Abstract
Brown adipose tissue (BAT) has abundant mitochondria with the unique capability of generating heat via uncoupled respiration. Mitochondrial uncoupling protein 1 (UCP1) is activated in BAT during cold stress and dissipates mitochondrial proton motive force generated by the electron transport chain to generate heat. However, other mitochondrial factors required for brown adipocyte respiration and thermogenesis under cold stress are largely unknown. Here, we show LETM1 domain-containing protein 1 (LETMD1) is a BAT-enriched and cold-induced protein required for cold-stimulated respiration and thermogenesis of BAT. Proximity labeling studies reveal that LETMD1 is a mitochondrial matrix protein. Letmd1 knockout male mice display aberrant BAT mitochondria and fail to carry out adaptive thermogenesis under cold stress. Letmd1 knockout BAT is deficient in oxidative phosphorylation (OXPHOS) complex proteins and has impaired mitochondrial respiration. In addition, BAT-specific Letmd1 deficient mice exhibit phenotypes identical to those observed in Letmd1 knockout mice. Collectively, we demonstrate that the BAT-enriched mitochondrial matrix protein LETMD1 plays a tissue-autonomous role that is essential for BAT mitochondrial function and thermogenesis.
Similar content being viewed by others
Introduction
Brown adipose tissue (BAT) differs from white adipose tissue (WAT) in that BAT is highly responsive to cold exposure for adaptive thermogenesis. BAT has abundant mitochondria that carry out its unique heat-generating function. Uncoupling protein 1 (UCP1), localized to the mitochondrial inner membrane of brown adipocytes, plays a major role in the thermogenic function of brown adipocytes. When BAT is activated by cold conditions, UCP1 dissipates proton (H+) motive force in the form of heat as a transporter of protons generated by the mitochondrial respiratory chain1. In this respect, mitochondrial respiration in BAT has an essential role in regulating whole-body energy homeostasis through adaptive thermogenesis in cold stress.
BAT glucose uptake and mitochondrial oxidative activity are increased by cold exposure in rodents2 and humans3. In activated BAT, a coordinated increase in aerobic energy metabolism and UCP1-mediated uncoupled respiration metabolize nutrients for heat production in non-shivering thermogenesis. BAT transplantation improves glucose tolerance and insulin resistance4, and counteracts obesity in mice5. Furthermore, in humans, BAT activation increases energy expenditure, reduces body fat mass, and improves whole-body glucose disposal and insulin sensitivity6,7. Thus, therapeutic manipulation of BAT activity has emerged as a promising strategy for the treatment of obesity and metabolic disorders8,9. To successfully implement this strategy, it is crucial to understand the molecular components that mediate the thermogenic function of BAT mitochondria induced by cold stimulus. However, aside from UCP1, other BAT mitochondrial factors required for uncoupled respiration and thermogenesis under cold stress are largely unknown.
In this study, we surveyed cold-inducible mitochondrial proteins in BAT and showed that LETM1 domain-containing protein 1 (LETMD1) is a BAT-enriched factor localized to the mitochondrial matrix of brown adipocytes. The Letmd1 gene was initially identified as an oncogene (HCCR1 or 2) associated with human cervical cancer and has been found to be highly expressed in various cancer cells, where it is linked to tumor suppressor function10,11,12,13,14. Our analysis of Letmd1 knockout (KO) mice demonstrated that LETMD1 plays an essential role in cold-stimulated respiration and adaptive thermogenesis of BAT. Furthermore, LETMD1-deficient brown adipocytes exhibited dysmorphic mitochondrial ultrastructure and a significant reduction in OXPHOS complex protein levels. Elucidating the role of LETMD1 in BAT thermogenesis contributes to a deeper understanding of molecular mechanisms that enable the functional specialization of mitochondria in thermogenic BAT.
Results
Identification of cold-inducible mitochondrial proteins in BAT
To identify candidate genes involved in the thermogenic function of BAT mitochondria, we analyzed publicly available gene expression data for genes that are enriched in BAT, as compared to epididymal WAT (eWAT) (GSE92844)15, and induced by cold stimulus (GSE70437)16 (Supplementary Fig. 1a). From this analysis, we uncovered a cluster of 144 genes that were both enriched in BAT and induced by cold stimulation (Supplementary Fig. 1a). Among these 144 genes, a subgroup of 31 genes were annotated as mitochondrial genes including Ucp1 (Supplementary Figs. 1b and 2).
Mitochondrial gene transcript levels and protein levels are frequently discordant, so we performed a complementary analysis with proteomics data17. By analyzing the intersection of proteins that are BAT-enriched proteins and also induced by cold, we identified 26 mitochondrial proteins that showed concordant expression at the transcript level (Supplementary Figs. 1c and 2). After combining the results of the transcriptomic and proteomic analyses, three candidate genes, Ucp1, Letmd1, and Acsl5 were identified (Supplementary Fig. 1d). UCP1 is a well-established mitochondrial protein required for BAT thermogenesis and ACSL5 has also been reported as an important regulator of whole-body energy metabolism in previous studies18. However, in contrast to UCP1 and ACSL5, the role of LETMD1 in BAT has not been explored.
LETMD1 is a BAT-enriched protein that is induced by cold exposure
To begin to understand the role of LETMD1 in BAT, we examined Letmd1 gene expression during brown adipocyte differentiation in an immortalized brown preadipocyte (iBPA) cell culture model19. During iBPA differentiation into brown adipocytes, Letmd1 gene expression increased at both RNA and protein levels (Fig. 1a, b). In adult mice, LETMD1 protein was highly enriched in BAT relative to WAT and other tissues, including mitochondria-rich muscle, suggesting that tissue mitochondrial content is not a determinant of Letmd1 gene expression (Fig. 1c). We further investigated the temporal regulation of Letmd1 gene expression in mice and found that Letmd1 transcript and protein levels increased during the early postnatal period, when thermogenic demand is known to be high (Fig. 1d, e).
a mRNA expression of Letmd1 during brown adipocyte (iBPA) differentiation. Expression is normalized to Rpl32. n = 3 per group. b Western blots of LETMD1 and UCP1 proteins during brown adipocyte (iBPA) differentiation. HSP90 is a loading control. c Western blot of LETMD1 protein in brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), epididymal white adipose tissue (eWAT), liver, and gastrocnemius muscle (GAS) from adult male mice. HSP90 is a loading control. Representative images from three independent repeats. d mRNA expression of Letmd1 from perinatal BAT from postnatal day 1 (P1), day 7 (P7), and day 14 (P14) male mice. nP1 = 4, nP7 = 5, nP14 = 5. Expression is normalized to Rpl32. e Western blots of LETMD1 and UCP1 proteins in BAT in the same sets as in (d). HSP90 is a loading control. Representative images from three independent repeats. f Representative western blots of LETMD1 and UCP1 proteins in BAT from adult male mice after 5 days of exposure to TN (thermoneutral, 30 °C), RT (room temperature, 23 °C), and cold (6 °C). α -Tubulin is a loading control. Representative images from three independent repeats. g mRNA expression of Letmd1 and thermogenic genes in the same sets as in (f). Expression is normalized to Rpl32. n = 3 per group. h Experimental scheme for a cold challenge in adult male mice. i H&E staining of BAT sections from mice exposed to cold (6 °C) and mice adapted to room temperature (23 °C) after cold stimulation. Scale bar, 200 μm. Representative images from three independent repeats. mRNA expression of Letmd1 (j) and Ucp1 (k) in the same sets as in (h). Expression is normalized to Rpl32. n = 3 mice per group. Data presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005. The significance of the results was assessed using one-way ANOVA. Source data are provided as a Source Data file.
