Abstract
The integrins and G protein-coupled receptors are both fundamental in cell biology. The cross talk between these two, however, is unclear. Here we show that β3 integrins negatively regulate G protein-coupled signaling by directly inhibiting the Gα13-p115RhoGEF interaction. Furthermore, whereas β3 deficiency or integrin antagonists inhibit integrin-dependent platelet aggregation and exocytosis (granule secretion), they enhance G protein-coupled RhoA activation and integrin-independent secretion. In contrast, a β3-derived Gα13-binding peptide or Gα13 knockout inhibits G protein-coupled RhoA activation and both integrin-independent and dependent platelet secretion without affecting primary platelet aggregation. In a mouse model of myocardial ischemia/reperfusion injury in vivo, the β3-derived Gα13-binding peptide inhibits platelet secretion of granule constituents, which exacerbates inflammation and ischemia/reperfusion injury. These data establish crucial integrin-G protein crosstalk, providing a rationale for therapeutic approaches that inhibit exocytosis in platelets and possibly other cells without adverse effects associated with loss of cell adhesion.
Similar content being viewed by others
Introduction
G protein-coupled receptors (GPCRs), a family of cell surface receptors with seven-transmembrane domains, detect molecular stimuli outside of the cell and activate intracellular responses1. GPCRs comprise the largest known gene family and are frequently targeted for drug development2,3. They transmit signals by coupling with intracellular heterotrimeric G proteins and upon receptor ligation, GPCRs function as guanine nucleotide exchange factors (GEFs), that convert the α subunit (e.g. Gαs, Gαi/o, Gαq/11 and Gα12/13) of a Gαβγ protein complex from the GDP-bound inactive form to the active GTP-bound form. Activated Gα subunits dissociate from the β/γ subunits, and both interact with their respective downstream targets to transmit GPCR signals4,5.
GPCR signaling stimulates and regulates cell adhesion, spreading, contractility, migration, phagocytosis, and exocytosis, in which the integrin family of α:β heterodimeric adhesion receptors play a crucial role6,7,8. Certain GPCRs, such as receptors for thrombin and thromboxane A2 (TXA2) stimulate Gα12/Gα13 binding to the RGS domain of Rho guanine nucleotide exchange factors (RhoGEF), such as p115RhoGEF. This activates the RhoGEF to convert inactive GDP-bound small GTPase RhoA into the active GTP-bound form9,10, and thus stimulates contractility, assembly of actin stress fibers11 and promotes exocytosis in platelets12,13 and other cells types14,15. However, Gα13 is dispensable for the activation of integrins16,17. GPCRs, via Gαq and Gαi signaling pathways, stimulate “inside-out” signaling, leading to the binding of talin and kindlins to the cytoplasmic domain of integrin β subunits and consequent activation of the ligand binding function of integrins, which mediate cell-matrix and cell-cell adhesion18,19,20. Ligand binding to integrins conversely induces “outside-in” signaling leading to cell spreading, retraction, migration, and exocytosis8,21 (Supplementary Fig. 1). Despite the importance of talin in inside-out signaling and in the late retraction phase of outside-in signaling, the early phase of integrin outside-in signaling leading to cell spreading does not require talin binding to integrins22,23, but requires Gα13, which interacts with the ExE motif conserved in the cytoplasmic domain of several integrin β subunits including β124, β225 and β317,23. This interaction is critical for the integrin-dependent activation of c-Src and c-Src-dependent downstream outside-in signaling pathways17,26. Thus, G proteins regulate the adhesion and signaling functions of integrins. However, it is unclear whether integrins or integrin signaling regulates GPCR-mediated intracellular signaling. In particular, it is unknown whether the direct interaction between integrins and Gα13 can regulate Gα13-coupled GPCR signaling.
In blood platelets, GPCR and integrin signaling pathways are both important in stimulating granule secretion of prothrombotic, proinflammatory, and pro-proliferation factors/receptors27. These factors and receptors are crucially involved in occlusive thrombosis and in facilitating inflammation, atherosclerosis28,29, and ischemia/reperfusion injury30. GPCRs stimulate integrin-independent granule secretion prior to integrin activation31. Following integrin ligation and primary aggregation, integrin outside-in signaling induces integrin-dependent granule secretion20. Whereas Gα13 mediates both GPCR-dependent RhoA signaling and integrin outside-in signaling, the relationship between Gα13 and the complex integrin-dependent and integrin-independent pathways of granule secretion is not clear. Understanding the role of Gα13 and the regulatory mechanisms of these pathways may facilitate innovative therapeutic approaches to selectively modulate platelet granule secretion independently of platelet adhesion/aggregation and hemostatic function, which current anti-platelet drugs fail to do.
In this work, we demonstrate that binding of the integrin β3 cytoplasmic domain to Gα13 through its ExE motif, directly disrupts the interaction between p115RhoGEF and Gα13, thus inhibiting Gα13-p115RhoGEF-mediated RhoA activation and signaling. In platelets, this inhibitory effect of integrins greatly reduces GPCR-mediated integrin-independent granule secretion. On the other hand, the Gα13-integrin interaction stimulates integrin outside-in signaling and integrin-dependent granule secretion. Furthermore, we demonstrate that the inhibitor peptide derived from the Gα13-binding β3 ExE motif disrupts the β3-Gα13 interaction to inhibit both GPCR-induced integrin-independent and integrin outside-in signaling-dependent granule secretion. This dual effect results in robust inhibition of granule secretion without affecting primary platelet aggregation, suggesting a new therapeutic approach for the development of selective inhibitors of platelet granule secretion for treating thrombo-inflammatory diseases.
Results
Direct inhibition of Gα13 binding to the RGS domain of p115RhoGEF by the integrin β3 cytoplasmic domain
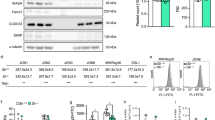
To explore a possible relationship between integrin-Gα13 interaction and p115RhoGEF-Gα13 interaction, we developed an in vitro binding assay in which the purified recombinant RGS domain of p115RhoGEF was allowed to bind to purified recombinant Gα13 in the absence or presence of increasing concentrations of purified recombinant β3 cytoplasmic domain (β3 CD)-GST fusion protein. β3 CD dose-dependently inhibited binding of the RGS domain of p115-RhoGEF to Gα13, whereas the control GST protein had no inhibitory effect (Fig. 1a). To determine whether this inhibition was due to the known β3 ExE motif interaction with Gα1323, we used a myristoylated synthetic peptide with a sequence identical to the β3 ExE motif, mP6 (Myr-FEEERA), and found that it dose-dependently inhibited the binding of the p115RhoGEF RGS domain to immobilized Gα13 (Fig. 1b). It also inhibited the binding of Gα13 to the immobilized p115 RGS domain as compared with the control peptide (Fig. 1c). These data demonstrate that the ExE motif in the cytoplasmic domain of β3 directly inhibits the binding of Gα13 to p115RhoGEF in in vitro assays using purified proteins.
