Abstract
Electrochemical conversion of nitrate to ammonia offers an efficient approach to reducing nitrate pollutants and a potential technology for low-temperature and low-pressure ammonia synthesis. However, the process is limited by multiple competing reactions and NO3− adsorption on cathode surfaces. Here, we report a Fe/Cu diatomic catalyst on holey nitrogen-doped graphene which exhibits high catalytic activities and selectivity for ammonia production. The catalyst enables a maximum ammonia Faradaic efficiency of 92.51% (−0.3 V(RHE)) and a high NH3 yield rate of 1.08 mmol h−1 mg−1 (at − 0.5 V(RHE)). Computational and theoretical analysis reveals that a relatively strong interaction between NO3− and Fe/Cu promotes the adsorption and discharge of NO3− anions. Nitrogen-oxygen bonds are also shown to be weakened due to the existence of hetero-atomic dual sites which lowers the overall reaction barriers. The dual-site and hetero-atom strategy in this work provides a flexible design for further catalyst development and expands the electrocatalytic techniques for nitrate reduction and ammonia synthesis.
Similar content being viewed by others
Introduction
Ammonia is a common and important chemical in agriculture, plastic, pharmaceutical industries, etc1,2. Since the invention of the Haber–Bosch process, large-scale synthesis and application of ammonia dramatically increase the crop yield and sustain the growing human population3,4. However, the Harber-Bosch process consumes extravagant resources and energy (1–2% of the annual global energy supply) and produces 1% of CO2 emission (1.8 tons CO2 for 1 ton NH3)5, causing severe environmental impact. Therefore, clean and energy-efficient technologies for ammonia synthesis receive increasing attention. At the same time, human activities have ceaselessly released reactive nitrogen into the environment, leading to an imbalance in the global nitrogen cycle6,7,8. The increasing concentration of nitrate (NO3–) in surface water and underground aquifer pollutes the water resources and poses severe threats to human health9,10,11. As NO3− is soluble and thermodynamically stable, removing NO3– from water is considered a challenging and long-standing task12,13. The direct conversion of nitrate to ammonia could reduce environmental pollution and concurrently save energy for sustainable ammonia production.
Hydrogenation of nitrate to ammonia is intrinsically a spontaneous energy-releasing process (∆G < 0) because nitrates are usually strong oxidative agents14. To pursue carbon neutrality, a thermocatalytic nitrate reduction requires hydrogen gas (H2) to be produced in a green and clean way without CO2 emission (most industrial H2 is generated from fossil fuels by steam reforming of hydrocarbons or coal gasification). By contrast, the electroreduction of nitrate to ammonia (as follows) is a promising green strategy because electrons are clean reducing agents without producing secondary wastes15,16.
The electrochemical nitrate reduction reaction (NO3−RR) involves an eight-electron transfer, which has slow kinetics17,18. In addition, the competitive hydrogen evolution reaction (HER) and a variety of byproducts complicate the reaction routes and render the NO3−RR less selective and efficient19,20. To date, achieving efficient catalysts for the NO3–RR remains a major challenge mainly because the catalytic mechanism and the structure–activity relationship of catalysts are poorly understood21.
Single-atom catalysts (SACs) have recently emerged as a new frontier in catalysis science owing to their convenient atomic design, easy structure–activity correlation, and maximum atom utilization efficiency22,23,24. Tunable local coordination and well-defined active sites25,26,27 make SACs an ideal platform to study the structure–activity relationship of catalytic NO3−RR. By engineering the coordination environments of active single atoms, the energy barriers of different proton-electron transfer steps could be selectively shifted, offering opportunities of increasing the selectivity of the NO3–RR. Sargent et al. reported that the appropriately optimized d-band center position could enhance the NO3−RR performance of Cu50Ni50 alloy as compared to pure Cu28. Guo et al. loaded a single transition metal atom on graphitic carbon nitrides and demonstrated efficient nitrate degradation and ammonia synthesis14. Fe SACs were reported to have high activity and selectivity toward the production of NH3 via the NO3–RR29. Yu et al. fabricated isolated Fe sites in FeN4 coordination and displayed twelve times higher turnover frequency than Fe nanoparticles30. These important studies imply that SACs including Fe/Cu may offer higher catalytic behavior. However, for multi-electron transfer reactions, one specific site makes SACs difficult to break the linear scaling relations of adsorption strength between catalysts and multiple similar intermediates31,32. Optimizing the interaction of a key intermediate in the rate-determining step (r.d.s) may turn another step to the r.d.s33. Dual-site catalysts could extend the catalysts with substantially different coordination environment34,35. However, the precise design and fabrication of dual-site catalysts remain a challenge and the catalytic mechanism of heteroatomic sites is elusive, especially for NO3–RR.
In this work, we propose a dual atoms catalyst for high-efficiency NO3–RR. Active metal atoms (Fe/Cu) are anchored to the holey edge sites of nitrogen-doped graphene (HNG), forming a “Y-type” ML3 coordination with two nitrogen atoms and one metal atom. Fe and Cu atoms bind together to form a dimer structure inside the holes (Fig. 1a). The resultant catalyst, Fe/Cu-HNG, demonstrates high activity (~38.5 mA cm−2 at −0.3 V vs reversible hydrogen electrode (RHE)) and selectivity (maximum Faradaic efficiency (FE) of 92.51%) for the reduction of NO3– towards NH3 under alkaline conditions. The maximum NH3 yield rate is as high as 1.08 mmol h−1 mg−1 at −0.5 V vs RHE. By combining operando differential electrochemical mass spectrometry (DEMS) and density functional theory (DFT) calculations, we reveal the reaction pathways and conversion mechanisms from NO3− to NH3. An in-depth analysis of electronic structure indicates that the strong coupling between NO3 and d-orbitals of dual metal atoms lowers the energy barrier of the first anionic adsorption step, which is the r.d.s at high current densities. The dual atoms heterostructure could further weaken N-O bonds, enabling low energy barriers for NH3 production. The synergistic effects of dual atoms offer an alternative approach to designing the NO3–RR catalysts.
