Abstract
Conductive metal-organic frameworks (MOFs) have performed well in the fields of energy and catalysis, among which two-dimensional (2D) and three-dimensional (3D) MOFs are well-known. Here, we have synthesized a one-dimensional (1D) conductive metal-organic framework (MOF) in which hexacoordinated 1,5-Diamino-4,8-dihydroxy-9,10-anthraceneedione (DDA) ligands are connected by double Cu ions, resulting in nanoribbon layers with 1D π-d conjugated nanoribbon plane and out-of-plane π-π stacking, which facilitates charge transport along two dimensions. The DDA-Cu as a highly conductive n-type MOF has high crystalline quality with a conductivity of ~ 9.4 S·m−1, which is at least two orders of magnitude higher than that of conventional 1D MOFs. Its electrical band gap (Eg) and exciton binding energy (Eb) are approximately 0.49 eV and 0.3 eV, respectively. When utilized as electrode material in a supercapacitor, the DDA-Cu exhibits good charge storage capacity and cycle stability. Meanwhile, as thse active semiconductor layer, it successfully simulates the artificial visual perception system with excellent bending resistance and air stability as a MOF-based flexible optoelectronic synaptic case. The controllable preparation of high-quality 1D DDA-Cu MOF may enable new architectural designs and various applications in the future.
Similar content being viewed by others
Introduction
One-dimensional (1D) materials such as nanofibers1, nanorods2,3, nanotubes4,5, and nanoribbons6,7,8 are an integral part of our technological society, which are widely utilized in several applications, including electronics, supercapacitors, membrane, catalysis, and sensors. In particular, inorganic 1D materials based on ZnO and carbon nanotubes have been intensively studied9,10,11. Graphene is a two-dimensional (2D) nanomaterial12. However, its nanoribbon allotrope constructed via covalent chemistry is a typical example of 1D conductive material13, whose band gap can be tuned as a function of the nanoribbon width and edge structure. Therefore, it has demonstrated exceptional electronic properties and several potential applications in electronic devices14. 1D quantum confinement and structural anisotropy can lead to interesting physical phenomena as well as applications in flexible electronic devices15,16. Recently, owing to their high structural regularity, chemically modular edges, and tunable structure–activity relationship, the construction of nanomaterials based on small molecular building blocks has garnered significant attention17. Although there are many reports on the synthesis of linear, planar, and stereoscopic polymers with diverse structures and properties, the synthesis of crystalline 1D organic nanomaterials, which includes repetition of highly conjugated structural units with stable ordered structure and excellent conductivity, has met with much less success. Therefore, the precise construction of 1D conductive nanomaterials through electronically conjugated metal-organic frameworks (MOFs) or covalent organic frameworks (COFs) is of immense significance to boost the applications of such materials.
Building effective charge transport pathways, such as through-bond pathways, extended conjugation pathways, and through-space pathways, plays a crucial role in the preparation of conductive MOFs18. For through-bond pathways (Fig. 1a), metal ions with different coordination numbers dominate the coordination bond paths of MOFs. Particularly, active d-electron transition metals, which have a suitable radius to obtain a better orbital overlap with the ligands, are conducive to generating a small band gap and high charge mobility. By utilizing ligands with aromatic organic cores, coordination bonds can evolve into extended π–d-conjugated planes (Fig. 1b), while π–π stacking of aromatic organic cores in the ligands causes the through-space pathways to be perpendicular to the conjugated planes in 2D materials (Fig. 1c). Especially, in some lanthanide MOFs19,20 and 2D COFs21,22,23, the significant contribution of π–π stacking to the conductivity has been confirmed. By constructing π–d-conjugated planes and π–π stacking paths, 2D MOFs can occupy the dominant position in conductive MOFs24,25,26. Based on the strong in-plane conjugation between the π-systems of the ligands and the d orbitals of the metals, the highest reported conductivity values of non-porous crystalline copper (II) benzenehexathiolate coordination polymers27 and porous Ni-based MOFs25 are 2500 and 40 S cm−1, respectively.
a–c Charge transport pathways through-bond (a), extended conjugation planes (b) and through-space (c). The “metal” means the d orbitals of metals, “ligand” is the p orbitals of ligand and “π core” represents the conjugated π plane of ligand. d Synthetic schematic diagram of DDA-Cu. e Crystal structure of DDA-Cu.