a Binding of purified GST-tagged RGS domain of p115RhoGEF (GST-p115RGS) to purified Histidine-tagged Gα13 (His-Gα13) coated on microtiter plates is inhibited by increasing concentrations of purified GST-β3 cytoplasmic domain fusion protein (β3CD) compared with control GST, 3 independent experiments. b, c Inhibition of the binding of purified GST-p115RGS to microtiter well-coated His-Gα13 (b) and the binding of His-Gα13 to microtiter well-coated GST-p115RGS (c) by increasing concentration of mP6 peptide or a scrambled peptide with the same amino acid composition (mP6 Scr), 3 independent experiments. d, e Co-immunoprecipitation of p115RhoGEF and Gα13 in WT (C57BL/6J) and β3-/- mouse platelets stimulated with thrombin (0.035 U/ mL) for increasing lengths of time. d A representative Western blot; e, quantification of data from 4 independent experiments. f, g Co-immunoprecipitation of p115RhoGEF and Gα13 in resting (0) and 0.03 U/mL thrombin-stimulated WT mouse platelets pre-treated with RGDS or control RGES peptide (2 mM). f A representative western blot; g quantification of data from four independent experiments. h, i Co-immunoprecipitation of p115RhoGEF and Gα13 in wild-type mouse platelets pre-treated with control peptide (myr-FAAAKL) or mP6 peptide (200 μM, DMSO), stimulated with thrombin (0.03 U/ mL) for 10 seconds. h A representative Western blot; i Quantification of co-immunoprecipitation of Gα13. Data are from three independent experiments. Platelets used in (d–g) were pre-treated with 500 μM aspirin to exclude the differential influence of secondary TXA2 production on Gα13 pathway of WT and β3-/- platelets. All data are shown as mean ± SEM. Statistical analysis was done using Student’s t-test, two tailed. Source data are provided as a Source Data file.
The inhibitory role of integrin β3 on GPCR-stimulated Gα13-p115RhoGEF interaction in platelets
To investigate whether the β3 inhibition of Gα13-p115RhoGEF interaction occurs in a cellular system, we compared the co-immunoprecipitation of Gα13 with p115RhoGEF in wild type (C57BL/6J) platelets and in integrin β3-/- platelets with or without stimulation of platelets with GPCR agonist thrombin. Thrombin is known to activate the Gα13-p115RhoGEF-RhoA signaling pathway10. To avoid possible effects of secondary TXA2 receptor-mediated Gα13 activation, platelets were pre-treated with aspirin to block TXA2 synthesis32. Only a low level of Gα13 was co-immunoprecipitated with p115RhoGEF in wild-type unstimulated platelets as compared with IgG control (Fig. 1d,e). Within 1 min following thrombin stimulation, Gα13 co-immunoprecipitation with p115RhoGEF was enhanced in wild-type platelets (Fig. 1d, e). Remarkably, co-immunoprecipitation of Gα13 with p115RhoGEF was almost 3-fold higher in β3-/- platelets as compared with wild-type platelets following thrombin-stimulated platelet activation (Fig. 1d, e). Of note, the total expression of Gα13 or p115-RhoGEF was not changed in β3-/- platelets as compared with wild-type platelets (Supplementary Fig. 2). Clearly, β3 expressed in wild-type platelets had a significant inhibitory effect on the Gα13-p115RhoGEF interaction, which was abolished in β3 knockout platelets. To further determine if and how ligand binding to integrin αIIbβ3 would affect Gα13-p115RhoGEF binding, we tested the effect of integrin antagonist RGDS peptide on the co-immunoprecipitation of Gα13 with p115RhoGEF, using RGES peptide as a control (Fig. 1f, g). Similar to β3 knockout, RGDS-treatment enhanced Gα13-p115RhoGEF co-immunoprecipitation following platelet activation, relative to control RGES-treated platelets (Fig. 1f, g). In control RGES-treated platelets, Gα13-p115RhoGEF interaction was reduced following the initial wave of binding (Fig. 1f, g). In contrast, Gα13-p115RhoGEF binding in RGDS-treated platelets was persistently high after the initial increase (Fig.1f, g). Similarly to RGDS, the clinically used integrin antagonist Eptifibatide (Integrilin) also enhanced thrombin-induced Gα13 binding to p115RhoGEF in platelets (Supplementary Fig. 3). These data suggest that blocking the ligand binding to integrin αIIbβ3 likely also diminished the ability of β3 to inhibit Gα13-p115RhoGEF interaction. Thus, the function of β3 in inhibiting Gα13-p115RhoGEF interaction is stimulated by ligand binding to integrin αIIbβ3.
We next determined whether the ExE peptide mP6 would inhibit the Gα13-p115RhoGEF interaction in thrombin-stimulated platelets similarly to the purified assay system. When mP6 was pre-incubated with wild-type mouse platelets followed by thrombin stimulation, co-immunoprecipitation of Gα13 with p115-RhoGEF was almost totally abolished (Fig. 1h, i). Thus, the ExE motif in the β3 cytoplasmic domain inhibits Gα13-p115RhoGEF interaction during thrombin-stimulated platelet activation.
The role of integrin β3 in inhibiting thrombin-induced RhoA activation
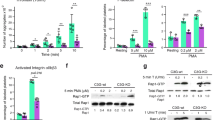
A direct downstream consequence of Gα13-p115 RhoGEF interaction is the activation of RhoA10,33,34. We thus investigated whether integrin β3 regulates Gα13/p115RhoGEF-mediated RhoA activation during GPCR-induced platelet activation using a GTP-bound RhoA pull down assay. As expected, thrombin-stimulated RhoA activation in wild-type platelets, which was transient, peaked after 1–2 min and then declined to near background level (Fig. 2a, b). Thrombin-stimulated RhoA activation was enhanced in β3-/- platelets compared to wild-type platelets (Fig. 2a, b), consistent with the enhanced Gα13-p115RhoGEF interaction shown in Fig. 1d. Importantly, β3-/- platelets stimulated with thrombin displayed prolonged RhoA activation persistent throughout the time course instead of the transient RhoA response observed in wild-type platelets. These data suggest that RhoA activity is negatively regulated by β3. To understand whether the inhibitory role of β3 in RhoA activation is a post-ligand binding function of αIIbβ3, we performed a RhoA pull-down assay in thrombin-stimulated platelets in the presence of integrin antagonist RGDS peptide or the control RGES peptide (Fig. 2c, d). RhoA activity in RGDS-treated platelets was already increased compared to the control RGES-treated platelets at the early time points following thrombin stimulation and was persistently high throughout the course of the experiment (10 min), despite the known inhibitory effect of RGDS on platelet aggregation (see text and figures below). This contrasts with the control (RGES treated) platelets in which RhoA activation was transient (Fig. 2c, d). These results confirm that ligand binding to integrin αIIbβ3 greatly enhances the inhibitory effects of β3 on RhoA activation. To further determine whether Gα13 binding to the ExE motif in β3 is responsible for the inhibitory effect of β3, we tested the effect of mP6 peptide on thrombin-induced RhoA activation. Indeed, mP6 significantly inhibited RhoA activation as compared with the control peptide throughout the course of the experiment, even at the very early time points (Fig. 2e, f). These data indicate that Gα13 binding to the ExE motif in the integrin β3 cytoplasmic domain is likely responsible for the inhibitory effect of β3 in Gα13-mediated RhoA activation. These results further suggest that the inhibitory effect of β3 on RhoA activation is independent of downstream integrin outside-in signaling. This is because mP6 diminishes integrin outside-in signaling23,24 and should thus reduce the inhibitory effect of β3 on GPCR-stimulated RhoA activation if it were dependent on outside-in signaling. Instead, mP6 mimicked the effect of β3 to inhibit GPCR-induced RhoA activation (Fig. 2e, f). Therefore, integrin β3 binding to Gα13 directly inhibits Gα13 binding to p115RhoGEF and downstream RhoA activation in platelets, independently of outside-in signaling.
a, b Rhotekin-RBD bead pull down assay was used to analyze RhoA activation in WT or β3-/- mouse platelets stimulated with thrombin (0.03 U/mL) at various time points, 4 independent experiments. c, d RhoA pull down assay with RGDS or RGES-pre-treated (2 mM, 3 min, 22 °C) mouse platelets stimulated with thrombin (0.03 U/mL) for the indicated time points, four independent experiments. e, f RhoA pull down assay with 0.03 U/ mL thrombin-stimulated mouse platelets pre-treated with control peptide or mP6 peptide (100 μM, DMSO) for 3 min at 22 °C, three independent experiments. a, c, e Representative western blots; b, d, f quantification of western blot images as analyzed using ImageJ. All data are shown as mean ± SEM. Statistical significance was determined using Student’s t test, (f) paired Student’s t test, two tailed. Source data are provided as a Source Data file.
