Abstract
Disease-causing mutations in genes encoding transcription factors (TFs) can affect TF interactions with their cognate DNA-binding motifs. Whether and how TF mutations impact upon the binding to TF composite elements (CE) and the interaction with other TFs is unclear. Here, we report a distinct mechanism of TF alteration in human lymphomas with perturbed B cell identity, in particular classic Hodgkin lymphoma. It is caused by a recurrent somatic missense mutation c.295 T > C (p.Cys99Arg; p.C99R) targeting the center of the DNA-binding domain of Interferon Regulatory Factor 4 (IRF4), a key TF in immune cells. IRF4-C99R fundamentally alters IRF4 DNA-binding, with loss-of-binding to canonical IRF motifs and neomorphic gain-of-binding to canonical and non-canonical IRF CEs. IRF4-C99R thoroughly modifies IRF4 function by blocking IRF4-dependent plasma cell induction, and up-regulates disease-specific genes in a non-canonical Activator Protein-1 (AP-1)-IRF-CE (AICE)-dependent manner. Our data explain how a single mutation causes a complex switch of TF specificity and gene regulation and open the perspective to specifically block the neomorphic DNA-binding activities of a mutant TF.
Similar content being viewed by others
Introduction
Deregulated transcription factor (TF) activities are major contributors towards malignant transformation, as particularly exemplified by various hematopoietic malignancies. One inherent feature of disturbed TF activities is the deregulation of cellular processes such as lineage maintenance, differentiation, growth, and survival, thus promoting oncogenic transformation1,2,3. Mutations targeting TF DNA-binding motifs can affect TF:DNA interaction and/or TF functionality4,5,6, but it is currently unclear whether such mutations can influence the interaction with other TFs and thus impact upon the nature of binding to TF Composite Elements (CEs). TF binding to DNA frequently involves the formation of multimeric complexes binding to CEs which display much higher affinity-binding compared to any of the partners binding alone7,8. For example, the Activator Protein-1 (AP-1) TF family typically binds as JUN/FOS dimer to the palindromic sequence 5′-TGASTCA-3′. However, the affinity of AP-1 DNA-binding is greatly increased when its binding is enhanced by contacts with the Nuclear Factor of Activated T cells (NFAT) at the respective CE. This CE reduces the extent to which AP-1 sites must conform to the consensus required for AP-1 alone, but also renders these sites dependent upon additional Ca2+ signaling9,10.
TF binding to DNA is a complex process, with arginine (Arg; R)-residues playing an important role in protein-DNA recognition11,12. For example, a cluster of loss-of-function TP53 mutations affects various R-residues central to TP53:DNA interaction13. Also, TF Interferon Regulatory Factor 4 (IRF4) contains an arginine at amino acid (AA) position 98 in the α3-helix of its DNA-binding domain (DBD), which is essential for its interaction with DNA14.
The expression of the IRF family member IRF4 is largely restricted to immune cells, where it exerts key regulatory functions15,16. IRF4 not only plays important roles in the activation of both B- and T-lymphoid cells, but is also required for the generation of germinal center (GC) B cells and orchestrates terminal B-cell differentiation, i.e., the formation of plasma cells17,18,19. Apart from these functions during normal lymphopoiesis, IRF4-dependency is a characteristic of various hematopoietic malignancies, including multiple myeloma, diffuse large B-cell lymphoma (DLBCL) subtypes, or T-cell lymphoma entities20,21,22. The strength of binding of IRF4 to its cognate DNA-binding motifs depends on the one hand on its expression levels23,24, but also on the interaction with other TFs binding to CEs, as shown for Erythroblast transformation-specific (ETS)-IRF (EICE)25 or AP-1-IRF CEs (AICE)26,27,28. The extent and nature of these interactions define the specificity and strength of IRF4-directed transcriptional regulation in a given cell type23,24. High-level IRF4 expression is characteristic for the Hodgkin/Reed-Sternberg (HRS) tumor cells of classic Hodgkin lymphoma (cHL), a common human B-cell-derived malignancy29. However, HRS cells lack the IRF4-instructed terminal B-cell differentiation gene expression program, including plasma cell genes30,31, and instead up-regulate genes characteristic of other cellular lineages30,32,33. Furthermore, HRS cells are surrounded by an inflammatory cellular infiltrate attracted by abundantly produced cytokines, chemokines and cell surface receptors30. Only very few genetic events driving these features of Hodgkin lymphoma cells are known.
Here, we describe a distinct mechanism of TF alteration in human lymphomas, particularly cHL, involving a recurrent somatic missense mutation c.295 T > C (p.Cys99Arg; p.C99R) that targets the center of the DNA-binding domain of IRF4. We show that IRF4-C99R results in fundamental changes in IRF4′s DNA-binding properties, combining loss-of-binding to canonical IRF motifs and neomorphic gain-of-binding to canonical and non-canonical IRF CEs. Functionally, we demonstrate that IRF4-C99R blocks IRF4-dependent plasma cell induction and up-regulates disease-specific genes in a non-canonical Activator Protein-1 (AP-1)-IRF-CE (AICE)-dependent manner. Our data explain how a single mutation causes a complex switch of TF specificity and gene regulation.
Results
The IRF4-C99R mutation is recurrent in human lymphoma
By mining and integrating both our own and additional published genomic and transcriptional data from well-characterized cHL cell lines, we identified and verified the same c.295 T > C (chr6:394,899 T > C; hg38) variant in the IRF4 gene in 2 of 7 HL cell lines, namely the B-cell-derived HRS cell lines L428 and U-HO1 (Fig. 1a and Supplementary Fig. 1a). Based on various in silico analyses integrated in ANNOVAR (including SIFT, Polyphen2, MutationTaster, FATHMM, CADD score), this variant was uniformly predicted to be deleterious (Supplementary Table 1) and is completely absent in germline genomic databases (gnomAD, accessed 2022/06/16). Furthermore, no germline nonsynonymous single nucleotide variants were collated in gnomAD affecting the neighboring AAs 90–104 with the exception of a singleton allele (1/251478) carrying a missense mutation in AA100. In the HL cell lines, the c.295 T > C mutant allele was accompanied by at least one wild-type (WT) copy of the IRF4 gene, and both WT and mutant IRF4 mRNA transcripts were equally detected (Fig. 1a and Supplementary Fig. 1a). Since HRS cells are rare in the affected lymph nodes, we validated the presence of IRF4 c.295 T > C in 4 of 20 primary cHL samples representing 3 of 19 cases (16%) by DNA-PCR of laser-microdissected HRS cells (Supplementary Table 2). The S104T mutation identified in L428 cells (Supplementary Fig. 1a) was not found in the primary cases, and thus not considered as recurrent. IRF4 c.295 T > C has recently been described in Primary Mediastinal B Cell Lymphoma (PMBCL)34, a lymphoma entity that shares distinct biological features with cHL. Parallel mining of targeted gene panel sequencing data from an unrelated large cohort of 486 PMBCL cases identified the same IRF4 c.295 T > C mutation in 29 of the 486 cases (5.9%) (Supplementary Fig. 1b). In contrast, IRF4 c.295 T > C is only rarely documented in other lymphoma types such as DLBCL (Supplementary Fig. 1b; refs. 35,36,37). Furthermore, the genomic location of the C99R c.295 T > C (chr6:394,899 T > C; hg38) mutation is within exon 3, and thus located >3 kb downstream of the transcription initiation site (TIS). In addition, it lacks the typical hotspot RGYW motif, indicating that this mutation is not caused by aberrant somatic hypermutation in B-cell lymphoma, which usually affects regions spanning about 2–2.5 kb downstream from the TIS38,39.
a Top, scheme of IRF4 with indication of p.Cys99Arg within the DBD. Bottom, IRF4 Sanger sequencing reads of L428 DNA (left) and cDNA (right). DBD DNA-binding domain, LD linker domain, IAD IRF association domain. b EMSA using ISRE probe and nuclear extracts of HEK293 cells transfected with IRF4-WT or mutants thereof. M, Mock control; C99A, R98A, and R98AC99A, loss-of-function mutants. Red arrow, IRF4–DNA complex. ss, supershift. ns nonspecific. Data are representative of at least three independent experiments. c BJAB cells were analyzed for gene expression changes following tet-induction of the respective IRF4 variants for the indicated times. Hierarchical clustering of 348 differentially expressed genes that change expression in either IRF4-WT or IRF4-C99R vs. Mock (M) cells. Dox doxycycline. Log2 fold changes (FC) of at least twofold are indicated in the heatmap. Experiments were performed in biologically independent duplicates and log2 FC values are from two averaged replicates per condition. d Nuclear extracts of HEK293 cells transfected with JUNB and BATF and increasing amounts of IRF4 variants were analyzed by EMSA for binding at AICE1 (AICE1 (Ctla4) and AICE1 (IL12Rb)) and AICE2 (AICE (Bcl11b)) probes. BP binding pattern. Red and blue arrows mark positions of IRF4-JUNB/BATF-DNA and JUNB/BATF-DNA complexes, respectively. EMSA of SP1 is shown as a control. Data are representative of at least three independent experiments. e EMSAs using wild-type AICE2 (BCL11b) probe (left) or variants thereof with reverse complement IRF motif (center left, AICE2FLIP), or IRF motifs positioned 3′ relative the AP-1 motif (center right and right). Extracts and complex positions are as in (d). Data are representative of at least three independent experiments. f Binding of JUNB/BATF together with increasing amounts of IRF4-WT or IRF4-C99R to AICE2 (Bcl11b) with a T > C mutant thereof at position -4 relative to the IRF motif. Extracts and complex positions are as in (d). Data are representative of at least three independent experiments. g IRF4-C99R docking to ISRE. This modeling is based on the previous crystal structure of IRF4-WT:ISRE DNA (PDB: 7JM4), in which C99 was substituted with R. ISRE-sequence is shown underneath; numbered bases of the upper strand shown in the image above. Blue/slate, IRF4 (both WT and C99R); orange, DNA phosphate backbone; pale green, dA and dT; gray, dG and dC. Source data for (b, d–f) are provided in the Source Data file.
IRF4 governs the plasma cell gene expression program at the stage of terminal B-cell differentiation40, which largely lacks in HRS cells31 despite high-level IRF4 expression across all subtypes (Supplementary Fig. 1c, d). In the IRF4 c.295 T > C mutation, the basic AA arginine replaces the neutral AA cysteine (Cys; C) (p.Cys99Arg; C99R) at position AA 99, which is highly conserved in IRF4 from humans to zebrafish and also within the DBD of most other IRF family proteins (Fig. 1a and Supplementary Fig. 1e). C99R is located in the center of the α3-recognition helix of the DBD of IRF4 and is positioned immediately adjacent to Arg98, which is essential for specific IRF4 DNA-binding14. This finding suggested that C99R might interfere with the formation of IRF4:DNA complexes and thus with IRF4′s transcriptional activity.
IRF4-C99R shows loss-of-function at ISRE but is functionally active
To characterize IRF4-C99R, we first explored its DNA-binding activity to the Interferon-Stimulated Response Element (ISRE) containing three consensus motifs 5′-GAAA-3′ (Fig. 1b and Supplementary Fig. 1f), one of the key motifs recognized by IRFs41,42. Unlike IRF4-WT, IRF4-C99R did not bind to the ISRE at all, as demonstrated by Electrophoretic Mobility Shift Assay (EMSA). However, the recurrent nature of IRF4-C99R mutation and high-level expression in cHL suggested that this mutation may not merely constitute a loss-of-function aberration, but could possess additional, de novo functions. To analyze IRF4-C99R functionality, we generated tetracycline (Tet)-inducible IRF4-C99R and IRF4-WT expressing bulk cultures of BJAB B-cell non-Hodgkin lymphoma cells, which express endogenous IRF4 only at a low level (Supplementary Fig. 2a). Time course gene expression analyses revealed that IRF4-C99R altered the expression of a distinct, albeit fewer set of genes compared to IRF4-WT (Fig. 1c, Supplementary Fig. 2b–e, and Supplementary Data 1). Notably, IRF4-C99R was unable to induce plasma cell-specific genes (Supplementary Fig. 2f), in agreement with its lost ability to bind the canonical ISRE motif. IRF4-C99R rescued HRS cells as efficiently as IRF4-WT from cell death induced by small-hairpin RNA (shRNA)-mediated knock-down of endogenous IRF4 (Supplementary Fig. 2g) thus corroborating its functionality.
