Abstract
Peptide-based therapeutics have gained attention as promising therapeutic modalities, however, their prevalent drawback is poor circulation half-life in vivo. In this paper, we report the selection of albumin-binding macrocyclic peptides from genetically encoded libraries of peptides modified by perfluoroaryl-cysteine SNAr chemistry, with decafluoro-diphenylsulfone (DFS). Testing of the binding of the selected peptides to albumin identified SICRFFC as the lead sequence. We replaced DFS with isosteric pentafluorophenyl sulfide (PFS) and the PFS-SICRFFCGG exhibited KD = 4–6 µM towards human serum albumin. When injected in mice, the concentration of the PFS-SICRFFCGG in plasma was indistinguishable from the reference peptide, SA-21. More importantly, a conjugate of PFS-SICRFFCGG and peptide apelin-17 analogue (N3-PEG6-NMe17A2) showed retention in circulation similar to SA-21; in contrast, apelin-17 analogue was cleared from the circulation after 2 min. The PFS-SICRFFC is the smallest known peptide macrocycle with a significant affinity for human albumin and substantial in vivo circulation half-life. It is a productive starting point for future development of compact macrocycles with extended half-life in vivo.
Similar content being viewed by others
Introduction
There are around 80 peptide drugs on the global market; more than 150 peptides are in clinical development, and another 400–600 peptides are undergoing preclinical studies1. In contrast to typical small molecule drugs, the large surface area of peptides allows them to interact with expanded binding interfaces commonly found in protein–protein interactions, protein–carbohydrate, and protein–DNA interactions. These proteins, classified as “undruggable targets,” have been difficult to target using conventional small molecule therapeutics but many of them have been addressed by peptide, proteins, or antibody therapeutics. Peptides are the smallest among the latter three modalities—2 to 10 kDa for peptides versus 150 kDa for full-sized antibodies—and they possess distinct pharmacokinetic (PK) properties. For example, bio-distribution of peptides and small proteins inside tumours and other non-vascularized tissues is improved compared to full-size antibodies. Several clinical candidates (TH1902, TH1904, BT5528, BT8009, BT1718, MMP-14) capitalize on such improved bio-distribution2,3,4. Unlike antibodies, which remain in circulation for 1–3 weeks due to the association with the neonatal Fc-receptor (FcRn) on the surface of endothelial cells5,6,7, peptide therapeutics clear within minutes to hours from plasma by renal filtration. Fast clearance is a beneficial property in several therapeutic applications, such as imaging (e.g., “tumour paint”), radionuclide delivery (e.g., LuratheraTM)8, and in administration of short-acting peptide hormones. However, for the more widespread adaptation of peptide modalities in diverse therapeutic applications, it is desired to tune the circulation lifetime of peptides from minutes to hours.
Only a few peptides exhibit a naturally extended circulation lifetime: a therapeutically relevant example is a natural venom, 39-residue peptide ‘exendin 4’, with low renal clearance in humans (5–7 h)9. This peptide gave rise to the FDA-approved drug exenatide for treating type 2 diabetes10,11. Despite favourable circulation half-life, modified derivatives of exenatide—liraglutide, albiglutide, dulaglutide, lixisenatide, and semaglutide1—have been developed to further tune circulation half-life and other PK properties. The majority of peptides and small proteins have to be modified as well to increase their circulation time. Such modification could be divided into several classes: Class 1: increase in size via covalent linkage to polyethylene glycol (PEG)12,13, polyglycerol14, and other synthetic macromolecules. Interestingly, steric hindrance by these size-increasing moieties also protects against proteolytic degradation15,16,17; Class 2: increase in size via controlled oligomerization18; Class 3: covalent linking to long-living serum protein (e.g., FDA-approved drugs albiglutide and dulaglutide, exenatide that conjugated to albumin and the IfG4 Fc domain)19,20,21 and Class 4: incorporation of moieties that bind non-covalently to serum proteins such as albumin22,23,24,25, immunoglobulin26,27, FcRn28, transthyretin29, and transferrin30,31. An important example in the last class is the lipidation of peptides to allow interaction with serum albumin. Lipidation has been one of the most successful strategies to prolong the half-life of peptides and small proteins such as insulin, giving rise to FDA-approved drugs such as Levemir®, Tresiba®, Victoza®, Saxenda®, and Ozempic® with extended serum half-life32,33. The improved properties of these and many other drugs stemming from their association with albumin mandate investigation of albumin as a carrier for therapeutic applications.
Albumin is the most abundant protein in plasma, with an average concentration of 600 µM and an average half-life of 19 days in humans34. The main mechanism leading to the long half-life of albumin and antibody are similar: both proteins interact with FcRns on the surface of endothelial cells6,7. This binding results in transient endocytosis of these proteins, and as a result, they are frequently sequestered from circulation and protected from clearance. At physiological pH, the binding affinity between albumin and FcRn is low; however, the interaction under acidic conditions in the endosome is strong to avoid lysosomal degradations and recycling of albumin to the extracellular space5. Albumin is a versatile carrier of essential fatty acids and diverse small organic molecules34. Among all the long-circulating serum proteins, albumin is considered to be one of the most important targets because of its ability to interact with hydrophobic small molecule drugs and enhance their pharmacokinetic properties. The recurrent therapeutic success of rationally lipidated peptides and proteins35 fuels interest in rational development of small molecules as well as non-lipidated proteins and peptides that bind to albumin.
Many FDA-approved small molecule drugs have an intrinsic affinity for human serum albumin (HSA). Targeted development of small molecules with high affinity for HSA has been a topic of research over the last 15 years (see recent review, ref. 25). Anti-HSA antibodies, nanobodies36, DARPins37, and other protein domains have been also developed. Such proteins can be fused to therapeutic proteins of interest to extend their in vivo circulation. Similarly, short peptides that bind to HSA could be used in tandem with therapeutic peptide or protein sequences to dial in predictable half-life for such therapeutics. Such short albumin-binding peptides could empower development of many future therapeutic peptides because they could be built into any genetically encoded peptide library (e.g., displayed on phage, RNA, and other platforms) to give rise to billion-scale libraries with predictable in vivo half-life. However, short HSA-binding peptides are scarce. A 31-mer peptide DX-236 (Ac-AEGTGDFWFCDRIAWYPQHLCEFLDPEGGGK-NH2) with a binding affinity of 1.9 µM was identified by Dyax Corp., and used to purify albumin (Fig. 1A)38. A 21-mer peptide SA-21 (Ac-RLIEDICLPRWGCLWEDD-NH2) with a binding affinity of ~0.5 µM to HSA was identified at Genentech (Fig. 1B)39 and subsequently conjugated to ligands for urokinase-type plasminogen activator22,40, Fab antibody fragments41,42 and small proteins43 to prolong their circulation half-lives. Heinis and co-workers developed a short heptapeptide modified by fluorescein isothiocyanide (FITC) and palmitic acid (FITC-EYEYKpalmESE-NH2) with a binding affinity of 39 nM to HSA (Fig. 1C)23, and the presence of both lipid moiety and fluorescein was critical for the binding of this peptide. This FITC-lipopeptide was fused with two different bicyclic peptides to boost the half-lives from minutes to hours23. Success of DX-236, SA-21, and FITC-lipopeptide, and other examples from the literature demonstrated the possibility of using HSA as a target for genetically-encoded selection to identify HSA-binding peptides with extended circulation half-life. Despite availability of generic lipid-based albumin binders, antibodies and protein-based binders to albumin, and small molecule-based human-albumin binders, there remains an interest in developing other classes to develop small peptide-based albumin-binding ligands with lower molecular weight23,39. In this work, we employ genetically encoded phage-displayed libraries of chemically modified macrocycles to develop albumin-binding mini scaffolds. To hone on the shortest possible peptide sequences, we employed a phage-displayed libraries SXCXnC, n = 3–5 modified with decafluorodiphenyl sulfone (DFS)44,45 where X is any amino acid except for cysteine (Fig. 1D). We hypothesized that a perfluoroaromatic linchpin might serve as a useful pharmacophore and it might be recognized by one of the binding sites of HSA similar to the binding of fatty acid; however, as we observed in the NMR studies and late-stage pharmacokinetic evaluation of albumin-binding macrocycles, the primary function of perfluoro-linchpin is to constrain the discovered peptide macrocycle in a productive albumin-binding conformation. While perfluoroaromatic linchpin alone does not equip a random peptide with albumin-binding properties, the change in the shape of perfluoroaromatic linchpin is detrimental to the albumin-binding properties and in vivo circulation.