The effect of integrin β3 on thrombin-induced platelet granule secretion
One function of the Gα13-p115RhoGEF-RhoA pathway is to stimulate granule secretion (exocytosis)13. To study whether and how integrin αIIbβ3 regulates GPCR-stimulated platelet dense granule secretion, we compared thrombin-stimulated ATP secretion from wild type and β3-/- mouse platelets and from human platelets pre-treated with control vehicle or Eptifibatide. As expected, β3 knockout or Eptifibatide inhibited platelet aggregation (Fig. 3a, c). However, β3 knockout or Eptifibatide enhanced ATP secretion at low concentrations of thrombin (Fig. 3b, d), despite the expectation that abolishing platelet aggregation should also inhibit integrin-dependent platelet granule secretion. The enhancement of ATP secretion in β3-/- platelets was not observed at high concentrations of thrombin (Fig. 3b) because granule secretion was already maximized and could not be further increased in control platelets. This rules out the possibility that the effect of β3 knockout was due to increased ATP concentration in platelets rather than enhanced ATP release (Fig. 3a, b). Similarly, total α-granule release of P-selectin during low-dose thrombin-stimulated platelet activation was also enhanced by Eptifibatide (Fig. 3e). These data show that integrin αIIbβ3 negatively regulates GPCR-induced, integrin-independent granule secretion.
a Aggregation and ATP secretion traces of washed WT and β3-/- platelets stimulated with thrombin. b Quantification of ATP secretion for a, three independent experiments. c Aggregation and ATP secretion traces of washed human platelets pre-incubated with vehicle or 10 μg/mL Eptifibatide for 3 min at 22 °C and stimulated with thrombin. d Quantification of ATP secretion for c, three independent experiments. e Flow cytometry analysis of P-selectin expression on mouse platelets pre-treated with control vehicle or Eptifibatide (20 μg/mL) for 3 min at 22 °C and stimulated with 0.03 U/mL thrombin at 37 °C with stirring at 1000 rpm in an aggregometer. Three independent experiments. All data are shown as mean ± SEM. Statistical significance was determined using Student’s t test, two tailed. Source data are provided as a Source Data file.
Integrin β3 negatively regulates integrin-independent secretion but stimulates integrin-dependent secretion
Platelet granule secretion can be mediated by integrin-independent and integrin-dependent signaling pathways. Thrombin stimulates a robust integrin-independent granule secretion and a relatively weak integrin-dependent secretion, which merge into one broad peak (Fig. 3c). However, a stable TXA2 analog, U46619, can distinctively induce a relatively weak integrin-independent first-wave secretion followed by a strong integrin-dependent second wave of granule secretion31 (Fig. 4a). Platelet aggregation stimulated by U46619 was inhibited in β3-/- platelets and in platelets treated with Eptifibatide (Fig. 4a, d), but the first wave of ATP secretion was enhanced in both (Fig. 4a, b, d, e). In contrast, the integrin-dependent second wave of secretion was diminished in β3-/- (Fig. 4a, c) and Eptifibatide-treated platelets (Fig. 4d, e). These data suggest that β3 negatively regulates integrin-independent early wave granule secretion but stimulates integrin-dependent second wave of granule secretion.
a Aggregation and ATP secretion traces of washed WT and β3-/- platelets induced by U46619 at 37 °C. Note the two waves of ATP secretion in WT platelets and a higher single (first) secretion wave in β3-/- platelets. b, c Quantification of the first (b) and second (c) waves of secretion as shown in a, three independent experiments. d Aggregation and ATP secretion traces of washed human platelets pre-incubated with vehicle or 10 μg/mL Eptifibatide for 3 min at 22 °C followed by stimulation with U46619 at 37 °C. e Quantification of secretion data from (d), 3 independent experiments. f U46619-induced ATP secretion traces of β3-/- platelets pre-incubated with control vehicle or 10 µM Y27632 for 3 min at 22 °C. All data are shown as mean ± SEM. Statistical significance was determined using Student’s t-test, two tailed. Source data are provided as a Source Data file.
Integrin-independent granule secretion is RhoA-dependent
To determine whether the Gα13-p115RhoGEF-RhoA pathway is involved in the integrin-independent secretion in β3-/- platelets, we measured U46619-induced ATP secretion in β3-/- platelets with or without Rho kinase inhibitor Y27632. Y27632 diminished U46619-induced granule secretion in β3-/- platelets (Fig. 4f), indicating that RhoA signaling is important in integrin-independent granule secretion. Previously, it was shown that RhoA also plays a key role in low-dose thrombin-induced platelet granule secretion12,13,35.
Gα13 plays stimulatory roles in both integrin-independent and integrin-dependent granule secretion
We used Gα13-/- platelets to investigate the role of Gα13 in integrin-independent and -dependent granule secretion (Fig. 5a). Platelet aggregation stimulated by thrombin was partially inhibited in Gα13-/- platelets compared with WT (Gα13fl/fl) (Fig. 5a, b), and platelet aggregation induced by high dose U46619 was only slightly affected by Gα13 knockout (Fig. 5d), confirming previous reports that Gα13 is not required for integrin activation and primary platelet aggregation16,17. Contrary to the enhancement of thrombin-induced granule secretion by β3 knockout/inhibition, Gα13-/- platelets exhibited diminished ATP secretion after low-dose thrombin stimulation (Fig. 5b, c), indicating Gα13 is needed for β3 integrin-independent granule secretion. Furthermore, both the first wave and second wave of ATP secretion induced by U46619 were reduced in Gα13-/- platelets (Fig. 5d, e). This contrasts with the effects of β3 knockout or inhibition, which selectively inhibited integrin-dependent second-wave granule secretion, but enhanced integrin-independent first wave secretion (Fig. 4a–e). This marked difference between Gα13 knockout and β3 integrin knockout/inhibition shows that Gα13 mediates both the GPCR-induced integrin-independent granule secretion via the Gα13-p115RhoGEF-RhoA pathway and integrin-dependent granule secretion via its role in mediating integrin outside-in signaling.
a Washed WT(Gα13fl/fl) and Gα13-/- mouse platelets were solubilized and immunoblotted with antibodies specifically recognizing mouse Gα13, and integrin β3. b Aggregation and ATP secretion traces of washed WT and Gα13-/- platelets stimulated with thrombin. c Quantification of secretion as shown in b, four independent experiments. d Aggregation and ATP secretion traces of WT and Gα13-/- platelets stimulated with 2 μmol/L U46619. e Quantification of ATP secretion as shown in d, six independent experiments. Aggregation assays were performed without exogenously added fibrinogen. All data are shown as mean ± SEM. Data were analyzed using Student’s t-test, (e) paired Student’s t-test, two tailed. Source data are provided as a Source Data file.
