Abstract
In hybrid organisms, genetically divergent homologous chromosomes pair and recombine during meiosis; however, the effect of specific types of polymorphisms on crossover is poorly understood. Here, to analyze this in Arabidopsis, we develop the seed-typing method that enables the massively parallel fine-mapping of crossovers by sequencing. We show that structural variants, observed in one of the generated intervals, do not change crossover frequency unless they are located directly within crossover hotspots. Both natural and Cas9-induced deletions result in lower hotspot activity but are not compensated by increases in immediately adjacent hotspots. To examine the effect of single nucleotide polymorphisms on crossover formation, we analyze hotspot activity in mismatch detection-deficient msh2 mutants. Surprisingly, polymorphic hotspots show reduced activity in msh2. In lines where only the hotspot-containing interval is heterozygous, crossover numbers increase above those in the inbred (homozygous). We conclude that MSH2 shapes crossover distribution by stimulating hotspot activity at polymorphic regions.
Similar content being viewed by others
Introduction
Crossover (CO) recombination plays a key mechanical role during the first meiotic division by ensuring proper chromosome segregation1,2. Together with the independent assortment of homologous chromosomes, COs are also the main source of genetic diversity in the population and are largely responsible for shaping adaptability to a changing environment3,4,5. In this context, the relationship between genetic diversity and meiotic recombination is particularly important. Despite many years of research, how CO distribution is shaped by DNA interhomolog polymorphisms remains poorly understood.
Meiotic recombination starts with the formation of programmed DNA double-strand breaks (DSBs)6. Following end resection, one DNA strand invades the homolog and is extended by DNA polymerase, forming a displacement loop. Multiple rounds of strand invasion, extension, and displacement often occur at this stage and create complex recombination intermediates7,8. Many of these intermediates are dissociated by DNA helicases and are repaired via synthesis-dependent strand annealing (SDSA). SDSA invariably leads to a noncrossover (NCO), where only a very short patch of DNA has been modified. Alternatively, second-end capture can form a double Holiday junction (dHJ), which is resolved via recombination pathways involving nucleases leading to either COs or NCOs2,9,10.
Usually, only a small fraction of meiotic DSBs is repaired by COs (5–10% in Arabidopsis thaliana), with most being repaired as NCOs. At least two recombination pathways can lead to crossover in most eukaryotes, including plants. The first pathway, called the ZMM pathway, is responsible for the formation of about 85% of all COs, which are referred to as Class I COs. Class I COs exhibit genetic interference, whereby the distance between two CO events on the same chromosome is greater than that expected from a random distribution. The frequency and placement of Class I COs are controlled by the abundance of the pro-crossover factor HEI10 and the synaptonemal complex (SC), a tripartite structure that forms between paired homologous chromosomes in prophase I11,12,13,14,15,16. The remaining crossovers, so-called Class II COs, show no interference and are largely dependent on the structure-specific nuclease MUS8117,18.
COs usually occur in narrow chromosomal regions called CO hotspots19,20,21,22,23. There are few studies on hotspots in plants, and new technical approaches would bring the field forward24. However, plant hotspots are known to be primarily located in gene promoters and terminators and are associated with active chromatin modifications, including low nucleosome density, the histone variant H2A.Z, tri-methylation at lysine 4 of histone H3 (H3K4me3), and low DNA methylation20,25. In line with these observations, CO hotpots are much less common in pericentromeric regions than along chromosome arms26. Importantly, pericentromeres tend to show a much higher level of polymorphisms, as manifested by both the presence of structural variants and a higher density of single nucleotide polymorphisms (SNPs)27,28,29.
Structural variation, including insertions, deletions, and loss of similarity, have been associated with local suppression of COs30,31,32. Sequence polymorphisms between homologous chromosomes may also result in base pair mismatches within recombination intermediates, which affects recombination outcomes33. The detection of such mismatches during meiotic recombination is often dependent on MutS-related heterodimers that contain MSH234,35,36,37,38,39. MSH2 heterodimers bind mismatches to prevent post-replicative mutations, while they often trigger heteroduplex rejection during recombination40,41,42,43. Interestingly, while the mitotic functions of MSH2 in heteroduplex rejection and correction of mismatches in heteroduplex DNA are conserved across eukaryotes, the role(s) of MSH2 in meiotic recombination may be different. For example, mismatches within recombination hotspots result in strand rejection and NCO repair in budding yeast34,35. However, loss of MSH2 function does not substantially change the meiotic CO frequency in mouse hotspots in crosses between strains exhibiting 0.6 and 1.8% sequence polymorphisms44. In Arabidopsis hybrids, CO midpoints are positively associated with interhomolog SNPs at the kilobase (kb) scale, and this association is significantly weakened in the msh2 background39. These observations suggest that MSH2 heterodimers may exert different functions in meiotic recombination. At a genomic scale, a positive correlation was observed between historical recombination as measured via linkage disequilibrium and sequence diversity in many species45,46,47,48,49. In addition, the pattern of heterozygosity along the chromosome determines CO distribution. In such a genetic context, wild-type Arabidopsis shifts COs from homozygous to heterozygous regions on a chromosome. This homozygosity–heterozygosity juxtaposition effect is interference dependent and disappears in the msh2 mutant background39,50. On the other hand, it has recently been reported that the megabase-scale crossover landscape is largely independent of sequence divergence, with recombination distributions in Arabidopsis pure lines being very similar to those of intra-specific hybrids51. This supports the notion that much of the association between CO rates and SNPs is due to the mutagenic effects of recombination rather than a pro-crossover influence of SNPs51.
Here, we develop the seed-typing method to study Arabidopsis crossovers at the hotspot scale. Seed-typing relies on the selection and targeted sequencing of recombinants within short genetic intervals. One of the intervals we developed, called Chili Pepper (ChP), includes three well-separated recombination hotspots. We observe that local structural polymorphisms have limited impact on a crossover, though deletions within the strongest hotspot cause a dramatic drop in CO rates. Based on seed-typing, we do not find evidence for a short-distance hotspot competition. Within a hotspot, crossovers tend to occur in regions devoid of polymorphism. However, we observe that hotspots with higher overall SNP levels show a reduction in CO activity in the msh2 mutant when compared to the wild type. In recombinant lines where only the ChP interval is heterozygous while the remainder of the genome is homozygous, ChP CO frequency is significantly higher than in fully homozygous inbreds. We do not observe these results in the msh2 mutant. We propose that MSH2 complexes detecting local polymorphisms stimulate Class I COs at the hotspot scale.
Results
Construction of extremely short interval lines applicable to seed-typing
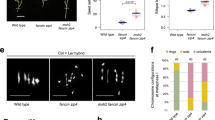
We developed a system for precise CO mapping that enables robust analysis of recombination at the hotspot scale. This method is based on extremely short interval lines (ESILs), which carry fluorescent reporters expressed in seeds, separated by no more than 50 kb. The fluorescently tagged intervals provide both precise CO frequency measurements11,50,52,53,54,55,56 and an easy assay for recombinants based on seed fluorescence. We used a recently developed large-scale collection of fluorescent reporter lines in Arabidopsis57 to construct five ESILs by crossing the selected single-color reporter lines in the Col-0 background (Supplementary Fig. 1). The intervals are located on the upper arm of chromosome 3 near the telomere (BB), interstitially forming adjacent intervals (Bow Tie [BT], TG, and BF), or in the pericentromeric region (ChP) (Fig. 1a and Supplementary Table 1).
a Col × Ler CO map26 for chromosome 3 with the location of the five ESILs marked in red. CO frequency (green) was plotted in 20-kb windows. The positions of pericentromeres and the genetically defined centromere are shown with light-green and blue rectangles, respectively. b CO frequency in the five ESILs, as measured by fluorescent seed scoring in inbreds (blue) and hybrids (red). The center line of a boxplot indicates the mean; the bounds indicate the 75th and 25th percentiles. Each dot represents measurements from one individual. The number of individuals are indicated above the boxplots. Two-sided P values indicate the statistical difference in CO rates in hybrids versus inbreds (Welch’s t-test). c Diagram of seed-typing. Crossing the Col-ESIL to a different accession (e.g., Ler) results in F1 hybrids. Segregation of fluorescent reporters in seeds obtained from F1 plants enables both CO frequency measurements in the interval (right panel) and the selection of single-color recombinant seeds, which are grown to isolate genomic DNA. Libraries for sequencing from all recombinants are prepared based on the amplicons obtained after LR-PCR. The CO landscape is determined by CO sites identified based on SNPs. d Selection of recombinants from Col-ChP × Ler F2 seeds based on fluorescence. Scale bars, 2 mm. Arrows indicate the location of --/-R (top) and G-/-- (bottom) seeds corresponding to Ler/Ler→Col/Ler and Col/Ler→Ler/Ler genotypes, respectively. e Genotypes of 243 Col-ChP × Ler recombinants. Each horizontal line corresponds to one recombinant with color-encoded genotype. The x-axis indicates coordinates within ChP. f CO landscape within ChP for a Col-ChP × Ler cross obtained by seed-typing. COs (blue) are normalized to the ChP CO frequency measured by seed scoring. SNPs spaced at least 100 bp apart, shown as vertical gray lines, were used to determine the CO topology. Black and red x-axis ticks correspond to all Col/Ler SNPs and InDels, respectively. Genes are shown as light-green (forward) and dark-green (reverse) rectangles, and transposons are shown as blue rectangles. Positions of three CO hotspots are indicated in colored rectangles below the plot. Source data are provided as a Source Data file.