Results
Selection of albumin binders
We devised and conducted three discovery campaigns that used different library architecture and selection strategies. In the first discovery campaign, we modified the phage libraries of structure SXCX4–5C with DFS following a previously published protocol and confirmed that 85% of the phage library is modified to yield octafluoro-diphenylsulfone-cross-linked macrocycles (OFS-SXCX4–5C-phage) (Fig. 2A, Supplementary Fig. 1A)44. We performed three rounds of phage selection using HSA coated to the surface of 96-well polystyrene plates as bait. In parallel, we screened the same library on polystyrene wells coated with Protein A (negative control) to distinguish specific HSA-binding sequences from poly-specific protein binding sequences (Supplementary Fig. 1A). In round 3, the recovery of the OFS-macrocycle library against HSA was 17-fold lower than the recovery of the unmodified library, indicating that the OFS linchpin contributes selection; however, the increase in round-to-round phage recovery was only modest. Despite depletion on protein A, the enriched population bound equally to HSA and protein A (Supplementary Fig. 1D). To mitigate these problems, the second discovery campaign increased the stringency and altered the immobilization of HSA between 96-well plate in rounds 1 and 3 and biotinylated HSA immobilized onto streptavidin beads in round 2 (Supplementary Fig. 2A). Such changes to selection stringency led to 200-fold increase in recovery in round 3 when compared to rounds 1 and 2. The recovery of the unmodified library panned against HSA was insignificant (Supplementary Fig. 2D). The binding of the OFS-macrocycle phage library recovered from round 3 to Protein A, ConA, and Casein was 2, 14, and 300-fold lower, respectively, compared to recovery on HSA-coated wells (Supplementary Fig. 4). These observations suggested that (i) specific albumin-binding sequences had been selected and (ii) the binding of these sequences to albumin required the presence of an OFS linchpin (Supplementary Fig. 4). Differential enrichment (DE) analysis of the next-generation sequencing (NGS) of all test and control experiments (Supplementary Table 1) identified families of peptide macrocycles that had statistically significantly higher (p < 0.05) enrichment in binding to HSA when compared to binding to unrelated protein (Supplementary Figs. 1 and 2). DE analysis of the first selection yielded three consensus motifs: STCHDITC (1), STCHYIGC (2) and STCHANC (3) (Supplementary Fig. 1E) whereas the second selection campaign yielded consensus motif: STCHTIYC (4) (Supplementary Fig. 2E). Although the original libraries were designed as SXCXnC where n = 4 and 5, and the SXCX3C sequences exist in such libraries only as rare deletions46, we still observed the enrichment of these rare sequences in the selection. Intrigued by the preference for smaller macrocycles, we devised a third selection campaign that employed only SXCX3C libraries modified with DFS (Fig. 2A). We used previously reported biotin capture44 to confirm that OFS linchpin can modify SXCX3C library to 88% conversion (Supplementary Fig. 3).
A Modification of phage-displayed SXCX3C disulfide library by DFS to yield OFS-SXCX3C library. B The OFS-SXCX3C library panned against a mixture of biotinylated HSA and His-tag expressed T4-GP in solution containing unlabelled milk proteins. Targets were captured separately with avidin beads and Ni-NTA beads affinity beads. In the negative control, OFS-SXCX3C library was panned against biotinylated ConA and captured with avidin beads. C Volcano plots and D Venn diagram visualizing the sequences from the OFS-SXCX3C phage-displayed library that were significantly enriched in the HSA screen compared to the naive library or selection against T4-GP, ConA. E A heat map display of the top 25 of 85 hits sequences from differential enrichment results. F Dipeptide motif analysis of all 85 hits. G Selected sequences for chemical synthesis of macrocycles for validation.
The SXCX3C library has been used in our previous publications successfully using only one round of sequencing46,47,48. It was sufficiently small (160,000 variants) to be fully covered by NGS and copy number of each member of the naive library was sufficiently large to afford quantification of the selection by NGS and DE analysis. To maximize the stringency of one-round-selection, we replaced immobilized albumin with soluble albumin as a screening bait. Soluble bait minimized preference of multivalent phage for multivalent display of albumin on the surface and avoided any detrimental change in albumin structure resulting from its immobilization on polystyrene surface. To mimic the complex serum environment, the panning mixture contained unlabelled milk proteins mixed with biotinylated HSA (Bio-HSA), His-tag fusion T4-PG protein (His6-T4-PG). After addition of phage, HSA and T4-PG were captured from this mixture with streptavidin or Ni-NTA affinity beads, respectively. Control selection employed the same mixture with biotinylated ConA (Bio-ConA) instead of Bio-HSA (Fig. 2B). The captured phage DNA was liberated from beads by treatment with hexane, amplified by PCR (Supplementary Fig. 6) and analysed by NGS (Fig. 2B). A DE analyses of selections against Bio-HSA, His6-T4-GP and Bio-ConA identified significantly enriched (p < 0.05, >3-fold) 85 hit sequences (Fig. 2C–E, Supplementary Fig. 4); their pairwise amino acid clustering identified FF and other motifs (Fig. 2F). From the combined analyses, we nominated sequences SICRFFC (5), SFCPMFC (6) and SLCKREC (7) as hits and STCQGEC (8) as a negative control for chemical synthesis and further validation (Fig. 2G). In summary, three selection strategies against HSA yielded divergent binding motifs. Such divergence of selection campaigns is not surprising because protein immobilization, depletion and amplification strategies were different among these strategies selection. To compare the sequences from these screens, we synthesized a series of hits and tested their binding to albumin using a battery of biochemical assays.
