Abstract
MHC class I-related protein 1 (MR1) is a metabolite-presenting molecule that restricts MR1-reactive T cells including mucosal-associated invariant T (MAIT) cells. In contrast to MAIT cells, the function of other MR1-restricted T cell subsets is largely unknown. Here, we report that mice in which a T cell-specific transcription factor, B-cell lymphoma/leukemia 11B (Bcl11b), was ablated in immature thymocytes (Bcl11b∆iThy mice) develop chronic inflammation. Bcl11b∆iThy mice lack conventional T cells and MAIT cells, whereas CD4+IL-18R+ αβ T cells expressing skewed Traj33 (Jα33)+ T cell receptors (TCR) accumulate in the periphery, which are necessary and sufficient for the pathogenesis. The disorders observed in Bcl11b∆iThy mice are ameliorated by MR1-deficiency, transfer of conventional T cells, or germ-free conditions. We further show the crystal structure of the TCR expressed by Traj33+ T cells expanded in Bcl11b∆iThy mice. Overall, we establish that MR1-reactive T cells have pathogenic potential.
Similar content being viewed by others
Introduction
Unlike conventional T cells, unconventional T cells such as invariant NKT (iNKT) cells and mucosal-associated invariant T (MAIT) cells display restricted TCR repertoires characterized by the expression of invariant TCRα chains. For example, MAIT cells express TRAV1-2-TRAJ33 in humans and the orthologous Trav1-Traj33 in mice. These TCRs recognize microbial riboflavin precursors such as 5-(2-oxopropylideneamino)−6-D-ribitylaminouracil (5-OP-RU) presented by MHC class I-related 1 (MR1) proteins1,2. Upon bacterial infection, MAIT cells are activated rapidly and elicit protective responses such as the production of IFNγ and IL-17A or cytotoxicity3. Although typical type 2 MAIT cells have not been reported, MAIT cells capable of producing type 2 cytokines were detected4. Recent reports have, in addition, identified non-canonical TRAV1neg MR1-reactive T cells that do not express the TCR or markers characteristic of MAIT cells5. The physiological/pathological functions of these non-canonical MR1-reactive T cells have not yet been fully elucidated.
Bcl11b is a T cell-specific transcription factor essential for the transition from double-negative (DN) to double-positive (DP) thymocytes6,7,8,9. Genetic deletion of Bcl11b in immature thymocytes (before the DP stage) (Bcl11bflox/flox × Rag1Cre/+; referred to as Bcl11bΔiThy mice) impaired the development of most T cell subsets. However, we previously observed that unconventional T cell subsets including Vγ1-bearing γδ T cells, develop from DN thymocytes in Bcl11bΔiThy mice10. Other similar “early developing” populations of lymphocytes have been recently identified9,11,12. We have herein used our Bcl11bΔiThy mice to characterize a population of these additional, as yet unappreciated, unconventional cells that develop from early thymocytes. In the present study, we demonstrate that Trav1neg atypical MR1-reactive T cells expand in Bcl11bΔiThy mice and acquire pathogenic potential, causing hypergammaglobulinemia and chronic inflammation in a process dependent on symbiotic bacteria.
Results
Bcl11b ΔiThy mice develop chronic inflammation
Bcl11bflox/flox × Rag1Cre/+ (Bcl11bΔiThy mice) were normal at birth10, but they did not gain weight at around 7 weeks of age and died by 15–20 weeks (Fig. 1a, b). Histological analysis of organs from Bcl11bΔiThy mice showed extensive infiltration of inflammatory cells into the periductal areas of the pancreas and disruption of acinar architecture (Fig. 1c). Bcl11bΔiThy mice also exhibited periductal fibrosis and atrophy of the exocrine portion of the pancreas (Fig. 1d). Serum lipase activity was elevated as inflammation progressed, indicating that Bcl11bΔiThy mice suffered severe pancreatic damage (Supplementary Fig. 1a). However, Bcl11bΔiThy mice did not develop diabetes (Fig. 1e), consistent with the observation that islets containing β cells were not damaged (Fig. 1c). In addition, we observed thickened lamina propria and shortened large intestines in Bcl11bΔiThy mice (Supplementary Fig. 1b, c). We also observed inflammation albeit less frequently in other organs such as the lung and lachrymal gland (Supplementary Fig. 1d). Thus, Bcl11bΔiThy mice develop spontaneous inflammation.
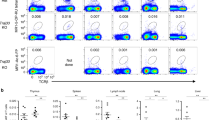
a, b Age-related changes of body weights (a) and survival rates (b) of WT (n = 8) and Bcl11bΔiThy mice (n = 8). Asterisks indicate statistical significance determined by Two-way ANOVA (a) (**p < 0.01, ***p < 0.001) and by logrank tests (***p = 0.0002) (b). c, d H&E staining (c) and sirius-red staining (d) of pancreatic sections from WT and Bcl11bΔiThy mice (15 weeks old). Scale bar shows 200 μm. c An arrow head indicates islet. ****p < 0.0001 by unpaired two-tailed Student’s t-tests. d Quantified data of inflammation scores based on histological analysis of pancreas of WT and Bcl11bΔiThy mice (15–20 weeks old) was shown on the right panel. ***p = 0.0001 by unpaired two-tailed Student’s t-tests. e Blood glucose levels in WT and Bcl11bΔiThy mice (n = 3 biologically independent animals) at different ages. Data are expressed as mean ± SEM. *p < 0.05 by two-way ANOVA tests. f, g Survival of the indicated strains of mice (Bcl11bΔiThy mice, n = 6; Bcl11bΔiThy × Rag2–/– mice, n = 10; Bcl11bΔiThy mice, n = 10; Bcl11bΔiThy × Tcrd–/– mice, n = 8; Bcl11bΔiThy × Ptcra–/– mice, n = 8) was monitored for 20 weeks. ****p < 0.0001, *p = 0.0149 by logrank tests. h, i, k, m Analysis of thymocytes (h, i, k, m) and splenocytes (m) in indicated mice (h, i, k: 1, 3, 7 weeks old, m: 9 weeks old). Representative dot plots are shown after gating on total lymphocytes (h, k, m). i Bar graphs show absolute numbers of total (left) and DP (right) thymocytes. ***p < 0.001 by unpaired two-tailed Student’s t-test. j αβ T cell frequencies in the peripheral blood lymphocytes (PBL) of WT and Bcl11bΔiThy mice (at least n = 3 in each group) over time. Data are expressed as mean ± SEM. *p < 0.05 by two-way ANOVA tests. l Western blot analysis of Bcl11b expression in sorted αβ T cells from two individual WT and Bcl11bΔiThy mice. β-actin: 42 kDa, Bcl11b: 96 kDa. Gating strategies are shown in Supplementary Fig. 6a for Fig. 1h, k and m (thymocytes), and Supplementary Fig. 6b for Fig. 1m (splenocytes). a, e, i, j Data are representative of three independent experiments. b, f, g Data are combined from at least two independent experiments.