IRF4-C99R fundamentally modifies IRF4′s DNA-binding specificity
In contrast to the formation of low-affinity homodimer or multimeric complexes on ISRE DNA motifs, efficient IRF4 DNA-binding requires distinct partners such as ETS and AP-1 proteins at CEs25,26,27. Given the broad absence of ETS TFs in cHL43,44, we considered the binding of IRF4 to EICE in HRS cells as being unlikely. However, constitutive AP-1 activity with high-level JUNB and BATF expression is a hallmark of HRS cells45,46. We therefore speculated that IRF4-C99R regulates gene expression by DNA-binding to the recently identified AICEs, either 5′-IRF(TTTC)/nnnn/AP-1(TGASTCA)-3′ with a spacing of 4 bp (AICE1) or 5′-IRF(GAAA)/AP-1(TGASTCA)-3′ with no spacing (AICE2)26,47, which both regulate key transcriptional programs in immune cells16. To evaluate this hypothesis, we monitored the formation of IRF4-JUNB/BATF-DNA complexes at strong (labeled as “AICE1 (Ctla4)”), weak (AICE1 (IL12Rb)) or intermediate (AICE2 (Bcl11b)) affinity AICE motifs26 (Fig. 1d and Supplementary Fig. 3a–c). While we observed a complete loss of IRF4-C99R binding at AICE1 (Ctla4) and AICE1 (IL12Rb) (designated as AICE Binding Pattern 1 (BP1)), IRF4-C99R-JUNB/BATF binding at AICE2 (Bcl11b) was enhanced compared to IRF4-WT (BP2) (Fig. 1d). IRF4-C99RS104T behaved similar to IRF4-C99R (Fig. 1b, d and Supplementary Fig. 3a), and, as it is not a recurrent mutation, it was not included in further experiments. Strikingly, reverse complementing the IRF motif in AICE2 (Bcl11b) from 5′-GAAA-3′ to 5′-TTTC-3′ (referred to as AICE2FLIP) revealed formation of mutant IRF4-C99R-JUNB/BATF-DNA complexes only (Fig. 1e, AICE2FLIP, BP3; Supplementary Fig. 3d). Moreover, formation of AICE complexes usually requires a thymine located at −4 bp (-4T) relative to AICE2 (referred to as AICE2-4T)47. IRF4-C99R overrides this restriction, as it forms strong DNA-binding complexes in the absence of -4T, which causes loss of IRF4-WT binding (Fig. 1f; AICE2-4C; BP4). Similarly, altered binding patterns of IRF4-C99R compared to IRF4-WT were observed together with c-JUN (JUN)/BATF heterodimeric AP-1 complexes (Supplementary Fig. 3e, f).
Furthermore, we performed structural modeling analysis to provide additional information on how the IRF4-C99R mutation influences the interaction with the ISRE and AICE1 DNA-binding motifs (Fig. 1g, Supplementary Fig. 4, and Supplementary Table 3). For the structural models, the initial structures of IRF4 and DNA were obtained from our previous crystal structure (PDB:7JM4), and the most viable models were considered based on resultant docking parameters such as HADDOCK score, cluster size and desolvation energy. As shown in Fig. 1g for the IRF4:ISRE interaction, a direct replacement of C99 with Arg resulted in a steric clash with DNA bases. To accommodate the interaction between C99R and ISRE, the dsDNA had to either bend and/or kink as shown in the overlay model of WT/C99R with ISRE (Supplementary Fig. 4a). For IRF4-C99R:AICE1 binding, the poor docking scores (Supplementary Table 3) indicated that it was highly unlikely that a significant interaction could take place as reflected by the poor energy-minimized structure (Supplementary Fig. 4b). Thus, even though our AICE1-modeling might be limited due to DNA distortions, it suggests that IRF4-C99R does not bind to AICE1, which is consistent with our EMSA results.
These patterns of alterations of the IRF4-C99R DNA-binding properties were also observed with recombinant proteins comprising just the DBDs of IRF4 (AA 20–139), JUNB (AA 269–329), and BATF (AA 28–87) only (Supplementary Fig. 5a–c). In addition, we visualized the DNA-bound fraction of IRF4-C99R or IRF4-WT by single-molecule fluorescence microscopy and interlaced time-lapse illumination48, which revealed comparable percentages of long-bound DNA contacts (>2 s) of IRF4-C99R compared to IRF4-WT molecules (Supplementary Fig. 5d). Together, our data demonstrate a unique combined loss-and-gain of DNA-binding preferences by IRF4-C99R, and, in particular, neomorphic binding activity to AICE2-like motifs.
Globally altered IRF4 DNA-binding patterns and cooperative activities in IRF4-C99R lymphoma cells
We next aimed to obtain global data supporting the above findings by interrogating our HL cell line models. To specifically map accessible chromatin in HRS cells in detail, we first generated high-resolution genome-wide DNaseI hypersensitive site (DHS) and digital footprinting data from the HRS cell lines L428, harbouring IRF4-C99R, and KM-H2, expressing IRF4-WT, as well as the non-Hodgkin, non-IRF4 expressing REH cells as a control (Supplementary Fig. 6a). The analyses of DNaseI cutting frequencies revealed protection against DNaseI digestion, indicative of occupancy by protein complexes, together with elevated accessibility of the flanking regions at AICE2 (BP2), AICE2FLIP (BP3), AICE2−4T and AICE2-4C (BP4) only in HRS cells (Fig. 2a). Notably, and in line with our DNA-binding experiments (see Fig. 1), these motifs were highest enriched and protected in L428IRF4-C99R (Fig. 2a). Co-localization analysis of these AICE2 motifs in L428IRF4-C99R cells revealed a specific cluster corresponding to mutant-specific sites co-localizing with AP-1 motifs but not with those of other TFs typically involved in B and HL cell gene regulation (Supplementary Fig. 6b, left), which was not observed in KM-H2IRF4-WT cells (Supplementary Fig. 6b, right). These findings again supported the idea of IRF4-C99R conferring cells with divergent expression profiles.
a Digital genomic footprinting (DGF) analyses showing occupancy at AICE2, AICE2FLIP, AICE2-4T, and AICE2-4C sites (horizontal) in L428IRF4-C99R HL cells, KM-H2IRF4-WT HL and REH non-Hodgkin cells (vertical). Red and blue lines represent the forward and reverse strands. b Correlation between L428IRF4-C99R/KM-H2IRF4-C99R DNaseI-Seq fold change defining three classes of elements, designated groups 1–3, (left) and log2 gene expression fold change (right). c Motif frequencies in DHSs defined in (b). d HOMER de novo motif discovery in specific DHSs defined in (b). e Heatmap showing Spearman′s correlation clustering from IRF4 and JUNB ChIP-Seq experiments on the union of IRF4 peaks from L428IRF4-C99R, KM-H2IRF4-WT and GM12878 cells. f Venn diagram-overlaps between IRF4 and JUNB ChIP peaks in GM12878, KM-H2IRF4-WT and L428IRF4-C99R cells. g L428/KM-H2 IRF4 ChIP peak fold change analyses (left) showing corresponding JUNB ChIP peaks (center), DHSs (right) as well as gene expression fold changes (far right). h Motif frequencies in KM-H2IRF4-WT-, shared and L428IRF4-C99R-specific ChIP peaks. i De novo motif discovery in specific ChIP-seq datasets using ExplaiNN. Motifs are ranked by their importance (left). When more than one motif of the same class was identified, the rank of the displayed motif is underlined. Only motifs that could be annotated with a biological representation are shown. All DGF/DNase-Seq/ChIP-Seq/RNA-Seq values are from two averaged replicates per condition. P values were determined by two-tailed unpaired t test without adjustment for multiple comparisons.
To define groups of L428 or KM-H2-specific DHSs, we determined the ratio of tag counts between L428IRF4-C99R and KM-H2IRF4-WT cells and ranked them according to their fold change in DNaseI-seq signal (Fig. 2b; groups 1–3). The L428IRF4-C99R-specific DHSs (i.e., group 3) correlated with upregulated gene expression in these cells (Fig. 2b). We then determined the enrichments of AICE2 (BP2), AICE2FLIP (BP3), AP-1 and ISRE motifs in the different DHS groups. L428IRF4-C99R-specific DHSs were enriched for AICE2, AICE2FLIP and AP-1 motifs, but depleted for ISRE motifs, whereas KM-H2IRF4-WT-specific DHSs were depleted for AICE2, AICE2FLIP and AP-1 motifs, but enriched for ISRE (Fig. 2c). An unbiased search for TF motifs in the cell line-specific DHSs using HOMER revealed AICE2 and AICE2FLIP as 2 of the most highly enriched motifs in L428IRF4-C99R-specific DHSs but not in KM-H2IRF4-WT-specific DHSs sites (Fig. 2d). Conversely, ISRE motifs were enriched in KM-H2IRF4-WT- but not L428IRF4-C99R-specific DHSs, again suggesting that IRF4-C99R shifts binding to distinct AICE2 motifs (Fig. 2d). Parallel DHS analyses comparing L428 versus REH (Supplementary Fig. 7a, b) or versus publicly available DHS data from lymphoblastoid GM12878 B cells (Supplementary Fig. 7c, d) also revealed specific enrichment of AICE2 motifs in L428IRF4-C99R-specific DHSs. Gene set enrichment analysis (GSEA) in DHSs from L428IRF4-C99R versus KM-H2IRF4-WT cells revealed an increased presence of footprinted AICE2 motif in upregulated genes (Supplementary Fig. 7e), arguing for the functional relevance of this motif in AICE2 L428IRF4-C99R cells.
A drawback of tools like HOMER is, that, although they are excellent at identifying global consensus binding motifs, they have difficulties in identifying different and slightly degenerate versions of the same motif, as found in CEs. In addition, these algorithms focus on the core motifs while ignoring the flanking nucleotides. To overcome these limitations, we used a novel deep-learning tool, ExplaiNN (explainable neural networks)49, to separately discover motifs de novo in the cell line-specific DHS datasets. This analysis confirmed that AICE1 (BP1) in KM-H2IRF4-WT and AICE2 (BP2) in L428IRF4-C99R were among the most important motifs (Supplementary Fig. 8a–c).
Finally, we performed genome-wide JUNB and IRF4 Chromatin Immunoprecipitation (ChIP)-Seq analyses in L428IRF4-C99R and KM-H2IRF4-WT cells (Fig. 2e–i and Supplementary Fig. 9a). We included publicly available IRF4 and JUNB ChIP-Seq data from GM12878 cells, since both IRF4 and JUNB are virtually not expressed in REH cells. Sequences within IRF4-JUNB ChIP peaks clustered closely together (Fig. 2e) and showed a greater overlap (Fig. 2f) in L428IRF4-C99R cells (Dice score: 0.7877) compared to KM-H2IRF4-WT and GM12878 cells (Dice scores: 0.4418 and 0.4478, respectively), in line with enforced binding of IRF4-C99R to IRF and AP-1 CEs. Although IRF4 ChIP peak frequency was higher in both HRS cell lines compared to GM12878 (Fig. 2f), the overlap with JUNB was much lower in KM-H2IRF4-WT cells. When individually ranked, IRF4 and JUNB showed highly similar binding patterns in L428IRF4-C99R but not in KM-H2IRF4-WT cells, corresponded to open chromatin regions and were associated with increased gene expression (Fig. 2g). Consistent with these analyses and motif discovery results from DHS datasets, de novo motif analyses by HOMER (Supplementary Fig. 9b) and supervised motif injection (Fig. 2h) showed increased frequencies of AICE2 (BP2) and AICE2FLIP (BP3) motifs in L428IRF4-C99R-specific IRF4 ChIP peaks, while conversely showing lower ISRE motif frequencies, when compared to KM-H2IRF4-WT specific ChIP peaks. These findings were also observed when IRF4 and JUNB chromatin binding patterns of L428 were compared against GM12878 cells (Supplementary Fig. 9c, d). Importantly, GSEA revealed that IRF4 and JUNB ChIP peaks were associated with increased gene expression in L428IRF4-C99R but not KM-H2IRF4-WT cells (Supplementary Fig. 9e).
