Abstract
Conversion into high-value-added organic nitrogen compounds through electrochemical C-N coupling reactions under ambient conditions is regarded as a sustainable development strategy to achieve carbon neutrality and high-value utilization of harmful substances. Herein, we report an electrochemical process for selective synthesis of high-valued formamide from carbon monoxide and nitrite with a Ru1Cu single-atom alloy under ambient conditions, which achieves a high formamide selectivity with Faradaic efficiency of 45.65 ± 0.76% at −0.5 V vs. RHE. In situ X-ray absorption spectroscopy, coupled with in situ Raman spectroscopy and density functional theory calculations results reveal that the adjacent Ru-Cu dual active sites can spontaneously couple *CO and *NH2 intermediates to realize a critical C-N coupling reaction, enabling high-performance electrosynthesis of formamide. This work offers insight into the high-value formamide electrocatalysis through coupling CO and NO2− under ambient conditions, paving the way for the synthesis of more-sustainable and high-value chemical products.
Similar content being viewed by others
Introduction
Amides and their derivatives represent commercially important category of organic compounds as witnessed by their widespread use as intermediates in the manufacture of chemical as well as polymer and biological compounds1,2,3,4,5,6,7. Among them, as an important chemical raw material, formamide (HCONH2) has been widely used in organic synthesis, pharmaceuticals, plastics, and pesticides, etc.5,6,7. At present, however, the industrial synthesis of formamide is generated by the reaction of fossil fuel-derived carbon monoxide (CO) and ammonia (NH3) under harsh reaction conditions, resulting in a large amount of energy consumption and emission of a large amount of greenhouse gases6,7. Moreover, special equipment and complicated synthetic processes are often required to improve the limited conversion efficiency2,4,6. Therefore, it is desirable to develop sustainable routes that enable efficient and low-cost synthesis of formamide under milder conditions.
Conversion of high value-added organonitrogen compounds by electrochemical C–N coupling reaction under environmental conditions is considered as a sustainable strategy to achieve carbon neutrality and high-value utilization of hazardous substances8,9,10,11,12,13,14,15. Recent advances demonstrate that electrochemical synthesis of organic amides from C–N coupling reaction by introducing an NH3 source during CO2/CO reduction reaction14,16. For example, Jiao et al. reported that nucleophilic addition of NH3 could boost the reduction of CO to *C=C=O, which in turn reacts with NH3 to form intermediates that proceed to form acetamide under strong basic conditions, demonstrating the potential for generating amide formation14. However, more advanced catalytic processes are still needed to expand the scope of possibilities of catalyzed C–N bond formation for generating more valuable products.
In this regard, nitrite/nitrate (NO2−/NO3−) is a highly abundant nitrogen source, particularly in industrial wastewater and polluted groundwater17,18. Moreover, the electrochemical NO2− reduction reaction (NO2−RR) offers a practical path to product NH3 with renewable electricity due to the lower dissociation energy for the N=O bond (204 kJ mol−1)19,20. Inspiring by this, electrocatalytic coupling NO2− with CO might be an alternative route to drive formamide synthesis by using abundant and cheap C- and N-containing feedstocks. The key challenges are the rational design of efficient and stable active sites for C/N precursor reduction and C–N coupling to improve formamide selectivity.
Herein, we realize the electrochemical coupling of CO with NO2− to product formamide with an electrocatalyst consisting of atomically dispersed Ru atoms on Cu nanoclusters single-atom alloy (denoted Ru1Cu SAA). Direct experimental evidence shows that isolated Ru atoms is incorporated into the lattice of Cu nanoclusters to generate Ru1Cu SAA. The Ru1Cu SAA affords an average formamide yield rate of 2483.77 ± 155.34 μg h−1 mgcat.−1, as well as ultra-high Faradaic efficiency (FE) of 45.65 ± 0.76% at −0.5 V vs. reversible hydrogen electrode (RHE). Furthermore, a series of in situ experimental studies and theoretical calculations unveil that the adjacent Ru–Cu dual active sites act as intrinsic active centers: the single Ru atom promotes the adsorption of NO2− and activates the deoxygenation hydrogenation process, while CO undergoes dissociation adsorption on adjacent Cu atoms. Consequently, the Ru–Cu synergistic catalysis promotes the formation of C–N bond through spontaneous coupling of *CO and *NH2 intermediates, resulting in high activity and selectivity toward electrosynthesis of formamide. Compared with monometallic catalysts, the dual-active-site catalyst can provide independent dual-site synergistic catalysis for C and N activation, thereby enhancing the C–N coupling efficiency.
Results
Structural characterization
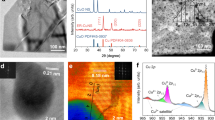
Figure 1a shows the schematic diagram of the fabrication process of Ru1Cu SAA (see “Methods”). First, the TiO2 nanowires with a diameter of ~10 nm were easily fabricated by chemical dealloying method served as catalyst supports (Supplementary Fig. 1 and Supplementary Fig. 2a)21. Subsequently, Cu nanoclusters (denoted as Cu NCs) were loaded onto the dealloyed TiO2 nanowires by thermal reduction. Afterward, Ru1Cu SAA was obtained by introducing Ru single atom onto the surface of Cu NCs through galvanic replacement reaction (Fig. 1b)22. Meanwhile, RuCu alloy (denoted as RuCu NPs) samples with higher Ru doping levels were synthetized as control samples. It is noted that Cu NCs and Ru1Cu SAA show similar X-ray diffraction (XRD) patterns without Ru or RuO2 phase, implying a high dispersion degree of Ru species (Supplementary Fig. 2a). In contrast, the lattice constants of RuCu NPs increase with the increase of Ru loading (Supplementary Fig. 2b) due to the substitution of Cu by Ru with a larger atomic radius. Low-magnification scanning transmission electron microscopy (STEM) image shows that homogeneous Ru1Cu SAA with an average size (~1.5 nm) are well dispersed and anchored onto the dealloyed TiO2 nanowires support (Supplementary Fig. 3). The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) (Fig. 1c) image clearly displays that number of bright and atom-sized features attributed to individual Ru atoms can be discerned on the crystal surface of Cu in Ru1Cu SAA. It is noteworthy that these isolated Ru atoms are surrounded by Cu atoms in different regions of the TiO2 nanowires without agglomerating into Ru nanoclusters. More importantly, the randomly magnified image further confirms the substitution of surface Cu atoms by isolated Ru atom (Fig. 1d), where the single Ru atoms (red circle) are located on Cu NCs, demonstrating the formation of Ru1Cu single-atom alloy. The STEM-coupled energy dispersive spectroscopic (EDS) elemental mapping reveals a uniform dispersion of Ru dopants in the Cu nanocluster matrix in Ru1Cu SAA (Fig. 1e), with a Ru:Cu atomic ratio of approximately 4:96 (Supplementary Fig. 4), which is consistent with inductively coupled plasm optical emission spectroscopy (ICP-OES) results (Supplementary Table 1). Furthermore, low-magnification STEM images show that the average size of RuCu NPs is ~1.1 nm, and the energy dispersive spectroscopy (EDS) confirmed that Cu and Ru are uniformly distributed in RuCu NPs (Supplementary Fig. 5).