To assess the applicability of the ESILs for fine-scale CO analysis, we used a published ultra-high-density CO map created for a cross between the Arabidopsis Col-0 and Ler−0 accessions26. To this end, we plotted the CO map for chromosome 3 with a window size of 20 kb and superimposed the location of the intervals from the five ESILs (Fig. 1a). Notably, the ChP interval coincided with one of the three most highly recombining sites on chromosome 3 out of 1169 windows (Fig. 1a). Next, we crossed the ESILs to the Arabidopsis accessions Col-0, Ct-1, and Ler−0 (hereafter Col, Ct, and Ler, respectively) to measure the CO frequency in the nearly isogenic lines, whose parents differ only in the presence of fluorescent reporters (for the sake of simplicity, we will call them inbreds) and in the hybrids, whose parents differ along the entire genome (Fig. 1b and Supplementary Table 1). In inbreds, all intervals showed a mean CO frequency of 3.5 cM/Mb or greater. The exception was TG, with a very low CO frequency. By contrast, the pericentromeric ChP interval exhibited the highest CO frequency (mean of 9.09 cM/Mb) (Fig. 1b). Measurements in hybrid contexts returned values similar to those observed in their respective inbreds, which was consistent with previous observations that the presence of polymorphisms does not inhibit COs (Fig. 1b)39,50,51. Again, the most interesting interval was ChP, which had a substantially higher CO frequency in hybrids (18.22 cM/Mb for Col-ChP × Ler and 14.52 cM/Mb for Col-ChP × Ct) relative to the genome average (3.2 cM/Mb for Col × Ler58).
Determination of CO hotspots in the highly polymorphic ChP interval
Due to its pericentromeric location, the ChP interval exhibited the highest density of Col × Ler interhomolog polymorphisms (18.7 polymorphisms/kb; Supplementary Table 2). Together with its very high CO frequency, this high degree of polymorphisms makes ChP an excellent system for the high-resolution mapping of CO events. To determine the positions of potential recombination hotspots within the ChP interval, we developed the seed-typing method, where F2 recombinant seeds from hybrid plants are selected based on the expression of a single fluorescent reporter indicating a CO event (Fig. 1c, d). Genomic DNA is isolated from seedlings obtained from preselected recombinants and used to amplify the entire interval by high-fidelity long-range PCR (LR-PCR; Fig. 1c) using three overlapping amplicons. We pooled the LR-PCR products for each recombinant and used them for library preparation using unique barcodes. We then pooled the libraries and sequenced them to a depth of ~1500×. For 81.5% recombinants (243 out of 298), we successfully and precisely determined the location of COs, defined as a transition between parental/heterozygous genotypes, based on the SNPs distinguishing Col and Ler sequences (Fig. 1e and Supplementary Fig. 2). By superimposing the location of all identified COs, we obtained a high-resolution map for ChP (Fig. 1f).
We identified 242 COs that are unevenly distributed across the 26.3-kb region, revealing the presence of three clearly separated hotspots, which we named Aro (8.8 kb), Coco (4.6 kb), and Nala (3.4 kb) (Fig. 1e, f and Supplementary Table 3). These hotspots were located within three of the four short genes present in ChP and differed in their recombination frequency: Aro accounted for 32% of the identified CO events, 60% for Coco, and only 8% for Nala. The activity of each hotspot can be determined by relating these values to the measurement of the CO frequency for the entire interval, which is performed by seed scoring (Fig. 1b, c). Each hotspot showed a CO frequency substantially higher than the genome average, with Coco reaching up to 62.14 cM/Mb (Supplementary Table 3).
Although ChP is highly polymorphic, COs occurred at places with relatively few polymorphisms. Hotspots did not show an increased number of DSBs relative to the rest of the interval (Fig. 2). We analyzed the profiles of various chromatin modifications over the ChP interval and observed that recombination hotspots are associated with an open chromatin structure, high histone acetylation, and the presence of H2A.Z (Fig. 2). Notably, the Coco hotspot showed high levels of H3K4me2, but not H3K4me3 (Fig. 2).
Histograms showing normalized coverage values for SPO11-1-oligonucleotides85, nucleosome occupancy (MNase-seq)86, H2A.Z86, H4K5ac78, H3K4me1/me387, H3K4me288, and DNA methylation (BSseq) in CG, CHG, and CHH sequence contexts89 were presented in relation to ChP CO landscape for Col-ChP × Ler. Source data are provided as a Source Data file.
Influence of structural polymorphisms on CO frequency within ChP
To assess the CO rate and enable recombinant selection, the eGFP and dsRed 3.2-kb reporter cassettes in the Col-ChP line are required in the hemizygous state. Genetically, in this state, they can be considered kilobase insertions. Since ChP is only 26.3 kb, the CO frequency in this interval may potentially be influenced by the presence of these two insertions. To test this possibility, we tried to construct a pseudoreporter line in which the reporter cassettes were preserved but produced nonfunctional fluorescent proteins. Crossing the Col-ChP line to such a Col-pseudo-ChP (Col-ΨChP) line would allow the measurement of recombination (with the Col-ChP fluorescent reporters) and quantify the effect of the T-DNA insertion when compared to Col-ChP × Col cross. To generate a Col-ΨChP line, we simultaneously targeted the dsRed and eGFP coding sequences by Cas9-mediated gene editing in the Col-ChP background and selected nonfluorescent seeds in successive generations of transformants having segregated out the CRISPR-Cas9 construct (Fig. 3a). In most cases, the loss of fluorescence was due to cassette silencing, which made them unsuitable for further experiments. However, we obtained one line in which the dsRed cassette carried a frameshift deletion while the eGFP cassette was largely removed (Supplementary Fig. 3). The cross of this Col-ΨChP line with Col-ChP only showed a slight decrease in ChP recombination (Fig. 3b), indicating that the high CO frequency in ChP was not due to the insertion of the dsRed cassette. Moreover, the ChP CO measurements for Col-ChP × Ler are consistent with the recombination estimates for this interval according to the Col × Ler F2 map26 (18.2 cM/Mb and 22.7 cM/Mb respectively; see also Fig. 1a, b). We concluded that DNA insertions have a limited effect on recombination within the interval.
a Generation of Col-pseudo-ChP (Col-ΨChP). Cas9 sgRNAs were used to simultaneously generate mutations in the dsRed and eGFP cassettes, leading to the loss of fluorescence. Nonfluorescent seeds were sequenced and crossed with Col-ChP to measure CO frequency. b CO frequency for Col × Col-ChP and Col × Col-ΨChP. Measurements for Col-ChP × Ler are shown for comparison. The center line of a boxplot indicates the mean; the upper and lower bounds indicate the 75th and 25th percentiles, respectively. Each dot represents a measurement from one individual. The number of individuals are also indicated above the boxplots. The two-sided P value was estimated by Welch’s t-test. c Alignments of the ChP region between different Arabidopsis accessions. The blue horizontal bar shows the query sequence (Col), red horizontal bars indicate homologous sequences in other accessions, colored vertical tick marks indicate short regions of lower similarity, and gray lines indicate nonhomologous regions. Insertion of an 8.7-kb fragment next to the ChP interval in Ler is also indicated. d CO frequency (in cM) for different crosses between Col-ChP and the accessions shown in (c). The center line of a boxplot indicates the mean; the upper and lower bounds indicate the 75th and 25th percentiles, respectively. Each dot represents a measurement from one or two pooled individuals. The numbers of individuals are indicated on the right from the boxplots. Two-sided P values indicate the statistical difference in ChP CO rate in hybrids versus inbreds (Welch’s t-test). Green triangles indicate the polymorphism level (SNPs+InDels) for each cross within the ChP interval. Source data are provided as a Source Data file.
