Abstract
In early type 2 diabetes, the strategy of “induction” with short-term intensive insulin therapy followed by “maintenance” with metformin can stabilize pancreatic beta-cell function in some patients but not others. We thus sought to elucidate determinants of sustained stabilization of beta-cell function. In this secondary analysis of ClinicalTrials.Gov NCT02192424, adults with ≤5-years diabetes duration were randomized to 3-weeks induction insulin therapy (glargine/lispro) followed by metformin maintenance either with or without intermittent 2-week courses of insulin every 3-months for 2-years. Sustained stabilization (higher beta-cell function at 2-years than at baseline) was achieved in 55 of 99 participants. Independent predictors of sustained stabilization were the change in beta-cell function during induction and changes in hepatic insulin resistance and alanine aminotransferase during maintenance. Thus, initial reversibility of beta-cell dysfunction during induction and subsequent preservation of hepatic insulin sensitivity during maintenance are associated with sustained stabilization of beta-cell function following short-term insulin and metformin.
ClinicalTrials.Gov NCT02192424
Similar content being viewed by others
Introduction
The natural history of type 2 diabetes (T2DM) is characterized by the progressive deterioration of pancreatic beta-cell function over time1. Despite the genetic underpinnings of beta-cell dysfunction, there exists a reversible component to this process early in the course of T2DM that may be responsive to interventions such as intensive lifestyle modification and short-term intensive insulin therapy (IIT)2,3. Indeed, by ameliorating reversible beta-cell dysfunction, these interventions can even induce a remission of diabetes2,3,4,5,6,7. Ultimately, however, the durability of such an outcome is dependent upon the ability to maintain the beneficial beta-cell effect that was induced with the initial intervention. Thus, there is currently interest in developing treatment strategies that can achieve the sustained stabilization of beta-cell function in early T2DM and understanding the underlying determinants of this effect8,9.
Here we show that one such strategy is the administration of initial short-term IIT (as induction therapy) followed by metformin (as maintenance therapy), an approach that has been shown to stabilize beta-cell function for 2-years in some patients but not others10,11. We recently reported the main findings of the REmission Studies Evaluating Type 2 Diabetes - Intermittent Insulin Therapy Main (RESET-IT Main) trial, which demonstrated that the addition of intermittent courses of IIT does not further enhance the beneficial effect on beta-cell function achieved with initial induction IIT followed by metformin maintenance11. Of note, this trial population underwent detailed characterization of metabolic function (including insulin sensitivity and beta-cell function) on 10 occasions over 2-years, thereby providing an opportunity to evaluate the metabolic response over time and determinants of its durability. Thus, in this context, we now sought to elucidate determinants of sustained stabilization of beta-cell function in response to short-term IIT and metformin in early T2DM. We demonstrate herein that (i) initial reversibility of beta-cell dysfunction during induction and (ii) subsequent preservation of hepatic insulin sensitivity during maintenance are associated with the sustained stabilization of beta-cell function in response to short-term IIT and metformin.
Results
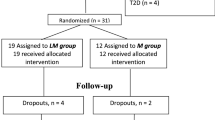
The RESET-IT Main trial (ClinicalTrials.Gov NCT02192424) was a multi-centre, parallel arm trial in which adults with T2DM were randomly assigned to receive induction therapy with a 3-week course of IIT, followed by maintenance therapy consisting of either (i) metformin alone or (ii) metformin with intermittent 2-week courses of short-term IIT administered every 3-months for 2-years. As recently reported11, the primary outcome of baseline-adjusted beta-cell function at 2-years (measured by Insulin Secretion-Sensitivity Index-2 (ISSI-2)) did not differ between the two treatment arms. The current secondary analysis focused on sustained stabilization of beta-cell function in this study population, defined by higher ISSI-2 at 2-years (or last study visit) than at baseline (i.e., reflecting an improvement in beta-cell function, in contrast to the deterioration over time that typically characterizes the natural history of T2DM).
