Abstract
Small interference RNAs are the key components of RNA interference, a conserved RNA silencing or viral defense mechanism in many eukaryotes. In Drosophila melanogaster, Dicer-2 (DmDcr-2)-mediated RNAi pathway plays important roles in defending against viral infections and protecting genome integrity. During the maturation of siRNAs, two cofactors can regulate DmDcr-2’s functions: Loqs-PD that is required for dsRNA processing, and R2D2 that is essential for the subsequent loading of siRNAs into effector Ago2 to form RISC complexes. However, due to the lack of structural information, it is still unclear whether R2D2 and Loqs-PD affect the functions of DmDcr-2 simultaneously. Here we present several cryo-EM structures of DmDcr-2/R2D2/Loqs-PD complex bound to dsRNAs with various lengths by the Helicase domain. These structures revealed that R2D2 and Loqs-PD can bind to different regions of DmDcr-2 without interfering with each other. Furthermore, the cryo-EM results demonstrate that these complexes can form large oligomers and assemble into fibers. The formation and depolymerization of these oligomers are associated with ATP hydrolysis. These findings provide insights into the structural mechanism of DmDcr-2 and its cofactors during siRNA processing.
Similar content being viewed by others
Introduction
RNA interference (RNAi) is a conserved post-transcriptional regulatory mechanism mediated by small RNAs. It plays vital roles in various biological processes such as gene regulation, tumor progression, antiviral defense, and controlling transposable elements in the genome across eukaryotic organisms1,2,3,4. In this pathway, Argonaute (Ago) proteins, which are components of the RNA-induced silencing complex (RISC), identify target RNAs by complementary base pairing using its bound small noncoding RNAs. The small RNAs can be microRNAs (miRNAs) generated from short hairpin RNA (pre-miRNA) or small interfering RNAs (siRNAs) from double-stranded RNA (dsRNA), both of which are 21–25 nucleotide (nt) duplexes with phosphate at 5’ end and a 2-nt overhang ended with hydroxyl termini at 3’ end5. In mammals, miRNA and siRNA are generated by the same RNase-III enzyme Dicer, while in Drosophila melanogaster, they are generally not only produced by different Dicer (DmDcr-1 and DmDcr-2) but also sorted to functionally distinct Argonaute proteins as DmAgo1 and DmAgo2, respectively6.
Dicer, a member of the RNase-III family of enzymes, is highly conserved across eukaryotes. It consists of several domains, including an N-terminal DExD/H helicase (Helicase) domain, cap domains (Platform domain and PAZ domain), a core region with two RNase-III domains, and C-terminal double-stranded RNA-binding domains (dsRBD). The arrangement and collaboration of these domains are crucial in determining the precise length and structural characteristics of the RNA molecules cleaved by RNase III7,8,9,10,11. The helicase domain in HsDicer (Human Dicer) and DmDcr-1 exhibits a low affinity for dsRNA-binding and lacks the capability to hydrolyze ATP. Consequently, their primary substrate is limited to short hairpin-shaped pre-miRNA. In contrast, the Helicase domain of DmDcr-2 differs from HsDicer or DmDcr-1 as it can bind to dsRNA with high affinity and facilitates ATP hydrolysis to transport dsRNA substrates to the RNA processing center12,13,14.
When the small RNA duplex precursors are processed by Dicer proteins, the cofactor dsRNA-binding proteins (dsRBPs) composed of multiple dsRBDs assist Dicer to complete the dicing process and transfer the products to Argonaute proteins15. Among these cofactors, TRBP/PACT in mammals and R2D2/Loquacious (Loqs) in Drosophila have been well-studied. These cofactors bind to Dicer at a stoichiometric ratio and affect its cleavage activity, product length, and product delivery etc.16,17,18,19. All dsRBDs adopt a common α–β–β–β–α-fold, but they can be divided into two distinct classes based on their dsRNA-binding ability. Type A dsRBDs share a similar dsRNA-binding way that is sequence-independent, whereas type B dsRBDs have no dsRNA-binding activity20,21. TRBP/PACT and Loqs-PA/Loqs-PB have two type A dsRBDs and one type B dsRBD that bind HsDicer and DmDcr-1, respectively22,23,24. R2D2 and Loqs isoform D (Loqs-PD) have two type A dsRBDs, serving as partner proteins of DmDcr-224,25,26.
It is generally believed that two kinds of siRNA are generated by DmDcr-2, exo-siRNA for defense against viruses and endo-siRNAs (or esiRNAs) for protecting genome integrity from the serious threat of transposable elements27. DmDcr-2 has been observed in several conformational states during the cleavage of dsRNA substrates, including the translocation state, active dicing state, and post dicing state, among others10,28. The presence of Loqs-PD has been found to enhance the processing activity of DmDcr-2 by increasing the initial dsRNA-binding rates of its helicase domain19,29,30. After the dsRNA substrate cleavage, R2D2 helps DmDcr-2 to direct the product siRNA duplex into the RNA interference effector Ago2 with the Hsp70/90 chaperone systems, rather than enhancing its cleavage activity7,31,32,33,34. Therefore, R2D2 serves as an important component of the RISC loading complex (RLC). It has been reported that Loqs-PD and R2D2 act sequentially in the siRNA pathway, and they are required in a common silencing mean that is triggered by either exogenous or endogenous dsRNAs35.
Like other cofactors, R2D2 and Loqs-PD interact with the Helicase domain of DmDcr-2 through their C-terminus. Recent studies have investigated the individual structures and functions of the DmDcr-2/Loqs-PD and DmDcr-2/R2D2 complexes, revealing that their binding sites do not overlap10,36. In addition, previous research has indicated the formation of a ternary complex involving DmDcr-2, Loqs-PD, and R2D2 in vivo37. However, the specific arrangement and synergistic interactions within this ternary complex remain poorly understood. In order to investigate the effects on DmDcr-2’s function in the ternary complex during siRNA generation, we constructed the DmDcr-2 mutant (D1217N/D1476N, DmDcr-2DDNN) which exhibited no RNA dicing activity but possessed helicase activity in this work. Using cryo-electron microscopy (cryo-EM), we determined several structures of the protein complexes with dsRNAs. The structures reveal that R2D2 and Loqs-PD can bind to DmDcr-2 simultaneously and induce the complex to form an oligomer in the presence of dsRNAs and ATP. These findings provide insights into the interactions between the components.