The ExE motif peptide mP6 inhibits both integrin-independent and integrin-dependent granule secretion
The mP6 peptide is a mimic of the β3 cytoplasmic ExE motif that binds to Gα13 to inhibit both the Gα13-p115RhoGEF interaction (Fig. 1) and the Gα13-β3 interaction23,36. We thus hypothesized that mP6 should inhibit both integrin-independent and integrin-dependent secretion. Indeed, mP6 inhibited low-dose thrombin-induced ATP secretion (Fig. 6a, b) and diminished both integrin-independent and dependent waves of ATP secretion (Fig. 6c, d) induced by thrombin and U46619. Furthermore, in the presence of extracellular fibrinogen, mP6 selectively reduced secretion-dependent secondary platelet aggregation without affecting primary aggregation (Supplementary Fig. 4). mP6 also inhibited low-dose thrombin-induced dense granule secretion of serotonin (Fig. 6e), α granule secretion of β-TG (Supplementary Fig. 5a), and surface expression of α granule membrane protein P-selectin (Fig. 6f) in platelets. Thus, dual blockage of both Gα13-p115RhoGEF and Gα13-integrin interaction by mP6 results in potent inhibition of both integrin-independent and dependent secretion of dense granules and α granules, but minimally affects primary platelet aggregation.
a, c ATP secretion traces of washed mouse platelets pre-incubated with 20 μM control FAAAKL or mP6 high loading peptide nanoparticles (HLPNs) and then stimulated with 0.03 U/mL thrombin (a) or 3 μmol/L U46619 (c). b, d Quantification of ATP secretion as shown in a, n = 3 or (c), n = 4, independent experiments. e, f Effects of control FAAAKL or mP6 HLPNs (20 μM) on serotonin secretion (e), or on P-selectin expression (f), in mouse platelets stimulated with 0.03U/ mL thrombin in an aggregometer stirring at 37 °C and 1000 rpm. n = 3, independent experiments. The assays were performed using washed platelets without adding fibrinogen. g Plasma serotonin levels in sham, saline, and M3mP6 HLPN treatment groups 24 h after myocardial infarction with reperfusion. Sham group, n = 12; Saline group, n = 14; M3mP6 group, n = 13, independent animals. Data in b, d–f are shown as mean ± SEM and in g are shown as median ± SEM. Statistical significance was determined using Student’s t-test, two tailed. h A schematic of crosstalk between the (G protein-coupled receptor) GPCR-coupled Gα13 pathway and integrin αIIbβ3 in regulating RhoA activation and platelet granule secretion. Left panel: GPCR agonists such as thrombin induce Gα13-p115RhoGEF-dependent activation of RhoA, which mediates integrin-independent granule secretion. Center panel: following integrin ligation, Gα13 interacts with the β3 cytoplasmic domain ExE motif, which inhibits Gα13 binding to p115RhoGEF, resulting in inhibition of p115RhoGEF-mediated RhoA activation and integrin-independent granule secretion. Gα13 binding to β3, however, also mediates integrin outside-in signaling leading to Src activation and integrin-dependent granule secretion. Right Panel: The β3-derived mP6 peptide binds to Gα13 to inhibit Gα13 interacting with both p115-RhoGEF and β3. Thus, mP6 potently inhibits both integrin-independent and integrin-dependent secretion without affecting integrin ligation and primary platelet adhesion/aggregation necessary for normal hemostasis. Source data are provided as a Source Data file.
The effect of ExE motif peptide on platelet granule secretion in vivo during myocardial ischemia/reperfusion injury in mice
Platelet granules contain high levels of platelet agonists, pro-coagulation molecules, pro-inflammatory cytokines and receptors, and growth factors28. Secretion of granule contents induced via activation of GPCRs and other platelet receptors results in not only great amplification of thrombosis, but also inflammation, cell proliferation, and atherosclerosis28. In this respect, the potent inhibition of platelet granule secretion and secretion-dependent secondary platelet aggregation as shown here provides an important mechanism underlying the strong anti-thrombotic effect of mP6 and the modified version (M3mP6) engineered into lipid-stabilized, high-loading peptide nanoparticles (HLPN) in vitro and in vivo17,36. However, it does not entirely explain why M3mP6 also protects the heart from myocardial ischemia–reperfusion injury (MI/RI) in a mouse model36, as current clinically used anti-thrombotic drugs are not effective in treating MI/RI37. MI/RI is a grave thrombo-inflammatory condition responsible for post-ischemia cardiac injury and heart failure, in which inflammation has been suggested to play an important role in addition to microvascular thrombosis38,39. Interestingly, secretion of serotonin from platelet granules was recently found to cause neutrophil degranulation and exacerbation of MI/RI in mouse models and in patients with acute coronary syndrome30, which is consistent with the observation that M3mP6 HLPN also inhibit neutrophil function during MI/RI36. Thus, we used the mouse left anterior descending (LAD) coronary artery ligation-reperfusion model to test whether blocking the Gα13-p115RhoGEF and Gα13-β3 interactions using M3mP6 HLPN would effectively inhibit serotonin release in vivo during myocardial ischemia-reperfusion. Indeed, M3mP6 HLPN significantly reduced plasma serotonin levels (Fig. 6g) and plasma levels of platelet α−granule proinflammatory chemokine protein β-TG (Supplementary Fig. 5b) in post-MI/R mice. Thus, in addition to its potent anti-thrombotic effects, blocking platelet secretion of serotonin and other anti-inflammatory factors enriched in platelet granules provides another key mechanism explaining the ability of M3mP6 to reduce cardiac injury and improve heart function and survival after MI/RI in mice36. Importantly, M3mP6 HLPN do not affect hemostasis nor increase bleeding risk36, despite the considerable anti-thrombotic/anti-inflammatory effects of M3mP6 resulting from inhibition of platelet secretion. These properties are uniquely advantageous over current integrin antagonists and other platelet inhibitors that primarily inhibit platelet adhesion/aggregation, leading to an enhanced risk for the severe adverse effect of bleeding.
Discussion
In this study, we discovered that integrin β3, by binding to Gα13, directly inhibits the binding of p115-RhoGEF to Gα13, and negatively regulates the GPCR-induced Gα13-p115RhoGEF-RhoA signaling pathway, demonstrating the direct cross-talk by which integrins regulate GPCR signaling. We further show that β3 integrins, by binding to Gα13, play dual roles of (1) negatively regulating Gα13-mediated, integrin-independent granule secretion and (2) transmitting integrin outside-in signaling to stimulate integrin-dependent granule secretion. Furthermore, we show that the ExE motif peptide mP6, derived from the sequence of the Gα13-binding site on β3, inhibits both Gα13-p115RhoGEF-RhoA-mediated integrin-independent granule secretion and integrin-dependent outside-in signaling-mediated granule secretion without compromising primary platelet aggregation. These data demonstrate an approach to robustly inhibit platelet granule secretion without compromising primary platelet thrombus formation and hemostasis (Fig. 6h).
