Abstract
The acquisition of germination and post-embryonic developmental ability during seed maturation is vital for seed vigor, an important trait for plant propagation and crop production. How seed vigor is established in seeds is still poorly understood. Here, we report the crucial function of Arabidopsis histone variant H3.3 in endowing seeds with post-embryonic developmental potentials. H3.3 is not essential for seed formation, but loss of H3.3 results in severely impaired germination and post-embryonic development. H3.3 exhibits a seed-specific 5′ gene end distribution and facilitates chromatin opening at regulatory regions in seeds. During germination, H3.3 is essential for proper gene transcriptional regulation. Moreover, H3.3 is constantly loaded at the 3′ gene end, correlating with gene body DNA methylation and the restriction of chromatin accessibility and cryptic transcription at this region. Our results suggest a fundamental role of H3.3 in initiating chromatin accessibility at regulatory regions in seed and licensing the embryonic to post-embryonic transition.
Similar content being viewed by others
Introduction
The formation of seeds is one of the most important innovations that contribute to the success of flowering plants. By seed formation, the embryo is protected in a desiccated and quiescent state, and, meanwhile, ready to swiftly initiate germination and post-embryonic development when optimal growth condition arises1. Producing highly vigorous seeds is thus key for plant reproduction and agriculture. Embryo obtains its germination capacity during the late seed maturation stage2. However, the underlying mechanisms establishing germination and post-embryonic developmental competence in seeds remain poorly understood.
The eukaryotic genome is tightly packed in the form of chromatin, which consists of DNA and histone-formed nucleosomes as basic subunits. Chromatin compaction restricts the access of transcription factors to cis-regulatory elements such as promoters and enhancers, and thus affecting gene transcription3,4. During cellular differentiation and developmental transitions, epigenetic reprogramming induces chromatin structure changes, thereby modifying the accessibility of regulatory elements to transcription machinery5,6. The establishment of chromatin accessibility is thus a pioneer event in developmental transition and lineage-specific transcriptional regulation. Several mechanisms have been identified to control chromatin structure dynamics, including ATP-dependent chromatin remodeling, post-translational modifications on histones, and the exchange of histone variants7,8. Besides their sequence divergence, histone variants often possess different expression patterns and in some cases unique modifications9,10,11. The deposition of histones variants can modify nucleosome stability, which differentiates chromatin into specialized property and cellular function4,12.
In animals, the histone H3 variant H3.3 differs from the replicative H3.1/H3.2 with only 4–5 amino acids13. Unlike H3.1, which is DNA replication-dependently expressed and incorporated into the chromatin, H3.3 is expressed and incorporated in a DNA replication-independent manner14,15,16,17,18. H3.3 is associated with transcribed regions and gene regulatory elements19,20. In Xenopus, H3.3 is essential for gastrulation, and it is proposed that H3.3 creates a permissive chromatin state critical for embryo development21,22. Knockout one of the two H3.3-coding genes in mouse causes reduced viability and infertility23,24,25, and complete deletion of H3.3 results in early embryonic lethality26. H3.3 is required to maintain a decondensed chromatin state and active histone modifications in mouse embryo27. Nevertheless, loss of H3.3 seems to have a limited impact on gene transcription, rather it causes abnormal chromosome segregation and impaired genome integrity26,27.
Although evolved independently in separate kingdoms, H3.1 and H3.3 in higher plants acquired comparable features as their counterparts in animals through convergent evolution28,29,30. For instance, in Arabidopsis H3.3 differs from H3.1 with four amino acids31, and H3.3 and H3.1 are DNA replication-independently and dependently expressed respectively32. Moreover, Arabidopsis H3.3 is mostly enriched at the body of transcribed genes32,33. Three genes HTR4, HTR5 and HTR8 in Arabidopsis encode H3.3. Knockdown of Arabidopsis H3.3 (h3.3kd) results in mild developmental defects, including leaf serration, early flowering and slightly reduced fertility34,35. H3.3 promotes permissive histone modifications at the floral repressor FLOWERING LOCUS C (FLC), leading to its activation to repress flowering35. Compared with the broad distribution of H3.3 on transcribed genes, only hundreds of genes are misexpressed in h3.3kd, indicating that H3.3 may not regulate transcription directly32. Hence, the role of H3.3 in plants remains to be investigated.
Here, we report the critical function of Arabidopsis H3.3 in the establishment of chromatin accessibility and endowing seeds with post-embryonic developmental competence. Complete deletion of H3.3 shows no obvious impact on embryogenesis, but results in severe defects in germination and vegetative growth. H3.3 is enriched around both 5′ and 3′ gene ends in mature seeds. The 5′ enrichment of H3.3 is seed-specific, and it facilitates chromatin opening, particularly at the regulatory regions of responsive and post-embryonic developmental genes, and H3.3 is essential for their transcriptional regulation during germination. On the other hand, 3′ localization of H3.3 represents a canonical H3.3 deposition pattern that correlates with gene body DNA methylation and the restriction of chromatin accessibility and cryptic transcription. Our results support a fundamental role of H3.3 in the establishment of chromatin accessibility and gene transcriptional regulation important for post-embryonic development.
Results
H3.3 is necessary for plant post-embryonic development
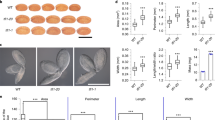
In a previous study, we created a complete H3.3 knockout mutant by combining a CRISPR-Cas9 generated htr4;htr5 double mutant with an htr8 mutant carrying a T-DNA insertion at HTR8. At that point, we did not obtain triple homozygous plants when screening the htr4/htr4;htr5/htr5;htr8/+ (h3.3ko/+) progenies, and thus we speculated that the h3.3ko mutant was embryonic lethal34. To analyze h3.3ko mutant phenotypes in detail, we examined the embryo development on h3.3ko/+ plants. Surprisingly, all seeds developed without abortion (Supplementary Fig. 1a). Around 7.5% to 11.5% of seeds collected from h3.3ko/+ plants could not germinate after 7 days of imbibition, and thus these were likely h3.3ko (Fig. 1a) (Supplementary Fig. 1b). This ratio is lower than 25% because knockout of H3.3 partially impairs male gametogenesis, and when crossing h3.3ko/+ with the wild-type (WT) Columbia (Col) mother, the transmission of h3.3ko was reduced to around 16% instead of the expected 50%34. These ungerminated seeds were then subjected to prolonged imbibition (up to two months), a portion of these seeds eventually germinated and produced small seedlings, and genotyping analysis confirmed that these seedlings were h3.3ko (Fig. 1b, c). Most of the h3.3ko seedlings stopped development immediately after germination without expanding cotyledons, and only a few of them could produce two true leaves (Fig. 1c, d). In rare cases, h3.3ko plants developed until the bolting stage and produced an inflorescence-like structure (Fig. 1c and Supplementary Fig. 1c). However, these plants ceased growth afterward and no silique was generated.
a The germination phenotype of h3.3ko/+ (htr4/htr4;htr5/htr5;htr8/+) progenies. Arrows indicate h3.3ko seeds that are strongly delayed in germination. b Days to germinate of Col, htr4/htr4;htr5/htr5, h3.3ko/+ (htr4/htr4;htr5/htr5;htr8/+) and h3.3ko (htr4/htr4;htr5/htr5;htr8/htr8) seeds. Seeds collected from h3.3ko/+ plants were sown on 1/2 MS, their genotypes were determined with seedlings formed after germination, and the first 12 germinated seeds were scored for each genotype. Values are means ± SD. (n = 12 biologically independent seeds). Radicle protrusion was considered as the completion of seed germination. c Percentages of h3.3ko seeds that germinated (within two months), produced true leaves and bolted. Values are means ± SD of three biological replicates. At least 110 seeds were used for each replicate. d The germination and growth phenotypes of Col, h3.3ko (segregated from h3.3ko;pHTR5::HTR5/-) and h3.3ko;pHTR5::HTR5. Scale bars = 1 mm (for seeds) or 1 cm (for seedlings). MS: mature seeds, G24h: subjected to germination for 24 h, G48h: subjected to germination for 48 h. e Days to germinate of Col, h3.3ko (segregated from h3.3ko;pHTR5::HTR5/-) and transgenic seeds expressing HTR5 or HTR13. The first 12 germinated seeds were scored. More than 110 seeds were sown on 1/2 MS. Values are means ± SD. (n = 12 biologically independent seeds). Radicle protrusion was considered as the completion of seed germination. For h3.3ko;pHTR5::HTR13 and h3.3ko;pHTR13::HTR5, the results from two independent transgenic lines are presented. f The germination and growth phenotypes of isolated Col and h3.3ko embryos. Scale bars = 1 mm. d: days.