Results
Structural characterization of Fe/Cu-HNG
To anchor dual atoms and form metal–metal dimers (as shown in Fig. 1a), we first engineered holes in graphene to create a great number of edge sites, which were further nitrified to bind Fe/Cu atoms. Holey graphene was fabricated by sonicating graphene oxide (GO) in nitric acid (68%). The strong oxidation capability of nitric acid would cut the C-C bonds of GO layers to yield epoxy chains (carboxyl and/or hydroxyl) and other defects36,37,38. During the following hydrothermal and annealing treatments, the above-mentioned functional groups and defects were substituted by nitrogen, forming HNG. Supplementary Fig. 1 shows the scanning electron microscope (SEM) images of HNG and reduced GO (rGO). An rGO layer exhibits a large and flat area whereas HNG becomes porous. The transmission electron microscope (TEM) images in Supplementary Fig. 2 further reveal the successful synthesis of micropores on HNG. When Fe/Cu precursors were added during the hydrothermal and annealing steps, Fe/Cu dual atoms could be loaded onto the N-edge of micropores. The Fe/Cu loadings in Fe/Cu-HNG are determined to be 3.3 and 2.8 wt%, respectively, by using an inductively coupled plasma method (Supplementary Table 1).
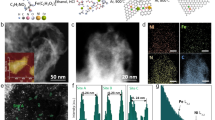
Figure 2a, b show the aberration-corrected high-angle annular dark-filed scanning TEM (HAADF-STEM) images of Fe/Cu-HNG. A great number of atom-sized bright dots are distributed on HNG. By zooming into the image, diatomic pairs (like dimers) could be mostly observed. Energy-dispersive X-ray spectroscopic (EDX) line scan through two bright dots indicates a distance of ~2.3 Å (Fig. 2c), which is in agreement with the bond length of Fe-Cu in the simulated model (Fig. 1a). The EDX elemental mapping images in Fig. 2d, e clearly indicate the uniform distribution of C, N, Fe, and Cu. Figure 2f presents a statistical distribution of the metal–metal pair length. The average bond length is 2.3 ± 0.2 Å. The electron energy loss spectrum (EELS) in Fig. 2g reveals the coexistence of Fe and Cu elements in Fe/Cu-HNG. More complementary characterizations and statistical analyses were shown in Supplementary Figs. 3, 4 to identify diatomic sites.
a HAADF-STEM image of Fe/Cu-HNG. b Zoomed-in HAADF-STEM image indicates the formation of dual atoms sites where two distinct adjacent bright dots were marked with blue dashed circles. c Corresponding intensity profiles of dual atoms pair. d STEM and e EDX elemental mapping images of C, N, Fe, and Cu in Fe/Cu-HNG. f Statistical distribution of Fe/Cu distance of the observed diatomic pairs. g EELS spectrum of Fe/Cu atomic sites. h N2 adsorption–desorption isotherms of Fe/Cu-HNG (inset: corresponding pore-size distribution). i XRD pattern of Fe/Cu-HNG.
The N2 adsorption–desorption isotherms of Fe/Cu-HNG (Fig. 2h) confirm the presence of highly mesoporous structures. The corresponding pore-size distribution has a major peak of 2–3 nm. The Brunauer–Emmett–Teller (BET) method is used to estimate the surface area to be 858 m2 g−1, which is five times higher than rGO (132 m2 g−1, Supplementary Fig. 5). A high surface area and porous structure could facilitate mass transport and improve the apparent activity of catalysts39,40,41. The X-ray diffraction (XRD) pattern (Fig. 2i) shows only two broad peaks, which are ascribed to stacking graphene layers (Supplementary Fig. 6)42,43. No other impurity peaks are observed. In conjunction with a wide-range SEM (Supplementary Fig. 7) and TEM (Supplementary Fig. 8) analysis, we may conclude that Fe/Cu diatomic structures were successfully constructed on HNG and no aggregation or nanoparticles of metals were observed. For comparison, homogenous diatomic materials (Fe/Fe-HNG and Cu/Cu-HNG) were also synthesized by adding Fe or Cu precursors, respectively. Detailed morphologic and structural characterizations of Fe/Fe-HNG and Cu/Cu-HNG were shown in Supplementary Figs. 9,10, respectively.
Figure 3a presents the X-ray photoelectron spectroscopic (XPS) analyses of the N 1s signal. The broad peak of the N 1s signal could be de-convoluted to pyridinic N (~398.3 eV), pyrrolic N (~400.6 eV), graphitic N (~401.4 eV), and Fe–N/Cu–N (~399.0 eV)44,45. The Fe 2p3/2 signals exhibit two peaks that could be assigned to the Fe2+ (709.9 eV) and Fe3+ (711.5 eV)46,47. By decreasing the number of metal precursors, we synthesized single-atom-loaded HNG (labeled as Fe-HNG or Cu-HNG,). As compared to single-atom Fe-HNG, the Fe 2p peaks of Fe/Cu-HNG shift toward high binding energy (Supplementary Fig. 11b). The Cu 2p3/2 spectrum in Supplementary Fig. 11c shows two peaks at 932.5 and 933.7 eV, which are assigned to Cu+ and Cu2+, respectively48,49. By contrast, the Cu 2p peaks of Fe/Cu-HNG have lower binding energy than those in single-atom Cu-HNG. The XPS peak shift of Fe/Cu dual atoms may imply electron transfer from Fe to Cu. The Raman spectra in Fig. 3b show two typical D and G bands of graphene at 1362 and 1576 cm−1, respectively. The intensity ratio of D to G is higher for Fe/Cu-HNG than that for rGO and HNG, indicating that the defective carbon nanosheets are formed owing to Fe/Cu dopants50,51.