Characteristics at Baseline and after 3-weeks Induction
Table 1 shows the baseline characteristics of the study population stratified into the following 2 groups: (i) participants who had sustained stabilization of beta-cell function for 2-years (n = 55) and (ii) those who did not have such stabilization (n = 44). At baseline, there were no differences between these groups in age, sex, ethnicity, duration of diabetes, metformin monotherapy before the trial, BMI, waist or insulin sensitivity/resistance (Matsuda index, Homeostasis Model of Assessment of Insulin Resistance (HOMA-IR)). However, those who went on to achieve stabilization had higher A1c (mean 6.7% vs 6.4%, p = 0.02) than their peers, coupled with greater glycemia on the oral glucose tolerances test (OGTT) (fasting glucose: p = 0.0003; 2 h glucose: p = 0.02). Consistent with these findings, the stabilization group had lower beta-cell function at baseline than the non-stabilization group (ISSI-2: p = 0.004; insulinogenic index/HOMA-IR: p = 0.04; ΔCpeptide0-120/Δglucose0-120 × Matsuda: p = 0.02; ΔISR0-120/Δgluc0-120 × Matsuda: p = 0.03).
During the 3-weeks of induction IIT, glycemic control was similar in the 2 groups, as evidenced by the profile of mean capillary glucose measurements across the day (Supplementary Fig. 1). Nevertheless, after 3-weeks of induction IIT, there were no longer significant differences between the two groups in either glycemic measures or beta-cell function (Table 2). Indeed, compared to their peers, those who went on to achieve stabilization had greater recovery of beta-cell function (change from baseline at 3-weeks: ISSI-2 p = 0.004; ΔCpeptide0-120/Δglucose0-120 × Matsuda p = 0.007; ΔISR0-120/Δgluc0-120 × Matsuda p = 0.02), coupled with greater decrease in fasting glucose (p = 0.02) and 2-h glucose on the OGTT (p = 0.0002). Thus, the stabilization group had poorer beta-cell function at baseline that was responsive to induction IIT, yielding no significant differences between the 2 groups at 3-weeks (Table 2).
Characteristics during maintenance phase
We next examined the maintenance phase (from 3-weeks to 2-years). Of note, the stabilization and non-stabilization groups did not differ in the maintenance therapy to which participants were randomized (Table 2). Similarly, time to initial loss of stabilization of beta-cell function did not differ between participants randomized to metformin alone and those randomized to metformin with intermittent IIT (log-rank p = 0.46; Supplementary Fig. 2). At 2-years, those who achieved stabilization had better beta-cell function than their peers (all 4 measures: p ≤ 0.02), coupled with better whole-body insulin sensitivity (Matsuda index: p = 0.0008), lower hepatic insulin resistance (HOMA-IR: p = 0.00004) and lower 2 h glucose on the OGTT (p = 0.03)(Table 3). Thus, 2 groups that were comparable at 3-weeks had significant differences at 2-years that did not appear to be attributable to their maintenance therapy, thereby raising the question of potential differential changes over the intervening time.
We first examined progressive changes over time in the glucose response to the OGTT in the 2 groups (Supplementary Fig. 3). At baseline, the stabilization group had higher glucose levels at all 5 timepoints on the OGTT (Panel A) but these differences were eliminated after 3-weeks of induction IIT (Panel B). The glucose profile then did not differ between the groups at 6-months (Panel C) and 12-months (Panel D). However, at 18-months and 24-months, the glucose response was now higher in the non-stabilization group than in those who achieved stabilization (Panels E and F, respectively), suggestive of underlying differential changes in metabolic function.