a Schematic illustration of preparation processes for Ru1Cu SAA catalysts, with Ti, O, Ru, and Cu atoms shown as pink, gray, blue, and orange, respectively. b SEM image of the Ru1Cu SAA. c HAADF-STEM image of Ru1Cu SAA. d Ru1Cu SAA enlarged images. e HAADF-STEM image and the corresponding elemental mapping. f Ru K-edge FT-EXAFS spectra of Ru1Cu SAA and reference samples (RuO2, and Ru foil), and corresponding Ru1Cu SAA fitting curves, inset showing the schematic model. g Ru K-edge EXAFS WT analysis of Ru1Cu SAA and Ru foil. h Cu K-edge FT-EXAFS spectra of Ru1Cu SAA and reference samples (Cu2O, CuO, and Cu foil), and corresponding Ru1Cu SAA fitting curves, inset showing the schematic model. Scale bars: b 200 nm, c 2 nm, d 1 nm, e 5 nm.
The electronic structure and surface composition of Cu NCs, Ru1Cu SAA, and RuCu NPs were investigated by X-ray photoelectron spectroscopy (XPS). The two paired peaks of Ru 3d XPS spectra are deconvoluted for Ru0+ and Ru4+ species (Supplementary Fig. 6a). Notably, the binding energy of Ru1Cu SAA shifts toward high binding energy by ~0.54 eV as compared with that of RuCu NPs, indicating that the Ru species carry more positive charges to assume the oxidation state23. Similarly, in the Cu 2p region, two paired peaks are associated with Cu0/1+ and Cu2+ species (Supplementary Fig. 6b). Moreover, the binding energy of Cu 2p3/2 in the Ru1Cu SAA (932.16 eV) shifts negatively (~0.37 eV) compared with the Cu NCs (932.53 eV). The shift of the binding energy is ascribed to the electronic interaction and charge transfer between Ru and Cu13,23. Furthermore, we used X-ray absorption spectroscopy (XAS) to further confirm the atomically dispersed Ru and probe the electronic and coordination structure of Ru1Cu SAA. The Ru K-edge extended X-ray absorption near-edge structure (XANES) spectrum of Ru1Cu SAA exhibits a distinct energy absorption edge profile compared with Ru foil (Supplementary Fig. 7), indicating the formation of Ru oxidized state in Ru 1Cu SAA due to slight oxidation of Ru atoms24,25. The corresponding Fourier-transformed extended X-ray absorption fine structure (FT-EXAFS) spectrum of the Ru1Cu SAA shows two distinct peaks (1.42 Å and 2.22 Å) (Fig. 1f). The obvious 1.42 Å peak ascribed to Ru–O scattering contributions, which is mainly caused by the inevitable oxidation of the material in the air and the loading of some Ru atoms on the TiO2 substrate during the material synthesis process24,26. While the peak at 2.22 Å in Ru1Cu SAA is distinct from that in Ru foil (2.39 Å), which could be tentatively assigned to Ru–Cu contribution27, indicating that the Ru dopants were atomically dispersed in Cu matrix after the galvanic replacement process and did not form nanocluster28. The presence of Ru–Cu scattering is further corroborated by wavelet transforms (WT) of Ru EXAFS oscillation in Fig. 1g. Besides the Ru–O bond (4.6 Å−1), one intensity maximum at near 8.1 Å−1 is exclusively observed, which is assigned to the Ru–Cu contribution in Ru1Cu SAA, clearly different from that in Ru foil, suggesting the formation of atomically dispersed Ru atoms on the Cu nanocluster22. Then according to a primitive model optimized with density functional theory (DFT, Supplementary Fig. 8), Ru1Cu SAA was constructed by replacing two Cu atoms with two Ru atoms on the Cu38 cluster model, where two Ru atoms are located at the centers of two adjacent Cu hexagons. A least-squares EXAFS fitting analysis for R-space spectrum of Ru in Ru1Cu SAA (Fig. 1f and Supplementary Table 2) indicates that the coordination number (CN) of the center Ru atom with surrounding Cu atoms on Ru1Cu SAA is approximately 4.8. This further confirms the presence of Ru–Cu coordination in Ru1Cu SAA, consistent with the WT analysis results29. The results suggest that predominant Ru are distributed as isolated and did not form nanoclusters. Additionally, Cu K-edge XANES spectra and the corresponding FT-EXAFS of Ru1Cu SAA show very similar absorption edge and peak positions with that of Cu foil (Supplementary Fig. 9 and Fig. 1h). However, the lower Cu–Cu/Cu–Ru scattering intensity of the Ru1Cu SAA compared to the Cu foil indicates the obvious local unsaturated coordination of the Ru1Cu SAA (Fig. 1h and Supplementary Table 2), which possesses higher catalytic activity30. Therefore, both HAADF-STEM, XAS characterizations, and structural modeling studies demonstrate the formation of Ru1Cu SAA with atomically dispersed Ru atoms on the surface of Cu nanoclusters. Such Ru1Cu SAA with dual sites is expected to provide flexible adsorption configurations for reaction intermediates and facilitate the coupling of C/N intermediates31,32.