The inflammation and decreased survival in Bcl11bΔiThy mice were absent on a Rag2-deficient background (Fig. 1f, Supplementary Fig. 1e); this suggests that T lymphocytes are required for these defects since Bcl11b is not expressed in B cells8,13. γδ T cells, which are present in Bcl11bΔiThy mice10, are not necessary for disease progression as Bcl11bΔiThy × Tcrd–/– mice still displayed this lethal disorder (Fig. 1g); in contrast, survival was significantly prolonged on a pre-TCRα-deficient background (Bcl11bΔiThy × Ptcra–/– mice) in which αβ T cells are absent (Fig. 1g). Notably, despite the DN2 arrest phenotype10 (Supplementary Fig. 2a, Fig. 1h, i), a few CD5+ αβTCR+ thymocytes were observed in 7-week-old Bcl11bΔiThy mice (Fig. 1k). In the periphery, αβTCR+ cells were extremely rare at an early age; however, the cell number gradually increased with age in Bcl11bΔiThy mice and exceeded that of WT mice (Fig. 1j). It is unlikely that this accumulation of mature T cells is due to incomplete deletion of Bcl11b in those cells, as Bcl11b protein could not be detected in peripheral αβ T cells from Bcl11bΔiThy mice (Fig. 1l). In the absence of preTCRα chains (Bcl11bΔiThy × Ptcra–/– mice), such peripheral αβ T cells were eliminated (Fig. 1m), indicating that these T cells must undergo pre-TCR-mediated β-selection. Furthermore, adoptive transfer of T cells from Bcl11bΔiThy mice into CD3ε-deficient mice was sufficient to induce the disorders (Supplementary Fig. 2b). These data suggest that αβ T cells that develop in the absence of Bcl11b possess pathogenic potential.
Pathogenic contribution of non-canonical MR1-reactive T cells
Most αβ T cells in Bcl11bΔiThy mice displayed a CD44highCD62LlowIL-18Rhigh phenotype, implying that they include unconventional T cells (Fig. 2a)3. However, CD1d-restricted iNKT cells were not detected in either the thymus or the periphery of Bcl11bΔiThy mice (Fig. 2b, c, Supplementary Fig. 2c, d). To evaluate the contribution of MR1-reactive αβ T cells to these disorders, Bcl11bΔiThy mice were crossed with MR1-deficient mice (Bcl11bΔiThy × Mr1–/– mice). Pancreatitis was ameliorated in the resulting Bcl11bΔiThy × Mr1–/– mice (Fig. 2e) and they survived longer (Fig. 2d). However, neither MR1 tetramer-positive (5-OP-RU-tet+) MAIT cells nor the canonical TCRα repertoire of MAIT cells (Trav1-Traj33) were detected in the spleens of Bcl11bΔiThy mice (Fig. 2b, c). Nevertheless, the proportion of CD44high IL-18Rhigh αβ T cells observed in Bcl11bΔiThy mice was reduced to a level comparable to that of WT mice in Bcl11bΔiThy × Mr1–/– mice (Fig. 2f). The T cells that accumulated in Bcl11bΔiThy mice did not express PLZF, which is a Bcl11b-dependent transcription factor characteristic of MAIT cells and iNKT cells (Supplementary Fig. 2e)14, confirming that they are not MAIT cells. Thus, the expansion of non-canonical MR1-reactive αβ T cells is associated with the development of chronic inflammation in Bcl11bΔiThy mice.
a Histograms of indicated surface markers (black line) on splenic CD4+ αβ T cells from indicated mice (10 weeks old) compared with isotype control (filled gray). b α-GC-loaded mCD1d tetramer+ cells and 5-OP-RU-loaded mMR1 tetramer+ cells in lymphocytes. c Each dot represents read count derived from transcript of Trav11-Traj18 and Trav1-Traj33 from the spleens of WT and Bcl11bΔiThy mice (n = 3 biologically independent animals). d Survival of Bcl11bΔiThy mice (n = 12) and Bcl11bΔiThy × Mr1–/– mice (n = 9). ***p = 0.0010 by logrank tests. e, f H&E staining of sections of pancreas with quantified data (e) and IL-18R and CD44 expressions on splenic αβ T cells (f) from indicated mice (15 weeks old). e Scale bar shows 50 μm. *p = 0.0420 by unpaired two-tailed Student’s t-tests. g–k BM cells from Bcl11bΔiThy mice (closed circle) or Rag1null Bcl11bΔiThy (Bcl11bflox/flox × Rag1Cre/Cre) mice (closed triangle) were transferred into Cd3e–/– mice. Bcl11bΔiThy BM cells were mixed with WT BM cells at 1:1 ratio before transfer (gray circle) (n = 3–5 biologically independent animals in each group). After transfer, body weights (g) and CD4+ αβ T cell frequencies (h) were monitored. g, h Data are expressed as mean ± SEM. i–k 26 weeks after transfer, frequencies of αβ T cells and B cells (i), CD4 and CD8α (upper) or IL-18R (lower) expressions (j), H&E staining of sections of pancreas with quantified data (k) in recipient mice were analyzed. k Scale bar shows 60 μm. *p = 0.0316, **p = 0.0089 by unpaired two-tailed Student’s t-test. l, m Whole T (dense gray square) or Treg-depleted T cells (light gray square) from WT mice or whole T cells from Bcl11bΔiThy mice (black circle) were transferred into 3-week-old Bcl11bΔiThy mice. l Survival of Bcl11bΔiThy mice receiving Bcl11bΔiThy T cells (n = 6), WT T cells (n = 5) and Treg-depleted T cells (n = 5). *p = 0.032 by logrank tests between mice receiving WT T cells and Treg-depleted T cells. m 16 weeks after transfer of the donor WT splenic T cells (CD45.1) to recipient Bcl11bΔiThy mice (3 weeks old), the numbers of splenic CD4+ T cells were analyzed. ****p < 0.0001 by unpaired two-tailed Student’s t-tests or **p = 0.0019 by paired two-tailed Student’s t-tests. Data are representative of (a, b, f) three and (c, i, j) two independent experiments. Gating strategies are shown in Supplementary Fig. 6b for Fig. 2b, and Supplementary Fig. 6c for Fig. 2f, i and j. Data are combined from three (d) or two (e, g, h, k, l, m) independent experiments.