The chromosomal region containing the ChP interval is structurally very polymorphic between different Arabidopsis accessions. To investigate how these differences might affect recombination frequency in ChP, we used accessions whose genomes were recently resequenced with high-quality chromosome-level assemblies59. Two of these accessions were structurally similar to Col over the ChP region, while four other accessions had a long highly diverged region of more than 7 kb located upstream of ChP (Fig. 3c and Supplementary Fig. 4). Moreover, the Ler accession also carried a large 8.7-kb diverged region downstream from the interval (Fig. 3c and Supplementary Fig. 4). Except for C24, all other hybrids obtained from a cross with Col-ChP had a significantly higher CO frequency than their corresponding inbred (Fig. 3d). Surprisingly, we noticed that large structural changes located on one (Sha, Cvi, and Kyo) or both (Ler) sides of the ChP interval did not result in a significantly different ChP CO frequency compared to accessions lacking these changes (An, Eri) (Welch’s t-test values indicated on Fig. 3d). Collectively, the data from crosses involving the pseudoreporter line and structurally diverged accessions show that even large structural alterations involving several kilobases do not have a significant effect on COs within ChP if they do not overlap with CO hotspots.
CO hotspots are highly conserved between Arabidopsis diverged accessions and show no competition
The presence of three distinct CO hotspots within the ChP interval makes it an ideal model for testing competition between hotspots and the conservation of the recombination landscape between Arabidopsis accessions. From the crosses analyzed by seed scoring, only Col-ChP × C24 showed a CO frequency that was not statistically different from the inbreds (mean 8.73 vs. 8.54, respectively, Fig. 3d). Sequence analysis showed that C24 carries a deletion of 1194 bp at the position overlapping the Coco hotspot (Fig. 3c). Using Col-ChP × C24 seed-typing, we measured a significant drop in the number of CO events within Coco (Fig. 4a, b and Supplementary Fig. 5). Although we observed a dramatic decrease in the CO number in the immediate vicinity of the deletion, we detected recombination events in the genomic neighborhood, indicating that the Coco hotspot still retains some of its activity. A CO landscape comparison between Col-ChP × C24 and Col-ChP × Ler revealed a nearly identical location for all three hotspots (Fig. 4a, b and Supplementary Fig. 5). Importantly, the CO activity of Aro and Nala, the two hotspots located on either side of Coco, did not increase due to lower Coco activity (Fig. 4c). This observation suggests that there is no competition between immediately adjacent hotspots; however, this conclusion may be obscured by variability in recombination between Ler and C24, e.g., the existence of trans-acting CO modifiers. To eliminate this possibility, we created de novo deletions within the Coco region in the Ler accession using a pair of single-guide RNAs (sgRNAs) to delete a central, hyperactive part of Coco. We obtained two independent lines with deletions of 714 bp (LerΔ#24) and 825 bp (LerΔ#76), respectively, which we then crossed to the Col-ChP line (Fig. 4d and Supplementary Fig. 6). Both crosses showed an approximately two-fold decrease in CO frequency compared to the Col-ChP × Ler control (Fig. 4e). Seed-typing of Col-ChP × LerΔ#24 revealed a dramatic reduction in CO events in the region neighboring the deletion, while we observed no other changes in CO topology (Fig. 4f, g). In addition, a calculation of hotspot activity showed no alteration in hotspot usage (Fig. 4h). This result confirms that Arabidopsis recombination hotspots operate independently of each other and that there is no short-distance hotspot competition.
a CO landscape within ChP for the Col-ChP × C24 (green) and Col-ChP × Ler (blue). COs are normalized to the measured ChP CO frequency. SNPs spaced at least 100 bp apart, shown as vertical gray lines, were used to determine the CO topology. Top and bottom x-axis tick marks correspond to Col/C24 and Col/Ler SNPs, respectively, while red tick marks correspond to InDels. Deletion within Coco is indicated by the pink rectangle. b Genotypes of 177 Col-ChP × C24 recombinants. Each horizontal line corresponds to one recombinant with color-encoded genotype. The x-axis indicates coordinates within ChP. c Hotspot activity for Col-ChP × C24 and Col-ChP × Ler. d Alignments of the ChP region between Col, C24, Ler, and LerΔ#24 and LerΔ#76 deletion lines. The blue horizontal bar shows the query sequence (Col); red horizontal bars indicate homologous sequences in other lines. Colored vertical tick marks indicate short regions of lower similarity, and gray lines indicate nonhomologous regions. Insertion of the 8.7-kb fragment next to the ChP interval in Ler, LerΔ#24, and LerΔ#76 is not shown for simplicity. e CO frequency for crosses between Col-ChP and the lines indicated in d. The center line of a boxplot indicates the mean; the upper and lower bounds indicate the 75th and 25th percentiles, respectively. Each dot represents a measurement from one individual. The numbers of individuals are also indicted above the boxplots. The two-sided P values were estimated by Welch’s t-test. f ChP CO landscape in Col-ChP × LerΔ#24 (red) and Col-ChP × Ler (blue). COs were normalized to the measured ChP CO frequency. SNPs spaced at least 100 bp apart, shown as vertical gray lines, were used to determine the CO topology. The black and red x-axis ticks correspond to all Col/Ler SNPs and InDels, respectively. The deletion LerΔ#24 is indicated by a pink rectangle. g Genotypes of 208 Col-ChP × LerΔ#24 recombinants. Each horizontal line corresponds to one recombinant with color-encoded genotype. h Hotspot activity for Col-ChP × LerΔ#24 and Col-ChP × Ler. Source data are provided as a Source Data file.
Loss of MSH2 function results in an increase or decrease of the CO rate depending on chromosomal location
As SNP-associated CO remodeling depends on MSH239, we checked how genetically inactivating MSH2 would affect recombination in the ChP and BT intervals, the latter being interstitial and with a lower SNP density than ChP (3.58 vs. 14.20 SNPs/kb; Fig. 1a, Supplementary Fig. 7, and Supplementary Table 2). We thus backcrossed the msh2-2 mutation to the Col-ChP line and measured CO frequency in the Col-ChPmsh2 × Colmsh2 inbred. CO frequency was identical to that in the wild type with functional MSH2 (Fig. 5a). We then used Cas9-mediated gene editing to introduce a null mutation at MSH2 in the Ler background (Supplementary Figs. 8, 9) and obtained the Col-ChPmsh2 × Lermsh2 hybrid. This msh2 hybrid showed a significantly lower CO rate than the corresponding wild-type hybrid (Col-ChP × Ler) but comparable to the rate observed in the inbred (Fig. 5a). Next, we investigated the BT CO rate in msh2 inbreds and hybrids (Fig. 5b). Similar to msh2 ChP, msh2 BT inbreds showed no change from the wild type. However, contrary to the Col-ChPmsh2 × Lermsh2 hybrid, the Col-BTmsh2 × Lermsh2 displayed a significantly higher CO frequency than its wild-type counterpart. These results suggest that MSH2-dependent changes in recombination levels observed in ChP and BT hybrids are affected by CO redistribution at the chromosome-scale and not the local effect of polymorphism.
a CO frequency for Col-ChP × Col inbreds and Col-ChP × Ler hybrids in the wild-type, msh2, and HEI10 overexpression (HEI10-OE) genetic contexts. The center line of a boxplot indicates the mean; the upper and lower bounds indicate the 75th and 25th percentiles, respectively. Each dot represents a measurement from one individual. The numbers of individuals are also indicated below the boxplots. The two-sided P values were estimated by Welch’s t-test. The chromosome 3 ideogram with the location of the ChP interval in relation to the pericentromere (gray box) is shown to the right. b As in a, but for the BT interval (Col-BT × Col inbreds and Col-BT × Ler hybrids). c Comparison of the CO landscape within the ChP interval for the Col-ChP × Ler wild type (blue) and Col-ChPmsh2 × Lermsh2 (red). COs are normalized to the measured ChP CO frequency. SNPs spaced at least 100 bp apart, shown as vertical gray lines, were used to determine the CO topology. The black and red x-axis ticks correspond to all Col/Ler SNPs and InDels, respectively. d Genotypes of 188 Col-ChPmsh2 × Lermsh2 recombinants. Each horizontal line corresponds to one recombinant with color-encoded genotype. The x-axis indicates coordinates within the ChP interval. e Relationship between SNP density and CO hotspot activity. Col-ChP × Ler CO hotspot activity for the msh2 mutant is represented as the proportion of the wild-type hotspot activity (blue bars). SNP density per each hotspot is shown as an orange line. Source data are provided as a Source Data file.
As the pro-CO function of MSH2 is related to the ZMM pathway, we also used a line overexpressing HEI10 (C2 line), in which the activity of the ZMM pathway is increased11,39,50. We observed that HEI10 overexpression does not cause a change in ChP CO rates, in the inbred or in the hybrid context, when compared to the wild type (Fig. 5a). However, when we repeated this experiment for the BT interval, we observed an increase in CO frequency for both the inbreds and hybrids (Fig. 5b). These results suggest that the increase in CO activity via the ZMM pathway triggered by HEI10 overexpression is not dependent on interhomolog polymorphisms but is dependent on the chromosomal location (e.g., centromere-proximity effect).