a High-resolution N 1s XPS spectrum of Fe/Cu-HNG. b Raman spectra of rGO, HNG, and Fe/Cu-HNG. c Cu K-edge XANES spectra of Fe/Cu-HNG, Cu/Cu-HNG, Cu-HNG, Cu foil, CuO, and Cu2O. d Fe K-edge XANES spectra of Fe/Cu-HNG, Fe/Fe-HNG, Fe-HNG, Fe foil, FeO, and Fe2O3. e k3-weighted FT of χ(k)-function from the Cu K-edge EXAFS. f k3-weighted FT of χ(k)-function from the Cu K-edge EXAFS. WT images of the Cu K-edge from g Cu-HNG h Fe/Cu-HNG, and the Fe K-edge from i Fe-HNG, and j Fe/Cu-HNG. k Proposed schematic model of Fe/Cu-HNG: Fe (aqua), Cu (orange), N (blue), and C (brown). l Fitting results of the EXAFS spectra of Fe/Cu-HNG at k-space and R space of Fe K-edge.
Figure 3c–f show the X-ray absorption near-edge spectra (XANES) and extended X-ray absorption fine structure (EXAFS). The Cu K-edge XANES of Fe/Cu-HNG (Fig. 3c) confirms that Cu has an oxidation state between Cu2O (1+) and CuO (2+)52. Similarly, the Fe K-edge XANES of Fe/Cu-HNG (Fig. 3d) resides between Fe (0) foil and Fe2O3 (3+), indicative of the oxidized Fe in Fe/Cu-HNG43,53. As compared to Fe-HNG, a minor shift of Fe K-edge in Fe/Cu-HNG toward high energy implies that Fe/Cu dimers in HNG slightly increase the oxidation state of Fe owing to Cu ligands. Fe atoms in Fe/Cu-HNG transfer electrons to Cu and slightly reduce Cu as compared to Cu-HNG. Such a trend obtained by the absorption spectra is in agreement with the XPS analyses. The k3-weighted Fourier transform (FT) from Cu K-edge EXAFS spectra (Fig. 3e) show that the major peaks of Fe/Cu-HNG, Cu-HNG, and Cu/Cu-HNG are located at ~1.45 Å, which corresponds to the first shell scattering of the Cu–N coordination54. Notably, the second peaks at 2.15 and 2.27 Å for Fe/Cu-HNG and Cu/Cu-HNG, respectively, are comparable to the first shell distance of Cu foil (2.24 Å), suggesting the presence of metal–metal diatomic configuration. Similarly, the major peaks at ~1.48 Å for Fe/Cu-HNG, Fe-HNG, and Fe/Fe-HNG in Fig. 3f are ascribed to Fe–N coordination55,56. The second peaks at 2.15 and 2.03 Å for Fe/Cu-HNG and Fe/Fe-HNG, respectively, further confirm the presence of metal–metal diatomic configuration. The k3-weighted FT spectra indicate that the metal–metal distance in Fe/Cu-HNG is shorter than Cu–Cu coordination in Cu/Cu-HNG and longer than Fe–Fe coordination in Fe/Fe-HNG, verifying the existence of heterogeneous Fe-Cu sites in Fe/Cu-HNG. Figure 3g–j present the wavelet transform (WT) of the EXAFS spectra. Both Cu-HNG and Fe/Cu-HNG exhibit the intensity maxima at ~4.8 Å−1 due to the Cu–N path in the Cu K-edge spectra, differing from the Cu–O path (~4.2 Å−1). In addition, Fe-HNG and Fe/Cu-HNG display the intensity maxima at 4.6 Å−1 due to the Fe–N path in the Fe K-edge spectra. For Fe/Cu-HNG, the WT of either Fe or Cu K-edge signals display a maximum at 2.15 Å, which is between the metal–metal bond length of Cu and Fe foils and different with Fe/Fe-HNG and Cu/Cu-HNG (see Supplementary Figs. 9–13), suggesting the formation of Fe–Cu bond (Fig. 3h, j). Therefore, the WT and FT-EXAFS analyses confirm the existence of metal–N coordination and metal–metal bonds in Fe/Cu-HNG57,58,59.
To further verify the coordination structure of Fe/Cu-HNG, we used the model in Fig. 3k to fit the FT-EXAFS curves in Fig. 3l (see other fittings in Supplementary Figs. 9–14). The fitted main peak around 1.97 Å and 2.05 Å originate from the first Fe–N and Cu–N coordination shell. The coordination numbers of Fe–N and Cu–N are around 2.16 and 2.23 (Supplementary Table 2), respectively. The second peak in Fig. 3l is fitted to be ~2.25 Å, which corresponds to the Fe–Cu path. The good agreement between the experimental and fitting results confirms the proposed structure that Fe/Cu dual atoms are anchored on MN2 sites and the neighboring Fe/Cu atoms bond together to form a metal–metal dimer structure60,61.
Electrocatalytic performance for the NO3 −RR
Figure 4a presents the linear sweep voltammetry (LSV) curves of electrochemical nitrate reduction in an electrolyte of 1 M KOH and 0.1 M KNO3. HNG delivers ultralow current density until the negative polarization reaches −0.4 V vs RHE. SACs (Fe-HNG and Cu-HNG) slightly push the on-set potential to positive voltages and modestly increase the current density. Furthermore, by increasing metal loading, we synthesized homogenous diatomic catalysts (Fe/Fe-HNG and Cu/Cu-HNG). Supplementary Fig. 15 shows that dual sites further improve the catalytic activity. Notably, hetero-atomic dual-site catalyst (Fe/Cu-HNG) dramatically boosts the current density as compared to single-atom, homogenous diatomic catalysts, and a mechanic mixture of Fe/Fe-HNG and Cu/Cu-HNG. In particular, the LSV curve of Fe/Cu-HNG exhibits a downward hump around −0.3 to −0.5 V vs RHE, which may result from the mass-transport-limited reduction of nitrates. To separate the contribution of NO3−RR from HER, we first measured the LSV in 1 M KOH without 0.1 M KNO3 (Supplementary Fig. 16). Supplementary Fig. 17a shows that Fe/Cu-HNG does not catalyze the HER well and delivers a nearly zero current density (at voltages higher than −0.35 V) in a pure KOH solution, implying a low contribution of the HER to the total current density in the nitrate solution. Given that the HER and NO3−RR are competitive reactions, we tentatively simulated the total reactions by simultaneously considering the HER with the Butler-Volmer equation and the NO3−RR with a mass-transport-limited LSV relation. Supplementary Fig. 17b shows that the NO3−RR accounts for 85.7% of total transferred electrons in the LSV measurement.
