Abstract
Intestinal barrier dysfunction leads to inflammation and associated metabolic changes. However, the relative impact of gut bacteria versus non-bacterial insults on animal health in the context of barrier dysfunction is not well understood. Here, we establish that loss of Drosophila N-glycanase 1 (Pngl) in a specific intestinal cell type leads to gut barrier defects, causing starvation and JNK overactivation. These abnormalities, along with loss of Pngl in enterocytes and fat body, result in Foxo overactivation, leading to hyperactive innate immune response and lipid catabolism and thereby contributing to lethality. Germ-free rearing of Pngl mutants rescued their developmental delay but not lethality. However, raising Pngl mutants on isocaloric, fat-rich diets partially rescued lethality. Our data indicate that Pngl functions in Drosophila larvae to establish the gut barrier, and that the lethality caused by loss of Pngl is primarily mediated through non-bacterial induction of immune and metabolic abnormalities.
Similar content being viewed by others
Introduction
Intestinal barrier dysfunction allows various pathogens and non-living stimuli to induce the innate immune response1,2,3,4,5. Although the goal of innate immune response induction is to restore intestinal homeostasis, its hyperactivation can have local and systemic adverse effects and is implicated in the pathogenesis of human diseases including autoimmune and neurodegenerative disorders6,7,8. In addition, hyperactive immune response can be accompanied by profound changes in metabolism including high energy demand and subsequent depletion of the nutrient depot9,10. For example, induction of chronic inflammation results in the aggravation of metabolic disorders in mouse models of obesity11. However, the relative contribution of infectious versus non-infectious mechanisms to detrimental consequences caused by gut barrier dysfunction is not well understood.
The gut mucus layer is one of the key components regulating intestinal barrier functions12. Equivalent to the mammalian gut mucus layer, a peritrophic matrix (PM) is present in the Drosophila intestine13. The PM is composed of highly glycosylated proteins and chitin, and is continuously secreted from a group of specialized cells called peritrophic matrix-forming ring (PR) cells in the proventriculus region at the junction of foregut and midgut13,14. There is strong evidence that in addition to chitins and mucin-type O-glycans, PM also contains N-glycoproteins15,16. Moreover, lectin-based studies in another insect suggest that N-glycoproteins might control the functional properties of PM like its permeability17. However, genetic evidence for the contribution of N-glycoproteins to gut barrier function in Drosophila is lacking.
Terminally misfolded proteins are detected by the endoplasmic reticulum-associated degradation (ERAD) pathway and retrotranslocated from ER to the cytosol for proteasomal degradation18. One of the major branches of ERAD uses N-glycans as a signal to recognize misfolded N-glycoproteins and shuttles them to the retrotranslocation machinery18. The cytosolic deglycosylating enzyme N-glycanase 1 (NGLY1) removes N-glycans from misfolded proteins and is thought to function in the ERAD pathway19,20,21. Recessive mutations in human NGLY1 cause a congenital disorder of deglycosylation named NGLY1 deficiency22,23,24. It is an ultra-rare disorder that leads to global developmental delay and affects multiple organ systems including the nervous system and the gastrointestinal system. Loss of the Drosophila homolog of human NGLY1 (PNGase-like or Pngl) results in semi-lethality, as less than 1% of homozygous mutant animals finish the larval and pupal development and eclose as an adult organism25,26. We have previously reported that loss of Pngl in the visceral mesoderm impairs signaling pathways mediated by decapentaplegic (Dpp; homolog of human bone morphogenetic protein 4, BMP4) and adenosine monophosphate-activated protein kinase (AMPK) in the larval intestine, which leads to structural and functional intestinal phenotypes and contributes to the lethality of Pngl–/– animals26,27. However, impaired BMP and AMPK signaling due to mesodermal loss of Pngl did not fully explain the lethality of Pngl mutants, suggesting critical roles for Pngl in other biological processes and potentially in other cell types.
Here, we report that loss of Pngl leads to increased expression of the innate immune genes in the Drosophila larval intestine and a systemic increase in lipid catabolism, compromising developmental progression and leading to lethality. We find that loss of Pngl in the PR cells leads to abnormalities in peritrophic matrix and impairment in the gut barrier function. Our data suggest that the gut barrier defects result in increased activation of Foxo in the gut epithelial cells, both via enhanced stress-induced JNK signaling and through a systemic starvation response. In addition, we observe that Pngl is required cell-autonomously in enterocytes and in the fat body to prevent aberrant Foxo activation and to repress lipid catabolism. Importantly, while germ-free rearing does not rescue the lethality of Pngl mutants, increasing the lipid content in isocaloric diets improves their survival to adulthood, suggesting that the mutant animals lack sufficient energy stores to reach the adult stage. Altogether, our data suggest that Pngl is required to establish the gut barrier in Drosophila larvae and that the lethality associated with gut barrier defects in Pngl mutants is primarily caused by non-bacterial insults.
Results
Pngl-mutant midguts show upregulation of immune genes
Animals homozygous for a Pngl null allele (Pnglex14/ex14, Pngl–/– hereafter) display severe developmental delay, with the majority of mutant animals not reaching the pupal stage26. Therefore, in this study, we analyzed age-matched third instar larvae. To determine the biological processes that contribute to lethality in Pngl mutants, we performed transcriptomic analysis using RNA sequencing (RNA-seq) on a mixed pool of male and female third instar larval midguts of Pngl–/– animals and three control strains: y w (Pngl+/+), Pngl+/–, and Pngl–/–; Pngl Dp/+, which lacks endogenous Pngl function but harbors one copy of a Pngl genomic duplication shown to fully rescue the lethality of Pngl–/– animals27. Principal component analysis (PCA) showed that the first two principal components drive the majority of the variance among the samples, and that all samples show strong distinctive clustering by genotype (Fig. 1a). We then identified the genes differentially expressed between Pngl–/-- and each control, as those showing an absolute fold-change of at least 1.5 and a False Discovery Rate (FDR) equal to or less than 0.05 (Supplementary Data 1). We generated a Shiny app that can be used to perform pairwise and three-way comparisons of these datasets and to download the corresponding differentially expressed gene lists (Supplementary Data 2; https://shiny-portal.embl.de/shinyapps/app/14_flyvenn). To increase the stringency of our analysis, we focused on those differentially expressed genes that were overlapping among these three pairwise comparisons: (1) Pngl–/– vs y w, (2) Pngl–/– vs Pngl+/–, and (3) Pngl–/– vs Pngl–/–; Pngl Dp/+. We found 459 upregulated and 469 downregulated genes in Pngl–/– larval midguts, when compared to all controls (Fig. 1b and Supplementary Data 3). Using the Database for Annotation, Visualization, and Integrated Discovery (DAVID)28,29, we performed functional gene ontology (GO) analysis on the differentially expressed genes and identified various biological processes significantly altered in Pngl–/– midguts (Fig. 1c and Supplementary Data 4). We found proteasome-mediated processes as the topmost significantly downregulated gene category (Fig. 1c; top panel), in agreement with previous reports on the regulation of proteasomal gene expression by Pngl and its homologs30,31,32,33,34.
a Principal component analysis (PCA) plot showing the variance among the genotypes used in RNA-seq. b Venn diagrams showing the overlap of differentially regulated genes in Pngl–/– with comparison to control (y w), Pngl+/– and Pngl–/–, Pngl Dp/+ in RNA-seq analysis. Number of both upregulated and downregulated genes shown in the Venn diagram were based on >1.5 fold-change. c Graph presents DAVID functional GO analysis of biological processes (BP) of downregulated (top), and upregulated (bottom) genes based on their –log10 of FDR. Numbers in parenthesis show the number of genes differentially expressed in each category and the numbers next to bars show the fold enrichment. d Graph showing expression of immune response-related genes in the indicated genotypes. Values are expressed as fold changes relative to control (y w). Mean ± standard deviation of three independent replicates is shown. Numbers on the bars indicate the P values. Significance is ascribed as P < 0.05 using one-way ANOVA with multiple comparisons followed by Šidák correction. Source data are provided as Source Data file.
