Abstract
Polymers with low ceiling temperatures (Tc) are highly desirable as they can depolymerize under mild conditions, but they typically suffer from demanding synthetic conditions and poor stability. We envision that this challenge can be addressed by developing high-Tc polymers that can be converted into low-Tc polymers on demand. Here, we demonstrate the mechanochemical generation of a low-Tc polymer, poly(2,5-dihydrofuran) (PDHF), from an unsaturated polyether that contains cyclobutane-fused THF in each repeat unit. Upon mechanically induced cycloreversion of cyclobutane, each repeat unit generates three repeat units of PDHF. The resulting PDHF completely depolymerizes into 2,5-dihydrofuran in the presence of a ruthenium catalyst. The mechanochemical generation of the otherwise difficult-to-synthesize PDHF highlights the power of polymer mechanochemistry in accessing elusive structures. The concept of mechanochemically regulating the Tc of polymers can be applied to develop next-generation sustainable plastics.
Similar content being viewed by others
Introduction
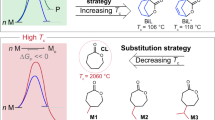
Synthetic polymers are among the most important materials in modern society due to their light weight, low cost, and robustness. However, the durability of polymers has caused environmental pollution and sustainability issues. The stable nature of conventional synthetic polymers can be partly ascribed to their high ceiling temperatures (Tc), which is defined as the temperature where polymerization and depolymerization reach an equilibrium. On the other hand, low-Tc polymers have metastable structures that are more susceptible to depolymerization. A low Tc is especially useful for triggered degradation in polymers. For example, polyphthalaldehyde, a well-known low-Tc (−40 °C) polymer, has been employed in photoresist and transient devices1,2,3,4,5. However, these materials are not competitive with industrially used polymers due to their metastable nature and are therefore not suitable for use as sustainable plastics. In addition, the conditions for synthesizing these polymers are typically demanding (e.g., low temperatures, ultrapure monomers, and a strict anhydrous and oxygen-free environment), and the synthesis often suffers from low yield and/or low molecular weight (Fig. 1a)6,7,8.
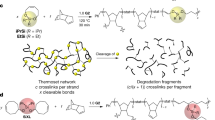
a Polymerization of low-Tc polymer requires demanding conditions and suffers from low yield and low molecular weight. b A high-Tc polymer is mechanochemically converted into a low-Tc polymer, which can undergo depolymerization. In this work, P1 is the high-Tc polymer, and poly(2,5-diydrofuran) is the low-Tc polymer.
Recently, our group and others have demonstrated the concept of locked degradability, aiming to address the stability issue of degradable polymers9,10,11,12. In the locked state, the polymer is nondegradable and highly stable. When recycling is needed, mechanochemical activation unlocks the degradability and converts the polymer into a degradable one. We envisioned that this strategy of locking and unlocking can be applied to address the stability issue of low Tc polymers: if a high-Tc polymer (locked state, for use and storage) can be converted into a low-Tc polymer (unlocked state, for recycling), the paradox between stability and depolymerization can be broken (Fig. 1b).
Here, we demonstrate the concept of mechanochemically regulating Tc with the 2,5-dihydrofuran (DHF) system, the ring-opening metathesis polymerization (ROMP) of which has proved challenging13. We designed a polymer P1 (Fig. 1b), which is an unsaturated polyether containing cyclobutane-fused THF in each repeat unit. Upon force-induced cycloreversion of cyclobutane, each repeat unit is converted into three repeat units of poly(2,5-dihydrofuran) (PDHF). The resulting PDHF undergoes quantitative depolymerization to form DHF. The mechanochemical generation of PDHF and its catalytic depolymerization reveal the potential of applying polymer mechanochemistry to address challenges in plastics sustainability.
Results and discussion
Revisiting PDHF synthesis
As five-membered cyclic olefins (such as cyclopentene and 2,3-dihydrofuran) have relatively low ring strain energies (RSE) that enable reversible polymerization, they have been leveraged recently to develop chemically recyclable polymers14,15. While successes have been achieved with cyclopentene6,14,16,17,18 and 2,3-dihydrofuran15,19,20, the ROMP of DHF has been less prolific. For example, Höcker’s attempts to polymerize DHF in bulk with a tungsten catalyst and with a chromium catalyst both failed19. Wagener reported the bulk ROMP of DHF using a molybdenum catalyst that yielded PDHF with a number-average molecular weight (Mn) >20 kDa, but the conversion was only 33%13. In addition, their acyclic diene metathesis polymerization using diallyl ether as the monomer afforded DHF and PDHF at conversions of 63% and 37%, respectively, and the latter had a Mn of 64013. Compared to cyclopentene and 2,3-dihydrofuran, the difficulty in the ROMP of DHF can be attributed to its even lower RSE (Fig. 2a; RSEs were calculated according to a previously described procedure17,21).