a LSV curves of Fe/Cu-HNG, Fe-HNG, Cu-HNG, and HNG in an electrolyte of 1 M KOH and 0.1 M KNO3. b NH3 FEs of Fe/Cu-HNG at varied potentials. c NH3 yield rates of Fe/Cu-HNG, Fe-HNG, and Cu-HNG. d 1H NMR spectra of the electrolytes after electrocatalysis at −0.3 V (vs RHE) using 0.1 M 15NH4+ or 0.01 M 14NH4+ in 1 M KOH as nitrogen source (1H NMR of the fresh electrolytes marked as 15NO3− and 14NO3− were provided as the controls). e Chronoamperometric curve of Fe/Cu-HNG at −0.3 V vs RHE for 24 h. f Cycling tests of Fe/Cu-HNG for the NO3−RR. g Comparison of the cathodic NH3 EEs obtained using the Fe/Cu-HNG, Fe-HNG, and Cu-HNG catalysts. h Time-dependent concentration changes of NO3− and ammonia during the NO3−RR using Fe/Cu-HNG. i DEMS analyses of nitrogen species during the NO3−RR (each cycle is one LSV scan from 0.1 to −0.6 V vs RHE).
To confirm the LSV analytic results, we quantified the Faraday efficiency (FE) and yield rate of major products at varied voltages (Eqs-1, 2 in Supplementary Methods) using standard curves of NH3, NO3−, and NO2− in Supplementary Figs. 18–20. In addition, a typical electrolysis curve and UV-vis testing curves confirm the increase in NH3 (Supplementary Fig. 21). Figure 4b demonstrates that the FE of NH3 initially increases with voltages and then decreases at high voltages. Specifically, Fe/Cu-HNG enables the higher FE maximum of NH3 (92.51%) at a more positive voltage (−0.3 V) than SACs Fe-HNG and Cu-HNG (Supplementary Figs. 22,23), suggesting that diatomic catalysts have higher catalytic activity than SACs. The yield rates of NH3 displayed in Fig. 4c further exemplify the much-enhanced activity and selectivity of Fe/Cu-HNG as compared to Fe-HNG and Cu-HNG. Supplementary Table 3 summarizes the performance of previously-reported catalysts. Fe/Cu-HNG delivers high yield rates (1.08 mmol h−1 mg−1 at −0.5 V vs RHE) and the ultralow energy consumption (8.76 Wh gNH3−1 mg−1), demonstrating the high catalytic activity of diatomic sites. To determine the N source of the detected ammonia and assess the yield rate of NH3 independently, a 1H nuclear magnetic resonance (NMR) test was employed to identify the NH3 generation of Fe/Cu-HNG in 1 M KOH with 0.1 M 15NO3− or 14NO3− (Fig. 4d and Supplementary Fig. 24). The typical 1H NMR spectra show two peaks due to 15NH4+ after electrolyzing 15NO3− and three peaks related to 14NH4+ after electrolyzing 14NO3−, confirming that the product NH3 actually originates from the NO3−RR rather than contaminations62. The 15NH3 and 14NH3 yields were quantified by the averaged NMR peak areas. The calibration curves of 1H NMR spectra are in good agreement with UV-vis spectrophotometry measurements by colorimetric methods, demonstrating the reliability of the ammonia production efficiency test (Supplementary Fig. 24c). The FEs of byproducts about NO2− and H2 were shown in Supplementary Fig. 25.
Figure 4e presents the chronoamperometric curve. Only a slight decrease in the current density is observed at a constant voltage. Continuous electrolysis for 24 h (Fig. 4f) shows that the FEs and yield rates of NH3 could be maintained at 90% and ~2400 μg h−1 cm−1, respectively, indicative of the electrochemical stability of Fe/Cu-HNG catalysts. The HAADF-STEM analyses indicate that the diatomic sites in Fe/Cu-HNG remain after 24 h (Supplementary Fig. 26). The distribution of diatomic sites is similar to that prior to the test. Furthermore, the k3-weighted FT of χ(k)-function in Supplementary Fig. 27 indicates the metal–N coordination and metal–metal remain similar as well. Supplementary Fig. 28 presents the LSV curves after 24 h cycles. The voltage curve nearly overlaps with that prior to the test, suggesting the stability of the Fe/Cu-HNG catalyst. Figure 4g displays the energy efficiency (EE) of NH3 production at varied concentrations (Eq-3 in Supplementary Methods). Owing to the lower overpotentials, Fe/Cu-HNG demonstrates much higher EEs than Fe-HNG and Cu-HNG. Supplementary Fig. 29 provides the influence of nitrate concentration on the NH3 yield rates and FEs. The maximal FEs of NO3– to NH3 in the tested concentration range were 83–93% at −0.3 V (vs RHE). Fe/Cu-HNG basically exhibits appreciable ammonia yield rates and high selectivity under varied nitrate concentrations. Figure 4h presents the concentration changes of NO3−-N and NH3-N in H-cell batch electrolysis (50 mL). Fe/Cu-HNG (1 × 1 cm2) could almost completely reduce 200 mg L−1 NO3−-N and generate 189.2 mg L−1 NH3-N in 180 min. The sum of NO3−-N and NH3-N is less than the initial NO3−. The imbalance of N implies the existence of byproducts beyond NH3.