Interestingly, a number of significantly upregulated gene categories were related to immune response (Fig. 1c; bottom panel). Many of the upregulated genes in the immune response gene categories encode for antimicrobial peptides (AMPs) and pattern recognition receptor proteins such as peptidoglycan recognition proteins (PGRPs). qRT-PCR analysis on 13 innate immune response genes from the list confirmed the RNA-seq results (Fig. 1d). Addition of a genomic copy of Pngl (Pngl Dp) significantly reduced the immune gene expression (Fig. 1d). We conclude that loss of Pngl leads to a significant increase in the expression of multiple immune response-related genes in the larval midgut. Importantly, impaired proteasomal gene expression or AMPKα signaling, both of which have been shown to be affected in Pngl-deficient animals27,33, cannot explain the severe increase in the expression of innate immunity genes in Pngl–/– larval midguts (Supplementary Figs. 1a, 1b and 2a).
Enhanced midgut immune response contributes to Pngl –/– lethality
Expression of AMPs and other innate immune genes in Drosophila is regulated by two signaling pathways, the Toll pathway and the immune deficiency (IMD) pathway35. Moreover, in the intestinal epithelium, the forkhead transcription factor Foxo can induce the expression of AMPs in response to starvation, energy deprivation, and infection36,37. To determine if the increased immune gene expression contributes to the lethality of Pngl mutants, we decreased the gene dosage of foxo, Rel (encodes Relish, which is the NF-κB transcription factor in the IMD pathway) and Tl (encodes the Toll receptor). Loss of one copy of each gene in Pngl–/– larvae resulted in a significant reduction in the midgut expression of the immune response genes, although in most cases the expression levels did not fully return to control levels (Fig. 2a). Notably, we observed a more robust rescue upon reducing foxo compared to Rel and Tl (Fig. 2a). Reducing the gene dosage of foxo rescued the lethality of Pngl mutants by 40%, while decreasing the gene dosage of Rel and Tl rescued the lethality of Pngl–/– animals by 19% and 21%, respectively (Fig. 2b). Moreover, combined heterozygosity for foxo and Rel or Tl in Pngl–/– animals did not further increase the degree of lethality rescue achieved by reducing foxo gene dosage alone (Fig. 2b). These observations suggest that these genes contribute to Pngl–/– lethality through a common mechanism, likely the induction of innate immune genes. Together, these observations suggest that foxo-mediated hyperactivation of innate immune genes is a major contributor to the lethality in Pngl mutants.
a Graph showing immune response gene expression (relative fold change to control, y w) in the midgut of age-matched larvae of the indicated genotypes (n = 3 independent replicate). b Graph showing % lethality rescue in Pngl mutants upon removing one copy (1X) of each immune gene activator (n = 4–6 independent replicates per genotype). c Graph showing survival upon Pngl knockdown driven by the indicated GAL4 drivers (n = 4 independent replicates). d Graph showing immune gene expression (relative fold change to genetic control) in the indicated genotypes (n = 3 independent replicates). e Graph showing % lethality rescue in Pngl mutants upon tissue-specific knockdown of immune activators (foxo, Rel and Tl) using the indicated GAL4 drivers (n = 3 independent replicates). f Graph showing % lethality rescue in Pngl mutants upon overexpression of wild-type (PnglWT) and catalytically inactivate (PnglC303A) Pngl using indicated GAL4 drivers (n = 4 independent replicates). g, h Graphs showing immune gene expression in the midgut of age-matched larvae of indicated genotypes (n = 3 independent replicates). In all panels, each circle represents an independent replicate, and mean ± standard deviation is shown. Numbers on bars indicate the P values. Significance is ascribed as P < 0.05 using one-way ANOVA with multiple comparisons followed by Šidák correction in (a–c, e–h) or two-tailed unpaired t-test (d). Source data are provided as Source Data file.
The Drosophila larval midgut epithelium is composed of enterocytes, enteroendocrine cells and adult midgut precursor cells38. Pngl knockdown in enterocytes, but not enteroendocrine or adult midgut precursor cells, led to partial lethality and immune gene induction (Fig. 2c, d). Enterocyte-specific knockdown of foxo, Rel, and Tl resulted in a lethality rescue of ~28%, 8%, and 3% in Pngl mutants, respectively (Fig. 2e). We also included the fat body in our analysis, given its prominent role in the systemic release of AMPs39. Fat body-specific knockdown of foxo, Rel, and Tl also led to modest but statistically significant rescue of lethality (Fig. 2e). Taken together, these data suggest that the detrimental effects of immune hyperactivation in Pngl mutants primarily results from Foxo-mediated induction of immune response genes in enterocytes and to some extent in the fat body.
Overexpression of wild-type Pngl (PnglWT) but not a catalytically-inactive form of Pngl (PnglC303A) using Myo1A-GAL4 and r4-GAL4 drivers led to ~19% and ~11% rescue of Pngl–/– lethality, respectively (Fig. 2f). These observations indicate that in addition to its role in mesoderm26, the enzymatic activity of Pngl is also required in enterocytes and fat body during Drosophila development. Notably, enterocyte- and fat body-specific overexpression of Pngl in Pngl mutants led to a significant reduction of innate immune gene expression in the midgut but did not fully rescue this phenotype (Fig. 2g, h). These data suggest that the hyperactive immune response in Pngl mutant midguts is due to the loss of Pngl in several cell types.
Pngl –/– larvae exhibit Foxo overactivation in intestine and fat body
Foxo is negatively regulated by insulin receptor (InR)/Akt signaling40. Under conditions of starvation or energy deprivation, decrease in insulin signaling allows the nuclear localization of Foxo and subsequent induction of its target genes41. We found increased nuclear localization of Foxo and a significant reduction in phospho-Foxo (pFoxo) levels in Pngl–/– midguts, which were rescued by Pngl Dp (Fig 3a, b), suggesting increased Foxo activation in midgut upon loss of Pngl. Western blot analysis showed a significant decrease in pAkt levels in Pngl–/– midguts (Fig. 3c). Moreover, we observed a lethality rescue of ~11% and ~17% by enterocyte-specific overexpression of AktWT and constitutively active Akt (AktCA), respectively, in Pngl mutants (Fig. 3d). Furthermore, overexpression of AktCA in enterocytes led to a significant reduction in innate immune gene expression in Pngl mutants (Fig. 3e). Western blot analysis showed that enterocyte-specific overexpression of Pngl rescues pAkt levels in Pngl–/– midguts (Fig. 3f). Together, these data suggest that reduced Akt activation in Pngl–/– enterocytes contributes to Foxo overactivation and increased immune gene expression in the midgut, and lethality.
a Confocal images showing DAPI (blue) and Foxo (red) staining in the larval midguts (96 hr after egg laying, AEL) of indicated genotypes. Scale bar is 50 µm. b Western blot images and quantification showing the Foxo and pFoxo levels in the larval midguts (96 hr AEL) of indicated genotypes. c Western blot images and quantification showing Akt and pAkt level in the larval midgut (96 hr AEL) of indicated genotypes. d Graph showing % lethality rescue of Pngl mutants in indicated genotypes. AktWT, wild-type Akt; AktCA, constitutively active Akt. e Graph showing immune gene expression in the larval midgut (96 hr AEL) of indicated genotypes. f Western blot images and quantification showing Akt and pAkt levels in the larval midgut (96 hr AEL) of indicated genotypes. g Graph showing the growth rate curve of control and Pngl–/– animals. h Images of larvae (96 hr AEL) and pupae of the indicated genotypes. Scale bars are 500 µm. i Graph showing quantification of larval body weight (96 hr AEL) in indicated genotypes. j Graph showing % lethality rescue of Pngl mutants in indicated genotypes. k Graph showing quantification of larval body weight (96 hr AEL) in indicated genotypes. l Graph showing the expression of InR in the midguts of age-matched control and Pngl–/– larvae. m Graph showing % rescue of Pngl–/– lethality in indicated genotypes. InRWT, wild-type InR; InRCA, constitutively active InR. n Graph showing larval body weight (96 hr AEL) in indicated genotypes. o Genes in the “Lipid catabolic process” category from the larval midgut RNA-seq, along with the fold increase in their expression level in Pngl–/– compared to y w control. p Graph showing the expression of lipases in Pngl–/– midgut. In all panels, the mean ± standard deviation of three independent replicates is shown. P values are indicated on bars. Significance is ascribed as P < 0.05 using one-way ANOVA followed by Šidák correction (b–f, i, k, m, n) or two-tailed unpaired student’s t-test (l, p). Source data are provided as Source Data file.