We next compared Letmd1 gene expression in BAT from mice housed at thermoneutrality (30 °C), room temperature (23 °C), or cold (6 °C) conditions and observed an inverse relationship between Letmd1 gene expression and ambient temperature (Fig. 1f, g). To further investigate the regulation of LETMD1 expression, we intraperitoneally injected mice with CL316,243, a selective β3-adrenoreceptor agonist, and found that LETMD1 protein levels increased in the BAT of CL316,243-treated mice compared to controls (Supplementary Fig. 3). Notably, when cold-exposed mice were re-acclimated to room temperature (Fig. 1h, i), cold-induced expression of Letmd1 mRNA in BAT reverted to room temperature levels (Fig. 1j), a pattern also seen for the expression of Ucp1 mRNA (Fig. 1k). Taken together, LETMD1 is enriched in mature brown adipocytes and exhibits dynamic regulation as a function of external cues determining the physiological demand for BAT thermogenesis.
LETMD1 is a mitochondrial matrix protein
Next, we investigated the subcellular localization of LETMD1 protein in brown adipocytes. Sequence analysis with MitoFates20 predicted a putative mitochondrial targeting sequence (MTS) (1-28aa) and a cleavage site by mitochondrial processing peptidases (MPP) (Fig. 2a). In agreement with sequence analysis, immunofluorescence imaging of LETMD1 overexpressing iBPA cells displayed a mitochondrial pattern of localization (Fig. 2b).
a Prediction of a putative mitochondrial targeting sequence (MTS) and cleavage site for mouse LETMD1 with MitoFates analysis. b Immunofluorescence image of Letmd1-Flag (Green), mitochondria (Red), and DAPI (Blue) in iBPA cells. Representative images from three independent repeats. Scale bar, 10 μm. c Experimental scheme of proximity labeling using APEX2 localized to the mitochondrial matrix (left) or intermembrane space (right). APEX2 enzyme (red) labels compartment-specific proteomes using desthiobiotin-phenol (D, DBP) as a substrate. Outer mitochondrial membrane, OMM; Intermembrane space, IMS; Inner mitochondrial membrane, IMM. d Electron micrographs of APEX2 staining patterns in untransfected HEK 293 cells and HEK 293 cells transfected with MTS-Letmd1-APEX2 (C-terminus fusion), MTS-APEX2-Letmd1 (N-terminus fusion), MTS-APEX2, and ∆MTS-Letmd1-APEX2 expression constructs. Representative images from two independent repeats. Scale bar, 1 μm. e TMHMM analysis of human LETMD1 protein sequence predicting two transmembrane domains (TM). f Position of amino acids fusing APEX2 with LETMD1 protein (black arrow). g Electron micrographs of APEX2 staining patterns in HEK 293 cells transfected with Letmd1(aa1 ~ 79)-APEX2-Letmd1(aa80 ~ ), Letmd1(aa1 ~ 115)-APEX2-Letmd1(aa116 ~ ) and Letmd1(aa1 ~ 148)-APEX2-Letmd1(aa149 ~ ) expression constructs. Representative images from two independent repeats. Scale bar, 1 μm. h Western blots of Flag-tagged LETMD1 protein and OMM-APEX2, IMS-APEX2, and matrix-APEX2 biotinylated proteins in mature brown adipocytes. Representative images from three independent repeats. Input is 3% of the total lysate prior to affinity purification of biotinylated protein species by streptavidin-beads. HSP90 is a loading control. Source data are provided as a Source Data file.
To gain more information on the domain structure and cellular localization of LETMD1 protein, we analyzed the activity of a series of APEX2 fusion expression constructs. APEX2 is an engineered peroxidase whose subcellular localization is visualized by electron microscopy (EM) of staining patterns generated by peroxidase activity21. High-resolution details offered by EM analysis of APEX2 staining patterns have been successful in determining mitochondrial compartment-specific localization and protein domain topology22,23,24,25 (Fig. 2c). EM imaging of HEK293 cells transfected with Letmd1-APEX2 (C-terminus APEX fusion) and APEX2-Letmd1 (N-terminus fusion) expression constructs both showed matrix staining patterns indicating that the N-terminus and C-terminus of LETMD1 are exposed to the matrix (Fig. 2d). While we observed a typical mitochondrial matrix pattern of peroxidase staining in cells expressing MTS-APEX2, APEX2 with an N-terminus MTS, cells expressing the Letmd1-APEX2 construct lacking the predicted MTS (1-28aa) abolished mitochondrial localization demonstrating the functionality of the LETMD1 MTS (Fig. 2d).
To further investigate the topological configuration of the LETMD1 protein, we performed TMHMM analysis26 which revealed two putative transmembrane domains (82-97aa and 139-161aa) within LETMD1 (Fig. 2e). To experimentally confirm the presence of these predicted transmembrane domains for LETMD1 within the context of a living cell, we analyzed three additional LETMD1-APEX fusion proteins in which APEX was positioned internally at different sites (F79, L115, P148) within LETMD1 (Fig. 2f). Contrary to the predictions from TMHMM analysis, all three internal LETMD1-APEX fusion proteins showed a matrix pattern by EM analysis (Fig. 2g). In another approach, we tested whether LETMD1 protein can be labeled by APEX2 localized to compartments of the mitochondria other than the matrix. APEX2 was expressed in the matrix, intermembrane space (IMS), outer membrane of mitochondria (OMM) compartments and the co-expressed LETMD1-Flag protein was tested for proximity labeling by each mitochondrial compartment-specific APEX. The results showed that the LETMD1-Flag protein was labeled by matrix-APEX but not by OMM-APEX or IMS-APEX (Fig. 2h). Taken together, these results strongly suggest that endogenous LETMD1 protein is a soluble protein residing in the matrix compartment of mitochondria and does not possess a transmembrane domain in its native folded state.