Results
The architecture of the overall structures
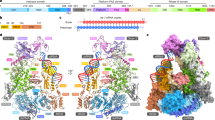
We co-expressed and purified the DmDcr-2DDNN and wild-type R2D2 in insect SF9 cells and then incubated them with purified full-length Loqs-PD expressed in E. coli. The size-exclusion chromatography (SEC) results showed that the three components can form a stable complex in vitro (Supplementary Fig. 1a, b). To mimic the substrates and products, we designed and transcribed two dsRNAs, one with a length of 50 bp and the other with a length of 19 bp (Fig. 1a). The protein complexes and RNAs were then incubated at equal molar ratios in buffer containing ATP and Magnesium ions. Then we use cryo-EM to analyze the structures of the ternary protein complex with 50-bp and 19-bp dsRNA substrates, respectively (termed 50-bp complex and 19-bp complex hereafter). Although large aggregates were not apparent in the original cryo-EM images (Supplementary Fig. 1c, f), all the 2D class averages revealed that protein-RNA complexes aggregate together and assemble into higher-order complexes (Supplementary Fig. 1d, h). From the 50-bp complex dataset, we acquired two structures: one in the dimer state with two DmDcr-2 molecules at a resolution of 3.72 Å, and another in the trimer state with three DmDcr-2 molecules at a resolution of 3.74 Å. In addition, from the 19-bp complex dataset, we obtained a trimer state structure with three DmDcr-2 molecules at a resolution of 3.70 Å. To facilitate clarity, we designated each monomer within the oligomer as 1st, 2nd, and 3rd from the bottom-up in the placement view (Fig. 1b–e). The protein components in both states displayed similar spatial arrangements, and the DmDcr-2 molecules were oriented in a similar direction. To our knowledge, this represents a novel organization of Dicer proteins (Fig. 1f). Notably, only the C-terminus region of R2D2 was well-defined in all the structures, serving as a scaffold between two adjacent DmDcr-2 molecules and indicating its crucial role in the formation and stabilization of these states. As in previous studies10, a small segment of the C-terminus of Loqs-PD could be identified in our maps. Interestingly, this density was only present in the 1st monomer (Fig. 1b–e).
a Schematic of 50-bp and 19-bp dsRNA. b–e The cryo-EM density map and atomic model of the complex in Dimer and Trimer state oligomer. f Color-coded domain architecture of the Trimer state oligomer. DmDcr-2, R2D2, and Loqs-PD are in the same color scheme is used as in (e). The intramolecular interactions are indicated as arrows. Domains participated in the intramolecular in DmDcr-2 are highlighted with Hel2i in yellow, Hel2 in cyan, Pincer in orange-red, RIIIDai in cornflower-blue, RIIIDb in forest-green, RIIIDbi in pink, and C-dsRBD in deep-sky-blue.
In the dimer state structure, the model of dsRNA could be well fitted into the continuous density; however, compared with free dsRNA, we observed significant distortion. The 1st and 2nd DmDcr-2 are linked by this dsRNA, and there are large angles of tilt and twist between the two Helicase domains (Supplementary Fig. 2a). This decoration pattern along dsRNA suggests that DmDcr-2’s Helicase domain can adopt a head-to-tail arrangement. Previous results have shown that double-stranded RNA can induce DmDcr-2/Loqs-PD to form a stable dimer of the DmDcr-2/Loqs-PD/dsRNA complex with C2 symmetry10. In that complex, two DmDcr-2 monomers form a helicase-to-cap arrangement with the C-terminus of Loqs-PD located between two DmDcr-2 molecules to stabilize the overall conformation (Supplementary Fig. 2b). In the same study, the “mid-translocation state” structure captured the state in which two DmDcr-2 molecules bind back-to-back via their Helicase domains with one dsRNA. We further analyzed the dataset of DmDcr-2/Loqs-PD/50-bp-dsRNA/ATP used in that work and got a low-resolution structure where one monomer in dimer state was connected back-to-back to the third DmDcr-2 via their Helicase domains (Supplementary Fig. 2b–d). Interestingly, when R2D2 was introduced into the same system, the arrangement of the two DmDcr-2 monomers transitioned to a head-to-tail configuration (Supplementary Fig. 2e). This indicates that R2D2 plays a distinct role from Loqs-PD in the complex, as it can alter the aggregation mode of DmDcr-2.
After processing the 50-bp complex dataset, we identified a trimeric structure which can be fitted well with the trimeric structure obtained from the 19-bp complex dataset. The correlation value between the two structures was 0.9503, indicating that the structures are highly similar (Supplementary Fig. 3a). It is worth noting that the 50-bp dsRNA might not be long enough to connect all three monomers in the trimeric structure. We propose that the presence of the trimeric state is likely attributed to the use of palindromic sequence RNA in the sample. This RNA can form short hairpin dsRNA structures resembling 25-bp dsRNA (Fig. 1a). Therefore, the formation mechanism of the two trimers is similar. The structural comparison revealed that the arrangement of the DmDcr-2 molecules in the trimeric state is very similar to that of the dimeric state, but the RNA densities are discontinuous (Supplementary Fig. 3b, c). This discontinuity resulted in variations in the bonding details between adjacent monomers. While dsRNA played a supportive role in the dimeric state, the DmDcr-2 monomers in the trimeric state exhibited much closer proximity to each other. This difference is further supported by the relative distances and angles between adjacent DmDcr-2 monomers, which were highly consistent in the trimeric state (Supplementary Fig. 3d).