Comparison of the absolute changes in metabolic parameters between 3-weeks and 2-years showed differences between the groups in adiposity (BMI: p = 0.004; waist circumference: p = 0.03), ALT (p = 0.01), glycemia (fasting glucose: p = 0.02; 2 h glucose: p = 0.008; A1c: p = 0.0001), HOMA-IR (p = 0.0008), ISSI-2 (p = 0.0004) and insulinogenic index/HOMA-IR (p = 0.00009) (Table 3). To determine whether these measures were changing differentially over time between the 2 groups, we next performed generalized least squares regression analyses (Fig. 1). As anticipated, there were differential changes over time in ISSI-2 (p = 0.0001) and insulinogenic index/HOMA-IR (p = 0.0004) during maintenance from 3-weeks to 2-years, with the non-stabilization group showing loss of beta-cell function (Fig. 1a, b). Of note, BMI (p = 0.033) also changed differentially between the groups, with decrease over time evident in the stabilization group, although waist circumference (p = 0.32) did not show such differential change (Fig. 1c, d). However, there were differential changes in both whole-body sensitivity (Matsuda index: p = 0.044) and hepatic insulin resistance (HOMA-IR: p = 0.001), with clear deterioration of each in the non-stabilization group (Fig. 1e, f).
(a) ISSI-2; (b) Insulinogenic index/HOMA-IR, (c) BMI, (d) Waist circumference, (e) Matsuda index; and (f) HOMA-IR, comparing those with stabilization of beta-cell function over 2-years and those without such stabilization. Post-IIT indicates the beginning of the maintenance phase that runs from 3-weeks to 24-months. P-values pertain to the interaction term between group and time during the maintenance phase from 3-weeks to 24-months. Data presented as mean ± standard error. Number of participants: no stabilization n = 44; stabilization n = 55.
Determinants of sustained beta-cell stabilization
Finally, we performed logistic regression analyses to identify independent determinants of the stabilization of beta-cell function at 2-years (Table 4). In a core model adjusted for age, duration of diabetes, baseline ISSI-2, and change in waist circumference from 3-weeks to 2-years, both change in ISSI-2 during induction and change in Matsuda index during maintenance emerged as independent predictors of stabilization of beta-cell function (Model I). Upon substitution of change in waist circumference with change in BMI from 3-weeks to 2-years, the latter measure (change in BMI) replaced the concurrent change in Matsuda index as a significant predictor (Model II). Compared to Model I, this change improved model performance (area-under-the-curve (AUC) increased and Akaike Information Criterion (AIC) decreased). These model parameters were further improved with replacement of the change in Matsuda index with the change in HOMA-IR from 3-weeks to 2-years (Model III). With this model, change in ISSI-2 during induction remained an independent predictor of stabilization (aOR = 1.02 [95%CI 1.00–1.03], p = 0.005) while the change in HOMA-IR during maintenance from 3-weeks to 2-years replaced the concurrent change in BMI as a significant predictor (aOR = 0.64 [0.49–0.83], p = 0.0007).
We next performed sensitivity analyses to evaluate the robustness of these findings from the core Model III (Table 4). These analyses revealed that the addition of total physical activity over the 2-years did not change the significant predictors (Model IV). However, upon its addition to the model (Model V), the change in ALT from 3-weeks to 2-years emerged as an additional predictor of stabilization of beta-cell function at 2-years (aOR = 0.92 [0.86–0.99], p = 0.02), coupled with the concomitant change in HOMA-IR during maintenance (aOR = 0.54 [0.38–0.77], p = 0.0007) and the change in ISSI-2 during induction (aOR = 1.01 [1.00–1.03], p = 0.02). Moreover, this model demonstrated better performance than the core Model III (likelihood ratio test p = 0.015).
On additional sensitivity analyses, we repeated the core logistic regression analyses by replacing ISSI-2 with each of the other 3 measures of beta-cell function. The significant predictors from the core model above persisted when ISSI-2 was replaced as the measure of beta-cell function with insulinogenic index/HOMA-IR, ΔCpeptide0-120/Δglucose0-120 × Matsuda and ΔISR0-120/Δgluc0-120 × Matsuda, respectively, with the sole exception being that the change in insulinogenic index/HOMA-IR during induction did not achieve significance. Recognizing that the current analysis was performed in the 99 study participants (out of 108) who completed at least 1-year of follow-up, we repeated the logistic regression analyses in all participants who completed at least 1 study visit after receiving any maintenance therapy (i.e., completed at least the 3-month visit). On these sensitivity analyses in 105 study participants, the significant variables from Table 4 persisted as predictors of stabilization of beta-cell function (defined by the last study visit, rather than at 2-years).