In order to efficiently obtain the h3.3ko mutant seeds, we transformed a construct carrying a WT pHTR5::HTR5 and a mature seed stage-specific mCherry fluorescence selection marker into h3.3ko/+36. The WT HTR5 successfully rescued the h3.3ko phenotypes (Fig. 1d, e). Arabidopsis H3.3 differs from H3.1 with only four amino acids. To examine whether the h3.3ko phenotypes were specifically caused by the loss of H3.3 or merely due to the reduced H3 supply, we attempted the h3.3ko complementation by expressing HTR13 (an H3.1-coding gene) under the HTR5 promoter (Supplementary Fig. 1d). Expressing HTR13 only slightly accelerated the germination of h3.3ko (Fig. 1e), suggesting that the h3.3ko defects were largely H3.3 specific. We then expressed HTR5 using the HTR13 promoter, which was successfully employed in driving HTR13 expression to complement the h3.1kd phenotypes37. Nevertheless, expressing HTR5 under the HTR13 promoter also failed to rescue the h3.3ko germination defects (Fig. 1e, Supplementary Figs. 1d and S1e). Together, we conclude that both the H3.3 protein sequences and its expression pattern are required for its function in seed germination.
We collected seeds from heterozygous pHTR5::HTR5 transgenic plants in the h3.3ko background, from which the h3.3ko seeds could be identified by selecting mature seeds without the mCherry signal (Supplementary Fig. 1f). The h3.3ko seeds selected in this way behaved the same as those segregated from h3.3ko/+ (Fig. 1a, b, d and e), and hence were used in all subsequent studies.
All germination tests were performed with seeds stored for three months after harvesting (after-ripened). Moreover, seeds were stratified for two days during germination, further excluding the possibility that the h3.3ko germination defects were due to its strong dormancy. Endosperm and testa (seed coat) are critical tissues in dormancy maintenance, and they also impose a physical constraint on the radicle protrusion38,39. To examine which tissues are responsible for the h3.3ko germination defects, we isolated h3.3ko embryos by removing endosperm and testa. The isolated h3.3ko embryos showed much slower growth compared with WT (Fig. 1f), indicating strong defects in h3.3ko embryos.
h3.3ko shows strong defects in transcriptome
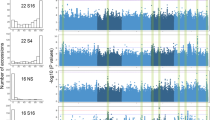
To search for a mechanism to explain the strongly impaired germination in h3.3ko, we profiled transcriptomes of WT and h3.3ko using RNA-seq. Seeds stored for three months after harvesting (hereinafter referred to as mature seeds) were subjected to imbibition and RNA was extracted from seeds collected at various time points (Fig. 2a). Principle component analysis (PCA) showed that compared with WT, the overall transcriptome changes in h3.3ko were less dynamic during imbibition (Fig. 2b). We combined all transcript level significantly decreased and increased genes in h3.3ko (fold change >2 and P adjust <0.05) identified from each time point during imbibition and performed gene ontology (GO) analysis, and found that responsive genes were especially enriched (Fig. 2c) (Supplementary Data 1), suggesting compromised imbibition response in h3.3ko compared with WT. GA and ABA are two major hormones that antagonistically regulate germination, during which GA biosynthesis and ABA catabolic genes need to be activated40,41. However, the activation of key GA synthetic and ABA catabolic genes such as GA3OX1, GA3OX2, GA20OX1, GA20OX2, and CYP707A2 was strongly abolished in h3.3ko (Fig. 2d and Supplementary Fig. 2a), in line with its impaired germination. Intriguingly, the variation between h3.3ko and Col transcriptomes was already notable at the mature seed stage and nearly 5000 genes were significantly misexpressed in mature h3.3ko seeds (Fig. 2b, e). Therefore, H3.3 is already required for the establishment of proper transcriptome in mature seeds.
a The germination time course in this study. MS: mature seeds, S12h/S24h/S48h: stratified for 12/24/48 h, G12h/G24h: subjected to germination for 12/24 h. At G24h, the germination of Col but not h3.3ko has been completed. b PCA plot of Col and h3.3ko seeds imbibed for different times showing their transcriptome differences determined by RNA-seq. PC1 covers the highest amount of variance between samples; PC2 covers most of the remaining variance. Three biological replicates were performed for each line at each time point. c GO analysis of a combined set of all significantly misexpressed genes in h3.3ko identified from each imbibition time point. Top 20 representative terms are listed and ranked by P value, which was calculated with hypergeometric test. d Transcript levels of GA3OX1, GA3OX2, GA20OX1, GA20OX2 and CYP707A2 during imbibition determined by RNA-seq (TPM) in Col and h3.3ko. Values are means ± SD of three biological replicates. e Number of transcript levels significantly decreased and increased genes in h3.3ko compared with Col at different imbibition time points.
A distinctive pattern of H3.3 enrichment over genes in mature seeds
To test whether the impaired germination of h3.3ko is due to defects in its mature seeds or is caused by the lack of H3.3 during imbibition, we introduced into h3.3ko/+ an HTR5 expression construct based on an estradiol inducible system (pER8::HTR5), which induces a strong uniform gene expression pattern similar to that of HTR532,42,43. Seeds collected from h3.3ko/+;pER8::HTR5 were subjected to imbibition with or without estrogen, and HTR5 transcription was successfully induced by estrogen (Fig. 3a). Interestingly, the induction of HTR5 expression during imbibition showed no impact on the germination of h3.3ko (Fig. 3b, c). The transcript levels of genes involved in H3.3 deposition were comparable in WT and h3.3ko, indicating that the H3.3 deposition pathway is generally intact in h3.3ko (Supplementary Fig. 2b). Therefore, it appears that loss of H3.3 already caused strong defects in mature seeds that affect germination, and a supply of H3.3 to h3.3ko during imbibition is not sufficient to complement the defects caused by the loss of H3.3 in seeds.
a The induction of HTR5 by estrogen during imbibition. Seeds were imbibed with or without estrogen for 48 h. Values are means ± SD of three biological replicates. PP2A was used as an endogenous control for normalization. b The germination phenotype of h3.3ko/+;pER8::HTR5 progenies treated with or without estrogen during imbibition. Arrows indicate h3.3ko;pER8::HTR5 seeds that are strongly delayed in germination. c Percentages of h3.3ko;pER8::HTR5 seeds that germinated within 2 month of imbibition with or without estrogen. Values are means ± SD of three biological replicates. 50 seeds were assessed for each replicate. d Heatmap showing the transcript levels of H3.1 and H3.3-coding genes in dissected embryos at different (early/middle/late) developmental stages. DAP: day after pollination. The expression data were extracted from the published RNA-seq datasets45. e The germination phenotypes of Col, h3.3ko, and h3.3ko;pH2B.S::HTR5 after subjected to germination for 84 h. f Percentages of h3.3ko;pH2B.S::HTR5 seeds that germinated within 10 days of imbibition. Values are means ± SD of three biological replicates. At least 38 seeds were assessed for each replicate. g Metaplot of HTR5-GFP ChIP-seq signals over all genes in mature seeds, during imbibition, and in the 10-day-old seedling. TSS: transcription start sites, TES: transcription end sites. h Heatmap of HTR5-GFP ChIP-seq signals over all genes in mature seeds, during imbibition, and in the 10-day-old seedling.