Validation of albumin binders
We observed non-specific reactivity of OFS-macrocyclic peptides with thiol nucleophiles such as glutathione (GSH) over several hours in basic pH (Supplementary Fig. 7). Replacing DFS with a less reactive pentafluorophenyl sulfide abolished the undesired reactivity: The resulting perfluorophenylsulfide (PFS)-macrocycles were unreactive to 2-mercaptoethanol over 3 weeks and unreactive towards free thiol on HSA (Supplementary Fig. 8). Molecular dynamics simulation suggested the OFS-macrocycles and the PFS-macrocycles exhibit similar ground-state conformational landscapes (Supplementary Fig. 9). Poor solubility of perfluoro-aryl cross-linked macrocycles 1–8 (STCHDITC, STCHYIGC, STCHANC, STCHTIYC, SICRFFC, SFCPMFC, SLCKREC, STCQGEC) made it difficult to evaluate their interaction with albumin in aqueous buffers. To increase their solubility, we synthesized these sequences with GGKKK or GGG tag at the C-terminus. The C-terminal tags provided sufficient solubility properties for downstream analyses. Some sequences were synthesized with both tags to verify that these tags did not affect binding to HSA.
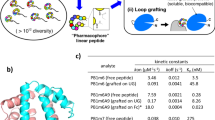
The unique fluorine handle in perfluoro-aryl-modified peptides made it possible to determine their binding to HSA using 19F NMR (Fig. 3A). In a typical experiment, we maintained peptide concentration at 50 µM and varied the concentration of HSA from 10 to 100 µM (4). The broadening of and disappearance of 19F signals that correspond to fluoroaromatic groups indicated the binding of the peptide to HSA (Fig. 3A and Supplementary Fig. 10A). It was not always possible to fit a definitive KD value to the binding response due to the complex binding behaviour and quality of the NMR signal. However, in an albumin titration series, it was straightforward to obtain a qualitative estimate such as the albumin concentration necessary to suppress 50% of the initial fluorine signal. After evaluation of eight sequences found in all discovery campaigns (Fig. 3A, Supplementary Figs. 10–11), we nominated PFS-SICRFFCGGG (14c) as the lead for further evaluation and PFS-STCQGECGGG (17c) as the negative control in further investigation.
A 19F NMR binding assay for macrocycles 12c (PFS-STCHANCGKKK), 14c (PFS-SICRFFCGGG), 15c (PFS-SFCPMFCGGG), 16c (PFS-SLCKRECGGG), and 17c (PFS-STCQGECGGG) at 20 µM against varying concentrations of HSA. B 19F NMR signals and C extrapolated binding curves for 14c (PFS-SICRFFCGGG) and five alanine mutants (21c, 22c, 23c, 24c, 25c) of this macrocycle.
A substantial change in measured KD values resulting from subtle amino-acid changes in PFS-macrocycles highlighted that the binding is not driven by the perfluoroaromatic linker alone. The peptide sequence plays a major role. Alanine scan of 14c further reinforced this observation (Fig. 3B, C). We observed that R4A or F5A changes in 14c ablated the binding between the perfluoro-macrocycles and HSA. In contrast, the S1A and I2A changes resulted in smaller changes in KD of 3- and 10-fold, respectively. The alanine scans indicate that variation to any of the four discovered amino acids in SXCXXXC sequence negatively affects the binding. Even serine, a constant residue in selection, becomes modestly important in albumin recognition as well. We titrated 14c and 17c against rat serum albumin and observed similar binding to rat and human albumin (Supplementary Fig. 12). We attempted to confirm the binding affinity of these sequences by isothermal titration calorimetry (ITC) using SA-21 as a control39; however, a complex multi-site binding behaviour for all peptides obscured the accurate evaluation of binding affinity by ITC (Supplementary Figs. 13–15), and this multi-step binding to albumin in ITC experiments has been observed in previous reports49. The 19F NMR assay, thus, was critically enabling for validation and ranking of the albumin binding leads.
A fluorescence polarization binding assay (FP) served as a complementary assay to confirm the binding affinities of the macrocycles labelled with the fluorophore BODIPY at the C- (18d) or N-terminus (14e). In a typical experiment, we used PFS-SICRFFCGGK (18d) or PFS-STCQGECGGK (19d) at 1 µM concentration and titrated HSA from 0.1 to 100 µM. The dose-response curve could fit a single-state binding model with a binding affinity of KD = 4–6 µM for 18d and at least 100 times weaker affinity for PFS-STCQGECGGK (19d) (Fig. 4A and Supplementary Fig. 16). BODIPY bound weakly to HSA with >300 µM binding affinity (Fig. 4A and Supplementary Fig. 16). The FP assay like the previous 19F NMR assay could also measure binding to other proteins or even complex mixtures (serum). A titration of the mouse serum (Supplementary Fig. 16C) yielded a similar binding profile to that observed in binding to pure albumin (Fig. 4A). Replacing HSA with lysozyme and RNAse, the assay detected no binding response, confirming that 18d binding was specific to HSA (Fig. 4B). Switching the location of the fluorescent probe from the N-terminus to C-terminus did not significantly change the affinity of 14c (KD = 4–6 µM, Supplementary Fig. 17). The switching from DFS (18f) to PFS (18d) exhibited no difference in FP experiment (Supplementary Fig. 18A), but minor differences were observed by NMR (Supplementary Fig. 18B). The results from FP were in the same order of magnitude as the semi-qualitative estimates acquired for BODIPY-free peptides by the 19F NMR binding assay, indicating that the presence of a fluorophore did not significantly increase the binding (Supplementary Fig. 19). Heinis and co-workers observed that fluorophores could dramatically increase binding affinity of peptides for albumin, and removing the fluorophore can be detrimental to the binding of the albumin ligand23. To exclude this possibility, we tested PFS-SICRFFCGG macrocycles with and without BODIPY in 19F NMR binding assay and observed similar binding affinity (Supplementary Fig. 19).
Elucidation of the binding pocket for perfluoro-macrocycles
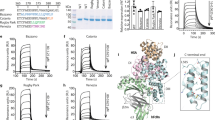
To identify the binding location of PFS-SICRFFCGGG (14c), we attempted to co-crystalize 14c with HSA but were not successful. As an alternative, we attempted to map the binding pocket of 14c by testing whether carbamazepine, diclofenac, or ibuprofen inhibit the binding of 14c (Supplementary Fig. 20). As none of these drugs influenced the binding of 14c, we concluded that primary binding pocket for 14c does not coincide with the primary binding pockets of carbamazepine, diclofenac, or ibuprofen. To identify the plausible binding location of 14c, we performed a series of docking calculations. Nine distinct sites on HSA bind to fatty acids50, some of which also accommodate ibuprofen and diclofenac51,52 (Supplementary Fig. 21, Fig. 5A). Docking of 14c to these nine sites identified binding site 1, which is close to Hemin-binding site, as the most favourable (Fig. 5A). The docking score for site 1 averaged over all the docking calculations across five distinct HSA structures (PDB 1E7E, 1E7F, 1E7G, 1E7H, 1E7I) was –8.95 ± 1.0 kcal/mol whereas the scores for the next sites were –6.6 ± 1.1, –6.2 ± 0.7, and –6.0 ± 1.2 kcal/mol (Supplementary Figs. 22 and 23). Observed docking preference away from known ibuprofen and diclofenac sites corroborated the experimental observation (Supplementary Fig. 20). As site 1 is hemin-binding site, a desired experimental validation of this prediction would be inhibition of HSA:14a interaction by hemin. We attempted to measure 14c:HSA interactions in the presence of hemin, but the results were inconclusive due to strong association of hemin and 14c in 19F NMR experiments. We note many drugs have primary and secondary (lower affinity) binding sites on HSA surface and further discussion on how multi-site binding affects prioritization of site 1 is provided Supplementary information section 28 “Docking Calculations”.