Small body size is a signature of decreased insulin signaling in flies42. Pngl–/– larvae showed growth retardation compared to controls (Fig. 3g). In agreement with a previous report43, we observed a small body size phenotype in Pngl–/– third instar larvae, which was rescued by decreasing foxo (Fig. 3h, i). Accordingly, our data suggest that reduced insulin signaling in the midgut might contribute to Pngl–/– lethality. The Drosophila insulin-like peptide 6 (Dilp6; official name Ilp6) is primarily expressed in third instar larval fat body and is essential for animal growth44. However, dilp6 overexpression in the fat body did not rescue the Pngl–/– lethality (Fig. 3j). We also overexpressed another insulin-like peptide (Dilp2) in the fat bodies of Pngl mutants but did not see any lethality rescue (Fig. 3j). Overexpression of dipl2 and dilp6 did not improve the small body size phenotype either (Fig. 3k). We next examined InR expression in midguts and found that it is significantly decreased in Pngl–/– midguts (Fig. 3l). Overexpression of InRWT and constitutively activate InR (InRCA) in enterocytes led to a modest but statistically significant rescue of the Pngl–/– lethality (Fig. 3m). A lower degree of rescue was also observed upon InRWT and InRCA overexpression in the fat body (Fig. 3m). Overexpression of InRWT and InRCA in enterocytes and fat body also exhibited a partial rescue of body size in Pngl mutants (Fig. 3n). These observations suggest that reduced InR signaling in enterocytes and potentially fat body contributes to the lethality of Pngl mutants.
Reduced InR signaling can result from starvation45. One of the hallmarks of starvation in Drosophila larvae is an increase in lipid catabolism. Importantly, one of the upregulated gene categories in the RNA-seq analysis was related to lipid catabolic process (Fig. 1c; bottom panel and Fig. 3o), which was confirmed by qRT-PCR experiments (Fig. 3p). These observations suggest that starvation contributes to reduced InR signaling and Foxo activation in Pngl mutant larvae.
Pngl –/– larvae exhibit gut barrier defects
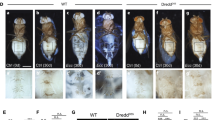
Gut barrier defects can lead to activation of the intestinal innate immune response2,3. To examine whether Pngl–/– larvae exhibited any gut barrier dysfunction, we fed the control and Pngl-mutant larvae on food containing FITC-labeled high molecular weight (500-kDa) dextran. In control midguts, the FITC signal was restricted to the central parts of the lumen area, but the Pngl–/– midguts failed to retain the FITC signal in the lumen area (Fig. 4a, b). Around 55% of the examined Pngl-mutant third instar larvae (but none of the WT) showed gut barrier defect, and providing one genomic copy of Pngl fully rescued the phenotype (Fig. 4a, c). Pngl–/– larvae show a gut clearance defect due to reduced AMPKα expression in visceral mesoderm27. However, adding an AMPKα Dp did not rescue the gut barrier defect in Pngl–/– larvae (Supplementary Fig. 2b). These observations indicate that Pngl is required for normal gut barrier formation in Drosophila larvae independently of the AMPKα defects previously reported in these animals.
a Low magnification fluorescent images of the midguts of indicated genotypes upon FITC-labeled dextran (yellow) feeding. b Confocal images showing phalloidin (magenta) and DAPI (blue) staining in the indicated genotypes upon FITC-labeled dextran (yellow) feeding. c Graph showing the quantification of gut barrier defect phenotype in the indicated genotypes (n = 60 for each genotype from three independent experiments). d Confocal images showing phalloidin (magenta), DAPI (blue), and HPA-lectin (yellow) staining for peritrophic matrix in control and Pngl mutant midguts. e Light microscopic images of cross-section of control and Pngl mutant midguts. L, lumen; PM, peritrophic matrix; PS, peritrophic space; EC, enterocytes. f Graph showing quantification of gut barrier defect phenotype in the indicated genotypes (n = 60 for each genotype from three independent experiments). g Graph showing % lethality rescue in the indicated genotypes. h Confocal images showing Phalloidin (magenta), DAPI (blue), and ConA (yellow) lectin staining in the proventriculus region of third instar larvae of indicated genotypes (n = 6–7 for each genotype). Insets show close-up of PM-secreting cells. i Graph showing the quantification of the number of intracellular puncta in the indicated genotypes. j Graph showing the size of intracellular puncta in the indicated genotypes (n = 12–30 puncta from three animals per genotype). k Confocal images showing Phalloidin (magenta), DAPI (blue), and ConA (yellow) lectin staining in the proventriculus region of third instar larvae of indicated genotypes (n = 4 for each genotype). Insets show a close-up of PM-secreting cells. l Graph showing the quantification of the number of intracellular puncta in the indicated genotypes. m Graph showing the size of intracellular puncta in the indicated genotypes (n = 9–14 puncta from four animals per genotype). The scale bar is 250 µm in (a) and 50 µm in (b, d, h, k). In all panels, each circle represents an independent replicate in (c, f, g, i) or a punctum in (j, m) and mean ± standard deviation is shown. P values are indicated on bars. Significance is ascribed as P < 0.05 using one-way ANOVA followed by Šidák correction. Source data are provided as Source Data file.
Given the impaired gut barrier function of Pngl–/– larvae, we examined the integrity of peritrophic matrix (PM) by marking the PM in Pngl–/– and control larvae with Helix pomatia agglutinin (HPA) lectin, which selectively binds to α-N-acetylgalactosamine residues and is specific for O-glycans46,47. In control larvae, PM separated a central luminal area from a peripheral “peritrophic” space adjacent to the apical surface of the midgut epithelium (Fig. 4d), an arrangement which is thought to ensure that abrasive food particles and microorganisms pass through the gut without contacting the epithelial cells13. However, although PM can clearly be observed in the midgut of Pngl–/– larvae, it is highly disorganized and appears to be collapsed on itself (Fig. 4d). This is accompanied by epithelial irregularities, as evidenced by defects in apical phalloidin staining and detachment of some epithelial cells (Fig. 4d). In addition, analysis of midgut sections by light microscopy showed the lumen area lined with intact PM and a peritrophic space between enterocytes and PM in control animals. In contrast, Pngl–/– midguts displayed a dense lumen content (potentially due to the gut clearance defect previously reported in these animals27), disorganized PM, and loss of peritrophic space (Fig. 4e). These observations indicate that impaired gut barrier function in Pngl–/– larvae is associated with PM abnormalities.
To determine if loss of Pngl in PR cells (present in proventriculus) contributes to the gut barrier defects observed in Pngl–/– larvae, we overexpressed Pngl in PR cells of Pngl mutants using path-GAL4 and fed the larvae with 500-kDa FITC-dextran. We found that the penetrance of gut barrier phenotype in Pngl–/– larvae was reduced from 55% to ~20% upon PR-specific overexpression of PnglWT, but not PnglC303A (Fig. 4f). This was accompanied by a lethality rescue of ~11% (Fig. 4g). In addition to proventriculus, path-GAL4 also drives expression in larval fat body, brain and salivary glands (Supplementary Fig 3a). However, Pngl overexpression in these tissues with specific drivers did not rescue the gut barrier defect of Pngl–/– larvae (Supplementary Fig. 3b). Additionally, Pngl knockdown driven by path-GAL4, but not by other drivers, recapitulates the gut barrier defect (Supplementary Fig 3c). Together, these data indicate that the deglycosylation activity of Pngl is required in PR cells to ensure the integrity of the PM and gut barrier and to promote the survival of Drosophila larvae.