The above results prompted us to examine if there were any morphological defects in h3.3ko seeds. The seed size and seed weight of mature h3.3ko seeds were comparable to that of WT (Supplementary Fig. 3a–c). In addition, mature embryos isolated from WT and h3.3ko seeds were morphologically similar (Fig. 1f and Supplementary Fig. 3d). Seed storage proteins (SSPs) are synthesized during the late stages of seed development and are the major nutritional sources for subsequent germination and early seedling development44. The abundance of SSPs was examined with mature seeds, and the major SSPs including 12S globulins and 2S albumins were normally accumulated in h3.3ko (Supplementary Fig. 3e), suggesting that H3.3 is not required for their synthesis. Hence, loss of H3.3 did not affect embryo morphology and the accumulation of SSPs.
In WT, the transcript levels of H3.3-coding genes markedly increased towards the end of embryo development, contrasting with the decreased expression of H3.1-coding genes (Fig. 3d, Supplementary Fig. 1d, e)45. Since H3.3 is not required for seed formation (Supplementary Figs. 1a and 3), we reasoned that H3.3 could become critical at the late seed maturation stage soon before seeds turn fully mature to prepare them for germination. To test this possibility, we expressed H3.3 in h3.3ko with the promoter of at2S3 (a seed storage protein gene) or H2B.S (a seed-specific H2B variant gene), both of which are expressed specifically at the late stages of seed development44,46. Expressing HTR5 under either promoter increased the germination rate of h3.3ko, with the H2B.S promoter showing stronger capability (Fig. 3e, f and Supplementary Fig. 4a). However, in both cases, most of the seedlings ceased development after germination, similar to the h3.3ko seedlings. This is probably because the seedling development also requires H3.3 expressed during and after germination. Nevertheless, these results further support the notion that H3.3 expressed during seed maturation is necessary for seed germination.
Ideally, a comparison of WT and h3.3ko at the molecular level (e.g. RNA-seq) during seed maturation would help the identification of the earliest stage when h3.3ko starts to show defects. However, the h3.3ko seeds were morphologically identical to WT (Supplementary Figs. 1a and 3), making them impracticable to isolate until they were fully mature (Supplementary Fig. 1f). To this end, we focused on the mature seed stage, and profiled the genome-wide accumulation of H3.3 with an HTR5-GFP reporter line using ChIP-seq32. H3.3 was localized at euchromatin regions and depleted over transposable elements (TEs) in mature seeds (Supplementary Fig. 4b, c), a pattern similar to that in vegetative tissues32,33,35. Metagene analyses showed enrichment of H3.3 at both 5′ gene ends/promoters and 3′ gene ends (Fig. 3g, h and Supplementary Fig. 4d, e). Notably, the strong accumulation of H3.3 around the 5′ gene ends was not detected previously in vegetative tissues32,33,35. We thus further analyzed H3.3 enrichment during imbibition and in 10-day-old seedlings and observed a change in H3.3 enrichment patterns. In particular, H3.3 accumulation was gradually reduced at the 5′ gene end but increased at the gene body and the 3′ gene end (Fig. 3g, h and Supplementary Fig. 4d, e). These results suggest that H3.3 supplied during imbibition could be predominantly deposited to the gene body and the 3′ gene end but not the 5′ gene end, and probably explain why induction of HTR5 expression during imbibition was not sufficient to rescue the germination defects of h3.3ko (Fig. 3a–c). Together, we conclude that H3.3 enrichment around the 5′ gene end is specific to seeds, suggesting its importance at this stage of development.
H3.3 regulates chromatin accessibility in mature seeds
The enrichment of H3.3 at the 5′ gene ends coincides with the localization of chromatin accessible regions around the gene transcription start sites47,48, we thus examined chromatin accessibility changes in h3.3ko mature seeds by Assay for Transposase Accessible Chromatin sequencing (ATAC-seq). Overall, accessible regions were mainly enriched at euchromatin in both WT and h3.3ko (Fig. 4a). Chromatin accessibility over TEs was not changed in h3.3ko (Supplementary Fig. 5a), in line with the low H3.3 abundance at TEs. At genes, the majority of the open chromatin regions localized at 5′ gene ends/promoters in mature WT seeds (Fig. 4b–d and Supplementary Fig. 5b, c), similar to findings in vegetative tissues47,48. However, compared with WT, the chromatin accessibility was overall lower around the 5′ gene ends in h3.3ko and higher towards the 3′ ends (Fig. 4b–e and Supplementary Fig. 5b, c). Hence, loss of H3.3 results in a global change of chromatin accessibility at genes in mature seeds.
a ATAC-seq signals in Col and h3.3ko mature seeds over Arabidopsis chromosomes. Signals were calculated in 100 kb bins. Pericentromeric heterochromatin regions are indicated with gray shading. b Metaplot of ATAC-seq signals in Col and h3.3ko mature seeds over all genes. c Heatmap of ATAC-seq signals in Col and h3.3ko mature seeds over all genes. d Genomic distribution of accessible regions in Col and h3.3ko mature seeds. e Genome browser view of ATAC-seq and HTR5-GFP ChIP-seq signals in Col and h3.3ko mature seeds over a few representative loci. AT1G72330: a gene mainly lost accessibility at the 5′ end/promoter region in h3.3ko, AT3G44110: a gene mainly gained accessibility around the 3′ end/genic region in h3.3ko, AT5G03730: a gene with both accessibility increased and decreased regions in h3.3ko. f, g Metaplot of HTR5-GFP ChIP-seq signals in mature seeds over accessibility significantly decreased (f) and increased (g) regions in mature h3.3ko seeds compared with Col. HTR5-GFP ChIP-seq signals over randomly selected regions are served as control.
We further identified regions that had significantly altered chromatin accessibility in h3.3ko (fold change >2 and P adjust <0.05). 3278 and 3436 regions significantly lost or gained accessibility respectively (Supplementary Fig. 6a, b) (Supplementary Data 2). Less accessible regions in h3.3ko were preferentially localized at promoters, but regions with increased chromatin accessibility were mainly enriched at genic regions and 3′ gene ends (Supplementary Fig. 6c), parallel with the chromatin accessibility changes in h3.3ko over genes (Fig. 4b–e). Accessibility increased and decreased regions in h3.3ko were both enriched with H3.3 in WT (Fig. 4e–g and Supplementary Fig. 6d, e), supporting the notion that H3.3 directly or indirectly regulates chromatin accessibility at these regions.
Notably, 5′ and 3′ localized H3.3 seems to have distinct impacts on chromatin accessibility. In animals, the coexistence of H3.3 and another histone variant H2A.Z destabilizes nucleosomes49. We thus profiled the enrichment of H2A.Z in mature seeds. Like in vegetative tissues, H2A.Z in mature seeds is enriched at the 5′ gene ends but depleted from the 3′ gene ends (Supplementary Fig. 7a)50,51. Moreover, accessibility decreased regions in h3.3ko carried higher levels of H2A.Z than accessibility increased regions (Supplementary Fig. 7b, c). To assess the requirement of H2A.Z in chromatin accessibility control, we performed ATAC-seq using mature seeds of WT and a hypomorphic h2a.z mutant, in which HTA9, one of the three H2A.Z-coding genes in Arabidopsis, is still expressed at low levels50,52. In h2a.z, a slight reduction of chromatin accessibility was observed at the 5′ gene end, while chromatin accessibility at the 3′ gene end was not changed compared with WT (Supplementary Fig. 8a). Importantly, regions with reduced accessibility in h3.3ko partially lost accessibility in h2a.z (Supplementary Fig. 8b), but loss of H2A.Z did not induce the chromatin accessibility at regions with increased accessibility in h3.3ko (Supplementary Fig. 8c). In addition, we observed moderately delayed germination of h2a.z compared with WT (Supplementary Fig. 8d). These results indicate that H2A.Z probably works together with H3.3 at the 5′ gene end to facilitate chromatin opening in mature seeds, while without H2A.Z at the 3′ gene end H3.3 somehow restricts chromatin accessibility. It is of note that the chromatin accessibility changes in the h2a.z mutant were less pronounced compared with that in h3.3ko. This could be because there is still residual H2A.Z in this h2a.z mutant, or other factors such as histone modifications enriched specifically at the 5′ or 3′ gene end may also contribute to the accessibility control together with H3.3.