R2D2 binds to the Hel2i of DmDcr-2, a similar binding position of TRBP on HsDicer
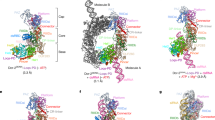
Although we used the full-length R2D2, only the C-terminal part (aa 186–311) of the structure could be well-modeled. This region consists of an α-helix and the C-terminal domain (Supplementary Fig. 4a, b). Despite the lack of sequence conservation, the C-terminal region adopts an α–β–β–β–α-fold and has a 3D structure similar to type B dsRBDs (Supplementary Fig. 4a). Besides, R2D2 binds to the Hel2i sub-domain of DmDcr-2 through this region, and both the binding position and binding mode are similar to those of TRBP on HsDicer38 (Supplementary Fig. 4c). Therefore, it can be inferred that R2D2 is also composed of two type A dsRBDs and one type B dsRBD. The well-modeled region of R2D2 includes a distinct α-helix that acts as a bridge between the two Helicase domains, preventing the DmDcr-2 monomers from approaching each other (Fig. 2a). Since the sequence of this helix is not conserved in common cofactors (Supplementary Fig. 4a), we refer to it as the “bridge helix” and will discuss it further later.
a Take Dimer state for example, R2D2 is located between the two DmDcr-2 monomers and inhibits the binding of the second DmDcr-2 to Loqs-PD. Two DmDcr-2 monomers are shown in surface mode. R2D2 and dsRNA are shown in cartoon mode. b Cryo-EM density and the fitted model of the Helicase domain of the 2nd DmDcr-2. The Loqs-PD binding position is occupied by R2D2. The cryo-EM map was shown at 70% transparency. c Cryo-EM density and the fitted model of the Helicase domain of the 1st DmDcr-2. The densities of Loqs-PD and R2D2 can be well-defined.
In the 1st monomer of the quaternary complex of DmDcr-2/R2D2/Loqs-PD/19-bp siRNA, the presence of R2D2 does not affect the interaction of DmDcr-2 with Loqs-PD, as they bind to different sites. The structures show that R2D2 and Loqs-PD bind to the Helicase domain of the 1st DmDcr-2 together, indicating that their functions are not mutually exclusive. This is in contrast to the functions of TRBP and PACT on HsDicer, where they need to bind to the same position10. However, it is evident that only the 1st DmDcr-2 binds with Loqs-PD, while the same position in 2nd and 3rd DmDcr-2 is occupied by the “bridge helix” of R2D2 (Fig. 2a–c). Therefore, although R2D2 and Loqs-PD do not overlap in binding position on a single DmDcr-2, R2D2 can occupy the binding position of Loqs-PD in the neighboring DmDcr-2 when the quaternary complexes form an oligomer. This may represent a new form of restraint for R2D2 and Loqs-PD, as R2D2 bound to the first DmDcr-2 can inhibit the second DmDcr-2 from binding to Loqs-PD.
Unique functions of the insertion regions in RNase-III domains of DmDcr-2
Within the RNase-III domain of proteins like Drosha or Dicer, there is often an insertion region of unknown function. Sequence alignment results show that both the sequences of insertion region of RNase IIIa (RIIIDai) and RNase IIIb (RIIIDbi) are not conserved10. The high-resolution structures of DmDcr-2 show that its RIIIDai consists of several α-helices (Supplementary Fig. 5a). Notably, this region is not modeled in other solved structures of Dicer proteins7,8,9,11,39, and the Alphafold2 prediction suggests that it is disordered40. Given its location at the interface of multiple oligomers (Fig. 3a, b), the RIIIDai may play important functional roles for DmDcr-2. This region contains many charged amino acids, so it may be essential for the formation and stabilization of different states by electrostatic interaction or hydrogen bonds (Fig. 3c, d).
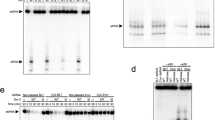
a, b The interface of PDB-7W0A and the Dimer state from 50-bp complex, one monomer shown in surface colored by electrostatic potential. The 1st monomer of DmDcr-2 in the Dimer state color with cyan, and another monomer in PDB-7W0A colored by tan. c, d Amino acid details at the interaction interface of boxed regions, partly charged or polar amino acids are labeled. e The DmDcr-2 monomer (lime) in the oligomer adopts the “translocation state” by comparing with PDB-7W0D (gray). R2D2 links the C-dsRBD and the Helicase domain of DmDcr-2, which may enhance the overall structural stability. f RIIIDbi forms a hydrophobic surface with C-terminal dsRBD of DmDcr-2 and binds to the “bridge helix” of R2D2. g The plot of cleavage efficiency of DmDcr-2, DmDcr-2/Loqs-PD, DmDcr-2/R2D2, DmDcr-2/R2D2/Loqs-PD with 106-bp blunt (BLT) termini dsRNA corresponding to Supplementary Fig. 6b (n = 3 each; means ± SD). Source data are provided as a Source Data file.
During the process of dsRNA cleavage, the ATP-dependent DmDcr-2 adopts various conformations to ensure efficient and precise dicing. In comparison to previously reported structures10, the state of DmDcr-2 monomer in the oligomer belongs to “translocation state”. In this state, the Helicase domain binds to the dsRNA and translocates along it, powered by the hydrolysis of ATP, before the dsRNA terminus reaches the PAZ-Platform domains. (Fig. 3e). In this oligomeric state, the previously unstructured loop (aa 1551–1608) as part of the insertion region of RNase IIIb (RIIIDbi) can be partially built (while aa 1560–1593 is still missing) and is sandwiched between the “bridge helix” of R2D2 and DmDcr-2’s C-terminal dsRBD (Fig. 3e, Supplementary Fig. 5b). The newly built loops (aa 1551–1559 and aa 1594–1608) form a hydrophobic surface with the dsRBD and interact with the “bridge helix” (Fig. 3f). Currently, the function of R2D2 is believed to serve as a component of RLC without enhancing the dicing activity. Compared to Loqs-PD, R2D2 has more interaction sites with DmDcr-2, which may stabilize the overall structure of DmDcr-2, thereby affecting the cleavage activity of DmDcr-2. To test this hypothesis, we analyzed the effects of Loqs-PD and R2D2 on DmDcr-2 cleavage activity. Cleavage assays show that R2D2 can weaken the dicing activity of DmDcr-2 in vitro (Fig. 3g and Supplementary Fig. 6). This may be attributed to the influence of R2D2 on the conformational changes that occur during DmDcr-2 dices its substrate.