Electrochemical performance
The electrocatalytic performance evaluation of Ru1Cu SAA was carried out under ambient conditions using the chronoamperometry method in a standard three-electrode electrochemical device. Gaseous products were quantified by gas chromatography (GC) (Supplementary Fig. 10) and liquid products were quantified using nuclear magnetic resonance (NMR) (Supplementary Figs. 11 and 12) and colorimetric analysis (Supplementary Fig. 13). The intrinsic CO reduction reaction (CORR) and NO2− reduction reaction (NO2−RR) performance of Ru1Cu SAA were firstly evaluated. As shown in Fig. 2a, under a pure CO gas feed, Ru1Cu SAA mainly produces hydrogen products and a small amount of acetate (Supplementary Fig. 14). In addition, Ru1Cu SAA exhibits high selectivity of NH3 with a FE of ~100% for NO2−RR (Fig. 2b), which might be attributed to the synergistic effect derived from SAA and optimization of the electronic structure22,27,33. Interestingly, when a CO-saturated aqueous solution containing 1 M KOH and 1 M KNO2 was used as the electrolyte, the linear sweep voltammetry (LSV) curve of Ru1Cu SAA exhibits an enhanced current density under mixed feed gas compared to pure CORR (Supplementary Fig. 15), which indicates the occurrence of the electrocatalytic C–N coupling reaction. Notably, new NMR peaks appearing in the 1H NMR spectrum of the electrolyte solution after electrolysis match the spectrum of formamide (Fig. 2c), which was not present in the sole CORR and NO2−RR. Furthermore, we further confirmed the production of formamide in testing the electrolyte solution by gas chromatography-mass spectrometry (GC-MS) (Fig. 2d). The experimental results show that formamide is only produced by the electrocatalytic co-reduction of CO and NO2−. In order to further clarify the sources of C and N in formamide, we carried out isotope labeling experiments using 15N-labeled NO2− and 13C-labeled CO as raw materials. The 1H NMR spectrum of the electrolyte after the coupling reaction of 15NO2− and 13CO shows typical H13CO15NH2 peaks (Fig. 2c). Meanwhile, GC-MS measurements further confirmed the production of H13CO15NH2 (Fig. 2d). These results demonstrate that the generated HCONH2 originated from the electrocatalytic coupling reaction of CO and NO2− on Ru1Cu SAA.
a, b Faradaic efficiencies of major reduction products on Ru1Cu SAA for a CORR and b NO2−RR. c 1H NMR spectra of standard references (HCONH2 and H13CO15NH2) and the electrolyte obtained after CORR, NO2−RR, and NO2− + CO/15NO2− + 13CO co-reduction. d GC-MS results of the electrolyte obtained after NO2− + CO and 15NO2− + 13CO co-reduction. e Products distribution at different applied potentials in a CO-saturated 1 M KOH + 1 M KNO2 solution on Ru1Cu SAA. f Highest formamide Faradaic efficiencies and Yield of Cu NCs, Ru1Cu SAA, and RuCu NPs. The error bars represent the standard deviation for at least three independent measurements.
The Cu NCs, Ru1Cu SAA, and RuCu NPs were further adopted as catalysts for formamide electrosynthesis by simultaneously reducing CO and NO2− (Supplementary Fig. 16). The Cu NCs shows high selectivity toward NH3, along with a small amount of formamide (Supplementary Fig. 17a). In stark contrast, Ru1Cu SAA delivers higher formamide FE and yield at various reduction potentials, with a maximum formamide FE of 45.65 ± 0.76% and yield of 2483.77 ± 155.34 μg h−1 mgcat.−1 at −0.5 V vs. RHE (Fig. 2e), respectively. When the Ru loading was further increased, the RuCu NPs exhibit higher hydrogen evolution reaction (HER) activity, thus resulting in lower the FE and yield of formamide (Fig. 2f and Supplementary Fig. 17b). These results suggest that the introduction of single-atom Ru can effectively improve the catalytic activity and selectivity of formamide synthesis while avoiding the promotion of competitive HER (Supplementary Fig. 18). To evaluate the intrinsic activities of the catalysts, we performed electrochemical double-layer capacitance (Cdl) measurements to normalize the electrochemically active surface area (ECSA) (Supplementary Fig. 19 and Supplementary Table 3)27,34. Although these catalysts present similar ECSA, Ru1Cu SAA still shows the best intrinsic activity for C–N coupling towards formamide, indicating that the atomic dispersion of Ru in Ru1Cu SAA enhances the intrinsic activity. Moreover, the durability of the Ru1Cu SAA is evaluated by chronoamperometry in CO-saturated aqueous solution containing 1 M KOH and 1 M KNO2 electrolyte. The current density, formamide FE, and yield of Ru1Cu SAA show a negligible change at constant applied potential after 52 h of continuous electrolysis (Supplementary Fig. 20). Furthermore, we further investigated the stability of the Ru1Cu SAA by using a membrane electrode assembly (MEA) electrolyzer. Remarkably, the Ru1Cu SAA that could maintain full-cell voltage durability at high current density (~250 mA cm−2) for 50 h, as well as structural and chemical stability (Supplementary Fig. 21). These results strongly suggest that the Ru1Cu SAA has excellent stability for formamide electrosynthesis.
