed via SCXRD, in which the guest EA molecules had been successfully removed to form permanent voids (Fig. 3a and Supplementary Fig. 7). Of note, heating under vacuum, the single crystal of Cu12a undergoes a phase transition at 338 K accompanied by a doubling of the crystallographic a axis and a change from the P21/c to C2/c space group. And the two-dimensional (2D) layers of Cu12a and Cu12a-π both adopt AA stacking mode, but the latter occurred with a distinct displacement between each layers along the ab plane, resulting in a slight reduction in the porosity (from 11.5% to 9.1%). Impressively, upon further soaking Cu12a-π in EA solvent for three days, the lattice again recovered to P21/c accompanied with the re-adsorption of the EA molecules. This indicates that the Cu12a and Cu12a-π as host matrixes can achieve reversible SC-SC transformation by removal and re-adsorption of the guest molecule, thus demonstrating the crystalline structural flexibility of Cu12a. Thermogravimetric analysis (TGA) (Fig. 3b) indicates that the EA molecules in Cu12a are completely removed after phase transition and the Cu12a-π still remains stable even up to 558 K (Fig. 3b). Besides that, the PXRD patterns of Cu12a and Cu12a-π exhibit distinct difference in peak positions and intensities, further verifying the occurrence of phase transition. Upon heating, the variable-temperature PXRD patterns of Cu12a-π show no change even up to 513 K, confirming its thermally robust structure (Fig. 3c), which is superior than most reported πOFs11,12,13,14,15,16. Furthermore, after soaking Cu12a-π in different solvents (such as methanol: MeOH, acetonitrile: CH3CN, ethanol: EtOH, acetone: ACE, n-butanol: nBuOH and n-propanol: nPrOH) for at least 72 h at room temperature, their PXRD patterns were highly consistent well with the theoretical patterns of Cu12a, which demonstrated the framework highly dynamic stability to chemical solvents (Fig. 3d). This excellence of thermal and chemical stabilities may be attributed to its unique molecular and packing structural features before and after phase transition.
a Reversible single-crystal-to-single-crystal transformation between Cu12a and Cu12a-π and corresponding unit cell parameters. b TGA and differential scanning calorimetry (DSC) curves of Cu12a and Cu12a-π. c Variable-temperature PXRD patterns of Cu12a-π. d PXRD patterns of Cu12a-π in different solvents. The guests in Cu12a are EA molecules.
In Fig. 4a, trumpet-type Cu12 cluster shows significant cavity structures above the poles of copper kernel, which is conductive to the close arrangement of cavity structures in one plane. And the higher rim of the cavity in Cu12 cluster shows a maximum pore diameter of ~8.9 Å. Therefore, Cu12 cluster can be regarded as a porous 0D cluster-based building unit, associated with two kinds of C-H···π interactions (C(sp3)-H···π(Cbz)=3.1 Å and C(sp2)-H···π(Cbz)=3.1 Å) between a carbazole ring and other carbazole ring, as well as between an allylene and a carbazole ring (Fig. 4b), together supramolecular self-assembly into an extended PSF. As shown in Fig. 4c, the Cu12 building units adopt 6-connected node to connect six adjacent building units to form a 2D ordered array, which undergoes obliquely slipped one-dimensional (1D) stacking via almost negligible interlayer H···H interactions (2.26 and 2.93 Å) of tertiary butyl to form a final 3D supramolecular framework (Fig. 4d). Of these, the intralayer C-H···π interactions contribute to the framework stability as the dominant linkages compared to the strength of weak interlayer H···H interactions37. By contrast, the supramolecular crystalline assembly of Cu12 cluster in Cu12a also exhibits a similar mode to Cu12a-π (Supplementary Fig. 8), except for the relative large diameter of pore ( ~ 9.3 Å) and length of intralayer bridges (C(sp3)-H···π(Cbz)=3.2 or 3.4 Å and C(sp2)-H···π(Cbz)=2.8 Å) due to the incorporation of guest EA solvents.
a The Cu12 cluster in Cu12a-π displays a trumpet-type motif. b The intralayer C-H···π interactions between Cu12 clusters. The top (c) and side (d) views of local PSF. e Left: N2 adsorption and desorption isotherms of Cu12a-π at 77 K; f The CH4, CO2, C2H4 and C2H6 adsorption-desorption isotherms of Cu12a-π at 273 (Left) and 293 K (Right). The sample weight of Cu12a-π in gas sorption measurements: 100 mg.