Again, we performed de novo motif discovery using ExplaiNN, but this time in the ChIP-seq datasets, and found that AICE1 (BP1) was the most important motif in KM-H2IRF4-WT cells, but was not identified in L428IRF4-C99R cells (Fig. 2i and Supplementary Figs. 8a and 10). AICE2 (BP2) emerged among the most important motifs in both datasets, with more importance in L428IRF4-C99R cells, wherein a total of five motif types (vs one in KM-H2IRF4-WT) were identified (Fig. 2i and Supplementary Fig. 10). The analyses also revealed the unique importance of AICE2FLIP (BP3) in L428IRF4-C99R cells. These results agree with our DNA-binding studies (Fig. 1), and further support the notion that IRF4-C99R fundamentally alters IRF4 genome-wide DNA-binding patterns in lymphoma cells and enforces cooperative binding with AP-1/JUN TFs at distinct neo-AICEs.
IRF4-C99R disrupts IRF4 function and reprograms gene expression in primary B cells
To further explore the functional consequences of IRF4-C99R expression in B cells, we retrovirally transduced primary mouse C57BL/6 splenic B cells with IRF4-WT, IRF4-C99R, or the loss-of-function (LOF) variant IRF4-R98AC99A as a control (Fig. 3a and Supplementary Fig. 11a). Within the known IRF4-R98AC99A LOF variant, the residues R98 and C99, which are critically involved in the formation of IRF4:DNA complexes, were both replaced by alanin (A) abolishing IRF(4) DNA-binding and function14,50,51,52. Culturing of B cells with LPS and IL−4 led to robust endogenous IRF4 expression (Supplementary Fig. 11a) and resulted in induction of around 30% plasmablasts, characterized by a CD138high and B220low phenotype (Fig. 3a). The same result was obtained after ectopic expression of the non-functional IRF4-R98AC99A variant (Fig. 3a). Following ectopic expression of IRF4-WT, ~70% of the cells converted to a plasmablast phenotype. In contrast, IRF4-C99R reduced the number of developing plasmablasts, i.e., blocked inherent plasmablast formation, arguing for a dominant-negative function of IRF4-C99R with respect to terminal B-cell differentiation (Fig. 3a). To examine alterations in gene expression, we isolated mouse C57BL/6 splenic B cells transduced with the different IRF4 variants followed by RNA-seq analyses (Fig. 3b–f and Supplementary Data 2). Overall, the data from the respective transfectants clustered separately, with IRF4-C99R showing a transcriptional profile more similar to the R98AC99A LOF variant than to IRF4-WT (Fig. 3b). IRF4-C99R regulated a reduced set of genes (Fig. 3c), encompassing a broad loss of IRF4-WT target gene expression along with a gain of novel targets (Fig. 3d, Supplementary Fig. 11b, and Supplementary Data 2). Integration of the mRNA expression profiles of the modified splenic B cells with those from various hematopoietic cell types showed an IRF4-C99R-regulated block of overall IRF4-WT-induced and plasma cell-specific gene expression (Fig. 3e), confirming that IRF4-C99R is unable to instruct the IRF4-directed plasma cell program. Concomitantly, IRF4-C99R upregulated myeloid-associated genes (Fig. 3e, f), phenocopying a central feature of cHL tumor cells30,32. Together, these data confirmed the fundamental changes in IRF4-C99R-dependent gene regulation and function compared to IRF4-WT.
a Following culture with LPS + IL-4, C57BL/6 mouse splenic B cells were transduced with MIG control retrovirus (MIG-ctrl; Mock), IRF4-WT, IRF4-C99R or, as a further control, IRF4-R98AC99A. Transduced GFP+ cells were analyzed by flow cytometry for expression of CD138 and B220. Top left panels, the indication of the percentage of living transduced cells in representative FACS profiles. Bottom left panels, analysis of CD138 and B220 in gated GFP+ cells. The percentage of CD138highB220low cells is indicated. Right, the mean ± SEM of n = 4 independent experiments is shown. P values were determined by two-tailed unpaired Student′s t test; ns, not significant. b–f Isolated murine splenic B cells transduced with IRF4-WT, IRF4-C99R, IRF4-R98AC99A, or MIG control (Mock) were analyzed by RNA-Seq. Experiments were performed in biologically independent triplicates (n = 3). b Spearman correlation of the various samples. Note, that the Mock and the IRF4-R98AC99A-LOF transduced cells cluster together, and that IRF4-C99R clusters in between these and IRF4-WT samples. c Volcano plots of genes differentially regulated between IRF4-WT versus Mock and IRF4-C99R versus Mock (MIG-ctrl). Note, that IRF4-C99R regulates less genes compared to IRF4-WT. d IRF4-C99R and IRF-WT regulated genes show only low overlap, as shown in UpSet plots for overall differentially regulated genes (left, top panel) and upregulated genes (left, bottom panel), as well as in overall comparisons of complete transcriptomes (right panel). e Differentially regulated genes by IRF4-C99R were compared to gene expression of lymphoid and myeloid cell types. Note, that the IRF4-C99R-dowregulated genes correspond to genes expressed in plasma cells or IRF4-WT-induced genes (green rectangle), whereas the IRF4-C99R-upregulated genes show specific expression in myeloid cells (red rectangle). GMP granulocyte/monocyte progenitor, BM bone marrow, PC plasma cell. f Comparison of IRF4-C99R fold change with ratio of gene expression from plasma cells and monocytes. The Spearman correlation between the log2 fold changes of plasma cells/blood monocytes versus C99R/WT was –0.39, with a P value less than 10−5. The number of genes compared was 12642. Source data for figure part 3a, right are provided in the Source Data file.
IRF4-C99R activates lymphoma-specific gene expression via non-canonical AICEs
To directly link IRF4-C99R-regulated genes to those specifically inherent to HRS cells of cHL, we integrated our RNA-seq data from the splenic B cells with HRS cell-specific gene expression profiles (Fig. 4a and Supplementary Fig. 12a). The latter were deduced from published microarray data as well as mRNA-seq-based gene expression profiles of cHL and non-Hodgkin lymphoma cells. Among the most HRS cell-specifically expressed genes which were upregulated exclusively by IRF4-C99R, but not by IRF4-WT, were GATA3, CCL5 (also called RANTES), and TNFRSF8 (CD30), all three being among the most prominent cHL hallmark genes31,53, together with CD80, PDE4D, and CASP6 (Fig. 4a and Supplementary Fig. 12a).
a Comparison of fold changes between IRF4-C99R-induced genes in mouse splenic B cells with differentially expressed genes of Hodgkin and non-Hodgkin cell lines based on microarray gene expression analyses. The Spearman correlation between the log2 fold changes of cHL (L428, L1236, KM-H2, HDLM-2)/non-cHL (REH, NAMALWA, SU-DHL-4) cell lines versus C99R/MIG was –0.25, with a P value less than 10−5. The number of genes compared was 434, comprising the most differentially expressed genes from microarray analyses between cHL and non-cHL cell lines. Note, that IRF4-C99R-induced genes include the known HL-hallmark genes GATA3, CCL5 (RANTES), and TNFRSF8 (CD30) (red rectangle). b Left, UCSC Genome Browser screenshot of REH, GM12878, KM-H2IRF4-WT and L428IRF4-C99R DHSs (red) as well as IRF4 (blue) and JUNB (green) ChIP peaks at the GATA3 gene locus. L428IRF4-C99R-specific ChIP peaks used for EMSA analyses are indicated by gray bars and designated as GATA3Peak_1, GATA3Peak_2, and GATA3Peak_3. Right, HEK293 cells were control transfected (–), or transfected with IRF4-WT, IRF4-C99R, JUNB and BATF, or combinations thereof, as indicated. Nuclear extracts were analyzed for DNA-binding activity at WT and ISRE-mutated GATA3Peak_1, GATA3Peak_2 and GATA3Peak_3 sites. EMSA data show one out of three independent experiments. c Information content (in bits; y axis) of half-ISRE motifs within motifs identified in the ChIP-seq data of L428IRF4-C99R (purple) or KM-H2IRF4-WT (green) cells present in any motif (i.e., all; left, WT n = 8; C99R n = 10), or annotated as AICE BP1−3 (center, WT n = 4; C99R n = 6) or ISRE (right, WT n = 4, C99R n = 4). The logos underneath of each plot represent the summary of the half-ISRE motif for each condition. All ChIP-Seq values are from two averaged replicates per condition. All box-whisker blots represent the median (central line), 25th–75th percentile (bounds of the box) and minimum–maximum (whiskers). Statistical significance was computed using the Welch′s t test (one-tailed). d HEK293 cells were transfected with reporter construct encompassing GATA3Peak_1 together with AP-1 (JUNB and BATF) and IRF4 variants. Luciferase activity is shown as fold activation compared to that of control transfected cells (far left), which is set as 1. Statistics were derived from n = 3 independent transfections, the mean ± SEM is shown. P values were determined by two-tailed unpaired Student′s t test. One out of three independent experiments is shown. e Comparison of gene expression changes between cell lines harboring C99R mutation (C99R/WT cHL) versus fold change between HL and non-Hodgkin cell lines based on RNA-seq analyses. The Spearman correlation between the log2 fold changes of cHL/non-cHL versus C99R cHL/WT cHL was 0.88, with a P value of less than 10−5. C99R-containing cHL cell lines were L428 and U-HO1, while the WT cell lines were L1236, KM-H2, HDLM-2 and L540Cy. Non-cHL cell lines were REH, NAMALWA, SU-DHL−4 and BJAB. Source data for (b–d) are provided in the Source Data file.
To further dissect the mechanism of the IRF4-C99R-specific induction of these genes, we reanalyzed our ChIP-Seq-data for IRF4-JUNB ChIP peaks specific to L428IRF4-C99R cells, but not found in KM-H2IRF4-WT cells. Focusing on regions regulating GATA3 expression, we identified several AICE2-like CEs among the L428IRF4-C99R-specific IRF4-JUNB ChIP peaks, designated as GATA3Peak_1 (5′-TGAGTCAGAGA-3′; the IRF-part of the binding motif is underlined), GATA3Peak_2 (5′-TAAATGAGTCA-3′) and GATA3Peak_3 (5′-GGAATGAGTCA-3′) (Fig. 4b, left). DNA-binding studies demonstrated that IRF4-C99R forms IRF-AP-1 composite complexes at these sites, whereas IRF4-WT did not bind to these sequences (Fig. 4b, right, AP-1 consisting of JUNB/BATF heterodimers; Supplementary Fig. 12b, c, AP-1 consisting of JUNB/BATF3 heterodimers). Of note, none of these sites contained canonical 5′-GAAA-3′ IRF motifs, but instead noncanonical degenerate variants thereof. These results pointed to the increased flexibility of IRF4 R99 compared to C99 at these motifs, similar to our observation made for the AICE2 variants described in Fig. 1e–g. The increased binding capacity of IRF4-C99R to degenerate half-ISRE containing motifs was mirrored in the observation that IRF-containing motifs identified in the ChIP-seq data using ExplaiNN were more degenerated in L428IRF4-C99R cells compared to KM-H2IRF4-WT cells (Fig. 4c), which was most pronounced for AICE motifs (Fig. 4c, center). We confirmed IRF4-C99R-mediated transcriptional activity of the GATA3Peak_1 element by the analysis of luciferase reporter constructs (Fig. 4d and Supplementary Fig. 12d). Here, IRF4-C99R specifically enhanced the luciferase activity in combination with the AP-1 TFs JUNB and BATF, whereas IRF4-WT did not. Finally, the comparison of expression profiles of HRS cell lines harboring an IRF4-C99R mutation with those lacking IRF4-C99R in relationship with cHL-specific genes showed that the cHL hallmark genes GATA3, CCL5 and TNFRSF8 were expressed at particularly high levels in the cell lines with IRF4-C99R (Fig. 4e). Thus, C99R is the primary inducer of the Hodgkin expression program in C99R bearing cells.
Discussion
The work described here presents evidence for a somatic mutation-induced fundamental shift in TF DNA-binding specificity and motif recognition, caused by a Cys-to-Arg substitution in the α3-recognition helix of the DNA-binding domain of the IRF4 protein (IRF4-C99R). This mutation is a hallmark of human lymphoma exhibiting perturbed B-cell identity, cHL and PMBCL. IRF4-C99R is to a large extent unable to regulate canonical IRF4 target genes, including those coordinating terminal B-cell differentiation40, and profoundly blocks plasmablast formation, i.e., terminal B-cell differentiation. Instead, it enforces an altered, disease-specific gene expression program driven by preferred binding to canonical AICE2 sites, and by compelling neomorphic binding to non-canonical AICEs. The IRF4-C99R-mediated altered DNA-binding preferences were not restricted to its interaction with JUNB/BATF-AP-1 heterodimers, but were also observed with other JUN and BATF family members, specifically c-JUN and BATF3, which are also known to be deregulated in cHL45,54,55. How direct protein-protein interactions, which have been suggested for binding to AICEs16,56, contribute to the cooperative binding patterns described here remains to be investigated in future studies.