Discussion
In this study, we show that 55 of 99 patients with T2DM achieved sustained stabilization of beta-cell function over 2-years in response to induction IIT followed by metformin maintenance. These individuals had poorer beta-cell function than their peers at baseline but experienced greater recovery thereof in response to 3-weeks of induction IIT. Over the subsequent 2-years, they had greater stability of weight/adiposity, hepatic insulin sensitivity and ALT compared to their peers, and ultimately exhibited better beta-cell function and glycemic control. Logistic regression analyses revealed 3 independent predictors of the sustained stabilization of beta-cell function: the change in ISSI-2 during induction IIT and the changes in HOMA-IR and ALT during maintenance. It thus emerges that the initial reversibility of beta-cell dysfunction during induction and the subsequent preservation of hepatic insulin sensitivity during maintenance are underlying determinants of sustained stabilization of beta-cell function in response to short-term IIT and metformin.
Two elements of these data point to the role of reversible beta-cell dysfunction. First, it is notable that those who ultimately achieved stabilization 2-years later had poorer (not better) beta-cell function than their peers at baseline. Second, in response to induction IIT, they experienced a greater improvement in beta-cell function than did their peers. Importantly, when both of these variables were included in the optimal model (Model V in Table 4 Panel B), only the change in ISSI-2 from baseline to 3-weeks was a significant independent predictor of sustained stabilization of beta-cell function. In other words, the initial recovery of beta-cell function following induction superseded the baseline measure in predicting stabilization 2-years later. These findings are consistent with the reported role of reversible beta-cell dysfunction in determining diabetes remission with lifestyle intervention2. Currently, the presence of reversible dysfunction is only identifiable retrospectively since existing measures of beta-cell function cannot distinguish between reversible and irreversible components. Growing recognition of the importance of reversible beta-cell dysfunction, as supported by the findings herein, underscores the need for the identification of phenotypic markers that may indicate its presence at baseline. Indeed, such insight could prospectively inform the judicious targeting of interventions that can ameliorate reversible beta-cell dysfunction (such as intensive lifestyle modification and short-term IIT).
After addressing reversible beta-cell dysfunction with induction therapy, the next challenge is maintaining this beneficial effect. In this regard, current thinking holds that improving insulin sensitivity (e.g. through weight loss) can off-load the secretory demands placed on the beta-cells and thereby reduce their functional deterioration in T2DM. Conversely, weight gain may increase insulin resistance and hasten beta-cell demise. In this context, it is notable that, during maintenance from 3-weeks to 2-years, there were differential changes over time in BMI between the stabilization and non-stabilization groups, with evidence of a decrease in the stabilization group (Fig. 1C). However, far more striking were the differential changes in insulin sensitivity/resistance, possibly reflecting downstream sequelae of the changes in BMI. Indeed, during this time, the non-stabilization group experienced deterioration of whole-body insulin sensitivity (Matsuda index) and hepatic insulin resistance (HOMA-IR), in contrast to the relative stability of these measures in the stabilization group (Fig. 1E, F). Furthermore, the sequential development of the core logistic regression model in Table 4 Panel A shows that the change in HOMA-IR from 3-weeks to 2-years supplanted the concomitant changes in Matsuda, BMI and waist in predicting sustained stabilization of beta-cell function. These data are suggestive of the specific importance of hepatic insulin sensitivity as a determinant of beta-cell function.
This emerging role of hepatic insulin sensitivity is supported by earlier observations. First, in women with recent gestational diabetes, worsening hepatic insulin resistance in the 1st year postpartum has been shown to independently predict declining beta-cell function, while concurrent changes in whole-body insulin sensitivity (Matsuda) and weight did not do so12. Second, in early T2DM, the change in HOMA-IR following 4-weeks of IIT has been associated with the concomitant change in beta-cell function13. Third, in the CORonary Diet Intervention with Olive oil and cardiovascular PREVention (CORDIOPREV) trial, higher baseline hepatic insulin sensitivity emerged as a predictor of the likelihood of achieving remission of diabetes with Mediterranean and low-fat diets14. The current study extends these findings by demonstrating the potential importance of preserving hepatic insulin sensitivity for the durability of the beneficial beta-cell effects of initial short-term IIT.