Investigation of formamide electrosynthesis mechanism
To elucidate the origins of the C–N coupling activity on the Ru1Cu SAA catalyst, in situ XAS measurements using a homemade cell were initially performed to probe the electronic structure and local atomic environment changes of Ru1Cu SAA during real electrosynthesis process35. During in situ XAS measurements, the applied potential was first increased from open circuit voltage (OCV) to −0.3 and −0.5 V vs. RHE, and then decreased back to OCV. Figure 3a shows the normalized operating Ru K-edge XANES at different applied potentials. Compared with the Ex situ condition, the absorption edge of the Ru K-edge XANES spectrum shifts toward the high-energy side (about 0.5 eV) (insert of Fig. 3a) under the OCV condition, while the white line peak broadens (Orange marked in Fig. 3a), implying an increase in the Ru oxidation state due to the binding of H2O and NO2−36. Furthermore, the absorption edge of Ru K-edge XANES shows a clear shift to the lower energies with increasing applied potential (inset of Fig. 3a), which is a combination of ligand effects and dissociation of the reactants or reaction intermediates. This probably results in Cu transfers electrons to Ru during C–N coupling, which in turn modulates the state of the adsorbed reactant intermediates and improves the catalytic activity of the alloy23,36. Meanwhile, the dissociation of reactants or reaction intermediates at Ru sites occurs and the recovery of the low oxidation state Ru reflects the rapid dissociation process of reactants or reaction intermediates on Ru atoms37. The results are further verified by corresponding FT-EXAFS spectra shown in Fig. 3b. In comparison with the Ex situ condition, an enhanced scattering peak of the Ru-C/N/O shell obtained under OCV condition displays a negative shift (0.12 Å) (Fig. 3b and Fig. 3c), which is ascribed to the adsorption of reactants on Ru atoms for the generation of C–N coupling38,39. With the increase of the potential, the scattering intensity of Ru–O/N/C shell gradually decreases (insert of Fig. 3b), indicating that a large amount of reactants (CO or NO2−) are consumed and oxidation state Ru reduction40,41. In addition, the scattering peaks of Ru–O/N/C shells gradually shift positively (Fig. 3c), which may be caused by the change of adsorbed species at Ru sites42. Meanwhile, the scattering peak of the Ru–Cu shell shows a low R shift and an enhanced intensity, which is caused by the reaction intermediates bound to Ru site and low oxidation state Ru recovery43. Moreover, the wavelet transforms (WT) spectrum of the corresponding Ru K-edge EXAFS oscillations further validates the above results (Fig. 3d). However, when the electrode potential was switched back to OCV, the Ru K-edges XANES, and FT-EXAFS spectra show signs of irreversible changes, possibly due to the strong adsorption of intermediate groups on Ru sites44. Figure 3e shows the in situ XANES spectra of Ru1Cu SAA at Cu K-edge. This is a remarkable positive shift under OCV condition compared with that under Ex situ condition, indicating that the reactants are adsorbed on the Cu atoms, which is also evidenced by the emerging Cu–O/N/C shell scattering peaks in the corresponding FT-EXAFS spectra (Fig. 3f). Notably, when the potential is applied, the intensity of Cu–O/N/C shell scattering decreases sharply, indicating that the reactants are rapidly consumed at the Cu sites40,41. Moreover, compared to Ex situ conditions, the lower intensity of Cu–Cu/Cu–Ru scattering under OCV and applied potential conditions is observed on the Ru1Cu SAA, especially the lowest intensity under −0.5 V vs. RHE conditions (inset of Fig. 3f), indicating the presence of a large amount of unsaturated coordination Cu under in situ conditions, which is favorable for the adsorption of reactants on Cu sites45. However, the majority of Ru1Cu SAA are Cu atoms, whose atomic structure is difficult to significantly change25,46. This results in Cu–Cu/Cu–Ru bond lengths with little variations during the entire in situ measurement (Fig. 3f). Thus, in situ XAS results give experimental evidence that C/N species adsorb and interact with surface Ru and Cu sites in Ru1Cu SAA.
a In situ XANES spectra of Ru1Cu SAA recorded at Ru K-edge. b Ru K-edge FT-EXAFS spectra for Ru1Cu SAA. c, The variation of the radial distance of Ru–O/C/N and Ru–Cu shells at different applied potentials. d Corresponding WT contour profiles. e Cu K-edge XANES spectra for Ru1Cu SAA. f Cu K-edge FT-EXAFS spectra for Ru1Cu SAA. g In situ Raman spectra of Ru1Cu SAA.
In situ Raman spectroscopy measurements were further conducted on Ru1Cu SAA to validate the plausibility of the mechanism of carboxamide formation by distinguishing intermediates at the molecular level (Supplementary Fig. 22). Figure 3g exhibits the in situ Raman spectra of the Ru1Cu SAA by utilizing an electrolyte containing CO-saturated 1 M KOH and 1 M KNO2 at different operated potentials. When the applied potential is increased to −0.5 V vs. RHE, a notable peak corresponding to the stretching vibration of the C–N bond was observed on Ru1Cu SAA47. Meanwhile, the C–H deformation at 1392 cm−1, NH2 at 1592 cm−1, and CO stretching at 1890 and 2190 cm−1 also started to appear47,48,49. These results further confirm the C–N bond formation and the occurrence of electrocatalytic processes. Moreover, the Raman signals of C–C, CH2, and CH3 stretching vibration were not observed, which indicates that the product of Ru1Cu SAA C–N coupling reaction is formamide, not other organic compounds.
To distinguish the roles of the individual components of Ru1Cu SAA in the C–N coupling process, we further investigated the formation of HCONH2 by C–N coupling at the Ru1Cu SAA interface by DFT calculations. The calculated adsorption energies of NO2− and CO on the optimized Ru1Cu SAA model (Supplementary Fig. 23 and Supplementary Fig. S24) confirm that Ru1Cu SAA is more favorable for the adsorption of NO2−, suggesting that NO2− will be enriched on the surface of Ru1Cu SAA. Moreover, Bader charge analysis determined that the adsorbed *NO2 species obtained −0.94 |e| from Ru1Cu SAA, while the adsorbed *CO species obtained −0.67 |e|. More charge transfer means that the interaction between Ru sites and *NO2 species is stronger than that of between Ru sites and CO* species (Supplementary Fig. 25). It can be inferred that the Ru sites are more favorable for the adsorption of NO2− during the reaction process, while CO is more inclined to adsorb on the Cu sites close to the Ru sites. Moreover, when CO was adsorbed on Cu site adjacent to Ru, the Gibbs free energy of NO2− on Ru site can be reduced from −1.80 eV (without *CO) to −1.83 eV (with *CO), indicating that the existence of activated CO molecules on Cu site can further promote the adsorption and activation of NO2− on adjacent metal sites (Supplementary Fig. 26). Based on the above analysis, we proposed two possible reaction paths (with *CO) of NO2−RR (Fig. 4a and Supplementary Fig. 27). The results show that the rate-determining step (RDS) energy barrier of path 2 (*CO*NOOH → *CO*NOHOH, ΔG = 0.31 eV) has a lower than that of path 1 (*CO*NO → *CO*NOH, ΔG = 1.28 eV). Therefore, it can be inferred that the formation of *NOHOH intermediate instead of *NO can reduce the RDS energy barrier of the whole reaction, which is beneficial to accelerate the subsequent protonation process and enhance the electrosynthesis performance of formamide. Interestingly, the assistance of CO did not alter the NO2−RR reaction pathway, but lowered the energy barrier of the RDS (Supplementary Fig. 28). It can be inferred that CO adsorbed at the Cu site accelerates NO2−RR, which promotes the production of nitrogen-containing intermediates at the Ru site. Furthermore, the RDS of Cu NCs is *CO*N → *CO*NH (ΔG = 0.76 eV), while that of Ru1Cu SAA is *CO*NOHOH → *CO*NH (ΔG = 0.31 eV) (insert of Fig. 4a and Supplementary Fig. 29). On the other hand, the kinetic barriers for the formation of *CO*NOHOH and *CO*NH on Ru1Cu SAA are 0.67 and 0.91 eV respectively (Supplementary Fig. 30). However, the energy barriers are 1.05 and 1.03 eV for Cu NC, which is higher than Ru1Cu SAA. Indicating that the synthesis of formamide is thermodynamically and kinetically preferred in Ru1Cu SAA, which is in good agreement with our experimental observations. The results indicate that the introduction of highly dispersed Ru atoms can effectively improve the adsorption and activation of NO2−.