The discovery of crosstalk between integrin and GPCR pathways to inhibit GPCR-mediated RhoA activation has significant implications in our understanding of how GPCRs and integrins may coordinate cell adhesion, spreading, retraction, and migration as well as exocytosis. An important role of GPCR signaling is to regulate the functions of the major adhesion receptor integrins. GPCR-coupled Gαq and Gαi pathways induce Rap1/talin/kindlin-dependent inside-out signaling, activating the ligand binding functions of integrins, and thus integrin-dependent cell adhesion/aggregation (Supplementary Fig. 1)40,41,42,43. GPCRs also stimulate Gα13-dependent RhoA activation important in cell retraction. Previous studies indicated that integrin-mediated cell spreading requires inhibition of RhoA-mediated cell retraction, and that coordinated cell spreading, and retraction drives cell migration8,24,44. Our finding that ligand-bound integrins may directly inhibit the Gα13-mediated RhoA activation pathway suggests a potential mechanism by which GPCR and integrins coordinate to stimulate cell spreading and inhibit cell retraction, only at locations where integrin-mediated adhesion occurs but allows cell retraction where integrins are not ligated, thus controlling the direction of cell movement.
In previous studies, we reported that Gα13 binding to the cytoplasmic domain of β3 integrin subunits mediate integrin outside-in signaling leading to cell spreading17,24,44. We also demonstrated that cell spreading induced by Gα13-dependent integrin outside-in signaling requires transient inhibition of RhoA17,23. The mechanism by which outside-in signaling mediates transient RhoA inhibition has not been clear, but there is evidence suggesting a potential role of integrin/Src-dependent p190RhoGAP activation45 and p190RhoGAP-mediated RhoA inactivation46,47. This mechanism was demonstrated in the experimental system of integrin-dependent cell spreading in the absence of GPCR agonist stimulation, and thus is a likely mechanism of inhibiting integrin-dependent RhoA activation, which occurs in the late retraction phase of integrin outside-in signaling following calpain-mediated abolishment of c-Src binding to β348. Here we show that the binding of an ExE motif in the cytoplasmic domain of β3 integrin to Gα13 directly inhibits Gα13 binding to p115RhoGEF and thus inhibits GPCR-mediated RhoA activation independently of the outside-in signaling pathway. Therefore, we demonstrate that integrins inhibit RhoA signaling by both direct inhibition of GPCR/Gα13-mediated RhoA activation and facilitating deactivation of RhoA via the Src-dependent early phase outside-in signaling pathway.
We demonstrate here that the cross-talk between integrin and GPCR pathways plays an important role in regulating exocytosis (granule secretion) in platelets. Platelet granule secretion can be stimulated by GPCR agonists, independent of integrins, which releases integrin ligands and other cargo molecules to facilitate platelet activation, adhesion, and aggregation. GPCR-stimulated granule secretion involves Gαq, Gαi, and Gα13 and their downstream signaling pathways, like phospholipase C (PLC) β-dependent calcium elevation and protein kinase C activation, the phosphoinositide 3-kinase-Akt pathway, and the p115-RhoGEF-RhoA pathway (Supplementary Fig. 1). Gαq and Gαi pathways are also critical in inside-out signaling leading to integrin activation. Ligand binding to integrins not only mediates platelet adhesion and aggregation, but also induces integrin-dependent granule secretion, critical for the second wave of platelet aggregation, thrombus expansion, and stabilization31. Here we show that ligand binding to integrin αIIbβ3 negatively regulates GPCR-stimulated, integrin-independent granule secretion by direct inhibition of the Gα13-p115RhoGEF-RhoA pathway. In contrast, integrin-dependent granule secretion is mediated via the Gα13-dependent integrin outside-in signaling pathway, which activates the PI3K-Akt3 signaling pathway and activation of immunoreceptor tyrosine-based activation motif (ITAM) signaling and thus PLCγ26,49,50. The finding that β3 integrins negatively regulate integrin-independent secretion while stimulating integrin-dependent granule secretion is likely to be physiologically relevant as it minimizes granule secretion in circulating platelets, even if stimulated by low concentrations of agonists, but greatly enhances platelet granule secretion only when platelets adhere and aggregate to form a thrombus. Importantly, platelet granules contain large amounts of growth factors and pro-inflammatory cytokines, which are known to stimulate inflammation, migration, and proliferation of vascular cells including endothelial cells and smooth muscle cells. Thus, platelet granule secretion promotes inflammation, atherosclerosis, and angiogenesis. However, patients with Glanzmann Thrombasthenia (with lack of expression or function of αIIbβ3)51 or β3 knockout mice52 were not protected but paradoxically showed enhanced development of atherosclerosis. These observations are not consistent with the current assumption that inhibition of β3-dependent platelet adhesion and secretion would reduce atherogenesis. Our finding that β3 knockout enhances integrin-independent exocytosis in platelets provides a potential mechanism for this pro-atherogenic effect. Thus, it would be interesting to investigate whether selectively targeting Gα13-integrin interaction would help prevent/reduce atherosclerosis and other vascular inflammatory conditions.
Most current anti-platelet drugs have inhibitory effects on granule secretion, but robustly inhibit platelet adhesion/ aggregation and have adverse effects of bleeding and vascular leakage, which may exacerbate inflammation. Additionally, integrin antagonists, as shown in this study, enhance integrin-independent granule secretion, even though they inhibit integrin-dependent granule secretion. In contrast, we show that the inhibitor peptide derived from the Gα13 binding sequence of integrin β3, mP6, has the dual effects of inhibiting both the Gα13-p115RhoGEF-RhoA pathway and integrin outside-in signaling, and thus inhibits both integrin-independent and dependent granule secretion as well as the second wave of platelet aggregation without affecting primary platelet adhesion and aggregation. This peptide also showed no effect on hemostasis in vivo23. This represents a new approach to effectively inhibit granule secretion both in and out of the localized thrombus without impairing hemostatic thrombus formation. Furthermore, the recent finding that platelet secretion of serotonin promotes the role of neutrophils in causing cardiac ischemia/reperfusion injury, provides a mechanism for the beneficial effects of inhibitors of Gα13-β3 integrin interaction in treating cardiac ischemia/reperfusion injury30,36.
In summary, our data suggest a new paradigm for cross-talk between GPCR and integrin signaling in which integrins directly inhibit Gα13-mediated GPCR signaling (Fig. 6h). We further demonstrate a unique strategy of targeting this G protein-integrin crosstalk to strongly inhibit both the GPCR- and integrin-mediated granule secretion in platelets without affecting the primary adhesive function of integrins (Fig. 6h). We also provide proof-of-concept in vivo data supporting a novel approach to target integrin-Gα13 crosstalk for the treatment of secretion-mediated thrombo-inflammatory conditions such as MI/RI. This contrasts with the lack of effectiveness of current anti-thrombotic (anti-platelet) therapies in treating MI/RI. In addition, current anti-platelet drugs strongly inhibit the adhesive function of platelet integrins, thus causing exacerbation of hemorrhage and vascular leakage, which is detrimental in the treatment of thrombo-inflammation. Our new approach and the underlying mechanisms uncovered in this study avoid this problem and suggest a promising future direction in treating MI/RI and possibly other thrombo-inflammatory diseases.
Methods
Ethics statement
All animal studies were approved by the Institutional Animal Care Committee of the University of Illinois at Chicago (animal protocol number: 22-184). For studies using human platelets, Institutional Review Board approval (1999-0610) was obtained from the University of Illinois at Chicago, and informed consent was obtained from all healthy donors in accordance with the Declaration of Helsinki.