To gain a better understanding of the reactivity and thermodynamics in the ROMP of DHF, we tested the polymerization in bulk using commercially available ruthenium catalysts. No polymer was observed when Grubbs 1st- and 2nd-generation catalysts (G1 and G2) were used (Supplementary Fig. 1 and Supplementary Fig. 2, respectively); a conversion of 23% was observed when G3 was used as the initiator, which yielded a PDHF with Mn = 14 kDa (Fig. 2b). Our RSE calculations show that the 10-membered ring DHF dimer has a RSE of 7.0 kcal/mol (Fig. 2a), which is 3.6 kcal/mol higher than that of DHF, suggesting more favorable ROMP thermodynamics. However, the attempts to polymerize DHF dimer using G1, G2, and G3 all failed, and they only yielded DHF as the product (Fig. 2c). Note that because DHF dimer is a solid, solvent was added to dissolve it for polymerization, which diluted the concentration. It is likely that the formed PDHF depolymerized in situ to DHF due to the more dilute concentration. Taken together, these trials further highlight the low Tc and proneness to depolymerization of PDHF due to the low RSE of DHF. The challenges in accessing PDHF motivated us to pursue a mechanochemical synthesis route.
Design and synthesis of PDHF-generating mechanophore
In PDHF, every two adjacent alkenes are separated by a -CH2-O-CH2-spacer (Fig. 3a). Since the cycloreversion of cyclobutane generates two alkenes, the force-induced cycloreversion of a cyclobutane-locked -CH2-O-CH2- unit, (i.e., a cyclobutane-fused THF; Fig. 3a) will generate a structure in which the -CH2-O-CH2- spacer is connected by two alkenes (Fig. 3a). A reasonable design of the polymer that can generate PDHF upon mechanochemical activation is shown in Fig. 3b: Each repeat unit contains a cyclobutane-fused THF and two -CH2-O-CH2- spacers that connect the cyclobutane and an alkene; force-induced cycloreversion of each repeat unit would result in three repeat units of PDHF. This design of polymer corresponds to a 10-membered ring monomer, M1, which was conveniently synthesized through nucleophilic substitution reactions between diol 121 and cis−1,4-dibromo-2-butene (Fig. 3c). The use of 15-crown-5 ether was found to be essential for the successful cyclization, likely due to the increased nucleophilicity of the alkoxide as the crown ether sequesters sodium cation22. The structure of M1 was confirmed by crystallography (Supplementary Fig. 34 and Supplementary Data 1). ROMP of M1 using G2 afforded the corresponding polymer P1 with a yield of 75%; different Mn were obtained by varying the monomer-to-initiator ratio (Supplementary Table. 1). According to the 1H NMR spectrum (Fig. 4b), the cis- and trans-alkenes in P1 have distinct proton peaks in both olefinic (5.9–5.6 ppm) and allylic (4.1–3.9 ppm) regions. Integration of the peaks show a cis-to-trans ratio of 1:5.3 (Supplementary Fig. 7). Thermogravimetric analysis of P1 showed a decomposition onset temperature (the temperature at which 5% weight loss occurs) of 332 °C (Supplementary Fig. 4). The glass transition temperature (Tg) of P1, measured by differential scanning calorimetry, is −17 °C (Supplementary Fig. 4).
Mechanochemical activation
To demonstrate the mechanochemical generation of PDHF, a THF solution of P1 with a Mn of 35 kDa was subjected to ultrasonication (power density: 9.26 W cm−2; temperature: 6–9 °C)23. Fig. 4b shows the 1H NMR spectra of P1 before and after 2 h of sonication. Observation of new olefinic (e) and allylic (f) peaks in the sonicated polymer (SP1) supports the formation of PDHF. Mechanochemical activation of cyclobutane has been extensively studied, and it is well accepted that the cycloreversion proceeds through a stepwise pathway involving the formation of a diradical intermediate followed by stereochemistry-determining alkene formation9,11,12,24,25,26,27. Analysis of the peaks revealed a mixture of E and Z alkenes in which more than 95% were E form (Fig. 4b), indicating that the initial stereochemistry is predominantly conserved. The retention in stereochemistry is in drastic contrast to previous sonication studies of ester-linked cyclobutane, where significant levels of inversion in the stereochemistry were observed11,24,25. The difference can be partly attributed to the different stability of the diradical intermediates between the alkyl- and ester-linked cyclobutanes27. According to a 2021 study by Liu et al. on the mechanochemical cycloreversion of cyclobutane-fused norbornene, the more stable ester-linked intermediate has greater susceptibility to relaxation, allowing for statistical dynamics to govern the product stereochemistry27. In their study, both cis- and trans-alkyl-substituted cyclobutane showed over 95% retention in stereochemistry. Nevertheless, in our recent study on a trans-cyclobutane-fused lactone, only 33% stereoretentive product was observed, despite the alkyl linker on cyclobutane9. To eliminate the effect from having different rings fused to cyclobutane (THF vs. lactone), we synthesized a polymer P2 that contains trans-cyclobutane-fused THF in its repeat unit (for details, see synthetic section of Supplementary Information). Sonication of P2 under otherwise identical conditions showed significant inversion in stereochemistry (>38%, Supplementary Table. 2), implying the impact of substituent stereochemistry on the stereochemistry of the ring-opening products. The different trends in stereochemistry retention/inversion observed between cyclobutane-fused norbornene and our system can be attributed to the different rings connected to cyclobutane, which is consistent with our previous observation that functional groups connected to C3 and C4 (Fig. 4a) can also impact the stereochemistry of the cycloreversion products9.