a Diagram of free energy changes for NO2− reduction on Ru1Cu SAA surface with the assistance of *CO. Insert shows free-energy diagram for NO2−RR on Ru1Cu SAA and Cu NCs. b Free energy diagrams of different reaction pathways for the synthesis of formamide on Ru1Cu SAA. Insert shows free-energy diagram for formamide production on Ru1Cu SAA and Cu NCs. c Diagram of the optimal energy pathway for the synthesis of formamide on Ru1Cu SAA and the corresponding atomic configurations for each step, Cu, Ru, C, O, N, and H atoms shown as orange, blue, gray, red, dark blue, and green, respectively.
The calculation results suggest that various N-containing intermediates can be generated during the NO2−RR process, so the adsorbed CO species may be coupled with various nitrogen-containing intermediates, such as *NOH, *N, *NH, and *NH2. Therefore, we have carried out a detailed study on the potential C–N coupling mechanism of electrosynthesis formamide. As shown in Fig. 4b, the coupling reaction of *CO with *NH2 intermediates to form *CONH2 (ΔG = −0.24 eV) is thermodynamically the most favorable compared to *CO*NOH → *CONOH (ΔG = 0.62 eV), *CO*N → *CO*NH (ΔG = 0.17 eV) and *CO*NH → *CONH (ΔG = −0.36 eV). The *CO*NH2 → *CO*NH2 process is an exothermic process, which indicates that the adjacent Ru–Cu dual sites can spontaneously couple *CO and *NH2 intermediates to achieve the critical C–N coupling reaction. In addition, it is generally known that the C–C coupling reaction is known to be an energy-absorbing step14,50, as is the coupling of carbon-containing intermediates with free NH314, while the coupling of *CO with *NH2 (*CO*NH2 → *CO*NH2, ΔG = −0.24 eV) is an energy-releasing step. Therefore, the C–N coupling synthesis of formamide is more favorable in terms of thermodynamics and kinetics. This confirms the poor catalytic activity of Ru1Cu SAA in the synthesis of multi-carbon organics. Notably, the energy barrier of *CO*N → *CO*NH (ΔG, 0.82 eV→0.17 eV) is significantly lower after the introduction of single atom Ru in the Cu nanoclusters (insert of Fig. 4b), suggesting that the introduction of highly dispersed Ru atoms can effectively promote NO2− protonation and participate in the subsequent C–N coupling process. These results indicate that formamide generation via coupling of *CO and *NH2 intermediates is thermodynamically and kinetically feasible. According to the above analysis, the overall reaction scheme can be best described as a suitable ten-step electrocatalytic cascade (Fig. 4c). With CO adsorption at Ru adjacent Cu sites, NO2− preferentially undergoes deoxy-hydrogenation at Ru sites until the key *NH2 intermediate is finally formed. Then, *CO adsorbed on Cu sites is spontaneously coupled with *NH2 on Ru sites to generate a critical C–N bond, enabling formamide electrosynthesis.
Discussion
In summary, we demonstrated a sustainable electrochemical approach to produce formamide through electrolysis co-reduction CO and NO2− pollutants at ambient conditions. Highly selective formamide production with a Faradaic efficiency of 45.65 ± 0.76% and a yield of 2483.77 ± 155.34 μg h−1 mgcat.−1 at −0.5 V vs. RHE are achieved on a Ru-dispersed Cu nanocluster electrocatalyst. As evidenced by in situ XAS, in situ Raman, and theoretical calculation, the key *CO and *NH2 intermediates tend to spontaneously couple with low energy barriers at adjacent Ru–Cu dual active sites, leading to highly selective synthesis of formamides. This work opens a avenue for sustainable formamide electrosynthesis from cheap CO and NO2− pollutant through the C–N coupling, providing a dual-sites design strategy based on single-atom alloy for the synthesis of various high-value-added chemicals.
Methods
Materials fabrication
The fabrication of TiO2 nanowires is described elsewhere21. 40 mg TiO2 nanowires was first dispersed in 10 mL of H2O with sonication to get well-dispersed TiO2 nanowires suspension. Subsequently, 200 μL Copper (II) Chloride Dihydrate solution (Adamas, 98%) (Cu2+: 10 mg mL−1, 2 mg) was added to the prepared TiO2 nanowires aqueous solution with stirring for 60 min at room temperature. The mixed solution was frozen in a liquid nitrogen environment and then freeze-dried. Then, TiO2 nanowires anchored with Cu NCs were obtained via a reduction treatment of the freeze-dried samples in a H2/Ar (H2: Ar = 20: 180 sccm) stream at 300 °C (heating rate: 5 °C min−1) for 2 h. Finally, the supported Ru1Cu SAA samples were prepared via a galvanic replacement method. The TiO2 nanowires anchored with Cu NCs sample was dispersed in deionized water (10 mL), followed by dropwise adding desired amount of RuCl3 solution (Adamas, 99.5%+, Ru: 47%) (Ru3+: 10 mg mL−1, 0.15 mg) in an ultrasonic water bath. The obtained slurry was centrifugated and washed with distilled water, and then dried. As control samples, RuCu NPs were prepared by the same method (The mass ratios of the precursor materials are TiO2: Cu: Ru = 40:3:1, respectively).