Altered chromatin accessibility in h3.3ko correlates with changes in transcription and gene body DNA methylation
As mentioned above, the mature seed stage is the earliest time point when we could distinguish h3.3ko from WT (Supplementary Figs. 1a, f and 3). At this stage, the transcriptome of h3.3ko was already drastically different from that of WT (Fig. 2b, e). We then analyzed the correlations between changes in chromatin accessibility and transcriptome in mature h3.3ko seeds. In general, the distribution patterns of transcripts on genes were slightly altered in h3.3ko compared with WT, that the transcript levels were reduced around the 5′ gene end, and were increased towards the 3′ gene end (Fig. 5a, b, Supplementary Fig. 9a, b).
a Metaplot of transcripts in Col and h3.3ko mature seeds over all genes. The profiles were generated after merging three biological replicates. b Metaplot of h3.3ko transcripts vs Col transcripts in mature seeds over all genes. The profiles were generated after merging three biological replicates. c Metaplot of transcripts in Col and h3.3ko mature seeds over accessibility significantly decreased and increased regions in mature h3.3ko seeds compared with Col. The profiles were generated after merging three biological replicates. d Metaplot of transcripts in Col and h3.3ko mature seeds over genes with accessibility significantly decreased and increased regions in mature h3.3ko seeds compared with Col. The profiles were generated after merging three biological replicates. e Venn diagrams of accessibility significantly decreased region-associated genes in mature h3.3ko seeds and transcript level significantly decreased and increased genes in mature h3.3ko seeds. P values are based on the hypergeometric test. f Genome browser view of ATAC-seq and RNA-seq signals in Col and h3.3ko mature seeds over a few representative loci showing increased chromatin accessibility and transcript levels around the 3′ gene end in h3.3ko compared with Col. Red lines indicate regions examined by ChIP-qPCR. g Relative RNA Pol II enrichment levels determined by ChIP-qPCR at AT1G36730, AT3G44110, and AT5G22000 in mature seeds. RNA Pol II enrichment levels were first normalized to input and then to that of Col. Values are means ± SD of three biological replicates. Statistical significance was determined by two-tailed Student’s t test (*P < 0.05; ns, P > 0.05). h, i Metaplot of CG methylation levels in Col and h3.3ko mature seeds over accessibility significantly decreased (h) and increased (i) regions in mature h3.3ko seeds compared with Col.
Next, we examined the transcripts of genes with significantly changed accessibility in h3.3ko. 4043 and 4388 genes were associated with accessibility decreased and increased regions respectively; with 920 genes associating with both (Supplementary Fig. 9c) (Supplementary Data 3). In h3.3ko, genes with accessibility decreased regions showed a strong reduction in chromatin accessibility around the 5′ end, and genes with accessibility increased regions mainly displayed a gain of accessibility towards the 3′ end (Supplementary Fig. 9d, e). These changes are consistent with the localization of accessibility decreased and increased regions in h3.3ko (Supplementary Fig. 6c). Overall, around accessibility decreased regions, fewer transcripts were detected in h3.3ko compared with WT (Fig. 5c), and genes associated with accessibility decreased regions showed an overall reduction in transcript levels (Fig. 5d). A detailed analysis revealed that a significant amount of them were indeed transcript level strongly decreased genes in mature h3.3ko seeds (Figs. 2e and 5e). Notably, some accessibility decreased region-associated genes showed increased transcript levels in h3.3ko compared with WT (Figs. 2e and 5e). Chromatin accessibility has also been found to link with gene repression53,54, which may involve chromatin binding proteins such as transcription factors acting as repressors55,56. 2928 accessibility decreased region-associated genes did not show strong transcript level changes in mature h3.3ko seeds compared with WT (Fig. 5e). Hence, these genes may bear regulatory capacity mainly necessary in later developmental stages.
At accessibility increased regions, transcript levels were overall higher in h3.3ko (Fig. 5c), and accessibility increased region-associated genes had more transcripts (Fig. 5d). Nevertheless, these transcripts produced in h3.3ko were unevenly distributed, with more being generated towards the 3′ gene end (Fig. 5d), indicating cryptic transcription. If this is the case, RNA Polymerase II (RNA Pol II) may randomly transcribe from both sense and antisense directions within these regions. We thus performed strand-specific RNA-seq to measure antisense transcripts in WT and h3.3ko mature seeds. The overall levels of antisense transcripts on genes were not increased in h3.3ko compared with WT (Supplementary Fig. 10a). However, at accessibility increased regions in h3.3ko, antisense transcripts were increased as well as sense transcripts (Supplementary Fig. 10b). Similarly, accessibility increased region-associated genes showed increased transcript levels from both sense and antisense, especially towards the 3′ gene end (Supplementary Fig. 10c). In mature seeds, RNA Pol II may remain at gene loci or still transcribe at a low rate57. We further selected a few genes showing increased chromatin accessibility and transcript levels towards the 3′ gene end and measured RNA Pol II enrichment levels at their loci by ChIP-qPCR in mature seeds (Fig. 5f). The results showed that the RNA Pol II enrichment levels were increased around the 3′ gene ends (Fig. 5g). Hence, the induction of transcripts at the 3′ gene end in h3.3ko is likely due to spurious transcription at both sense and antisense directions by RNA Pol II.
Knockdown of H3.3 induces the loss of CG DNA methylation at the gene body, especially towards the 3′ gene end34. Using genome-wide bisulfite sequencing (BS-seq), we confirmed a strong reduction of gene body CG methylation in mature h3.3ko seeds. In addition, CHG and CHH methylation levels at the gene body were also slightly reduced (Supplementary Fig. 11a). At TE regions, only a reduction of CHH methylation levels was observed in h3.3ko (Supplementary Fig. 11b). H3.3 is largely absent from TEs, and therefore the loss of CHH methylation at TEs could be caused by an indirect effect. Accessibility increased but not decreased regions in h3.3ko showed reduced CG methylation levels (Fig. 5g, h). Consistently, hypo CG differentially methylated regions (DMR) in h3.3ko displayed increased chromatin accessibility (Supplementary Fig. 11c). It is unlikely that loss of gene body CG methylation in h3.3ko causes increased chromatin accessibility since a complete loss of CG methylation in met1 fails to induce chromatin accessibility at the gene body58. Together, these results suggest that H3.3-mediated chromatin accessibility control is probably required for proper transcriptional regulation and gene body DNA methylation.
Loss of chromatin accessibility in h3.3ko is associated with gene misregulation during germination
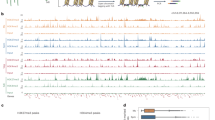
The above results indicate that H3.3 in seeds regulates chromatin accessibility and impacts on transcription. The enrichment of H3.3 around the 5′ gene end was gradually diminished along germination and vegetative growth (Fig. 3f, g). We thus examined chromatin accessibility in WT during germination and in seedlings, and found that the overall chromatin accessibility at the 5′ gene end was not strongly changed (Supplementary Fig. 12a). We then focused on the H3.3-dependent open chromatin regions in mature seeds (accessibility significantly decreased regions in mature h3.3ko seeds that preferentially enriched around the 5′ gene end). At these regions, H3.3 enrichment levels were drastically reduced during germination and in seedlings, yet this was only coupled with a moderate reduction in chromatin accessibility (Fig. 6a, b). In h3.3ko, the accessibility at these regions remained low after imbibition (Fig. 6c and Supplementary Fig. 12b). These results indicate that the H3.3-dependent chromatin accessibility in seeds is largely maintained during germination and subsequent development.
a, b Metaplot of HTR5-GFP ChIP-seq (a) and ATAC-seq (b) signals in mature seeds, during imbibition and in the 10-day-old seedlings over accessibility decreased regions in mature h3.3ko seeds compared with Col. c. Metaplot of ATAC-seq signals in imbibed Col and h3.3ko seeds over accessibility significantly decreased regions in mature h3.3ko seeds compared with Col. d Heatmap showing expression profiles (z-normalized) of genes associated with accessibility significantly decreased regions in mature h3.3ko seeds compared with Col. Genes were grouped into five clusters based on their expression patterns during imbibition in Col. e GO analysis of genes associated with accessibility significantly decreased regions in mature h3.3ko seeds compared with Col. Top 15 representative terms are listed and ranked by P value, which was calculated with hypergeometric test. f Metaplot of ATAC-seq signals in imbibed Col and h3.3ko seeds over accessibility significantly increased regions in mature h3.3ko seeds compared with Col.