Factors that affect the assembly of oligomers
According to the structure of the oligomer, RIIIDai, RIIIDbi, and R2D2 are all potential stabilizers of the oligomer structure, as they are located at the interface of the oligomer. In order to demonstrate the effect of R2D2 on the oligomer, we introduced two constructs of R2D2: the sequence (aa 188–209) including the “bridge helix” replaced with 8*GS (R2D28*GS); or completely remove the “bridge helix” and its preceding region (R2D2(215-311)). The BS3-cross-linked results showed that both the mutation and the truncation constructs significantly reduced the oligomerization rate of the complex (Fig. 4a, b and Supplementary Fig. 7a). Negative staining 2D averages showed that there were still some oligomers in R2D28*GS, but the high-resolution structure was not obtained from the more cryo-EM data, probably because the overall stiffness was reduced without this helix (Supplementary Fig. 7b, c). Moreover, R2D2(215-311) lost the ability to promote oligomer formation (Fig. 4b and Supplementary Fig. 7a), which also suggests that the two tandem dsRBDs of R2D2 might help initiate the oligomerization.
a Schematic of the R2D2WT, R2D28*GS, and R2D2(215-311) constructs. b Size-exclusion chromatography results of BS3-cross-linked DmDcr-2/R2D2/Loqs-PD/19-bp dsRNA complexes with three different R2D2 constructs using Superose 6 10/300 increase. c The statistic of the ratio of oligomeric particles in different conditions (19-bp dsRNA used here), corresponding to Supplementary Fig. 8. After oligomer formation, the concentration was diluted to 1 μM (corresponding to DmDcr-2/R2D2/Loqs-PD complexes) and then exposed to buffers containing final concentrations of 5 mM ADP, 5 mM ATP, 5 mM ATP with 0.1 μM RNase A, 5 mM ATPγS, 5 mM ATPγS with 0.1 μM RNase A, respectively. Negative staining samples were prepared after incubation at 25 °C for 1 h, and the ratio of oligomers was counted (n = 3 each; means ± SD). Source data are provided as a Source Data file. d The spring-like helical fiber model of the oligomer of the quaternary complexes based on the Trimer state structure.
Environmental factors from the surrounding milieu can also influence oligomer formation. The negative staining EM results indicate that both dsRNA and ATP are required for oligomer formation, as shown by the 2D averages (Supplementary Fig. 7d, e). To investigate the oligomerization state under different conditions, we determined the ratio of oligomeric particles based on 2D averages of negative staining electron microscopy (Supplementary Fig. 8). The results show that ADP induces oligomer dissociation, while ATPγS maintains the oligomeric state (Fig. 4c). Therefore, after oligomer formation, DmDcr-2 can still hydrolyze ATP, and the proportion of oligomers gradually decreases with ATP consumption.
The oligomeric mode of trimeric state provides a further extension of the assembly mechanism that is not influenced by the length of dsRNA. In the structure of the 19-bp complex, we also observe some density of the fourth DmDcr-2 (Fig. 1c). Structural analysis indicates that the oligomeric mode of DmDcr-2 primarily depends on the dsRNA-induced conformational changes of the Helicase domain, independent of the length of dsRNA (19 bp or longer is sufficient). The oligomers can theoretically extend to form fibers, which is confirmed by negative stain electron microscopy (Fig. 4d and Supplementary Fig. 7a, d). To further analyze whether oligomerization exists in other arthropods, we compared the amino acid sequences of RIIIDai and R2D2 in several insect species, as they located at the interface of the oligomer. The alignment results showed that RIIIDai of Dcr-2 and R2D2 are only conserved in Drosophilidae (Supplementary Fig. 9), suggesting that oligomerization of Dcr-2 may not be a common feature among insects.
Discussion
We used cryo-EM to determine the structures of DmDcr-2 and its cofactors, Loqs-PD and R2D2, in complex with dsRNA of varying lengths in the presence of ATP and Mg2+ ion. Surprisingly, we found that these components can form oligomers, enabling us to identify the fractions of R2D2 and Loqs-PD bound to DmDcr-2. The different binding sites revealed the compatibility relationship between R2D2 and Loqs-PD and their distinct effects on DmDcr-2. While the previous DmDcr-2/R2D2 dimer structure showed that dsRBD1-2 of R2D2 were positioned near the active dicing center mainly by the hydrophobic interactions between R2D2’s dsRBD2 and DmDcr-2’s central linker36. The C-terminal loop of Loqs-PD interact with DmDcr-2 turns out to give the dsRBDs of Loqs-PD a wider range for their stochastic movement and increase their dsRNA-binding probability10,29. It has been reported that Loqs-PD can promote the processing activity by increasing the initial dsRNA-binding rates of DmDcr-2’s helicase domain instead of changing the processivity of DmDcr-2, suggested that Loqs-PD probably dissociated from dsRNA after the initial dsRNA-binding state formation30. Thus, the dsRBDs’ density of Loqs-PD was missing in all reported structures. The multi-site interaction between R2D2 and DmDcr-2 limited the spatial location of R2D236, in contrast to the wider range of freedom of the two tandem dsRBDs of Loqs-PD. This difference is reflected in their respective functions, with Loqs-PD recruiting surrounding dsRNA substrates and loading them into the Helicase domain to accelerate dicing activity30, while R2D2 is poised to capture product siRNA RNA duplex and load it onto Ago2. However, further investigation is needed to elucidate the inhibitory effect of R2D2 on DmDcr-2’s dicing activity.