A Macrocycle 14c shows the lowest binding energy when binding to pocket 1 near the Hemin-binding site of HSA; three helical domains (DI, DII, DIII), the subdomains (A and B) and binding sites for ibuprofen and diclofenac are mapped on the same HSA structure. B SMD and umbrella sampling trajectories in site 1 revealed that ARG4 of the 14c forms stable salt bridges with ASP183 of the albumin whereas this salt bridge is absent in other binding modes (C, D). E KD values measured by 19F NMR. F ΔΔG for alanine point mutations calculated by free energy perturbation (FEP). G Correlation of experimental ΔΔG calculated from KD and ΔΔG from FEP calculation for the alanine mutants.
To further evaluate prioritization of albumin site 1 as a plausible binding site for 14c, we performed NVT (constant temperature, constant volume) and NPT (constant temperature, constant pressure) simulations of 14c in site 1 of HSA solvated with explicit TIP3P water molecules and calculated binding free energy using steered molecule dynamics (SMD) and umbrella sampling techniques. We then calculated the potential of mean force (PMF) of the unbinding process in biased MD simulations with a harmonic potential whose interaction center is located at a specific distance between the binding pocket and the center of mass of 14c. The ΔG was about –7.0 kcal/mol for pocket 1, while the ΔG for the other pockets was less than –4.2 kcal/mol. The PMF corroborated that pocket 1 has significantly stronger binding energy than the others (Fig. 5A, Supplementary Fig. 26). Furthermore, the calculated ΔΔG of free energy perturbation calculations (FEP) for 14c and five Ala mutants of 14c were in alignment with KD measured for the same alanine mutants in the 19F NMR assay (Fig. 3B, C). The biggest loss of function for R4A mutant highlighted the importance of Arginine interaction for albumin binding (Fig. 5E–G); in contrast, both NMR and FEP agreed on a relatively minor role of N-terminal Ser and minor loss for S1A mutant.
In summary, while we failed to co-crystalize 14c with HSA, the combined findings from FP and NMR, drug inhibition, docking/MD, and aligned performance of Ala-mutants in FEP and NMR studies offer a useful guide for optimization of 14c and its use in delivery. For example, both the binding pose in site 1, and NMR/FP studies suggest that both C- and N-termini of the 14c might be accessible as a plausible location for the attachments of payloads to 14c. We followed up on these suggestions in pharmacokinetic studies.
Circulation lifetime of 14c derivatives in mice
We evaluated the half-life of 14c, its derivatives, and SA-21 peptide as a positive control in mice by administrating a mixture of peptides into mice’s tail vein followed by HPLC/MS analysis of plasma samples at 2 and 60 min. The concentration of SA-21 in plasma at 2 and 60 min decreased subtly from 4 × 104 to 3 × 104 ng/mL whereas concentration of macrocycle 14c in plasma was 3 and 1.5 × 104 ng/mL at the same time points (Fig. 6B). In contrast, four derivatives of 14c were excreted at 2 min or were not detectable at 60 min. The fast-clearing analogues were linear SICRFFCGGG with the cysteines alkylated by iodoacetamide (14l), the alanine mutants PFS-SICAFFCGGG (23c) and PFS-SACRFFCGGG (21c), and the control PFS-STCQGECGGG sequence (17c); poor plasma retention of the last three analogues was in agreement with their significantly decreased association with albumin in the NMR studies (Fig. 3). Replacing PFS by hexafluorobenzene (HFB, 14j) and decafluorobiphenyl (DFB, 14k) did not ablate retention in plasma completely (Fig. 6A); however, concentration of HFB- and DFB-modified macrocycles after 60 min were factor of 10 lower when compared to PFS-modified 14c parent. These observations reinforced the important structural features of 14c: both the amino acids sequence of 14c and specific conformational constraint of this sequence imposed by PFS linker are critical for even a short-term retention in circulation.
A Lead sequence SICRFFC modified with N- and C-terminal extensions and different linchpins. B A mixture of compounds, including SICRFFC modifications, alanine scans, together with SA-21 injected into mice and monitored for their retention up to 1 h (n = 3 mice for each peptide). C N- and C-terminal extensions on PFS-SICRFFC macrocycle for payload attachments monitored for circulation time. n = 4 mice for each peptide. D Monitoring the effects of alanine mutants and C-terminal propargyl glycine on retention time. n = 4 mice for each peptide. E Effects of different linchpins on circulation time. n = 4 mice for each peptide. F 14c conjugated to therapeutically relevant payload, apelin-17 analogue (N3-PEG6-NMe17A2). Bars and boxes represent mean values ± SD.
In the same short-term circulation study, we examined the several N- and C-terminal derivatives of 14c to identify the permissive location in 14c molecule for the immobilization of the cargo. The two N-terminal derivatives of the 14c were macrocycle PFS-AICRFFCGGG (21c) with Ser to Ala replacement on N-terminus and macrocycle 14g synthesized from 14c by acylation of N-terminal Ser with azido-PEG6-carboxylic acid. Both 21c and exhibited a factor of 10 decrease in concentration after 60 min when compared to 14c parent. In NMR studies, modifications at the N-terminus led to a factor of 3 decrease in albumin binding capacity (Fig. 3) and the combined observations suggest that N-terminus is not an optimal location for modification of 14c. We then examined the retention of three C-terminal derivatives: PFS-SICRFFCGGK(N3) (26c) where K(N3) denotes a γ-azidolysine, PFS-SICRFFCGGGZ (20c), where Z indicated propargyl glycine and 20h synthesized by Cu-catalysed 1,3-dipolar cycloaddition (CuAAC) of 20c and di-azido-PEG3 linker. The concentration of 26c decreased in plasma by factor of >10 after 60 min but we were delighted to observe that plasma concentration of C-terminal conjugates 20c and 20h at 60 min was indistinguishable from that of the parent 14c macrocycles.
Encouraged by the initial evaluation of serum retention, we extended the study to 3 h. Gratifyingly we observed that the concentration of 14c and SA-21 in plasma after 3 h were indistinguishable (Fig. 6C). Analogues of 14c in which PFS was replaced by HFB (14j) or DFB (14k) perfluoroarenes consistently showed over 10x decrease in plasma concentration; a decrease of nearly 100x was observed for 14m analogue in which perfluoroarene was substituted by metabromoxylene linker (MBX) (Fig. 6C). As expected, macrocycle 25c, the F6A mutant of 14c, was not detectable in serum at or after 60 min and the concentration of macrocycle 21c S1A mutant of 14c was significantly lower than 14c at 3 h (Fig. 6D). After 3 h of circulation plasma concentrations of PFS-SICRFFCGGGZ (20c) at 3 h were indistinguishable from 14c (Fig. 6E) whereas the PFS-SICRFFCGGK(N3) 26c showed a suboptimal long-term performance in plasma (Supplementary Fig. 32). Similarly to the short-term observations (Fig. 6B), it appeared that the molecules with longer (Gly)3-linker at the C-terminus provided better plasma retention than those with (Gly)2 linker. We did not investigate the linker requirements in detail but we attached all subsequent payloads to the C-terminal end via (Gly)3-linker.