MSH2 stimulates crossovers in hotspots in response to local polymorphism
If the loss of MSH2 function causes a change in CO in hybrids but not in inbreds, does the activity of individual hotspots also change? To answer this question, we investigated the CO landscape in Col-ChPmsh2 × Lermsh2. The resulting CO profile was similar to that of the wild type (Fig. 5c, d). Although we observed fewer CO events throughout the ChP interval, all three hotspots were active in the msh2 background (Fig. 5c). However, the activity of the Aro hotspot showed the largest percentage decrease in the msh2 background compared to the wild-type background (34.5% activity of the wild type) (Fig. 5e). In turn, the activity of Nala dropped only to 70.8% of its wild-type levels (Fig. 5e). Considering that Aro and Nala are the most and the least polymorphic hotspots in ChP, respectively (Supplementary Table 3), we conclude that MSH2-dependent CO stimulation in polymorphic regions can be observed at the scale of individual hotspots.
Next, we determined the precise CO sites for 185 and 196 recombinants from the Col-BT × Ler and Col-BTmsh2 × Lermsh2 crosses, respectively. For this purpose, we developed seed-typing for the BT interval using five overlapping LR-PCR amplicons. The BT CO landscape was very different from that of the ChP interval, as it included many hotspots close to each other (Fig. 6a–c). Due to the much lower SNP density and high gene density, individual hotspots overlapped in SNP-SNP sections, as approximated by H2A.Z peaks, making it impossible to clearly distinguish between them (Fig. 7 and Supplementary Fig. 10). Therefore, we divided the BT interval into 13 nonoverlapping sections of 2.6–5.2 kb each based on the pattern of polymorphisms and the occurrence of H2A.Z peaks (Fig. 6d and Supplementary Table 4). We excluded three sections from the analysis because they contained no CO events. We then calculated the Col/Ler polymorphism density and msh2 crossover activity relative to the wild type for each section. As with ChP, we also observed a clear relationship for BT between the level of polymorphism and the change in CO activity in msh2 (Fig. 6e). In the highly polymorphic sections 11 and 9, the msh2 mutant showed fewer CO events than the wild type, whereas the mutant had more COs in sections containing fewer than eight SNPs/kb. Interestingly, in section 10, with only 0.6 SNP/kb, the number of CO events was more than four times higher in msh2 than in the wild type, suggesting that without functional mismatch detection, CO events mostly occur in polymorphism-free hotspots. The relationship between the change in msh2 recombination frequency versus the wild type and the polymorphism density was statistically significant (two-sided P value = 2.37 × 10–4, Spearman rho = –0.912). The results for ChP and BT show that MSH2 increases the chance of CO in hotspots surrounded by more SNPs. Importantly, this effect is independent of whether there is an increase (ChP) or a decrease (BT) in CO frequency for a given chromosomal interval in the background defective in mismatch recognition (Fig. 5a, b).
a CO landscape within the BT interval for the Col-BT × Ler wild type (blue) and Col-BTmsh2 × Lermsh2 (red). COs were normalized to the measured BT CO frequency. SNPs spaced at least 400 bp apart, shown as vertical gray lines, were used to determine the CO topology. The black and red x-axis ticks correspond to all Col/Ler SNPs and InDels, respectively. Gene models are shown by green (forward) or purple (reverse) lines above the plot. b Genotypes of 185 Col-BT × Ler recombinants. Each horizontal line corresponds to one recombinant with color-encoded genotype. The x-axes indicate coordinates within the BT interval. c As in b, but for 196 Col-BTmsh2 × Lermsh2 recombinants. d Col-BT × Ler COs (blue; cM/Mb), Col-BTmsh2 × Lermsh2 COs (red; cM/Mb), and polymorphism density (green; SNPs + indels/kb) plotted in 13 sections of the BT interval. COs were normalized to BT CO frequency measured for the wild type and msh2. The separation into 13 sections (numbers) based on polymorphism and CO pattern is indicated by vertical dashed lines. e Relationship between SNP density and CO activity. Col-BT × Ler CO activity for msh2 is represented as the proportion of the wild-type activity for the sections shown in d (blue bars). SNP density per section, is shown as an orange line. Sections #7, #12, and #13 were excluded from the analysis as they did not have COs in the wild type and/or msh2. The Spearman’s rank correlation coefficient (rho) between SNP density and relative crossover activity with the two-sided P value is printed inset. Source data are provided as a Source Data file.
Histograms showing normalized coverage values for SPO11−1-oligonucleotides85, nucleosome occupancy (MNase-seq)86, H2A.Z86, H4K5ac78, H3K4me1/me387, H3K4me288, and DNA methylation (BSseq) in CG, CHG, and CHH sequence contexts89 were presented in relation to BT CO landscape for Col-BT × Ler. Source data are provided as a Source Data file.
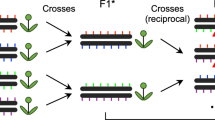
Our comparison of the recombination for ChP and BT in the wild type and msh2 was based on the analysis of F1 individuals that are polymorphic along the entire genome. Consequently, CO measurements reflect both the local and chromosomal-scale effects of polymorphisms. To investigate only the direct influence of interhomolog polymorphisms on CO formation in the ChP interval, we took advantage of a unique feature of our system that allows the use of recombinants in downstream analyses via successive generations (Fig. 1c). For seed-typing, we sampled 3-week-old plants, which allows recombinants to further grow and produce offspring. Based on the identified CO sites, we selected Col-ChPmsh2 × Lermsh2 recombinants with green or red fluorescence and crossed them (Fig. 8a). From their offspring, we selected F2 seeds, where a new CO event occurred between fluorescent reporters, thus bringing the two reporters onto the same chromosome, with an intervening Ler fragment in between them. We then backcrossed these lines (3×) to the Col accession. As a result, we obtained R2 (Recombinant × Recombinant) lines in which only selected fragments of ChP carried Col/Ler polymorphisms, while the remaining genome was homozygous Col (Fig. 8b and Supplementary Tables 5, 6). Importantly, all R2 lines that carried Col/Ler polymorphisms across the entire interval (R2-1; ~240 SNPs) showed ChP CO frequencies significantly higher than both ChP inbreds and hybrids (Welch’s t-test; P ≤ 1.8 × 10–2) (Fig. 8c). Plants heterozygous only within the Coco hotspot (R2-2 lines; ≥44 SNPs) also had a significantly higher ChP CO rate than pure inbreds (P ≤ 3.6 × 10–2). The siblings of R2-1 and R2-2 plants in the msh2 background exhibited a CO rate that was not different from that of the msh2 inbred or hybrid (Fig. 8d). This result indicates that MSH2 promotes crossovers in a single hotspot based on locally occurring interhomolog polymorphisms.
a Diagram illustrating the isolation of the R2 lines. Double green (G-/G-) and double red (-R/-R) seeds were selected from the progeny of sequenced recombinants and crossed (F1). Recombinant GR/-R F2 seeds were visually selected and backcrossed to wild-type or msh2 plants; at each generation, GR/-- seeds were selected. The BC3 plants were used to measure ChP CO frequency. b Polymorphism pattern for different R2 crosses. The chromosome carrying fluorescent reporters (green and red arrows) contains a segment inherited from the Ler parent (gray). The Col-ChP × Ler F1 cross is shown for comparison. Hotspot positions are indicated in rectangles. c ChP CO frequency in R2 lines. Each dataset corresponds to an independent R2 line; The numbers below each bar indicate the number of SNPs differentiating homologous chromosomes. WG whole-genome. The center line of a boxplot indicates the mean; the upper and lower bounds indicate the 75th and 25th percentiles, respectively. Each dot represents one individual. The two-sided P values were estimated by Welch’s t-test. d As in c, but for the siblings of R2 lines in msh2. e ChP crossover frequency in Col/Col inbreds, Col/Ler hybrids, lines Col/Ler heterozygous (HET) in ChP while Col/Col homozygous (HOM) elsewhere, and lines Ler/Ler homozygous in ChP while Col/Ler heterozygous elsewhere, in either wild type or msh2. A boxplot is defined as in c. Data for different R2-1 from c and d were presented together. The two-sided P values were estimated by Welch’s t-test. f, g Model showing the effect of MSH2 on Arabidopsis crossovers. f At the chromosome-scale, COs are stimulated in polymorphic regions, close to the centromere (Cen). This crossover redistribution is specific for Class I COs and thus also depends on interference (blue arrow). Blue bars represent the local SNP density. g At the kilobase scale, hotspots located in SNP-rich regions are more active than neighboring hotspots at less polymorphic sites due to MSH2-dependent stimulation (blue arrows). However, COs occur mostly at polymorphism-free sites within each hotspot (orange arrows). Source data are provided as a Source Data file.