Theoretically, 1D MOFs have a higher structural degree of freedom than 2D and 3D MOFs, providing more opportunities for creating conductive paths. However, for 1D MOFs, the chain stacking is adjusted by van der Waals or hydrogen bond interactions with only one dimension restricted by the coordination bonds. This creates several challenges in designing the spatial arrangement of 1D chains to improve conductivity. Here, focusing on the construction of effective charge transport pathways, we have designed and synthesized a 1D DDA-Cu MOF with nanoribbon layers based on the self-assembly of Cu2+ and DDA ligand with an extended π plane of the anthraquinone nucleus (Fig. 1d). Surprisingly, in the plane, the DDA-Cu chains, called nanoribbons, form extended π–d-conjugated planes with the configuration restrictions of the bimetallic Cu2+ sites, and the chains arrange in a nanoribbon layer by van der Waals interaction similar to the 2D MOF layer. In addition, the DDA-Cu exhibits a tight π–π stacking outside the plane. As expected, the DDA-Cu exhibits excellent conductivity of 9.4 S m−1, one of the highest reported values for 1D conductive MOFs. Density functional theory (DFT) calculations are utilized to confirm that the constructed out-of-plane charge transport pathways are responsible for excellent conductivity. Further, the good electrical performance of the DDA-Cu is validated by employing it as an active material for symmetrical supercapacitors. The mass capacitance and areal capacitance reach 118 F/g and 236 mF/cm2, respectively, exceeding the values of many recently reported carbon materials (carbon nanotubes, graphene, MOFs, etc.). In addition, a flexible photoelectric synaptic device based on DDA-Cu films is also manufactured, and it effectively mimics biological synapse-like signals, including postsynaptic current, paired-pulse facilitation (PPF), short-term plasticity (STP), long-term plasticity (LTP), and STP-to-LTP transformation. Interestingly, the flexible device array with good air stability and bending resistance achieves an imaging function similar to human visual perception.
Results
Synthesis and characterization
Figure 1d displays a schematic of the synthetic route of DDA-Cu and the detailed reaction mechanism is presented in Supplementary Fig. 1. We selected hexacoordinated DDA as the organic building block for the coordination reaction with Cu2+ ions and obtained crystalline DDA-Cu MOF with isolated yields of 85–92% in a solution of ethanol and water. Due to the high restriction of bimetallic sites on the structure planarity, the DDA-Cu grows linearly along the coordination nodes, and the 1D nanoribbons stack through non-bonding interactions to form bulk materials (Fig. 1e).
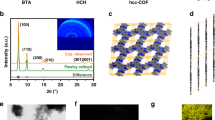
As shown in Fig. 2a and b, the DDA-Cu MOF has a needle-like crystalline morphology with a maximum length of more than 100 μm. The elemental mapping (Fig. 2c) demonstrates a homogeneous distribution of O, Cu, N and C at the end of the crystal. In the X-ray photoelectron spectra (XPS) (see Supplementary Fig. 2a), the two main peaks at 953.8 and 933.9 eV are ascribed to Cu 2p1/2 and Cu 2p3/2, respectively, which are consistent with the values reported for Cu-based frameworks28. The electron paramagnetic resonance (EPR) spectrum of DDA-Cu displays a clear signal (g = 2.12), which is attributed to the single electron of 3d9 orbital of Cu2+ (see Supplementary Fig. 3). The line intensity for DDA-Cu in the X-ray absorption near edge spectrum (XANES; Fig. 2d) is closer to that for CuO, indicating higher energy than Cu2O and Cu, which demonstrates the formation of Cu2+ species in the MOF. The extended X-ray absorption fine structure (EXAFS) reveals the presence of metal coordination structure in the DDA-Cu (Fig. 2e). The EXAFS fitting curves show that the coordination number of the Cu site is 4 with the distance of 1.82 Å for Cu–O and 1.96 Å for Cu–N. Furthermore, the maximum intensity at ~5 Å–1 in the wavelet transform (WT) of the EXAFS indicates the Cu–O/N contribution (see Supplementary Fig. 4).
a Optical image of DDA-Cu crystals. Scale bar 25 μm. b SEM image of a DDA-Cu crystal. The scale bar is 25 μm. c Element mapping of the DDA-Cu crystal. Scale bar 5 μm. d XANES profiles of DDA-Cu, CuO, Cu2O, and Cu. e EXAFS curves of DDA-Cu, Cu2O, CuO and Cu. f 13C CP/MAS NMR spectra for DDA-Cu and DDA. g–i Partial enlarged view of carbon atoms 4, 1 and 7 in DDA-Cu and DDA. j PXRD results of DDA–Cu. k, l TEM images of DDA–Cu with different magnifications. Scale bars 10 nm in (k) and 1 nm in (l). Source data are provided as a Source Data file.
The solid-state 13C cross-polarization/magic angle spinning nuclear magnetic resonance (13C CP/MAS NMR) spectra demonstrate the organic structure of DDA-Cu. As shown in Fig. 2f, the organic C skeleton of DDA-Cu is consistent with that of the DDA ligand, and the chemical shifts of C connected to the amino group (4), carbonyl group (1), and hydroxyl group (7) have a significant change (Fig. 2g–i), causing the transfer of 2p electrons in N/O to the 3d orbital of Cu2+. This is also supported by the shift of N/O toward higher binding energy in XPS of DDA-Cu (see Supplementary Fig. 2). The elemental analysis by inductively coupled plasma-optical emission spectrometry (ICP-OES) suggests that the content of Cu in DDA-Cu is 30.05%, which is consistent with the theoretical value of 32.5%.