Structural characterization
XRD patterns of the samples were taken by using a Rigaku MiniFlex X-ray diffraction. The surface topography of the samples was characterized with a Tescan MIRA3 SEM, equipped with an Oxford energy-dispersive X-ray spectroscope. The transmission electron microscopy (TEM), high-angle annular dark field-scanning TEM (HAADF-STEM), and element mapping were taken by a Thermo scientific Themis Z (3.2) with double spherical aberration (Cs) correctors for both the probe-forming. The chemical state and composition of the samples were characterized using X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250Xi). X-ray absorption near-edge structure (XANES) and Fourier transform (FT) curve of extended X-ray absorption fine structure (EXAFS) spectra were measured at beamline BL01C1 of Taiwan light source. The contents of Ru and Cu were obtained via the inductively coupled plasma-optical emission spectrometry (ICP-OES) (Agilent 730). In situ Raman spectroscopy was performed by a WITec Alpha300R (WITec GmbH, Germany) confocal spectrometer equipped with a 532 nm single longitudinal-mode laser at room temperature.
Preparation of the electrode
To prepare the catalyst ink, the catalyst was ultrasonically dispersed in solution (40 μL of Nafion solution (Adamas, RG, 5 wt%), 960 μL of absolute ethanol), and ultrasonicated for 30 min to form a uniform ink. The homogeneous ink was loaded onto the gas diffusion layer (Sigraset 29 BC) electrodes and dried under ambient conditions. The catalyst loading was estimated to be ~0.6 mg cm−2.
Electrochemical formamide synthesis
The electrochemical experiments were performed on an electrochemical workstation (CHI660E) using an H-Cell with a three-electrode configuration (working electrode, Pt plate counter electrode, and Hg/HgO/saturated 1 M KOH reference electrode). the cathode and anode chambers were separated by an anion exchange membrane (FAB-PK-130). All of the potential measurements were converted to the RHE using the following formula: ERHE = EHg/HgO + 0.097 + 0.0591 × pH.
For electrochemical formamide synthesis, potentiostatic tests were carried out in CO-saturated 1 M KNO2 + 1 M KOH (CO, Changsha Gaoke Gas Co., 99.9999%; KNO2, Adamas, RG; KOH, Greagent, AR), which was bubbled with CO for 20 min before the measurement. During the experiment, CO enters the cathode chamber continuously at a constant flow rate.
For the stability tests in MEA electrolyzers, the experimental set-up used was a commercial MEA electrolyzer (4 cm2). The MEA consisted of a cathode electrode, anion-exchange membrane (FAB-PK-130), and anode electrode (IrO2-Ti mesh).
Electrochemical CORR measurements
For electrochemical CORR tests, potentiostatic tests were carried out in 1 M KOH without NO2−.
Electrochemical NO2 −RR measurements
For electrochemical NO2−RR tests, potentiostatic tests were carried out in 1 M KNO2 + 1 M KOH without feeding CO.
Product qualitative and quantification
A gas chromatograph equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD) was used for the quantification of the gaseous products. The gas chromatography used high purity argon (99.999%) as carrier gas. Organic liquid products were quantified by Bruker 400 MHz NMR spectrometer. The NMR samples were prepared by mixing 0.5 mL of electrolyte with 0.1 mL of deuterated water (D2O), and 0.02 μL of dimethyl sulfoxide (DMSO) was added as an internal standard. Unusually, the test solution of NO2-RR was acidified (pH adjusted to ~2) before NMR testing. In addition, the production of formamide was further verified by GC-MS (gas chromatography-mass spectrometry) (Agilent 59771A).
Determination of NH3
After diluting the post-test electrolyte solution to the appropriate concentration, the NH3 concentration in the electrolyte solution was detected spectrophotometrically modified by the modified indophenol blue method51. 2 mL of the diluted electrolyte solution was taken and 2 mL of NaOH (1 M) solution containing salicylic acid (5 wt%) (99%, Adamas) and sodium citrate dihydrate (5 wt%) (AR, Greagent), 1 mL of 0.05 M NaClO (Active chlorine ≥7.5%: Greagent), and 0.2 mL of a 1 wt% C5FeN6Na2O (sodium nitroferricyanide, 99%+, Adamas) aqueous solution were added sequentially. After being left at room temperature for 2 h, the absorption spectra of the developed solutions were detected by UV-Vis spectrophotometer (Shimadzu, UV-2600). The formation of indophenol blue was determined by absorbance at a fixed wavelength of 655 nm. The concentration-absorbance curves were calibrated using standard ammonia sulfate solutions ((NH4)2SO4, Greagent, ≥99.5%), as shown in Supplementary Fig. 13.
Isotope labeling experiments
Isotope labeling experiments were performed using Na15NO2 (99 at.% of 15N, Shanghai Macklin Biochemical Technology Co., Ltd.) as the N source and 13CO (99 at.% of 13C, Wuhan Newradar Special Gas Co. Ltd) as the C source. After potentiostatic electrolysis at −0.5 V (vs. RHE), the catholyte was collected and concentrated for NMR and GC-MS analysis.
Calculation of Faradaic efficiency for corresponding product
The Faradaic efficiency of each gas product was calculated by the equation:
where n is the number of electrons transferred, F is Faraday’s constant, x is the mole fraction of product, V is the total molar flow rate of gas and j is the total current.
The Faradaic efficiency for liquid products generation was calculated as follows:
Where n is the number of electrons transferred; V is electrolyte volume; C is the concentration of liquid products; F is Faraday’s constant; Q is the electric quantity.
Calculation of yield rate for corresponding product
The average yield rate was calculated as follows:
where cformamide is corresponding product concentration (μg mL−1), V is the total volume of electrolyte (mL), t is time (h) for electrocatalysis and m is the catalyst loadings (mg).