By crossing the R2-1 lines to Ler, we obtained plants, where only the ChP interval were Ler/Ler homozygous while the remainder of the genome was Col/Ler heterozygous (Fig. 8b). Interestingly, ChP CO frequencies were significantly lower in these plants than in ChP hybrids (Welch’s t-test; P = 4.5 × 10–3; Fig. 8e). This effect disappeared in siblings carrying the msh2 mutations (Fig. 8e). Hence, ChP shows a clear heterozygosity-homozygosity juxtaposition effect: When ChP is heterozygous in a fully homozygous genome, it shows an increase in CO frequency compared to hybrids and inbreds, while a decrease in CO frequency is observed when homozygous ChP is contrasted against the otherwise heterozygous genome (Fig. 8e and Supplementary Fig. 11).
In general, our study demonstrates that although crossovers tend to occur in hotspot centers that are devoid of polymorphism, SNPs in the immediate vicinity of hotspots are detected by MSH2, which stimulates the CO activity of these hotspots.
Discussion
Here, we describe the seed-typing method for investigating the CO distribution with unprecedented resolution in two intervals located in the interstitial and pericentromeric regions of the chromosome. Particularly noteworthy is that we identified the pericentromeric interval, ChP, as one of the hottest regions in the entire genome based on the high-density CO map generated for the Col × Ler cross (Fig. 1a)26. ChP is also the first pericentromeric region in Arabidopsis to be explored for high-resolution CO topology. ChP contains three gene-located hotspots that are clearly separated from each other (Fig. 1f). The central hotspot, Coco, had a recombination rate above 60 cM/kb and, to the best of our knowledge, is hotter than any other hotspot described in Arabidopsis to date60,61,62,63,64,65. A chromatin landscape analysis showed that Coco coincided with a region enriched for H3K4me2 but not H3K4me3 marks and that it was located within a long noncoding RNA gene. However, it remains unclear what makes this hotspot so active.
Structural rearrangements such as large insertions and deletions are often associated with local CO suppression26,30,31,32. However, this study dissects the influence of such big structural variations adjacent to meiotic hotspots on recombination. Surprisingly, we determined that a variation of <7 kb surrounding the hotspots did not significantly affect their activity (Fig. 3). By contrast, we showed that deletions within a hotspot cause its partial inactivation (Fig. 3). This result was similar to the observation made for the mouse A3 hotspot, where the presence of three closely spaced small deletions define the CO refractory zone32. These results suggest that structural changes affect the frequency of recombination only when they are located within recombination hotspots. However, to what extent this is a universal phenomenon for all CO hotspots remains to be explored.
The ChP interval allowed us to investigate competition between hotspots, a phenomenon that has been reported in budding yeast, fission yeast, and mammals66,67,68,69,70. In Arabidopsis, silencing of the CO hotspot 3a via RNA-dependent DNA methylation (RdDM) results in a lack of compensatory effect in the neighboring hotspot 3b63. Similarly, the silencing of 3b does not affect the activity of 3a63. However, RdDM-mediated silencing induces chromatin changes that may have secondary consequences for hotspot activity and should therefore be interpreted with caution. Our results based on the inactivation of the hotspot by sgRNA-induced deletions support the lack of competition between immediate adjacent hotspots in Arabidopsis (Fig. 4).
Although the entire ChP interval is characterized by a high density of polymorphisms (18.7 InDels and SNPs per kilobase), COs mainly occurred in short sections ranging in length from several dozen to several hundred base pairs, which are almost identical between the two parents (Fig. 1f). This feature may be universal, as it was previously reported for other Arabidopsis hotspots and in other species such as mice32,64,65. Analysis of CO distribution in the Col-ChP × C24 cross with a slightly different polymorphism pattern revealed that the CO sites shifted to new, conserved (that is, lacking polymorphisms) chromosome sections, albeit within the same hotspots (Fig. 4a). Two important conclusions can be drawn from these observations: (1) The location of hotspots is independent of the presence of SNPs and (2) a chromosome section with a very limited number of interhomolog polymorphisms is required to efficiently form COs. The first of these conclusions is not surprising given previous reports indicating that the main determinants of CO sites in plants are open chromatin structure, active chromatin marks, and lack of DNA methylation20,51,71,72. However, the second conclusion is somehow unexpected given that Arabidopsis COs have been shown to have a local preference for heterozygous regions when juxtaposed to homozygous regions39,50.
The fact that the detection of interhomolog polymorphisms during meiotic recombination depends on the mismatch detection system, the key element of which is the MSH2 protein39, helped explain this phenomenon. Using the msh2 mutant, we examined CO repair without polymorphisms (inbreds) and with polymorphisms (hybrids) for the centromere-proximal ChP interval and the interstitial BT interval. The effect of the msh2 mutation on CO frequency was different between the two intervals (Fig. 5a, b), likely reflecting a CO redistribution along the chromosome in msh2, which is consistent with our previous findings39. Seed-typing revealed that hotspots in regions with a lower SNP density were used more frequently in msh2 than in the wild type (Figs. 5c–e, 6). This observation indicates that MSH2 complexes actively stimulate CO repair in polymorphic hotspots.
To differentiate the polymorphism effect from potential chromosome-scale effects, we used a set of R2 lines that carry heterozygous fragments only within ChP while the rest of the genome is homozygous. Surprisingly, we observed that the presence of polymorphisms in a single Coco hotspot (≤3.3 kb, 44 SNPs) was sufficient to stimulate COs over the entire ChP interval (26.3 kb; Fig. 8c). This result is similar to the effect described earlier in which the juxtaposition of heterozygous and homozygous regions is associated with increased CO frequency in heterozygous regions at the expense of neighboring homozygous regions (Supplementary Fig. 11)39,50. Significantly increased recombination was also reported in heterozygous regions of maize plants selfed for six generations73. Our results show that the heterozygosity/homozygosity juxtaposition effect is reflected at the scale of a single hotspot. Importantly, these data offer a mechanical insight into this effect and provide an explanation for the apparent inconsistency with the observed preference of COs to occur in nonpolymorphic intervals65. We observed that CO breakpoints invariably occur in the SNP-free sections of the hotspot in both the wild type and the msh2 mutant. However, in the wild type, CO repair takes place more often in a hotspot whose immediate vicinity is SNP-rich. We propose that MSH2 heterodimers bind mismatches, forming heteroduplexes that then recruit MLH1 complexes to induce a second cut, which triggers a CO repair (Fig. 8e–g). Other scenarios are also possible, including MSH2 heterodimers regulating branch migration74 or stabilizing D-loops. Furthermore, interference causes significant CO redistribution at the chromosome-scale, as visualized by comparing wild-type and msh2 CO patterns39 (Fig. 8e).
As many as 20% of all plants are estimated to be predominantly selfing, including the model plant Arabidopsis and important monocot and eudicot crops like wheat, rice, and tomato75,76. Heterozygous regions in principally homozygous genomes that emerge from occasional outcrossing followed by many rounds of inbreeding are the only available sources of variation, both at the single individual and population scales77. Perhaps this is why meiotic recombination is targeted to heterozygous regions in selfing plants, as now evidenced in Arabidopsis. Our analysis shows how MSH2-dependent detection of interhomolog polymorphism shapes the chromosomal CO pattern at the scale of single recombination hotspots. This feature may be of crucial importance in the evolution of self-pollinating plant species.
Methods
Plant material
Traffic lines used to obtain ESILs were created by ref. 57. Arabidopsis accessions (Ler-0, An-1, Eri-1, Sha, Kyo, C24, Cvi-0, and Ct-1) and the msh2 T-DNA insertion line (SALK_002708) was obtained from the Nottingham Arabidopsis Stock Centre (NASC).
CRISPR-Cas9 mutagenesis
CRISPR-Cas9 technique was used to generate desirable genome modifications in ChP and in Ler. It was based on designing a pair of sgRNAs to induce a more efficient mutagenesis78,79. Constructs carrying cassettes with sgRNAs cloned under AtU3C or AtU6-26 promoters and Cas9 under ICU2 promoter were introduced to plants via Agrobacterium-mediated transformation. Constructs used for the transformation of Ler plants additionally contained a seed-expressed dsRed reporter marker for easier preselection of transformants78,79. Transformants were genotyped for the desired modifications and in the next generation construct-free plants with the heritable modifications were sequenced to confirm the length and the exact positions of the generated deletions.