Functionally, IRF4-C99R combines distinct LOF- and gain-of-function (GOF) properties, which are directly related to its switch in DNA-binding specificities. The block in B-cell differentiation in cHL involves genetic and epigenetic alterations as well as lineage-inappropriate gene expression30,32,33,57,58. Our data demonstrate how IRF4-C99R′s LOF properties contribute to the block in differentiation, one of the hallmarks of malignant transformation59. In line, IRF4-C99R is mostly associated with cHL and PMBCL and is rarely found in other mature B-cell malignancies with maintained B-cell phenotype. Whether IRF4-C99R actively represses lineage differentiation remains to be clarified. On the other hand, IRF4-C99R′s GOF properties are exemplified by the distinct activation of lymphoma-specific gene expression, including GATA3, CCL5, and TNFRSF8. Expression of these genes is stongly associated with the cHL phenotype and they play important roles not only for the tumor cells themselves but also for their interaction with the microenvironment31,53.
Overall, our data demonstrate that AICE motifs are not only key regulatory elements of cellular differentiation and activation processes in immune cells such as T cells or dendritic cells26,27,28,56, but that they can non-canonically interact with mutant TFs to establish malignancy-associated gene expression. The arginine mutation-induced shift in DNA-binding and gene regulation highlights the critical role of arginine residues in determining interactions with the DNA interface11,12. Moreover, we provide a prominent example how nucleotide sequences flanking the TF core DNA-binding motif modify TF:DNA interactions. Such an Arg-induced shift in TF binding specificity to distinct CEs is difficult to predict with current methodologies and distinguishes IRF4-C99R from most other mutations affecting TF:DNA interactions reported previously4,5,6,60,61 and might also operate in other diseases. Indeed, within the framework of the IRF4 International Consortium, we recently reported a complementary recurrent heterozygous p.T95R mutation targeting IRF4′s DNA-binding domain as the cause of an autosomal dominant combined immunodeficiency (CID)62. However, while both the mutated C99R- and T95R-IRF4 proteins have lost the ability to regulate canonical IRF4 target genes and to exert IRF4′s physiological function to coordinate plasma cell differentiation, they also remarkably differ. IRF4-C99R is a somatic mutation, IRF4-T95R a germline mutation. In addition, whilst IRF4-T95R shows an overall broadly increased DNA-binding affinity to canonical and non-canonical DNA motifs, IRF4-C99R displays a unique and distinct loss-and-gain pattern of DNA-binding and regulates different gene sets. We suggest to name such diseases ‘Mutation-Induced Neomorphic Transcription factor Binding’ (MINTraB)-induced diseases.
Disease-causing TF activities can in principle be therapeutically targeted, which has been shown for a few examples like MYC or NOTCH1 (refs. 63,64,65). For this purpose, small compound or peptide-based inhibitors have been reported. Thus, the data presented here open the possibility of designing inhibitors that specifically block the neomorphic DNA-binding activity of a mutant TF, without modulating the activities of its normal counterpart.
Methods
Statement on ethical regulations
We confirm that our study complies with all relevant ethical regulations. Human patient material was analyzed retrospectively. Samples were provided by the University Cancer Center Frankfurt (UCT, Germany), the Hematopathology Section of Christian-Albrechts-University Kiel (Germany), and the Lymphoma Reference Centre at the Institute of Pathology, University of Würzburg (Germany). We used archived anonymized specimens from patients with diagnosed cHL. The use of human material was approved by the institutional review boards and local Ethics Committees of Charité and University Cancer Center Frankfurt (SHN-06-2018; 15-6184-BO; EA2/087/16), and an individual informed consent for the use of these anonymized specimens is not required. All animal experiments were approved by the local authority Landesamt für Gesundheit und Soziales (LAGeSo; X9027/11).
Sex and gender reporting
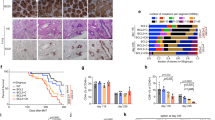
Classic Hodgkin lymphoma, the main lymphoma entity for which we show data in our manuscript, shows only minor differences in its sex distribution (ratio male:female—1,3: 1), with an even equal distribution between men and women in young adulthood66,67. Thus, a clear-cut sex-based phenotypic bias in unlikely. In our study, we thus did not specifically select for sex or perform gender-based analyses. Primary lymphoma samples were selected on a random base for the immunohistochemistry analyses. Given the large number of cases analyzed, it is conceivable that our data reflect the overall distribution of male and female cases within the general population. Selection of materials for lymphoma single-cell analyses as well as of the cell lines was primarily determined by the limited availability of respective materials within the community.
Cell lines, culture conditions, and transfections
HRS [L428, L1236, KM-H2, L591 (EBV+), U-HO1 (all of B-cell origin); HDLM-2, L540, L540cy (all of T-cell origin)], pro-B lymphoblastic leukemia (REH), Burkitt′s lymphoma (NAMALWA, BL-60, BJAB), diffuse large B-cell lymphoma (SU-DHL−4), and HEK293 cell lines were cultured as previously described32,68. Cell lines used in our study were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ), the American Tye Culture Collection, and other investigators (L1236 and L591 from V. Diehl, Cologne, Germany; L540cy from A. Engert, Cologne, Germany; BJAB from P. Krammer, Heidelberg, Germany). Cell lines were regularly tested negative for mycoplasma contamination, and their authenticity was verified by STR fingerprinting. For preparation of nuclear extracts for DNA-binding studies, HEK293 cells were transfected by electroporation in OPTI-MEM I using Gene-Pulser II (Bio-Rad) with 960 μF and 0.18 kV with 10 μg pcDNA3-FLAG-JUNB, 10 μg pcDNA3-FLAG-BATF, or increasing amounts, ranging from 0.5 to 10 μg, of the respective pHEBO-IRF4 variants. For analysis of luciferase activity, HEK293 cells were transfected with 15 μg of pGL3_GATA3-3P_AICE_long reporter construct, together with 150 ng pRL-TKLuc as an internal control, where indicated together with 5 μg pcDNA3-FLAG-JUNB, 5 μg pcDNA3-FLAG-BATF, or 40 μg of the respective pHEBO-IRF4 variants. Forty-eight hours after transfection, the ratio of the two luciferases was determined (Dual luciferase kit, Promega). For generation of inducible BJAB cells, cells were electroporated with 40 μg of pRTS1-IRF4-WT or -IRF4-C99R or pRTS1 control plasmid in OPTI-MEM I using Gene-Pulser II with 50 μF and 0.5 kV. Twenty-four hours after transfection, 28 μg/mL Hygromycin B (Sigma-Aldrich, Taufkirchen, Germany) were added. After 21–28 days of culture in the presence of Hygromycin B, cells were suitable for functional assays. The respective IRF4 variants were induced by the addition of 100 ng/mL doxycycline (D9891; Sigma-Aldrich).
Preparation of whole cell and nuclear extracts, immunoblotting, and electrophoretic mobility shift assays (EMSA)
Preparation of whole cell and nuclear extracts as well as immunoblotting and EMSA were performed as previously described32,33,68. For EMSA analyses, we used 3–5 μg nuclear extracts per lane. EMSA buffer contained 10 mM HEPES, pH 7.9, 70 mM KCl, 5 mM dithiothreitol, 1 mM EDTA, 2.5 mM MgCl2, 4% Ficoll, 0.5 mg/ml BSA, 0.1 μg/ml poly-deoxyinosinic-deoxycytidylic acid (poly[(dI)•(dC)]). The double-stranded oligonucleotides used for EMSA are indicated in Supplementary Data Table 4. After annealing, oligonucleotides were end-labeled with [α-32P]dCTP with Klenow fragment. Positions of the complexes were visualized by autoradiography. Antibodies used for supershift analyses and for immunoblotting are indicated in Supplementary Table 5. If not validated by the manufacturer, we validated antibodies with respective positive and negative controls (cell lines, transfected cells).
DNA constructs
The pHEBO-IRF4-HAtag expression construct and its control pHEBO-CMV-HAtag were kindly provided by L. Pasqualucci (New York). The R98A, C99A, C99R, and S104T mutations were introduced by use of the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene) into the pHEBO-IRF4-HAtag expression construct according to the manufacturer′s recommendations and by use of primers indicated in Supplementary Table 4. For the retroviral transduction experiments of C57BL/6 splenic B cells, the coding sequences for human IRF4 (WT, C99R, R98AC99A) were amplified from the pHEBO-constructs using the IRF4_XhoI_forw 5′-ACCTCGAGGCCACCATGAACCTGGAGGGCGGCGGCCGA-3′ and IRF4_EcoRI_rev 5′-ACGAATTCTTAAGGCCCTGGACCCAAAGAAGCGTAATC-3′ primers and cloned in front of the IRES sequence of the MSCV-IRES-GFP (MIG) plasmid (kindly provided by F. Rosenbauer, Münster, Germany) via XhoI and EcoRI. For the pRTS1-based inducible expression constructs69 of the IRF4-WT and IRF4-C99R variants, IRF4-WT and IRF4-C99R were amplified using the respective pHEBO-IRF4 expression constructs as templates. The amplified IRF4-WT- and IRF4-C99R-products were ligated via XbaI into pUC19-Sfi, respectively, and mobilized by SfiI digestion for cloning into pRTS1. For the pcDNA3-FLAG-JUNB expression construct, full-length human JUNB was amplified from cDNA of the human L428 cell line, and cloned via BamHI and XhoI into pcDNA3-FLAG (Invitrogen). Full-length human c-JUN (JUN) was amplified from cDNA of the human cell line L1236 by use of primers JUN_FLAG_BamHI s 5′-GCGGATCCACTGCAAAGATGGAAACG-3′ and JUN_STOP_XhoI as 5′-GCCTCGAGTCAAAATGTTTGCAACTG-3′, and cloned via BamHI and XhoI into pcDNA3-FLAG. pcDNA3-based expression constructs for BATF and BATF3 were previously described54. For cloning of the pGL3_GATA3-3P_AICE_long reporter construct encompassing GATA3Peak_1, DNA from My-La cells was amplified by use of primers GATA3_AICE_KpnI s 5′-GCGGTACCATACAGACCCTTCCAGCCAC-3′ and GATA3_AICE_XhoI as 5′-GCCTCGAGAACAGATGTGGGGAGTCAGA-3′ and cloned via KpnI and XhoI into the multiple cloning site (MCS) of pGL3 (Promega). All constructs were verified by sequencing.
Sanger sequencing (cell lines)
Primer sequences for the validation of IRF4 mutations IRF4-C99R and S104T identified by whole exome sequencing in cHL cell lines were designated using the Primer3 software (version 4.1.0; http://frodo.wi.mit.edu/primer3/) (Supplementary Table 4). cDNA for RT-PCR was synthesized using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific). Sanger sequencing was performed according to standard procedures.
Laser microdissection and PCR analyses of primary HRS cells
Tissue samples used for laser microdissection were provided by the University Cancer Center Frankfurt (UCT; Germany) and by the Hematopathology Section of Christian-Albrechts-University Kiel (Germany). Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and the study was approved by the institutional review board and local Ethics Committee of University Cancer Center Frankfurt (SHN-06-2018; 15-6184-BO). Pools of 10 HRS cells or pools of 10 non-tumor cells, and membrane sections without tissue as controls were laser-microdissected as previously described70. Following digestion with proteinase K for 3 h at 55 °C and heat inactivation for 10 min at 95 °C, a semi-nested, two-rounded PCR with exon-spanning primers was performed to amplify exon 3 of IRF4. PCR products were separated on a 1% agarose gel. Gel-purified products were sequenced on an ABI3130 (Applied Biosystems) and evaluated with SeqScape software v2.5 (Applied Biosystems). For the assessment of mutations, forward and reverse sequences were mandatory. Primer sequences were (always 5′–3′): IRF4_E3_fw 5′-TCGTGCCACTGTACTCTAGCC-3′; IRF4_E3_rv1 5′-ATCTGGCTGCCTCTGTTAGGT-3′; IRF4_E3_rv2 5′-AGCTAGAAAGTGATGCTCAGAATG-3′; IRF4_E3_fw_II 5‘-AGTTCCGAGAAGGCATCGAC-3′; IRF4_E3_rv1_II 5′-ATTGGCTCCCTCAGGAACAA-3′; IRF4_E3_rv2_II 5′-TGTACGGGTCTGAGATGTCCA-3′. For DNA from frozen tissue sections, the primers IRF4_E3_fw and IRF4_E3_rv2 were used in the first round of PCR (product size 389 bp), and the primers IRF4_E3_fw and IRF4_E3_rv1 in the second round (product size 346 bp). For DNA from paraffin sections, primers IRF4_E3_fw_II and IRF4_E3_rv1_II were used in the first round (fragment size 160 bp), and primers IRF4_E3_fw_II and IRF4_E3_rv2_II in the second round (fragment size 129 bp). PCR conditions were 98 °C 4 min, 40 cycles of 98 °C 30 s, 62 °C 20 s, 72 °C 20 s, final elongation 72 °C 3 min.