On the basis of packing characteristic of Cu12a-π, it is clearly seen that the small cavities provided by the thiacalix[4]arene and permanent voids between each layers effectively endow the Cu12a-π with certain porosity (Supplementary Fig. 7). Correspondingly, analysis of N2 adsorption isotherms of Cu12a-π gives rise to a Brunauer-Emmett-Teller (Langmuir) surface area of 31.93 (45.29) m2·g−1, and a wide pore size distribution in the range of 0.8–1.6 nm calculated by Horvath-Kawazoe (HK) model (Fig. 4e and Supplementary Fig. 9). These results are thought to reflect the small cavities provided by the thiacalix[4]arene and the voids between the 2D layers. Meanwhile, Fig. 4f shows the pure-component equilibrium adsorption isotherms for the CH4, CO2, C2H4, and C2H6 collected at different temperatures. The maximum C2H6 uptake reaches 30.48 cm3·g−1 (273 K) at normal pressure, further confirming the real existence of permanent porosity in Cu12a-π.
Transformed into NACs
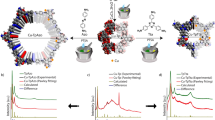
Remarkably, post-modification has been one of the research hotspots for MOFs in recent years, facilitating the acquisition of anticipant structures and properties38,39,40,41. Inspired by this, the Cu12 nanocluster acting as a building unit may feature ligand-exchange to remodel the PSFs. Therefore, we tried to transform Cu12a-π by dissolving crystalline samples in an isopropanol/tetrahydrofuran (v:v = 3:3) mixture, to which an excess of H4PTC4A (p-phenyl-thiacalix[4]arene) was added, and then the above solution continued to react under solvothermal condition to generate red block crystals of Cu12b. Except for the p-phenyl-thiacalix[4]arene (Supplementary Fig. 5d), the monomer Cu12 cluster in Cu12b has similar compositions to that of Cu12a, and the four phenyl groups act as the up rim of cavity structure (Fig. 5a). In view of the significantly different optical absorbance between the two Cu12 clusters (Supplementary Fig. 10a), we carried out the time-course optical absorptions to monitor the conversion process (Fig. 5b). With time going on, the intensity of its characteristic absorption peak at ~300 nm increased significantly upon stirring, as well as the peak at ~340 nm gradually changed from a prominent peak to a shoulder peak. Meanwhile, the color of the mixture solution transformed from yellow to pale yellow and finally to brownish yellow (Supplementary Fig. 10b). So as to intuitively visualize the trend of the two characteristic peaks, the correlation between the absorbance ratio of the two peaks and reaction time was illustrated. As the time went from 0 to 105 min, the value of A300 nm/A340 nm was raised from 1.563 to a maximum of 2.361, and then maintained around 2.335 for Cu12b (Supplementary Fig. 10c). Considering the following factors: (i) the extra charge transition of IL(Cbz-PrA+PTC4A)CT in Cu12b leading to the variation in the absorption intensity of the two nanoclusters at ~300 nm (Please see details in the Supplementary Methods); (ii) the position of the maximum absorption peaks of the two ligands (H4TC4A and H4PTC4A) (Supplementary Fig. 10d); (iii) dynamic characteristics of coordination bonds, we tentatively concluded that the increase of absorption strength in A300 nm/A340 nm may be attributed to the dissociation of TC4A4‒ and bonding of PTC4A4‒ ligands in Cu12a, leading to the increase in the absorption intensity of whole system near 300 nm. When the dissociation and bonding were in equilibrium, the A300 nm/A340 nm reached its crest values.
a Structural transformation of monomer Cu12 cluster from Cu12a to Cu12b. b UV‒Vis absorption spectra of the conversion from Cu12a to Cu12b over the time course at 293 K. c, d Positive-ion mode ESI-MS of the crystals of Cu12a (a) and Cu12b (d) dissolved in mixed CHCl3 and CH3OH. Inset: The zoom-in mass spectrum of experimental (green line) and simulated (red line) isotope-distribution patterns of each labeled species. e Time-course ESI-MS of transformation from Cu12a to Cu12b induced by adding H4PTC4A.