To assess whether there is an accumulation of N-glycoproteins in the PR cells of Pngl mutant larvae, we stained the proventricular regions of these animals and control third instar larvae with two lectins: wheat germ agglutinin (WGA), which recognizes N-acetylglucosamine in O-glycans, chitins, and N-glycans, and concanavalin A (Con A), which primarily binds high-mannose N-glycans15,48. Notably, Pngl mutants showed a significant increase in the number and size of ConA+ intracellular puncta in the PR cells, which was rescued by Pngl Dp, suggesting that loss of Pngl might affect the trafficking and/or secretion of some N-glycoproteins (Fig. 4h–j). A similar increase was observed in the number of WGA+ puncta in Pngl–/– PR cells (Supplementary Fig. 3d, e). Importantly, overexpression of PnglWT, but not PnglC303A, in PR cells rescued the number and size of the ConA+ intracellular puncta in Pngl mutants (Fig. 4k–m), suggesting a key role for the enzymatic activity of Pngl in PR cells. These observations provide a potential mechanism for the PM defects observed in Pngl–/– larvae.
Germ-free rearing does not rescue the Pngl –/– lethality
The gut barrier defects and upregulation of multiple microbial recognition peptides in Pngl–/– larvae (Figs. 4 and 1d) suggest that the gut microbiota might contribute to some of the Pngl–/– phenotypes. Moreover, altered gut microbiota can precede intestinal barrier defects in aging adult Drosophila49. To directly examine the role of gut microbiota in Pngl-mutant phenotypes, we generated germ-free (GF) Pngl–/– and control larvae. GF Pngl mutants did not show any improvement in their gut barrier phenotype (Fig. 5a), suggesting that the PM defects caused by loss of Pngl are not due to altered gut microbiota.
a Graph showing quantification of gut barrier defect phenotype in germ-free control and Pngl–/– larvae (60 animals for each group from four independent experiments). b Graph showing the percentage of germ-free and conventionally reared control and Pngl–/– animals that reached the pupal stage (120 animals for each group from four independent experiments). c Graph showing % lethality rescue in germ-free and conventionally reared Pngl–/– and Pngl–/–; foxo+/– animals (n = 4 independent replicates). d Graph showing immune gene expression in germ-free control and Pngl–/– larvae (n = 3 independent replicates). In all panels, each circle represents an independent replicate, and mean ± standard deviation is shown. P values are indicated on bars. Significance is ascribed as P < 0.05 using two-way ANOVA followed by Šidák correction (b, c) or two-tailed unpaired student’s t-test (a, d). Source data are provided as Source Data file.
The GF Pngl–/– larvae showed a significant increase in the percentage of larvae that reach the pupal stage compared to conventionally-reared Pngl–/– larvae (Fig. 5b), suggesting an important role for gut microbiome in the developmental delay of Pngl mutants. However, GF rearing did not lead to any rescue in the Pngl–/– lethality (Fig. 5c). Of note, GF rearing of Pngl–/–; foxo+/– larvae resulted in further improvement in their survival compared to conventionally-reared Pngl–/–; foxo+/– animals (Fig. 5c, also compare to Fig. 2b) suggesting that the contribution of gut microbiome to Pngl–/– lethality is redundant to that of Foxo hyperactivation. Expression of the genes involved in the recognition of microorganisms is not statistically different between GF Pngl–/– and GF y w larval midguts (Fig. 5d). However, the GF Pngl–/– larval midguts still exhibit a significant increase in the expression of most AMPs (Fig. 5d). Together, these data suggest that although gut microbiota plays an important role in the developmental delay of Pngl–/– larvae, it is not the major inducer of immune gene expression and lethality in these animals.
Gut barrier defects lead to starvation and Pngl –/– lethality
To gain mechanistic insight into the role of gut barrier defects in the lethality of Pngl–/– larvae and to separate the effects of gut barrier defect from the cell-autonomous effects of loss of Pngl in the enterocytes and fat body, we induced gut barrier defects in control (Pngl+/+) animals by feeding them with polyoxin D (Poly D), a chitin synthase inhibitor that disrupts PM formation in insects50,51. Poly D feeding for 48 hours resulted in a 43%-penetrant gut barrier phenotype in y w animals (Fig. 6a), confirming that this strategy can impair PM formation in Drosophila larvae. Poly D feeding also led to ~19% lethality, and increased expression of immune genes and lipases (Fig. 6b–d), indicating gut barrier defect can cause immune gene induction, starvation and lethality in Drosophila larvae.
a Confocal images showing FITC-labeled dextan (yellow) in midguts from control and Poly D-fed larvae of indicated genotypes and quantification of gut barrier defect in these animals (n = 3 independent replicates). Scale bar is 250 µm. b Graph showing % adult eclosion in Poly D-fed control and foxo+/– animals (n = 4 independent replicates). c Graph showing immune response gene expression in control and Poly D-fed y w and foxo+/– midguts (n = 3 independent replicates). d Graph showing gene expression of lipases in control and Poly D-fed midguts of y w larvae (n = 3 independent replicates). e Western blot images and quantification graph showing Foxo and pFoxo levels in the midguts of Poly D-fed larvae of indicated genotypes (n = 3 independent replicates). f Confocal images showing DAPI (blue) and pJNK (red) staining and quantification in control and Poly D-fed y w midguts. The scale bar is 50 µm. n = 69 nuclei from four animals for each group. g Confocal images showing DAPI (blue) and pJNK (red) staining and quantification in control and Pngl–/– midguts. The scale bar is 50 µm. n = 56 (y w) and 66 (Pngl–/–) nuclei from four animals for each group. h Graph showing % lethality rescue upon enterocyte-specific knockdown of Drosophila JNK (bsk) in Pngl mutants (n = 3 independent replicates). i Graph showing innate immune gene expression in the indicated genotypes (n = 3 independent replicates). j Confocal images showing DAPI (blue) and Foxo (red) staining and quantification of Foxo nuclear localization in the indicated genotypes. The scale bar is 50 µm. n = 35–36 nuclei from three animals for each group. Each circle represents an independent replicate in (a–e, h, i) or a nucleus in (f, g, j). Mean ± standard deviation is shown in all graphs. P values are indicated on bars. Significance is ascribed as P < 0.05 using one-way ANOVA followed by Šidák correction (e, I, j), two-way ANOVA followed by Šidák correction (c) or two-tailed unpaired student’s t-test (a, b, d, f–h). Source data are provided as Source Data file.
Poly D-fed foxo+/– larvae showed a 38%-penetrant gut barrier phenotype, indicating that as expected, loss of one copy of foxo does not affect the ability of Poly D to impair gut barrier integrity (Fig. 6a). However, removing one copy of foxo rescued the lethality caused Poly D feeding and significantly reduced the expression of most immune genes induced by this chemical (Fig. 6b, c). Further, western blot analysis revealed a significant reduction in the relative levels of pFoxo in midguts of Poly D-fed larvae, suggesting Foxo activation (Fig. 6e). Importantly, quantification of the number of mandibular movements per second suggests a comparable feeding behavior in control and Pngl-mutant larvae (Supplementary Fig. 4). Therefore, the starvation signature observed in these animals is not likely to be due to reduced feeding. Together, these observations indicate that gut barrier defects can lead to a starvation-like condition in Drosophila larvae and suggest that Foxo likely operates downstream of this starvation-like phenotype to mediate the lethality associated with gut barrier defects.
Overexpression of InR or Akt in the midgut was less effective in rescuing the Pngl–/– lethality compared to foxo knockdown by using the same driver (compare Fig. 3d, m to Fig. 2f). Therefore, in addition to reduced InR signaling, other pathways are likely to contribute to Foxo activation and lethality in Pngl–/– midguts. The Jun-N-terminal Kinase (JNK) pathway is a potential candidate for these effects, as it is activated in epithelial tissues by a variety of extrinsic and intrinsic stressors and can induce the nuclear localization and activation of Foxo52,53. Indeed, feeding Poly D to control larvae led to the activation of JNK signaling in larval midgut, as evidenced by the accumulation of phospho-JNK (pJNK) in their midgut epithelial cells (Fig. 6f). Western blot analysis showed that enterocyte-specific knockdown of the Drosophila JNK homolog basket (bsk) suppressed the Foxo activation in the midgut of Poly D-fed larvae (Fig. 6e). Pngl–/– larvae also accumulated pJNK in their midgut epithelial cells (Fig. 6g). Moreover, bsk knockdown in enterocytes led to a lethality rescue of 22% in Pngl–/– larvae (Fig. 6h), accompanied by a significant decrease in immune gene expression and a significant decrease in Foxo nuclear localization in midguts (Fig. 6i, j). Altogether, these observations suggest that gut barrier defects lead to increased JNK signaling in Pngl–/– midgut epithelial cells, which itself contributes to Foxo activation and lethality in these animals.