In order to generate a deletion within the Coco hotspot in Ler, a pair of sgRNAs (Coco_del1: 5′-gcctttgtgatttagtggcag-3′; Coco_del2: 5′-ccggttaaaagttttaagac-3′) were designed to target cleavages at desired positions within the highest peak of recombination. To obtain a knock-out msh2 mutant in Ler, a pair of sgRNAs (msh2_1: 5′-agaacatgtaccgcagggat-3′; msh2_2: 5′-gcacctagagcaggagtcgca-3′) were designed to target the fourth exon of MSH2. The mutant was verified by RT-PCR amplification of the MSH2 transcript from wild-type and mutant plants. Furthermore, the RT-PCR products from the msh2-6 mutant was Sanger sequenced to confirm that the deletion produces a premature stop codon. CRISPR-Cas9 was also used to create pseudoreporter lines of ChP. To inactivate the fluorescence of the reporter cassettes, two pairs of sgRNAs were designed to target both dsRed (dsRed_1a: 5′- atcctgcaaactggaatcct-3′; dsRed_1b: 5′- aggattccagtttgcagga-3′ and eGFP (GFP_1a: 5′-aagttcagcgtgtccggcg-3′; GFP_1b: 5′-ggcgagggcgatgccaccta-3′) coding sequences.
Generation of extremely short interval lines
To obtain extremely short interval lines (ESILs), two lines carrying coding sequences of the seed-expressed fluorescent transgenes (eGFP and dsRed) at the defined regions57 were chosen and crossed with each other (Supplementary Table 1). The F1 plants were selfed and the F2 seeds were harvested. Seeds obtained from gametes in which a CO occurred between the reporters were preselected using epifluorescent stereomicroscope Lumar version 12 (Zeiss) equipped with dsRed and green fluorescent protein filters. As a result of CO, those seeds show the homozygous state of one reporter and a hemizygous state of the second one (GR/-R or GR/G-). The presence of the fluorescent cassettes in the plants grown from preselected seeds were confirmed by genotyping using two primers flanking the reporter cassette mixed with the primer binding to the eGFP or dsRed coding sequences (Supplementary Table 7). If the lines are devoid of the particular cassette, the amplicons are obtained only with the left and right primers, and are between 300-600 bp. The F2 plants were selfed and in the next generation, the homozygous ESILs were preselected under the epifluorescent stereomicroscope.
Measurement of crossovers using a seed-based system
The recombination within the obtained ESILs was measured using a seed-based system, which was already described50,80. First, the homozygous ESILs were crossed with the non-color accessions and the pictures of F2 seeds, derived from the entire plant, were acquired using an epifluorescent stereomicroscope (Zeiss). Each set of seed pictures included pictures in brightfield, and ultraviolet through filters for red and green fluorescence. The number of seeds was counted using CellProfiler program according to the protocol80. Due to low recombination rates within ESILs, the number of single-color recombinants was detected manually. To increase the accuracy of crossover measurements for such short intervals, all seeds from each plant were analyzed, which gives an average measurement of ~3000–5000 seeds per each biological replicate. The recombination frequency was presented in cM according to the formula: RF = 100 × ((NG + NR)/NT), where NG is the number of only green seeds, NR is the number of only red seeds, and NT is the total number of seeds derived from the entire plant. Raw seed scoring data for all the measurements are presented in Supplementary Data 1.
Preselection of recombinants based on the fluorescence
Recombinants, those seeds that have experienced the crossover between two reporter genes, were preselected manually based on the fluorescence intensity of the seeds. As a result of crossover, the recombinant seeds present single-color fluorescence (-R/-- or G-/--) or they are homozygous for one reporter and hemizygous for the second one (GR/-R or GR/G-).
Seed-typing
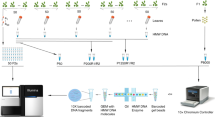
DNA was extracted from the leaves of recombinant plants grown from preselected recombinant seeds. DNA extraction was performed as described in ref. 58. To provide only non-degraded, high-quality DNA for further steps of the seed-typing method, the extracted DNA was additionally purified using AMPure XP magnetic beads (Beckman-Coulter). The extracted DNA was mixed in a 1:1 ratio with magnetic beads and incubated for 5 min at room temperature. After the incubation, the samples were placed on the magnetic stand and incubated for 5 min. The supernatant was discarded and the beads were washed twice with 80% ethanol. DNA was eluted in the same volume of 10 mM Tris (pH 8.0) as the starting volume of the DNA. The quality of the DNA was checked in 1% agarose gel. Such prepared DNA was used as a template for long-range PCR (LR-PCR).
In LR-PCR, the whole ChP (26.3 kb) interval was amplified in three reactions (due to deletion at the 5′-end in Ler and C24 when compared to Col, it was necessary to use different forward primers, therefore the interval in crosses with those accessions was amplified in four reactions) resulting in amplicons of 9–11 kb long. BT (49.1 kb) was amplified in five reactions with amplicons 10–11 kb long. The sequences of the primers used for the amplifications are described in Supplementary Table 7. LR-PCR was performed using PrimeSTAR GXL Polymerase (TaKaRa Bio, Shiga, Japan). The PCR conditions were as followed: 0.2-10 ng of template DNA, 1.2 µL of 2.5 mmol/L primers, 3 µL buffer, 1.2 µL dNTP, 0.3 µL Polymerase, and distilled water to 15 µL, 30 cycles 98 °C 10 s, 68 °C 12 min. The efficiency of the amplification was verified in 1% agarose gel. Next, 10 µL of each PCR product amplified using the same DNA sample were pooled together. The DNA was purified (Clean-Up Concentrator, A&A Biotechnology) and eluted in 20 µL of 10 mM Tris (pH 8.0).
For library preparation, tagmentation was performed by mixing 1 µL of the pooled, purified PCR products with 1 µL of Tagmentation Buffer (40 mM Tris-HCl pH = 7.5, 40 mM MgCl2), 0.5 µL of DMF (Sigma), 2.35 µL of Nuclease-free water (Thermo Fisher) and 0.15 µL of loaded, in-house produced Tn5 (Tn5 Transposase was prepared according to the protocol81). Loading Tn5 with the annealed linker oligonucleotides was followed54. The tagmentation reaction was incubated at 55 °C for 2 min and then stopped by adding 1 µL 0.1% SDS and incubation at 65 °C for 10 min. Amplification of the tagmented DNA was performed using the KAPA2G Robust PCR kit (Sigma) and custom P5 and P7 indexing primers. Each sample was amplified with the unique set of P5 and P7 primers as described in ref. 26. Subsequently, the libraries were visualized in a 2% agarose gel to group samples into pools of the comparable intensity of sheared DNA fragments between 450–700 bp. Each pool contained the same number of libraries. The concentrated libraries (Clean-Up Concentrator, A&A Biotechnology) were size selected in 2% agarose gel, performing electrophoresis for 2 h. Subsequently, DNA fragments of the desired size were excised and extracted from the gel using Gel Extraction Kit. The quality and quantity of the libraries were verified using TapeStation system (Agilent) and Qubit 2.0 fluorometer. Paired-end sequencing of 576 libraries per lane was performed on HiSeq X−10 instrument (Illumina).
To identify SNPs within the ChP or BT intervals between Col and other Arabidopsis accessions (Ler, C24), demultiplexed paired-end forward and reverse reads have been pooled and aligned to Col reference sequence with the use of bwa-mem algorithm from Burrows-Wheeler Aligner (BWA) software82. The Resulting BAM files have been sorted and indexed with the use of SAMtools v1.283. SNPs were called using SAMtools and BCFtools84. Subsequently, SNPs with low coverage (<30 reads) and poor quality have been filtered out from the list. Individual sequencing libraries (243 samples from F2 Ler × Col-ChP population, 177 samples from F2 C24 × Col-ChP population, 209 samples from the F2 Col-ChP × LerΔ#24 population, 187 samples from F2 Lermsh2 × Col-ChPmsh2 population, 160 samples from F2 Ler × Col-BT population, 196 samples from F2 Lermsh2 × Col-BTmsh2 population) have been aligned to Col-0 ChP or BT sequences with the use of bwa-mem algorithm with default parameters and compared to previously generated SNP list with SAMtools and BCFtools. Characteristics of sequencing results for libraries in which COs were identified are shown in Supplementary Table 8. Custom R script enabled comparison of the percentage of reads associated with reference (Col) or variant nucleotides in Ler and C24 accessions. This has further allowed for genotype determination at particular SNP location and manual designation of CO breakpoint.