The PK properties of the analogues that contained polyethylene glycol (PEG) moieties at N- or C-termini reinforced the above observation and identified the optimal location for attachment of therapeutically-relevant payloads such as peptide apelin analogues53,54,55,56. The macrocycle 14g synthesized from 14c by acylation of N-terminal Ser with azido-PEG6-carboxylic acid was not detectable in plasma after 3 h whereas the concentration of C-terminal PEG-ylated compounds 20h and 14i after 3 h of circulation was indistinguishable from unmodified PFS-SICRFFCGGG. Compounds 14i is a product of C-terminal amidation of PFS-SICRFFCGGG-COOH by NH2-PEG5-N3, whereas in compound 20h di-azido-PEG3 linker was ligated to PFS-SICRFFCGGGZ (20c) by CuAAC. An indistinguishable plasma concentration of 14c, 14i, 20c, and 20h after 3 h suggested that structural changes after the (Gly)3-linker are permissive for albumin recognition and do not affect long-term circulation in plasma. Gratifyingly we observed that conjugation of PFS-SICRFFCGGG to apelin-17 analogue (N3-PEG6-NMe17A) increased its plasma retention significantly. We could not detect the apelin-17 analogue in plasma even 2 min after injection and such rapid clearance of apelin analogues is consistent with previous observations57. In contrast, plasma concentration of compound 20n, produced by CuAAC ligation of N3-PEG6-NMe17A53 to PFS-SICRFFCGGGZ (20c) by Cu-catalysed 1,3-dipolar cycloaddition was indistinguishable from the plasma concentration of 20c after 3 h. Such enhanced circulation of apelin-17 analogue promoted by a relatively small albumin-binding peptide is a promising starting point for more in-depth investigation and benchmarking to previously reported conjugates of apelin and albumin binding antibody58 or apelin and 40 kDa PEG group59.
Discussion
Late-stage modification of peptides and genetically-encoded (GE) libraries of peptides by cross-linkers (linchpins) is one of the common approaches to incorporate beneficial attributes to their properties60. Alkylation of cysteine residues in peptides via an SN2 reaction using bi- or tri-dentate alkyl halides has been used for cyclization of peptides, incorporation of unnatural fragments into the resulting macrocycles47,61,62, and late-stage modification of phage- and mRNA-displayed libraries to yield billion-scale GE libraries. Peptide cyclization via SNAr reaction with perfluoroarenes popularized by the Pentelute group forms alkyl-aryl thioethers45; other classes reactions have been developed to form aryl63,64,65, alkenyl and alkynyl thioethers66 in unprotected peptides. Aryl and perfluoroaryl thioethers are more resistant toward oxidation when compared to traditional bis-alkyl thioesters44. Decreased conformational mobility or aryl-thioether bond has been proposed to equip the resulting macrocycles with favourable properties such as cell permeability and proteolytic stability50,67. Our report described the selection from perfluoro-aryl macrocyclic GE libraries. There exists only one example of GE selection from SNAr-modified phage libraries: Lu and co-workers recently employed 2,4-difluoro-6-hydroxy-1,3,5-benzenetricarbonitrile (DFB) as a reagent that can modify phage libraries in water68. Chen and co-workers also used Pd-catalysed SNAr reaction to yield DNA-encoded libraries69. Both SNAr reaction yield macrocycles do not contain any fluorine atoms. On the other hand, fluorine handles present in perfluoroaryl-cross-linked macrocycles offer a unique possibility to use of 19F NMR to measure protein-macrocycle interactions. Interaction of perfluorinated aryls with proteins is also electronically distinct from non-fluorinated aromatic residues and in some cases it can offer uniquely advantageous interactions70.
An interesting observation in selection of GE OFS-macrocycle libraries is mild reactivity of these structures towards thiol nucleophiles71. Libraries of mild electrophiles72,73,74 and phage-displayed libraries with built-in electrophiles75 have emerged as an important starting point for discovery of covalent and reversibly covalent inhibitors. While we do not show it in our report, it is possible that an attenuated reactivity of OFS-macrocycles towards thiols can be used as an advantageous feature in discovery of inhibitors that form covalent bonds with thiol residues in proteins. If reactivity of the selected macrocycles is not desired, one can perform late-stage replacement of OFS moiety in the identified hits with nearly isosteric perfluorophenyl-sulfide. The PFS linchpin is not sufficiently reactive for direct modification of phage-displayed libraries in water, but replacement of DFS linchpin by PFS “post-discovery” maintains the conformation and binding affinity of the discovered macrocycles while alleviating their undesired electrophilicity. Our report, thus, suggests a general approach for the future utility of perfluoroaryl-modified libraries: Step1: select phage-displayed libraries of OFS-macrocycles against the desired target. Step 2: evaluate PFS-modified synthetic macrocycles for their ability to bind to these targets.
Human serum albumin target used in this publication is a commonly employed model target in screen of phage-displayed or DNA-encoded libraries (DEL) and traditional high-throughput screening (HTS). Albumin is a complex multi-pocket receptor with regions that can bind to fatty acid-like moieties, dicarboxylic acids as well a wide variety of aromatic and heterocyclic compounds and large dye molecules51,76. Albumin also contains several binding sites for peptides as well as small proteins that have been utilized for half-life extension strategies23,77. Small footprint peptide macrocycles discovered in this report add to a diverse set of known albumin binders. Small-molecules have been developed with astonishing affinity for human albumin, but such molecules exhibit no binding to mouse albumin and no retention in mice, unless such mouse is engineered to express human albumin78. Mid-size peptide macrocycle described in this report might provide an interesting opportunity for sufficient affinity but also much desired cross-reactivity between the species. The affinity of PFS-SICRFFCGGG (14c) (1–4 µM confirmed in FP and 19F NMR assays) is less than an order of magnitude away from the affinity of benchmark SA-21 (0.6 µM). An interesting role for (Gly)3 linker at the C-terminus in PK studies suggests that a C-terminal augmentation to this scaffold is a productive avenue for fine-tuning of the affinity without increasing the size of the peptide. Remarkably, 14c and its analogues with C-terminal extensions beyond (Gly)3 linker exhibited virtually identical retention times, mirroring the benchmark albumin binder SA-21. This compelling finding underscores their potential as highly promising tools for albumin-binding applications. The small size of such peptide-macrocycle families makes it trivial to make them by solid-phase synthesis or incorporate them a part of another sequence produced by solid phase synthesis. More importantly, the SICRFFC motif can be easily re-introduced into phage-displayed libraries to serve as a constant N-terminal albumin binding motif and giving rise to libraries with predictable circulation half-life. The compact nature of the macrocycle allows introducing this albumin-binding moiety to diverse display platforms within the initial stages of screening of genetically-encoded libraries, making it possible to perform de-novo discovery of peptide and macrocyclic modalities with predictable circulation lifetime in animal models.