Correction of T cell insufficiency ameliorates chronic inflammation
Bcl11bΔiThy mice lack most conventional αβ T cells10. We therefore speculated that this T cell imbalance may allow the development/expansion of pathogenic innate-type T cells. After the transfer of bone marrow (BM) cells from Bcl11b∆iThy mice into CD3ε-deficient mice, recipient mice experienced body weight loss in a lymphocyte-dependent (Rag1-dependent) manner (Fig. 2g). However, when BM cells from Bcl11bΔiThy and WT mice were mixed at a 1:1 ratio and the combination transferred, the body weights of recipient mice remained normal (Fig. 2g). Consistent with this, expansion of pathogenic CD4+ Bcl11bΔiThy T cells (Supplementary Fig. 2b) was constrained in the presence of WT cells (Fig. 2h–j), possibly due to the survival disadvantages15. Furthermore, chronic inflammation induced by Bcl11bΔiThy BM cells was eliminated by co-transfer with WT BM cells (Fig. 2k, Supplementary Fig. 2f). The effect of the presence of balanced T cells was also confirmed by using non-irradiated Bcl11bΔiThy recipient mice, as transfer of WT T cells limited the expansion of donor T cells (Fig. 2m), inflammations (Fig. 2k, Supplementary Fig. 2f) and fatal disorder independently of regulatory T cells (Treg) (Fig. 2l). These results suggest that the imbalance in T cells observed in the Bcl11bΔiThy genetic environment contributes to the pathogenesis.
MR1-dependent expansion of Traj33-bearing T cells in Bcl11b ΔiThy mice
To characterize the profiles and clonotypes of the pathogenic T cells at a clonal level, we performed single-cell-based TCR and RNA-sequencing (scTCR-RNAseq). In Bcl11bΔiThy mice, the TCR repertoire was highly skewed with small number of clones dominating (Fig. 3a). The most frequent clone in Bcl11bΔiThy mice accounted for 15% of total αβ T cells and expressed a Trav7-6-Traj33/Trbv13-2-Trbj1-1 TCR (designated as clonotype #1) with identical CDR3α and CDR3β sequences (Fig. 3a–c). Cells expressing clonotype #1 occupied 87% of all Traj33+ T cells in Bcl11bΔiThy mice (Fig. 3c, d, left panels). Furthermore, the TCRα and β chains of clonotype #1 preferentially paired with each other (Supplementary Fig. 3a, b). Although the Vα usage of clonotype #1 (Trav7-6) differed from the canonical Trav1+ MAIT TCR, the Traj33 Jα, which provides the critical amino acids for MR1 contact, was identical to typical MAIT cells16 (Fig. 3b, Supplementary Fig. 3c, d). We therefore solved the crystal structure of the apo form of the clonotype #1 TCR (Fig. 3e) and superimposed this structure onto the complex structure of the MR1-6-formylpterin (6FP)-MAIT TCR17. The comparison suggested that the Tyr residue in the SNYQ sequence, particularly Y97, provided by the Jα33 chain, was located in a similar but different position to the Y95 of canonical MAIT TCR, suggesting that it pointed toward the ligand binding pocket of MR1 in a slightly different manner18 (Fig. 3f, g, Supplementary Fig. 3e). Indeed, the frequency of Traj33+ T cells, including those expressing clonotype #1, was dramatically decreased on an MR1-deficient background (Fig. 3c, d, right panels), indicating that MR1 promotes the development and/or expansion of Traj33+ non-MAIT cells in Bcl11bΔiThy mice.
a–d, h Sorted T cells from Bcl11bΔiThy mice or Bcl11bΔiThy × Mr1–/– mice (11 weeks old) were analyzed by single-cell TCR and transcriptome analysis. a Frequencies of the top 200 clones in T cells from Bcl11bΔiThy mice. b Junctional amino acid sequences of the TCRα and β chains of the top 1 clonotype (clonotype #1). c, d tSNE plots generated by data based on single cell transcriptome and TCR analysis of the indicated strains of mice; clonotype #1 (red) or Traj33+ clonotypes (wine red) and other clonotypes (gray) are shown. e Crystal structure of clone #1 TCRαβ ectodomain. f Closeup view of CDR3α regions. g Putative interaction between Traj33 and MR1. Crystal structure of MAIT TCR-MR1-6-FP ternary complex is superposed onto clone #1 TCRαβ heterodimer. The positions of Traj33 are highlighted (green). h Colored dots indicate clonotype #1 with significantly high expression levels of indicated genes. i Seven clusters of clonotype #1 cells after analysis by mclust. j Three distinct lineages (Lin1-3) of clonotype #1 cells after analysis of slingshot. The pseudotime can be arbitrarily “stretched” by factors such as the magnitude of differential expression or the density of cells, depending on the algorithm for each lineage. The absolute values of the pseudotime in a lineage make little sense. Hence, the magnitude of the pseudotime has little comparability across lineages. In each panel, the values of pseudotime were normalized to 0–100 corresponding to orange-red and black, respectively. k Heat map graphs of expressions of indicated genes involved in TFH cell differentiation in clonotype #1 cells. Source data are provided as Source Data files; Supplementary_code.text and Supplementary_data.RData.