Estimation of heterozygosity level in R2 lines
To estimate the heterozygosity level in R2 lines, genomic DNA from two randomly picked individuals representing each R2 line was extracted as described (see Seed-typing) and used as a template to prepare libraries for sequencing (see Seed-typing). Paired-end sequencing was performed on the HiSeq X-10 instrument (Illumina). Demultiplexed raw data from paired-end sequencing have been aligned to the Col-0 reference. SNPs were called using SAMtools and BCFtools and compared to the list of SNPs from Col × Ler. Based on the results, sizes of Col/Ler heterozygous blocks for each chromosome were deduced. The A. thaliana genome size estimate of 125 Mb was used to calculate percent heterozygosity (Supplementary Table 6).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The seed-typing sequence data generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject accession PRJNA882919. The Col-0 TAIR10 reference genome is downloaded from the TAIR database [https://www.arabidopsis.org/]. Genomic sequences of Arabidopsis thaliana accessions (An-1, C24, Cvi-0, Eri-1, Kyo, Ler, Sha) used in this study can be downloaded from https://1001genomes.org/projects/MPIPZJiao2020/index.html]. Col × Ler F2 high-density crossover data were downloaded from FigShare [https://doi.org/10.25386/genetics.9733838]. Seed scoring raw data were provided in Supplementary Data 1. Source data are provided with this paper.
Code availability
The related code is available at GitHub [https://github.com/LabGenBiol/ESILs].
References
Hunter, N. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7, a016618 (2015).
Mercier, R., Mézard, C., Jenczewski, E., Macaisne, N. & Grelon, M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 66, 297–327 (2015).
Dapper, A. L. & Payseur, B. A. Connecting theory and data to understand recombination rate evolution. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160469 (2017).
Stapley, J., Feulner, P. G. D., Johnston, S. E., Santure, A. W. & Smadja, C. M. Variation in recombination frequency and distribution across eukaryotes: patterns and processes. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160455 (2017).
Veller, C., Kleckner, N. & Nowak, M. A. A rigorous measure of genome-wide genetic shuffling that takes into account crossover positions and Mendel’s second law. Proc. Natl Acad. Sci. USA 116, 1659–1668 (2019).
Keeney, S. & Neale, M. J. Initiation of meiotic recombination by formation of DNA double-strand breaks: mechanism and regulation. Biochem. Soc. Trans. 34, 523–525 (2006).
Marsolier-Kergoat, M.-C., Khan, M. M., Schott, J., Zhu, X. & Llorente, B. Mechanistic view and genetic control of DNA recombination during meiosis. Mol. Cell 70, 9–20.e6 (2018).
Ahuja, J. S., Harvey, C. S., Wheeler, D. L. & Lichten, M. Repeated strand invasion and extensive branch migration are hallmarks of meiotic recombination. Mol. Cell 81, 4258–4270.e4 (2021).
Wang, Y. & Copenhaver, G. P. Meiotic recombination: mixing it up in plants. Annu. Rev. Plant Biol. 69, 13.1–13.33 (2018).
Pyatnitskaya, A., Borde, V. & De Muyt, A. Crossing and zipping: molecular duties of the ZMM proteins in meiosis. Chromosoma 128, 181–198 (2019).
Ziolkowski, P. A. et al. Natural variation and dosage of the HEI10 meiotic E3 ligase control Arabidopsis crossover recombination. Genes Dev. 31, 306–317 (2017).
Serra, H. et al. Massive crossover elevation via combination of HEI10 and recq4a recq4b during Arabidopsis meiosis. Proc. Natl Acad. Sci. USA 115, 2437–2442 (2018).
France, M. G. et al. ZYP1 is required for obligate cross-over formation and cross-over interference in Arabidopsis. Proc. Natl Acad. Sci. USA 118, 1–11 (2021).
Capilla-Pérez, L. et al. The synaptonemal complex imposes crossover interference and heterochiasmy in Arabidopsis. Proc. Natl Acad. Sci. USA 118, 1–11 (2021).
Morgan, C. et al. Diffusion-mediated HEI10 coarsening can explain meiotic crossover positioning in Arabidopsis. Nat. Commun. 12, 4674 (2021).
Durand, S. et al. Joint control of meiotic crossover patterning by the synaptonemal complex and HEI10 dosage. Nat. Commun. 13, 5999 (2022).
Berchowitz, L. E., Francis, K. E., Bey, A. L. & Copenhaver, G. P. The role of AtMUS81 in interference-insensitive crossovers in A. thaliana. PLoS Genet. 3, e132 (2007).
Higgins, J. D., Buckling, E. F., Franklin, F. C. H. & Jones, G. H. Expression and functional analysis of AtMUS81 in Arabidopsis meiosis reveals a role in the second pathway of crossing-over. Plant J. 54, 152–162 (2008).
Myers, S., Bottolo, L., Freeman, C., McVean, G. & Donnelly, P. A fine-scale map of recombination rates and hotspots across the human genome. Science 310, 321–324 (2005).
Choi, K. et al. Arabidopsis meiotic crossover hot spots overlap with H2A.Z nucleosomes at gene promoters. Nat. Genet. 45, 1327–1336 (2013).
Pan, J. et al. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144, 719–731 (2011).
Smagulova, F. et al. Genome-wide analysis reveals novel molecular features of mouse recombination hotspots. Nature 472, 375–378 (2011).
Pratto, F. et al. Recombination initiation maps of individual human genomes. Science 346, 826–835 (2014).
Choi, K. & Henderson, I. R. Meiotic recombination hotspots—a comparative view. Plant J. 83, 52–61 (2015).
Kianian, P. M. A. et al. High-resolution crossover mapping reveals similarities and differences of male and female recombination in maize. Nat. Commun. 9, 2370 (2018).
Rowan, B. A. et al. An ultra high-density Arabidopsis thaliana crossover. Genetics 213, 771–787 (2019).
Ziolkowski, P. A., Koczyk, G., Galganski, L. & Sadowski, J. Genome sequence comparison of Col and Ler lines reveals the dynamic nature of Arabidopsis chromosomes. Nucleic Acids Res. 37, 3189–3201 (2009).
Cao, J. et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 43, 956–963 (2011).
Zapata, L. et al. Chromosome-level assembly of Arabidopsis thaliana L er reveals the extent of translocation and inversion polymorphisms. Proc. Natl Acad. Sci. USA 113, E4052–E4060 (2016).
Dooner, H. K. Genetic fine structure of the BRONZE locus in maize. Genetics 113, 1021–1036 (1986).
Jeffreys, A. J. & Neumann, R. Factors influencing recombination frequency and distribution in a human meiotic crossover hotspot. Hum. Mol. Genet. 14, 2277–2287 (2005).
Cole, F., Keeney, S. & Jasin, M. Comprehensive, fine-scale dissection of homologous recombination outcomes at a hot spot in mouse meiosis. Mol. Cell 39, 700–710 (2010).
Ziolkowski, P. A. & Henderson, I. R. Interconnections between meiotic recombination and sequence polymorphism in plant genomes. N. Phytol. 213, 1022–1029 (2017).
Borts, R. H. & Haber, J. E. Meiotic recombination in yeast: alteration by multiple heterozygosities. Science 237, 1459–1465 (1987).
Chen, W. & Jinks-Robertson, S. The role of the mismatch repair machinery in regulating mitotic and meiotic recombination between diverged sequences in yeast. Genetics 151, 1299–1313 (1999).
Cooper, T. J. et al. Mismatch repair disturbs meiotic class I crossover control. Preprint at bioRxiv https://doi.org/10.1101/480418 (2021).
Elliott, B. & Jasin, M. Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol. Cell. Biol. 21, 2671–2682 (2001).
Emmanuel, E., Yehuda, E., Melamed-Bessudo, C., Avivi-Ragolsky, N. & Levy, A. A. The role of AtMSH2 in homologous recombination in Arabidopsis thaliana. EMBO Rep. 7, 100–105 (2006).
Blackwell, A. R. et al. MSH2 shapes the meiotic crossover landscape in relation to interhomolog polymorphism in Arabidopsis. EMBO J. 39, e104858 (2020).
Borts, R. H., Chambers, S. R. & Abdullah, M. F. The many faces of mismatch repair in meiosis. Mutat. Res. 451, 129–150 (2000).
Spies, M. & Fishel, R. Mismatch repair during homologous and homeologous recombination. Cold Spring Harb. Perspect. Biol. 7, a022657 (2015).
Tham, K.-C., Kanaar, R. & Lebbink, J. H. G. Mismatch repair and homeologous recombination. DNA Repair . 38, 75–83 (2016).
Myung, K., Datta, A., Chen, C. & Kolodner, R. D. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 27, 113–116 (2001).
Peterson, S. E., Scott, K. & Jasin, M. Mechanistic insight into crossing over during mouse meiosis. Mol. Cell 78, 1252–1263.e3 (2020).
Begun, D. J. & Aquadro, C. F. Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356, 519–520 (1992).
Nordborg, M. et al. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3, e196 (2005).
Spencer, C. C. A. et al. The influence of recombination on human genetic diversity. PLoS Genet. 2, e148 (2006).
Gore, M. A. et al. A first-generation haplotype map of maize. Science 326, 1115–1117 (2009).
Cutter, A. D. & Payseur, B. A. Genomic signatures of selection at linked sites: unifying the disparity among species. Nat. Rev. Genet. 14, 262–274 (2013).