LETMD1 is required for adaptive thermogenesis
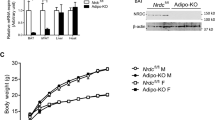
To study the in vivo function of LETMD1, we generated Letmd1 knockout (KO) mice that are homozygous for a Letmd1 null allele. Compared to control mice, Letmd1 KO mice had increased body weight (Fig. 3a) in the absence of significant changes in food intake or physical activity (Fig. 3b, c). Gross examination of Letmd1 KO BAT revealed a striking whitened appearance and histological analysis revealed dramatically enlarged lipid droplets in Letmd1 KO BAT compared to littermate controls (Fig. 3d). Furthermore, the expression of Ucp1 and thermogenic genes was markedly reduced in Letmd1 KO BAT from adult mice (Fig. 3e, f). This reduction in UCP1 protein levels in Letmd1 KO BAT was observed immediately after birth and persisted through the perinatal period, when thermogenic demand first emerges (Fig. 3g). These observations led us to examine whether the thermogenic function of BAT is altered in Letmd1 KO mice. Indeed, we found that Letmd1 KO mice failed to maintain core body temperature during a cold challenge, indicating a defect in adaptive thermogenesis (Fig. 3h). Infrared imaging of Letmd1 KO mice further confirmed the inability of Letmd1 KO mice to activate BAT thermogenesis and maintain body temperature during a cold challenge (Fig. 3i). Although LETMD1 protein was induced in BAT during perinatal stages when thermogenic BAT is highly active, we did not observe any effect on neonatal survival rates due to LETMD1 deficiency under standard housing conditions (Supplementary Fig. 4).
Body weight (a), food intake (b), and locomotor activity, (c) of 13-week-old wild-type (WT) and Letmd1 KO (KO) male mice. nbody weight = 4, nfood intake = 3, nactivity = 4. d Gross image of brown adipose tissue (BAT) and H&E stained BAT tissue sections from 13-week-old WT and Letmd1 KO male mice. Representative images from five independent repeats. Scale bar, 200 μm. e Western blots of LETMD1 and UCP1 proteins in BAT of 13-week-old male mice. HSP90 is a loading control. f mRNA expression of Letmd1 and brown adipocyte marker genes in the same sets as in (e). Expression is normalized to Rpl32. n = 5 per group. g Western blots of LETMD1 and UCP1 proteins in BAT from male mice on postnatal day 1 (P1), day 2 (P2), and day 3 (P3). HSP90 is loading control. Representative images from three independent repeats. h Core body temperature of adult male mice during cold (8 °C) challenge. n = 6 per group. i Images of surface temperature using infrared thermography after 5 h cold challenge. Images were quantified for the region of interest corresponding to interscapular BAT. n = 4 per group. Oxygen consumption rates (j) and carbon dioxide production (k) rates of WT and Letmd1 KO male mice exposed to cold (4°C). n = 5 per group. l Representative H&E staining and UCP1 protein immunostaining of BAT from WT and Letmd1 KO male mice exposed to cold (4 °C) for 5 h. Representative images from five independent repeats. Scale bar, 200 μm. Data presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005. The significance of the results was assessed using a two-tailed Student’ t test. Source data are provided as a Source Data file.
To further characterize physiological alterations in Letmd1 KO mice, we performed indirect calorimetry of control and Letmd1 KO mice during a cold challenge. At 25 °C, we did not observe any difference in oxygen consumption (VO2) and carbon dioxide production (VCO2) between the groups. However, although both wild-type and Letmd1 KO mice initially increased VO2 and VCO2 in response to a cold challenge, Letmd1 KO mice failed to sustain elevated levels of VO2 and VCO2 which is necessary for a prolonged thermogenic response (Fig. 3j, k). This impaired response to cold stress in Letmd1 KO mice was accompanied by a failure to upregulate UCP1 protein in BAT tissue (Fig. 3l). These results demonstrate an obligate in vivo requirement for LETMD1 function in the adaptive thermogenic response of BAT upon a cold challenge.
LETMD1 is essential for BAT mitochondrial integrity and function
To investigate the molecular role of LETMD1 in brown adipocyte function and adaptive thermogenesis, we performed RNA-seq analysis of Letmd1 KO and wild-type BAT. Gene ontology (GO) analysis of transcriptome changes revealed down-regulation of a broad array of genes involved in processes that occur in the mitochondrial matrix compartment in Letmd1 KO BAT (Fig. 4a). At the ultrastructural level, Letmd1 KO BAT contained dysmorphic mitochondria with sparse distended cristae, a principal site of OXPHOS complex (Fig. 4b). These data led us to directly examine OXPHOS complex components and functions in Letmd1 KO BAT. At embryonic day 16.5, when thermogenic demand is absent, BAT OXPHOS complex proteins were unaffected by LETMD1 deficiency (Fig. 4c). However, during the early postnatal period, control BAT upregulated OXPHOS complex proteins in response to thermogenic demand but Letmd1 KO BAT failed to upregulate OXPHOS proteins during this critical period for BAT thermogenesis (Fig. 4d). The defective OXPHOS protein expression in Letmd1 KO BAT was further exacerbated in adult stages and showed a near complete loss of complex I and IV proteins (Fig. 4e).
a Gene ontology analysis of BAT transcriptomes from wild-type (WT) and Letmd1 KO (KO) male mice. n = 3 per group. b Electron micrographs of BAT showing mitochondrial structure. Scale bar, 1 μm. Representative images from three independent repeats.c Western blots of OXPHOS complex and LETMD1 proteins from E16.5 embryonic BAT. Representative images from two independent repeats. HSP90 is a loading control. d Western blots of OXPHOS complex proteins from perinatal BAT from WT and Letmd1 KO male mice. Representative images from three independent repeats. HSP90 is a loading control. e Western blots of OXPHOS complex proteins from BAT from adult WT and Letmd1 KO male mice. Representative images from five independent repeats. HSP90 is a loading control. f Oxygen consumption rates of control (SCR) and Letmd1 knockdown (shLetmd1) brown adipocytes in response to 4 h pre-treatment of 10 µM forskolin (FSK). nSCR = 3, nSCR-FSK = 5, nshLetmd1 = 4, nshLetmd1-FSK = 3. g Basal respiration, maximal respiration, proton leak, and ATP production in the same sets as (f). The OCR values were normalized to the protein levels of each well. Data presented as mean ± SEM. h Western blots of LETMD1, OXPHOS complex, and UCP1 proteins in the same sets as (f). Representative images from three independent repeats. *p < 0.05, **p < 0.005, ***p < 0.0005. The significance of the results was assessed using one-way ANOVA. Source data are provided as a Source Data file.