To decipher the intermediate byproducts and reaction pathway, we conducted a DEMS analysis for multiple cycles63,64 (see the schematic setup in Supplementary Fig. 30). During each cycle, the applied voltage was scanned from 0.1 to −0.6 V (vs RHE). Figure 4i presents the mass-to-charge (m/z) ratio signals of 46, 30, 33, and 17, which correspond to NO2, NO, NH2OH, and NH3, respectively. In addition to the major product (NH3), NO has two orders of magnitude higher fraction than NH2OH and NO2.
Theoretical analysis of NO3 −RR mechanism
There exist four possible pathways65,66 from NO3− to NH3 as shown in Supplementary Fig. 31. The DEMS measurements reveal the appearance of NO2, NO, and NH2OH, implying the most possible reaction pathway as illustrated in Fig. 5a. NO3− is first adsorbed and discharged to form *NO3, which plays an important role because the poor affinity of NO3− make the discharge difficult (Fig. 1b). Once absorbed, *NO3 is then hydrogenated to form *NO3H, which is further attacked by protons to release H2O and yield *NO2. The hydrogenation/dehydration cycle reduces *NO2 following the sequence: *NO2H → *NO → *NOH → *NHOH → *NH2OH → *NH2 → *NH3. The last step is the desorption of NH3 off catalysts.
a Reaction pathway of the NO3−RR and adsorption models of intermediates. b MO theory analysis of the splitting of metal 3d orbitals in a “Y-type” triple coordination and the interaction diagram between Fe/Cu 3d orbitals and Op orbitals of NO3. c PDOS of Fe/Cu 3d orbitals. d Interacting Wannier orbitals of NO3 on Fe/Cu-HNG. e Electron density difference diagram in the sliced plane through the Fe/Cu dimer. f Free energy diagram of each intermediate state on the metal atom sites in Fe/Cu at U = −0.3 V vs RHE.
To understand the catalytic mechanism of the NO3−RR, we first analyzed the structure and bonding of Fe/Cu-HNG and the influence of diatomic sites on the reaction routes. The geometric model of Fe/Cu-HNG is constructed from the EXAFS fitting result (Fig. 3k). Based on the molecular orbital (MO) theory understanding of “Y-type” ML3 coordination (see discussion below Supplementary Fig. 32), we plotted the energy level splitting of 3d orbitals and their interactions with NO3 in Fig. 5b. Figure 5c shows the calculated partial density of states (PDOS) of d-orbitals of Fe/Cu-HNG. By integrating these 3d orbitals, we obtained the relative energy level of each d-orbital. Their relative positions are in agreement with the MO theory analysis in Fig. 5b.
Furthermore, NO3 on Fe/Cu-HNG was modeled and analyzed. After geometric optimization, two oxygen atoms of NO3 are attracted to Fe/Cu dual sites. Figure 5d presents the interacting Wannier orbitals of NO3 on Fe/Cu-HNG, where the 3dxz orbitals of Fe/Cu form bonds with 2px orbitals of two oxygen of NO3, which basically agree with the MO theory analysis. The binding energy of NO3 on Fe/Cu-HNG is −1.19 eV, which is stronger than that of single-atom sites (−0.89 eV for Fe-HNG and −0.56 eV for Cu-HNG, Supplementary Fig. 33). Strong adsorption of NO3 could lower the energy barrier of the first discharge step (\({ \ast+{{{{{{\mathrm{NO}}}}}}}}_{3}^{-}{\to {{{{{{\mathrm{NO}}}}}}}}_{3}^{*}+{e}^{-}\)). NO3−RR differs from other electroreduction reactions (like the HER) because the planar symmetrical (D3h) resonant structure of NO3− and a strong hydrogen bond with water weaken the interaction between NO3− and electrodes and thus limits electron transfer67,68. Once absorbed, negative polarization will accelerate the following hydrogenation and/or dehydration steps. Diatomic sites facilitate the first discharge step owing to the strong adsorption to NO3 and however, may increase the energy barriers of the following steps because other intermediates may also strongly bind to the diatomic sites.
An efficient catalyst has to make a balance between the need for strongly adsorbing NO3 and modestly adsorbing other intermediates. Furthermore, we analyzed the adsorption configuration and energy change of intermediates on diatomic sites. Supplementary Fig. 34 presents the optimized geometric models of *NO3 on these diatomic sites. The binding energy of *NO3 on Fe/Fe is −1.89 eV, which is stronger than that on Cu/Cu (−0.21 eV) because the Fe \(3{d}_{{z}^{2}}\) and 3dxz states have a higher level than those of Cu. In addition, it is noted that the Fe-O bond length of *NO3 on Fe/Fe is 1.823 Å and the Cu-O bond length of *NO3 on Cu/Cu is 1.945 Å, implying that Fe interacts more strongly with intermediates than Cu. Supplementary Fig. 35 shows that most intermediates (except *NO2 and *NH2) on Fe/Cu-HNG have medium binding energies as compared to them on Fe/Fe-HNG or Cu/Cu-HNG. It is understandable from the viewpoint of the d-band center that higher 3d orbitals of Fe than Cu may lead to stronger adsorption of most intermediates. The exceptions may mainly result from hetero-atomic dimer configuration, which is also demonstrated by the electron density difference diagram in Fig. 5e. Fe transfers electrons to Cu, forming a polarized metal–metal dimer. Such a Fe/Cu hetero-atomic dimer tunes the adsorption energy slightly off the general trend.
Supplementary Fig. 36 presents the energy diagram at the equilibrium potential (U = 0.69 V). The second step (*NO3 → *NO3H) has the highest energy barrier at equilibrium. When the voltage is polarized to −0.3 V vs RHE (Fig. 5f), all the steps are downhill and the NO3−RR occurs spontaneously. Therefore, the diatomic sites of Fe/Cu-HNG appropriately reconcile the conflicting requirements on reducing energy barriers of both the initial discharge and following hydrogenation/dehydration steps, thereby dramatically enhancing the catalytic activity of NO3−RR from NO3− to NH3.