To verify the assumption on the transformation mechanism, we monitored the ligand-exchange process in the structural transformation from Cu12a to Cu12b by electrospray ionization mass spectrometry (ESI-MS). As shown in Fig. 5c, d, the Cu12a and Cu12b in mixed CH3OH and CHCl3 exhibit a series of +1 species (1a-1e for Cu12a; 2a-2i for Cu12b) at the m/z range of 3010-3090 and 3170-3345, respectively, which correspond to their intact cluster bound with different cations or solvents. The most dominant peak 1b at m/z 3036.9040 and peak 2b at m/z 3196.6478 can be attributed to [Cu12(TC4A)2(Cbz-PrA)4Na]+ (Cal. 3036.8907) and [Cu12(PTC4A)2(Cbz-PrA)4Na]+ (Cal. 3196.6410), respectively. However, as the reaction of H4PTC4A and Cu12a proceeded in CHCl3, the parent peaks of Cu12a (1a−1e) progressively faded away within 20 min (Fig. 5e), which involves the dissociation of the cluster. Of note, an intermediate species (3a) appeared at 30 min, and can be assigned to [Cu12(PTC4A)(TC4A)(Cbz-PrA)4H]+. This species 3a is the mono-substituted product of Cu12a, in which one of the TC4A4- ligands is replaced by PTC4A4-. After 80 min, we observed a species 2j, [Cu12(PTC4A)2(Cbz-PrA)4(CH3OH)(H2O)2K]+, which can be identified as the bis-substituted product of Cu12a. This result indicates that Cu12b is more thermodynamically stable than Cu12a, which also was confirmed by DFT calculations (Supplementary Fig. 11). Moreover, the collision-induced dissociation (CID) mass further demonstrated the higher stability of Cu12b than Cu12a in gas phase (Please see details in the Supplementary Figures). More formula assignments for the above species in ESI-MS were performed as listed in Supplementary Table 3.
The transformation between the two Cu12 clusters was initially presumed to be a simple ligand-exchange process based on the single crystal structure and optical monitoring. However, further investigation using ESI-MS tracking revealed that this transformation was not a straightforward ligand-substitution, but rather a kinetically driven dissociation of the initial cluster, followed by a reassembly to form a thermodynamically stable new cluster. Thus, we have established the dissociation-reassembly (DR) mechanism to describe above transformation process, highlighting the dynamic nature of the dissociation and subsequent reassembly steps. The DR mechanism provides a conceptual framework to understand the intricate dynamics involved in the transformation of Cu12a to the more stable Cu12b42,43,44,45,46,47,48,49,50.
Considering the deep cavity of PTC4A4‒, we further checked the packing formed by Cu12 cluster in Cu12b. There is one THF molecule residing in the cavity of each PTC4A4‒ in Cu12b, which reduces the void produced by PTC4A4‒ ligand itself, so the Cu12b is a nonporous structure. The activated experiment of Cu12b was further carried out, and the activated sample namely Cu12b-NACs had been successfully removed the guest THF solvents (Supplementary Fig. 12). Of note, Cu12b-NACs also undergoes a SC-SC phase transition accompanied by a trebling of the crystallographic volume. Due to the removal of THF molecules in both lattice and the cavity of PTC4A4‒, the Cu12 clusters are shifted to more closer, producing deeper interpenetration between the upper phenyl rings of adjacent PTC4A4‒ compared to those in Cu12b, where the THF molecules block interpenetration (Supplementary Fig. 13). Thus, the Cu12b-NACs can be viewed as cluster-based nonporous adaptive crystals (NACs), which are relatively nonporous but with medium surface areas51,52,53,54,55,56. In light of this, the analysis of N2 adsorption isotherm of Cu12b-NACs gives an experimental Brunauer-Emmett-Teller (Langmuir) surface area of 14.88 (12.63) m2·g-1, which is less than half of that of Cu12a-π, and the pore-size distribution exhibits broad and ambiguous peak (Supplementary Fig. 14), which further verifies that Cu12b-NACs acting as the NAC features medium surface area and non-porosity. We also performed the gas adsorption experiments of Cu12b-NACs at 273 K, and the uptake amounts of CH4, CO2, C2H4 and C2H6 decreased a half compared to that of Cu12a-π. This observation fits the fact of condensed stacking of crystallographic structure.