Simultaneous single-cell RNA-seq analysis implied that clonotype #1 was detected in different clusters including Mki67high proliferating cells, and Mki67neg cells expressing Il4, Ifng or Gzmb (Fig. 3h). No IL-17A-positive cluster was detected. Pseudotime analysis of clonotype #1 allowed us to visualize three distinct differentiation pathways when Mki67high cells were defined as a root for the analysis (Fig. 3i, j). Along the pseudotime dimension, the proliferative ability identified by Mki67 expression gradually decreased to the end, suggesting that functional differentiation occurred after proliferation (Fig. 3k). One of the three computationally predicted lineages (Lin 1) was characterized by Il4 expression and gradually acquired expression of TFH marker genes such as Bcl6, Cxcr5, Pdcd1, Il21, Sostdc1 and Rgs1619,20,21 (Fig. 3k, Supplementary Fig. 3g). Another lineage (Lin 2) (Fig. 3j) overlapped with a CTL-like cluster (Supplementary Fig. 3h). Importantly, the T cells bearing Trav7-6-Traj33 was also detected in other individual Bcl11bΔiThy mice and even in WT mice at a low frequency (Supplementary Fig. 3f), suggesting that these T cells are not arisen sporadically and aberrantly under Bcl11b-deficient conditions.
Thus, Traj33+ T cells bearing non-MAIT TCRs are clonally expanded in the presence of MR1 and proceed through functional differentiation in Bcl11bΔiThy mice.
Elevated serum immunoglobulin and autoantibodies in Bcl11b ΔiThy mice
Consistent with the expansion of TFH-like cells expressing IL-4, IgG levels in the serum of Bcl11bΔiThy mice began to increase at about the same age as when αβ T cells began to be detected (Fig. 4a). All IgG subclasses were significantly higher in Bcl11bΔiThy mice (Fig. 4b). Sera from individual Bcl11bΔiThy mice reacted to lysates from mouse pancreas, suggesting the presence of autoantibodies (Fig. 4c). Furthermore, IgG1 antibodies were deposited around pancreatic ductal sites in Bcl11bΔiThy mice; this was not observed on an MR1-deficient background (Fig. 4d). Both the number of IgG1+ B cells and IgG1 production were increased in the peripheral blood of Bcl11bΔiThy mice in the presence of MR1 (Fig. 4e, f) and pre-TCRα (Supplementary Fig. 4a). These data suggest that Bcl11bΔiThy mice develop hyperimmunoglobulinemia including autoantibodies in an MR1-restricted T cell-dependent manner. Indeed, preventing the expansion of such pathogenic T cells by adoptive transfer of WT T cells recovered Bcl11bΔiThy mice from hyperimmunoglobulinemia (Supplementary Fig. 4b).
a, b Productions of total immunoglobulin (n = 3 biologically independent animals in each group) (a) and immunoglobulin subclass proteins at 12 weeks of age (b) in the sera of WT and Bcl11bΔiThy mice analyzed by ELISA. Asterisks indicate statistical significance determined by Two-way ANOVA (a) or by unpaired two-tailed Student’s t-test (b) (*p < 0.05, **p < 0.01, ***p < 0.001). c Pancreatic homogenates from Rag1–/– mice were blotted with sera from six individual sick Bcl11bΔiThy mice (10–15 weeks old) in the lane-separated membrane. d IgG1 depositions determined by immunohistochemical analysis of sections of pancreas from the indicated strains of mice (15 weeks old). Dotted circles indicate pancreatic ducts. Scale bar: 100 μm. Quantified data of antibody deposited areas was shown on the right panel. *p = 0.0121 and 0.0387 by unpaired two-tailed Student’s t-tests. e Graphs show the percentage of MR1-dependent IgG1+ B cells (n = 6–10 biologically independent animals at each time point). f IgG1 production in 12-week-old indicated mice. *p = 0.0149 by two-way ANOVA (e) or *p = 0.0234 by unpaired two-tailed Student’s t-tests (f). g Percentages of germinal center (GC) B cells and TFH cells in WT and Bcl11bΔiThy mice (13–15 weeks old) analyzed by mass cytometry (n = 4 biologically independent animals). The center of the box is the median, bounds are the 75% and 25% percentiles. The whisker bounds are 1.5*the interquartile range past the 25% or 75% percentiles. All values are shown so the minima and maxima are just the top and bottom data points. h–j Surface CD40L expression (h), IL-4 production (i) and IL-4-GFP reporter fluorescence (j) in CD4+ αβ T cells of WT, Bcl11bΔiThy, Il4GFP/+ and Bcl11bΔiThy × Il4GFP/+ mice (10 weeks old). Numbers in dot plots show positive cells among total lymphocytes (h–j). k, l IgG1 productions (k) and histological analysis of pancreas by H&E staining (l) in Bcl11bΔiThy mice after steroid treatment with dexamethasone (Dex). k, l *p = 0.0138 (k), *p = 0.0421 (l) by unpaired two-tailed Student’s t-tests. l Scale bar: 100 μm. a, e Data are expressed as mean ± SEM. b, e, f, g Data are combined from at least two independent experiments. Data are representative of two (a) and three (h–j) independent experiments.
Consistent with the high IgG1 production in Bcl11bΔiThy mice, mass cytometric analysis showed increases in germinal center (GC) B cells and TFH-like cells in Bcl11bΔiThy mice (Fig. 4g, Supplementary Fig. 4c, d). We also observed peanut agglutinin positive (PNA+) germinal center-like structures in the enlarged pancreatic lymph nodes of Bcl11bΔiThy mice, which was not observed in WT mice (Supplementary Fig. 4e). Consistent with these observations, CD4+ αβ T cells in Bcl11bΔiThy mice expressed CD40L (Fig. 4h) and produced IL-4 (Fig. 4i). IL-4-GFP reporter fluorescence was also detected in pancreatic LN T cells from Bcl11bΔiThy × Il4GFP/+ mice (Fig. 4j). On either a B cell-deficient (μMT) or IL-4-deficient (Il4GFP/GFP) background, Bcl11bΔiThy mice did not survive longer (Supplementary Fig. 4f). The inflamed pancreases of Bcl11bΔiThy mice were infiltrated with some IgG1+ B cells and CD4+ αβ T cells in an MR1-dependent manner (Supplementary Fig. 4g, h). These clinical features are similar to those in human autoimmune pancreatitis (AIP)22, which has been characterized by the high levels of serum human IgG4 (a putative counterpart of mouse IgG123), periductal pancreatitis, lymphocyte infiltration and steroid responsiveness24. Indeed, steroid treatment ameliorated elevation of serum IgG and pancreatitis in Bcl11bΔiThy mice (Fig. 4k, l).