To examine the functional consequence of OXPHOS complex deficiency in Letmd1 KO BAT, we measured respiratory function in scrambled control (SCR) and Letmd1 knockdown brown adipocytes (shLetmd1) in the presence or absence of forskolin stimulation. We found that there were no significant differences in basal respiration between control (SCR) and shLetmd1 brown adipocytes in the unstimulated state (Fig. 4f, g). However, upon forskolin (FSK) stimulation, we observed a dramatic reduction in both basal and maximal respiration of shLetmd1 cells, in contrast to control (SCR) cells (Fig. 4f, g). In forskolin-stimulated conditions, the proton leak of Letmd1 knockdown cells was also significantly reduced, indicating that Letmd1 knockdown leads to impaired UCP1 activity (Fig. 4g). Analysis of OXPHOS and UCP1 expression in Letmd1 knockdown cells showed a slight decrease in OXPHOS complex I and IV, as well as UCP1 protein levels (Fig. 4h). Additional experiments showed that Letmd1 knockdown impairs mitochondrial respiration and thermogenesis in brown adipocytes stimulated by isoproterenol (Supplementary Fig. 5a, b). Conversely, Ucp1 knockdown had no effect on either Letmd1 mRNA or protein expression levels but did result in decreased expression of OXPHOS components (Supplementary Fig. 6). These findings suggest that LETMD1 functions upstream of UCP1 in regulating mitochondrial function in brown adipocytes. Taken together, these results demonstrate the crucial role of LETMD1 in supporting brown adipocyte OXPHOS function in a stimulation-dependent manner.
LETMD1 functions in a BAT-autonomous manner
We next examined whether the structural and functional defects observed in Letmd1 KO mice were specific to BAT. To this end, we analyzed mitochondrial ultrastructure in metabolic tissues with high mitochondrial content such as heart and soleus muscle, and found no significant differences between Letmd1 KO and control (Fig. 5a, b). We also quantified OXPHOS complex proteins in metabolic tissues including iWAT, eWAT, liver, and muscle tissue lysates by western blot. In contrast to the reduced OXPHOS complex levels observed in BAT, the results show that other Letmd1 KO tissues have normal levels of OXPHOS complex expression (Fig. 5c–f). To thoroughly investigate the specific requirements of LETMD1 in BAT, we generated mice with a Letmd1 conditional knockout allele and crossed them with Ucp1-Cre mice to obtain Ucp1-Cre; Letmd1flox/flox (Letmd1 BKO) (Fig. 6a), which were confirmed to have BAT-specific deletion of Letmd1 (Fig. 6b, c). Similar to Letmd1 KO BAT, gross examination of BAT from Letmd1 BKO BAT showed a whitened appearance (Fig. 6d) and histological analysis of BAT showed brown adipocytes with enlarged lipid droplets (Fig. 6e). There were no differences in body weight (Fig. 6f), fat and liver tissue weights (Fig. 6g), fat mass/lean mass ratios (Fig. 6h), and fasting blood glucose (Fig. 6i) between Letmd1 BKO and control mice. However, the key molecular phenotypes observed in Letmd1 KO mice such as mRNA expression of BAT thermogenic genes (Fig. 6j) and reduced expression of OXPHOS complex proteins and UCP1 (Fig. 6k) were recapitulated in Letmd1 BKO mice. These results strongly support the notion that LETMD1 plays a tissue-autonomous role that is specific to BAT mitochondria function and thermogenesis.
Electron micrographs of mitochondria from heart (a) and soleus muscle (b) of adult wild-type (WT) and Letmd1 KO (KO) male mice. Representative images from three independent repeats. Scale bar, 5 μm and 1 μm respectively. Western blots were performed to evaluate mitochondrial OXPHOS complex expression in inguinal white adipose tissue (iWAT) (c), epididymal white adipose tissue (eWAT) (d), liver (e), and gastrocnemius muscle (f) from 13-week-old male WT and Letmd1 KO mice. n = 3 per group. HSP90 is a loading control. Representative images from three independent repeats. Source data are provided as a Source Data file.
a Strategy for generating Letmd1 conditional alleles. See “Materials and Methods” for details. LETMD1 protein (b) and mRNA (c) expression levels were measured in tissues (BAT brown adipose tissue, iWAT inguinal white adipose tissue, eWAT epididymal white adipose tissue, liver, heart, GAS gastrocnemius muscle, SOL soleus muscle) from 12-week-old male Letmd1flox/flox mice (Con) and Ucp1-Cre; Letmd1flox/flox mice (BKO). n = 3 per group. Representative results from three independent repeats. d Gross images of brown adipose tissue from 12-week-old male Letmd1flox/flox mice (Con) and Ucp1-Cre; Letmd1flox/flox mice (BKO). Scale bar, 1 cm. Representative images from three independent repeats. e Representative hematoxylin and eosin (H&E) staining of BAT, iWAT, eWAT, and liver from 12-week-old male Letmd1flox/flox mice (Con) and Ucp1-Cre; Letmd1flox/flox mice (BKO). Scale bar, 60 µm. Representative images from three independent repeats. Body weight (f), tissue weight (g), fat mass/lean mass (h), and blood glucose levels (i) of 12-week-old male Letmd1flox/flox mice (Con) and Ucp1-Cre; Letmdflox/flox mice (BKO). n = 3 per group. j mRNA expression of thermogenic genes. mRNA expression is normalized to Rpl32. n = 3 per group. Data presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 k Western blots of LETMD1 protein, mitochondrial complex components, and UCP1 protein in the BAT from 12-week-old male Letmdflox/flox mice (Con) and Ucp1-Cre; Letmd1flox/flox mice (BKO). HSP90 is loading control. Representative images from three independent repeats. The significance of the results was assessed using a two-tailed Student’ t test. Source data are provided as a Source Data file.
Discussion
Adaptive thermogenesis of BAT is critical for defending against cold stress and maintaining body temperature in mammals. A mitochondrial protein, UCP1, plays a key role in this process by uncoupling ATP production from the proton motive force generated by OXPHOS complex. However, additional mitochondrial factors required for BAT thermogenesis are largely unknown. In this study, we uncover an essential role for the BAT-enriched protein LETMD1 in mitochondrial respiration and adaptive thermogenesis.
LETMD1 is annotated as a mitochondrial protein in MitoCarta 1.027, MitoCarta 2.028 and MitoCarta 3.029. Furthermore, LETMD1 was categorized as one of the mitochondrial uncharacterized proteins (MXPs) in humans30 but its specific molecular and physiological function in BAT has not been explored. Here, we demonstrate that LETMD1 has critical functions in regulating mitochondrial which is essential for maintaining BAT activation during adaptive thermogenesis.