Pngl mutants exhibit an increase in lipid catabolism
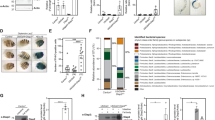
Starvation can lead to lipid mobilization45,54. In line with the significant increase in the expression of multiple lipase genes in Pngl–/– midguts (Fig. 3o, p), we observed a significant reduction in the lipid storage in fat body and midgut of Pngl mutants (Fig. 7a). We also found a significant decrease in triacylglycerol (TAG) level in hemolymph and midgut of Pngl mutants throughout the third instar larval period (Fig. 7b), accompanied by a significant increase in free fatty acid levels in hemolymph of early third instar Pngl mutants (Fig. 7c, 72 hr). By mid-third instar larval stage, free fatty acid levels return to normal (Fig. 7c, 108 hr), potentially suggesting the depletion of energy reserve as the development of Pngl–/– larvae proceeds. Loss of one copy of foxo improved all of these phenotypes (Fig. 7a–c). Together, these data indicate that Pngl–/– larvae experience a significant degree of starvation associated with a Foxo-dependent increase in lipid catabolism.
a Images showing Nile red (red) and DAPI (blue) staining in fat body and midgut of the indicated genotypes and their quantification (n = 4 independent replicates). Scale bars are 50 µm. b Triacylglycerol (TAG) level in hemolymph and midgut of indicated genotypes (n = 3 independent replicates). c Free fatty acid (FFA) level in the hemolymph of indicated genotypes (n = 3 independent replicates). d Pie charts showing % carbohydrates, proteins and fats in different diet compositions. e Graph showing % animals that reach the pupal stage upon feeding on the indicated diets (n = 3 independent replicates). f Graph showing lethality rescue in Pngl mutants upon feeding on the indicated diets (n = 3 independent replicates). g Schematic model showing that loss of Pngl in several cell types of the Drosophila larvae results in Foxo overactivation and subsequent innate immune gene expression and lipid catabolism, leading to lethality. In all panels, each circle represents an independent replicate, and mean ± standard deviation is shown. P values are indicated on bars. Significance is ascribed as P < 0.05 using one-way ANOVA followed by Šidák correction (a, e, f) or two-way ANOVA followed by Šidák correction (b, c). Source data are provided as Source Data file.
Dietary lipid supplementation partially rescues the Pngl –/– lethality
In the standard fly food used in our experiments, only 2% of the total energy contents is provided by lipids. The depletion of lipid storage in Pngl–/– midgut and fat body prompted us to examine whether increasing the fat content of the food can promote the survival of Pngl–/– larvae. To this end, we used two additional isocaloric food compositions with different fat contents in our experiments: a “high-protein intermediate-fat diet” (HPIFD) and a “high-fat diet” HFD (Fig. 7d and Supplementary Table 1). On our standard diet (SD), on average 19.6% of Pngl–/– larvae reached the pupal stage (Fig. 7e). However, when grown on isocaloric HPIFD and HFD, 37.3% and 77.2% of Pngl–/– larvae reached the pupal stage, respectively (Fig. 7e). Further analysis indicated that the developmental delay of Pngl–/– larvae was partially rescued by HPIFD and fully rescued by HFD (Supplementary Fig. 5a). Remarkably, HPIFD and HFD also led to Pngl–/– lethality rescues of 19.2% and 41.1%, respectively (Fig. 7f). Immunostaining indicated that HFD significantly reduced the Foxo nuclear localization in midgut and fat body of Pngl mutants (Supplementary Fig. 5b), and partially rescued innate immune gene overexpression in Pngl mutants (Supplementary Fig. 5c). These data underscore the contribution of metabolic abnormalities and energy depletion to the developmental delay and lethality in Pngl mutants.
Discussion
We previously reported that Pngl is required in the midgut visceral mesoderm for the regulation of Dpp and AMP kinase signaling in Drosophila26,27,55. However, impairment of these two pathways only partially explained the lethality of Pngl–/– animals. Here, we report that Pngl plays critical roles in several other cell types (PR cells, enterocytes, and fat body cells). Loss of Pngl leads to gut barrier defects, as well as suppression of InR signaling and activation of JNK signaling in enterocytes. These alterations result in overactivation of Foxo in enterocytes and fat body, which in turn leads to hyperactivation of intestinal innate immune genes and increased lipid catabolism, ultimately causing lethality (Fig. 7g). Pngl mutants show significant developmental delay and less than 1% of the animals reach adulthood25,26. Intriguingly, growing Pngl mutants on an isocaloric high-fat diet fully rescued their developmental delay and allowed ~41% of the animals to reach adulthood, strongly suggesting that depletion of energy depot during the larval and pupal development plays a critical role in the lethality of Pngl mutants. We note that a high-fat diet also suppressed Foxo overactivation in the fat body and midgut of Pngl–/– larvae. This observation suggests that by worsening the starvation status, enhanced lipid catabolism engages in a vicious cycle with Foxo overactivation and negatively impacts the survival of Pngl–/– animals. We note that nutrient deprivation can also suppress lipogenesis in enterocytes and the fat body through enhanced secretion of Tachykinins by enteroendocrine cells56. It remains to be examined whether reduced lipogenesis also contributes to low lipid content in Pngl–/– larvae.
Our data provide strong evidence that hyperactivation of innate immune genes contributes to lethality in Pngl mutants. First, decreasing the gene dosage of foxo, Rel and Tl, which are the known regulators of innate immune gene expression, partially rescued the lethality in Pngl mutants. In addition, in all of our other genetic and diet-induced rescue experiments, rescue of lethality was accompanied by reduced immune gene expression. It has recently been reported that overexpression of AMPs results in cytotoxicity and cell death in aging Drosophila7. Our data suggest immune hyperactivation can also induce lethality during development and that Pngl prevents immune hyperactivation in part by helping establish a normal gut barrier.
Although foxo heterozygosity had the biggest rescue effect in Pngl mutants, loss of one copy of Rel and Tl also showed some degree of lethality rescue. Since pathogens are thought to be the main inducers of the Imd and Toll pathways in Drosophila57, we anticipated that gut microbiota contributes to the lethality of Pngl–/– animals. Rather surprisingly, GF rearing of Pngl mutants did not rescue lethality at all, even though it dramatically rescued the developmental delay in these animals. How can one reconcile these observations? Since GF rearing also removes commensal bacteria, one possibility is that commensal bacteria are important for gut homeostasis in Pngl mutants. Regardless of the reason for this discrepancy, our data indicate that upon gut barrier defect in Pngl mutants, gut microbiota are not the primary mediators of hyperactive immune response.
Accumulation of ConA+ and WGA+ puncta in PM-secreting cells in Pngl–/– larvae suggests that loss of Pngl might affect the secretion of some N-glycoproteins. Components of the Drosophila larval PM have not been systematically identified. However, studies in other insects indicate that some PM components are N-glycoproteins16,17. It remains to be studied whether the gut barrier defects observed in Pngl–/– larvae result from a failure in the deglycosylation and secretion of key N-glycoprotein components of the PM. Notably, a number of putative PM components (Muc68Ca, Muc26B, Muc96D, Muc11A, and Muc68E) were downregulated in Pngl–/– midguts based on our RNA-seq analysis (Supplementary Data 3), suggesting an alternative mechanism for PM abnormalities in Pngl mutants.
Human insulin receptors harbors 19 predicted N-glycosylation sites, 14 of which have been experimentally verified58. Importantly, Drosophila InR is predicted to have 12 N-glycosylation sites (NetNGlyc 1.0 server prediction59). Therefore, while the reduction in InR signaling in Pngl–/– larvae can be explained by starvation and reduced expression of InR, it is also plausible that the InR protein itself is a direct target of Pngl and shows abnormal trafficking and/or function upon loss of Pngl-mediated deglycosylation. Further studies are required to identify and characterize the biologically relevant targets of NGLY1/Pngl in the Drosophila larval intestine and fat body.