To evaluate the mechanochemical reactivity of the cis-cyclobutane-fused THF, a kinetic study was performed on the sonication of P1. Polymers of two different initial Mn, 89 kDa and 58 kDa, were subjected to sonication. During sonication, aliquots were taken periodically from the reaction solution for 1H NMR and GPC analyses to monitor the process of mechanochemical activation. A reduction in molecular weight was observed with increased sonication time (Fig. 5a). At 90 min, both the 89 kDa and 58 kDa polymers reached Mn ~40 kDa. Meanwhile, the percentage of cyclobutane ring opening increased over time, and after 90 min of sonication, P1 with initial Mn of 89 kDa and 58 kDa reached ring-opening percentages of 53% and 38%, respectively (Fig. 5b). Here, the greater extent of ring opening in the 89 kDa polymer can be ascribed to a higher accessible force for the long polymer chain. In addition, for the similar initial Mn (~89 kDa), the ring opening in P1 occurred faster than those for the trans-cyclobutane-fused THF polymer P2 (Supplementary Table. 2) and the previously reported trans-cyclobutane-fused lactone polymer (Supplementary Fig. 12). The preferential reactivity of cis-cyclobutane over its trans analog is consistent with what was observed in other cyclobutane-based mechanophores and can be attributed to more efficient coupling of the force to the mechanophore in the cis configuration24,28,29,30.
A typical control experiment to validate mechanochemical activation is to sonicate a polymer with a molecular weight below the limiting Mn31. Our recent study suggests that the limiting Mn for the cyclobutane mechanophore under similar experimental conditions is ~10 kDa9. A P1 of 5 kDa was prepared and sonicated for 30 min. The 1H NMR spectrum indicated no reaction had occurred (Supplementary Fig. 10), and the polymer retention time remained unchanged (Supplementary Fig. 11), supporting the mechanical nature of the generation of PDHF. In addition, a 154 kDa P1 was subjected to sonication for 1 h, resulting in an SP1 with 70 kDa Mn and 54% PDHF. Notably, the size of PDHF in the SP1 is 38 kDa, which has not been achieved by the ROMP of DHF. This serves as another example of using polymer mechanochemistry to access chemical structures that are otherwise challenging to make32,33.
Depolymerization studies
SP1 of 44 kDa with 68% PDHF (generated from sonication of 154 kDa for 4 h), was dissolved in CDCl3 at a concentration of [olefin] = 0.06 M, and 0.8 mol% G2 was added. The reaction was allowed to proceed under 30 °C for 6 h (Fig. 6a). 1H NMR of the resulting solution clearly showed olefinic peak of DHF in the range of 5.92–5.86 ppm (Fig. 6b), and integration of the peak correspond to a 98% conversion of PDHF to DHF (Supplementary Table. 3). The efficient depolymerization of PDHF also implies that SP1 is mostly a block copolymer of P1 and PDHF, similar to what was observed in sonication studies of other multi-mechanophore polymers9,10,11,12,32,34. In contrast, when P1 was subjected to a similar condition ([olefin] = 0.025 M; 2 mol% G2), no formation of M1 was observed from the 1H NMR spectrum (Fig. 6c), indicating a relatively high Tc; the mass spectrometry analysis of the products showed a mixture of cyclic dimer, trimer, and tetramer of M1 (Supplementary Fig. 18).