Our proximity labeling enzyme-assisted topology studies demonstrate that LETMD1 is a mitochondrial matrix protein, which contradicts a previous study suggesting LETMD1 protein as an outer mitochondrial membrane protein31 (Q924L1, UniProt). In our experiments, LETMD1 proteins fused with APEX revealed that LETMD1 protein lacks transmembrane domains, and N and C termini are exposed to the mitochondrial matrix (Fig. 2). In Letmd1 knockdown iBPA brown adipocytes, LETMD1-APEX2 fusion protein restored UCP1 expression functionally like LETMD1 protein (Supplementary Fig. 7). While APEX insertion is unlikely to cause misfolding, it cannot be completely excluded. Despite these concerns, Letmd1-APEX proteins with APEX insertion demonstrate both catalytic activity and matrix-targeted localization, suggesting that both Letmd1 and APEX are properly folded in the Letmd1-APEX fusion protein (Fig. 2g).
Our results also reveal significant differences in topology between LETMD1 protein and the related LETM1. Unlike LETM1, which is reported to have two transmembrane domains and both termini are in the mitochondrial matrix25, our results indicate that LETMD1 has a fully matrix-localized topology that suggests its role in mitochondrial function may differ from that claimed for LETM1. Despite these differences, LETMD1 and LETM1 function show similarities as LETM1 regulates mitochondrial swelling32, assembly of the respiratory chains33, and cristae organization34. Several studies have reported that LETM1 (SLC55A1) functions as a K+/H+ exchanger (KHE)35,36,37 or Ca2+/H+ antiporter (CHE)38,39,40,41. However, it is important to note that the proposed topology of these LETM1 and LETMD1 were derived from different analyses, and additional investigations will be required to comprehensively evaluate the functional and mechanistic differences between these two proteins.
The mitochondrial matrix is the key locale for coordinating the activity of the OXPHOS complex and UCP1 protein-mediated uncoupling, which together maintain the thermogenic program of brown adipocytes. We observed a slight decrease in OXPHOS complex I and IV associated with Letmd1 knockdown in cultured brown adipocytes (Fig. 4h), but this difference was not as pronounced as what was seen in the adult BAT of Letmd1 KO mice (Fig. 4e). These results suggest that the severely impaired OCR response in brown adipocytes by Letmd1 knockdown under forskolin or isoproterenol-treated conditions cannot be fully explained by decreased expression of OXPHOS complex proteins. Nevertheless, we consistently observed pronounced reduction of OXPHOS complex I and IV protein in Letmd1 KO BAT which would contribute to impaired BAT thermogenesis upon acute cold challenge (Fig. 3h, j). The reason for the difference between the effects of LETMD1 deficiency on OXPHOS complex protein levels in in vivo BAT versus in vitro brown adipocytes is an important topic for future research.
A notable observation from previous studies is that Ucp1 KO mice exhibit a marked decrease in OXPHOS complex expression, indicating a unique interdependence between UCP1 protein and OXPHOS function in BAT42. In the setting of UCP1 deficiency, cold-induced activation of BAT metabolism leads to a ~ 95% decrease in OXPHOS complex I and IV subunits. The study also demonstrates that environmental cold stimulus, rather than innate abnormalities in BAT development, is responsible for the diminished expression of electron transport chain components in Ucp1 KO mice42, which parallels the phenotype we observed in the BAT of Letmd1 KO mice (Figs. 3 and 4). In our study, we have demonstrated that UCP1 protein levels decrease in the BAT of Letmd1 KO mice and Letmd1 knockdown brown adipocytes (Fig. 3g and 4h), while a global proteomics analysis by Spiegelman and colleagues indicates that Ucp1 KO has no effect on LETMD1 protein levels42. Interestingly, we also found that Ucp1 knockdown in differentiated iBPA brown adipocytes had no effect on LETMD1 expression, but did lead to decreased OXPHOS protein levels (Supplementary Fig. 6). These results provide strong evidence that LETMD1 acts upstream of UCP1, although the precise mechanism through which LETMD1 regulates UCP1 expression remains unclear and warrants further investigation. However, we propose that the decrease in OXPHOS complex levels, particularly complex I and IV, in Letmd1 KO BAT is likely due to the downregulation of UCP1. Furthermore, we find that the observed effect of LETMD1 deficiency on OXPHOS complex levels is specific to BAT (Figs. 4 and 5), thereby highlighting a BAT-specific and BAT-autonomous role for LETMD1 in mitochondrial OXPHOS and uncoupled respiration. Finally, our demonstration that mice with a BAT-specific deletion of Letmd1 exhibit phenotypes identical to those observed in whole-body Letmd1 KO mice provides additional evidence for the BAT-autonomous requirement of LETMD1 (Fig. 6).
Increasing the activity of BAT in humans has emerged as a promising strategy to prevent obesity and metabolic disorders by enhancing energy expenditure8,9. Consequently, the identification of novel molecular regulators of mitochondrial metabolism and BAT thermogenesis presents a significant opportunity for preventive and therapeutic interventions for obesity43,44. In this context, we have demonstrated that LETMD1, a mitochondrial matrix protein that is specifically enriched in BAT and dynamically regulated in response to thermogenic stimuli, is a crucial component required for thermogenic BAT function. Moreover, analysis using our Gene-Module Association Determination (G-MAD)45 approach reveals that LETMD1 is strongly associated with mitochondrial function in both mouse and human adipose tissues (Supplementary Fig. 8). These findings provide further support for the role of LETMD1 in regulating mitochondrial metabolism and BAT thermogenesis and highlight its potential as a therapeutic target for the treatment of obesity and metabolic diseases.
During the process of revising our manuscript, we came across several recently published reports that have also investigated the role of LETMD1 in BAT function46,47,48,49. While these studies consistently demonstrated the upregulation of LETMD1 in response to cold stimulation and impaired thermogenic function in Letmd1 KO mice, each report describes distinct aspects of LETMD1 function. For instance, Choi et al.46 demonstrated an interaction between LETMD1 and Brg1 in the nucleus, suggesting a potential link to transcriptional regulation of BAT thermogenesis. Snyder et al.47 demonstrated that LETMD1 is essential for BAT structure and OXPHOS expression, even under thermoneutral conditions. Song et al.48 observed perturbations in mitochondrial calcium homeostasis in brown adipocytes lacking LETMD1, while Xiao et al.49 reported the localization of LETMD1 to the mitochondrial inner membrane. In contrast, our study provides conclusive evidence that LETMD1 functions autonomously within brown adipocytes, as demonstrated by the phenotype of brown adipocyte-specific Letmd1 KO mice. Moreover, our results show that LETMD1 is a mitochondrial matrix protein using proximity labeling techniques, providing a more accurate representation of LETMD1 protein topology in a live cell. Our findings complement and converge upon the growing body of literature underscoring the critical role of LETMD1 and its function in BAT thermogenesis.
Methods
Ethics statement
All animal studies were performed in compliance with the institutional guidelines of the Korean Research Institute of Biotechnology and Bioscience, and all mouse experiments were approved and performed under institutional guidelines.