In situ XAS measurements
The corresponding in situ XAS spectroscopic measurements (Ru K- and Cu K-edges XAS) were performed at the BL01C1 beamline at the National Synchrotron Radiation Research Center (NSRRC, Taiwan). The in situ XAS measurements were performed in a customized three-electrode cell with a carbon rod and a saturated glycury electrode (SCE) as the counter and reference electrode, respectively. Catalyst ink was dropped on a carbon cloth substrate as the working electrode. Potentiostatic tests were performed in a CO-saturated 1 M KNO2 + 1 M KOH electrolyte with CO bubbling for 20 min before measurement. The working electrode was covered with Kapton film on one side facing the incident X-rays, while the other side was in contact with the electrolyte. XAS spectra were measured in fluorescence mode at room temperature. The obtained XAS data were processed with the ATHENA program.
Electrochemical in situ Raman measurements
The in situ Raman measurement tests were performed using a customized three-electrode cell with a platinum wire and an Ag/AgCl electrode as the counter and reference electrode, respectively. The catalyst ink was dropped onto a glassy carbon substrate as a working electrode. In situ Raman spectra were recorded using a Thermo Fisher DXR2 Raman microscope with DXR 532 nm laser as the excitation source.
Computational method
All the density functional theory (DFT) calculations were performed by using the Vienna ab initio Simulation Package (VASP) with the projector augmented wave (PAW) potentials52,53. The generalized gradient approximation (GGA)/Perdew-Burke-Ernzerhof (PBE) level were adopted54. All the atomic positions were allowed to relax until the forces were less than 0.02 eV/Å, and the electron convergence energy was set to 10−5 eV. The cutoff was set to 400 eV to expand wave function. The boxes were set to 15 × 15 × 15 for all the Ru1Cu SAA structures.
In order to simplify the model, we adopted the Cu38 cluster model as the candidate catalyst substrate, where the Ru1Cu SAA (Cu36Ru2) can be achieved by replacing two Cu atoms with two Ru atoms. To screen out the stable Ru1Cu SAA structure, the formation energies can be considered as follows:
where the \({E}_{{{\mbox{C}}}{{{\mbox{u}}}}_{36}{{\mbox{R}}}{{{\mbox{u}}}}_{2}}\), \(\,{E}_{{{\mbox{C}}}{{{\mbox{u}}}}_{1}}\), \(\,{E}_{{{\mbox{C}}}{{{\mbox{u}}}}_{38}}\), and \(\,{E}_{{{\mbox{R}}}{{{\mbox{u}}}}_{1}}\) are the energies for Ru1Cu SAA, single Cu atom, Cu38 cluster, and single Ru atom, respectively.
In this work, the adsorption energies of NO2− and/or CO on Ru1Cu SAA interface were calculated, respectively, can be defined as follows:
where the \({E}_{{{\mbox{C}}}{{{\mbox{u}}}}_{36}{{\mbox{R}}}{{{\mbox{u}}}}_{2}+{{\mbox{N}}}{{{\mbox{O}}}}_{2}/{{\mbox{CO}}}}\), \({E}_{{{\mbox{C}}}{{{\mbox{u}}}}_{36}{{\mbox{R}}}{{{\mbox{u}}}}_{2}}\), and \({E}_{{{\mbox{N}}}{{{\mbox{O}}}}_{2}/{{\mbox{CO}}}}\) are the energies for NO2− and/or CO adsorption on Ru1Cu SAA interface, Ru1Cu SAA, NO2− or CO in gas, respectively.
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.
References
Pattabiraman, V. R. & Bode, J. W. Rethinking amide bond synthesis. Nature 480, 471–479 (2011).
Sabatini, M. T., Boulton, L. T., Sneddon, H. F. & Sheppard, T. D. A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2, 10–17 (2019).
de Figueiredo, R. M., Suppo, J.-S. & Campagne, J.-M. Nonclassical routes for amide bond formation. Chem. Rev. 116, 12029–12122 (2016).
Ke, Z. & Yeung, Y.-Y. Unearthing the mechanism of umpolung amide synthesis. Chem 5, 1014–1016 (2019).
Meng, N. et al. Electrosynthesis of formamide from methanol and ammonia under ambient conditions. Nat. Commun. 13, 5452 (2022).
Bada, J. L., Chalmers, J. H. & Cleaves, H. J. Is formamide a geochemically plausible prebiotic solvent? Phys. Chem. Chem. Phys. 18, 20085–20090 (2016).
Bredehöft, J. H., Böhler, E., Schmidt, F., Borrmann, T. & Swiderek, P. Electron-induced synthesis of formamide in condensed mixtures of carbon monoxide and ammonia. ACS Earth Space Chem. 1, 50–59 (2017).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C-N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Tao, Z., Rooney, C., Liang, Y. & Wang, H. Accessing organonitrogen compounds via C-N coupling in electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 19630–19642 (2021).
Huang, Y., Wang, Y., Wu, Y., Yu, Y. & Zhang, B. Electrocatalytic construction of the C-N bond from the derivates of CO2 and N2. Sci. China Chem. 65, 204–206 (2022).
Wu, Y., Jiang, Z., Lin, Z., Liang, Y. & Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 4, 725–730 (2021).
Guo, C. et al. Electrochemical upgrading of formic acid to formamide via coupling nitrite Co-reduction. J. Am. Chem. Soc. 144, 16006–16011 (2022).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Jouny, M. et al. Formation of carbon-nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Tang, C., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Electrocatalytic refinery for sustainable production of fuels and chemicals. Angew. Chem. Int. Ed. 60, 19572–19590 (2021).
Li, J. & Kornienko, N. Electrochemically driven C-N bond formation from CO2 and ammonia at the triple-phase boundary. Chem. Sci. 13, 3957–3964 (2022).
Su, H. et al. Soil nitrite as a source of atmospheric HONO and OH radicals. Science 333, 1616–1618 (2011).
Pye, V. I. & Patrick, R. Ground water contamination in the United States. Science 221, 713–718 (1983).
Wang, J. et al. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy 86, 106088 (2021).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Gao, J. J. et al. Platinum single atoms/clusters stabilized in transition metal oxides for enhanced electrocatalysis. Electrochim. Acta 297, 155–162 (2019).