The previously reported structure of the DmDcr-2/R2D2/siRNA complex demonstrated that two dsRNA duplexes bind to the Hel1 sub-domain and R2D236. However, in comparison to the apo state structure, the helicase domain remained unaltered (resembling the letter “C”). This implies that the mere presence of the dicing product dsRNA duplex is insufficient to induce conformational changes in the helicase domain of the DmDcr-2/R2D2 complex. Nevertheless, the structures of DmDcr-2 bound with both the 50-bp substrate and 19-bp duplex product demonstrated that these dsRNAs are incorporated into the helicase domain, prompting the transformation from a stretched C-shape to the activated O-shape state (Supplementary Fig. 10a–d). This conformational change is crucial both for DmDcr-2 to process its substrates and for oligomer formation (Fig. 5). Although only a small proportion of Loqs-PD was identified at the periphery of all the oligomers, it is highly probable that it plays an important role in the proper assembly of this oligomer. The absence of Loqs-PD resulted in only a small percentage of DmDcr-2/R2D2 with dsRNA complexes forming oligomers when following the same experimental procedure. However, due to the low oligomeric ratio and the dominant orientation problem, the three-dimensional structure of the complex could not be obtained (Supplementary Fig. 10e). Therefore, the synergistic functions of Loqs-PD and R2D2 promote the formation of oligomers.
Loqs-PD can recruit dsRNA substrates from the surrounding environment and then load them into the Helicase domain of DmDcr-2. This process triggers conformational changes in the Helicase domains, which ultimately promote oligomerization. Once oligomerization occurs, Loqs-PD is released. While each of these steps is reversible, the oligomers tend to depolymerize when the ATP concentration becomes low.
Previous study shows that DmDcr-2 and R2D2 are co-localized in vivo37. Our fluorescence results also support the co-localization of DmDcr-2, R2D2, and Loqs-PD in S2 cells, suggesting the presence of a ternary complex and oligomers in vivo (Supplementary Fig. 11). Understanding the biological function of DmDcr-2 oligomerization is of great significance. Based on our structural analysis, we propose several hypotheses regarding its potential functions. The trimeric structure of the complex allows for the full loading of siRNA duplexes into the Helicase domains, providing temporary protection against degradation until Ago2 is in close proximity. Although the loading of these siRNA duplexes onto Ago2 could not be confirmed, the addition of RNaseA decreased the ratio of oligomers in the presence of ATP compared to ATPγS, suggesting that the siRNA duplexes could be released (Fig. 4c). Oligomer formation restricts the function of Loqs-PD through R2D2, ensuring that released siRNA is loaded onto R2D2 and subsequently onto Ago2, rather than being captured by Loqs-PD and the Helicase domain (Fig. 5). The dependence of oligomerization on dsRNA and ATP implies that this phenomenon may exist in vivo. While attempts to observe the presence of these oligomers in the S2 cell line were unsuccessful. Future research will be needed to investigate the role of these oligomers in vivo, especially their unique functions for Drosophila.
Methods
Protein purification and dsRNA preparation
Plasmids construction, protein expression and purification of full-length DmDcr-2, DmDcr-2DDNN, Loqs-PD were same as described previously10. Briefly, DmDcr-2 or DmDcr-2DDNN were expressed using the Bac-to-Bac baculovirus expression system (Invitrogen) in sf9 cells. The collected cells were resuspended in buffer A (150 mM NaCl, 20 mM Tris-HCl pH 8.0, 10% glycerol, 20 mM imidazole) with 0.5 mM PMSF and protease inhibitors, and lysed by adding 0.5% Triton X-100 and shaken gently for 30 min at 4 °C. DmDcr-2 or DmDcr-2DDNN were purified to homogeneity using Ni-NTA affinity, Hitrap Q column (Cytiva), 2nd Ni-NTA affinity and size-exclusion chromatography using the Superdex 200 10/300 Increase column (Cytiva) (in that order). The peak fractions corresponding to target proteins were collected and concentrated to about 10 mg/ml (50 μM) and stored at −80 °C in SEC buffer (100 mM NaCl, 20 mM Tris-HCl pH 8.0, 1 mM DTT). Loqs-PD was expressed in Escherichia coli BL21 (DE3). Loqs-PD was first purified by Ni-NTA affinity chromatography. Using protease ULP1 to remove the 6×His–SUMO tag, and dialysis was applied to remove imidazole. The sample was then applied to a second Ni-NTA chromatography and the flow-through was collected for size-exclusion chromatography using the Superdex 200 16/600 column (Cytiva). Fractions corresponding to Loqs-PD were collected and concentrated to about 10 mg/ml (250 μM) and stored at −80 °C in buffer containing 500 mM NaCl, 20 mM Tris-HCl pH 8.0, and 1 mM DTT.
Full-length R2D2 (UniProtKB: Q9VLW8) was PCR amplified from Drosophila cDNA and cloned into pFastBac (with 6×HIS affinity tag). The other mutations were generated using a standard PCR-based cloning strategy and cloned into the same vectors. All plasmids’ identities were confirmed by sequencing analysis. Expression and purification of DmDcr-2/R2D2 and DmDcr-2/R2D2-mutions were followed the same protocol. Take DmDcr-2/R2D2, for example, DmDcr-2/R2D2 was co-expressed using the Bac-to-Bac baculovirus expression system (Invitrogen) in sf9 cells at 27 °C. One liter of cells (2 × 106 cells per ml, medium from Sino Biological) was infected with 20 ml baculovirus at 27 °C. After growth at 27 °C for 48 h, the cells were collected, resuspended in buffer A with 0.5 mM PMSF and protease inhibitors, and lysed by adding 0.5% Triton X-100 and shaken gently for 30 min at 4 °C. DmDcr-2/R2D2 was purified to homogeneity using Ni-NTA affinity (GE Healthcare), anion exchange (Hitrap Q column, Cytiva), 2nd Ni-NTA affinity, and size-exclusion chromatography (Superdex 200 10/300 Increase column, Cytiva). Fractions corresponding to target complexes were collected and concentrated to about 10 mg/ml (42 μM) and stored at −80 °C in SEC buffer.