Animal experiments
The ES cell clone 84278, which was generated by the International Knockout Mouse Consortium (IKMC) and contains a knockout-first allele of the Letmd1 gene, was purchased from The European Conditional Mouse Mutagenesis Program (EUCOMM). The Letmd1 knockout-first targeting vector contains a lacZ and a neomycin resistance gene cassette that is flanked by Flp recombinase target (FRT) sequences. This cassette was inserted into an intron between exon 2 and exon 3 of Letmd1 gene to disrupt endogenous LETMD1 expression. Exon 3 and exon 4 of Letmd1 gene are also flanked by loxP sites and there is a loxP site between the lacZ and neomycin resistance gene. Letmd1 heterozygous null mice (Letmd1 +/−) were obtained by microinjection of this ES cell clone into C57BL6/N mice (Macrogen). To generate Letmd1 conditional knockout mice that harbor a Letmd1 floxed allele, we bred mice with the Letmd1 knockout-first allele with ACTB-Flpe mice (JAX 003800). Mice containing the Letmd1 floxed allele were further bred to Ucp1-Cre mice (JAX 024670) to generate mice with BAT-specific deletion of the Letmd1 gene (Ucp1-Cre; Letmdflox/flox). Unless otherwise specified, all experiments involving mice were conducted using male mice between 12 and 17 weeks of age. Mice were maintained in a specific pathogen-free animal facility under institutional guidelines of the Korean Research Institute of Biotechnology and Bioscience.
Cell culture, adipogenic differentiation, and Oil-Red-O staining
An immortalized brown preadipocyte (iBPA) cell line was kindly provided by Dr. Shingo Kajimura (UCSF, San Francisco, CA, USA) and grown in high glucose Dulbecco’s modified Eagle medium (DMEM, Gibco) containing 10% fetal bovine serum (Gibco) and 1% antibiotics at 37 °C in a humidified atmosphere with 5% CO2. For brown adipocyte differentiation, cells were induced as previously described19. In brief, cells were cultured to confluence (day 0) in a maintenance medium containing 10% FBS, and 1% antibiotics, and then induced to differentiate in a differentiation medium (DMEM containing 10% FBS) supplemented with 0.5 mM isobutylmethylxanthine (IBMX, Sigma I5879), 0.5 µM dexamethasone (Sigma D1756), 20 nM insulin (Santa cruz sc-360248), 1 nM 3,3′,5-Triiodo-L-thyronine (T3, Sigma T2877), and 125 µM indomethacin (Sigma I7378) for 2 days, after which (day 2) the cells were further induced to differentiate and maturate for 4 days in the growth medium composed of DMEM,10% FBS, 20 nM insulin and 1 nM T3.
Generation of stable overexpression or knockdown cell lines
To construct iBPA cells stably expressing FLAG-tagged mouse LETMD1, a retroviral infection system was used. For LETMD1 expression, DNA encoding the FLAG-tagged Letmd1 was inserted into the pRetroX-IRES-ZsGreen1 vector (Clontech Laboratories, Mountain View, CA, USA). For virus production, GP2-293 packaging cells (Clontech, 631458) were transfected using TransIT®-LT1 Transfection Reagent (Mirus, MIR2300), and infected cells were selected using a FACSAria cell sorter (BD Biosciences) and maintained in high glucose Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (Gibco). To knockdown endogenous LETMD1 expression, we used a retrovirus-mediated shRNA system. Short-hairpin shRNAs were designed by selecting a target sequence for the mouse Letmd1 gene according to Knockout RNAi systems user manual (Clontech). The shRNA against Letmd1 was inserted into the multi-cloning site of the pSIREN-RetroQ-DsRed vector (Clontech). The following shRNA sequences targeting the coding region of Letmd1 mRNA were used to knockdown Letmd1 expression: 5ʹ- GATCCGCAACTGCTAGTCAAGCATTTCAAGAGAATGCTTGACTAGCAGTTGCTTTTTTG-3ʹ and 5ʹ- AATTCAAAAAAGCAACTGCTAGTCAAGCATTCTCTTGAAATGCTTGACTAGCAGTTGCG-3ʹ. Control shRNA (scrambled) vector was provided by Clontech.
Indirect calorimetry
For indirect calorimetry studies, mice were housed individually in metabolic cages (CLAMS12, Columbus Instruments) with ad libitum access to food and water. Oxygen consumption rates and carbon dioxide production rates were measured for 48 h. The activity was monitored simultaneously with metabolic measurements.
Core body temperature measurement and Infrared camera imaging
For measuring core temperature of cold exposed mice, mice were exposed to 8 °C in slow-temperature chamber (DHIN02-0034, DBL) and core body temperature was monitored using a rectal thermometer (Testo 925, Testo). The surface temperature of mice was measured with an infrared camera (Fortric 228, Fortric precision instruments) and analyzed with AnalyzIR (Fortric precision instruments) software.
Histology analysis
Mouse tissues were fixed in 10% neutral buffered formalin (Sigma, HT501128) for 24 h and embedded in paraffin by an automated tissue processor (Leica, TP1020). In total, 4 μm-thick tissue sections were obtained, deparaffinized, rehydrated, and stained with hematoxylin and eosin.
Extracellular flux assays
Oxygen consumption rate (OCR) was measured using the XFe96 extracellular flux analyzer (Seahorse Bioscience). On the day before the experiment, the sensor cartridge was placed into the calibration buffer (Seahorse Bioscience) and incubated at 37 °C in a non-CO2 incubator. Prior to measurement, cells were equilibrated in an assay medium containing 25 mM D-glucose, 4 mM L-glutamine, and 1 mM sodium pyruvate for 1 h. To measure OCR by mitochondrial respiration, cells were treated sequentially with 2.5 µM oligomycin, 5 µM Carbonyl cyanide m-chlorophenyl hydrazone (CCCP), and 2 µM rotenone/5 µM antimycin A. OCR was measured 4 h after 10 µM forskolin or 1 h after 1 µM isoproterenol treatment. At the end of each assay, cells were lysed with ice-cold RIPA buffer, and the protein contents were measured by Bradford assay. The OCR values were normalized to the protein levels of each well. The data were analyzed using Wave 2.6.1 software (Agilent).