Iodine adsorption
It is well known that capturing iodine with high adsorption capacity from aqueous solution remains a challenge for most porous materials57,58,59,60,61,62,63,64. Benefiting from the permanent porosity of Cu12a-π and the potentially adaptive absorption capacity of Cu12b-NACs, the capacities of iodine uptake would be worthy of further investigation, especially in aqueous solution. Hence, adsorption experiments involved with saturated aqueous solutions of iodine were performed. In this case, the UV‒Vis spectra of this aqueous solutions show a weakening of the absorption profile with time in the presence of Cu12a-π and Cu12b-NACs (Fig. 6a, b and Supplementary Fig. 15–17). The Cu12a-π needs much more time to reach equilibrium, but shows higher uptake efficiency compared to Cu12b-NACs. Wherein, Cu12a-π shows an iodine adsorption efficiency of 97.2% from aqueous solution within 60 min, with a total iodine uptake of 2.96 g·g‒1, which was superior to most reported iodine adsorbents (Supplementary Table 4). Although, because of the relatively nonporous feature caused by the offset of p-phenyl-thiacalix[4]arene on the packing of Cu12b-NACs, the uptake efficiency of iodine was about 89.42%, with an equilibrium uptake of 2.73 g·g‒1. Therefore, we can draw a preliminary conclusion that the iodine adsorption sites of the Cu12a-π and Cu12b-NACs are not only the cavity of the supramolecular framework, but also are related to the surface areas of crystal. The scanning electron microscopy (SEM) image of I2@Cu12a-π showed the crystal-like morphology (Fig. 6c), and the PXRD pattern of Cu12a-π soaked in aqueous solution shows similar diffraction peaks to Cu12a-π, indicating its excellent framework stability. Of note, I2@Cu12a-π still shows obvious diffraction peaks, suggesting that the framework of Cu12a-π was not interrupted after iodine adsorption (Fig. 6d). Besides, the Cu12a-π also shows some degree of recyclable ability and can be further reused for additional iodine adsorption tests via soaking I2@Cu12a-π in EA solvent (Supplementary Fig. 18).
a Time-dependent adsorption profiles for the Cu12a-π and Cu12b-NACs (0.1 mg·mL‒1) when immersed in 30 mL iodine aqueous solutions (1.2 mM). b UV‒Vis spectra of saturated iodine aqueous solutions before and after adsorption by Cu12a-π and Cu12b-NACs, respectively; the inset shows the color changes of the iodine aqueous solution after adsorption by Cu12a-π and Cu12b-NACs, respectively. c SEM and EDX images of I2@Cu12a-π, scan bar = 100 μm. d PXRD patterns of Cu12a-π, Cu12a-π in H2O and I2@Cu12a-π. e UV‒Vis spectra of Cu12a-π, I2@Cu12a-π and I2. The inset shows color change of the Cu12a-π before (left) and after (right) adsorbing iodine. f XPS spectrum of I2@Cu12a-π.
Due to its relatively high iodine adsorption capacity, Cu12a-π was chosen as the ideal sample to verify that the iodine was present as polyiodide ions after adsorption. For this, the UV‒Vis absorption, energy-dispersive x-ray spectroscopy were carried out. The UV‒Vis absorption spectra of Cu12a-π and the pristine iodine showcase a significantly different absorption profile compared to I2@Cu12a-π (Fig. 6e) in the solid state. Both the Cu12a-π and pristine iodine exhibit weak absorption intensity beyond 900 nm, yet the latter exhibits a broad tail peak over the 1000 nm. The strong absorption of I2@Cu12a-π in near-infrared region may be attributed to the charge transfer between polyiodide anions (mainly I3− and I5−) and Cu12a-π, which has been reported previously for iodine adsorption materials65,66. Moreover, the I3d X-ray photoelectron spectrum (XPS) of I2@Cu12a-π was measured, and two broad peaks at ~620 and ~632 eV indicate the presence of the I3‒ and I5‒ anions, respectively (Fig. 6f).