A kinetic study on the depolymerization of SP1 was performed by taking aliquots at different time points for 1H NMR and GPC characterization. At 5 min, depolymerization of the PDHF segment reached 33% (Fig. 7), and the depolymerization was complete at 2 h. Compared to previous studies on polypentenamer by Tuba et al., which showed complete depolymerization within 4 min at ambient temperature35, the slower depolymerization kinetics of PDHF could be due to the weak coordination of the oxygen atoms (on the polyether and on the generated DHF) to the ruthenium catalyst center, an effect that has been observed previously in ruthenium-catalyzed olefin metathesis when solvents such as THF and diethyl ether were used36. In addition, GPC traces were found to gradually shift to a longer retention time (Supplementary Fig. 16), and Mn of the residual polymer decreased to 6.3 kDa at 2 h of depolymerization, at which point the extent of PDHF depolymerization reached 96%. From 2 h to 6 h, although PDHF block had been mostly consumed, the molecular weight of the residual polymer continued decreasing to 4.9 kDa; the further reduction in molecular weight could be due to the intramolecular secondary metathesis on the P1 block.
Bulk mechanochemical activation
While solution-phase ultrasonication is suitable for testing the reactivity of mechanophores on a laboratory scale, the use of solvents diminishes its practicality as a technique for polymer degradation and recycling. In order to make the mechanically active degradable/depolymerizable polymers a real consideration as next-generation sustainable materials, activating mechanophores in bulk materials is essential. To test the feasibility of activating the cyclobutane-fused THF mechanophore in bulk, we first conducted an extrusion experiment on P1. 200 mg of P1 (Mn = 69 kDa, Đ = 1.80) was blended with 20 g of polyethylene glycol (PEG, Mn ~ 2 kDa), and the blend was subjected to extrusion on a twin screw extruder with the processing temperature set at 70 °C. The extrusion product was precipitated in water to remove PEG. 1H NMR of the resulting product showed minimal change compared to the original polymer (Fig. 8), indicating an insignificant extent of mechanophore activation. GPC trace of the extruded product showed a reduction in molecular weight (Supplementary Fig. 22), likely due to thermomechanical degradation, which has been observed during the extrusion of polyolefins37. The collected sample was then stirred with G2 in CDCl3 overnight, and 1H NMR of the resulting product showed an olefinic peak of DHF in the range of 5.92–5.86 ppm. Integration of the peaks showed 3% DHF relative to the total olefins, corresponding to 1% ring opening of the cyclobutane-fused THF mechanophore, considering that the activation of each mechanophore generates three PDHF units (Fig. 3b). GPC trace of the G2-treated sample showed no polymer peak left (Supplementary Fig. 22), indicating that the polymer was converted into oligomers through secondary metathesis.
To further explore the activation of the cyclobutane-fused THF mechanophore in bulk materials, the mechanophore was incorporated into a polymer network PN1 by copolymerizing M1 with a bis-cyclooctene crosslinker (Fig. 9a), which gave a transparent film (Fig. 9b). The film PN1 was subjected to uniaxial compression testing, which showed Young’s modulus of 1.03 ± 0.19 MPa. The film was compressed until 90% compressive strain, and the resulting material and 2 mol% G2 catalyst were added to CDCl3, and the mixture was stirred at 30 °C for 16 h. The sol fraction of the resulting mixture showed no DHF formation on 1H NMR (Fig. 9c).
a Synthetic scheme of PN1. b Picture of PN1 thin film and the stress–strain curve obtained from compressive testing of the film (sample 1, black; sample 2, red; sample 3, blue). c Partial 1H NMR spectra for ball milled (top, red) and compressed (bottom, black) PN1 after being stirred with G2 catalyst overnight. d Partial 1H NMR spectra for P1 (top, black), SP1 (middle, blue), and hydrolyzed PN1 (bottom, red).
While ball milling has been extensively applied to mechanochemically degrade commercial polymers38,39,40,41,42, such as polystyrene, poly (ethylene terephthalate), and epoxy resin, its use in the context of mechanophore activation is rare. To test the feasibility of activating the mechanophore through ball mill grinding, the film PN1 was subjected to ball milling (25 Hz, 10 min) followed by depolymerization (2 mol% G2, CDCl3, 30 °C, 16 h). 1H NMR of the sol fraction showed a pronounced peak in the range of 5.92–5.86 ppm, indicating the formation of a significant amount of DHF (Fig. 9c). Integration of the peaks showed 6 mol% DHF compared to the original olefin in M1 (Supplementary Fig. 23). To quantify the extent of mechanophore activation, the ball milled film (prior to depolymerization) was subjected to hydrolysis in 0.1 M KOH solution in H2O/DMSO (5/95) to degrade the ester group in the crosslinker. The de-crosslinked linear polymer sample was extracted with CDCl3 and characterized with 1H NMR. As shown in Fig. 9d, both olefinic and allylic protons of PDHF were observed, and integration of the peaks showed 17% of mechanophore activation (Supplementary Fig. 26.). The 17% mechanophore activation should give ~40% DHF generation if all the PDHF units generated were converted into DHF. The substantially lower (only 6%) fraction of DHF generation could be due to the presence of the nondepolymerizable polyoctenamer units and the statistical (instead of blocky) distribution of PDHF along the polymer backbone. Nevertheless, the significant level of mechanophore activation achieved here sets a promising direction of mechanically activating and degrading this class of polymers in bulk using ball milling.