IRF4 mutation analysis in PMBCL patients
To generate a custom cRNA bait library (SureSelect, Agilent Technologies) for targeted gene capture, a total of 106 genes (including IRF4) that have been reported to be affected by genetic aberrations in PMBCL were selected. To ensure high quality, only samples that had a coverage of 100× in ≥80% of the exonic regions were included. The median and mean sequencing coverages were 830× and 666×, respectively. Variant calling and filtering was performed as described earlier71 with the following adaptations as no germline controls were included: (i) 10%_posterior_quantile >0.1; (ii) 10%_posterior_quantile(realignment) >0.1; (iii) VAF for synonymous and nonsynonymous SNVs <0.45, >0.55, and >0.95 for regions that were not affected by SCNA. Over 3000 mutations were extensively inspected for artifacts and mapping errors through visual inspection with the Integrative Genomics Viewer (IGV). A detailed description of the PMBCL patient cohort, applied sequencing workflow, and corresponding bioinformatical analysis are described in ref. 72, and in Noerenberg et al. (J Clin Oncol, in press). This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics review committee of the Charité—Universitätsmedizin Berlin (EA2/087/16) and of every participating center.
IRF4 shRNA-mediated cytotoxicity assay of L428 cells
For efficient retroviral transductions, L428 cells were engineered to express a murine ecotropic receptor as previously described73. In addition, the cells were also engineered to express a bacterial tetracycline repressor allowing doxycycline-inducible small-hairpin RNA (shRNA) or cDNA expression. The retroviral transduction experiments, shRNA-mediated RNA interference and cytotoxicity assays were performed as described elsewhere22,73,74,75. In brief, to assess toxicity of an shRNA, retroviruses that co-express green fluorescent protein (GFP) were used as described22,73,74,75. Flow cytometry was performed two days after shRNA transduction to determine the initial GFP-positive proportion of live cells for each shRNA. Subsequently, cells were cultured with doxycyline (40 ng/ml) to induce shRNA expression, and the proportion of GFP-positive cell was measured at the indicated time points. The GFP-positive proportion at each time point was normalized to that of the negative control shRNA and further normalized to the day two fraction. The targeting sequence of IRF4 shRNAs #1 and #2 were 5′-CCGCCATTCCTCTATTCAAGA and 5′-GTGCCATTTCTCAGGGAAGTA as described20,22. As a negative control shRNA, a previously described shRNA against MSMO1 was used22. Each shRNA experiment was reproduced at least two times. For the IRF4 rescue experiments, IRF4 (NM_002460.3) single mutant IRF4C99R and double mutant IRF4C99RS104T cDNAs were created and the experiment was performed as previously described20,22. In brief, to assess rescue effect of an IRF4 cDNA, L428 cells were transduced with an IRF4#1 or #2 shRNA, followed by retroviral ectopic expression of either an empty vector or an IRF4 cDNA that co-expresses GFP. We compared cell growth for each overexpression relative to the growth for the empty vector which is normalized to the 100% line, and further normalized to the day two fraction. Each experiment was reproduced at least two times. Combining the four curves (both shRNAs and their replicates) for each cDNA, aggregated curves show mean viabilities (markers) ± standard errors (transparent tunnels). At day 11, we statistically compared with 100%, i.e., with our null hypothesis for zero rescue effect (one-sample one-tailed t tests).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue specimens from cases diagnosed as classic Hodgkin lymphoma (30 cHL mixed cellularity subtype; 30 cHL nodular sclerosis subtype; 30 cases lymphocyte-rich subtype) were retrieved from the files of the Lymphoma Reference Centre at the Institute of Pathology, University of Würzburg, Germany. For this retrospective study we used archived anonymized tissue specimens, and there was no participant compensation. From each paraffin block 2-μm sections were cut and subjected to immunohistochemical stainings. Immunostains were performed in an automatic immunostainer using program ER2 (Bond III, Leica Biosystems, Nussloch, Germany) using the manufacturer′s protocols and detection reagents. Detection of IRF4 employed the monoclonal antibody MUM1P (M725929; dilution 1:400; DAKO/Agilent, Waldbronn, Germany).
Cloning and purification of recombinant proteins
Codon-optimized sequences encoding the DNA-binding domains of human BATF (AA 28–87) and JUNB (AA 269–329) were cloned into pMAL-C2X (NEB). The sequence encoding human IRF4 DBD (AA 20–139) was cloned into pGEX6P1 (Cytivia). IRF4 mutations were introduced by QuikChange Site-Directed Mutagenesis Kit (Agilent) according to the manufacturer′s recommendations. All constructs were verified by sequencing. Plasmids were separately transfected into BL21-DE3-Rosetta (Novagen). Proteins were expressed overnight at 18 °C in TB medium (Melford) after induction with 40 mM Isopropyl-β-D-thiogalactopyranosid (IPTG). Cells were resuspended in 50 mM HEPES pH 7.5, 300 mM NaCl, 2.5 dithiothreitol (DTT), 1 μM DNase, 200 μM Pefablock (Carl Roth) and lysed in a microfluidizer (Microfluidics). Eluates containing MBP-fusions were applied to 5 mL amylose resin (NEB) columns and extensively washed with 20 mM HEPES pH 7.5, 150 mM NaCl, 2.5 mM DTT. Proteins were eluted in the same buffer containing additional 10 mM maltose. Eluates containing GST-IRF4 protein were applied to a 5 mM GSH sepharose (Cytivia) column and extensively washed with 20 mM HEPES pH 7.5, 150 mM NaCl, 2.5 mM DTT. Proteins were eluted in the same buffer conaining additionally 20 mM glutathione (pH 7.5) (Sigma-Aldrich). GST was removed by the addition of PreScission Protease in a ratio of 1:100. All proteins were separately concentrated using 10 kD cut-off Amicon Ultra-15 Centrifugal filters (Millipore) and applied to a final gel filtration run on a Superdex 75 column (Cytivia) using 20 mM HEPES, pH 7.5, 150 mM NaCl, 2 mM DTT as running buffer. Peak fractions containing the protein of interest were concentrated and flash-frozen in small aliquots.
DNase-seq
DNaseI-seq was essentially performed as previously described68 with slight modifications. Briefly, cells were washed and resuspended at 108 cells/ml in ice-cold ψ buffer (11 mM KPO4, pH 7.4, 108 mM KCl, 22 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 1 mM dithiothreitol, 1 mM ATP). 1 Mio REH, KM-H2 or L428 cells were treated with 12 U/mL DNaseI (Worthington) for 3 min at 22 °C. Digestion was stopped with the addition of 200 μl lysis buffer (100 mM Tris, pH 8.0, 40 mM EDTA, 2% SDS, 200 μg/ml proteinase K) overnight at 37 °C. DNase digestion efficiency was checked via low-voltage overnight electrophoresis (10 V) on a 0.5% TAE agarose gel. Short-fragment size selection was performed by cutting out gel bands between 100–200 bp and subsequent purification using the QiaQuick gel extraction kit (Qiagen) according to the manufacturer′s instructions. Library preparation was performed using the KAPA hyperprep kit (Roche) following the manufacturer′s guidelines. Library quality was checked via qPCR using TBP, ACTB and gene desert control oligonucleotides76. Libraries were sequenced at 400 million reads per library in single-end mode on separate lanes using an Illumina HiSeq 2000 system according to the manufacturer′s instructions.
ChIP-seq
ChIP was performed as previously described77 using double-crosslinking. Cells were resuspended at 3.3 × 106 cells/mL in PBS and first crosslinked with 8.3 μl/ml DSG (Sigma) for 45 min at room temperature, subsequently washed 4× and crosslinked in 1% formaldehyde for 10 min at room temperature, with both crosslinking methods entailing sustained tube rotation. Crosslinking was quenched in 0.2 M glycine and cells were washed 2×. Cells were lysed in Buffer A (10 mM Hepes, 10 mM EDTA, 0.5 mM EGTA, 0.25% Triton X100), then in Buffer B (10 mM Hepes, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.01% Triton X100), at 1 × 107 cells/ml and 4 °C with rotation for 10 min for both stages. Nuclei were resuspended at 2 × 107 cells/ml in 4 °C IP Buffer I (25 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% Triton X100, 0.25% SDS) and sonicated in 6 × 300 μl per reaction using a Picoruptor sonicator (Diagenode) at 240 W with 30 cycles of 30 s on, 30 s off at 4 °C. Cell debris was pelleted via 10 min 16,000×g centrifugation and diluted in IP Buffer II (8.33 mM Tris, 50 mM NaCl, 6.33 mM EDTA, 0.33% Triton X100, 0.0833% SDS, 5% glycerol final concentration). 5% of chromatin was saved as input control. Immunoprecipitation was carried out overnight using Maximum Recovery tubes (Axigen) with rotation at 4 °C in 50 μl PBS + 0.02% Tween 20 with 15 μl protein G dynabeads that were washed, blocked with 0.5% BSA and conjugated with either IRF4 (sc-6059-X, Santa Cruz) or JUNB (sc−46-X, Santa Cruz) antibodies for 4 h ar 4 °C with rotation. Beads were subsequently washed on ice by magnetic separation using 1× PBS + 0.02% Tween 20, 2× Wash Buffer 1 (20 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% Triton X100, 0.1% SDS), 1× Wash Buffer 2 (20 mM Tris, 500 mM NaCl, 2 mM EDTA, 1% Triton X100, 0.1% SDS), 1× with LiCL Buffer (10 mM Tris, 250 mM LiCl, 1 mM EDTA, 0.5% NP40, 0.5% Na-deoxycholate), 2× with TE/NaCL Buffer (10 mM Tris, 50 mM NaCl, 1 mM EDTA). Beads were eluted using 2 × 50 μL Elution Buffer (100 mM NaHCO3, 1% SDS) with shaking for 15 min at RT, and eluates were pooled. Chromatin was reverse-crosslinked overnight at 65 °C in Elution Buffer + 200 mM NaCl, followed by 100 μg/ml RNase A and 0.25 mg/ml proteinase K digestion for 1 h at 37 °C and 55 °C, respectively. DNA was purified via phenol chloroform extaction. ChIP efficiency was checked using IL3–40, CSF1R FIRE, and gene desert control oligonucleotides76. Library preparation was performed using the KAPA hyperprep kit (Roche) following the manufacturer′s guidelines. Libraries were sequenced in single-end mode at 50 million reads per library using an Illumina HiSeq 2000 system according to the manufacturer′s instructions.