Figure 2j shows the powder X-ray diffraction (PXRD) pattern of the crystalline DDA-Cu. The PXRD pattern indicates that DDA-Cu is a crystalline material with a long-range structure. The experimental (blue curve) and theoretical (grey curve) results are in good agreement with each other. The Pawley refinement result (Rp = 2.5%, and Rwp = 3.5%) proves that the cell parameters of DDA-Cu are a = 11.8 Å, b = 7.9 Å, c = 3.2 Å, α = β = 90.0°, and γ = 87.7° in the P2/M space group (the crystal structure is presented in Table S1). Five diffraction peaks at 2θ = 7.4°, 11.2°, 18.4°, 22.5°, 127.9° are observed, which can be indexed as the (1 0 0), (0 1 0), (2 1 0), (0 2 0), and (0 0 1) reflections. The presence of a high-intensity peak at 7.4° is associated with the periodicities in the (1 0 0) plane and indicates a well-defined ordered columnar array. In addition, for comparison, aligned, dislocated and rotation structures are also predicted in which one Cu2+ ion is coordinated with two organic ligands (see Supplementary Figs. 5 and 6). For the dislocated one (Model A), two small peaks are located at 9.2° and 9.5°. For the aligned one (Model B), there is no peak in the range from 8° to 12°. These features are not observed in our samples, indicating the sole formation of crystalline DDA-Cu with bimetallic Cu2+ coordination at the joint point (Fig. 1e).
The N2/Ar adsorption/desorption isotherms of DDA-Cu are shown in Supplementary Fig. 7. The calculated Brunauer–Emmett–Teller surface area is ~127.3 m2/g (N2). The high-resolution transmission electron microscopy (HRTEM) image demonstrates the 1D periodicity of DDA-Cu, and the lattice fringe spacing is ~1.18 nm, which corresponds to the (1 0 0) plane (Fig. 2k and l). Further, the lattice fringe spacing of ~0.32 nm corresponding to the (0 0 1) plane demonstrates its layered structure, which is similar to the 2D graphene structure (see Supplementary Fig. 8). The chemical stability of DDA-Cu was evaluated in Supplementary Fig. 9. In addition, thermal gravimetric analysis reveals an initial decomposition temperature of approximately 320 °C (see Supplementary Fig. 10), suggesting good thermal stability of the DDA-Cu.
Similar to the 2D π-conjugated COFs and MOFs systems, the 1D DDA-Cu displays strong charge delocalization across the entire nanoribbon plane, which is caused by the good energy overlap between the bimetallic nodes and the organic ligands in oxidized form. This property, along with the layered structure, makes the 1D DDA-Cu an ideal candidate to produce high-quality films by a liquid/liquid interface method that can preserve the electronic properties of the ribbons. DDA-Cu films can be transferred on sapphire and Si/SiO2 substrates (see Fig. 3a and Supplementary Fig. 11), and their thickness can be controlled by the growth time (see Supplementary Fig. 12). The film is ~8 nm thick after 12 h growth, and after a longer growth time of 72 h, the thickness can reach nearly 245 nm. Further, its morphology changes from an early dense film to a loose leaf stacked on the surface (Fig. 3b). For a 25-nm-thick film, the 2D grazing-incidence wide-angle X-ray scattering (GIWAXS) image (Fig. 3c) and the integral curve (Fig. 3d) prove the crystallinity of the film, and the four diffraction signals at q = 0.44, 0.81, 1.6, and 1.9 Å–1 are identified, which are in accordance with the PXRD results. According to the intensity variation of the (0 0 1) diffraction ring (1.9 Å–1) in the in-plane and out-of-plane direction of GIWAXS profiles, a preferentially oriented film is formed on the substrate surface (see Supplementary Fig. 13). The structure of DDA-Cu film is also investigated by high-resolution atomic force microscopy (HRAFM; see Fig. 3e and Supplementary Fig. 14), where parallel patterns with a width of ~1.2 nm are observed. In addition, HRTEM images show that the film consists of some highly ordered, perfectly aligned small domains that correspond to the (1 0 0) plane (see Supplementary Fig. 15), indicating the high-crystalline quality of the DDA-Cu film.
a Optical image of DDA-Cu film transferred on sapphire. b SEM images of DDA-Cu films grown at different reaction times. Scale bar 1 μm. c 2D GIWAXS image of a DDA-Cu film. Scale bar 4 nm. d The integral curve of GIWAXS result. e HRAFM of DDA-Cu film. Scale bar 4 nm. Source data are provided as a Source Data file.
Electrical properties
The conductivity of the DDA-Cu film transferred on a Si/SiO2 substrate was measured through two-terminal devices (see Supplementary Fig. 16), and the results are shown in Supplementary Fig. 17. The linear and symmetric current–voltage (I–V) curves suggest that ohmic contacts are formed at the source and drain electrodes. The obtained conductivity is ~9.4 S m−1, which is at least two orders of magnitude higher than that of conventional 1D MOFs29,30 (Table S2) and is comparable to that of some conductive MOF and COF films23,28 (Table S3). The impressive conductivity makes it a promising candidate for energy storage applications. To this end, we studied its capacitance performance through a three-electrode system in a 3 M KOH aqueous solution. The cyclic voltammetry (CV) curves have no typical redox waves under both cathodic (−0.4 to 0.0 V) and anodic (0.0–0.6 V) cycling (see Supplementary Fig. 18a), suggesting a double-layer capacitance behavior31 in a wide voltage window of 1 V. The charge storage mechanism is discussed in detail in Supplementary Figs. 19 and 20. Subsequently, we fabricated a symmetrical supercapacitor with DDA-Cu as the active electrode material. The CV curves exhibit good reversibility at different scan rates (see Supplementary Fig. 18b) similar to the curves of 2D MOF electrode32, demonstrating a stable charge storage behavior of this device. The calculated specific gravimetric capacitance (Cg) using the galvanostatic charge–discharge (GCD) curves at different current densities is shown in Supplementary Fig. 18c. At a low charge–discharge rate of 0.1 A/g, the Cg is 118 F/g, which is superior to that of many reported activated carbons, carbon nanotubes, graphene, and MOFs (Table S4). Furthermore, under a current density of 0.4 mA/cm2, the calculated maximum areal capacitance (Cs) is 236 mF/cm2, which is significantly better than the reported values for some MOF-based supercapacitors33,34,35,36. The ion and charge transport kinetics of the device was examined by electrochemical impedance spectroscopy (EIS). As displayed in the Nyquist plots (see Supplementary Fig. 18d), the small semicircle at a high frequency indicates low charge transfer resistance (Rct, 1.3 Ω), and low equivalent resistance (Rs, 3.2 Ω), demonstrating efficient charge transfer and electrolyte ion transport24,32.