We have demonstrated the mechanochemical generation of a low-Tc polymer PDHF that can depolymerize into DHF in the presence of a ruthenium catalyst. The mechanochemical activation enabled depolymerization demonstrated here can enrich the growing toolbox for force-induced small molecule release43,44,45,46. The utility of cyclobutane in controlling depolymerization of polymers again captures the versatility of this mechanophore, as it has been used to toughen hydrogels47, access conjugated polymers32,33, regulate reactivity25, and control polymer degradation9,10,11,12. In previous works on mechanically active degradable polymers, cyclobutane was used to lock the degradable functionalities9,10,11,12; here, the function of cyclobutane is distinct in that the two alkenes generated from the cycloreversion of cyclobutane are used for olefin metathesis. In addition to the generation of olefins, cyclobutane also regulates the number of spacer atoms between the generated alkenes, which determines the size of the corresponding monomer, and in doing so, adjusts the Tc of the resulting polymer. Since the connection between the ring size of monomers and the Tc of polymers generally exists in all ring-opening polymerization systems, the strategy of mechanochemically regulating the number of spacer atoms and Tc of polymers can be applied to other types of chemically recyclable polymers48,49.
Methods
Materials
Tetrahydrofuran (THF), N,N-dimethylformamide (DMF) were obtained from Sigma-Aldrich and dried by immersing in 4 Å molecular sieves desiccant (beads, 4–8 mesh, Sigma-Aldrich) overnight before use. CDCl3 and DMSO-d6 were purchased from Cambridge Isotope Laboratories. Other solvents were purchased from Fisher Chemical and used without further purification. All other chemicals were purchased from Sigma-Aldrich or Fisher Chemical without further purification before use unless specified.
Instruments
All GPC experiments were carried out using Tosoh EcoSEC HLC-8320 GPC with two 17393 TSKgel columns (7.8 mm ID × 30 cm, 13 µm) and one 17367-TSKgel Guard Column (7.5 mm ID × 7.5 cm, 13 µm) using preservative-free HPLC grade THF (obtained from Fisher Chemical) at a flow rate of 1 mL min−1 at 40°C. Purification by preparative GPC were done by LaboACE LC-5060 which was connected to two JAIGEL-2HR columns using HPLC grade chloroform with 0.75% ethanol as preservative (obtained from Fisher Chemical) at a flow rate of 10 mL min−1. 1H and 13C NMR spectra were collected on a Varian 500 MHz spectrometer using CDCl3 or DMSO-d6 as the solvent and referenced to residual solvent peak (δ = 7.26 in 1H, 77.16 in 13C for CDCl3; δ = 2.5 in 1H, 39.52 in 13C for DMSO-d6). X-ray crystallographic study was carried out using Bruker ApexII Duo with comounted Mo and Cu microfocus radiation sources and Quazar optics for increased source brightness. ESI-MS spectra were recorded on a Waters Synapt HDMS quadrupole/time-of-flight (Q-ToF) mass spectrometer (Waters, Beverly, MA) in positive ion mode. Samples were prepared to a final concentration of 1 μg mL−1 in methanol. Each sample was introduced to the electrospray source via direct infusion at a flow rate of 5 μL/min, with source parameters as follows: capillary voltage: 3.15 kV; cone voltage: 35 V; sampling cone voltage: 3.2 V; source temperature: 90 °C; desolvation temperature: 150 °C. High resolution-atmospheric pressure chemical ionization mass spectra were acquired using a Waters Synapt HDMS Q-ToF Mass Spectrometer (Waters, Beverly, MA) equipped with an atmospheric solids analyses probe in positive ion mode.
Calculations of RSEs
The RSEs of the cyclic olefins were calculated by following a method demonstrated by Grubbs and coworkers17. This method involves the calculation of the enthalpy change for the ring-closing metathesis of the acyclic diene that forms the cyclic olefin and ethylene. The ring-closing metathesis is an isodesmic reaction—the numbers and types of bonds of the reactant are identical to those of the products. Since there is no ring strain in the acyclic diene reactant or the ethylene products, the enthalpy change of the reaction should originate in the ring strain of the cyclic olefin (Eq. (1)).