Remission of pancreatitis in Bcl11b ΔiThy mice under germ free condition
A canonical MAIT cell antigen, 5-OP-RU, is provided mainly by microbiota1,25,26. We thus assessed whether symbiotic microbes affect the pathogenic T cells and disorders observed in Bcl11bΔiThy mice by generating germ-free (GF) Bcl11bΔiThy mice. Under germ-free conditions, we did not observe weight loss (Fig. 5a), a survival defect (Fig. 5b), T cell expansion (Fig. 5c), pancreatitis (Fig. 5d) or hypergammaglobulinemia (Fig. 5e) in Bcl11bΔiThy mice. Furthermore, the number of peripheral αβ T cells expressing high levels of IL-18R was reduced in GF Bcl11bΔiThy mice compared with Bcl11bΔiThy mice under specific pathogen-free (SPF) conditions (Fig. 5f–h). We also performed scTCR-RNA-seq on T cells from GF Bcl11bΔiThy mice and did not detect clonotype #1 (Fig. 5i), although this clonotype accumulated in SPF Bcl11bΔiThy mice (Fig. 3c). These results suggest that symbiotic microbes are required for the fatal disorders in Bcl11bΔiThy mice. Indeed, like germ-free conditions, treatment with antibiotics also ameliorated chronic inflammation (Fig. 5j) and improved survival (Fig. 5k) in Bcl11bΔiThy mice.
a–c Age-related changes in body weight (a), survival rates (b) and CD4+ T cell frequencies (c) for WT and Bcl11bΔiThy mice under SPF (WT mice (white), n = 5; Bcl11bΔiThy mice (black), n = 8) or GF conditions (WT mice (gray), n = 18; Bcl11bΔiThy mice (red), n = 13). d H&E staining of sections of pancreas of WT and Bcl11bΔiThy mice (18 weeks old) under SPF or GF conditions. Scale bar shows 100 μm. e Each plot represents amount of IgG1 present in the serum of the indicated mice (18 weeks old). f Frequencies of B cells (B220+) and αβ T cells (TCRβ+) within splenocytes from the indicated mice (16 weeks old) after gating on lymphocytes. g Each plot represents CD4+ T cell number in the spleens (spl) and mesenteric lymph nodes (mLN) from the indicated mice (18 weeks old). h Histograms show IL-18R expression on αβ T cells from WT and Bcl11bΔiThy mice (18 weeks old) under SPF or GF conditions. i Single cell transcriptome and TCR analysis of sorted αβ T cells (n = 7551 cells) from Bcl11bΔiThy mice (18 weeks old) maintained under GF conditions. Numbers in the tSNE plot show the frequency of clonotype #1. j H&E staining of sections of pancreas of mice (16 weeks old) after treatment with or without antibiotics (Abx). Scale bar shows 60 μm. k Graphs show survival of Bcl11bΔiThy mice after treatment with (red) or without (black) antibiotics (Abx) (n = 9 and n = 6 biologically independent animals respectively). Asterisks indicate statistical significance between GF and SPF conditions of Bcl11bΔiThy mice determined by two-way ANOVA test (a, c) (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001), unpaired two-tailed Student’s t-test (****p < 0.0001 (e); ****p < 0.0001, **p = 0.0033 (spl), **p = 0.0082, ***p = 0.0009 (mLN) (g)), and logrank test (p = 0.0339) (k) respectively. c Data are expressed as mean ± SEM. Gating strategies are shown in Supplementary Fig. 6c for Fig. 5h, and Supplementary Fig. 6d for Fig. 5f. d, f, h, j Data are representative of three independent experiments. a, b, c, e, g, k Data are combined from at least two independent experiments.
Clonotype #1 responds to fecal components
The above observations suggest that symbiotic microbes may provide an antigen(s) to clonotype #1 to support clonal expansion and/or activation. We thus reconstituted the expression of clonotype #1 TCRα (Trav7-6-Traj33) and TCRβ (Trbv13-2-Trbj1-1) in a TCRαβ-deficient reporter cell line (Fig. 6a). To identify antigenic components, fecal extracts from SPF mice were fractionated and tested for stimulatory activity towards reporter cells expressing the clonotype #1 TCR in the presence of MR1-expressing antigen-presenting cells. Fraction #54 was found to induce NFAT reporter activity in an MR1-dependent manner (Fig. 6b), as it was suppressed in the presence of Ac-6-FP (Fig. 6c). To determine the active component(s) in fraction #54, we analyzed these seventy fractions by non-targeted metabolomics27. Twenty-two candidates were selectively and reproducibly present in fraction #54 (Fig. 6d, Supplementary Fig. 5a, b). Among them, only one candidate (#54-6) had similar m/z value to the exact mass of a known metabolite (human metabolome database, https://hmdb.ca), riboflavin (RF) (Supplementary Fig. 5b). MS/MS analysis confirmed that this precursor ion is derived from RF (Fig. 6e). Indeed, synthetic RF stabilized the surface expression of MR1 (Fig. 6f), suggesting that RF specifically binds to MR1 as recently reported28. Although RF was not recognized by MAIT TCRs (Fig. 6g, blue)28, it activated cells expressing the clonotype #1 (Fig. 6g, red) and this activation was blocked by both Ac-6-FP and anti-MR1 antibody (Fig. 6g, black). As predicted from its TCR sequence (Fig. 3b, Supplementary Fig. 3c), clonotype #1 did not react with an authentic MAIT cell antigen, 5-OP-RU (Fig. 6h). These results suggest that non-canonical MR1-reactive T cells recognize metabolites presented by MR1 that are distinct from MAIT cell antigens.