RNA sequencing
Total RNA was isolated using Trizol reagent (Invitrogen). RNA quality was assessed by Agilent 2100 bioanalyzer using the RNA 6000 Nano Chip (Agilent Technologies, Amstelveen, The Netherlands), and RNA quantification was performed using ND-2000 Spectrophotometer (Thermo Inc., DE, USA). For control and test RNAs, the construction of library was performed using QuantSeq 3ʹ mRNA-Seq Library Prep Kit (Lexogen, Inc., Austria) according to the manufacturer’s instructions. In brief, each 500 ng total RNA were prepared and an oligo-dT primer containing an Illumina-compatible sequence at its 5ʹ end was hybridized to the RNA and reverse transcription was performed. After degradation of the RNA template, second strand synthesis was initiated by a random primer containing an Illumina-compatible linker sequence at its 5ʹ end. The double-stranded library was purified by using magnetic beads to remove all reaction components. The library was amplified to add the complete adapter sequences required for cluster generation. The finished library is purified from PCR components. High-throughput sequencing was performed as single-end 75 sequencing using NextSeq 500 (Illumina, Inc., USA). QuantSeq 3ʹ mRNA-Seq reads were aligned using Bowtie250. Bowtie2 indices were either generated from genome assembly sequence or the representative transcript sequences for aligning to the genome and transcriptome. The alignment file was used for assembling transcripts, estimating their abundances and detecting differential expression of genes. Differentially expressed gene were determined based on counts from unique and multiple alignments using coverage in Bedtools51. The RC (Read Count) data were processed based on quantile normalization method using EdgeR within R52 using Bioconductor53. Gene classification was based on searches done by DAVID (http://david.abcc.ncifcrf.gov/) and Medline databases (http://www.ncbi.nlm.nih.gov/). Data mining and graphic visualization were performed using ExDEGA (Ebiogen Inc., Korea).
Biotin-phenol labeling in live cells
APEX2-fusion expression vectors were introduced into HEK 293 T (CRL-3216, ATCC), or iBPA cells for biotin-phenol labeling experiments. Viral expression of either the LETMD1-APEX fusion protein or the wild-type LETMD1 protein in 3ʹ UTR-targeted Letmd1 shRNA knockdown iBPA cells restored Ucp1 expression to a similar extent, indicating that LETMD1-APEX fusion protein retains the functional properties of the wild-type LETMD1 protein (Supplementary Fig. 7). To prepare iBPA cells for biotin-phenol labeling, we introduced APEX2-fusion expression constructs to iBPA cells through electroporation and induced their differentiation into brown adipocytes. At day 6 of differentiation, the culture medium was replaced with fresh growth medium containing 500 µM desthiobiotin-phenol (DBP), followed by incubation at 37 °C under CO2 for 30 min. The cells were then treated with 1 mM H2O2 and gently agitated for 1 min at room temperature. To terminate the reaction, the cells were washed thrice with ice-cold DPBS containing 5 mM trolox, 10 mM sodium ascorbate, and 10 mM sodium azide. Subsequently, the cells were lysed for western blot analysis or fixed for imaging analysis. We performed western blot analysis, fluorescence microscope imaging, and transmission electron microscope (TEM) imaging of DBP-labeled cells as described previously54,55.
Western blot analysis
Homogenized tissue or cells were lysed with ice-cold RIPA buffer (Pierce, 89900) containing 1X protease inhibitor cocktail (GenDEPOT, P3100) and incubated at 4 °C for 30 min. After centrifugation at 15,000 × g for 15 min, the supernatant was moved to a new tube. Protein concentrations were measured using the Bradford assay (Bio-Rad, 5000001). Protein samples were directly analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with SDS sample buffer (60 mM Tris-Cl (pH 6.8), 10% sodium lauryl sulfate, 25% glycerol, 100 mM dithiothreitol, 0.04% Bromophenol blue) without boiling. Western blot analysis was performed according to standard methods. Antibodies used in immunoblot analyses included those against LETMD1 (LSBio, LS-C384640, 1:1000); UCP1 (Abcam, ab10983, 1:1000); HSP90 (Santa Cruz, sc-7947, 1:2000); α-Tubulin (Sigma-Aldrich, sc-8035, 1:3000); OXPHOS cocktail (Abcam, ab110413, 1:1000); Streptavidin-HRP (Thermo Scientific, S911, 1:3000); and Flag (Sigma-Aldrich, F3165, 1:3000). The specific signals were amplified by horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibody (Santa Cruz, 1:3000).
mRNA expression analysis by qRT-PCR
Total RNA was extracted from tissue or cultured cells using TRIzol Reagent (Invitrogen, 15596026) according to manufacturer instructions, and first-strand cDNA was synthesized from total RNA using the reverse transcriptase M-MLV (Promega, M1701) and a random primer (Promega, C1181) according to manufacturer protocols. Real-time PCR was performed using 2X Real-time PCR Smart mix kit (SolGent, SRH91-R500) according to the manufacturer protocols. The data were analyzed using CFX Maestro software 2.0 (Bio-Rad). Gene expression levels were normalized to 60 S ribosomal protein L32 (Rpl32). Primers used for qRT-PCR are listed in Supplementary Table 1.
Statistics and reproducibility
Statistical analysis was performed using Prism 8.4 software (GraphPad). All statistics are described in figure legends. In general, comparisons between two groups were performed using the two-tailed Student’s t test and multiple group comparisons were performed by one-way ANOVA followed by Tukey-Kramer post-hoc test. Data are expressed as mean ± standard error of the mean (SEM). Results with P values less than 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
We analyzed publicly available gene expression data for genes that are enriched in BAT, as compared to epididymal WAT (eWAT) (GSE92844) and induced by cold stimulus (GSE70437). RNA sequencing data are deposited in Gene Expression Omnibus (GEO) under accession number GSE232223. The source data for this study are provided with this paper. Source data are provided with this paper.
References
Klingenberg, M. Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem. Sci. 15, 108–112 (1990).
Labbé, S. M. et al. In vivo measurement of energy substrate contribution to cold‐induced brown adipose tissue thermogenesis. FASEB J. 29, 2046–2058 (2015).
Blondin, D. P. et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J. Clin. Endocrinol. Metab. 99, E438–E446 (2014).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Liu, X. et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 156, 2461–2469 (2015).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014).
Chechi, K., Nedergaard, J. & Richard, D. Brown adipose tissue as an anti‐obesity tissue in humans. Obes. Rev. 15, 92–106 (2014).
Saito, M. Human brown adipose tissue: regulation and anti-obesity potential. Endocr. J. 61, 409–416 (2014).
Ko, J. et al. Identification and differential expression of novel human cervical cancer oncogene HCCR-2 in human cancers and its involvement in p53 stabilization. Oncogene 22, 4679–4689 (2003).
Xu, Z. et al. Epidermal growth factor induces HCCR expression via PI3K/Akt/mTOR signaling in PANC-1 pancreatic cancer cells. BMC Cancer 10, 161 (2010).
Meng, K. et al. Human cervical cancer oncogene-1 over expression in colon cancer and its clinical significance. Int. J. Clin. Exp. Med. 8, 939–943 (2015).
Ha, S. A. et al. Oncoprotein HCCR-1 expression in breast cancer is well correlated with known breast cancer prognostic factors including the HER2 overexpression, p53 mutation, and ER/PR status. BMC Cancer 9, 51 (2009).
Zhu, L. F. et al. HCCR-1 is a novel prognostic indicator for gastric cancer and promotes cell proliferation. J. Cancer 10, 3533–3542 (2019).