Discussion
In summary, we have successfully developed a dynamic and transformable 3D PSF (Cu12a-π) with cluster-based building units held together through intralayer non-covalent C-H··π interactions. The Cu12a-π is successfully constructed through the SC-SC transformation by the removal of guest molecules from pristine Cu12a, showing unexpected structural dynamics and superior thermal stability than most reported π-stacked frameworks. Moreover, the changing in upper rim of thiacalix[4]arene, Cu12a-π can be transformed into the nonporous adaptive crystal Cu12b-NACs following a DR mechanism, realizing the structural and performance updates. Based on the permanent porosity, Cu12a-π can absorb iodine in aqueous solution reaching a high capacity of 2.96 g·g‒1. Overall, all unique structural features as well as the iodine adsorption performance of such cluster-based PSF are indicative of a bright future of this type of porous materials.
Methods
Syntheses of the As-prepared PSFs
Cu12a
Cbz-PrACu (5.00 mg, 0.02 mmol) and H4TC4A (13.0 mg, 0.02 mmol) were mixed in 6.00 mL methanol/ethyl acetate (v:v = 3:3). The resulting suspension was sealed and heated at 80 °C for 2000 min. After cooling to room temperature, red crystals of Cu12a were formed (4.57 mg; yield: 91%). Selected IR peaks (cm−1): 2950 (s), 2906 (w), 2860 (w), 1732 (s), 1584 (m), 1450 (s), 1364 (m), 1315 (m), 1257 (s), 1208 (m), 1037 (m), 926 (w), 885 (w), 840 (s), 751 (s), 711 (s), 625 (w), 531 (m).
Cu12b
The crystals of Cu12a (4.57 mg, 1.52 μmol) were dissolved in mixed solvents of 6.00 mL isopropanol/tetrahydrofuran (v:v = 3:3), then H4PTC4A (16.0 mg, 0.02 mmol) was added successively. The resulting suspension was sealed and heated at 80 °C for 2000 min. After cooling to room temperature, red crystals of Cu12b were formed (2.07 mg; yield: 43%). Selected IR peaks (cm−1): 2955 (s), 2901 (w), 2865 (w), 1777 (w), 1589 (m), 1454 (s), 1364 (m), 1311 (s), 1255 (s), 1202 (m), 916 (w), 876 (w), 831 (s), 741 (s), 714 (s), 616 (w), 526 (m).
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information files. Other relevant data are available from the corresponding author upon request. The raw data for the TGA, DSC, N2 adsorption and desorption isotherms are provided with Supplementary Data 1-3. Source data depicting the coordinates of the optimized structures are present Source Data file. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC: 2234029-2234033 for Cu12a, Cu12a-π, Cu12a-readsorption, Cu12b and Cu12b-NACs. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Kitagawa, S. Future porous materials. Acc. Chem. Res. 50, 514–516 (2017).
Li, Z., He, T., Gong, Y. & Jiang, D. Covalent organic frameworks: pore design and interface engineering. Acc. Chem. Res. 53, 1672–1685 (2020).
Yu, J., Corma, A. & Li, Y. Functional porous materials chemistry. Adv. Mater. 32, e2006277 (2020).
Huang, S., Chen, G. & Ouyang, G. Confining enzymes in porous organic frameworks: from synthetic strategy and characterization to healthcare applications. Chem. Soc. Rev. 51, 6824–6863 (2022).
Serrano-Plana, J. Double-walled design for tetrahedral cages. Nat. Chem. 13, 729 (2021).
Armstrong, G. The Li-ions share. Nat. Chem. 11, 1076–1078 (2019).
di Nunzio, M. R., Hisaki, I. & Douhal, A. HOFs under light: relevance to photon-based science and applications. J. Photochem. Photobiol. C. 47, 100418 (2021).
Hisaki, I., Xin, C., Takahashi, K. & Nakamura, T. Designing hydrogen-bonded organic frameworks (HOFs) with permanent porosity. Angew. Chem. Int. Ed. 58, 11160–11170 (2019).
Lin, R. B. et al. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 48, 1362–1389 (2019).
Zhang, H. et al. NIR-II hydrogen-bonded organic frameworks (HOFs) used for target-specific amyloid-β photooxygenation in an alzheimer’s disease model. Angew. Chem. Int. Ed. 61, e202109068 (2022).