Band structure
Characterizing the band structure through experimental and theoretical methods can be useful to comprehensively explore the electronic structure of the DDA-Cu and understand its intrinsic conduction mechanism. Figure 4a shows the ultraviolet photoelectron spectrum (UPS) of DDA-Cu, where the highest occupied molecular orbital (HOMO) is 0.37 eV below the Fermi level (Ef). Traditionally, the lowest unoccupied molecular orbital (LUMO) of organic materials is estimated based on the optical band gap (Egopt) because it is challenging to determine a reliable LUMO value through experiments37. However, via low-energy inverse photoelectron spectroscopy (LEIPS) with low damage to film materials, we show an example that the experimental LUMO of MOFs can be measured directly, which is 4.40 eV below the vacuum level (Evac) for DDA-Cu (Fig. 4b). Therefore, the electrical band gap (Eg) of DDA-Cu is calculated to be 0.49 eV, where the Ef is set as 0 (Fig. 4c). This is one of the typical narrow band gaps reported for MOFs19,20,38, which facilitates easy charge transportation. And we performed variable temperature conductance tests (see Supplementary Fig. 21), Hall effect tests (see Supplementary Fig. 22a) by van der Pauw technique, and Motto–Schotky curve test (see Supplementary Fig. 22b) proving the n-type semiconducting properties of DDA-Cu. In addition, the Egopt of DDA-Cu is estimated through infrared diffuse reflection spectra (see Supplementary Fig. 23), and the indirect Egopt is obtained to be 0.19 eV. Then, the exciton binding energy (Eb = Eg–Egopt) is ~0.3 eV (see Supplementary Fig. 24), which represents the energy binding an electron–hole pair through the electrostatic Coulomb force in DDA-Cu39,40. The Eb is close to the minimum value achieved for 2D metal halide perovskites and transition-metal dichalcogenides (TMDCs)41,42, which facilitates high-efficiency charge separation in DDA-Cu with potential application in optoelectronic devices.
a The UPS spectroscopy of DDA-Cu. b The LEIPS spectroscopy of DDA-Cu. c The electrical band gap characterization combined by UPS and LEIPS. d A view of the Brillouin zone paths used in the DFT calculations. e The band structure (the left part) and density of states (the right part) for DDA-Cu by HSE06 method. The Fermi energy has been corrected to 0 eV. The orange and blue branches correspond to spin-up and spin-down bands in the band structure, respectively. For the density of states, the arrow up represents the spin-up part and the arrow down represents the spin-down part. Source data are provided as a Source Data file.
Further, DFT calculations based on HSE06 method were utilized to examine the band structure of the DDA-Cu. The Brillouin zone paths are shown in Fig. 4d. The band structure and projected density of states (PDOS) of DDA-Cu are shown in Fig. 4e. Specifically, in the band structure, along the direction of interlayer π–π stacking (G–Y, A–B, and D–E), the band crosses the Fermi level, which suggests metallic characteristics of the MOF as the optimal charge transport path. By contrast, the Fermi level is located inside the bands along the in-plane direction (Z–G, Y–A, B–D and E–C), indicating a semiconductor behavior. An in-plane indirect band gap of 0.34 eV at the G and Z points (see Supplementary Fig. 25) was observed. In fact, in the in-plane direction, the band also presents significant anisotropy: significant dispersion occurs along the chain direction (Z–G and B–D) in the band; however, the band is almost flat in the direction perpendicular to the DDA-Cu chain (Y–A and E–C) as the most unfavorable charge transport path. These results indicate that DDA-Cu is rich in anisotropic transport behavior19.