To further elucidate the subsequent deoxygenation/hydrogenation, we calculated the crystal orbital Hamilton population (COHP) of NO molecules adsorbed on Fe/Cu, Fe/Fe, and Cu/Cu diatomic sites. The integrated COHP (ICOHP) in Fig. 6a can be used as a quantitative indicator of the N-O activation. Compared to a free NO molecule, NO molecules on Fe/Fe and Cu/Cu sites were activated to varying degrees. A relatively more positive ICOHP (−14.04 eV) of NO molecules on Fe/Cu sites indicates a substantially weakened N-O bond because of the hetero-atomic structure. The activated N-O bond will facilitate the subsequent hydrogenation69. Operando DEMS measurements were conducted to verify the calculation results. Figure 6b shows that the NO yield ratio of Fe/Cu to Cu/Cu (or Fe/Fe) is around 2. Owing to the significant activation of Fe/Cu sites, the NH2OH/NH4 yield of Fe/Cu-HNG is dramatically increased to 8–10 times higher than those of Cu/Cu or Fe/Fe catalysts. It should also be noted that the intensity scale of the NH3 signal is two orders of magnitude higher than intermediates. These experimental and theoretical results confirm that Fe/Cu-HNG could lower the energy barrier of NO3−RR and activate NO molecules, leading to a high NH3 yield and selectivity.
Discussion
Electrochemical reduction of nitrate to ammonia could reduce nitrate pollution and concurrently realize low-temperature and low-pressure ammonia synthesis. The slow kinetics of the nitrate-to-ammonia reaction requires efficient catalysts. We synthesized a Fe/Cu diatomic catalyst on holey nitrogen-doped graphene for nitrate reduction. Fe/Cu is coordinated with two nitrogen atoms and one metal, which is similar to a “Y-type” ML3 structure. Dual metal sites are bonded to form a metal–metal dimer with a local configuration of N2Fe-CuN2. Owing to the relatively strong adsorption to NO3, the resultant Fe/Cu diatomic catalyst enhances the first rate-determining step, which is used to limit the approach of NO3– to the cathode because of its poor affinity with electrodes. Operando DEMS and DFT calculations reveal the reaction pathway and conversion mechanisms from NO3− to NH3. Compared to Fe/Fe and Cu/Cu configurations, Fe/Cu diatomic sites provide medium interactions toward most other intermediates and lower the overall energy barriers for the conversion from NO3− to NH3. In brief, Fe/Cu dual sites reconcile the opposite requirements of molecule-catalyst interaction and realize a low energy consumption and high activity synthesis of ammonia. Specifically, the resultant catalyst demonstrates the high activity of ~38.5 mA cm–2 (at −0.3 V vs RHE) and selectivity of 92.51% FE for the reduction of NO3– to NH3. The high NH3 yield rate of 1.08 mmol h−1 mg−1 is achieved at −0.5 V. Overall, this work provides an alternative opportunity for both nitrate abatement and ammonia synthesis and expands the rational design of atomically dispersed catalysts and their applications.
Methods
Synthesis of catalyst
GO were prepared via a modified Hummers method70. Typically, 3.0 g graphite (Aladdin, 99.9% metals basis) and 18.0 g potassium permanganate (KMnO4, Sinopharm Chemical Reagents Co., Ltd., AR, ≥99.5%) were slowly added into the solution of sulfuric acid (H2SO4, Sinopharm Chemical Reagents Co., Ltd., GR, 95.0–98%, 360 mL) and phosphoric acid (H3PO4, Sinopharm Chemical Reagents Co., Ltd., GR, ≥85.0%, 40 mL) under stirring. The mixture was heated to 50 °C for 12 h. After cooling down to room temperature by pouring onto ice (~400 mL), a 3.0 mL hydrogen peroxide solution (H2O2, Aladdin, AR, 30% in H2O) was added dropwise. The resultant solid was washed sequentially by de-ionized water, hydrochloric acid (HCl, Sinopharm Chemical Reagents Co., Ltd., AR, 36.0–38.0%), and ethanol (Sinopharm Chemical Reagents Co., Ltd., AR, ≥99.5%). Finally, the GO product was collected by vacuum freeze drying for 24 h. Approximately 100 mg GO was dispersed in an aqueous solution of 200 mL nitric acid (HNO3, Sinopharm Chemical Reagents Co., Ltd., GR, 65.0–68.0%). After ultrasonicated for 3 h, the dispersion was centrifuged and the solid phase was cleaned with de-ionized water. Iron chloride hexahydrate (FeCl3·6H2O, Aladdin, 99%, 9.0 mg), cupric chloride dihydrate (CuCl2·2H2O, Aladdin, AR, 6.0 mg), and urea (Aladdin, ≥99.5%, 100 mg) were added in the re-dispersed GO suspension (100 mL, ~2.0 mg L−1) and then ultrasonicated for 2 h. The mixed suspension was stirred for 12 h and then transferred into a Teflon-lined autoclave. After hydrothermally treated at 180 °C for 12 h, a porous hydrogel was formed. The hydrogel was washed and freeze-dried. The resultant powder was annealed at 800 °C for 2 h at a flowing gas of argon (Ar, Nanjing Special Gas Factory Co., Ltd., 99.999%, 100 sccm) and ammonia (NH3, Nanjing Special Gas Factory Co., Ltd., 99.999%, 50 sccm) to yield Fe/Cu-HNG powder.