Human patients with NGLY1 deficiency display an array of symptoms including global developmental delay, lack of tears, and chronic constipation60. Some of the NGLY1 deficiency patients were reported to have recurrent, severe respiratory infections, while others were reported to have higher than expected antibody titers against rubella and rubeola after Measles, Mumps, and Rubella (MMR) vaccination23. Transcriptome profiling indicated significant upregulation of genes involved in immunity in NGLY1 deficiency patient fibroblasts compared to control fibroblasts34. Moreover, global gene profiling in Ngly1-deficient melanoma cells showed upregulation of cytokines such as interferon β1 and interleukin 29 (ref. 61), and Ngly1–/– mouse embryonic fibroblasts exhibit increased expression of the interferon genes31. In addition, RNA-seq in adult flies with RNAi-medicated Pngl knockdown also showed an increase in the expression of the innate immune genes33. Although these reports linked NGLY1 deficiency and altered immune response in various contexts, the current study is the first report on the role of NGLY1/Pngl in gut immunity and systemic metabolism in an in vivo model. Of note, altered glycosylation in intestinal epithelial cells is implicated in chronic inflammatory diseases including inflammatory bowel disease62. Considering these reports, the critical roles uncovered here for Drosophila Pngl in regulating the gut mucus barrier, innate immune response, and metabolic homeostasis warrant further studies to explore whether loss of NGLY1 in other systems or alterations in N-glycosylation machinery exert similar immune and metabolic effects.
Methods
Drosophila strains and culture
Animals were reared at room temperature on standard food containing cornmeal, molasses, and yeast in all experiments except for those involving high-protein, intermediate-fat diet (HPIFD) and high-fat diet (HFD). The detailed composition of these diets is listed in Supplementary Table 1. The following Drosophila strains were used in the study: (1) y w, (2) foxoΔ94/TM6B, Tb1, (3) Tl/TM3, Sb1, (4) RelE38, (5) UAS-foxoRNAi, (6) UAS-TlRNAi, (7) y1 sc* v1 sev21;UAS-RelRNAi, (8) Myo1A-GAL4 (enterocytes), (9) r4-GAL4 (fat body), (10) c135-GAL4 (path-GAL4), (11) UAS-dipl2, (12) UAS-InRWT, (13) UAS-InRA1325D (constitutively activated UAS-InRCA), (14) UAS-AktWT, (15) UAS-AktΔPH (constitutively activated UAS-AktCA), (16) UAS-mCherrynls, (17) pros-GAL4 (enteroendocrine cells), (18) esg-GAL4 (adult midgut precursors), (19) elav-GAL4 (neurons), (20) C147-GAL4 (salivary glands) and (21) UAS-bskRNAi (Bloomington Drosophila Stock Center); (22) Mef2-GAL4 (mesoderm; ref. 63); (23) Pnglex14, (24) UAS-PnglWT and (25) UAS-PnglC303A (ref. 25); (26) PBac{Pnglwt}VK31 (Pngl duplication; ref. 27); (27) Dp(1;3)DC102, PBac{DC102}VK33 (AMPKα duplication; ref. 64); and (28) UAS-dipl6 (ref. 65).
Transcriptomic analysis/RNA sequencing
Midgut tissues from a mixed pool of male and female third instar larvae at 96 hours after egg laying were dissected and homogenized in groups of 25 in a cold solution of Tri-reagent (Sigma-Aldrich, T9424); the amount of RNA in each sample was determined by Nanodrop, and RNA quality was analyzed using agarose gel electrophoresis (1.2%). Two biological replicates were used for each genotype. The samples were prepared on a Beckman FXP using the Illumina TruSeq stranded mRNA chemistry and sequenced on a NextSeq 500 (Mid Output flowcell) in paired-end mode. Raw paired-end sequencing reads were trimmed using cutadapt v1.12 to remove Illumina adapters and low-quality bases. The processed reads were then aligned to the Drosophila dm6 genome using STAR v2.5.3a. Gene-level counts, based on GTF annotations from FlyBase Release 6.20, were tabulated by totaling all reads overlapping the collapsed set of exons for each gene following previously published methods66. Genes with a counts per million reads mapped (CPM) of less than 5 in less than two samples were excluded. The principal component analysis (PCA) plot was generated using the log-transformed CPM. Differentially expressed genes were identified by using the edgeR glmFit model. The false discovery rate (FDR) was controlled by applying the Benjamini–Hochberg procedure to the P values. Significantly expressed genes were defined as those exhibiting an absolute fold-change of at least 1.5 and an FDR of less than or equal to 0.05. The raw data have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE206229.
Developmental assay
For developmental assay, the expected ratio was calculated based on Mendelian inheritance for genotypic classes and the observed/expected ratio is reported as a percentage. Crosses made up of 5 virgin females and 5 males were set in each tube after a period for sexual maturation and housing to obtain the maximum fitness level during the three days of egg deposition in a non-overcrowded environment. Crosses were set in triplicate and flies were transferred to fresh vials every 3 days for three times. The total number of pupae for genotypic classes produced over 20 days in each vial was scored.
Quantification of lethality rescue
In lethality rescue experiments, the number of genetic siblings and their Mendelian ratio were used to calculate the expected number of the target progeny. Lethality rescue in Pngl mutants was examined as described previously26. We scored the number of adult progeny including Pngl homozygous and heterozygous animals. The number of homozygous animals is expected to be 50% of the number of heterozygous animals. Accordingly, we calculated the expected number of homozygous animals based on the observed number of heterozygous animals. For example, if in a genetic cross, we observe 50 heterozygotes, we would expect 25 homozygotes. Considering this expected homozygous number as 100%, we calculated the % homozygotes eclosed. We can formulate the calculation as % lethality rescue = Observed number of homozygotes x 100 / Observed number of heterozygotes x 0.5. For all genetic crosses, 3-6 independent replicates were used.
Real time quantitative RT-PCR analysis
Total RNA was extracted from 5 larval midguts with Trizol (Invitrogen) and dissolved in 25 μL of RNase-free water. cDNA was then synthesized from 1 μg total RNA using amfiRivert II cDNA Synthesis Master Mix (R5500, GenDEPOT), and qPCR was carried out using amfiSure qGreen Q-PCR Master Mix, Low ROX (Q5601, GenDEPOT). Expression levels were normalized to Actin (endogenous control). Relative gene expression was calculated as fold change using the 2-ΔΔCt method. The oligonucleotides used to assess target genes expression are listed in Supplementary Table 2.
Dissections, staining, image acquisition, and processing
Larval midgut and fat body tissues were dissected and fixed in 4% paraformaldehyde. Antibodies were rabbit anti-dFoxo 1:250 (ab195977, abcam) and rabbit anti-SAPK/JNK 1:500 (Cat No. 559309, Sigma-Aldrich). Lectin staining in the proventriculus region was performed using helix pomatia agglutinin (HPA), Alexa FluorTM 488 conjugate 1:1000 (cat No. L11271, Invitrogen), wheat germ agglutinin (WGA) CF®488 A 1:1000 (Cat No. 29022, Biotium), and concanavalin A (ConA) CF®488 A 1:1000 (Cat No. 29016, Biotium). Confocal images were acquired using a Leica TCS-SP8 microscope using LAS X 3.1.5 software and processed with Amira5.2.2. Images were generated as a limited projection view of 5-10 optical sections. Quantifications were performed using ImageJ. All images were processed in Adobe Photoshop CC. Figures were assembled in Adobe Illustrator CC. For comparison between different groups, color intensity per nucleus was calculated in 3-4 independent replicates.
Western blotting
Protein samples were prepared from larval midguts in lysis buffer containing Halt™ Phosphatase Inhibitor Single-Use Cocktail (Thermo Fisher Cat. No. 78428) and Protease Inhibitor Cocktail (Promega Cat. No. G6521). The following antibodies were used: rabbit anti-pFoxo1 1:1000 (Cat No. 9461, Cell Signaling Technology), rabbit anti-dFoxo1 1:1000 (ab195977, Abcam), rabbit anti-Akt 1:1000 (Cat No. 4691, Cell Signaling Technology), rabbit anti-pAkt 1:1000 (cat No. 4060, Cell Signaling Technology) and mouse anti-actin 1:1000 (DSHB Cat. No. 224236-1). Western blots were developed using Clarify ECL Western Blotting Substrates (BioRad). The bands were detected using an Azure Biosystems c280 digital imager using chemiluminescent detection of HRP. Three independent immunoblots were performed for each experiment.