a Histograms show clonotype #1 TCR expression as compared with the parental cell line (Mock). These cells were stained with isotype control (closed) and anti-TCRβ antibody (open). b, c Graph shows NFAT-GFP expressions in the reporter cell line expressing clonotype #1 TCR after cocultured with fractionated fecal homogenates in the presence of mouse MR1-expressing NIH3T3 cells. c Ac-6-FP (10 μM) was used to block interactions between MR1 and TCR. ***p = 0.0004 by unpaired two-tailed Student’s t-test. NS indicates not significantly different. d Plots of the ion intensity of precursor ions contained in the fraction of #54. #54-6, having similar m/z value to exact mass of known metabolites in the human metabolome database, was highlighted in red. e HRMS/MS spectra of riboflavin; fraction #54-6 (upper) and authentic standard (lower) in the positive ion mode. f MR1 expressions on MR1-expressing thymoma 15 h after co-culture in the presence of riboflavin were analyzed using a PE-labeled anti-MR1 antibody. MFI indicates mean fluorescence intensity of the MR1 expression. g, h GFP-reporter activities of cells expressing the clonotype #1 TCR or MAIT TCR after stimulation with riboflavin (g) or 5-OP-RU (h) in the presence of mouse MR1-expressing NIH3T3 cells with or without Ac-6-FP (100 μM) or anti-MR1 antibody (10 μg/ml). 5-OP-RU concentrations are indicated as described in the Methods section. g Asterisks indicate statistical significance determined by unpaired two-tailed Student’s t-test (**p = 0.0024, *p = 0.0241). c, f, g, h Data are expressed as mean ± SEM. Gating strategies are shown in Supplementary Fig. 6e for Fig. 6a. a, c, f, g, h Data are representative of two independent experiments.
Discussion
In the present study, we demonstrate that non-canonical MR1-reactive αβ T cells acquire pathogenic potential through microbiota-dependent clonal expansion. RF metabolites, such as 5-OP-RU, are agonistic antigens for MAIT cells2, whereas RF itself is a weak antagonist of MAIT cells28. Considering its different action on expanded clonotypes in our study, RF might regulate MR1-reactive T cells as both an agonist and an antagonist depending on the TCR repertoire28. RF may contribute to the maintenance of MR1-reactive T cells in normal setting; however, excessive intake of RF might influence inflammation in particular T-imbalanced settings. It is also possible that, in addition to RF, the active fraction #54 contains an antigen(s) which has not yet been annotated (Fig. 6d and Supplementary Fig. 5b). Taken together with recent reports28,29,30,31,32, more diverse metabolites than previously demonstrated may regulate various mouse and human MR1-reactive T cells.
An important question arising is whether pathogenic T cells, such as clonotype #1, are also detected in Bcl11b-sufficient mice. CDR3-matched Trav7-6-Traj33 TCRs were identified by bulk TCR sequencing of WT mice; however, such cells exist at low frequency (Supplementary Fig. 3f) and are not pathogenic. Thus, a normal environment in which innate T cell subsets are balanced is likely to restrict excessive expansion of such clonotypes, thus preventing pathogenic conversion10,33. Development of probes, such as specific tetramers or clonotypic mAbs, would enable us to monitor the status of these clonotypes during various experimental settings.
It should be noted that Bcl11b is critical for the development and function of Treg cells, since CD4-Cre-driven Bcl11b-deficient mice develop inflammatory diseases due to the dysregulated activation of effector Th17 cells via defective Treg function34. This scenario, however, may not explain the symptoms observed in Rag1-Cre-driven Bcl11b-deficient (Bcl11b∆iThy) mice, as the correction of T-imbalance could suppress the disease in the absence of Treg (Fig. 2l). Furthermore, most conventional αβ T cells do not develop in these mice and thus effector Th17 cells were extremely rare in Bcl11b∆iThy mice (Fig. 3i). Rather, MR1-reactive T cells possessing TFH potential accumulated (Fig. 3i, l). It is possible that these cells may provide B cell help for autoantibody production in a non-cognate manner as recently reported35.
It is interesting that canonical type 2 MAIT cells have not been clearly defined36, given that type 2 lymphocytes are a well-established and conserved subset across the wide variety of T lymphocytes and innate lymphocyte lineages37,38,39. The identification of Trav1negTraj33pos non-canonical MR1-reactive T cells that produce IL-4 might represent MR1-reactive type 2 T cells, together with recent reports on IL-4-producing MAIT cells4. Still, we cannot exclude the possibility that the absence of Bcl11b results in the derepression of IL-4-related genes in these cells40. Detailed immune profile analysis of clonotype #1 T cells present in WT mice would clarify this issue.
The observation of incomplete symptom suppression in Bcl11b∆iThy mice on a μMT- or Il4–/–-background suggests that antibodies are not the sole effectors contributing to these disorders. As previously proposed, the cytotoxic effector molecules, granzymes, could be an additional candidate for causing direct tissue damage41. The immune profile of the second most frequent T cell clone (clonotype #2) expanding in Bcl11b∆iThy mice might support this assumption; these cells included a granzyme B+ population whose development was also dependent on MR1 and microbiota (Supplementary Fig. 3i, j).
Although Bcl11b is required for early T cell development, Bcl11b-null mice are neonatal lethal due to its importance in neural development13. Thus, genetic modification of Bcl11b specifically in immature thymocytes within ‘viable’ mice allowed us to detect previously unappreciated αβ T cell subsets. Such type 2 skewing of the remaining T cells may partly explain the exacerbated type 2 immunity in humans bearing hypomorphic Bcl11b mutations42. Currently, whether αβ T cells in Bcl11bΔiThy mice develop through the DP stage is unclear; it is possible that they develop into mature T cells from the DN2 stage, in a process similar to an IFNγ-producing γδ T subset10,43. The reported “DN2 arrest phenotype of Bcl11b-deficient cells6,7,8” and the “requirement of pre-TCR-mediated β-selection (generally thought to accompany the DN to DP transition)” in the present study are apparently contradictory observations. Still, these T cells in Bcl11bΔiThy mice express CD5, a marker indicative of having experienced TCR engagement during positive selection. Typical MAIT and iNKT cells are positively selected by DP thymocytes and express PLZF, a lineage-defining transcription factor for these innate T lymphocytes3. However, a subpopulation of MR1-restricted T cells is selected by non-hematopoietic cells and is PLZF-negative26, as observed in the T cells of Bcl11bΔiThy mice (Supplementary Fig. 2e). Given that antigen-presenting DP cells are lacking in the Bcl11b∆iThy thymus, T cells might be selected by thymic epithelial cells. The introduction of fine fate-mapping alleles in mixed chimera experiments may clarify this issue.