Ziolkowski, P. A. et al. Juxtaposition of heterozygous and homozygous regions causes reciprocal crossover remodelling via interference during Arabidopsis meiosis. Elife 4, e03708 (2015).
Lian, Q. et al. The megabase-scale crossover landscape is largely independent of sequence divergence. Nat. Commun. 13, 3828 (2022).
Melamed-Bessudo, C., Yehuda, E., Stuitje, A. R. & Levy, A. A. A new seed-based assay for meiotic recombination in Arabidopsis thaliana. Plant J. 43, 458–466 (2005).
Lawrence, E. J. et al. Natural variation in TBP-ASSOCIATED FACTOR 4b controls meiotic crossover and germline transcription in Arabidopsis. Curr. Biol. https://doi.org/10.1016/j.cub.2019.06.084 (2019).
Zhu, L. et al. Natural variation identifies SNI1, the SMC5/6 component, as a modifier of meiotic crossover in Arabidopsis. Proc. Natl. Acad. Sci. USA 118, e2021970118 (2021).
Nageswaran, D. C. et al. HIGH CROSSOVER RATE1 encodes PROTEIN PHOSPHATASE X1 and restricts meiotic crossovers in Arabidopsis. Nat. Plants 7, 452–467 (2021).
Kim, J. et al. Arabidopsis HEAT SHOCK FACTOR BINDING PROTEIN is required to limit meiotic crossovers and HEI 10 transcription. EMBO J. https://doi.org/10.15252/embj.2021109958 (2022).
Wu, G., Rossidivito, G., Hu, T., Berlyand, Y. & Poethig, R. S. Traffic lines: new tools for genetic analysis in Arabidopsis thaliana. Genetics 200, 35–45 (2015).
Rowan, B. A., Patel, V., Weigel, D. & Schneeberger, K. Rapid and inexpensive whole-genome genotyping-by-sequencing for crossover localization and fine-scale genetic mapping. G3 5, 385–398 (2015).
Jiao, W. B. & Schneeberger, K. Chromosome-level assemblies of multiple Arabidopsis genomes reveal hotspots of rearrangements with altered evolutionary dynamics. Nat. Commun. 11, 1–10 (2020).
Drouaud, J. & Mézard, C. Characterization of meiotic crossovers in pollen from Arabidopsis thaliana. Methods Mol. Biol. 745, 223–249 (2011).
Yelina, N. E. et al. Epigenetic remodeling of meiotic crossover frequency in Arabidopsis thaliana DNA methyltransferase mutants. PLoS Genet. 8, e1002844 (2012).
Drouaud, J. et al. Contrasted patterns of crossover and non-crossover at Arabidopsis thaliana meiotic recombination hotspots. PLoS Genet. 9, e1003922 (2013).
Yelina, N. E. et al. DNA methylation epigenetically silences crossover hot spots and controls chromosomal domains of meiotic recombination in Arabidopsis. Genes Dev. 29, 2183–2202 (2015).
Choi, K. et al. Recombination rate heterogeneity within Arabidopsis disease resistance genes. PLoS Genet. 12, 1–30 (2016).
Serra, H. et al. Interhomolog polymorphism shapes meiotic crossover within the Arabidopsis RAC1 and RPP13 disease resistance genes. PLoS Genet. 14, 1–28 (2018).
Fan, Q. Q., Xu, F., White, M. A. & Petes, T. D. Competition between adjacent meiotic recombination hotspots in the yeast Saccharomyces cerevisiae. Genetics 145, 661–670 (1997).
Robine, N. et al. Genome-wide redistribution of meiotic double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 27, 1868–1880 (2007).
Acquaviva, L. et al. The COMPASS subunit Spp1 links histone methylation to initiation of meiotic recombination. Science 339, 215–218 (2013).
Billings, T. et al. DNA binding specificities of the long zinc-finger recombination protein PRDM9. Genome Biol. 14, R35 (2013).
Fowler, K. R., Hyppa, R. W., Cromie, G. A. & Smith, G. R. Physical basis for long-distance communication along meiotic chromosomes. Proc. Natl Acad. Sci. USA 115, E9333–E9342 (2018).
Wijnker, E. et al. The genomic landscape of meiotic crossovers and gene conversions in Arabidopsis thaliana. Elife 2, e01426 (2013).
He, Y. et al. Genomic features shaping the landscape of meiotic double-strand break hotspots in maize. Proc. Natl Acad. Sci. USA 114, 12231–12236 (2017).
Roessler, K. et al. The genome-wide dynamics of purging during selfing in maize. Nat. Plants 5, 980–990 (2019).
Duroc, Y. et al. Concerted action of the MutLβ heterodimer and Mer3 helicase regulates the global extent of meiotic gene conversion. Elife 6, e21900 (2017).
Barrett, S. C. H. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284 (2002).
Zohary, D., Hopf, M. & Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin (Oxford Univ. Press, 2012).
Ziolkowski, P. A. Why do plants need the ZMM crossover pathway? A snapshot of meiotic recombination from the perspective of interhomolog polymorphism. Plant Reprod. https://doi.org/10.1007/s00497-022-00446-3 (2022).
Bieluszewski, T. et al. NuA4 and H2A.Z control environmental responses and autotrophic growth in Arabidopsis. Nat. Commun. 13, 277 (2022).
Bieluszewski, T., Szymanska-Lejman, M., Dziegielewski, W., Zhu, L. & Ziolkowski, P. A. Efficient generation of CRISPR/Cas9-based mutants supported by fluorescent seed selection in different Arabidopsis accessions. Methods Mol. Biol. 2484, 161–182 (2022).
Kbiri, N., Dluzewska, J., Henderson, I. R. & Ziolkowski, P. A. Quantifying meiotic crossover recombination in Arabidopsis lines expressing fluorescent reporters in seeds using SeedScoring pipeline for CellProfiler. Methods Mol. Biol. 2484, 121–134 (2022).
Hennig, B. P. et al. Large-scale low-cost NGS library preparation using a robust Tn5 purification and tagmentation protocol. G3 8, 79–89 (2018).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The sequence alignment / map format and SAMtools. Bioinformatics 25, 2078–2079 (2018).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011).
Choi, K. et al. Nucleosomes and DNA methylation shape meiotic DSB frequency in Arabidopsis transposons and gene regulatory regions. Genome Res. 28, 532–546 (2018).
Sura, W. et al. Dual role of the histone variant H2A.Z in transcriptional regulation of stress-response genes. Plant Cell 29, 791–807 (2017).
Potok, M. E. et al. The role of ATXR6 expression in modulating genome stability and transposable element repression in Arabidopsis. Proc. Natl. Acad. Sci. USA 119, e2115570119 (2022).
Yu, X. et al. The BORDER family of negative transcription elongation factors regulates flowering time in Arabidopsis. Curr. Biol. 31, 5377–5384.e5 (2021).
Stroud, H., Greenberg, M. V. C., Feng, S., Bernatavichute, Y. V. & Jacobsen, S. E. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152, 352–364 (2012).
Acknowledgements
We thank Charles Underwood and Bertrand Llorente for discussions and comments on this manuscript and Beth Rowan for sharing a Col × Ler F2 high-density crossover dataset. Natalia Glumińska is acknowledged for technical assistance. This work was supported by the Polish National Science Center (NCN) grants 2016/22/E/NZ2/00455 and 2020/39/I/NZ2/02464 to P.A.Z., 2021/41/N/NZ2/00340 to M.S.-L., the Foundation for Polish Science grant (POIR.04.04.00-00-5C0F/17-00) to P.A.Z., and the National Science Foundation grant (MCB-1614191) to R.S.P.
Author information
Authors and Affiliations
Contributions
M.S.-L. and P.A.Z. designed research; M.S.-L., W.D., J.D., and N.K. performed research; A.B. contributed new reagents; R.S.P. contributed new materials; W.D. performed the computational analyses; M.S.-L., W.D., J.D., N.K., and P.A.Z. analyzed data; and M.S.-L., W.D., and P.A.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Andrew Lloyd and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szymanska-Lejman, M., Dziegielewski, W., Dluzewska, J. et al. The effect of DNA polymorphisms and natural variation on crossover hotspot activity in Arabidopsis hybrids. Nat Commun 14, 33 (2023). https://doi.org/10.1038/s41467-022-35722-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35722-3
This article is cited by
-
Structural variation and DNA methylation shape the centromere-proximal meiotic crossover landscape in Arabidopsis
Genome Biology (2024)
-
Meiotic recombination dynamics in plants with repeat-based holocentromeres shed light on the primary drivers of crossover patterning
Nature Plants (2024)
-
MSH2 stimulates interfering and inhibits non-interfering crossovers in response to genetic polymorphism
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.