In mature seeds, many genes associated with the H3.3-dependent open chromatin regions did not show strong transcript level changes in h3.3ko compared with WT (Fig. 5e). To understand the importance of these H3.3-dependent open chromatin regions in transcriptional regulation during germination, we compared the transcript levels of their associated genes in WT and h3.3ko during imbibition. These genes were grouped into five clusters based on their expression patterns during imbibition in WT (Fig. 6d). Importantly, in h3.3ko, most of the genes in clusters 1–4 failed to fully activate during imbibition, while those in cluster 5 were not sufficiently repressed (Fig. 6d). GO analysis of these genes revealed mainly responsive processes and post-embryonic development (Fig. 6e). Hence, H3.3-dependent chromatin accessibility at these responsive and developmental genes is likely necessary for their transcriptional control during germination.
At regions where the accessibility was repressed by H3.3 in mature seeds (mainly localized at gene body and 3′ gene end), H3.3 remained deposited in WT during imbibition and in seedlings and is required to maintain their low chromatin accessibility and to repress cryptic transcription at these regions (Fig. 6f and Supplementary Fig. 12c–e), indicating a canonical H3.3 distribution pattern around the 3′ gene end that constantly prevents chromatin opening and cryptic transcription at genic regions.
Discussion
In Arabidopsis, both paternal and maternal-derived H3.3 is removed from the zygote genome a few hours after fertilization, followed by de novo synthesis of H3.3 before the zygote divides. This process was speculated to prevent the transmission of epigenetic memory from parents and meanwhile establish the zygotic epigenome critical for embryogenesis42,59,60. Interestingly, our genetic evidence suggests that H3.3 is not essential for early plant embryonic development. It is likely that H3.1 or other H3 proteins largely compensate for the loss of H3.3 at this stage, especially considering that H3.1 is abundantly produced during cell division, a dominant event in early embryogenesis61. After pattern formation at the torpedo stage, the embryo gradually ceases cell division and initiates maturation2. This is coupled with a strong reduction in H3.1 expression and an increase in H3.3 expression (Fig. 3d), which coincident with the extensive H3.1 replacement by H3.3 during embryo maturation62. Thus, H3.3 likely starts to be critical at embryo maturation stages, and reprograms the embryo epigenome for the establishment of post-embryonic developmental potentials. Consistently, embryos before torpedo stages are not capable to germinate when excised from seeds, while later stage embryos could germinate2. This role of H3.3 is comparable to the function of a specialized form of non-replicative H3 variant H3.10 in sperm that establishes chromatin accessibility at promoters of genes expressed in early embryogenesis9,59. It is unknown by which mechanism H3.3 is deposited to the 5′ gene ends in seeds but removed during germination and seedling development. This possibly involves the H3.3 deposition chaperone HIRA63 that plays a moderate function in seed germination64, or ATRX involved in the deposition of H3.365,66. In addition, specific chromatin remodelers might recruit H3.3 deposition machinery. Alternatively, removal of H3.1 from this region during embryo maturation may allow H3.3 to accumulate.
Several pieces of evidence suggest that seed germination is largely programmed during seed maturation67. Protein synthesis from stored mRNA in mature seeds is required for the completion of germination68,69,70. Moreover, mRNA abundance changes are observed within 1–2 h after imbibition without de novo protein synthesis required, highlighting the function of seed-stored transcription machinery in quickly adjusting transcription71. Our results suggest that H3.3 deposited in seeds is vital for their acquisition of germination capacity. Loss of H3.3 causes reduced and increased chromatin accessibility at 5′ and 3′ gene ends respectively, and results in impaired regulation of responsive and post-embryonic development genes and increased cryptic transcription. Since H3.3 is part of the chromatin core (nucleosome), it is unlikely that H3.3 impacts on other physiological processes that would in turn affect chromatin accessibility. Therefore, we propose the following model (Fig. 7): H3.3 is loaded at the 5′ gene ends/promoters in mature seeds, facilitating chromatin opening (Figs. 3f, g and 4). During germination and subsequent seedling development, 5′ enriched H3.3 is gradually lost. However, the chromatin accessibility is still maintained (Fig. 6). A plausible explanation is that the 5′ enriched H3.3 may initiate chromatin opening to enable transcription factor binding in seeds and during germination. Afterward, the binding of transcription factors could sustain chromatin accessibility without H3.3. We found that the H3.3-established open chromatin regions are enriched with the transcription factor binding motifs especially those of bZIP, bHLH and BZR/BES family members (Supplementary Fig. 13 and Supplementary Data 4). On the other hand, H3.3 is constantly deposited around the 3′ gene ends and prevents chromatin accessibility and cryptic transcription. Nevertheless, it is still unclear whether the altered chromatin accessibility in h3.3ko is the direct cause of transcription changes and the impaired post-embryonic development, and the exact role of H3.3 in chromatin regulation and post-embryonic development remains to be investigated.
In maturing and mature seeds, H3.3 is loaded at the 5′ gene ends/promoters and establishes chromatin accessibility. During germination and seedling development, H3.3 is gradually removed from the 5′ gene ends, but the accessibility established by H3.3 is largely maintained probably by the binding of transcription factors (as indicated by the dashed border), and this may allow proper gene transcriptional regulation (activation or repression) during post-embryonic development. On the other hand, H3.3 is constantly deposited around the 3′ gene ends and prevents chromatin opening and cryptic transcription. Loss of H3.3 causes reduced and increased chromatin accessibility at 5′ and 3′ gene ends respectively, and results in compromised transcriptional regulation and severely impaired post-embryonic development. H2A.Z and the 5′ and 3′ specific histone modifications may also contribute to the H3.3-mediated chromatin accessibility control.
As a replacement histone, H3.3 deposition has been proposed to alter the nucleosome property4,12. In human cells, the presence of H3.3 destabilizes nucleosome, especially when co-existed with H2A.Z, leading to a nucleosome-free region at the 5′ gene end for transcription factor binding49,72. In mouse embryonic stem cell, H3.3 and H2A.Z collaborate around gene transcription start sites to set up a poised chromatin state for gene activation during cell differentiation73. Despite a predominant localization of H3.3 at the 3′ gene end in Arabidopsis vegetative tissues32,33,35, our findings show that at certain developmental stages it is highly enriched around the 5′ gene end and facilitates chromatin opening. With fully developed leaves that have ceased cell division and expansion, a small fraction of H3.3 is also detected at the 5′ gene ends/promoters74. Besides in mature embryo, a considerable amount of H3.3 is deposited to replace H3.1 in plant cells that have exited proliferation and become terminal differentiated62. This implies a broad role of H3.3 in reprogramming the epigenome for cell fate determination. Further study of detailed mechanisms might provide a potential to manipulate chromatin accessibility and cellular differentiation by engineered H3.3 deposition.
Methods
Plant materials and growth conditions
Arabidopsis h3.3ko and HTR5-GFP lines were described previously32,34. Plants were grown in long-day conditions (16 h light/8 h dark) at 22 °C. For seed germination, after-ripened mature seeds (seeds harvested and stored at room temperature for three months) were sown on 1/2 MS medium. After being kept at 4 °C for two days (stratification), seeds were subjected for germination in long days at ~22 °C. Three replicates were performed to assess the germination and growth of h3.3ko, at least 110 seeds were used for each replicate. h3.3ko seeds germinated within two months after sowing were considered as germinated, and the rest seeds were discarded. The germination date of the first 12 germinated seeds was scored.
For dissected embryo growth analysis, embryos from after-ripened Col and h3.3ko seeds were isolated by manually removing endosperm and testa. The isolated embryos were cultivated on 1/2 MS medium under long-day conditions.