Flexible organic optoelectronic synapse
Narrow Eg and small Eb are conducive to achieving charge excitation and efficient charge separation39. Thus, based on the DDA-Cu films coated on Si/SiO2, we fabricated optoelectronic devices and explored their potential as an active semiconductor layer in the simulation of human brain synapses (Fig. 5a). The current variation caused by light excitation can simulate postsynaptic current change (∆PSC) during the nerve signal transmission process of the human brain. The proposed photo-synaptic mechanism is presented in Supplementary Fig. 26 and we also analyzed the influence of the thermal effect of lighting (see Supplementary Fig. 27). We investigated the ∆PSC characteristics under different excitation wavelengths in the device. The ∆PSC of red light is higher than that of yellow light (see Supplementary Fig. 28), which is consistent with the ultraviolet–visible–near-infrared (UV–vis–NIR) absorption spectrum (see Supplementary Fig. 29), and white light corresponds to the most significant excitation current. Therefore, white light was used to investigate the performance of the device. As shown in Supplementary Fig. 30a, since the photogenerated electron–hole pairs in the DDA-Cu cannot recombine immediately after the light is removed43, the current decreases slowly (∆PSC = 5.0 nA) after the light source is removed. Further, ∆PSC increases to a higher value of 5.5 nA after two light pulses (see Supplementary Fig. 30b).
a Schematic diagram of synapses in the human brain. b The optical image of a DDA-Cu-based flexible device on PET substrate. c Synaptic signals of the flexible devices based on PET substrate stimulated by one light pulse (red) and two light pulses (blue) under white light (excitation time = 10 s, interval time = 15 s between two light pulses, 20 mW/cm2). The VDS is 5 V. d Variation curve of PPF with △t. e The transition from STP to LTP by the duration of different light stimuli. f The transition of STP to LTP is introduced by different light intensities. g Diagram of the image memory of the number “21”. Source data are provided as a Source Data file.
The ever-increasing demand for flexible electronics around the world prompted us to evaluate the application of DDA-Cu film in flexible devices (Fig. 5b). Compared with the devices using the Si/SiO2 as substrate, the synaptic response has only a slight decrease (∆PSC = 3.6 nA by one light pulse and ∆PSC = 4.1 nA by two light pulses in Fig. 5c and Supplementary Fig. 30c,d) in the devices based on polyethylene terephthalate (PET). The reproducibility of the photo-synaptic behavior described above is shown in Supplementary Figs. 31 and 32. PPF is an important manifestation of synaptic STP, which is defined as the ratio of the second ∆PSC to the first ∆PSC after two light pulses. Figure 5d shows the variation in the PPF as a function of the time interval (∆t). As the ∆t increases, the PPF value gradually decreases from 121% to 112%, and this is similar to the positive correlation between memory strength and memory interval in the human brain learning process, which is essential for recognizing and decoding time-resolved information (Table S5)44,45,46. Repeated rehearsal of STP gradually leads to LTP. The transformable characteristics of synaptic plasticity enable synapses to provide specific responses to complex external stimuli. When the light lasts for 1 s, the ∆PSC rapidly decreases to 0 after the light is removed (Fig. 5e). However, when the illumination time increases to 30 s, the ∆PSC increases significantly (∆PSC = 4.8 nA). After the light is turned off, the current remains at a high value and it takes more time to decay to 0, which demonstrates the typical transition from STP to LTP by radiation time. We also fitted the decay curves of different irradiation times and studied the effect of the duration time of light on the time constant (see Supplementary Fig. 33). Meanwhile, after the light is turned off, the device exhibits higher current as the frequency gradually increases from 0.02 to 0.25 Hz (see Supplementary Fig. 34), which proves that the radiation frequency can also effectively realize the transition from STP to LTP. The light spike number can also accomplish the device transition STP to LTP (see Supplementary Fig. 35). When the spike number is increased from 3 to 16, ∆PSC reaches a larger value, and a higher steady-state current is obtained after the light is turned off. Similarly, the light intensity can also trigger this transition (Fig. 5f). These pieces of evidence of the transition from STP to LTP imply that the devices are beneficial to simulate the human-like short-term memory and long-term memory behaviors for application in smart microelectronic devices.
Furthermore, we integrated a 4 × 10 photo synapse array to simulate the human-like visual perception function, and the image recognition and memory functions of the devices are evaluated by irradiating the patterned light (see Supplementary Fig. 36). The number pattern “21” is successfully resolved after the light is turned on (Fig. 5g), demonstrating the reliable imaging function of the device. Additionally, after the light source is removed, the “21” pattern is memorized and remains clearly visible until 50 s later, verifying that the memory and memory loss behaviors are successfully imitated. It may be noted that the ∆PSC distribution of the imaging point under and after the illumination is narrow with small fluctuations, proving the high stability and uniformity of the human-like visual perception system. Specifically, after being exposed to air for 45 days, the ∆PSC of the device shows a minor decrease (see Supplementary Fig. 37), and after 500 bending experiments (see Supplementary Fig. 38), ∆PSC still remains at 70% (see Supplementary Fig. 39), indicating the excellent bending resistance and air stability. The results suggest that the flexible DDA-Cu-based devices can effectively simulate the artificial vision system with excellent stability.
Discussion
We fabricated a conductive 1D DDA-Cu MOF with extended π–d-conjugated nanoribbon layers. The experimental results showed that the DDA-Cu is a typical narrow band gap semiconductor (0.49 eV) with a small Eb of 0.3 eV. It exhibited an impressive conductivity of ~9.4 S m−1, which was attributed to the efficient charge transport along the DDA-Cu chains and the π–π stacking direction, as confirmed by DFT calculations. When used as the active material for a supercapacitor, the DDA-Cu exhibited excellent charge storage capacity and stability. Furthermore, as the active layer of photosynaptic device, it successfully simulated the artificial visual perception system with an imaging function, excellent bending resistance, and air stability, which has immense potential in human-like visual simulation.