Abramov, P. A., Novikov, A. S. & Sokolov, M. N. Interactions of aromatic rings in the crystal structures of hybrid polyoxometalates and Ru clusters. CrystEngComm 23, 6409–6417 (2021).
Deng, J.-H. et al. π-π stacking interactions: non-negligible forces for stabilizing porous supramolecular frameworks. Sci. Adv. 6, eaax9976 (2020).
Fukunaga, H. & Miyasaka, H. Magnet design by integration of layer and chain magnetic systems in a π-stacked pillared layer framework. Angew. Chem. Int. Ed. 54, 569–573 (2015).
Gass, I. A., Moubaraki, B., Langley, S. K., Batten, S. R. & Murray, K. S. A π-π 3D network of tetranuclear μ2/μ3-carbonato Dy(III) bis-pyrazolylpyridine clusters showing single molecule magnetism features. Chem. Commun. 48, 2089–2091 (2012).
Cai, S. et al. Hydrogen-bonded organic aromatic frameworks for ultralong phosphorescence by intralayer π-π interactions. Angew. Chem. Int. Ed. 57, 4005–4009 (2018).
Sozzani, P., Comotti, A., Bracco, S. & Simonutti, R. A family of supramolecular frameworks of polyconjugated molecules hosted in aromatic nanochannels. Angew. Chem. 116, 2852–2857 (2004).
Whitesides, G. M. et al. Noncovalent synthesis: using physical-organic chemistry to make aggregates. Acc. Chem. Res. 28, 37–44 (1995).
Zang, L., Che, Y. & Moore, J. S. One-dimensional self-Assembly of planar π-conjugated molecules: adaptable building blocks for organic nanodevices. Acc. Chem. Res. 41, 1596–1608 (2008).
Qi, X.-L. et al. A flexible metal azolate framework with drastic luminescence response toward solvent vapors and carbon dioxide. Chem. Sci. 2, 2214–2218 (2011).
Wei, Y.-S. et al. Turning on the flexibility of isoreticular porous coordination frameworks for drastically tunable framework breathing and thermal expansion. Chem. Sci. 4, 1539–1546 (2013).
Yot, P. G. et al. Large breathing of the MOF MIL-47(VIV) under mechanical pressure: a joint experimental-modelling exploration. Chem. Sci. 3, 1100–1104 (2012).
Meng, D. et al. Non-covalent π-stacked robust topological organic framework. PNAS 117, 20397 (2020).
Yang, Y. et al. Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism. Nat. Chem. 13, 933–939 (2021).
Chen, T. et al. Adsorptive separation of aromatic compounds from alkanes by π–π interactions in a carbazole-based conjugated microporous polymer. ACS Appl. Mater. Interf. 12, 56385–56392 (2020).
Bi, Y., Du, S. & Liao, W. p-tert-Butylthiacalix[4]arene-supported high-nuclearity {Co24M8} (M = Mo or W) nanospheres and the hybrids with keggin polyoxometalates. Chem. Commun. 47, 4724–4726 (2011).
Bi, Y., Wang, S., Liu, M., Du, S. & Liao, W. A tetragonal prismatic {Co32} nanocage based on thiacalixarene. Chem. Commun. 49, 6785–6787 (2013).
Bi, Y. et al. Making a [Co24] metallamacrocycle from the shuttlecock-like tetranuclear cobalt-calixarene building blocks. Chem. Commun. 46, 6362–6364 (2010).
Liu, M., Liao, W., Hu, C., Du, S. & Zhang, H. Calixarene-based nanoscale coordination cages. Angew. Chem. Int. Ed. 51, 1585–1588 (2012).
Xiong, K. et al. Truncated octahedral coordination cage incorporating six tetranuclear-metal building blocks and twelve linear edges. Chem. Sci. 3, 2321–2325 (2012).
Hietsoi, O., Filatov, A. S., Dubceac, C. & Petrukhina, M. A. Structural diversity and photoluminescence of copper(I) carboxylates: from discrete complexes to infinite metal-based wires and helices. Coord. Chem. Rev. 295, 125–138 (2015).
Zhang, C. et al. Solvent-induced isomeric Cu13 nanoclusters: chlorine to copper charge transfer boosting molecular oxygen activation in sulfide selective oxidation. ACS Nano 16, 9598–9607 (2022).