Plasmid construction and h3.3ko complementation
For h3.3ko complementation constructs, HTR5 or HTR13 coding sequences were placed under the control of HTR5 or HTR13 promoter in the binary vector pBlligatorR43, which contains a mature seed stage-specific mCherry fluorescence selection marker36. For the estradiol induction, HTR5 coding sequences were placed under the pER8 promoter in the binary vector pMDC743,75. To express HTR5 with at2S3 or H2B.S promoter, the at2S3 or H2B.S promoter sequences were first cloned into pGWB51076, followed by the insertion of HTR5 coding sequences.
For the germination test of h3.3ko;pER8::HTR5 seeds with or without estrogen (10 μM) treatment during imbibition, 50 seeds were assessed in each replicate and seeds were transferred onto a new 1/2 MS plate supplemented with or without estrogen every 5 days in case of estrogen degradation. The germination rates were scored after 2 month of imbibition. For the germination test of h3.3ko;pat2S3::HTR5 and h3.3ko;pH2B.S::HTR5 seeds, germination rates were scored after 1 month and 10 days imbibition respectively. At least 38 seeds were assessed in each replicate.
Seed size measurement
Complete dried mature seeds were spread on a white background. After the image taken, seed size was measured with ImageJ. At least 47 seeds were scored for each genotype.
Seed storage protein analysis
One hundred and fifty Col or h3.3ko mature seeds were homogenized in protein extraction buffer (100 mM Tris-HCl pH 8.0, 0.5% SDS, 10% glycerol, 2% β-ME). Extracted proteins were resolved on 15% SDS-PAGE gel and stained with Coomassie Blue.
RNA-seq
Total RNA was extracted from mature seeds (seeds stored for three months after harvesting) or imbibed seeds using Minibest plant RNA extraction kit (Takara). Three independent biological replicates were performed. Sequencing libraries were prepared with BGISEQ-500 RNA-seq library preparation kit according to the manufacturer’s instruction. For strand-specific RNA-seq, total RNA extracted from Col and h3.3ko mature seeds were subjected to library preparation with MGIEasy RNA directional library preparation kit. Prepared libraries were sequenced on a DNBSEQ-T7 platform and paired-end 150 bp reads were generated.
RNA-seq data analysis
Adapter trimming was performed and low-quality reads were filtered with fastp version 0.20.177. Reads were mapped to the Arabidopsis genome (TAIR10) using Hisat2 version 2.1.1078. The numbers of mapped reads were determined with SAMtools version 1.979, and are listed in Supplementary Table 1. Reads per gene were counted by HTseq version 0.11.280. Transcripts per million (TPM) values were generated using R. Differential gene expression analysis was performed using DESeq2 version 1.26.081. Genes that displayed a more than two-fold expression change and had a P adjust value <0.05 were considered as differentially expressed. For data visualization, bigwig coverage files were generated using deepTools utility bamCoverage82 with a bin size of 10 bp and normalized with CPM. The PCA plot was generated with plotPCA in DESeq2 version 1.26.0. The expression of HTR4, HTR5 and HTR8 during embryogenesis was extracted from the published datasets45. Gene ontology analysis was performed with DAVID (https://david.ncifcrf.gov)83. Clustering of genes associated with accessibility decreased regions in mature h3.3ko seeds compared with Col was performed based on their expression changes during germination in Col using K-Means clustering in R.
RT-qPCR
Total RNA was extracted from mature seeds (seeds stored for three months after harvesting), imbibed seeds or 7-day-old seedlings using Minibest plant RNA extraction kit (Takara). Reverse transcription was performed using HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, R312-02). Real-time quantitative PCR was conducted on an Applied Biosystems QuantStudio 6 Flex Real-Time PCR System using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Q711-02). PP2A was used as an endogenous control for normalization. Three independent biological replicates were performed for each line and condition. Primers used to amplify HTR5, HTR13, GA3OX1, GA3OX2, GA20OX1, GA20OX2, CYP707A2, and PP2A are listed in Supplementary Table 2. The HTR5 amplification primers were designed to only amplify the WT but not Crispr-mutated HTR5. Primers amplifying HTR5 and HTR13 were tested to have similar amplification efficiency.
ChIP-seq
ChIP was performed with mature HTR5-GFP seeds (seeds stored for three months after harvesting) or seeds imbibed for different times. Materials were ground with liquid nitrogen into fine powder and immediately fixed with 1% formaldehyde32,33,35,50,51,52. Nuclei were extracted and mononucleosomes were generated with micrococcal nuclease (MNase) (Sigma, N5386) digestion as previously described52. Immunoprecipitation was conducted with anti-GFP (Thermo Fisher Scientific, A-11122) or anti-H2A.Z51 antibody. After antibody incubation, Protein A Dynabeads (Thermo Fisher Scientific, 10002D) were added to collect the immunocomplexes. After washing, elution, reverse cross-linking and DNA purification, recovered DNA was subjected to library preparation with VAHTS universal DNA library prep kit for Illumina (Vazyme, ND607) according to the manufacturer’s instruction and sequenced with Illumina NovaSeq 6000 to generate paired-end 150 bp reads. ChIP-seq experiments were performed with two independent biological replicates.
ChIP-seq data analysis
Adapter trimming was performed and low-quality reads were filtered with fastp version 0.20.177. Reads were mapped to the Arabidopsis genome (TAIR10) with Bowtie2 version 2.4.284, and filtered for duplicated reads by using Picard version 2.24.0 MarkDuplicates (https://github.com/broadinstitute/picard). The numbers of mapped reads were determined with SAMtools version 1.979, and are listed in Supplementary Table 1. For data visualization, bigwig coverage files were generated using deepTools utility bamCoverage82 with a bin size of 10 bp and normalized to input or sequencing depth using RPKM. Visualization was performed with IGV version 2.7.285. Heatmaps and average normalized ChIP-seq profiles were generated using deepTools utilities plotHeatmap and plotProfile. Published HTR5-GFP ChIP-seq data in seedlings were processed in the same manner35.
ChIP-qPCR
Mature seeds were ground with liquid nitrogen into fine powder and immediately fixed with 1% formaldehyde. Chromatin was sheared by sonication and immunoprecipitation was performed with anti-RNA Pol II antibody (abcam, ab26721). The amounts of immunoprecipitated DNA were quantified by real-time quantitative PCR. Three independent biological replicates were performed. Primers used for PCR amplification are listed in Supplementary Table 2.
ATAC-seq
Mature seeds (seeds stored for three months after harvesting), seeds imbibed for different times or 10-day-old seedlings were chopped in lysis buffer (15 mM Tris-HCl pH 7.5, 20 mM NaCl, 80 mM KCl, 0.5 mM Spermine, 0.2% Triton X-100, 5 mM β-ME, protease inhibitor cocktail). After being filtered with a 30μm filter (CellTrics), nuclei were stained with DAPI and subjected to fluorescence-activated cell sorting (FACS). 50,000 nuclei per sample were collected and mixed with Tn5 transposase (Mei5bio, MF650-01), after 20 min of incubation at 37 °C, tagmented DNA was recovered with ChIP DNA Clean & Concentrator kit (ZYMO, D5205). After PCR amplification, sequencing libraries were purified with VAHTS DNA Clean Beads (Vazyme N411) and sequenced with Illumina NovaSeq 6000 to generate paired-end 150 bp reads. ATAC-seq experiments were performed with two independent biological replicates.
ATAC-seq data analysis
Adapter trimming was performed and low-quality reads were filtered with fastp version 0.20.177. Reads were mapped to the Arabidopsis genome (TAIR10) with Bowtie2 version 2.4.284. Reads mapped to chloroplast and mitochondria genome were removed and duplicated reads were filtered with Picard version 2.24.0 MarkDuplicates (https://github.com/broadinstitute/picard). The numbers of mapped reads were determined with SAMtools version 1.979, and are listed in Supplementary Table 1. ATAC-seq open chromatin peaks were identified using MACS2 version 2.1.2 with default parameters86. The q-value cutoff for peak calling was 0.05. Only peaks identified from both biological replicates were kept. Peak distributions were analyzed with ChIPseeker version 1.22.187. Promoter refers to accessible regions located <2 kb upstream of the transcription start sites (TSS), downstream refers to accessible regions located <1 kb downstream of the transcription end sites (TES), Intergenic region refers to accessible regions located >2 kb upstream of TSS and more than 1 kb downstream of TES. To identify differentially enriched peaks, a common peak set was created by merging peaks in Col and h3.3ko, scores for open chromatin peak regions were calculated with deepTools utility multiBigwigSummary82, and differential peaks were called by requiring more than two-fold difference and P adjust <0.05. Genes were assigned to the peaks when the genic and 1 kb upstream regions overlapped with the peak regions for at least one base pair. For data visualization, bigwig coverage files were generated using deepTools utility bamCoverage82 with a bin size of 10 bp and normalized with RPKM. DNA motif analysis was performed with HOMER (version 4.11) using “findMotifs.pl“88.