Under GF conditions, inflammatory symptoms in many autoimmune models worsen44,45,46. In contrast, the complete remission of inflammation in GF Bcl11b∆iThy mice indicates that the effector mechanisms are distinct from typical autoimmune diseases. Indeed, the generation of pathogenic T cells and inflammation in the current model require microbiota, presumably because microbiota provide antigens for MR1-restricted T cells, as was also recently shown for MAIT cells2,26,41,47. Similarly, as yet unidentified “mobile” metabolites from symbiotic bacteria may also activate the MR1-reactive T cells in the intestine and pancreas, which are anatomically connected via pancreatic ducts48,49. It is also possible that cytokine milieu, such as IL-12 or IL-1/18, present under SPF conditions might activate MR1-reactive T cells independently of the TCR50.
A link between lymphopenia and autoimmunity has been reported51. Under lymphopenic conditions, the compensatory expansion of T cells exhibiting innate T cell/TFH cell profiles, converts these cells into pathogenic effectors52,53,54. Taken together, the present study suggests a possible contribution of non-canonical innate T cells to autoimmune diseases under T cell-imbalanced conditions and/or dysbiosis.
Methods
Mice
This study was approved by the Committee of Ethics on Animal Experiments in the Faculty of Medicine of Kyushu University, Science Research Center of Institute of Life Science and Medicine of Yamaguchi University and Research Institute for Microbial Diseases of Osaka University. Experiments were carried out under the control of the Guidelines for Animal Experiments. Mice were maintained under 12 h dark/light cycle and constant conditions of temperature (18–23 °C) and humidity (40–60%). C57BL/6 mice were purchased from SLC (Shizuoka, Japan). Bcl11bflox/flox mice were provided by R. Kominami (Niigata University, Japan). Rag1Cre/+ mice were provided by T. Rabbitts (Leeds Institute of Molecular Medicine, United Kingdom) by courtesy of K. Akashi (Kyushu University, Japan). Il4GFP/GFP mice were provided by Dr. W.E. Paul (National Institute for Health, USA). μMT mice were provided by D. Kitamura (Tokyo University of Science, Japan). Bcl11bflox/flox, Rag1Cre/+ Il4GFP/GFP. and μMT mice were backcrossed to C57BL/6 mice more than ten generation. Mr1–/– mice provided by Dr. Susan Gilfillan (Washington University, USA) on the courtesy of Dr. K. Iwabuchi (Kitazato University, Japan) were backcrossed to C57BL/6 mice for at least twenty generations. Throughout the study, we mostly used littermate mice for control groups and designated Rag1-, Bcl11b- and MR1-sufficient mice (Rag1Cre/+ or +/+ × Bcl11b+/+ × Mr1+/–, Rag1Cre/+ or +/+ × Bcl11bflox/+ × Mr1+/–, Rag1+/+ × Bcl11bflox/flox or flox/+ × Mr1+/+, Rag1+/+ × Bcl11bflox/flox × Mr1+/+ or +/–, Rag1+/+ × Bcl11bflox/+ × Mr1+/+ mice) as WT mice. Germ-free Bcl11bΔiThy mice are generated by in vitro fertilization at the CLEA Japan. Germ-free mice were bred and maintained in vinyl isolators within the facility in CLEA Japan. For steroid treatment, Bcl11bΔiThy mice were sequentially administrated from 5 weeks of age with an increased amount of dexamethasone (Cat. No.D4902, Sigma) (1, 3, 10 mg/kg) three times for every two weeks intraperitoneally, in accordance with human therapy55 and analyzed at 12 weeks of age. For treatment with antibiotics, mice (5 weeks old) were given for 13 weeks with ampicillin (Cat. No.19769-64) (1 g/L), vancomycin (Cat. No.35575-94) (500 mg/L), neomycin sulfate (Cat. No.19767-42) (1 g/L), and metronidazole (Cat. No.23254-64) (1 g/L) in drinking water. All antibiotics were purchased from Nakarai Tesque, Inc.
Cell lines
Reporter cell line expressing clonotype #1 αβTCR or MAIT TCR was generated by retroviral gene transduction to TCR-negative mouse T cell hybridoma with an NFAT-GFP reporter gene, a kind gift from Dr. H. Arase (Osaka University, Japan). Mouse MR1-expressing antigen-presenting cells were generated by retroviral gene transduction of mouse MR1 to mouse fibroblastic cell line NIH3T3 (Cat. No.CRL1658, ATCC).
Compounds
Ac-6-FP (Cat.No.11.418) was purchased from Schircks laboratories. 5-A-RU (Cat.No.A629245) was purchased from Toronto Research Chemicals. Methylglyoxal solution (Cat.No.M0252) and riboflavin (Cat.No.R9504) were purchased from Sigma-Aldrich. 5-OP-RU was generated by reacting 5-A-RU with equal molar ratio of methylglyoxal. 5-OP-RU concentrations are shown under assumption that all 5-A-RU is converted to 5-OP-RU.