Regents and materials
Myristoylated peptides, mP6 (Myr-FEEERA), mP6Scr (Myr-ERAFEE), M3mP6 (Myr-FEEERL), and control peptide (Myr-FAAAKL) were synthesized and purified by the Research Resources Center of the University of Illinois at Chicago, CPC Scientific Inc. (San Jose, CA) or Peptide 2.0 Inc. (Chantilly, VA). These peptides were encapsulated into high-loading peptide nanoparticles (HLPN) to allow efficient entry into cells36. RGDS and RGES peptides were synthesized by Peptide 2.0 Inc. (Chantilly, VA). Apyrase, prostaglandin E1 (PGE1) and aspirin were from Sigma-Aldrich (St Louis, MO). α-Thrombin and fibrinogen were from Enzyme Research Laboratories (South Bend, IN). Luciferase/luciferin reagent was from Chrono-log (Havertown, PA). U46619 and Y27632 were from Calbiochem. Anti-Gα13 (67188-1-lg, 1:1000 for western blot), anti-p115 RhoGEF (11363-1-AP, 2 μg per 500 μL cell lysate), anti-GAPDH (60004-1-lg, 1:50,000 for western blot) and anti-integrin β3 (18309-1-AP, 1:1000 for western blot) were from Proteintech (Rosemont, IL). Rabbit IgG (2729S, 2 μg per 500 μL cell lysate) and anti-p115 RhoGEF (D25D2, 1:1000 for western blot) were from Cell Signaling technology Inc. (Danvers, MA). Protein A/G PLUS-Agarose (sc-2003) beads were from Santa Cruz Biotechnology Inc (Dallas, TX). FITC-conjugated rat anti-mouse CD62P antibody (553744, 1 μg/ 105 platelets) and FITC-conjugated rat IgG1 λ isotype control (553995, 1 μg/ 105 platelets) were from BD Pharmingen. Eptifibatide was from Merck & Co., Inc. (White house station, NJ). RhoA activation assay Biochem Kit (BK036) was from Cytoskeleton, Inc (Denver, CO). Serotonin Research ELISA kit (BA-E-5900R) was from LDN Labor Diognostika (Nordhorn, Germany). Mouse beta-TG (beta-Thromboglobulin) ELISA Kit (MBS2510762) was from MyBiosource, Inc (San Diego, CA). K2EDTA tubes (367842) were from BD Vacutainer (Boston, MA).
Animals
Gα13flox/flox mice were generous gifts from Dr. Stefan Offermanns, Max Planck Institute for Heart and Lung Research (Bad Nauheim, Germany)16. Generation of platelet-specific Gα13 knockout (Gα13fl/fl, PF4-Cre+, referred to as Gα13-/-) mice were previously described25,53. Integrin β3 knockout (β3-/-) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and were maintained on a C57BL/6J background. C57BL/6J and Gα13fl/fl (referred to as Gα13+/+) mice were used as their respective controls. Mice used in this study were 8 to 16-weeks-old with an equal sex ratio. Animals were housed and bred in the Biologic Resources Laboratory at the University of Illinois at Chicago under 12 h light-dark cycles, controlled temperature (~23°) and 40–50% humidity with free access to food and water. A randomized approach of choosing mice was used throughout the study, using all mice with the correct genotype without bias.
Preparation of platelets
Washed mouse and human platelets were prepared as previously described17,23,26. Fresh mouse blood was drawn from the inferior vena cava and added with one-ninth volume of anti-coagulant solution (85 mM trisodium citrate, 83 mM d-glucose, and 21 mM citric acid). After adding a final concentration of 0.1 μg/mL prostaglandin E1 and 1 U/mL apyrase, platelet-rich plasma (PRP) was isolated by centrifugation of whole blood at 200×g at 22 °C for 10 min, and mouse platelets were further isolated by centrifugation at 870×g at 22 °C for 10 min. In some experiments, an additional 1 U/ mL apyrase and 1/100 volume of 0.5 M EDTA (pH 8.0) was added to PRP before centrifugation to minimize platelet activation. For human blood, a one-seventh volume of ACD (85 mM trisodium citrate, 110 mM D-glucose, and 78 mM citric acid) was used as anticoagulant and centrifuged at 300 g for 20 min, 22 °C, to obtain PRP, which was further centrifuged at 700×g at 22 °C for 10 min to isolate platelets. Thereafter, mouse or human platelets were washed once with CGS buffer (sodium chloride 0.12 M, d-glucose, 0.03 M, trisodium citrate 0.0129 M, pH 6.5) and resuspended in modified Tyrode’s buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 0.42 mM NaH2PO4, 10 mM N-2-hydroxyethylpiperazine-N′−2-ethanesulfonic acid, 1 mM MgCl2, pH 7.4). Washed platelets were then allowed to rest at room temperature for at least 1 h. CaCl2 (1 mM) was added to platelet suspensions 10 minutes before use.
Platelet aggregation and granule secretion
Platelet aggregation and dense granule secretion were measured in a turbidimetric platelet lumi-aggregometer (Chrono-log) at 37 °C with stirring (1000 rpm), and the real-time traces were recorded using Aggro/Link8 (version 1.2.9) from CHRONO-LOG CORP25,54,55. Peptides, (mP6 or control peptide), encapsulated into HLPN36, were added to the platelet suspension for 3 minutes at room temperature prior to assay. Platelet dense granule secretion of ATP was assessed by using luciferin-luciferase reagent in the aggregometer31,56. Quantification was performed using an ATP standard curve. Mouse platelet secretion of serotonin from dense granules was assessed by a Serotonin Research ELISA kit. The total α granule of β-TG secretion was assessed by mouse β-TG ELISA kit. Specifically, platelet suspensions (with or without agonist stimulation) were placed on ice to stop the secretion and were immediately spun down at 10,000×g at 4 °C, for 10 min. Supernatants were saved for ELISA analysis following manufacturer’s instructions.
Platelet α-granule secretion was also analyzed by estimating the surface expression of P-selectin57. Mouse platelets isolated from freshly drawn blood as described above were washed in CGS buffer (with 0.1% BSA, pH6.5) and resuspended in modified Tyrode’s buffer (with 0.1% BSA, pH 7.4) containing 1 mM CaCl2 and 1 mM MgCl2. Washed platelets in Tyrode’s buffer were pre-incubated with control vehicle or 20 μg/mL Eptifibatide, 20 μM control peptide or mP6 HPLNs for 3 min at room temperature, and then stimulated with thrombin in an aggregometer at 37 °C at 1000 rpm stirring rate for the indicated time points. After fixing with 2% paraformaldehyde, platelets were incubated with an FITC-conjugated rat anti-mouse P-selectin antibody or rat IgG control for 30 min at 22 °C in the dark. After dilution 10-fold in PBS (containing 1% BSA), platelet P-selectin expression was analyzed using a Accuri C6 flow cytometer with CFlow Plus software (version 1.0.227.4) (BD Biosciences). The Gating strategy for flow cytometry analysis is shown in Supplementary Fig. 6.