BS-seq
Genomic DNA was extracted from mature seeds (seeds stored for three months after harvesting) with Quick-DNA Plant/Seed Miniprep Kit (ZYMO, D6020). 200 ng fragmented genomic DNA (200 bp–500 bp) was used for library preparation with VAHTS universal Pro DNA library prep kit for Illumina (Vazyme, ND608). NEBNext Multiplex Oligos for Illumina (NEB, E7535) were used for adapter ligation. After purification with VAHTS DNA Clean Beads (Vazyme N411), bisulfite conversion was performed with EZ DNA Methylation-Gold Kit (ZYMO, D5005), followed by DNA purification and PCR amplification. Libraries were sequenced with Illumina NovaSeq 6000 to generate paired-end 150 bp reads. BS-seq experiments were performed with two independent biological replicates.
BS-seq data analysis
Adapter trimming was performed and low-quality reads were filtered with fastp version 0.20.177. Reads were mapped to the Arabidopsis genome (TAIR10) with BS-Seeker2 version 2.1.8 using default parameters89. Duplicated reads were filtered with Picard version 2.24.0 MarkDuplicates. The numbers of mapped reads are listed in Supplementary Table 1. CG, CHG, and CHH methylation levels were calculated with CGmaptools (version 0.1.2)90. Methylation data were visualized with ggplot2 in R (version 1.1.1106). CG DMRs were called with CGmaptools using dmr function with default parameters.
Statistical analysis
Statistical tests performed are indicated in the figure captions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated during the current study are available in the GEO repository GSE209645. The ChIP-seq data of HTR5-GFP in seedlings were downloaded from GSE167384. Source data are provided with this paper.
References
Leprince, O., Pellizzaro, A., Berriri, S. & Buitink, J. Late seed maturation: drying without dying. J. Exp. Bot. 68, 827–841 (2017).
Raz, V., Bergervoet, J. H. & Koornneef, M. Sequential steps for developmental arrest in Arabidopsis seeds. Development 128, 243–252 (2001).
Lai, W. K. M. & Pugh, B. F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat. Rev. Mol. Cell Biol. 18, 548–562 (2017).
Venkatesh, S. & Workman, J. L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 16, 178–189 (2015).
Krishnakumar, R. & Blelloch, R. H. Epigenetics of cellular reprogramming. Curr. Opin. Genet. Dev. 23, 548–555 (2013).
Li, D., Shu, X., Zhu, P. & Pei, D. Chromatin accessibility dynamics during cell fate reprogramming. EMBO Rep. 22, e51644 (2021).
Swygert, S. G. & Peterson, C. L. Chromatin dynamics: interplay between remodeling enzymes and histone modifications. Biochim. Biophys. Acta 1839, 728–736 (2014).
Talbert, P. B. & Henikoff, S. Histone variants on the move: substrates for chromatin dynamics. Nat. Rev. Mol. Cell Biol. 18, 115–126 (2017).
Borg, M., Jiang, D. & Berger, F. Histone variants take center stage in shaping the epigenome. Curr. Opin. Plant Biol. 61, 101991 (2021).
Martire, S. & Banaszynski, L. A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 21, 522–541 (2020).
Probst, A. V., Desvoyes, B. & Gutierrez, C. Similar yet critically different: the distribution, dynamics and function of histone variants. J. Exp. Bot. 71, 5191–5204 (2020).
Henikoff, S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat. Rev. Genet. 9, 15–26 (2008).
Filipescu, D., Muller, S. & Almouzni, G. Histone H3 variants and their chaperones during development and disease: contributing to epigenetic control. Annu. Rev. Cell Dev. Biol. 30, 615–646 (2014).
Ahmad, K. & Henikoff, S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200 (2002).
Gaillard, P. H. L. et al. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell 86, 887–896 (1996).
Ray-Gallet, D. et al. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol. Cell 9, 1091–1100 (2002).
Smith, S. & Stillman, B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58, 15–25 (1989).
Tagami, H., Ray-Gallet, D., Almouzni, G. & Nakatani, Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61 (2004).
Goldberg, A. D. et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691 (2010).
Wirbelauer, C., Bell, O. & Schubeler, D. Variant histone H3.3 is deposited at sites of nucleosomal displacement throughout transcribed genes while active histone modifications show a promoter-proximal bias. Genes Dev. 19, 1761–1766 (2005).
Sitbon, D., Boyarchuk, E., Dingli, F., Loew, D. & Almouzni, G. Histone variant H3.3 residue S31 is essential for Xenopus gastrulation regardless of the deposition pathway. Nat. Commun. 11, 1256 (2020).
Szenker, E., Lacoste, N. & Almouzni, G. A developmental requirement for HIRA-dependent H3.3 deposition revealed at gastrulation in Xenopus. Cell Rep. 1, 730–740 (2012).
Bush, K. M. et al. Endogenous mammalian histone H3.3 exhibits chromatin-related functions during development. Epigenet. Chromatin 6, 7 (2013).
Couldrey, C., Carlton, M. B., Nolan, P. M., Colledge, W. H. & Evans, M. J. A retroviral gene trap insertion into the histone 3.3A gene causes partial neonatal lethality, stunted growth, neuromuscular deficits and male sub-fertility in transgenic mice. Hum. Mol. Genet. 8, 2489–2495 (1999).
Tang, M. C. et al. Contribution of the two genes encoding histone variant h3.3 to viability and fertility in mice. PLoS Genet. 11, e1004964 (2015).
Jang, C. W., Shibata, Y., Starmer, J., Yee, D. & Magnuson, T. Histone H3.3 maintains genome integrity during mammalian development. Genes Dev. 29, 1377–1392 (2015).
Lin, C. J., Conti, M. & Ramalho-Santos, M. Histone variant H3.3 maintains a decondensed chromatin state essential for mouse preimplantation development. Development 140, 3624–3634 (2013).
Loppin, B. & Berger, F. Histone variants: the nexus of developmental decisions and epigenetic memory. Annu. Rev. Genet. 54, 121–149 (2020).
Lu, L., Chen, X., Qian, S. & Zhong, X. The plant-specific histone residue Phe41 is important for genome-wide H3.1 distribution. Nat. Commun. 9, 630 (2018).
Talbert, P. B. et al. A unified phylogeny-based nomenclature for histone variants. Epigenet. Chromatin 5, 7 (2012).
Jiang, D. & Berger, F. Histone variants in plant transcriptional regulation. Biochim. Biophys. Acta Gene Regul. Mech. 1860, 123–130 (2017).
Wollmann, H. et al. Dynamic deposition of histone variant H3.3 accompanies developmental remodeling of the Arabidopsis transcriptome. PLoS Genet. 8, e1002658 (2012).
Stroud, H. et al. Genome-wide analysis of histone H3.1 and H3.3 variants in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 109, 5370–5375 (2012).
Wollmann, H. et al. The histone H3 variant H3.3 regulates gene body DNA methylation in Arabidopsis thaliana. Genome Biol. 18, 94 (2017).
Zhao, F., Zhang, H., Zhao, T., Li, Z. & Jiang, D. The histone variant H3.3 promotes the active chromatin state to repress flowering in Arabidopsis. Plant Physiol. 186, 2051–2063 (2021).
Kawashima, T. et al. Dynamic F-actin movement is essential for fertilization in Arabidopsis thaliana. eLife https://doi.org/10.7554/eLife.04501 (2014).