Materials characterization
Morphologic and EDS mapping images were collected using a Zeiss Ultra 55 field emission scanning electron microscope. TEM analyses were conducted with an FEI Tecnai G2 20 microscope at 200 kV. Atomic-resolution STEM-HAADF images and EELS spectra were obtained on an FEI Titan G2 60-300 STEM/TEM at 300 kV with a field emission gun or on a JEOL Grand ARM with double spherical aberration correctors. XRD patterns were collected using Rigaku D/MAX 2500 V with Cu Kα radiation (1.5418 Å). XPS analysis was performed on an ESCALab MKII spectrometer with Mg Kα X-ray as the excitation source. Raman spectroscopic characterizations (Renishaw inVia Raman spectroscope) experiments were performed using a 514 nm laser. N2 adsorption–desorption isotherms were recorded on an ASAP 2020 accelerated surface area and porosimetry instrument (Micromeritics), equipped with automated surface area. Barrett–Emmett–Teller methods were used to calculate the surface area. The XAS spectra of Fe and Cu K-edge were measured in a fluorescence mode at the beamline BL14W1 of the Shanghai Synchrotron Radiation Facility in China. The concentrations of ions were analyzed by a Shimadzu UV-3600 plus spectrophotometer. The detailed measuring processes are described in detail in the Supplementary Information.
Data availability
All data are available from the authors upon request. Source data are provided with this paper.
References
Van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
Suryanto, B. H. R. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2, 290–296 (2019).
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, 6611 (2018).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Wang, J. et al. Electrocatalytic reduction of nitrate to ammonia on low-cost ultrathin CoOx nanosheets. ACS Catal. 11, 15135–15140 (2021).
Wang, J. et al. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy 86, 106088 (2021).
Yu, Y. et al. Promoting selective electroreduction of nitrates to ammonia over electron-deficient Co modulated by rectifying Schottky contacts. Sci. China Chem. 63, 1469–1476 (2020).
Li, Y. et al. Molybdenum sulfide: A bioinspired electrocatalyst for dissimilatory ammonia synthesis with geoelectrical current. J. Phys. Chem. C. 121, 2154–2164 (2016).
Wei, L. et al. Mild and selective hydrogenation of nitrate to ammonia in the absence of noble metals. ACS Catal. 10, 3618–3628 (2020).
Wang, Z., Young, S. D., Goldsmith, B. R. & Singh, N. Increasing electrocatalytic nitrate reduction activity by controlling adsorption through PtRu alloying. J. Catal. 395, 143–154 (2021).
Li, J. et al. Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J. Am. Chem. Soc. 142, 7036–7046 (2020).
McEnaney, J. M. et al. Electrolyte engineering for efficient electrochemical nitrate reduction to ammonia on a titanium electrode. ACS Sustain. Chem. Eng. 8, 2672–2681 (2020).
Duca, M. & Koper, M. T. M. Powering denitrification: the perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 5, 9726–9742 (2012).
Niu, H. et al. Theoretical insights into the mechanism of selective nitrate-to-ammonia electroreduction on single-atom catalysts. Adv. Funct. Mater. 31, 2008533 (2021).
Ye, S. et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 15, 760–770 (2022).
Kani, N. C. et al. Solar-driven electrochemical synthesis of ammonia using nitrate with 11% solar-to-fuel efficiency at ambient conditions. Energy Environ. Sci. 14, 6349–6359 (2021).
Daiyan, R. et al. Nitrate reduction to ammonium: from CuO defect engineering to waste NOx-to-NH3 economic feasibility. Energy Environ. Sci. 14, 3588–3598 (2021).
Jia, R. et al. Boosting selective nitrate electroreduction to ammonium by constructing oxygen vacancies in TiO2. ACS Catal. 10, 3533–3540 (2020).
Garcia-Segura, S., Lanzarini-Lopes, M., Hristovski, K. & Westerhoff, P. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl. Catal. B 236, 546–568 (2018).
Fang, Z. et al. Porous two-dimensional iron-cyano nanosheets for high-rate electrochemical nitrate reduction. ACS Nano 16, 1072–1081 (2022).
Choi, J. et al. Electroreduction of nitrates, nitrites, and gaseous nitrogen oxides: a potential source of ammonia in dinitrogen reduction studies. ACS Energy Lett. 5, 2095–2097 (2020).
Gu, J. et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Jung, E. et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Luo, F. et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 19, 1215–1223 (2020).
Zhou, M. et al. Single-atom Ni-N4 provides a robust cellular NO sensor. Nat. Commun. 11, 3188 (2020).
DeRita, L. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019).
Liu, J. C. et al. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 9, 1610 (2018).
Wang, Y. et al. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 142, 5702–5708 (2020).
Wu, Z.-Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Li, P., Jin, Z., Fang, Z. & Yu, G. A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 14, 3522–3531 (2021).
Zhang, W. et al. Emerging dual-atomic-site catalysts for efficient energy catalysis. Adv. Mater. 33, 2102576 (2021).
Khorshidi, A., Violet, J., Hashemi, J. & Peterson, A. A. How strain can break the scaling relations of catalysis. Nat. Catal. 1, 263–268 (2018).
Nwaokorie, C. F. & Montemore, M. M. Alloy catalyst design beyond the volcano plot by breaking scaling relations. J. Phys. Chem. C. 126, 3993–3999 (2022).
Darby, M. T., Stamatakis, M., Michaelides, A. & Sykes, E. C. H. Lonely atoms with special gifts: breaking linear scaling relationships in heterogeneous catalysis with single-atom alloys. J. Phys. Chem. Lett. 9, 5636–5646 (2018).
Hannagan, R. T. et al. First-principles design of a single-atom-alloy propane dehydrogenation catalyst. Science 372, 1444–1447 (2021).
Zhang, S. et al. High-performance supercapacitor of graphene quantum dots with uniform sizes. ACS Appl. Mater. Interfaces 10, 12983–12991 (2018).
Zhao, X., Hayner, C. M., Kung, M. C. & Kung, H. H. Flexible holey graphene paper electrodes with enhanced rate capability for energy storage applications. ACS Nano 5, 8739–8749 (2011).
Sun, H. et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 356, 599–604 (2017).
Yang, Y. et al. O-coordinated W-Mo dual-atom catalyst for pH-universal electrocatalytic hydrogen evolution. Sci. Adv. 6, 6586 (2020).