Purified DmDcr-2 DDNN/R2D2 and Loqs-PD were mixed at a molar ratio of 1:1.5 and incubated on ice for 1 h before the size-exclusion chromatography (Superose 6 10/300 Increase column, Cytiva) equilibrated with SEC buffer. Fractions corresponding to a three-component complex were collected for further experiments.
Preparation of dsRNAs
The sense and antisense strands of 19-bp siRNA were ordered from Dharmacon. The 50-bp and 106-bp dsRNAs were in vitro transcribed using T7 RNA polymerase. The pUC19 plasmids containing target sequences with 3′-HDV ribozyme sequences were linearized by EcoRI, extracted with phenol–chloroform, and precipitated with isopropanol. The in vitro transcription reaction was performed at 37 °C for 5 h in the buffer containing 100 mM HEPES-K (pH 7.9), 10 mM MgCl2, 10 mM DTT, 6 mM NTP each, 2 mM spermidine, 200 μg/ml linearized plasmid and 100 μg/ml T7 RNA polymerase. The transcription products were purified by 8% denaturing urea PAGE, eluted from gel slices, and precipitated with isopropanol. After centrifugation, the RNA precipitant was collected, washed twice with 70% ethanol and air-dried, and the RNA was dissolved in ultrapure water. We next used T4 PNK (NEB, M0201) to remove the 2′,3′ cyclic phosphate at the 3′ end of the RNA. The sense and antisense strands of 19-bp and 106-bp dsRNA were annealed by heated to 95 °C for 3 min and then slowly cooled to room temperature in the buffer containing 50 mM HEPES-Na pH 8.0, 100 mM NaCl, 1 mM MgCl2.
In vitro dsRNA cleavage assays
DmDcr-2 and its cofactor complexes cleavage assays were performed in cleavage assay buffer (100 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM DTT) with an equal molar ratio of unlabeled dsRNA (1.2 μM protein and 1.2 μM dsRNA). Protein complexes and dsRNA were preincubated at 25 °C for 15 min, then added ATP and MgCl2 to a final concentration of 5 mM to start the reaction. The reactions were stopped with an equal volume of 2× formamide loading buffer (95% formamide, 20 mM EDTA, 0.1% SDS, 0.005% xylene cyanol, 0.005% bromophenol blue). Samples were separated by 12% denaturing PAGE, post-stained with Gelred, and visualized on Typhoon FLA-9000 (GE Healthcare) system.
Size-exclusion chromatography after BS3-crosslinking
In all, 900 pmol of the purified Dcr-2/R2D2/Loqs-PD complexes with 1080 pmol 19-bp siRNA were preincubated in reaction buffer containing 50 mM HEPES-Na pH 8.0, 100 mM NaCl 5 mM ATP, 5 mM MgCl2 and 5% glycerol at 25 °C for 1 h. Then added 1 mM bis(sulfosuccinimidyl)-suberate (BS3; Thermo Fisher Scientific, 21580) and incubated at 25 °C for 2 h and quenched by 20 mM Glycine. Cross-linked complexes were analyzed by negative staining EM and size-exclusion chromatography using Superose 6 10/300 increase column.
Negative staining EM data collection
The DmDcr-2/R2D2/Loqs-PD complex with dsRNA samples were diluted to a final concentration of ~100 nM (corresponding to DmDcr-2/R2D2/Loqs-PD complexes) using SEC buffer (with additional 5 mM ATP and 5 mM MgCl2 if needed) immediately before the negative-stained specimen was prepared. In total, 4 μl of the solution was applied to a glow-discharged holey carbon grid with a thin layer of continuous carbon over the holes. After adsorption on the grid for 1 min, the grid was negatively stained with three droplets of 2% (w/v) uranyl acetate solution for total of 1 min, the residual stain was blotted off, and the grid was air-dried. The specimen prepared as above was inserted into an FEI F20 transmission electron microscope equipped with a FEG and operated at 200 kV acceleration voltage for examination. We examined the specimen and collected digital micrographs at a nominal magnification of 50,000× with a total dose of about 20 e–/Å2 and a defocus value ranging from −2.0 to −1.5 μm on a Gatan Ultra4000 CCD camera, with a final pixel size of 2.2 Å.
Image processing of negative staining
All the image processing was performed in CryoSPARC41. For each kind of samples, 20 micrographs were collected and imported into CryoSPARC. As many particles as possible were picked automatically using blob picker. Particles were extracted using a box size of 200 × 200 and then resized to 100 × 100. Then two rounds of reference-free 2D classification were performed. Contaminants were removed for the first round, and the ratio of oligomers is determined from the two-dimensional averaged images of the second round.
Immunofluorescence staining
The S2 cells were cultured in Schneider Drosophila Medium with 10% fetal bovine serum (FBS). Transfections were performed with Fugene HD (Promega) according to the manufacturer’s instructions. The fluorescent protein-tagged DmDcr-2/Loqs-PD/R2D2 were constructed in the pUAST vector. All DNA plasmids were cleared of endotoxin. For living cell imaging experiment, 1 μg of plasmids for each protein were co-transfected in a 35-mm dish with a glass bottom (Nest, 801001). Cells were washed twice with PBS and fixed in 500 μL 4% Paraformaldehyde (PFA, sigma, F8775). After that, cells were incubated in permeabilization buffer (0.1% Triton X-100) in PBS for 20 min at room temperature.