It is notable that, while the change in HOMA-IR from 3-weeks to 2-years superseded the changes in Matsuda, BMI and waist, consideration of the concomitant change in ALT yielded the optimal model of sustained stabilization of beta-cell function (Model V in Table 4 Panel B). Moreover, the change in ALT from 3-weeks to 2-years emerged as a significant predictor in this model, alongside the change in HOMA-IR. These data further implicate the role of the liver in determining beta-cell health and suggest the presence of other relevant elements beyond hepatic insulin resistance as reflected by HOMA-IR. One possibility in this regard is hepatic fat, the impact of which warrants evaluation in future studies of the long-term trajectory of beta-cell function. Indeed, the emergence of the change in ALT as a significant predictor in Table 4 potentially may be pointing to the importance of regional fat deposition in determining this outcome. It is a limitation of this study is that participants were not assessed for concomitant metabolic dysfunction-associated fatty liver disease.
An additional limitation is the relatively modest sample size (n = 99), which precluded adjustment for multiple comparisons such that these analyses should be interpreted cautiously as hypothesis-generating. With a larger sample size, we speculate that the change in BMI during the maintenance phase may have emerged as an additional significant negative predictor of sustained stabilization of beta-cell function over two years in the logistic regression analyses in Table 4, along with its downstream implications for insulin sensitivity as reflected in the concomitant change in HOMA-IR. Another limitation is that insulin sensitivity/resistance and beta-cell function were measured with OGTT-based surrogate indices rather than clamp studies. However, it should be noted that these indices are all validated and established measures that have been widely applied in previous studies3,11,15,16,17,18,19,20,21. Moreover, the demands of clamp studies would have made it difficult for participants to undergo such assessments on 10 occasions over 2-years. In the current study, these serial assessments provided unique insight into the changes over time in metabolic function.
In conclusion, treatment with 3-weeks of induction IIT followed by metformin maintenance achieved stabilization of beta-cell function over 2-years in 55 of 99 participants. These individuals had poorer beta-cell function than their peers at baseline but experienced greater functional recovery thereof in response to induction IIT, suggestive of reversibility of beta-cell dysfunction. Their subsequent changes in HOMA-IR and ALT during the ensuing 2 years emerged as significant predictors of sustained stabilization of beta-cell function, coupled with the initial beta-cell response to induction IIT. Thus, the initial reversibility of beta-cell dysfunction during induction and the subsequent preservation of hepatic insulin sensitivity during maintenance are physiologic changes associated with the sustained stabilization of beta-cell function in response to short-term IIT and metformin.
Methods
This multi-centre clinical trial was approved by the research ethics boards of Mount Sinai Hospital (Toronto, ON), Western University (London, ON), and Hamilton Health Sciences (Hamilton, ON), which were sites where the study took place. The trial was registered at ClinicalTrials.Gov NCT02192424. It was conducted in accordance with Good Clinical Practice and the principles of the Declaration of Helsinki, and all participants provided written informed consent. The study protocol has been previously described in detail, along with reporting of the primary outcome11.
Study population
Inclusion criteria included age 30–80 years and duration of T2DM ≤ 5 years. Before the trial, participants could be treated with either metformin or lifestyle only. Exclusion criteria included treatment with anti-diabetic medication other than metformin, estimated glomerular filtration rate <50 ml/min, and known liver disease or transaminases > 2.5-fold above normal.
Randomization and Interventions
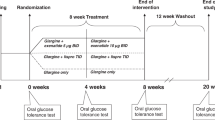
Participants stopped metformin (if applicable) and fasted overnight prior to the baseline visit, at which they completed a 2 h 75 g oral glucose tolerance test (OGTT). At this visit, they received instruction on healthy lifestyle practices for managing T2DM22,23 (which they were encouraged to continue throughout the trial) and were randomized (1:1) by computer-generated random allocation sequence to one of the following regimens: (i) 3-weeks of induction IIT followed by maintenance therapy with daily metformin thereafter for 2-years or (ii) the same regimen but with maintenance metformin supplemented with 2-week courses of IIT every 3- months (i.e. at 3-, 6-, 9-, 12-, 15-, 18- and 21-months).