Hosono, N., Terashima, A., Kusaka, S., Matsuda, R. & Kitagawa, S. Highly responsive nature of porous coordination polymer surfaces imaged by in situ atomic force microscopy. Nat. Chem. 11, 109–116 (2019).
Carrington, E. J. et al. Solvent-switchable continuous-breathing behaviour in a diamondoid metal-organic framework and its influence on CO2 versus CH4 selectivity. Nat. Chem. 9, 882–889 (2017).
Mason, J. A. et al. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015).
Férey, G. & Serre, C. Large breathing effects in three-dimensional porous hybrid matter: facts, analyses, rules and consequences. Chem. Soc. Rev. 38, 1380–1399 (2009).
Schneemann, A. et al. Flexible metal-organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Huang, J.-H., Wang, Z.-Y., Zang, S.-Q. & Mak, T. C. W. Spontaneous resolution of chiral multi-thiolate-protected Ag30 nanoclusters. ACS Cent. Sci. 6, 1971–1976 (2020).
Liao, X., Fu, H., Yan, T. & Lei, J. Electroactive metal-organic framework composites: design and biosensing application. Biosens. Bioelectron. 146, 111743 (2019).
Zhang, L. et al. Cooperative sieving and functionalization of Zr metal-organic frameworks through insertion and post-modification of auxiliary linkers. ACS Appl. Mater. Interfaces 11, 22390–22397 (2019).
Yin, W. et al. The tuning of optical properties of nanoscale MOFs-based thin film through post-modification. Nanomaterials 7, 242–253 (2017).
Zhang, L. et al. Post-modification of a MOF through a fluorescent-labeling technology for the selective sensing and adsorption of Ag+ in aqueous solution. Dalton Trans. 41, 10153–10155 (2012).
Guan, Z. J. et al. Isomerization in alkynyl-protected gold nanoclusters. J. Am. Chem. Soc. 142, 2995–3001 (2020).
Kang, X. & Zhu, M. Transformation of atomically precise nanoclusters by ligand-exchange. Chem. Mater. 31, 9939–9969 (2019).
Carducci, T. M., Blackwell, R. E. & Murray, R. W. Charge-transfer effects in ligand exchange reactions of Au25 monolayer-protected clusters. J. Phys. Chem. Lett. 6, 1299–1302 (2015).
Chen, Y. X. et al. Isomerism in Au28(SR)20 nanocluster and stable structures. J. Am. Chem. Soc. 138, 1482–1485 (2016).
Dhayal, R. S. et al. Diselenophosphates-induced conversion of an achiral [Cu20(H)11{S2P(OiPr)2}9] into a chiral [Cu20(H)11{Se2P(OiPr)2}9] polyhydrido nanocluster. Angew. Chem. Int. Ed. 54, 13604–13608 (2015).
Heinecke, C. L. et al. Structural and theoretical basis for ligand exchange on thiolate monolayer protected gold nanoclusters. J. Am. Chem. Soc. 134, 13316–13322 (2012).
Liu, C. et al. Establishing porosity gradients within metal–organic frameworks using partial postsynthetic ligand exchange. J. Am. Chem. Soc. 138, 12045–12048 (2016).
Wang, Y. et al. An intermetallic Au24Ag20 superatom nanocluster stabilized by labile ligands. J. Am. Chem. Soc. 137, 4324–4327 (2015).
Yang, S. et al. In situ two-phase ligand exchange: a new method for the synthesis of alloy nanoclusters with precise atomic structures. J. Am. Chem. Soc. 139, 5668–5671 (2017).
Li, B. et al. Capture of sulfur mustard by pillar[5]arene: from host-guest complexation to efficient adsorption using nonporous adaptive crystals. iScience 23, 101443 (2020).
Luo, D., He, Y., Tian, J., Sessler, J. L. & Chi, X. Reversible iodine capture by nonporous adaptive crystals of a bipyridine cage. J. Am. Chem. Soc. 144, 113–117 (2022).
Luo, D., Tian, J., Sessler, J. L. & Chi, X. Nonporous adaptive calix[4]pyrrole crystals for polar compound separations. J. Am. Chem. Soc. 143, 18849–18853 (2021).