Feeding behavior assay
The larval feeding behavior method was adapted from ref. 67. Age-matched third instar larvae (96 hours after egg laying or AEL) were placed in sucrose-agar plates (5% sucrose mixed in 3% agar medium) and allowed to settle for 15 min. The number of mouth-hook contractions per minute were counted. A total number of 30 larvae were scored for each group.
Generation of germ-free animals
GF animals were generated by following the previously published method68. Flies were kept for egg laying in grape juice agar plate for 3-4 hr. Eggs were collected and dechorionated with 2.7% sodium hypochlorite for 2-3 minutes. Dechorionated eggs were washed twice in 70% ethanol and thrice in water, and then transferred to sterile fly food containing tetracycline (50 µg/mL). Flies were reared on sterile food vials for three generations. The absence of bacteria in GF flies was confirmed by 16 S rRNA amplification using the 27 F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492 R (5’-GGTTACCTTGTTACGACTT-3’) primers.
Growth rate curve
To examine larval growth, we obtained a pool of 20-30 larvae per group and scored their weight at 24, 48, 72, 96, 120, 144, and 168 hours AEL. Average larval weight (mg) was then calculated to generate the growth curve.
Nile red staining
Midgut and fat body tissues from third-instar larvae were dissected and fixed in 4% paraformaldehyde. Fixation and washing of tissues were followed by the incubation in with 1:2500 dilution of 0.5 mg/mL Nile red (Cat No. 19123, Sigma Aldrich) for half an hour. Upon incubation, tissues were rinsed in water and mounted in 80% glycerol followed by image acquisition. Four animals were used per group. Images were generated as a projection view of 5-10 optical sections. Quantification of Nile red staining in fat body images was done by measuring the red intensity in individual cells from four independent replicates. For comparison between different groups, intensity per cell was divided by the cell area. In midgut images, total red intensity was measured in four independent replicates. For comparison between different groups, intensity was divided by the number of nuclei.
Triacylglycerol and free fatty acid estimation
Triacylglycerol and free fatty acid levels were estimated from larval midgut and hemolymph. Midgut samples were prepared by homogenizing ~15 midguts in cold 1X PBST (0.05% tween). Hemolymph was collected from 15 larvae and mixed in cold 1X PBST. Triacylglycerol levels were estimated using the manufacturer’s protocol (InfinityTM Triglycerides Liquid stable reagent, Thermo scientific #TR22421). Free fatty acid levels were estimated using the manufacturer’s protocol (Sigma Aldrich # MAK044). Triacylglycerol and free fatty acid levels were normalized with the protein content determined by Bradford assay.
Dextran feeding assay and gut barrier defect quantification
Third instar larvae were fed on semi-solid drops of 500 kDa FITC-labelled dextran (Sigma-Aldrich, St. Louis, MO, USA), diluted to 1 mg/mL in sucrose-agar medium (5% sucrose mixed in 3% agar medium) on a petri dish. Larvae were allowed to feed for 15 minutes. Larvae were washed with cold 1X PBS to remove any excess FITC-dextran on the surface. Midguts were dissected out and fixed in 4% paraformaldehyde followed by imaging under a fluorescent microscope. For gut barrier defect quantification, larval guts that failed to retain FITC-signal (green) in their lumen were scored. Pharmacological induction of gut barrier defects was achieved by rearing third instar larvae on food containing 100 µM polyoxin D (Sigma Aldrich # 529313) for 48 hr. Gut barrier defects were examined and quantified using the dextran feeding assay described above.
Larval midgut sectioning
Intact midguts from age-matched larvae at 96 hours AEL were dissected and fixed in 4% paraformaldehyde, 2% glutaraldehyde, and 0.1 M cacodylic acid. Post-fixation in 2% osmium tetraoxide (OsO4) was followed by dehydration in graded series of alcohol and propylene oxide. The midgut tissues were then embedded and blocked in Embed-812 resin. The resin blocks were sectioned using an ultramicrotome. Thin sections (5 µm) of the anterior midgut regions were obtained and stained with toluidine blue. Images were acquired using light microscopy and presented as greyscale.
Statistics and reproducibility
Two-tailed unpaired Student’s t-test, one-way ANOVA and two-way ANOVA with Šídák’s multiple comparisons test, were used for statistical analyses. Significance was ascribed as P < 0.05. P values are indicated in all figures. P values falling between 0.99 and 0.0001 are mentioned as exact numbers, while those more than 0.99 or less than 0.0001 are indicated as >0.99 or <0.0001, respectively. Statistical tests and parameters including the sample sizes and the number of independent biological replicates are mentioned in Figure Legends. All experiments, including staining and imaging, were performed at least three times with comparable results. All statistical analyses were performed using GraphPad Prism 9.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data are available within the article and supplementary information. Source data are provided as Source Data file with this paper. RNA-seq data generated in this study are available in the National Center for Biotechnology Information Gene Expression Omnibus database under the accession number GSE206229. Source data are provided with this paper.
References
Kvidera, S. K. et al. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 100, 4113–4127 (2017).
Pan, Z., Yuan, X., Tu, W., Fu, Z. & Jin, Y. Subchronic exposure of environmentally relevant concentrations of F-53B in mice resulted in gut barrier dysfunction and colonic inflammation in a sex-independent manner. Environ. Pollut. 253, 268–277 (2019).
Rera, M., Clark, R. I. & Walker, D. W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl Acad. Sci. USA 109, 21528–21533 (2012).
Zhu, R. & Ma, X. C. Role of metabolic changes of mucosal layer in the intestinal barrier dysfunction following trauma/hemorrhagic shock. Pathol. Res. Pract. 214, 1879–1884 (2018).
Takiishi, T., Fenero, C. I. M. & Camara, N. O. S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 5, e1373208 (2017).
Blach-Olszewska, Z. & Leszek, J. Mechanisms of over-activated innate immune system regulation in autoimmune and neurodegenerative disorders. Neuropsychiatr. Dis. Treat 3, 365–372 (2007).
Badinloo, M. et al. Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Arch. Insect Biochem. Physiol. 98, e21464 (2018).
Cao, Y., Chtarbanova, S., Petersen, A. J. & Ganetzky, B. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proc Natl Acad Sci USA 110, E1752–E1760 (2013).
Kominsky, D. J., Campbell, E. L. & Colgan, S. P. Metabolic shifts in immunity and inflammation. J. Immunol. 184, 4062–4068 (2010).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Zhao, L. et al. Chronic inflammation aggravates metabolic disorders of hepatic fatty acids in high-fat diet-induced obese mice. Sci. Rep. 5, 10222 (2015).
Paone, P. & Cani, P. D. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69, 2232–2243 (2020).
Lehane, M. J. Peritrophic matrix structure and function. Annu. Rev. Entomol. 42, 525–550 (1997).
Rizki, M. T. M. The secretory activity of the proventriculus of Drosophila melanogaster. J. Exp. Zool. 131, 203–221 (1956).
Lehane, M. J., Allingham, P. G. & Weglicki, P. Composition of the peritrophic matrix of the tsetse fly, Glossina morsitans morsitans. Cell Tissue Res. 283, 375–384 (1996).
Liu, D. et al. The N-glycan profile of the peritrophic membrane in the Colorado potato beetle larva (Leptinotarsa decemlineata). J. Insect. Physiol. 115, 27–32 (2019).
Eisemann, C. H. et al. Larvicidal activity of lectins on Lucilia cuprina: mechanism of action. Entomol. Exp. Appl. 72, 1–10 (1994).
Sun, Z. & Brodsky, J. L. Protein quality control in the secretory pathway. J. Cell Biol. 218, 3171–3187 (2019).
Suzuki, T., Park, H. & Lennarz, W. J. Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. FASEB J. 16, 635–641 (2002).
Hosomi, A., Fujita, M., Tomioka, A., Kaji, H. & Suzuki, T. Identification of PNGase-dependent ERAD substrates in Saccharomyces cerevisiae. Biochem. J. 473, 3001–3012 (2016).