Jiang, D. & Berger, F. DNA replication-coupled histone modification maintains Polycomb gene silencing in plants. Science 357, 1146–1149 (2017).
Debeaujon, I., Leon-Kloosterziel, K. M. & Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 122, 403–414 (2000).
Holdsworth, M. J., Bentsink, L. & Soppe, W. J. J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. N. Phytologist 179, 33–54 (2008).
Ogawa, M. et al. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15, 1591–1604 (2003).
Okamoto, M. et al. CYP707A1 and CYP707A2, which encode abscisic acid 8 ‘-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141, 97–107 (2006).
Ingouff, M. et al. Zygotic resetting of the HISTONE 3 variant repertoire participates in epigenetic reprogramming in Arabidopsis. Curr. Biol. 20, 2137–2143 (2010).
Zuo, J., Niu, Q. W. & Chua, N. H. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J.: Cell Mol. Biol. 24, 265–273 (2000).
Fujiwara, T., Nambara, E., Yamagishi, K., Goto, D. B. & Naito, S. Storage proteins. Arabidopsis book / Am. Soc. Plant Biologists 1, e0020 (2002).
Schneider, A. et al. Potential targets of VIVIPAROUS1/ABI3-LIKE1 (VAL1) repression in developing Arabidopsis thaliana embryos. Plant J. 85, 305–319 (2016).
Jiang, D. et al. The evolution and functional divergence of the histone H2B family in plants. PLoS Genet. 16, e1008964 (2020).
Lu, Z., Hofmeister, B. T., Vollmers, C., DuBois, R. M. & Schmitz, R. J. Combining ATAC-seq with nuclei sorting for discovery of cis-regulatory regions in plant genomes. Nucleic Acids Res. 45, e41 (2017).
Maher, K. A. et al. Profiling of accessible chromatin regions across multiple plant species and cell types reveals common gene regulatory principles and new control modules. Plant Cell 30, 15–36 (2018).
Jin, C. Y. & Felsenfeld, G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 21, 1519–1529 (2007).
Coleman-Derr, D. & Zilberman, D. Deposition of histone variant H2A.Z within gene bodies regulates responsive genes. PLoS Genet. 8, e1002988 (2012).
Yelagandula, R. et al. The histone variant H2A.W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell 158, 98–109 (2014).
Xue, M. et al. The INO80 chromatin remodeling complex promotes thermomorphogenesis by connecting H2A.Z eviction and active transcription in Arabidopsis. Mol. Plant 14, 1799–1813 (2021).
Maezawa, S., Yukawa, M., Alavattam, K. G., Barski, A. & Namekawa, S. H. Dynamic reorganization of open chromatin underlies diverse transcriptomes during spermatogenesis. Nucleic Acids Res. 46, 593–608 (2018).
Starks, R. R., Biswas, A., Jain, A. & Tuteja, G. Combined analysis of dissimilar promoter accessibility and gene expression profiles identifies tissue-specific genes and actively repressed networks. Epigenet. Chromatin 12, 16 (2019).
Gaston, K. & Jayaraman, P. S. Transcriptional repression in eukaryotes: repressors and repression mechanisms. Cell Mol. Life Sci. 60, 721–741 (2003).
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011).
Comai, L. & Harada, J. J. Transcriptional activities in dry seed nuclei indicate the timing of the transition from embryogeny to germination. Proc. Natl Acad. Sci. USA 87, 2671–2674 (1990).
Zhong, Z. et al. DNA methylation-linked chromatin accessibility affects genomic architecture in Arabidopsis. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2023347118 (2021).
Borg, M. et al. Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat. Cell Biol. https://doi.org/10.1038/s41556-020-0515-y (2020).
Ingouff, M., Hamamura, Y., Gourgues, M., Higashiyama, T. & Berger, F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr. Biol. 17, 1032–1037 (2007).
ten Hove, C. A., Lu, K. J. & Weijers, D. Building a plant: cell fate specification in the early Arabidopsis embryo. Development 142, 420–430 (2015).
Otero, S., Desvoyes, B., Peiro, R. & Gutierrez, C. Histone H3 dynamics reveal domains with distinct proliferation potential in the Arabidopsis root. Plant Cell 28, 1361–1371 (2016).
Nie, X., Wang, H., Li, J., Holec, S. & Berger, F. The HIRA complex that deposits the histone H3.3 is conserved in Arabidopsis and facilitates transcriptional dynamics. Biol. open 3, 794–802 (2014).
Layat, E. et al. The Histone chaperone HIRA is a positive regulator of seed germination. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22084031 (2021).
Duc, C. et al. Arabidopsis ATRX modulates H3.3 occupancy and fine-tunes gene expression. Plant Cell 29, 1773–1793 (2017).
Wang, H. et al. LHP1 Interacts with ATRX through plant-specific domains at specific loci targeted by PRC2. Mol. Plant 11, 1038–1052 (2018).
Rajjou, L. et al. Seed germination and vigor. Annu. Rev. Plant Biol. 63, 507–533 (2012).
Dure, L. & Waters, L. Long-lived messenger RNA: evidence from cotton seed germination. Science 147, 410–412 (1965).
Rajjou, L. et al. The effect of alpha-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol. 134, 1598–1613 (2004).
Nakabayashi, K., Okamoto, M., Koshiba, T., Kamiya, Y. & Nambara, E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J.: Cell Mol. Biol. 41, 697–709 (2005).
Kimura, M. & Nambara, E. Stored and neosynthesized mRNA in Arabidopsis seeds: effects of cycloheximide and controlled deterioration treatment on the resumption of transcription during imbibition. Plant Mol. Biol. 73, 119–129 (2010).
Jin, C. et al. H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat. Genet. 41, 941–945 (2009).
Yang, Y. et al. HIRA complex presets transcriptional potential through coordinating depositions of the histone variants H3.3 and H2A.Z on the poised genes in mESCs. Nucleic Acids Res. https://doi.org/10.1093/nar/gkab1221 (2021).
Shu, H. et al. Arabidopsis replacement histone variant H3.3 occupies promoters of regulated genes. Genome Biol. 15, R62 (2014).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Nakagawa, T. et al. Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in Transgenic analysis of plants. Biosci. Biotech. Bioch 71, 2095–2100 (2007).
Chen, S. F., Zhou, Y. Q., Chen, Y. R. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, 884–890 (2018).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907-+ (2019).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ramirez, F., Dundar, F., Diehl, S., Gruning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–U354 (2012).
Thorvaldsdottir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Yu, G., Wang, L. G. & He, Q. Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Guo, W. et al. BS-Seeker2: a versatile aligning pipeline for bisulfite sequencing data. BMC Genomics 14, 774 (2013).
Guo, W. et al. CGmapTools improves the precision of heterozygous SNV calls and supports allele-specific methylation detection and visualization in bisulfite-sequencing data. Bioinformatics 34, 381–387 (2018).
Acknowledgements
We thank Dr. Hidenori Takeuchi for providing CRISPR-Cas9 generated htr4;htr5 double mutant. Work in D.J. laboratory was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Precision Seed Design and Breeding, XDA24020303), the National Natural Science Foundation of China (31970527, 32170545, and 32150610472) and the National Key R&D Program of China (2019YFA0903903). Work in F.B. laboratory was supported by FWF grant (P30802).
Author information
Authors and Affiliations
Contributions
F.B. and D.J. conceived the research. T.Z., J.L., M.X., J.P., L.M., and D.J. performed experiments. H.Z. performed bioinformatics analysis. F.B. and D.J. wrote the paper with inputs from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yuda Fang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, T., Lu, J., Zhang, H. et al. Histone H3.3 deposition in seed is essential for the post-embryonic developmental competence in Arabidopsis. Nat Commun 13, 7728 (2022). https://doi.org/10.1038/s41467-022-35509-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35509-6
This article is cited by
-
In vitro co-expression chromatin assembly and remodeling platform for plant histone variants
Scientific Reports (2024)
-
Interplay between coding and non-coding regulation drives the Arabidopsis seed-to-seedling transition
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.