Sheng, X. et al. Separation of 2-chloropyridine/3-chloropyridine by nonporous adaptive crystals of pillararenes with different substituents and cavity sizes. J. Am. Chem. Soc. 142, 6360–6364 (2020).
Wu, J. R. & Yang, Y. W. Synthetic macrocycle-based nonporous adaptive crystals for molecular separation. Angew. Chem., Int. Ed. 60, 1690–1701 (2021).
Zhou, J., Yu, G., Li, Q., Wang, M. & Huang, F. Separation of benzene and cyclohexane by nonporous adaptive crystals of a hybrid[3]arene. J. Am. Chem. Soc. 142, 2228–2232 (2020).
Wang, G.-Q. et al. A hydrolytically stable cage-based metal–organic framework containing two types of building blocks for the adsorption of iodine and dyes. Inorg. Chem. Front. 8, 1083–1092 (2021).
Qu, G. et al. Rapid iodine capture from radioactive wastewater by green and low-cost biomass waste derived porous silicon–carbon composite. RSC Adv. 11, 5268–5275 (2021).
Alsalbokh, M., Fakeri, N., Lawson, S., Rownaghi, A. A. & Rezaei, F. Adsorption of iodine from aqueous solutions by aminosilane-grafted mesoporous alumina. Chem. Eng. J. 415, 128968–128975 (2021).
Jie, K. et al. Reversible iodine capture by nonporous pillar[6]arene crystals. J. Am. Chem. Soc. 139, 15320–15323 (2017).
Li, B. et al. Terphen[n]arenes and quaterphen[n]arenes (n=3-6): one-pot synthesis, self-assembly into supramolecular gels, and iodine capture. Angew. Chem., Int. Ed. 58, 3885–3889 (2019).
Lin, Y. et al. An elastic hydrogen-bonded cross-linked organic framework for effective iodine capture in water. J. Am. Chem. Soc. 139, 7172–7175 (2017).
Xie, L. et al. Calix[4]pyrrole-based crosslinked polymer networks for highly effective iodine adsorption from water. Angew. Chem., Int. Ed. 134, e202113724 (2022).
Yin, Z., Wang, Q.-X. & Zeng, M.-H. Iodine release and recovery, influence of polyiodide anions on electrical conductivity and nonlinear optical activity in an interdigitated and interpenetrated bipillared-bilayer metal–organic framework. J. Am. Chem. Soc. 134, 4857–4863 (2012).
Xia, Y., Wiesinger, J. M., MacDiarmid, A. G. & Epstein, A. J. Camphorsulfonic acid fully doped polyaniline emeraldine salt: conformations in different solvents studied by an ultraviolet/visible/near-infrared spectroscopic method. Chem. Mater. 7, 443–445 (1995).
Tigelaar, D. M. et al. Role of solvent and secondary doping in polyaniline films doped with chiral camphorsulfonic acid: preparation of a chiral metal. Chem. Mater. 14, 1430–1438 (2002).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 22171164, 22325105 and 52261135637 to D.S., 22201159 to Z.W.), the Fok Ying Tong Education Foundation (171009), the Natural Science Foundation of Shandong Province (Nos. ZR2019ZD45, ZR2020ZD35, JQ201803, and ZR2017MB061), the Taishan Scholar Project of Shandong Province of China (Nos. tsqn201812003 and ts20190908), the National Postdoctoral Innovative Talents Support Program (No. BX2021171), China Postdoctoral Science Foundation (No. 2021M700081).
Author information
Authors and Affiliations
Contributions
Original idea was conceived by D.S., experiments and data analyses were performed by C.Z., Z.W., W.-D.S., H.C., L.Z., T.L., X.-Q.H., Z.-Y.G., C.-H.T., M.A., P.C. and D.S., structure characterizations were performed by C.Z. and D.S., manuscript was drafted by C.Z. and D.S. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiaodong Chi, You-Gui Huang, Sergei Tretiak and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, C., Wang, Z., Si, WD. et al. Dynamic and transformable Cu12 cluster-based C-H···π-stacked porous supramolecular frameworks. Nat Commun 14, 6413 (2023). https://doi.org/10.1038/s41467-023-42201-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42201-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.