Methods
Materials
Cu(CH3COO)2·H2O, 1,5-Diamino-4,8-dihydroxyanthraquinone (DDA) were purchased from SAAN Chemical Technology Co. Ltd. Ethanol, and tetrahydrofuran and dichloromethane (CH2Cl2) were purchased from Concord Technology Co., Ltd. All reagents were utilized without purification.
Synthesis of DDA-Cu MOF crystals
30 mg of DDA was added 400 mL ethanol and 100 mL of water (solution A). 22.2 mg Cu(CH3COO)2·H2O was dissolved in a mixed solution of 400 mL ethanol and 100 mL water (solution B). Solution A was sonicated for 30 min to ensure dissolution. Solution A was stirred while adding solution B into solution A (about 2 drops per second). Then the mixed solution was stirred at 90 °C under normal pressure for 24 h. After centrifugation at 9000 rpm, the blue-black precipitate was collected and centrifuged repeatedly with ethanol and water until the supernatant was clear. The blue-black product was dried in a vacuum drying oven at 90 °C for 12 h.
Synthesis of DDA-Cu MOF film
A liquid–liquid interface is manufactured for the interface coordination reaction of Cu2+ and DDA. The bottom layer is 200 mL CH2Cl2 containing 5.5 mg DDA and the top layer is 100 mL H2O solution containing 13.5 mg Cu(CH3COO)2·H2O. After standing for 3 days, a stable large-area DDA-Cu MOF film can be obtained at the interface.
Characterization of DDA-Cu MOF
13C CP/MAS NMR spectroscopy was recorded on Bruker AVANCE III spectrometer (300 MHz). All chemical shifts are reported in parts per million (ppm). PXRD data were recorded on a powder x-ray diffractometer (Empyrean). TEM observations were conducted on a Cryo-transmission electron microscope (Themis 300). XPS measurements were finished with the ESCALAB250Xl spectrometer using Al Kα X-rays. The UV−vis−NIR absorption spectrum was taken on an ultraviolet, visible spectrophotometer (SHIMADZU, UV-2600). SEM images and element mapping were performed on the Hitachi S-4800 microscope. Thermogravimetric analysis was conducted in a nitrogen atmosphere with a heating rate of 5 oC/min on a Pyris 1 TGA. The GIWAXS measurements were conducted at the synchrotron XRD facility at beamline No. 14B/15U at Shanghai Synchrotron Radiation Facility (SSRF). Elemental analysis of Cu in DDA-Cu MOF was tested by an Agilent 5110 inductively coupled plasma-optical emission spectrometer (ICP-OES). For XAS measurements, the DDA-Cu were analyzed by Cu K-edge XANES at the XAS beamline of the Australian Synchrotron. Energy in spectra has been calibrated, averaged and normalized utilizing the Athena software package47. EPR spectra were collected on a Bruker E500 electron paramagnetic resonance spectrometer at room temperature. N2 adsorption isotherm curves are obtained from the micromeritics ASAP 2460 3.01. Atomic force microscopy tests were conducted using a NanoMan VS AFM under the tapping mode. HRAFM images were obtained using CyPher ES AFM.
Computation method
All the above calculations are conducted using the density functional theory with the projector-augmented-wave (PAW) method, as adopted in the Vienna ab initio simulation package (VASP). For the initial structure optimization of DDA-Cu, the generalized gradient approximation raised by Perdew, Burke, and Ernzerhof (GGA-PBE) is applied for the exchange-correlation potential. For an exact depiction of the localized d electrons of DDA-Cu, the on-site Coulomb interaction was considered to the d orbitals of Cu employing an appropriate U value (4.0 eV)48. The energy convergence criterion was 1 × 10–6 eV and the Hellmann Feynman force convergence criterion was 0.03 eV/Å. The kinetic energy cut-off of 500 eV was applied. Subsequently, via the HSE06 method, a hybrid functional with 1/4 exact Harteen–Fock exchange term, more accurate structural relaxation of DDA-Cu and band structure calculation with the equilibrium geometry of DDA-Cu were applied. It needs to be clarified that the calculation results are based on the perfect crystal model. When complex situations such as crystal defects (vacancy, distortion, etc.)/doping (caused by impurities, etc.)/possible oxidation forms must be considered, the applicability of the above results should be reassessed.
Electrochemical measurement
To prepare the electrode, DDA-Cu crystals, polytetrafluoroethylene, and conductive carbon black with a mass ratio of 7:1:2 were mixed and ground into jelly and coated on 1 × 1 cm nickel foams. The electrode is dried at 90 °C for 8 h and fully dried, and the mass of the active materials is about 4 mg for one single electrode using all measurements. The prepared DDA-Cu on nickel foams as the working electrode is used for a three-electrode test in a 3 M KOH aqueous solution with a Pt plate as the counter electrode and Ag/AgCl as the reference electrode using a CHI 760E electrochemical equipment. Two pieces of DDA-Cu electrodes are separated by a diaphragm impregnated with 3 M KOH to prepare the symmetrical supercapacitors and wrapped plastic wrap on the outside to reduce electrolyte volatilization. According to the GCD curves, the specific capacitance is calculated according to the following formulas:
where \(\frac{I}{m}\) (A/g) and \(\frac{I}{S}\) (mA/cm2) are the current density,\(\,\triangle t\) represents the discharge time,\(\,\triangle V\) means the voltage window. For the electrochemical impedance spectroscopy (EIS) test, the frequency was changed from 105 to 0.01 Hz at open circuit potential with an amplitude of 10 mV.