Co-immunoprecipitation
Washed mouse platelets resuspended in modified Tyrode’s buffer (3 × 108 /mL) were stimulated with thrombin for the indicated time points in an aggregometer (37 °C, 1000 rpm), and then were solubilized in HEPES-NP40 Buffer (20 mM HEPES, 10 mM MgCl2, 150 mM NaCl, 1% NP-40,1 mM EGTA, 10% Glycerol, 1 mM sodium orthovanadate, 1 mM NaF, pH 7.4), with complete protease inhibitor cocktail (1 tablet/10 ml buffer, Roche Diagnostics, Mannheim), on ice for 30 min. Platelet lysates were then cleared at 14,000×g for 10 min at 4 °C and the supernatants incubated with 4 μg/ml of anti-p115RhoGEF (11363-1-AP) or IgG over night at 4°C. They were then further incubated with Protein A/G PLUS-Agarose beads, rotating at 4°C for 2 h. After 3 washes with lysis buffer, immunoprecipitants were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotted with antibodies against p115-RhoGEF (D25D2) or Gα13. When comparing Gα13-p115 RhoGEF interactions in WT (C57BL/6 J) or β3-/- platelets, and in RGES or RGDS (2 mM) pre-treated platelets17, and in control vehicle or 40μM Eptifibatide pre-treated platelets, 500 µM aspirin31 was also added to platelets to block and exclude the differential influence of secondary TXA2 production on the Gα13 pathway. In some experiments, a control peptide or mP6 peptide (200 μM in DMSO) was used to pretreat platelets for 3 minutes prior to assay. Quantitation of western blots was performed using ImageJ. The data were normalized relative to the values obtained from platelets in the resting state (0 min).
GTP-RhoA pull down assay
For the GST-RhoA pull down assay, all necessary regents including lysis buffer, washing buffer, Rhotekin-RBD protein beads, protease inhibitor cocktail, and anti-RhoA monoclonal antibody were those included in the RhoA activation assay Biochem Kit. Briefly, isolated mouse platelets (3 × 108 /mL) resuspended in modified Tyrode’s buffer were lysed quickly with RhoA lysis buffer (added with 1× protease inhibitor cocktail) after thrombin stimulation for the indicated time points in an aggregometer. Lysates were cleared by centrifugation at 17,000×g for 2 min at 4 °C, and the supernatants were incubated for 1 h with 50 µg Rhotekin-RBD protein beads/sample. Samples were washed once using washing buffer and then immunoblotted with anti-RhoA antibody (ARH05, 1:500 for western blot). Cell lysates were also immunoblotted with anti-RhoA as loading control. In some experiments, integrin inhibitor RGDS or control RGES (2 mM), and mP6 or control peptide (100 µΜ in DMSO) were used to treat platelets for 3 minutes before thrombin stimulation for the indicated time points. Quantitation of western blots was performed using ImageJ. The data are normalized relative to the values obtained from platelets in the resting state (0 min).
In vitro competition assay
GST-β3CD was purified as described before17,23. For GST-β3CD cDNA construction, integrin β3 cytoplastic domain (716-762) cDNA was amplified by PCR and cloned into pGEX-4T2 vector using Bam HI and Xho I restriction sites. The forward primer is 5′-CGTGGATCCAAACTCCTCATCACCATCCACGACC-3′; the reverse primer is 5′-GCGCTCGAGTTAAGTGCCCCGGTACGTGATATTG-3′. GST-β3CD was purified from BL21 (DE3) E. coli with IPTG induction and using glutathione-conjugated beads17. Histidine-tagged Gα13(His-Gα13) was previously provided by Dr. Tohru Kozasa (University of Illinois at Chicago, Chicago, IL)58. GST-RGS was purified from BL21 cells transformed with vector PGEXKG p115-RGS. His-Gα13 (5 μg/mL) or 2.5 μg/mL GST-RGS were coated onto 96-well microtiter plates overnight. After blocking with 5% BSA overnight at 4 °C, plates were washed three times with PBS. Increasing concentrations of purified GST-β3CD protein/ GST control protein, or inhibitor peptide mP6 or control peptide were then added to the plates in Tris-NP40 buffer (50 mM Tris, 10 mM MgCl2, 150 mM NaCl2, 1% NP-40)23. After incubation for 1 h at room temperature, 0.25 μg/mL GST-RGS or 2.5 μg/mL His-tagged recombinant Gα13 was added to the plates for another 2 h. Bound RGS of p115 or Gα13 was estimated with anti-p115 RhoGEF (D25D2) or anti-Gα13 antibodies, anti-rabbit, or anti-mouse HRP-conjugated secondary antibodies and 3,3′,5,5′-tetramethylbenzidine substrates (Thermo Fisher Scientific Pierce Biotechnology, Rockford, IL). The wells were washed three times with PBST between each step. The reactions were terminated with 0.16 M sulfuric acid and measured for OD450nm with a FlexStation3 Multi-mode Microplate reader (Molecular Devices, LLC).
Mouse myocardial ischemia and reperfusion model
The MI/RI model was performed as previously described36. C57BL/6J mice were randomly assigned to sham and surgical groups. Following induction of ischemia, M3mP6 HLPN or saline was injected at a bolus dose of 5 μmol/kg through the jugular vein cannula. After 1 h of ischemia, the suture was cut and removed, followed by M3mP6 HLPN (2.5 μmol/kg/h) or saline infusion through the jugular vein cannula for 24 h. To obtain plasma, blood was drawn via the vena cava into K2EDTA tubes and centrifuged at 2000 g within 30 min of collection. Plasma serotonin30 and β-TG were quantified using ELISA kits according to manufacturer’s instructions.
Statistical analysis
Data are expressed as means (or median) ± SEM. For parametric data, differences between groups of samples were evaluated with Student’s t-test, with GraphPad Prism software. For nonparametric data, statistical significance was determined using the Mann–Whitney test. A p-value ≤ 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data generated in this study are provided in the Supplementary Information/Source Data file. Additional information can be obtained from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Wootten, D., Christopoulos, A., Marti-Solano, M., Babu, M. M. & Sexton, P. M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 19, 638–653 (2018).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schioth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 (2017).
Hauser, A. S. et al. Pharmacogenomics of GPCR drug targets. Cell 172, 41–54.e19 (2018).
Hilger, D., Masureel, M. & Kobilka, B. K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 25, 4–12 (2018).
Oldham, W. M. & Hamm, H. E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 (2008).
Wickstrom, S. A. & Fassler, R. Regulation of membrane traffic by integrin signaling. Trends Cell Biol. 21, 266–273 (2011).
Bergmeier, W. & Hynes, R. O. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring. Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a005132 (2012).
Shen, B., Delaney, M. K. & Du, X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr. Opin. Cell Biol. 24, 600–606 (2012).
Kozasa, T. et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280, 2109–2111 (1998).
Hart, M. J. et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280, 2112–2114 (1998).
Ridley, A. J. & Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399 (1992).
Delaney, M. K. et al. Agonist-induced platelet procoagulant activity requires shear and a Rac1-dependent signaling mechanism. Blood 124, 1957–1967 (2014).
Pleines, I. et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 119, 1054–1063 (2012).
Price, L. S., Norman, J. C., Ridley, A. J. & Koffer, A. The small GTPases Rac and Rho as regulators of secretion in mast cells. Curr. Biol. 5, 68–73 (1995).
Miklavc, P. et al. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion-a role for Rho, formins and myosin II. J. Cell Sci. 125, 2765–2774 (2012).
Moers, A. et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat. Med. 9, 1418–1422 (2003).
Gong, H. et al. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science 327, 340–343 (2010).