Mo, Q. et al. Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight 2, e93166 (2017).
Marcher, A. B. et al. RNA-seq and mass-spectrometry-based lipidomics reveal extensive changes of glycerolipid pathways in brown adipose tissue in response to cold. Cell Rep. 13, 2000–2013 (2015).
Forner, F. et al. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab. 10, 324–335 (2009).
Bowman, T. A. et al. Acyl CoA synthetase 5 (ACSL5) ablation in mice increases energy expenditure and insulin sensitivity and delays fat absorption. Mol. Metab. 5, 210–220 (2016).
Uldry, M. et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 3, 333–341 (2006).
Fukasawa, Y. et al. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol. Cell. Proteom. 14, 1113–1126 (2015).
Martell, J. D. et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat. Biotechnol. 30, 1143–1148 (2012).
Rhee, H. W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339, 1328–1331 (2013).
Hung, V. et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 55, 332–341 (2014).
Hung, V. et al. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. Elife 6, e24463 (2017).
Lee, S. Y. et al. Architecture mapping of the inner mitochondrial membrane proteome by chemical tools in live cells. J. Am. Chem. Soc. 139, 3651–3662 (2017).
Krogh, A., Larsson, B., Von Heijne, G. & Sonnhammer, E. L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, D1251–D1257 (2016).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Floyd, B. J. et al. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol. Cell 63, 621–632 (2016).
Cho, G. W. et al. HCCR-1, a novel oncogene, encodes a mitochondrial outer membrane protein and suppresses the UVC-induced apoptosis. BMC Cell Biol. 8, 50 (2007).
Hasegawa, A. & van der Bliek, A. M. Inverse correlation between expression of the Wolfs Hirschhorn candidate gene Letm1 and mitochondrial volume in C. elegans and in mammalian cells. Hum. Mol. Genet. 16, 2061–2071 (2007).
Tamai, S. et al. Characterization of the mitochondrial protein LETM1, which maintains the mitochondrial tubular shapes and interacts with the AAA-ATPase BCS1L. J. Cell. Sci. 121, 2588–2600 (2008).
Nakamura, S. et al. The mitochondrial inner membrane protein LETM1 modulates cristae organization through its LETM domain. Commun. Biol. 3, 99 (2020).
Nowikovsky, K. et al. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 279, 30307–30315 (2004).
Froschauer, E. et al. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim. Biophys. Acta 1711, 41–48 (2005).
Austin, S. et al. LETM1-mediated K+ and Na+ homeostasis regulates mitochondrial Ca2+ efflux. Front. Physiol. 8, 839 (2017).
Jiang, D. et al. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326, 144–147 (2009).
Jiang, D. et al. Letm1, the mitochondrial Ca2+/H+ antiporter, is essential for normal glucose metabolism and alters brain function in Wolf–Hirschhorn syndrome. Proc. Natl. Acad. Sci. USA 110, E2249–E2254 (2013).
Doonan, P. J. et al. LETM1-dependent mitochondrial Ca2+ flux modulates cellular bioenergetics and proliferation. FASEB J. 28, 4936 (2014).
Shao, J. et al. Leucine zipper-EF-hand containing transmembrane protein 1 (LETM1) forms a Ca 2+/H+ antiporter. Sci. Rep. 6, 34174 (2016).
Kazak, L. et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc. Natl. Acad. Sci. USA 114, 7981–7986 (2017).
Kajimura, S. & Saito, M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu. Rev. Physiol. 76, 225–249 (2014).
Hussain, M. F. et al. Regulation of adipocyte thermogenesis: mechanisms controlling obesity. FEBS J. 287, 3370–3385 (2020).
Li, H. et al. Identifying gene function and module connections by the integration of multispecies expression compendia. Genome Res. 29, 2034–2045 (2019).
Choi, K. M. et al. Defective brown adipose tissue thermogenesis and impaired glucose metabolism in mice lacking Letmd1. Cell Rep. 37, 110104 (2021).
Snyder, M. M. et al. LETMD1 is required for mitochondrial structure and thermogenic function of brown adipocytes. FASEB J. 35, e21965 (2021).
Song, R. et al. Deletion of Letmd1 leads to the disruption of mitochondrial function in brown adipose tissue. Biochimie 201, 100–115 (2022).
Xiao, H. et al. Architecture of the outbred brown fat proteome defines regulators of metabolic physiology. Cell 185, 4654–4673 (2022).
Langmead, B. et al. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Quinlan, A. R. et al. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Georges, N. T. et al. Frequentist model averaging and applications to bernoulli trials. Open J. Stat. 6, 545–553 (2016).
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Lee, S. Y. et al. APEX fingerprinting reveals the subcellular localization of proteins of interest. Cell Rep. 15, 1837–1847 (2016).
Hung, V. et al. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 11, 456–475 (2016).
Acknowledgements
We gratefully acknowledge Hye-Young Um, Hee-Saeng Jung, and Ju Eun Kim for administrative and technical support. We thank Shingo Kajimura for providing iBPA cells and Hail Kim, Seyun Kim, and Min-Woo Lee for providing reagents and helpful discussion. The J.A. lab received funding from the EPFL, the European Research Council under the European Union’s Horizon 2020 research and innovation program (ERC-AdG-787702), the Swiss National Science Foundation (SNSF 31003A_179435 and Sinergia CRSII5_202302), and a GRL grant from the National Research Foundation of Korea (NRF 2017K1A1A2013124). W.K.K. was supported by the Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN22C0120), and National Research Foundation of Korea (2020R1A2C2007111, 2019R1A2C1006035). J.M.S. was supported by the KRIBB (KGM5392212, CRC22011), KAIST (N11230003), Korea Evaluation Institute of Industrial Technology (1415181231), and National Research Foundation of Korea (2021R1A2C2007573).
Author information
Authors and Affiliations
Contributions
A.P., K.K., I.P., H.-W.R., W.K.K., K.-H.B. and J.M.S. conceived the study and interpreted data. A.P., K.K., I.P., S.H.L., K.Y.P. and S.-J.L. performed experiments. M.J. and J.Y.M. performed electron microscopy analysis. X.L., M.B.S. and J.A. performed systems genetics analysis. A.P, K.K, W.K.K, K.-H.B. and J.M.S. wrote the manuscript. D.-S.K., J.K., D.-S.L., J.Y.M., H.-W.R., E.-J.W., K.-J.O., E.W.L., B.S.H. and S.C.L. critically reviewed the study and provided helpful discussion. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, A., Kim, Ke., Park, I. et al. Mitochondrial matrix protein LETMD1 maintains thermogenic capacity of brown adipose tissue in male mice. Nat Commun 14, 3746 (2023). https://doi.org/10.1038/s41467-023-39106-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39106-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.