Methods
Preparation of SXCX3C phage-displayed library
The procedures have been adopted and modified as previously described in publications that produced the M13-displayed SXCXXXC library46 and M13-SDB vector79 and SXCX4C and SXCX5C libraries80. In short, the vector SB4 QFT*LHQ was digested with Kpn I HF (NEB Cat# R3142S) and Eag I HF (NEB Cat# R3505S). A primer/template pair consisting of primer 5′-AT GGC GCC CGG CCG AAC CTC CAC C-3′ and template 5′-CC CGG GTA CCT TTC TAT TCT CAC TCT TCT X TGT XXX TGT GGT GGA GGT TCG GCC GGG CGC TTG ATT-3′ with ‘X’ representing a trinucleotide formed by annealing. The primer/template was then extended using Klenow DNA polymerase (NEB) according to the manufacturer’s instructions. The insert fragment was then digested with Kpn1 HF and Eag1 HF, gel purified, and ligated into the cut vector. The ligation products were then transformed into electrocompetent E. coli cells, and the transformants were grown overnight on E. coli TG1 to allow for phage production. Phage cultures were then centrifuged to remove cells and debris, and then the phage was precipitated by PEG precipitation (5% PEG 0.5 M NaCl). Other SDB vectors have been processed identically. We sequenced the naive libraries by Illumina sequencing, and the naive library of SXCXnC (n = 3–5) composition is publicly available at the following link: https://48hd.cloud/file/1470.
Panning campaigns
Three panning campaigns were conducted to discover binders for Human Serum Albumin (HSA) (Sigma-Aldrich, Cat# A4327-1G) with Protein A (Sigma-Aldrich, cat# P6031-1MG), Concanavalin A (Sigma-Aldrich, Cat# C2010-100MG) or T4-Gp as the negative controls. The proteins were immobilized on polystyrene plates or magnetic beads and panned against OFS-macrocyclic libraries or unmodified libraries. The details on panning experiments are discussed in supplementary information sections 3–5 entitled “Panning Strategy”. The analyses were performed by next-generation sequencing (NGS) of phage DNA similarly to previous publications47,62 and the details are described in Supplementary Information section 9 “Illumina Sequencing […]” and Section 31 “MATLAB Script for DE analysis”.
Preparation of Illumina sequencing samples
Similar to previous reports47,62, phage eluted from the target was subjected to PCR amplification to append Illumina multiplexing barcodes, and sequencing adaptors to randomized library regions (see Supplementary Information section 7 entitled “7. PCR amplification protocol for Illumina deep sequencing”). All PCR products were quantified by 2% (w/v) agarose gel in Tris-Borate-EDTA buffer at 100 volts for ~35 min using a low molecular weight DNA ladder as standard (NEB, Cat# N3233S). PCR products that contain different indexing barcodes were pooled, allowing 10 ng of each product in the mixture. The mixture was purified by eGel, quantified by quBit and sequenced using the Illumina NextSeq paired-end 500/550 High Output Kit v2.5 (2 × 75 Cycles). Data were automatically uploaded to BaseSpace™ Sequence Hub.
Processing of Illumina data
The Gzip compressed FASTQ files were downloaded from BaseSpace™ Sequence Hub. The files were converted into tables of DNA sequences and their counts per experiment. Briefly, FASTQ files were parsed based on unique multiplexing barcodes within the reads discarding any reads that contained a low-quality score. Mapping the forward (F) and reverse (R) barcoding regions, mapping of F and R priming regions allowing no more than one base substitution each and F-R read alignment allowing no mismatches between F and R reads yielded DNA sequences located between the priming regions as described in previous publications62. The files with DNA reads, raw counts, and mapped peptide modifications were uploaded to http://48hd.cloud/ server. Each experiment has a unique alphanumeric name and unique static URL in Supplementary Tables 1–3.
General protocol for cyclization with decafluorodiphenylsulfone
Procedure was analogous to previously published methods44,45. In short, linear peptide (10 mM) was dissolved in 50% acetonitrile and Tris buffer (50 mM Tris-HCl, pH 8.5), then 2 equivalents of DFS in 50% acetonitrile and Tris buffer (50 mM Tris-HCl, pH 8.5) was added to the mixture. The mixture was vortexed for 30 s, incubated for 2 h at room temperature, purified by HPLC and further lyophilized to yield product.
General protocol for cyclization with pentaflurophenyl-sulfide
Procedure was analogous to previously published methods44,45. In short, linear peptide (10 mM) was dissolved in 50 mM Tris in DMF, then 2 equivalents of PFS (Matrix Scientific, Cat# 009083) was added to the mixture. The mixture was vortexed for 30 s and allow to react for 1 h at RT. The reaction mixture was purified by HPLC and lyophilized to yield the product.
In vivo pharmacokinetic experiment
All the procedures and experiments involving animals were carried out using a protocol approved by the Health Sciences Laboratory Animal Services (HSLAS), University of Alberta. The protocol was approved as per the Canadian Council on Animal Care (CCAC) guidelines. Approved protocol # AUP00002467. All mice (Strain: C57BL/6J) were maintained in pathogen-free conditions at the University of Alberta breeding facility. Housed on ventilated caging (Tecniplast); Temperature: 21 +/− 2 degrees Celcius; Humidity: 30–70% rH; Light Cycle: 12 L:12D. Caging: 1 cm of aspen chip, 1 cotton Nestlet, 1 Bed’r’nest, 1 polycarbonate tube; Water: ad lib, deionized UV filtered water; Food: ad lib, irradiated Lab Diet 50LD Rodent Chow (4.5% fat). Peptide mixtures of 100 µM were prepared in PBS. Mice were administered with 200 µL of the peptide mixture solution with tail veil injection. A series of 6 blood samples were collected at time points from 2 min up to 240 min (Fig. 6B: 2 min, 60 min; Fig. 6C–E: 2 min, 60 min, 180 min; Supplementary Fig. 32: 2 min, 5 min, 30 min, 60 min, 120 min, 240 min). Samples were collected in tubes that contained sodium citrate as an anticoagulant and then centrifuged at 5 min at 2000 × g to collect the blood plasma. 10 µL of plasma portion were transferred into a tube containing 40 µL of 8:2 acetonitrile/water to precipitate proteins. The samples were centrifuged at 17,000 × g for 10 min at 4 °C. Supernatants were then transferred to new tubes and subjected to analysis by LC-MS.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supplementary information document contains Supplementary Figs. 1–99, Tables 1–10, synthetic methods and characterization of compounds, details of phage display selection, next-generation sequencing and bioinformatics analysis, and all biochemical assays. PDB files produced by docking, data for the MATLAB script and the MATLAB scripts are available at Supplementary data.zip. The DNA reads, raw counts, and mapped peptide modifications are available publicly at http://48hd.cloud/ server and the links to individual files are listed in Supplementary Tables 1–3. The HSA molecular docking scripts are available at https://doi.org/10.5281/zenodo.8165764.
Change history
21 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41467-023-43517-3
References
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug. Discov. 20, 309–325 (2021).
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Dreher, M. R. et al. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl Cancer Inst. 98, 335–344 (2006).
Firer, M. A. & Gellerman, G. Targeted drug delivery for cancer therapy: the other side of antibodies. J. Hematol. Oncol. 5, 70 (2012).
Andersen, J. T. et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat. Commun. 3, 610 (2012).
Latvala, S., Jacobsen, B., Otteneder, M. B., Herrmann, A. & Kronenberg, S. Distribution of FcRn across species and tissues. J. Histochem. Cytochem. 65, 321–333 (2017).
Rudnik-Jansen, I. & Howard, K. A. FcRn expression in cancer: mechanistic basis and therapeutic opportunities. J. Control. Release 337, 248–257 (2021).