Hethene, Hcyclic olefin, and Hdiene are the calculated enthalpies at 298.15 K and 1.0 atm for ethene, the cyclic olefin, and the diene, respectively. The structures were first optimized with the Universal Force Field as implemented in the auto-optimization tool in Avogadro, followed by a conformer search with Avogadro. The optimized conformation was subjected to geometry optimization and vibrational frequency calculations at the level of B3LYP/6-31 G(d,p) using the Gaussian 16 revision C.01 software.
Polymerization
P1 of different molecular weights were made by ROMP of M1. The following is an example of the procedure: To a vial was added M1 (100 mg, 0.48 mmol, 1 equiv.) and G2 (0.4 mg, 0.000476 mmol, 0.001 equiv., in 480 μL of DCM stock solution). The reaction was gently stirred for 3 h, and excess amount of ethyl vinyl ether (150 μL) was added and stirred for 30 min to terminate the polymerization. The solvent was then removed on a rotavaper and subjected to prepGPC for further purification to yield transparent viscous oil, which was dried under vacuum overnight to afford 75 mg of polymer P1. (75 mg, yield: 75%, Mn = 154 kDa, Đ = 1.97)
Sonication
Sonication experiments were conducted using a Vibracell model VCX500 (Sonics & Materials) with a standard solid probe (tip diameter = 13 mm, titanium alloy Ti-6Al-4V) operated at 20 kHz. 1 mg/1 mL THF solution of P1 was made and transferred to a reaction vessel (made by U Akron Glassblowing Shop) and was then deoxygenated by bubbling through N2 for 30 min. The solution was sonicated under N2 using a pulse sequence 1 s on/1 s off at an energy density of 9.3 W/cm2 (AMPL = 25%). The temperature of the solution was maintained by placing the vessel in an ice/water bath. Ring-opening percentage and molecular weight changes were tracked by 1H NMR and GPC, respectively.
Depolymerization
The sonicated sample SP1 (33 mg, %RO = 68%) was dried and reacted with 0.8 mol% of G2 (2.7 mg, 0.0031 mmol, 0.008 equiv., in 6.3 mL of degassed CDCl3 stock solution) to olefin (0.06 M, 1.0 equiv) under the protection of N2 for 6 h at 30 °C. Aliquots were taken out at different reaction periods, and excess amount of ethyl vinyl ether (100 μL) was added and left standing for 30 min before 1HNMR and GPC analysis.
Data availability
Crystallographic data for the structures in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition no. 2179611 (M1). All other data supporting the findings of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Tsuda, M., Hata, M., Nishida, R. & Oikawa, S. Acid-catalyzed degradation mechanism of poly(phthalaldehyde): unzipping reaction of chemical amplification resist. J. Polym. Sci., Part A: Polym. Chem. 35, 77–89 (1997).
Kaitz, J. A., Diesendruck, C. E. & Moore, J. S. End group characterization of poly(phthalaldehyde): surprising discovery of a reversible, cationic macrocyclization mechanism. J. Am. Chem. Soc. 135, 12755–12761 (2013).
Diesendruck, C. E. et al. Mechanically triggered heterolytic unzipping of a low-ceiling-temperature polymer. Nat. Chem. 6, 623–628 (2014).
Wang, F. & Diesendruck, C. E. Polyphthalaldehyde: synthesis, derivatives, and applications. Macromol. Rapid. Commun. 39, 1700519 (2018).
Feinberg, A. M. et al. Triggered transience of plastic materials by a single electron transfer mechanism. ACS Cent. Sci. 6, 266–273 (2020).
Myers, S. B. & Register, R. A. Synthesis of narrow-distribution polycyclopentene using a ruthenium ring-opening metathesis initiator. Polymer 49, 877–882 (2008).
Tang, X. et al. The quest for converting biorenewable bifunctional α-methylene-γ-butyrolactone into degradable and recyclable polyester: controlling vinyl-addition/ring-opening/cross-linking pathways. J. Am. Chem. Soc. 138, 14326–14337 (2016).
Neary, W. J. & Kennemur, J. G. Variable temperature romp: leveraging low ring strain thermodynamics to achieve well-defined polypentenamers. Macromolecules 50, 4935–4941 (2017).
Hsu, T.-G. et al. A polymer with “locked” degradability: superior backbone stability and accessible degradability enabled by mechanophore installation. J. Am. Chem. Soc. 142, 2100–2104 (2020).
Lin, Y., Kouznetsova, T. B., Chang, C.-C. & Craig, S. L. Enhanced polymer mechanical degradation through mechanochemically unveiled lactonization. Nat. Commun. 11, 4987 (2020).