Hirsch, C., Blom, D. & Ploegh, H. L. A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J 22, 1036–1046 (2003).
Enns, G. M. et al. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet. Med. 16, 751–758 (2014).
Lam, C. et al. Prospective phenotyping of NGLY1-CDDG, the first congenital disorder of deglycosylation. Genet. Med. 19, 160–168 (2017).
Need, A. C. et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J. Med. Genet. 49, 353–361 (2012).
Funakoshi, Y. et al. Evidence for an essential deglycosylation-independent activity of PNGase in Drosophila melanogaster. PLoS One 5, e10545 (2010).
Galeone, A. et al. Tissue-specific regulation of BMP signaling by Drosophila N-glycanase 1. Elife 6, e27612 (2017).
Han, S. Y. et al. A conserved role for AMP-activated protein kinase in NGLY1 deficiency. PLoS Genet. 16, e1009258 (2020).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Tomlin, F. M. et al. Inhibition of NGLY1 inactivates the transcription factor nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent. Sci. 3, 1143–1155 (2017).
Yang, K., Huang, R., Fujihira, H., Suzuki, T. & Yan, N. N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J. Exp. Med. 215, 2600–2616 (2018).
Lehrbach, N. J. & Ruvkun, G. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife 5, e17721 (2016).
Owings, K. G., Lowry, J. B., Bi, Y., Might, M. & Chow, C. Y. Transcriptome and functional analysis in a Drosophila model of NGLY1 deficiency provides insight into therapeutic approaches. Human molecular genetics 27, 1055–1066 (2018).
Rauscher, B. et al. Patient-derived gene and protein expression signatures of NGLY1 deficiency. J. Biochem. 171, 187–199 (2022).
Buchon, N., Silverman, N. & Cherry, S. Immunity in Drosophila melanogaster-from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 14, 796–810 (2014).
Becker, T. et al. FOXO-dependent regulation of innate immune homeostasis. Nature 463, 369–373 (2010).
Fink, C. et al. Intestinal FoxO signaling is required to survive oral infection in Drosophila. Mucosal. Immunol. 9, 927–936 (2016).
Takashima, S., Younossi-Hartenstein, A., Ortiz, P. A. & Hartenstein, V. A novel tissue in an established model system: the Drosophila pupal midgut. Dev. Genes Evol. 221, 69–81 (2011).
Hanson, M. A. et al. Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. Elife 8, e44341 (2019).
Eijkelenboom, A. & Burgering, B. M. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Junger, M. A. et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2, 20 (2003).
Rulifson, E. J., Kim, S. K. & Nusse, R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 (2002).
Rodriguez, T. P. et al. Defects in the neuroendocrine axis contribute to global development delay in a drosophila model of ngly1 deficiency. G3 (Bethesda) 8, 2193–2204 (2018).
Okamoto, N. et al. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev. Cell 17, 885–891 (2009).
Britton, J. S., Lockwood, W. K., Li, L., Cohen, S. M. & Edgar, B. A. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2, 239–249 (2002).
Zhang, L., Turner, B., Ribbeck, K. & Ten Hagen, K. G. Loss of the mucosal barrier alters the progenitor cell niche via Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling. J. Biol. Chem. 292, 21231–21242 (2017).
Zhang, L. et al. O-glycosylation regulates polarized secretion by modulating Tango1 stability. Proc. Natl Acad. Sci. USA 111, 7296–7301 (2014).
Rudin, W. & Hecker, H. Lectin-binding sites in the midgut of the mosquitoes Anopheles stephensi Liston and Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 75, 268–279 (1989).
Clark, R. I. et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality. Cell Rep. 12, 1656–1667 (2015).
Rodgers, F. H., Gendrin, M., Wyer, C. A. S. & Christophides, G. K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog. 13, e1006391 (2017).
Shahabuddin, M., Kaidoh, T., Aikawa, M. & Kaslow, D. C. Plasmodium gallinaceum: mosquito peritrophic matrix and the parasite-vector compatibility. Exp. Parasitol. 81, 386–393 (1995).
Biteau, B., Karpac, J., Hwangbo, D. & Jasper, H. Regulation of Drosophila lifespan by JNK signaling. Exp. Gerontol. 46, 349–354 (2011).
Wang, M. C., Bohmann, D. & Jasper, H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125 (2005).
Butterworth, F. M., Bodenstein, D. & King, R. C. Adipose tissue of drosophila melanogaster. i. an experimental study of larval fat body. J. Exp. Zool. 158, 141–153 (1965).
Galeone, A. et al. Regulation of BMP4/Dpp retrotranslocation and signaling by deglycosylation. Elife 9, e55596 (2020).
Song, W., Veenstra, J. A. & Perrimon, N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 9, 40–47 (2014).
De Gregorio, E., Spellman, P. T., Tzou, P., Rubin, G. M. & Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21, 2568–2579 (2002).
Sparrow, L. G. et al. N-linked glycans of the human insulin receptor and their distribution over the crystal structure. Proteins 71, 426–439 (2008).
Blom, N., Sicheritz-Ponten, T., Gupta, R., Gammeltoft, S. & Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649 (2004).
Pandey, A., Adams, J. M., Han, S. Y. & Jafar-Nejad, H. NGLY1 deficiency, a congenital disorder of deglycosylation: from disease gene function to pathophysiology. Cells 11, 1155 (2022).
Zolekar, A. et al. Stress and interferon signalling-mediated apoptosis contributes to pleiotropic anticancer responses induced by targeting NGLY1. Br. J. Cancer 119, 1538–1551 (2018).
Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 15, 346–366 (2019).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Venken, K. J. et al. A molecularly defined duplication set for the X chromosome of Drosophila melanogaster. Genetics 186, 1111–1125 (2010).
Slaidina, M., Delanoue, R., Gronke, S., Partridge, L. & Leopold, P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell 17, 874–884 (2009).
Story, B. et al. Defining the expression of piRNA and transposable elements in Drosophila ovarian germline stem cells and somatic support cells. Life Sci. Alliance 2, e201800211 (2019).
Shen, P. Analysis of feeding behavior of Drosophila larvae on solid food. Cold Spring Harb. Protoc. 2012, pdb.prot069328 (2012).
Ryu, J. H. et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319, 777–782 (2008).
Acknowledgements
We acknowledge support from the NIH (R35GM130317 to H.J.N.), the Mizutani Foundation for Glycoscience (grant #220061 to H.J.N.), the Grace Science Foundation (research grants to H.J.N. and L.M.S.), AIRC (Investigator grant 20661 to T.V), MUR (PRIN investigator grant 2020CLZ5XW to T.V.) and Telethon Italia (Investigator grant GMR22T1078 to T.V.). We thank the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Developmental Studies Hybridoma Bank for reagents; the Microscopy Core of the Baylor College of Medicine (BCM) Intellectual and Developmental Disabilities Research Center (IDDRC) (supported by P50 HD103555 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD/NIH]), the BCM Integrated Microscopy Core (supported by NCI-CA125123, NIDDK-56338-13/ 15, and CPRIT-RP150578 and the John S. Dunn Gulf Coast Consortium for Chemical Genomics), Debra Townley for assistance with sectioning midguts for light microscopy, and Professor Pierre Leopold for generously providing UAS-dilp6 strain and Drosophila anti-Foxo antibody. We also thank members of the Jafar-Nejad and Vaccari labs for discussions.
Author information
Authors and Affiliations
Contributions
A.P., A.G., S.Y.H, T.V., and H.J.N. designed and conceived the project and interpreted. the data. W.F.M., B.S., and L.M.S. performed RNA-seq and transcriptomic analysis. A.P., A.G., S.Y.H., and G.C. performed the experiments. A.P., A.G., T.V., and H.J.N. wrote the manuscript. All authors read, edited, and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Cathy Ramos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandey, A., Galeone, A., Han, S.Y. et al. Gut barrier defects, intestinal immune hyperactivation and enhanced lipid catabolism drive lethality in NGLY1-deficient Drosophila. Nat Commun 14, 5667 (2023). https://doi.org/10.1038/s41467-023-40910-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40910-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.