These 2 treatment protocols have been described in detail previously in ref. 11. In brief, in both groups, induction IIT was administered as multiple daily injections consisting of bedtime insulin glargine and pre-prandial lispro. Doses were titrated to target fasting glucose between 4.0–6.0 mmol/l and 2 h postprandial glucose < 8 mmol/l on self-monitoring. On the last day of the 3-week induction, the final insulin dose was lispro prior to dinner (no bedtime glargine). Participants then fasted overnight and completed their 3-week OGTT the next morning.
Following the 3-week OGTT, participants began maintenance therapy consisting of either (i) metformin or (ii) metformin + intermittent IIT. In both arms, metformin was initiated at 1000 mg/day for 2 weeks, followed by 2000 mg/day or maximal tolerated dose thereafter. In the intermittent IIT arm, a 2-week course of IIT was administered every 3-months with the same glucose targets as during induction, as previously described in detail in ref. 11. These 2-week courses of IIT were started at study visits at 3-, 6-, 9-, 12-, 15-, 18- and 21-months, following completion of an OGTT at each of these visits. A final OGTT was done at 24-months.
Physiologic Indices on OGTT
The serial 2 h 75 g OGTTs enabled assessment of physiologic indices reflecting insulin sensitivity/resistance and beta-cell function. Participants fasted overnight prior to each OGTT, with metformin held on the morning of the test. During each OGTT, venous blood samples were drawn at fasting and at 30-, 60-, 90- and 120-min post-challenge. Glucose, insulin and C-peptide were measured from these samples, as previously described in ref. 11. Whole-body insulin sensitivity was assessed by Matsuda index15 and hepatic insulin resistance was assessed by Homeostasis Model Assessment (HOMA-IR)16. Beta-cell function was assessed in 4 ways, with the primary measure being the Insulin Secretion-Sensitivity Index-2 (ISSI-2) (the baseline-adjusted value of which at 2-years was the primary outcome of the trial). ISSI-2 is a validated OGTT-based measure of beta-cell compensation that is analogous to the disposition index from the intravenous glucose tolerance test, against which it has been directly validated17,18. The other 3 measures of beta-cell function were (i) insulinogenic index/HOMA-IR, (ii) ΔCpep0-120/Δgluc0-120 × Matsuda index and (iii) ΔISR0-120/Δgluc0-120 × Matsuda index (where ISR is the pre-hepatic insulin secretion rate determined by C-peptide deconvolution), with these indices calculated as previously described in refs. 3,11.
Outcomes
The primary and secondary outcomes of the trial have been previously reported in detail11. The current secondary analysis focused on sustained stabilization of beta-cell function, which was defined by having higher ISSI-2 at 2-years (or last study visit) than at baseline. Of the 108 study participants, there were 9 individuals whose last study visit occurred at <12-months, representing a duration of follow-up that was considered insufficient for determination of sustained stabilization of beta-cell function. The current analysis was thus performed in the 99 participants in whom sustained stabilization of beta-cell function could be assessed.