Yang, H. B. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Ren, W. et al. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew. Chem. Int. Ed. 58, 6972–6976 (2019).
Chen, Y. et al. Atomic Fe dispersed on N-doped carbon hollow nanospheres for high-efficiency electrocatalytic oxygen reduction. Adv. Mater. 31, 1806312 (2019).
Pan, Y. et al. A bimetallic Zn/Fe polyphthalocyanine-derived single-atom Fe-N4 catalytic site:A superior trifunctional catalyst for overall water splitting and Zn-air batteries. Angew. Chem. Int. Ed. 57, 8614–8618 (2018).
Zeng, Z. et al. Orbital coupling of hetero-diatomic nickel-iron site for bifunctional electrocatalysis of CO2 reduction and oxygen evolution. Nat. Commun. 12, 4088 (2021).
Gan, G. et al. Ultrathin Fe-N-C single-atom catalysts with bifunctional active site for simultaneous production of ethylene and aromatic chlorides. Nano Energy 80, 105532 (2021).
Wan, X. et al. Iron atom-cluster interactions increase activity and improve durability in Fe-N-C fuel cells. Nat. Commun. 13, 2963 (2022).
Xu, J. et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 4, 233–241 (2021).
Shang, H. et al. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun. 11, 3049 (2020).
Xia, C. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13, 887–894 (2021).
Kawai, S. et al. Three-dimensional graphene nanoribbons as a framework for molecular assembly and local probe chemistry. Sci. Adv. 6, 8913 (2020).
Cheng, H. et al. Atomically dispersed Ni/Cu dual sites for boosting the CO2 Reduction Reaction. ACS Catal. 11, 12673–12681 (2021).
Han, J. et al. Single-atom Fe-Nx-C as an efficient electrocatalyst for zinc-air batteries. Adv. Funct. Mater. 29, 1808872 (2019).
Li, Y. et al. Synergistic effect of atomically dispersed Ni-Zn pair sites for enhanced CO2 electroreduction. Adv. Mater. 33, 2102212 (2021).
Ying, Y., Luo, X., Qiao, J. & Huang, H. “More is different:” synergistic effect and structural engineering in double‐atom catalysts. Adv. Funct. Mater. 31, 2007423 (2020).
Han, X. et al. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 31, 1905622 (2019).
Pan, Y. et al. Regulating the coordination structure of single-atom Fe-NxCy catalytic sites for benzene oxidation. Nat. Commun. 10, 4290 (2019).
Wang, J. et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 11, 3375–3379 (2018).
Chen, J. et al. Dual single-atomic Ni-N4 and Fe-N4 sites constructing janus hollow graphene for selective oxygen electrocatalysis. Adv. Mater. 32, 2003134 (2020).
Gu, J. et al. Synergizing metal-support interactions and spatial confinement boosts dynamics of atomic nickel for hydrogenations. Nat. Nanotechnol. 16, 1141–1149 (2021).
Lu, X. et al. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv. Mater. 32, 2002246 (2020).
Chen, F. Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Yao, D. et al. In situ fragmented bismuth nanoparticles for electrocatalytic nitrogen reduction. Adv. Energy Mater. 10, 2001289 (2020).
Wang, Y. et al. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 59, 5350–5354 (2020).
Hu, T. et al. Theoretical insights into superior nitrate reduction to ammonia performance of copper catalysts. ACS Catal. 11, 14417–14427 (2021).
Liu, J.-X., Richards, D., Singh, N. & Goldsmith, B. R. Activity and selectivity trends in electrocatalytic nitrate reduction on transition metals. ACS Catal. 9, 7052–7064 (2019).
Courtney, L. F., Yun, J. P., Ellen, M. M., Zachary, G. & Alison, R. F. A bioinspired iron catalyst for nitrate and perchlorate reduction. Science 354, 741–743 (2016).
He, W. et al. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 13, 1129 (2022).
Niu, H. et al. A feasible strategy for identifying single-atom catalysts toward electrochemical NO-to-NH3 conversion. Small 17, 2102396 (2021).
Daniela, C. et al. Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010).
Acknowledgements
The authors acknowledge the financial support of the National Key R&D Program of China (No. 2020YFA0406104), the National Natural Science Foundation of China (Nos. 22075131), and the State Key Laboratory of Multiphase Complex Systems (No. MPCS-2021-A). The numerical calculations were carried out at the computing facilities in the High-Performance Computing Center (HPCC) of Nanjing University. S.Z thanks Program B for Outstanding PhD candidate at Nanjing University.
Author information
Authors and Affiliations
Contributions
Shu.Z. and H.Z. conceived and designed this work. Shu.Z., J.W., X.J., Z.L., and Z.S. synthesized materials and conducted characterizations. J.W. and P.W. carried out microscopic analyses. Y.W., Q.W. X.W., and H.W. conducted surface analyses and N quantification. Shu.Z. performed the DFT simulation. X.J., M.Z., Sha.Z., L.Y., L.D., and J.Z characterized and analyzed the X-ray adsorption data. J.L., H.Z., and Shu.Z. wrote the paper. H.Z. and Q.Z. supervises the work. All authors have discussed the results and commented on the manuscript. Shu.Z. and J.W. contributed equally to the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Huiyuan Zhu, Wenhui He and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, S., Wu, J., Zheng, M. et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat Commun 14, 3634 (2023). https://doi.org/10.1038/s41467-023-39366-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39366-9
This article is cited by
-
Self-propelled assembly of nanoparticles with self-catalytic regulation for tumour-specific imaging and therapy
Nature Communications (2024)
-
Mechanochemical route to fabricate an efficient nitrate reduction electrocatalyst
Nano Research (2024)
-
Current Status and Perspectives of Dual-Atom Catalysts Towards Sustainable Energy Utilization
Nano-Micro Letters (2024)
-
Efficient nitrate electroreduction over Mn-doped Cu catalyst via regulating N-containing intermediates adsorption configuration
Science China Chemistry (2024)
-
Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia
Nature Water (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.