Lin, Y., Kouznetsova, T. B. & Craig, S. L. Mechanically gated degradable polymers. J. Am. Chem. Soc. 142, 2105–2109 (2020).
Yang, J. & Xia, Y. Mechanochemical generation of acid-degradable poly(enol ether)s. Chem. Sci. 12, 4389–4394 (2021).
Wagener, K. B., Brzezinska, K. & Bauch, C. G. Acyclic diene metathesis (admet) polymerization. The preparation of sym -unsaturated polyethers by the use of mo(ch-t-bu)(n-2,6-c6h3-i-pr2)[occh3(cf3)2]2 catalyst. Makromol. Chem. Rapid Commun. 13, (1992).
Neary, W. J., Isais, T. A. & Kennemur, J. G. Depolymerization of bottlebrush polypentenamers and their macromolecular metamorphosis. J. Am. Chem. Soc. 141, 14220–14229 (2019).
Feist, J. D. & Xia, Y. Enol ethers are effective monomers for ring-opening metathesis polymerization: synthesis of degradable and depolymerizable poly(2,3-dihydrofuran). J. Am. Chem. Soc. 142, 1186–1189 (2020).
Trzaska, S. T., Lee, L.-B. W. & Register, R. A. Synthesis of narrow-distribution “perfect” polyethylene and its block copolymers by polymerization of cyclopentene. Macromolecules 33, 9215–9221 (2000).
Hejl, A., Scherman, O. A. & Grubbs, R. H. Ring-opening metathesis polymerization of functionalized low-strain monomers with ruthenium-based catalysts. Macromolecules 38, 7214–7218 (2005).
Neary, W. J. & Kennemur, J. G. Polypentenamer renaissance: challenges and opportunities. ACS Macro Lett. 8, 46–56 (2019).
Thu, C. T., Bastelberger, T. & Höcker, H. On the polymerization of 2,3-dihydrofuran with tungsten and chromium carbenes. Makromol. Chem. Rapid Commun. 2, 383–386 (1981).
Feist, J. D., Lee, D. C. & Xia, Y. A versatile approach for the synthesis of degradable polymers via controlled ring-opening metathesis copolymerization. Nat. Chem. 14, 53–58 (2022).
Sathe, D. et al. Olefin metathesis–based chemically recyclable polymers enabled by fused-ring monomers. Nat. Chem. 13, 743–750 (2021).
Aspinall, H. C., Greeves, N., Lee, W.-M., McIver, E. G. & Smith, P. M. An improved williamson etherification of hindered alcohols promoted by 15-crown-5 and sodium hydride. Tetrahedron Lett. 38, 4679–4682 (1997).
May, P. A. & Moore, J. S. Polymer mechanochemistry: techniques to generate molecular force via elongational flows. Chem. Soc. Rev. 42, 7497–7506 (2013).
Kean, Z. S., Niu, Z., Hewage, G. B., Rheingold, A. L. & Craig, S. L. Stress-responsive polymers containing cyclobutane core mechanophores: Reactivity and mechanistic insights. J. Am. Chem. Soc. 135, 13598–13604 (2013).
Wang, J., Kouznetsova, T. B., Boulatov, R. & Craig, S. L. Mechanical gating of a mechanochemical reaction cascade. Nat. Commun. 7, 13433 (2016).
Chen, Z. et al. The cascade unzipping of ladderane reveals dynamic effects in mechanochemistry. Nat. Chem. 12, 302–309 (2020).
Liu, Y. et al. Flyby reaction trajectories: chemical dynamics under extrinsic force. Science 373, 208–212 (2021).
Kryger, M. J., Munaretto, A. M. & Moore, J. S. Structure-mechanochemical activity relationships for cyclobutane mechanophores. J. Am. Chem. Soc. 133, 18992–18998 (2011).
Zhang, H. et al. Multi-modal mechanophores based on cinnamate dimers. Nat. Commun. 8, 1147 (2017).
Klein, I. M., Husic, C. C., Kovacs, D. P., Choquette, N. J. & Robb, M. J. Validation of the cogef method as a predictive tool for polymer mechanochemistry. J. Am. Chem. Soc. 142, 16364–16381 (2020).
Caruso, M. M. et al. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798 (2009).
Chen, Z. et al. Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene. Science 357, 475–479 (2017).
Boswell, B. R. et al. Mechanochemical synthesis of an elusive fluorinated polyacetylene. Nat. Chem. 13, 41–46 (2021).
Black Ramirez, A. L. et al. Microstructure of copolymers formed by the reagentless, mechanochemical remodeling of homopolymers via pulsed ultrasound. ACS Macro Lett. 1, 23–27 (2012).