Electronic device
DDA-Cu film was transferred on SiO2/Si substrates (300 nm SiO2 as a dielectric layer), which were coated with interdigital electrodes. The thickness of Au electrodes is 30 nm. The I–V curve measurements were conducted in an N2 atmosphere at room temperature. The conductivity (k, S m–1) was evaluated as
where \(I\) is the channel current (A), \(E\) is the source–drain voltage, \(l\) (m) is the channel length, \(d\) (m) is the channel width and \(h\) (m) is the thickness of DDA-Cu film, respectively. The size of l and d used for devices is 50 and 1400 μm. Da-Cu films for I–V tests were obtained at 12 h (growth time) and the thickness is about 8 nm. The flexible organic optoelectronic synapse devices were built by transferring the film on PET substrates. Si/SiO2 and PET substrates have been cleaned by immersion in water and ethanol and dried with nitrogen before use. All device performance tests are done on Keithley 4200SC semiconductor parameter analyzer.
Data availability
We have declared that all data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
Change history
12 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41467-023-35884-8
References
Jin, X. H. et al. Long-range exciton transport in conjugated polymer nanofibers prepared by seeded growth. Science 360, 897–900 (2018).
Steimle, B. C., Fenton, J. L. & Schaak, R. E. Rational construction of a scalable heterostructured nanorod megalibrary. Science 367, 418–424 (2020).
Xie, X., Li, Y., Liu, Z. Q., Haruta, M. & Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458, 746–749 (2009).
Liu, L. et al. Aligned, high-density semiconducting carbon nanotube arrays for high-performance electronics. Science 368, 850–856 (2020).
Korde, A. et al. Single-walled zeolitic nanotubes. Science 375, 62–66 (2022).
Slota, M. et al. Magnetic edge states and coherent manipulation of graphene nanoribbons. Nature 557, 691–695 (2018).
Yano, Y. et al. Living annulative π-extension polymerization for graphene nanoribbon synthesis. Nature 571, 387–392 (2019).
Li, X., Wang, X., Zhang, L., Lee, S. & Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 319, 1229–1232 (2008).
Kong, X. Y., Ding, Y., Yang, R. & Wang, Z. L. Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts. Science 303, 1348–1351 (2004).
Niyogi, S. et al. Chemistry of single-walled carbon nanotubes. Acc. Chem. Res. 35, 1105–1113 (2002).
Fujimori, T. et al. Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun. 4, 2162 (2013).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Chen, Z., Narita, A. & Mullen, K. Graphene nanoribbons: on-surface synthesis and integration into electronic devices. Adv. Mater. 32, 2001893 (2020).
Salehi-Khojin, A. et al. Polycrystalline graphene ribbons as chemiresistors. Adv. Mater. 24, 53–57, 52 (2012).
Son, Y. W., Cohen, M. L. & Louie, S. G. Half-metallic graphene nanoribbons. Nature 444, 347–349 (2006).
Zhu, C. et al. Stretchable temperature-sensing circuits with strain suppression based on carbon nanotube transistors. Nat. Electron. 1, 183–190 (2018).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Xie, L. S., Skorupskii, G. & Dinca, M. Electrically conductive metal-organic frameworks. Chem. Rev. 120, 8536–8580 (2020).
Skorupskii, G. et al. Efficient and tunable one-dimensional charge transport in layered lanthanide metal-organic frameworks. Nat. Chem. 12, 131–136 (2020).
Dong, R. et al. High-mobility band-like charge transport in a semiconducting two-dimensional metal-organic framework. Nat. Mater. 17, 1027–1032 (2018).
Guo, J. et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4, 2736 (2013).
Wang, M. et al. High-mobility semiconducting two-dimensional conjugated covalent organic frameworks with p-type doping. J. Am. Chem. Soc. 142, 21622–21627 (2020).
Yang, C. et al. Chemically stable polyarylether-based metallophthalocyanine frameworks with high carrier mobilities for capacitive energy storage. J. Am. Chem. Soc. 143, 17701–17707 (2021).
Du, X. et al. Solid-solid interface growth of conductive metal-organic framework nanowire arrays and their supercapacitor application. Mater. Chem. Front. 4, 243–251 (2020).
Sheberla, D. et al. High electrical conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a semiconducting metal-organic graphene analogue. J. Am. Chem. Soc. 136, 8859–8862 (2014).
Huang, X. et al. A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 6, 7408 (2015).
Huang, X. et al. Superconductivity in a copper(II)-based coordination polymer with perfect kagome structure. Angew. Chem. Int. Ed. Engl. 57, 146–150 (2018).
Liu, Y. et al. Electrochemical synthesis of large area two-dimensional metal-organic framework films on copper anodes. Angew. Chem. Int. Ed. Engl. 60, 2887–2891 (2021).