Single-molecule fluorescence microscopy
(A) Cloning of IRF4 plasmids for fusion proteins: cDNAs encoding human IRF4 and IRF4-C99R were cloned into the LV-tetO-HaloTag plasmid using EcoRI and XbaI restriction sites and One Shot Stbl3 chemically competent E. coli (Thermo Fisher Scientific, USA)78. Coding regions of the plasmids were verified by Sanger sequencing. (B) Generation of stable cell lines: Lentiviral transduction was used to generate HeLa cells which stably express IRF4-WT- or IRF4-C99R-HaloTag fusion proteins78. In brief, HEK293T cells were transiently transfected with psPAX2 (Addgene #12260), PMD2.G (Addgene #12259), and the respective pLV-tetO IRF4-HaloTag variants using JetPrime (PolyPlus). Supernatants containing viruses were harvested through a 0.45 μm filter after 48 h. HeLa cells were infected at 37 °C and 5% CO2 for 72 h. (C) Preparation of cells for imaging: One day before imaging, cells were seeded on a heatable glass bottom dish (DelaT, Bioptechs), and 15 min prior to imaging 3 pM silicon rhodamine (SiR) HaLoTag ligand (kindly provided by K. Johnson, Heidelberg, Germany) were added according to the HaloTag staining protocol (Promega). Thereafter, cells were washed with PBS and placed for 30 min at 37 °C and 5% CO2 in DMEM. Before imaging, cells were washed three times with PBS and imaged in 2 mL OptiMEM. (D) Microscope setup: A custom-built fluorescence microscope for single-molecule fluorescence imaging was used as described48. (E) Interlaced time-lapse illumination and data analysis: Cells were illuminated with a highly inclined light beam79 using an interlaced time-lapse illumination scheme48. In ITM, we repeated a pattern of two consecutive images with 50 ms camera integration time followed by a dark time of 2 s. Localization of fluorescent molecules within an image and tracking of molecules across consecutive images was performed by use of Tracklt v1.0.1 (ref. 80). Detection and tracking parameters were: threshold factor′ 3, ‘tracking radius′ 2, ‘min. track length′ 2, ‘gap frames′ 0, ‘min. track length before gap frame′ 0. Molecules only detected within a single image were classified as unbound, the ones detected in two consecutive images within an area of 0.35 μm2 as short-bound, and those tracked over at least one dark time as long-bound. For each imaged cell, the ratio of all bound molecules (including short- and long-bound molecules) to all molecules (including long-, short-, and unbound molecules) and of long-bound molecules to all molecules was calculated. The significance between IRF4-WT and IRF4-C99R was tested with an unpaired, non-parametric t test (Mann–Withney test) using Graphpad prism 9.0.1.
Reference-free DNA modeling and IRF4 docking studies
The structural modeling is designed to provide insight as to how the IRF4-C99R mutation can influence binding to the different DNA motifs and to complement the functional data observed in this study. To model the structural basis for the interaction of IRF-WT or IRF4-C99R with different DNA elements, unbiased random docking and interaction studies were examined using HADDOCK 2.2 (ref. 81). The initial structures of IRF4-WT and the ISRE DNA were obtained from our previous crystal structure (PDB: 7JM4). In generating the annealed AICE1 DNA motif, template-based free annealing and ternary structure of the DNA fragment were obtained using HNADOCK DNA program82. As the option to generate a DNA structure does not exist in HNADOCK, ssDNA structures were initially generated in PyMOL v2.5 and then imported, and energy was minimized in HNADOCK to generate the dsDNA. Deprived annealing of DNA ends was noted due to low Tm in the modeling at the 5′ or 3′ ends. All docking studies and modeling were performed using standard HADDOCK 2.2. The HADDOCK program differs from ab initio docking methods by utilizing information from known or predicted protein interfaces for ambigous interaction restraints (AIRs) and utilizes flexible docking. The default HADDOCK program was used without additional restraints. No MD simulations were used, and flexible docking was performed as a default option in HADDOCK with default energy minimization. Furthermore, all the hydrogen atoms in the initial PDB structures were retained, thereby simulating more realistic results. Using the Kyte-Doolittle mode in the HADDOCK 2.2, in silico water molecules were added while docking the IRF4 with DNA to distinguish any water-mediated solvation contacts. As shown in the modeling analysis report in Supplementary Table 3, to assess the quality and confidence in the modeled structures, several modeling outcome parameters were analyzed as per the HADDOCK guidelines (https://www.bonvinlab.org/software/haddock2.2/analysis/). Primarily the lower values of HADDOCK score, Z-score, Van der Waals and electrostatic energy were all considered to denote better models. In fact, for all parameters, apart for the buried interface and desolvation energy, lower values signified greater confidence in the proposed model. The modeled structures and the interaction interface of IRF4-WT or IRF4-C99R with different DNA elements (ISRE or AICE1) were assessed for their quality using HADDOCK. Analysis of output parameters of 5 different clusters obtained from the docked models (HADDOCK 2.2) was used to validate that the models were true and consistent across all clusters. The binding free energies were also taken into consideration in selecting the best possible models. Further validation and refinement was undertaken by ensuring that the residues occupied Ramachandran favored positions using Coot (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/). The figures were generated using PyMol v2.5.
RNA-seq of human lymphoma cell lines
RNA-seq analyses of L428, L1236, KM-H2, U-HO1, L591, HDLM-2, L540, L540cy, REH, NAMALWA, BJAB and SU-DHL-4 cells was performed in duplicates. In brief, barcoded mRNA-seq cDNA libraries were prepared from 600 ng of total RNA using Illumina′s TruSeq Stranded RNA Sample Preparation Kit. mRNA was isolated using Oligo(dT) magnetic beads. Isolated mRNA was fragmented using divalent cations and heat. Fragmented mRNA was converted into cDNA using random primers and SuperScriptII (Invitrogen). This was followed by second-strand synthesis. cDNA was repaired and 3′ adenylated. 3′ single T-overhang Illumina multiplex specific adapters were ligated on the cDNA fragments, and these fragments were enriched by PCR. All cleanups were done using Agencourt XP magnetic beads. Barcoded RNA-seq libraries were clustered on the cBot using the Truseq PE cluster kit V3 using 10 pM and 2 × 50 bps were sequenced on the Illumina HiSeq2500 using a Truseq SBS V3 kit.
Generation of retroviral particles, mouse B-cell isolation, retroviral transduction
By use of calcium-phosphate buffer, 10 μg of retroviral plasmids (MSCV-based) encoding human IRF4-WT, IRF4-C99R, or IRF4-R98AC99A were transfected into the Plat-E packing cell line83, together with packaging plasmids pGagpol (10 μg) and pEnv (2 μg) (both courtesy of A. Leutz, Berlin) and 25 μM chloroquin (#6628, Sigma). Thereafter, cells were incubated for 6–8 h at 37 °C and 5% CO2, followed by change of medium to B-cell medium (DMEM high glucose (4,5 g/l) supplemented with 10% FCS, 1% sodium pyruvate, 1% penicillin-streptomycin, 1% HEPES, 1% l-glutamine, 1% non-essential amino acids and 0.05% β-mercaptoethanol) and further cultivation. 48 h after transfection, cell culture supernatants were harvested, filtered (0.45 μm) and frozen at –80 °C. Splenic B cells were isolated from 8- to 12- week-old C75BL/6 mice (originally obtained from Jackson Laboratories) by CD43 depletion with magnetic anti-mouse CD43 microbeads (#130-049-801, Milenyi Biotech) according to the manufacturer′s instructions. Purified B cells (density 1 × 106 cells/ml; 4 × 106 cells per well) were cultured in the presence of recombinant mouse IL-4 (25 ng/ml; #404-ML, R&D) and LPS (20 μg/ml; #L2880, Sigma) overnight to induce B-cell activation and terminal differentiation. 24 h after isolation, B cells were collected (300 × g, 5 min, 4 °C) and resuspended in B-cell medium supplemented with 8 μg/ml polybrene (#TR-1003, EMD Millipore) at a density of 2 × 106 cells/ml. To introduce the IRF4 variants, 4 × 106 B cells per well were plated in 2 ml on 6-well plates that had been coated with RetroNectin (25 μg/ml, 4 °C, overnight; #T100B, Takara), blocked with 2% BSA in PBS (1 h) and pre-loaded with the respective retroviral particles (1 h, 37 °C). Retroviral transduction was performed by the addition of 2 ml of the respective retroviral supernatant and subsequent centrifugation (800 × g, 90 min, 32 °C). 24 h after transduction, B cells were collected (300 × g, 5 min, 4 °C), resuspended in B-cell medium and cultured (density 1 × 106 cells/ml; 4 × 106 cells per well) for another 72 h (FACS for RNA-seq, flow cytometric analysis of plasma cell differentiation) in the presence of recombinant mouse IL-4 and LPS. Animal experiments were approved by the local authorities (Landesamt für Gesundheit und Soziales, LAGeSo; X9027/11).
Flow cytometry of C57BL/6 splenic B cells
Retrovirally transduced B cells were harvested, blocked with TruStain FcX (α-mouse CD16/32; 10 min, 4 °C; #101320, BioLegend) and stained (20 min, 4 °C) with B220-PerCP/Cyanine5.5 (#103235; BioLegend) and CD138-PE (#142504; BioLegend) in PBS, pH 7.2, supplemented with 3% FCS and 1 mM EDTA. Analysis of the samples was performed on a FACSCantoII instrument (BD BioSciences) or sorted on a FACSAria (BD BioSciences). FlowJo software (BD FlowJo, RRID:SCR_008520; v9.9.6) was used to generate plots.
Bioinformatics analyses of HL RNA-seq, DNase-seq, and ChIP-seq data
HL cell line RNA-seq processing
Reads were aligned in paired-end mode to the hg19 genome using STAR v2.3.0 (ref. 84) using --outSAMattributes Standard --outSAMunmapped None --outReadsUnmapped Fastx --outFilterMismatchNoverLmax 0.02 as parameters. Counts were obtained using featureCounts v2.0.0 (ref. 85) with -p -B -C -Q 10 --primary -s 0 as parameters. Normalization and differential gene expression analysis were performed using DESeq2 v1.14.1 (ref. 86) using the standard analysis protocol, performing variance stabilization transform normalization. Gene set enrichment analyses were performed using GSEA v3.0 (ref. 87).
DNase-seq and ChIP-seq processing
Base-calling was carried out using HiSeq Analysis Software v2.0 (Illumina). Reads were demultiplexed using bcl2fastq v2.16.0 (Illumina). As libraries were sequenced in separate lanes, unassigned reads/unreadable indexes were assigned to their respective lane. Reads were subsequently aligned in single-end mode to the hg19 genome using bowtie2 v2.1.0 (ref. 88) using --very-sensitive-local as a parameter and sorted by coordinate ordering using samtools sort v1.1 (ref. 89). Peak calling and depth coverage track generation were carried out using macs2 v2.1.0 (ref. 90) using callpeak -g hs -q 0.001 -B --SPMR --trackline <trackline> as parameters, with --keep-dup all and --keep-dup auto for DNaseI- and ChIP-Seq assays, respectively, to account for high depth sequencing of DNase-Seq libraries. Peak calling yielded 61983, 65612, and 68370 peaks for Reh, KM-H2, and L428 DNase-Seq datasets, respectively, 33082 and 30022 peaks for KM-H2 and L428 IRF4 ChIP-Seq datasets, respectively, as well as 24914 and 24886 peaks for KM-H2 and L428 JUNB ChIP-Seq datasets, respectively.
DNase-seq and ChIP-seq processing
For pairwise comparisons, the union of peak summits was obtained as previously described91 and masked against blacklisted and simple repeat regions92 using bedtools intersect v2.19.0 (ref. 93) with -v as a parameter. Corresponding depth coverages were obtained using Homer annotatePeaks v4.6 (ref. 94) with -hist 10 -ghist -size 2000 as parameters and subsequently log2 fold-changed ranked on total signal [−100 bp; +100 bp] around peak summits as previously described77. Heatmaps were obtained using Java TreeView v1.1.4 (ref. 95). Venn diagramme overlaps and specific peak populations were computed using ChIPpeakAnno makeVennDiagram v.1.12.0 (ref. 96), and bedtools intersect with totalTest = <sum of ChIP-Seq peak numbers> and -u as a parameter, respectively. Spearman correlation clustering heatmaps were obtained via gplots heatmap.2 v2.17.0 using total ChIP-Seq signal [−100 bp; +100 bp] around the union of GM12878, KM-H2 and L428 IRF4 ChIP-Seq peak summits. For motif, DNase-Seq and ChIP-Seq average profile significance testing, signal [−200 bp; +200 bp] from summits were averaged per region, split into three classes and tested for significance using t tests. For GSEA analyses, peaks were annotated to the closest gene using bedtools closest using -t first as a parameter; the top 1000 specific peaks sorted by decreasing signal were used. Footprinted motif co-occurrence clustering was performed as previously described77,91 with specific peaks and 1000 similarly-sized samplings of control peaks being annotated with 16 motifs using Homer annotatePeaks. Intersection matrices were computed using pyBedtools intersection_matrix97. Enrichment z-scores were computed by subtracting mean co-occurrences between the specific peaks and control peaks, dividing by the standard deviation of control peaks.
Public dataset processing
Reads for IRF4 and JUNB GM12878 ENCODE ChIP-Seq datasets98,99 and GM12878 ENCODE DNase-Seq100 were retrieved from the Sequence Read Archive (SRA) and processed as ChIP-Seq datasets above. L428 and REH DNase-seq datasets generated in this study were complemented in reads from corresponding previously published, lower sequencing depth datasets68.