Ginsberg, M. H. Integrin activation. BMB Rep. 47, 655–659 (2014).
Plow, E. F., Qin, J. & Byzova, T. Kindling the flame of integrin activation and function with kindlins. Curr. Opin. Hematol. 16, 323–328 (2009).
Estevez, B. & Du, X. New concepts and mechanisms of platelet activation signaling. Physiology 32, 162–177 (2017).
Durrant, T. N., van den Bosch, M. T. & Hers, I. Integrin alphaIIbbeta3 outside-in signaling. Blood 130, 1607–1619 (2017).
Arias-Salgado, E. G., Lizano, S., Shattil, S. J. & Ginsberg, M. H. Specification of the direction of adhesive signaling by the integrin beta cytoplasmic domain. J. Biol. Chem. 280, 29699–29707 (2005).
Shen, B. et al. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 503, 131–135 (2013).
Shen, B. et al. The interaction of Gα13 with integrin β1 mediates cell migration by dynamic regulation of RhoA. Mol. Biol. Cell 26, 3658–3670 (2015).
Cheng, N. et al. Targeting Gα13-integrin interaction ameliorates systemic inflammation. Nat. Commun. 12, 3185 (2021).
Xu, Z. et al. Shear and integrin outside-in signaling activate NADPH-oxidase 2 to promote platelet activation. Arterioscler. Thromb. Vasc. Biol. 41, 1638–1653 (2021).
Coorssen, J. R., Davidson, M. M. & Haslam, R. J. Factors affecting dense and alpha-granule secretion from electropermeabilized human platelets: Ca(2+)-independent actions of phorbol ester and GTP gamma S. Cell Regul. 1, 1027–1041 (1990).
Golebiewska, E. M. & Poole, A. W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 29, 153–162 (2015).
Gawaz, M. Platelets in the onset of atherosclerosis. Blood Cells Mol. Dis. 36, 206–210 (2006).
Mauler, M. et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation 139, 918–931 (2019).
Li, Z. et al. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J. Biol. Chem. 278, 30725–30731 (2003).
Fontana, P., Zufferey, A., Daali, Y. & Reny, J. L. Antiplatelet therapy: targeting the TxA2 pathway. J. Cardiovasc. Transl. Res. 7, 29–38 (2014).
Kozasa, T., Hajicek, N., Chow, C. R. & Suzuki, N. Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13. J. Biochem. 150, 357–369 (2011).
Siehler, S. Regulation of RhoGEF proteins by G12/13-coupled receptors. Br. J. Pharmacol. 158, 41–49 (2009).
Akbar, H., Duan, X., Saleem, S., Davis, A. K. & Zheng, Y. RhoA and Rac1 GTPases differentially regulate agonist-receptor mediated reactive oxygen species generation in platelets. PLoS ONE 11, e0163227 (2016).
Pang, A. et al. High-loading Gα13-binding EXE peptide nanoparticles prevent thrombosis and protect mice from cardiac ischemia/reperfusion injury. Sci. Transl. Med. 12, eaaz7287 (2020).
Allencherril, J. et al. Do we need potent intravenous antiplatelet inhibition at the time of reperfusion during ST-segment elevation myocardial infarction? J. Cardiovasc. Pharmacol. Ther. 24, 215–224 (2019).
Ziegler, M., Wang, X. & Peter, K. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovasc. Res. 115, 1178–1188 (2019).
Kalogeris, T., Baines, C. P., Krenz, M. & Korthuis, R. J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229–317 (2012).
Zhu, L. et al. Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat. Commun. 8, 1744 (2017).
Ma, Y. Q., Qin, J., Wu, C. & Plow, E. F. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J. Cell Biol. 181, 439–446 (2008).
Moser, M., Nieswandt, B., Ussar, S., Pozgajova, M. & Fässler, R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330 (2008).
Lagarrigue, F. et al. Talin-1 is the principal platelet Rap1 effector of integrin activation. Blood 136, 1180–1190 (2020).
Chang, C. W., Cheng, N., Bai, Y., Skidgel, R. A. & Du, X. Galpha13 mediates transendothelial migration of neutrophils by promoting integrin-dependent motility without affecting directionality. J. Immunol. https://doi.org/10.4049/jimmunol.2001385 (2021).
Arthur, W. T., Petch, L. A. & Burridge, K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722 (2000).
Arthur, W. T. & Burridge, K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12, 2711–2720 (2001).
Ren, X. D., Kiosses, W. B. & Schwartz, M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Flevaris, P. et al. A molecular switch that controls cell spreading and retraction. J. Cell Biol. 179, 553–565 (2007).
Boylan, B. et al. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood 112, 2780–2786 (2008).
Estevez, B. et al. LIM kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood 121, 4586–4594 (2013).
Shpilberg, O. et al. Patients with Glanzmann thrombasthenia lacking platelet glycoprotein alpha(IIb)beta(3) (GPIIb/IIIa) and alpha(v)beta(3) receptors are not protected from atherosclerosis. Circulation 105, 1044–1048 (2002).
Weng, S. et al. Beta3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc. Natl Acad. Sci. USA 100, 6730–6735 (2003).
Pang, A. et al. Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood 132, 533–543 (2018).
Delaney, M. K., Liu, J., Zheng, Y., Berndt, M. C. & Du, X. The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation. Arterioscler. Thromb. Vasc. Biol. 32, 2761–2768 (2012).
Estevez, B. et al. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood 127, 626–636 (2016).
Li, Z., Zhang, G., Marjanovic, J. A., Ruan, C. & Du, X. A platelet secretion pathway mediated by cGMP-dependent protein kinase*. J. Biol. Chem. 279, 42469–42475 (2004).
Flevaris, P. et al. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK–dependent integrin outside-in retractile signaling pathway. Blood 113, 893–901 (2009).
Tanabe, S., Kreutz, B., Suzuki, N. & Kozasa, T. Methods in Enzymology Vol. 390 (ed D. P. Siderovski) p. 285–294 (Academic Press, 2004).
Acknowledgements
We thank Dr. Stefan Offermanns, Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany, for providing Gα13 knockout mice. This work is supported in part by grants from the National Heart, Lung, and Blood Institute grants R35HL150797 (X.D.), RO1HL080264 (X.D), RO1HL125356 (X.D.), R44HL156560-01A1 (R.A.S.), and R44 HL142396 (R.A.S), and by the Lilly Research Award Program (G.D. and X.D.).
Author information
Authors and Affiliations
Contributions
Y.Z., X.Z. and B.S. contributed equally to experimentation, data analysis, and manuscript writing. Y.B., C.C., A.S., C.W. and A.M. performed some experiments and data analysis. G.D. contributes to important discussions and editing. R.A.S. contributed to experimental planning, analysis, and manuscript writing, and N.C. contributed to some experiments, technical supervision, data analysis, and manuscript writing. X.D. led the project, designed research, analyzed/interpreted data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
University of Illinois at Chicago holds patents related to this study. X.D. holds equity interests in DMT, Inc., which licenses UIC technology. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tatiana Byzova, Suzanne Scarlata, Nanpeng Chenfor, and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Zhao, X., Shen, B. et al. Integrin β3 directly inhibits the Gα13-p115RhoGEF interaction to regulate G protein signaling and platelet exocytosis. Nat Commun 14, 4966 (2023). https://doi.org/10.1038/s41467-023-40531-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40531-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.