Live-cell imaging
S2 cell line was transiently transfected with fluorescent protein-tagged DmDcr-2/R2D2/Loqs-PD for 48hrs and imaged on an Olympus FV3000 confocal microscope using a ×60 oil objective.
Cryo-EM sample preparation and data collection
Protein complex and two kinds of dsRNA were mixed at a molar concentration of 1:1, respectively, and incubated at 25 °C for 2 h before sample preparation. For each sample, an aliquot of 4 μl of purified samples was diluted to 1 μM using SEC buffer with an additional 5 mM ATP and 5 mM MgCl2 and applied to a reduced graphene grid42 (Shuimu BioSciences Ltd.), which were glow-discharged (in a HARRICK PLASMA), for 10 s at middle level after 2 min evacuation. The grids were then blotted by 55 mm filter paper (Ted Pella) for 0.5 s at 22 °C and 100% humidity, then flash-frozen in liquid ethane using FEI Vitrobot Marke IV. Cryo-EM data were collected on different Titan Krios electron microscopes, all of which were operated at 300 kV, equipped with a Gatan K3 direct electron detector and a Gatan Quantum energy filter. Data were automatically recorded using AutoEMation43 (50-bp dsRNA dataset) or EPU (19-bp dsRNA dataset) in counting mode and defocus values varied from −1.5 to −2.0 μm. For other parameters of the two datasets, see Supplementary Table 1.
Image processing and 3D reconstruction
For different cryo-EM datasets, the image processing was adopted in similar steps. All the raw dose-fractionated image stacks were two times Fourier binned, aligned, dose-weighted, and summed using MotionCor244. The contrast transfer function (CTF) parameters were estimated using CTFFIND445. Particles were picked automatically using blob picker in CryoSPARC41. After one round of reference-free 2D classification, and several rounds of 3D reference-based classification in RELION46, using the initial 3D reference models obtained by Ab-initio reconstruction in CryoSPARC, particles from good 3D classes, with better overall structure features, were selected for 3D refinement. The final high-resolution homogeneous refinement and local resolution distribution were performed in CryoSPARC. The resolutions were determined by gold-standard Fourier shell correlation. For the details of each dataset, please see Supplementary Fig. 1.
Model building and refinement
The initial atomic model of R2D2 was predicted using Alphafold2. DmDcr-2, Loqs-PD, and dsRNA were separated from PDB-7W0A. The models were docked into EM density maps in UCSF ChimeraX47 and then adjusted manually in COOT48. Finally, all the models were refined against the EM map by PHENIX49 in real space with secondary structure and geometry restraints. All figures in the manuscripts were illustrated by UCSF ChimeraX47 and UCSF Chimera50. The final models were validated in PHENIX software package. The model statistics are summarized in Supplementary Table 1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the cryo-EM maps and models have been deposited in the wwPDB OneDep System. The EMD accession codes of Dimer state from 50-bp-oligomer, Trimer state 19-bp-oligomer, and Trimer state 50-bp-oligomer are 34707, 34708, 34709. The PDB ID codes of Dimer state from 50-bp-oligomer, Trimer state 19-bp-oligomer are 8HF0 and 8HF1, respectively. Other parameters are listed in Supplementary Table 1. Other structural models cited in this study for analysis (7W0A, 7W0D, 4WYQ, and 7V6C) are also accessible through the PDB. All other data or materials can be obtained from the corresponding author upon request. Source data are provided with this paper.
References
Malone, C. D. & Hannon, G. J. Small RNAs as guardians of the genome. Cell 136, 656–668 (2009).
Nicoloso, M. S., Spizzo, R., Shimizu, M., Rossi, S. & Calin, G. A. MicroRNAs–the micro steering wheel of tumour metastases. Nat. Rev. Cancer 9, 293–302 (2009).
Ivey, K. N. & Srivastava, D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 7, 36–41 (2010).
Alberti, C. & Cochella, L. A framework for understanding the roles of miRNAs in animal development. Development 144, 2548–2559 (2017).
Tomari, Y. & Zamore, P. D. Perspective: machines for RNAi. Genes Dev. 19, 517–529 (2005).
Carthew, R. W. & Sontheimer, E. J. Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009).
Liu, Z. et al. Cryo-EM structure of human dicer and its complexes with a pre-miRNA substrate. Cell 173, 1191–1203 e12 (2018).
Wang, Q. et al. Mechanism of siRNA production by a plant Dicer-RNA complex in dicing-competent conformation. Science 374, 1152–1157 (2021).
Jouravleva, K. et al. Structural basis of microRNA biogenesis by Dicer-1 and its partner protein Loqs-PB. Mol. Cell 82, 4049–4063 (2022).
Su, S. et al. Structural insights into dsRNA processing by Drosophila Dicer-2-Loqs-PD. Nature 607, 399–406 (2022).
Lee, Y. Y., Lee, H., Kim, H., Kim, V. N. & Roh, S. H. Structure of the human DICER-pre-miRNA complex in a dicing state. Nature 615, 331–338 (2023).
Cenik, E. S. et al. Phosphate and R2D2 restrict the substrate specificity of Dicer-2, an ATP-driven ribonuclease. Mol. Cell 42, 172–184 (2011).
Welker, N. C. et al. Dicer’s helicase domain discriminates dsRNA termini to promote an altered reaction mode. Mol. Cell 41, 589–599 (2011).
Aderounmu, A. M., Aruscavage, P. J., Kolaczkowski, B. & Bass, B. L. Ancestral protein reconstruction reveals evolutionary events governing variation in Dicer helicase function. eLife 12, e85120 (2023).
Wilson, R. C. & Doudna, J. A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 42, 217–239 (2013).