Statistical Analyses
Statistical analyses were conducted with R 4.2.2, with two-tailed P-values < 0.05 considered statistically significant. Continuous variables were tested for normality of distribution, with natural log transformation of skewed variables conducted where necessary. The study population was first stratified into those who did and did not achieve stabilization of beta-cell function. Characteristics of the stabilization and non-stabilization groups at baseline, after 3-weeks of induction IIT and after 2-years of maintenance therapy were compared by Analysis of Variance (normally distributed variables) or Kruskal-Wallis test (skewed variables), or Chi-Square test (categorical variables)(Tables 1–3). The daily profile of mean capillary blood glucose during induction IIT was determined for each group (Supplementary Fig. 1). The time to initial loss of stabilization of beta-cell function (defined by 2 consecutive visits with ISSI-2 lower than at baseline) was compared between treatment arms by log-rank test (Supplementary Fig. 2). The glucose response on the OGTT was compared between the stabilization and non-stabilization groups at 6 study visits, with differences at each timepoint on the test assessed by t-test (Supplementary Fig. 3). The longitudinal changes over time in ISSI-2, insulinogenic index/HOMA-IR, BMI, waist circumference, Matsuda index, and HOMA-IR were compared between the groups by generalized least squares (GLS) regression, wherein the interaction effect between group and time was examined (Fig. 1). Logistic regression analyses were performed to develop a core model of predictors of stabilization of beta-cell function over 2-years amongst age, duration of diabetes, log baseline ISSI-2, change in ISSI-2 during induction, and changes in waist/BMI and Matsuda/HOMA-IR during maintenance (Table 4 Panel A). Area-under-the-curve (AUC) and Akaike Information Criterion (AIC) enabled comparison of non-nested models I, II and III with respect to prediction accuracy and goodness-of-fit, with highest AUC and lowest AIC identifying the core model. Sensitivity analyses were performed by adding total physical activity over 2 years (assessed by Baecke questionnaire24,25) or change in ALT during maintenance to the core model, with likelihood ratio tests performed to compare nested regression models (Table 4 Panel B).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
De-identified data can be made available under restricted access from the corresponding author (Ravi.Retnakaran@SinaiHealth.ca), for academic purposes, subject to a material transfer agreement and approval of the Mount Sinai Hospital Research Ethics Board. Individual participant data that underlie the results reported in this article can be made available by this mechanism, after de-identification, to achieve the aims in the approved proposal. Access is controlled in this way because of the clinical nature of the data. The study protocol can also be made available in this way. This data access mechanism will be available beginning 9 months and ending 36 months following publication of this article. We will attempt to respond to requests within 3 months, pending Research Ethics Board capacity to do so within this time frame. Source data for figures have been provided with this paper. Source data are provided with this paper.
Code availability
The code used for the statistical analyses is available at Github at the following link: https://github.com/rretnakaran/Determinants-of-Beta-cell-Function.git
References
Halban, P. A. et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37, 1751–1758 (2014).
Taylor, R. et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab. 28, 547–556 (2018).
Retnakaran, R., Ye, C., Emery, A., Kramer, C. K. & Zinman, B. The metabolic effects of adding exenatide to basal insulin therapy when targeting remission in early type 2 diabetes in a randomized clinical trial. Nat. Commun. 13, 6109 (2022).
Goldenberg, J. Z. et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 372, m4743 (2021).
Kramer, C. K., Zinman, B. & Retnakaran, R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 1, 28–34 (2013).
Gregg, E. W. et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 308, 2489–2496 (2012).
Weng, J. et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicenter randomised parallel-group trial. Lancet 371, 1753–1760 (2008).
Salunkhe, V. A., Veluthakal, R., Kahn, S. E. & Thurmond, D. C. Novel approaches to restore beta cell function in prediabetes and type 2 diabetes. Diabetologia 61, 1895–1901 (2018).
Harrison, L. B., Adams-Huet, B., Raskin, P. & Lingvay, I. Beta-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 35, 1406–1412 (2012).
Retnakaran, R., Choi, H., Ye, C., Kramer, C. K. & Zinman, B. Two-year trial of intermittent insulin therapy vs metformin for the preservation of β-cell function after initial short-term intensive insulin induction in early type 2 diabetes. Diabetes Obes. Metab. 20, 1399–1407 (2018).
Retnakaran, R. et al. Short-term intensive insulin as induction and maintenance therapy for the preservation of beta-cell function in early type 2 diabetes (RESET-IT Main): A 2-year randomized controlled trial. Diabetes Obes. Metab. 23, 1926–1935 (2021).
Retnakaran, R. et al. Hepatic insulin resistance is an early determinant of declining β-cell function in the first year postpartum after glucose intolerance in pregnancy. Diabetes Care 34, 2431–2434 (2011).
Kramer, C. K., Choi, H., Zinman, B. & Retnakaran, R. Determinants of reversibility of β-cell dysfunction in response to short-term intensive insulin therapy in patients with early type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 305, E1398–E1407 (2013).