Motifs discovery, average profiles, and heatmaps
Motif discovery was performed using Homer findMotifsGenome using default parameters. Motif average profiles and heatmaps were generated using Homer annotatePeaks using -hist 10 -ghist -size 2000 as parameters and plotted using Java TreeView. Statistics were done in R v4.0.3.
Digital genomic footprinting
Digital genomic footprinting was performed using pyDNase wellington_footprints v0.2.6 (ref. 101) using -A as a parameter, yielding 60,669, 75,813, and 75,755 footprints for REH, KM-H2 and L428 cells, respectively. Individual motif bed files were obtained from union of REH, KM-H2 and L428 footprints annotated for each motif using Homer annotatePeaks -mbed -size given as parameters and subsequently plotted as motif footprint profiles using pyDNase dnase_average_profiles with -A -n as parameters. AICE2 and AICE2FLIP motifs were obtained from ref. 47. Motif footprinting scores were obtained using wellington_score_heatmap using -A as a parameter, with scores at the footprint centre being log2 transformed and used for t test significance testing102.
Bioinformatic analysis of mouse splenic B-cell and lymphoma cell line RNA-seq data
RNA-seq of splenic B cells
RNA prepared from isolated murine splenic B cells transduced with MIG control virus or IRF4-WT, IRF4-C99R, or IRF4-R98AC99A variants was processed by use of the KAPA mRNA Hyper Prep Kit for Illumina Platforms (KK8580; Roche) and KAPA Single-Indexed Adapter Kit (KK8700; Roche). Libraries were sequenced by use of Illumina HighSeq 4000. RNA-seq data from mouse splenic B cells was processed using PiGx-RNA-seq103 pipeline. In short, the data was mapped onto the GRCm38/mm10 version of the mouse transcriptome (downloaded from the ENSEMBL database104) using SALMON (v0.9.1)105. The quantified data was processed using tximport (v1.22.0)106, and the differential expression analysis was done using DESeq2 v1.34.0 (ref. 86). Genes with less than 5 reads in all biological replicates of one condition were filtered out before the analysis. Two groups of differentially expressed genes were defined—a relaxed set containing genes with an absolute log2 fold change of 0.5, and a stringent set containing games with an absolute log2 fold change of 1. The fold change was deemed significant if the adjusted P value was less than 0.05 (Benjamini–Hochberg corrected).
RNA-seq of lymphoma cell lines
RNA-seq data of the Hodgkin and non-Hodgkin cell lines was processed using the PiGx-RNA-seq103 pipeline. In short, the data were mapped onto the GRCh38/hg38 version of the human transcriptome using SALMON (v0.9.1)105. Differential gene expression results were integrated with the previously analyzed microarray data from cHL cell lines107.
Data integration and visualization
All analyses were done in R v4.1 using custom scripts. Quantified genes were imported into R using the tximport package (v1.22.6). Splenic B-cell per-sample heatmap was constructed by calculating the Pearson correlation coefficient DESeq2 normalized expression values. The heatmap was visualized using the ComplexHeatmap package (v2.10.0)108. The number of stringently differentially expressed genes in each condition was visualized using UpSet diagrams (UpSetR v1.4.0)109. Human and mouse genes were mapped through the orthologous assignment using the ENSEMBL database. Monocyte, B cell, and plasma cell expression profiles were extracted from the ARCHS4 database110. Samples with the following keywords in the Sample_source_name_ch1 field were used in the analysis: “granulocyte-monocyte progenitor (GMP) cells”, “Blood-derived monocyte”, “Bone marrow, plasma cells, WT”; “Splenic B cells, Wild type”; “WT, B cells”; “LPS activated B cells”. If multiple samples corresponded to one condition, their expression values were averaged.
Microarray analyses of inducible BJAB cells
For microarray gene expression analyses of BJAB cells following tet-induction of IRF4-WT or IRF4-C99R, mRNA was processed by use of the Illumina Total Prep RNA Amplification Kit (AMIL1791; Invitrogen) and use of Human HAT-12_v4 Bead Chips (Affimetrix). The microarray gene expression data from a total of 24 arrays were analyzed in GenomeStudio software (Illumina, Little Chesterford, UK) with background subtraction from experiments that were performed with Tet-inducible BJAB cells expressing Mock control, IRF4-WT or the IRF4-C99R mutant. The raw data output by GenomeStudio was analyzed using the Lumi R package111 with quantile normalization. The 10% threshold (P value <= 0.1) was applied to all samples. Genes with at least twofold-change in expression (either up or down) were selected in either IRF4-WT versus Mock and IRF4-C99R vs Mock in different time courses. Principle component analysis and hierarchical clustering were carried out using all expressed genes across all replicate samples to show that replicates are highly correlated, and then hierarchical clustering of differentially expressed genes was carried out only for genes associated with at least a twofold change in one condition in either IRF4-WT versus Mock and IRF4-C99R vs Mock. Hierarchical clustering was used with Euclidean distance and average linkage clustering.
ExplaiNN models and calculation of information content
Deep-learning models
Four different ExplaiNN models49, each with 100 units, were trained on either IRF4-C99R or IRF4-WT ChIP-/DNase-seq data. The architecture of each unit was as follows: • 1st convolutional layer with 1 filter (26 × 4), batch normalization, exponential activation to improve the representation of the learnt sequence motifs112 and max pooling (7 × 7); • 1st fully connected layer with 100 nodes, batch normalization, ReLU activation and 30% dropout; and • 2nd fully connected layer with 1 node, batch normalization and ReLU activation. For training the models, ChIP-/DNase-seq peaks were resized to 201 bp by extending their summits 100 bp in each direction using BEDTools slop (version 2.30.0)93. Negative sequences were obtained by dinucleotide shuffling each dataset using BiasAway (version 3.3.0)113. Sequences were randomly split into training (80%), validation (10%) and test (10%) sets using the “train_test_split” function from scikit-learn (version 0.24.2) (https://jmlr.csail.mit.edu/papers/v12/pedregosa11a.html). Models were trained as described in ExplaiNN. Briefly, using the Adam optimizer (https://arxiv.org/abs/1412.6980) and binary cross entropy as loss function, applying one-hot encoding, setting the learning rate to 0.003 and batch size to 100, and using an early stopping criteria to prevent overfitting. Models were also interpreted following the specifications from ExplaiNN. The filter of each unit was converted into a motif by aligning all sub-sequences activating that filter’s unit by ≥50% of its maximum activation value in correctly predicted sequences. The importance of each motif was calculated as the product of the activation of its unit for each correctly predicted sequence activating that unit by ≥50% of its maximum activation value times the weight of the final layer of that unit.
Information content
For each ChIP-seq motif annotated as AICE1, AICE2, or AICE2FLIP, the information content for the four bp corresponding to the half-ISRE site was calculated using Biopython114. Summary motifs of the half-ISRE sites in present in these motifs were obtained by aligning the individual 4-mers corresponding to the half-ISRE sites. Statistical significance was computed using the Welch’s t test (one-tailed) as implemented in SciPy (version 1.7.1)115.
Statistics and reproducibility
Statistical analyses were mainly performed in R v4.0.3, R v4.1 and Prism v9.0.2. The correlation of data was determined by the Pearson correlation coefficient. Other data are presented as mean ± SEM or as box-whisker blots showing median, 25th–75th percentile, and minimum–maximum as stated in the respective figure legends. P values were determined by two-tailed unpaired Student′s t test without adjustment for multiple comparisons if not indicated otherwise in the figure legends. No statistical method was used to predetermine sample size. No data were excluded form the analyses apart from technical failures. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data presented in this study are available at the Gene Expression Omnibus116 under superseries accession GSE211445. Deposited datasets are DNaseI-Seq: GSE211441; ChIP-Seq: GSE211443; HL and NHL cell line RNA-Seq: GSE211444; BJAB cells with Tet-inducible control, IRF4-WT, and IRF4-C99R Illumina BeadChip HT-12 V4.0 expression arrays: GSE211913. RNA-Seq data of mouse splenic B cells are deposited in ArrayExpress database under ID E-MTAB-12522. High-througput sequencing data of the PMBCL cohort is in part publicly available72, and in part deposited (BioProject PRJNA851197 and EGAS00001006452) (Noerenberg et al., J Clin Oncol, in press) but currently only accessible upon request from F. Damm (frederik.damm@charite.de). Source data are provided with this paper.
References
Brady, S. W. et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat. Genet. 54, 1376–1389 (2022).
Rosenbauer, F. & Tenen, D. G. Transcription factors in myeloid development: balancing differentiation with transformation. Nat. Rev. Immunol. 7, 105–117 (2007).
Rui, L., Schmitz, R., Ceribelli, M. & Staudt, L. M. Malignant pirates of the immune system. Nat. Immunol. 12, 933–940 (2011).
Roos-Weil, D. et al. A recurrent activating missense mutation in Waldenström macroglobulinemia affects the DNA binding of the ETS transcription factor SPI1 and enhances proliferation. Cancer Discov. 9, 796–811 (2019).
Lazarian, G. et al. A hotspot mutation in transcription factor IKZF3 drives B cell neoplasia via transcriptional dysregulation. Cancer Cell 39, 380–393.e8 (2021).
Hayatsu, N. et al. Analyses of a mutant Foxp3 allele reveal BATF as a critical transcription factor in the differentiation and accumulation of tissue regulatory T cells. Immunity 47, 268–283.e9 (2017).
Morgunova, E. & Taipale, J. Structural perspective of cooperative transcription factor binding. Curr. Opin. Struct. Biol. 47, 1–8 (2017).
Lambert, S. A. et al. The human transcription factors. Cell 172, 650–665 (2018).
Chen, L., Glover, J. N., Hogan, P. G., Rao, A. & Harrison, S. C. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature 392, 42–48 (1998).
Cockerill, P. N. et al. Human granulocyte-macrophage colony-stimulating factor enhancer function is associated with cooperative interactions between AP-1 and NFATp/c. Mol. Cell. Biol. 15, 2071–2079 (1995).
Luscombe, N. M., Laskowski, R. A. & Thornton, J. M. Amino acid-base interactions: a three-dimensional analysis of protein-DNA interactions at an atomic level. Nucleic Acids Res. 29, 2860–2874 (2001).
Rohs, R. et al. The role of DNA shape in protein-DNA recognition. Nature 461, 1248–1253 (2009).
Baugh, E. H., Ke, H., Levine, A. J., Bonneau, R. A. & Chan, C. S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 25, 154–160 (2018).
Brass, A. L., Zhu, A. Q. & Singh, H. Assembly requirements of PU.1-Pip (IRF-4) activator complexes: inhibiting function in vivo using fused dimers. EMBO J. 18, 977–991 (1999).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Murphy, T. L., Tussiwand, R. & Murphy, K. M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 13, 499–509 (2013).
Matsuyama, T. et al. Molecular cloning of LSIRF, a lymphoid-specific member of the interferon regulatory factor family that binds the interferon-stimulated response element (ISRE). Nucleic Acids Res. 23, 2127–2136 (1995).
Huber, M. & Lohoff, M. IRF4 at the crossroads of effector T-cell fate decision. Eur. J. Immunol. 44, 1886–1895 (2014).
De Silva, N. S., Simonetti, G., Heise, N. & Klein, U. The diverse roles of IRF4 in late germinal center B-cell differentiation. Immunol. Rev. 247, 73–92 (2012).
Shaffer, A. L. et al. IRF4 addiction in multiple myeloma. Nature 454, 226–231 (2008).
Yang, Y. et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 21, 723–737 (2012).
Weilemann, A. et al. Essential role of IRF4 and MYC signaling for survival of anaplastic large cell lymphoma. Blood 125, 124–132 (2015).
Ochiai, K. et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 38, 918–929 (2013).
Krishnamoorthy, V. et al. The IRF4 gene regulatory module functions as a read-write integrator to dynamically coordinate T helper cell fate. Immunity 47, 481–497.e7 (2017).
Eisenbeis, C. F., Singh, H. & Storb, U. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 9, 1377–1387 (1995).
Glasmacher, E. et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science 338, 975–980 (2012).
Li, P. et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Falini, B. et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 95, 2084–2092 (2000).
Küppers, R. The biology of Hodgkin’s lymphoma. Nat. Rev. Cancer 9, 15–27 (2009).