Lee, H. Y., Zhou, K., Smith, A. M., Noland, C. L. & Doudna, J. A. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 41, 6568–6576 (2013).
Fukunaga, R. et al. Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell 151, 533–546 (2012).
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Sinha, N. K., Trettin, K. D., Aruscavage, P. J. & Bass, B. L. Drosophila dicer-2 cleavage is mediated by helicase-and dsRNA termini-dependent states that are modulated by Loquacious-PD. Mol. Cell 58, 406–417 (2015).
Tian, B., Bevilacqua, P. C., Diegelman-Parente, A. & Mathews, M. B. The double-stranded-RNA-binding motif: interference and much more. Nat. Rev. Mol. Cell Biol. 5, 1013–1023 (2004).
Masliah, G., Barraud, P. & Allain, F. H. RNA recognition by double-stranded RNA binding domains: a matter of shape and sequence. Cell Mol. Life Sci. 70, 1875–1895 (2013).
Haase, A. D. et al. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 6, 961–967 (2005).
Lee, Y. et al. The role of PACT in the RNA silencing pathway. EMBO J. 25, 522–532 (2006).
Hartig, J. V., Esslinger, S., Böttcher, R., Saito, K. & Förstemann, K. Endo‐siRNAs depend on a new isoform of loquacious and target artificially introduced, high‐copy sequences. EMBO J. 28, 2932–2944 (2009).
Liu, Q. et al. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science 301, 1921–1925 (2003).
Hartig, J. V. & Forstemann, K. Loqs-PD and R2D2 define independent pathways for RISC generation in Drosophila. Nucleic Acids Res. 39, 3836–3851 (2011).
Golden, D. E., Gerbasi, V. R. & Sontheimer, E. J. An inside job for siRNAs. Mol. Cell 31, 309–312 (2008).
Sinha, N. K., Iwasa, J., Shen, P. S. & Bass, B. L. Dicer uses distinct modules for recognizing dsRNA termini. Science 359, 329–334 (2018).
Trettin, K. D., Sinha, N. K., Eckert, D. M., Apple, S. E. & Bass, B. L. Loquacious-PD facilitates Drosophila Dicer-2 cleavage through interactions with the helicase domain and dsRNA. Proc. Natl. Acad. Sci. USA 114, E7939–E7948 (2017).
Naganuma, M., Tadakuma, H. & Tomari, Y. Single-molecule analysis of processive double-stranded RNA cleavage by Drosophila Dicer-2. Nat. Commun. 12, 4268 (2021).
Miyoshi, K., Miyoshi, T., Hartig, J. V., Siomi, H. & Siomi, M. C. Molecular mechanisms that funnel RNA precursors into endogenous small-interfering RNA and microRNA biogenesis pathways in Drosophila. Rna 16, 506–515 (2010).
Liu, X., Jiang, F., Kalidas, S., Smith, D. & Liu, Q. Dicer-2 and R2D2 coordinately bind siRNA to promote assembly of the siRISC complexes. Rna 12, 1514–1520 (2006).
Iwasaki, S. et al. Defining fundamental steps in the assembly of the Drosophila RNAi enzyme complex. Nature 521, 533–536 (2015).
Tsuboyama, K., Tadakuma, H. & Tomari, Y. Conformational activation of argonaute by distinct yet coordinated actions of the Hsp70 and Hsp90 chaperone systems. Mol. Cell 70, 722–729. e4 (2018).
Marques, J. T. et al. Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila. Nat. Struct. Mol. Biol. 17, 24–30 (2010).
Yamaguchi, S. et al. Structure of the Dicer-2-R2D2 heterodimer bound to a small RNA duplex. Nature 607, 393–398 (2022).
Nishida, K. M. et al. Roles of R2D2, a cytoplasmic D2 body component, in the endogenous siRNA pathway in Drosophila. Mol. Cell 49, 680–691 (2013).
Wilson, R. C. et al. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol Cell 57, 397–407 (2015).
Wei, X. et al. Structural basis of microRNA processing by Dicer-like 1. Nat. Plants 7, 1389–1396 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Nan, L. et al. Reduced graphene oxide membrane as supporting film for high-resolution cryo-EM. Biophys. Rep. 7, 227 (2021).
Lei, J. & Frank, J. Automated acquisition of cryo-electron micrographs for single particle reconstruction on an FEI Tecnai electron microscope. J. Struct. Biol. 150, 69–80 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank Hong-Wei Wang at Tsinghua University for the support, guidance, and suggestion on this work. We thank Qinghua Liu from the National Institute of Biological Sciences, Beijing for the DmDcr-2 plasmid. We would like to thank Yan Dong’s lab for providing the S2 cell line. We thank Jianlin Lei, Xiaomin Li, and Fan Yang at Tsinghua University for assistance of data collection. We acknowledge the Tsinghua University Branch of the China National Center for Protein Sciences (Beijing) and Shuimu BioSciences Ltd. for providing the cryo-EM facility support and computational facility support. This research is supported by the National Key Research and Development Program of China (2018YFC1003800 to J.M.) and the National Natural Science Foundation of China (31971130 and 31230041 to J.M., 32000849 to J.W.).
Author information
Authors and Affiliations
Contributions
J.M. and J.W. conceived the study. J.H. and X.Y. initiated the project. T.D., S.S., J.W., and J.M. designed experiments. T.D. and S.S. prepared the samples and performed the biochemical experiments. J.W. and S.S. performed cryo-EM experiments and structure determination. J.W. and S.S. built and refined the models. T.D., S.S., J.W., Y.H., and J.M. analyzed the data, T.D., S.S., J.W., and J.M. wrote the manuscript with input from other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, T., Su, S., Yuan, X. et al. Structural mechanism of R2D2 and Loqs-PD synergistic modulation on DmDcr-2 oligomers. Nat Commun 14, 5228 (2023). https://doi.org/10.1038/s41467-023-40919-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40919-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.