Horwitz, N. E. et al. Redox-active 1D coordination polymers of iron-sulfur clusters. J. Am. Chem. Soc. 141, 3940–3951 (2019).
Qu, L. et al. Porous molecular conductor: Electrochemical fabrication of through-space conduction pathways among linear coordination polymers. J. Am. Chem. Soc. 141, 6802–6806 (2019).
Sheberla, D. et al. Conductive mof electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 16, 220–224 (2017).
Wu, H. et al. Conductive metal-organic frameworks selectively grown on laser-scribed graphene for electrochemical microsupercapacitors. Adv. Energy Mater. 9, 1900482 (2019).
Zhang, Y. Z. et al. A simple approach to boost capacitance: Flexible supercapacitors based on manganese oxides@MOFs via chemically induced in situ self-transformation. Adv. Mater. 28, 5242–5248 (2016).
Wang, L. et al. Flexible solid-state supercapacitor based on a metal-organic framework interwoven by electrochemically-deposited pani. J. Am. Chem. Soc. 137, 4920–4923 (2015).
Pu, X. et al. A self-charging power unit by integration of a textile triboelectric nanogenerator and a flexible lithium-ion battery for wearable electronics. Adv. Mater. 27, 2472–2478 (2015).
Su, F., Lv, X. & Miao, M. High-performance two-ply yarn supercapacitors based on carbon nanotube yarns dotted with Co3O4 and NiO nanoparticles. Small 11, 854–861 (2015).
Li, C. et al. Two-dimensional conjugated polymer synthesized by interfacial Suzuki reaction: towards electronic device applications. Angew. Chem. Int. Ed. 59, 9403–9407 (2020).
Pathak, A. et al. Integration of a (–Cu–S–)n plane in a metal-organic framework affords high electrical conductivity. Nat. Commun. 10, 1721 (2019).
Passarelli, J. V. et al. Tunable exciton binding energy in 2D hybrid layered perovskites through donor–acceptor interactions within the organic layer. Nat. Chem. 12, 672–682 (2020).
Zhang, G. et al. Determination of layer-dependent exciton binding energies in few-layer black phosphorus. Sci. Adv. 4, eaap9977 (2018).
Chen, X. et al. Tuning spin-polarized lifetime in two-dimensional metal-halide perovskite through exciton binding energy. J. Am. Chem. Soc. 143, 19438–19445 (2021).
Raja, A. et al. Coulomb engineering of the bandgap and excitons in two-dimensional materials. Nat. Commun. 8, 15251 (2017).
Liu, Y. et al. Two-dimensional metal-organic framework film for realizing optoelectronic synaptic plasticity. Angew. Chem. Int. Ed. Engl. 60, 17440–17445 (2021).
Zhu, L. Q., Wan, C. J., Guo, L. Q., Shi, Y. & Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 5, 3158 (2014).
Kim, K., Chen, C. L., Truong, Q., Shen, A. M. & Chen, Y. A carbon nanotube synapse with dynamic logic and learning. Adv. Mater. 25, 1693–1698 (2013).
Liu, Y. H., Zhu, L. Q., Feng, P., Shi, Y. & Wan, Q. Freestanding artificial synapses based on laterally proton-coupled transistors on chitosan membranes. Adv. Mater. 27, 5599–5604 (2015).
Ravel, B. & Newville, M. Athena, Artemis, Hephaestus: data analysis for x-ray absorption spectroscopy using Ifeffit. J. Synchrotron Radiat. 12, 537–541 (2005).
Jiang, Y. et al. Synthesis of a copper 1,3,5-triamino-2,4,6-benzenetriol metal-organic framework. J. Am. Chem. Soc. 142, 18346–18354 (2020).
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (52173211), the Chinese Academy of Sciences (Hundred Talents Plan and the Strategic Priority Research Program of Sciences (Grant No. XDB30000000)), CAS Project for Young Scientists in Basic Research (No. YSBR-053) and the Beijing National Laboratory for Molecular Sciences (BNLMS). The theoretical calculations were carried out on TianHe-1(A) at National Supercomputer Center in Tianjin. The authors acknowledge the Shanghai Synchrotron Radiation Facility (SSRF) for providing precious time and the synchrotron XRD facility at beamline No. 14B/15U. The authors thank Xiaojing Ren from Shiyanjia Lab (www.shiyanjia.com) for the N2 adsorption isotherm measurement and analysis. The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
J.Y.C. and Y.Q.L. designed and supervised the project. S.C.S. carried out the experiments and theoretical calculations. S.C.S., C.S.D., Y.X.L., M.H.L., X.Y.W., W.Q.G., and Y.Z. performed characterization experiments. J.Y.C. conceptualized the work and analyzed the data. S.C.S. and J.Y.C. wrote the manuscript. J.C.D. provided suggestions for theoretical calculations. All the authors participated in discussions of the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shang, S., Du, C., Liu, Y. et al. A one-dimensional conductive metal-organic framework with extended π-d conjugated nanoribbon layers. Nat Commun 13, 7599 (2022). https://doi.org/10.1038/s41467-022-35315-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35315-0
This article is cited by
-
Ultra-selective uranium separation by in-situ formation of π-f conjugated 2D uranium-organic framework
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.