Tiacci, E. et al. Analyzing primary Hodgkin and Reed-Sternberg cells to capture the molecular and cellular pathogenesis of classical Hodgkin lymphoma. Blood 120, 4609–4620 (2012).
Lamprecht, B. et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat. Med. 16, 571–9 (2010).
Mathas, S. et al. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat. Immunol. 7, 207–215 (2006).
Mottok, A. et al. Integrative genomic analysis identifies key pathogenic mechanisms in primary mediastinal large B-cell lymphoma. Blood 134, 802–813 (2019).
Mareschal, S. et al. Whole exome sequencing of relapsed/refractory patients expands the repertoire of somatic mutations in diffuse large B-cell lymphoma. Genes Chromosomes Cancer 55, 251–267 (2016).
Schmitz, R. et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. New Engl. J. Med. 378, 1396–1407 (2018).
Reddy, A. et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell 171, 481–494.e15 (2017).
Storb, U. et al. Cis-acting sequences that affect somatic hypermutation of Ig genes. Immunol. Rev. 162, 153–160 (1998).
Pasqualucci, L. et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 412, 341–346 (2001).
Klein, U. et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 7, 773–782 (2006).
Tanaka, N., Kawakami, T. & Taniguchi, T. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol. Cell. Biol. 13, 4531–4538 (1993).
Levy, D. E., Kessler, D. S., Pine, R., Reich, N. & Darnell, J. E. Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 2, 383–393 (1988).
Jundt, F. et al. Loss of PU.1 expression is associated with defective immunoglobulin transcription in Hodgkin and Reed-Sternberg cells of classical Hodgkin disease. Blood 99, 3060–3062 (2002).
Overbeck, B. M. et al. ETS1 encoding a transcription factor involved in B-cell differentiation is recurrently deleted and down-regulated in classical Hodgkin’s lymphoma. Haematologica 97, 1612–1614 (2012).
Mathas, S. et al. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-kappa B. EMBO J. 21, 4104–4113 (2002).
Küppers, R. et al. Identification of Hodgkin and Reed-Sternberg cell-specific genes by gene expression profiling. J. Clin. Investig. 111, 529–537 (2003).
Iwata, A. et al. Quality of TCR signaling determined by differential affinities of enhancers for the composite BATF-IRF4 transcription factor complex. Nat. Immunol. 460, 405 (2017).
Reisser, M. et al. Single-molecule imaging correlates decreasing nuclear volume with increasing TF-chromatin associations during zebrafish development. Nat. Commun. 9, 5218–11 (2018).
Novakovsky, G., Fornes, O., Saraswat, M., Mostafavi, S. & Wasserman, W. W. ExplaiNN: interpretable and transparent neural networks for genomics. Genome Biol. 24, 154–24 (2023).
Escalante, C. R., Yie, J., Thanos, D. & Aggarwal, A. K. Structure of IRF-1 with bound DNA reveals determinants of interferon regulation. Nature 391, 103–106 (1998).
Escalante, C. R. et al. Crystal structure of PU.1/IRF-4/DNA ternary complex. Mol. Cell 10, 1097–1105 (2002).
Sciammas, R. et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25, 225–236 (2006).
Schwab, U. et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature 299, 65–67 (1982).
Schleussner, N. et al. The AP-1-BATF and -BATF3 module is essential for growth, survival and TH17/ILC3 skewing of anaplastic large cell lymphoma. Leukemia 12, 933–2007 (2018).
Lollies, A. et al. An oncogenic axis of STAT-mediated BATF3 upregulation causing MYC activity in classical Hodgkin lymphoma and anaplastic large cell lymphoma. Leukemia 66, 848–101 (2017).
Tussiwand, R. et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature 490, 502–507 (2012).
Ushmorov, A. et al. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood 107, 2493–2500 (2006).
Seitz, V. et al. Classical Hodgkin’s lymphoma shows epigenetic features of abortive plasma cell differentiation. Haematologica 96, 863–870 (2011).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Lui, J. C. et al. A neomorphic variant in SP7 alters sequence specificity and causes a high-turnover bone disorder. Nat. Commun. 13, 700–714 (2022).
Arruabarrena-Aristorena, A. et al. FOXA1 mutations reveal distinct chromatin profiles and influence therapeutic response in breast cancer. Cancer Cell 38, 534–550.e9 (2020).
IRF4 International Consortium et al. A multimorphic mutation in IRF4 causes human autosomal dominant combined immunodeficiency. Sci. Immunol. 8, eade7953 (2023).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Moellering, R. E. et al. Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188 (2009).
Bushweller, J. H. Targeting transcription factors in cancer—from undruggable to reality. Nat. Rev. Cancer 19, 611–624 (2019).
Jameson, J. L. et al. Harrison′s Principles of Internal Medicine, Vol. 20e, Chapter 105 (McGraw Hill, 2020).
Hermann, S. & Kraywinkel, K. Faktenblatt: epidemiologie der hodgkin-lymphome in Deutschland. Der Onkol. 24, 280–285 (2018).
Kreher, S. et al. Mapping of transcription factor motifs in active chromatin identifies IRF5 as key regulator in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 111, E4513–E4522 (2014).
Bornkamm, G. W. et al. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 33, e137–e137 (2005).
Küppers, R., Schneider, M. & Hansmann, M.-L. Laser-based microdissection of single cells from tissue sections and PCR analysis of rearranged immunoglobulin genes from isolated normal and malignant human B cells. Methods Mol. Biol. 1956, 61–75 (2019).
Yoshida, K. et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 (2011).
Briest, F. et al. Frequent ZNF217 mutations lead to transcriptional deregulation of interferon signal transduction via altered chromatin accessibility in B cell lymphoma. Leukemia https://doi.org/10.1038/s41375-023-02013-9 (2023).
Ngo, V. N. et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 (2006).
Pfeifer, M. et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 110, 12420–12425 (2013).
Dai, B. et al. B-cell receptor-driven MALT1 activity regulates MYC signaling in mantle cell lymphoma. Blood 129, 333–346 (2017).
Bevington, S. L. et al. Inducible chromatin priming is associated with the establishment of immunological memory in T cells. EMBO J. 35, 515–535 (2016).
Cauchy, P. et al. Chronic FLT3-ITD signaling in acute myeloid leukemia is connected to a specific chromatin signature. Cell Rep. 12, 821–836 (2015).
Hipp, L. et al. Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc. Natl. Acad. Sci. USA 116, 880–889 (2019).
Popp, A. P., Hettich, J. & Gebhardt, J. C. M. Altering transcription factor binding reveals comprehensive transcriptional kinetics of a basic gene. Nucleic Acids Res. 49, 6249–6266 (2021).
Kuhn, T., Hettich, J., Davtyan, R. & Gebhardt, J. C. M. Single molecule tracking and analysis framework including theory-predicted parameter settings. Sci. Rep. 11, 9465–12 (2021).
van Zundert, G. C. P. et al. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 428, 720–725 (2016).
He, J., Wang, J., Tao, H., Xiao, Y. & Huang, S.-Y. HNADOCK: a nucleic acid docking server for modeling RNA/DNA-RNA/DNA 3D complex structures. Nucleic Acids Res. 47, W35–W42 (2019).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 (2000).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550–21 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137–R139 (2008).
Cauchy, P. et al. Dynamic recruitment of Ets1 to both nucleosome-occupied and -depleted enhancer regions mediates a transcriptional program switch during early T-cell differentiation. Nucleic Acids Res. 44, 3567–3585 (2016).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE Blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354–9355 (2019).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Saldanha, A. J. Java Treeview-extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004).
Zhu, L. J. et al. ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinforma. 11, 237–10 (2010).
Dale, R. K., Pedersen, B. S. & Quinlan, A. R. Pybedtools: a flexible Python library for manipulating genomic datasets and annotations. Bioinformatics 27, 3423–3424 (2011).
Gertz, J. et al. Distinct properties of cell-type-specific and shared transcription factor binding sites. Mol. Cell 52, 25–36 (2013).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Thurman, R. E. et al. The accessible chromatin landscape of the human genome. Nature 489, 75–82 (2012).
Piper, J. et al. Wellington: a novel method for the accurate identification of digital genomic footprints from DNase-seq data. Nucleic Acids Res. 41, e201 (2013).
Piragyte, I. et al. A metabolic interplay coordinated by HLX regulates myeloid differentiation and AML through partly overlapping pathways. Nat. Commun. 9, 3090–17 (2018).
Wurmus, R. et al. PiGx: reproducible genomics analysis pipelines with GNU Guix. Gigascience 7, giy123 (2018).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Köchert, K. et al. High-level expression of Mastermind-like 2 contributes to aberrant activation of the NOTCH signaling pathway in human lymphomas. Oncogene 30, 1831–1840 (2011).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Conway, J. R., Lex, A. & Gehlenborg, N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940 (2017).
Lachmann, A. et al. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 9, 1366–10 (2018).
Du, P., Kibbe, W. A. & Lin, S. M. lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 (2008).
Koo, P. K. & Ploenzke, M. Improving representations of genomic sequence motifs in convolutional networks with exponential activations. Nat. Mach. Intell. 3, 258–266 (2021).
Khan, A., Riudavets Puig, R., Boddie, P. & Mathelier, A. BiasAway: command-line and web server to generate nucleotide composition-matched DNA background sequences. Bioinformatics 37, 1607–1609 (2021).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Acknowledgements
The authors like to thank Natalia Soloch (Poznan), Sabine Werner (Berlin) and Brigitte Wollert-Wulf (Berlin) for excellent technical assistance, and P. Rahn (Berlin) for cell sorting. Support for infrastructure has been provided by the KinderKrebsInitiative Buchholz/Holm-Seppensen. We thank Wolfram Klapper (Kiel) for providing HL tissue samples, and Patrick Sorn (Mainz) and the Team Medical Genomics (TRON gGmbH, Mainz) for RNA-seq processing and data analysis. R.S. and M.G. received funding from the European Union′s Horizon 2020 research and innovation program under grant agreement No 952304. O.F. and W.W.W. were supported by grants from the Canadian Institutes of Health Research (PJT-162120), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2017-06824), and BC Children′s Hospital Foundation and Research Institute. R.K. and M.-L.H. were supported by the Wilhelm Sander Foundation (2018.101.1). This study was in part supported by grants from the Brigitte and Dr. Konstanze Wegener-Stiftung (#55), the Deutsche Krebshilfe (#70113148, #70113643) awarded to F.D., funds of the Max Planck Society (K150) to R.G., the Deutsche Forschungsgemeinschaft to M.J. and S.M. (MA 3313/2-1 and JA 1847/2-1). Work in the lab of C.B. and P.N.C. was funded by a Blood Cancer Research UK program grant (15001), the Kay Kendall Leukemia Fund (KKL725) and a studentship donation from Arthur D. Riggs from City of Hope for B.E.-W.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
V.F. and M.G. contributed equally to this work as co-second authors. N.S., P.C., V.F., M.G., and O.F. designed and performed experiments, interpreted data and wrote the manuscript; N.V., M.G.C., and S.S. performed and interpreted structure modeling; S.A.A. analyzed and interpreted microarray data; M.A.W., M.-L.H., S.H., and R.K. designed, performed, and interpreted HRS single-cell analyses; I.A. and A.R. performed and interpreted IHC analyses; F.D. and D.N. performed and interpreted PMBCL analyses; O.D. and SG designed and performed production of recombinant proteins; J.C.M.G. and A.Re. designed, performed and interpreted single-molecule fluorescence microscopy; T.B. analyzed RNA-seq data; E.K. and S.L. performed experiments and interpreted data; U.P., W.W., and M.C. performed experiments, interpreted data and contributed to writing of the MS; A.F. interpreted the data; B.E.-W., L.H., P.C., and N.O. designed, perfomed and interpreted DNase and ChIP experiments; A.W., W.X., M.Gr., and G.L. designed, performed and interpreted shRNA experiments; K.S., K.R., G.L., A.A., and W.W.W. interpreted the data and contributed to writing of the manuscript; P.N.C., C.S., R.S., R.K., R.G., M.J., and C.B. designed research, interpreted data and wrote the manuscript, S.M. designed research, interpreted data, wrote the manuscript and supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Vlad Cojocaru, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schleussner, N., Cauchy, P., Franke, V. et al. Transcriptional reprogramming by mutated IRF4 in lymphoma. Nat Commun 14, 6947 (2023). https://doi.org/10.1038/s41467-023-41954-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41954-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.