Zhang, X. et al. Platinum-copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun. 10, 5812 (2019).
Wu, Q. et al. Identifying electrocatalytic Sites of the nanoporous copper-ruthenium alloy for hydrogen evolution reaction in alkaline electrolyte. ACS Energy Lett. 5, 192–199 (2020).
Ji, K. Y. et al. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural promoted by a Ru1Cu single-atom alloy catalyst. Angew. Chem. Int. Ed. 61, e202209849 (2022).
Liu, X. et al. Dynamic surface reconstruction of single-atom bimetallic alloy under operando electrochemical conditions. Nano Lett. 20, 8319–8325 (2020).
Yao, Y. et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2, 304–313 (2019).
Chen, F. Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Pietrowski, M., Zieliński, M. & Wojciechowska, M. High-selectivity hydrogenation of chloronitrobenzene to chloroaniline over magnesium fluoride-supported bimetallic ruthenium-copper catalysts. ChemCatChem 3, 835–838 (2011).
Huang, H. W. et al. Activation of inert copper for significantly enhanced hydrogen evolution behaviors by trace ruthenium doping. Nano Energy 92, 106763 (2022).
Li, P. et al. In situ dual doping for constructing efficient CO2-to-methanol electrocatalysts. Nat. Commun. 13, 1965 (2022).
Fu, J., Yang, Y. & Hu, J.-S. Dual-sites tandem catalysts for C-N bond formation via electrocatalytic coupling of CO2 and nitrogenous small molecules. ACS Mater. Lett. 3, 1468–1476 (2021).
Zhang, X. et al. Identifying and tailoring C-N coupling site for efficient urea synthesis over diatomic Fe-Ni catalyst. Nat. Commun. 13, 5337 (2022).
Cai, J. et al. Electrocatalytic nitrate-to-ammonia conversion with ~100% Faradaic efficiency via single-atom alloying. Appl. Catal. B-Environ. 316, 121683 (2022).
Shi, J. et al. Promoting nitric oxide electroreduction to ammonia over electron-rich Cu modulated by Ru doping. Sci. China Chem. 64, 1493–1497 (2021).
Jiang, K. et al. Rational strain engineering of single-atom ruthenium on nanoporous MoS2 for highly efficient hydrogen evolution. Nat. Commun. 12, 1687 (2021).
Cao, L. et al. Identification of single-atom active sites in carbon-based cobalt catalysts during electrocatalytic hydrogen evolution. Nat. Catal. 2, 134–141 (2018).
Wang, X. et al. Efficient upgrading of CO to C3 fuel using asymmetric C-C coupling active sites. Nat. Commun. 10, 5186 (2019).
Yu, Y. et al. Self-activated catalytic sites on nanoporous dilute alloy for high-efficiency electrochemical hydrogen evolution. ACS Nano 15, 5333–5340 (2021).
Zhao, Y. et al. Surface-exposed single-Ni atoms with potential-driven dynamic behaviors for highly efficient electrocatalytic oxygen evolution. Angew. Chem. Int. Ed. 61, e202212542 (2022).
Su, X. et al. Complementary operando spectroscopy identification of in-situ generated metastable charge-asymmetry Cu2-CuN3 clusters for CO2 reduction to ethanol. Nat. Commun. 13, 1322 (2022).
Li, Y. et al. Interstitial boron-triggered electron-deficient Os aerogels for enhanced pH-universal hydrogen evolution. Nat. Commun. 13, 1143 (2022).
Su, H. et al. In-situ spectroscopic observation of dynamic-coupling oxygen on atomically dispersed iridium electrocatalyst for acidic water oxidation. Nat. Commun. 12, 6118 (2021).
Cao, L. et al. Dynamic oxygen adsorption on single-atomic Ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat. Commun. 10, 4849 (2019).
Li, P. et al. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat. Commun. 10, 1711 (2019).
Lei, W. et al. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 4, 1777 (2013).
Liu, S. et al. Unidirectional thermal diffusion in bimetallic Cu@Au nanoparticles. ACS Nano 8, 1886–1892 (2014).
Ojha, A. K. et al. Investigation of hydrogen bonding and self-association in neat HCONH2 and the binary mixture (HCONH2 + CH3OH) by concentration dependent Raman study and ab initio calculations. J. Mol. Struct. 689, 127–135 (2004).
Lan, J. et al. Nanoporous B13C2 towards highly efficient electrochemical nitrogen fixation. Small 17, 2102814 (2021).
Chang, X. et al. Understanding the complementarities of surface-enhanced infrared and Raman spectroscopies in CO adsorption and electrochemical reduction. Nat. Commun. 13, 2656 (2022).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu-Pd sites. Nat. Catal. 5, 251–258 (2022).
Zhu, D. et al. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 12, 836–841 (2013).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Hafner, J. Abinitio molecular-dynamics for liquid-metals. Phys. Rev. B 47, 558–561 (1993).
Hamann, D. R. Generalized gradient theory for silica phase transitions. Phys. Rev. Lett. 76, 660–663 (1996).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51771072 (Y.W.T.)), the Youth 1000 Talent Program of China (Y.W.T.), the Outstanding Youth Scientist Foundation of Hunan Province (no. 2020JJ2006 (Y.W.T.)), the Fundamental Research Funds for the Central Universities, Hunan University State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body Independent Research Project (no. 71860007), and Hunan Provincial Innovation Foundation for Postgraduate (no. CX20220415 (J.L.)).
Author information
Authors and Affiliations
Contributions
Y.W.T. conceived and directed the project. J.L. carried out key experiments. Z.X.W. and S.L.Z. performed theoretical calculations. J.L., D.C.C., Y.R.L., and T.S.C. contributed to the XAS measurements and analyses of the XAS experiment results. Y.W.T. and J.L. wrote the manuscript with input from all other authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, J., Wei, Z., Lu, YR. et al. Efficient electrosynthesis of formamide from carbon monoxide and nitrite on a Ru-dispersed Cu nanocluster catalyst. Nat Commun 14, 2870 (2023). https://doi.org/10.1038/s41467-023-38603-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-38603-5
This article is cited by
-
Prospects of formamide as nitrogen source in biotechnological production processes
Applied Microbiology and Biotechnology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.