Roncero-Ramos, I. et al. Beta cell functionality and hepatic insulin resistance are major contributors to type 2 diabetes remission and starting pharmacological therapy: from CORDIOPREV randomized controlled trial. Transl. Res. 238, 12–24 (2021).
Matsuda, M. & DeFronzo, R. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Retnakaran, R. et al. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 16, 1901–1907 (2008).
Retnakaran, R., Qi, Y., Goran, M. I. & Hamilton, J. K. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet. Med. 26, 1198–1203 (2009).
Defronzo, R. A. et al. Prediction of diabetes based on baseline metabolic characteristics in individuals at high risk. Diabetes Care 36, 3607–3612 (2013).
Zinman, B. et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): A double-blind randomized controlled study. Lancet 376, 103–111 (2010).
Stancáková, A. et al. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 58, 1212–1221 (2009).
Diabetes Canada Clinical Practice Guidelines Expert Committee. et al. Physical activity and diabetes. Can. J. Diabetes 42, S54–S63 (2018).
Diabetes Canada Clinical Practice Guidelines Expert Committee. et al. Nutrition therapy. Can. J. Diabetes 42, S64–S79 (2018).
Baecke, J. A., Burema, J. & Frijters, J. E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 36, 936–942 (1982).
Pereira, M. A. et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci. Sports Exerc. 29, S1–S205 (1997).
Acknowledgements
RR holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at Mount Sinai Hospital. SBH holds the Diabetes Canada Chair in Diabetes Management at Western University. SMR holds the Brian W. Gilbert Chair in Primary Health Care at Western University. HCG holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. This study was supported by the Canadian Institutes of Health Research (CIHR) (MOP 133701) (RR). The funder had no role in study design, data collection and analysis, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
R.R. and B.Z. designed the study. R.R., A.E., S.B.H., S.R., H.C.G., N.M., C.K.K. and B.Z. implemented the study and acquired the data. R.R. led overall study implementation. A.E. oversaw research coordination. J.P. performed the statistical analyses. R.R. wrote the manuscript. R.R. and J.P. verified the data. All authors contributed to interpretation of the data and critical revision of the manuscript. All authors approved the manuscript. R.R. is the guarantor of this work.
Corresponding author
Ethics declarations
Competing interests
R.R. reports grants from Boehringer Ingelheim, grants and personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Eli Lilly, outside the submitted work. SBH reports grants and personal fees from Sanofi, grants and personal fees from Novo Nordisk, grants and personal fees from AstraZeneca, personal fees from Eli Lilly, personal fees from Janssen, grants and personal fees from Abbott, outside the submitted work. S.M.R. reports personal fees and non-financial support from Boehringer Ingelheim, Janssen, Eli Lilly, Sanofi, Bayer and AstraZeneca; grants, personal fees and non-financial support from Abbott, and Novo Nordisk; personal fees from Western University, Schulich School of Medicine and Dentistry and The Federation of Medical Women of Canada; grants from the Canadian Institute of Health Research, outside the submitted work. N.M. reports grants and non-financial support from AstraZeneca, grants and non-financial support from Merck, grants and non-financial support from Sanofi, outside the submitted work. H.C.G. reports research grants from Eli Lilly, AstraZeneca, Merck, Novo Nordisk and Sanofi; honoraria for speaking from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, DKSH, and Zuellig; and consulting fees from Abbott, Covance, Eli Lilly, Novo Nordisk, Sanofi, Pfizer and Kowa. C.K.K. reports research grants from Boehringer Ingelheim. J.P., A.E., and B.Z. have nothing to disclose.
Peer review
Peer review information
Nature Communications thanks Jianping Weng, Jochen Seufert and Jingwei Wu for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Retnakaran, R., Pu, J., Emery, A. et al. Determinants of sustained stabilization of beta-cell function following short-term insulin therapy in type 2 diabetes. Nat Commun 14, 4514 (2023). https://doi.org/10.1038/s41467-023-40287-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40287-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.