Tuba, R. et al. Synthesis of recyclable tire additives via equilibrium ring-opening metathesis polymerization. ACS Sustain. Chem. Eng. 4, 6090–6094 (2016).
Sanford, M. S., Love, J. A. & Grubbs, R. H. Mechanism and activity of ruthenium olefin metathesis catalysts. J. Am. Chem. Soc. 123, 6543–6554 (2001).
Hinsken, H., Moss, S., Pauquet, J.-R. & Zweifel, H. Degradation of polyolefins during melt processing. Polym. Degrad. Stab. 34, 279–293 (1991).
Pas, T. et al. Mechanodegradation of polymers: a limiting factor of mechanochemical activation in the production of amorphous solid dispersions by cryomilling. Mol. Pharmaceutics 17, 2987–2999 (2020).
Peterson, G. I., Ko, W., Hwang, Y.-J. & Choi, T.-L. Mechanochemical degradation of amorphous polymers with ball-mill grinding: Influence of the glass transition temperature. Macromolecules 53, 7795–7802 (2020).
Jimenez-Francisco, M., Carrillo, J. G. & Garcia-Cerda, L. A. Mechanochemical tuning of molecular weight distribution of styrene homopolymers as postpolymerization modification in solvent-free solid-state. J. Appl. Polym. Sci. 138, 49628 (2021).
Kang, P., Yang, S., Bai, S. & Wang, Q. Novel application of mechanochemistry in waste epoxy recycling via solid-state shear milling. ACS Sustain. Chem. Eng. 9, 11778–11789 (2021).
Štrukil, V. Highly efficient solid-state hydrolysis of waste polyethylene terephthalate by mechanochemical milling and vapor-assisted aging. ChemSusChem 14, 330–338 (2021).
Larsen, M. B. & Boydston, A. J. “Flex-activated” mechanophores: using polymer mechanochemistry to direct bond bending activation. J. Am. Chem. Soc. 135, 8189–8192 (2013).
Hu, X., Zeng, T., Husic, C. C. & Robb, M. J. Mechanically triggered small molecule release from a masked furfuryl carbonate. J. Am. Chem. Soc. 141, 15018–15023 (2019).
Shi, Z., Wu, J., Song, Q., Göstl, R. & Herrmann, A. Toward drug release using polymer mechanochemical disulfide scission. J. Am. Chem. Soc. 142, 14725–14732 (2020).
Versaw, B. A., Zeng, T., Hu, X. & Robb, M. J. Harnessing the power of force: development of mechanophores for molecular release. J. Am. Chem. Soc. 143, 21461–21473 (2021).
Wang, Z. et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 374, 193–196 (2021).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Shi, C. et al. Hybrid monomer design for unifying conflicting polymerizability, recyclability, and performance properties. Chem 7, 670–685 (2021).
Acknowledgements
This material is based on work supported by the University of Akron and the National Science Foundation under grant no. CHE-2204079. T.-G.H. acknowledges Sigma Xi Grants-in-Aid of Research (G03152021120132966) for financial support. The authors thank Kayla Williams Pavlantos and Prof. Chrys Wesdemiotis for mass spectrometry analysis. The single-crystal structures were characterized with an X-ray diffractometer supported by the National Science Foundation (CHE-0840446 to C.J.Z.). The computational resources were provided by Extreme Science and Engineering Discovery Environment (TG-CHE220003). The authors thank The Ohio Board of Regents and The National Science Foundation (CHE-0341701 and DMR-0414599) for the funds used to purchase the NMR instrument used in this work.
Author information
Authors and Affiliations
Contributions
J.W. conceived the project and directed the research. H.C. and J.W. performed the density functional theory calculations and analyzed the computational data. T.-G.H., S.L., G.X., J.Z., J.R., J.S., and R.M. conducted the monomer and polymer syntheses. W.-Y.C., S.G., and C.J.Z. collected and analyzed the single-crystal data. S.Y., Z.W., and T.-G.H. conducted the thermal characterization of the polymers. T.-G.H. conducted solution-phase polymer mechanochemical activation and depolymerization. Z.W., L.W. S.Y., and T.-G.H. conducted bulk mechanochemical activation and depolymerization studies. T.-G.H. and J.W. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Robert Göst and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsu, TG., Liu, S., Guan, X. et al. Mechanochemically accessing a challenging-to-synthesize depolymerizable polymer. Nat Commun 14, 225 (2023). https://doi.org/10.1038/s41467-023-35925-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-35925-2
This article is cited by
-
Leveraging mechanochemistry for sustainable polymer degradation
Polymer Journal (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.