Li, X., Sun, Y., Ma, L., Liu, G. & Wang, Z. The renal clearable magnetic resonance imaging contrast agents: state of the art and recent advances. Molecules 25, 5072 (2020).
Eng, J., Kleinman, W. A., Singh, L., Singh, G. & Raufman, J. P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 267, 7402–7405 (1992).
Nielsen, L. L., Young, A. A. & Parkes, D. G. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul. Pept. 117, 77–88 (2004).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Turecek, P. L., Bossard, M. J., Schoetens, F. & Ivens, I. A. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 105, 460–475 (2016).
Wu, L. et al. Precise and combinatorial PEGylation generates a low-immunogenic and stable form of human growth hormone. J. Control. Release 249, 84–93 (2017).
Tully, M. et al. Polyglycerol for half-life extension of proteins—alternative to PEGylation. Biomacromolecules 22, 1406–1416 (2021).
Ilyas, H. et al. Effect of PEGylation on host defense peptide complexation with bacterial lipopolysaccharide. Bioconjug. Chem. 32, 1729–1741 (2021).
Lawrence, P. B. et al. Criteria for selecting PEGylation sites on proteins for higher thermodynamic and proteolytic stability. J. Am. Chem. Soc. 136, 17547–17560 (2014).
Xiao, Q., Ashton, D. S., Jones, Z. B., Thompson, K. P. & Price, J. L. Long-range PEG stapling: macrocyclization for increased protein conformational stability and resistance to proteolysis. RSC Chem. Biol. 1, 273–280 (2020).
Dwyer, J. J. et al. Design of helical, oligomeric HIV-1 fusion inhibitor peptides with potent activity against enfuvirtide-resistant virus. Proc. Natl Acad. Sci. USA 104, 12772–12777 (2007).
Arslan, F. B., Ozturk Atar, K. & Calis, S. Antibody-mediated drug delivery. Int. J. Pharm. 596, 120268 (2021).
Richards, D. A. Exploring alternative antibody scaffolds: antibody fragments and antibody mimics for targeted drug delivery. Drug Discov. Today Technol. 30, 35–46 (2018).
Duivelshof, B. L. et al. Therapeutic Fc-fusion proteins: current analytical strategies. J. Sep. Sci. 44, 35–62 (2021).
Angelini, A., Morales-Sanfrutos, J., Diderich, P., Chen, S. & Heinis, C. Bicyclization and tethering to albumin yields long-acting peptide antagonists. J. Med. Chem. 55, 10187–10197 (2012).
Zorzi, A., Middendorp, S. J., Wilbs, J., Deyle, K. & Heinis, C. Acylated heptapeptide binds albumin with high affinity and application as tag furnishes long-acting peptides. Nat. Commun. 8, 16092 (2017).
Bern, M., Sand, K. M., Nilsen, J., Sandlie, I. & Andersen, J. T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release 211, 144–162 (2015).
Zorzi, A., Linciano, S. & Angelini, A. Non-covalent albumin-binding ligands for extending the circulating half-life of small biotherapeutics. Medchemcomm 10, 1068–1081 (2019).
Menegatti, S., Hussain, M., Naik, A. D., Carbonell, R. G. & Rao, B. M. mRNA display selection and solid-phase synthesis of Fc-binding cyclic peptide affinity ligands. Biotechnol. Bioeng. 110, 857–870 (2013).
Sockolosky, J. T., Kivimae, S. & Szoka, F. C. Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice. PLoS ONE 9, e102566 (2014).
Sockolosky, J. T. & Szoka, F. C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 91, 109–124 (2015).
Penchala, S. C. et al. A biomimetic approach for enhancing the in vivo half-life of peptides. Nat. Chem. Biol. 11, 793–798 (2015).
Wang, Z. et al. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci. Rep. 5, 12651 (2015).
Kuang, Y. et al. Dual functional peptide-driven nanoparticles for highly efficient glioma-targeting and drug codelivery. Mol. Pharm. 13, 1599–1607 (2016).
Bech, E. M., Pedersen, S. L. & Jensen, K. J. Chemical strategies for half-life extension of biopharmaceuticals: lipidation and its alternatives. ACS Med. Chem. Lett. 9, 577–580 (2018).
van Witteloostuijn, S. B., Pedersen, S. L. & Jensen, K. J. Half-life extension of biopharmaceuticals using chemical methods: alternatives to PEGylation. ChemMedChem 11, 2474–2495 (2016).
Peters, T. All About Albumin: Biochemistry, Genetics, and Medical Applications (Elsevier Science, 1995).
Zaykov, A. N., Mayer, J. P. & DiMarchi, R. D. Pursuit of a perfect insulin. Nat. Rev. Drug Discov. 15, 425–439 (2016).
Tijink, B. M. et al. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: taking advantage of modular Nanobody technology. Mol. Cancer Ther. 7, 2288–2297 (2008).
Steiner, D. et al. Half-life extension using serum albumin-binding DARPin® domains. Protein Eng. Des. Sel. 30, 583–591 (2017).
Sato, A. K. et al. Development of mammalian serum albumin affinity purification media by peptide phage display. Biotechnol. Prog. 18, 182–192 (2002).
Dennis, M. S. et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J. Biol. Chem. 277, 35035–35043 (2002).
Pollaro, L. et al. Bicyclic peptides conjugated to an albumin-binding tag diffuse efficiently into solid tumors. Mol. Cancer Ther. 14, 151–161 (2015).
Nguyen, A. et al. The pharmacokinetics of an albumin-binding Fab (AB.Fab) can be modulated as a function of affinity for albumin. Protein Eng. Des. Sel. 19, 291–297 (2006).
Dennis, M. S. et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 67, 254–261 (2007).
Langenheim, J. F. & Chen, W. Y. Improving the pharmacokinetics/pharmacodynamics of prolactin, GH, and their antagonists by fusion to a synthetic albumin-binding peptide. J. Endocrinol. 203, 375–387 (2009).
Kalhor-Monfared, S. et al. Rapid biocompatible macrocyclization of peptides with decafluoro-diphenylsulfone. Chem. Sci. 7, 3785–3790 (2016).
Spokoyny, A. M. et al. A perfluoroaryl-cysteine S(N)Ar chemistry approach to unprotected peptide stapling. J. Am. Chem. Soc. 135, 5946–5949 (2013).
He, B. et al. Compositional bias in naive and chemically-modified phage-displayed libraries uncovered by paired-end deep sequencing. Sci. Rep. 8, 1214 (2018).
Ekanayake, A. I. et al. Genetically encoded fragment-based discovery from phage-displayed macrocyclic libraries with genetically encoded unnatural pharmacophores. J. Am. Chem. Soc. 143, 5497–5507 (2021).
Triana, V. & Derda, R. Tandem Wittig/Diels-Alder diversification of genetically encoded peptide libraries. Org. Biomol. Chem. 15, 7869–7877 (2017).
Callmann, C. E. et al. Antitumor activity of 1,18-octadecanedioic acid-paclitaxel complexed with human serum albumin. J. Am. Chem. Soc. 141, 11765–11769 (2019).
Bhattacharya, A. A., Grune, T. & Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 303, 721–732 (2000).
Ghuman, J. et al. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 353, 38–52 (2005).
Zhang, Y. et al. Structural basis of non-steroidal anti-inflammatory drug diclofenac binding to human serum albumin. Chem. Biol. Drug Des. 86, 1178–1184 (2015).
Fernandez, K. X. et al. Metabolically stable apelin-analogues, incorporating cyclohexylalanine and homoarginine, as potent apelin receptor activators. RSC Med. Chem. 12, 1402–1413 (2021).
Tatemoto, K. et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 251, 471–476 (1998).
de Oliveira, A. A., Vergara, A., Wang, X., Vederas, J. C. & Oudit, G. Y. Apelin pathway in cardiovascular, kidney, and metabolic diseases: Therapeutic role of apelin analogs and apelin receptor agonists. Peptides 147, 170697 (2022).
Fischer, C. et al. Optimizing PEG-extended apelin analogues as cardioprotective drug leads: importance of the KFRR motif and aromatic head group for improved physiological activity. J. Med. Chem. 63, 12073–12082 (2020).
Japp, A. G. et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 121, 1818–1827 (2010).
Read, C. et al. Apelin peptides linked to anti-serum albumin domain antibodies retain affinity in vitro and are efficacious receptor agonists in vivo. Basic Clin. Pharmacol. Toxicol. 126, 96–103 (2020).
Jia, Z. Q. et al. Cardiovascular effects of a PEGylated apelin. Peptides 38, 181–188 (2012).
Derda, R. & Jafari, M. R. Synthetic cross-linking of peptides: molecular linchpins for peptide cyclization. Protein Pept. Lett. 25, 1051–1075 (2018).
Heinis, C., Rutherford, T., Freund, S. & Winter, G. Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 5, 502–507 (2009).
Wong, J. Y. et al. Genetically-encoded discovery of proteolytically stable bicyclic inhibitors for morphogen NODAL. Chem. Sci. 12, 9694–9703 (2021).
Hanaya, K. et al. Rapid nickel(ii)-promoted cysteine S-arylation with arylboronic acids. Chem. Commun. 55, 2841–2844 (2019).
Messina, M. S. et al. Organometallic gold(III) reagents for cysteine arylation. J. Am. Chem. Soc. 140, 7065–7069 (2018).
Zhang, C., Vinogradova, E. V., Spokoyny, A. M., Buchwald, S. L. & Pentelute, B. L. Arylation chemistry for bioconjugation. Angew. Chem. 58, 4810–4839 (2019).
Ceballos, J., Grinhagena, E., Sangouard, G., Heinis, C. & Waser, J. Cys-Cys and Cys-Lys stapling of unprotected peptides enabled by hypervalent iodine reagents. Angew. Chem. 60, 9022–9031 (2021).
de Araujo, A. D., Hoang, H. N., Lim, J., Mak, J. Y. W. & Fairlie, D. P. Tuning electrostatic and hydrophobic surfaces of aromatic rings to enhance membrane association and cell uptake of peptides. Angew. Chem. 61, e202203995 (2022).
Zheng, X., Liu, W., Liu, Z., Zhao, Y. & Wu, C. Biocompatible and rapid cyclization of peptides with 2,4-difluoro-6-hydroxy-1,3,5-benzenetricarbonitrile for the development of cyclic peptide libraries. Bioconjug. Chem. 31, 2085–2091 (2020).
Yang, P. et al. Streamlined construction of peptide macrocycles via palladium-catalyzed intramolecular S-arylation in solution and on DNA. Chem. Sci. 12, 5804–5810 (2021).
Dougherty, D. A. The cation-pi interaction. Acc. Chem. Res. 46, 885–893 (2013).
Ngambenjawong, C., Pineda, J. M. & Pun, S. H. Engineering an affinity-enhanced peptide through optimization of cyclization chemistry. Bioconjug. Chem. 27, 2854–2862 (2016).
Abbasov, M. E. et al. A proteome-wide atlas of lysine-reactive chemistry. Nat. Chem. 13, 1081–1092 (2021).
Brighty, G. J. et al. Using sulfuramidimidoyl fluorides that undergo sulfur(VI) fluoride exchange for inverse drug discovery. Nat. Chem. 12, 906–913 (2020).
Kuljanin, M. et al. Reimagining high-throughput profiling of reactive cysteines for cell-based screening of large electrophile libraries. Nat. Biotechnol. 39, 630–641 (2021).
Chen, S. et al. Identification of highly selective covalent inhibitors by phage display. Nat. Biotechnol. 39, 490–498 (2021).
Sudlow, G., Birkett, D. J. & Wade, D. N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 11, 824–832 (1975).
Li, Y. et al. Versatile protein recognition by the encoded display of multiple chemical elements on a constant macrocyclic scaffold. Nat. Chem. 10, 441–448 (2018).
Vantourout, J. C. et al. In vivo half-life extension of BMP1/TLL metalloproteinase inhibitors using small-molecule human serum albumin binders. Bioconjug. Chem. 32, 279–289 (2021).
Sojitra, M. et al. Genetically encoded multivalent liquid glycan array displayed on M13 bacteriophage. Nat. Chem. Biol. 17, 806–816 (2021).
Tjhung, K. F. et al. Silent encoding of chemical post-translational modifications in phage-displayed libraries. J. Am. Chem. Soc. 138, 32–35 (2016).
Acknowledgements
This research was supported by a research contract from the Ferring Research Institute, Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2016-402511 to R.D.), NSERC Accelerator Supplement (to R.D.), and Canadian Institutes of Health Research grant (CIHR FRN 168961, to R.D.). This work was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM124160 (PI: Y.-S.L.) Infrastructure support was provided by CFI New Leader Opportunity (to R.D.). We thank Dr. Randy Whittal for assistance with LCMS, and Mark Miskolzie for assistance with 19F NMR kinetics.
Author information
Authors and Affiliations
Contributions
R.D. conceived, designed, and supervised the overall project. E.R.A.-P., S.K.-M. and J.W. performed chemical modifications on phage display libraries and selection against albumin under the supervision of R.D., J.J.D. and J.M.N. W.C.K.P. designed and supervised all 19F NMR assays, J.W., A.E., R.Q., S.E.K. and J.L. performed the 19F NMR binding assays. J.W. performed and analysed all fluorescence polarization assays. J.W. and E.R.A.-P. performed and analysed all isothermal titration calorimetry assays. J.W., A.E., S.K., R.Q., E.R.A.-P. and S.K-M. chemically synthesized and characterized all phage display-derived peptides conjugates and K.F. synthesized and characterized all apelin conjugates under supervision of J.C.V. M.M., R.D. and A.E. designed in vivo studies. S.S. performed in vivo mice studies and A.E. performed analysis of all samples from in vivo studies. P.K., L.V., J.M., Y.-S.L., H.K. and H.C. performed computational studies. R.D., J.W. and A.E. wrote the manuscript and edited the final manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing financial interests. Ratmir Derda is the founder and CEO of 48Hour Discovery Inc. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong, J.Y.K., Ekanayake, A.I., Kharchenko, S. et al. Genetically encoded discovery of perfluoroaryl macrocycles that bind to albumin and exhibit extended circulation in vivo. Nat Commun 14, 5654 (2023). https://doi.org/10.1038/s41467-023-41427-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41427-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.