Flowcytometric analysis
αGC-loaded CD1d tetramers were prepared following the manufacturer’s instructions (Cat No.TS-MCD-1, Medical & Biological Laboratories co., LTD). For staining of mouse MAIT cells, APC-labeled mouse MR1 tetramer provided by NIH Tetramer core facility was used2. FITC-conjugated anti-mouse (m) CD45.1 (A20, 1:40 dilution, Cat No.110705), anti-mCD45.2 (104, 1:100 dilution, Cat No.109805) mAbs, PE-conjugated anti-mCD5 (53-7.3, 1:100 dilution, Cat No.100607), anti-mCD8α (53-6.7, 1:80 dilution, Cat No.100707), anti-mCD40L/CD154 (MR1, 1:100 dilution, Cat No.106505), anti-mCD117 (2B8, 1:40 dilution, Cat No.105807), and anti-MR1 (26.5, 1:40 dilution, Cat No.361105) mAbs, PECy7-conjugated anti-mCD4 (RM4-5, 1:200 dilution, Cat No.100527) mAb, PerCP-Cy5.5-conjugated anti-mI-A/I-E (M5/114.15.2, 1:200 dilution, Cat No.107623) and anti-mCD44 (IM7, 1:200 dilution, Cat No.103031) mAbs, APC-conjugated anti-mTCRβ (H57-597, 1:40 dilution, Cat No.109211), anti-mCD4 (RM4-5, 1:100 dilution, Cat No.100515), anti-mIL-18Rα (A17071D, 1:100 dilution, Cat No.157905), anti-mCD25 (PC61, 1:200 dilution, Cat No.102011), anti-mCD44 (IM7, 1:200 dilution, Cat No.103011), anti-mCD45.2 (104, 1:40 dilution, Cat No.109813) and anti-mCD62L (MEL-14, 1:100 dilution, Cat No.104411) mAbs, AF700-conjugated mCD8 (53-6.7, 1:100 dilution, Cat No.100729) mAb, APCCy7-conjugated mTCRβ (H57-597, 1:40 dilution, Cat No.109219) mAb, BV421-conjugated mTCRβ (H57-597, 1:40 dilution, Cat No.109229) and mCD8α (53-6.7, 1:80 dilution, Cat No.100737) mAbs, BV510-conjugated mB220 (RA3-6B2, 1:100 dilution, Cat No.103247) and anti-mCD4 (RM4-5, 1:100 dilution, Cat No.100553) mAbs were purchased from BioLegend. FITC-conjugated anti-mIgG1 (A85-1, 1:50 dilution, Cat No.553443) mAb was purchased from BD Biosciences. PE-conjugated anti-mIL-18R (P3TUNYA, 1:80, Cat No.12-5183-82) and APC-eFluor780 conjugated anti-mTCRβ (H57-597, 1:20, Cat No.47-5961-82) mAbs were purchased from Thermo Fisher Scientific. Dead cells were stained with 7AAD (7-amino-actinomycin D, Cat No.420403, BioLegend) and viable cells were analyzed by FACS Verse (BD Biosciences) or Gallios flow cytometer (Beckman Coulter). The data were analyzed using FlowJo software (TreeStar).
Mass cytometry analysis
Antibodies were conjugated with the MaxPar conjugation kit according to the manufacturer’s instructions with the exception of cisplatin-labeled antibodies. Metal isotopes were obtained from Standard BioTools with the exception of Indium 113 and 115 (Trace Sciences). Conjugated antibodies were stored in PBS‐based Ab stabilizer (Candor Biosciences) and all antibodies were titrated for optimal staining concentrations with control mouse tissues. Full details of the staining panel are provided in Supplementary Table 1. For staining, 1 × 106 cells per sample were initially barcoded with metal-conjugated CD45 antibodies in the presence of Fc blocker for 30 min at RT and then washed twice in CyFACS buffer (PBS with 0.1% BSA and 2 mM EDTA) and then barcoded cells were pooled. Pooled cells were stained with a metal-conjugated surface stain antibody cocktail for 45 min at RT. Cells were then washed twice in CyFACS buffer, stained for viability with the cisplatin analogue dichloro-(ethylenediamine) palladium (II) in PBS for 5 min at RT and then fixed and permeabilized using the Foxp3 Transcription Factor Staining Buffer Set according to the manufacturer’s protocol (Thermo Fisher Scientific). Cells were subsequently stained with an intracellular antibody cocktail for 45 min at 4 °C. Cells were then washed twice in CyFACS buffer and once in PBS. Cells were then fixed overnight in 2% formaldehyde solution containing DNA Cell-ID Intercalator-103Rh (Standard BioTools). Prior to acquisition, cells were washed once in CyFACS buffer and twice in H2O. Cells were then diluted to 1 × 106 cells/ml in H2O containing 15% EQ Four Element Calibration Beads (Standard BioTools) and filtered. Cells were acquired at a rate of 200–300 cells/s using a Helios mass cytometer (Standard BioTools). Flow Cytometry Standard files were normalized to EQ bead signal. Initial gating and debarcoding was performed in CytoBank software. Clustering was then performed after gating on B cells (B220+CD3–) or CD4 T cells (CD4+CD3+TCRβ+B220–) by FlowSOM within the CATALYST Bioconductor package referring to CyTOF workflow (version 3)56. Differential abundance analysis of clusters was performed with edgeR, calculating adjusted p-values.
Bone marrow and T cell transplantation
BM cells from CD45.1+ WT or CD45.2+ Bcl11bΔiThy mice were suspended in phosphate-buffered saline (PBS) and 1 × 107 cells were injected into 8 Gy-irradiated Cd3e–/– mice. For mixed BM transfer, Cd3e–/– mice received 1:1 ratio of CD45.1+ WT and CD45.2+ Bcl11bΔiThy BM cells. The body weight and the frequency of CD4+ T cells in the peripheral blood were analyzed weekly. For T cell transfer, 1 × 107 cells of CD4+ T cells or CD4+ T cells lacking CD25+ Treg cells from CD45.1+ WT mice were transferred into CD45.2+ Bcl11bΔiThy mice.
Single cell-based transcriptome and TCR analysis
Libraries for mouse T cells were prepared using following reagents; Chromium Single Cell 5′ Library & Gel Bead Kit, PN-1000014; Chromium Single Cell A Chip Kit, PN-1000009; Chromium i7 Multiplex Kit, PN-120262; Chromium Single Cell V(D)J Enrichment Kit, Mouse T Cell, PN-1000071. Approximately 2 × 104 cells are loaded into Chromium microfluidic chips to generate single-cell gel-bead emulsions using the Chromium controller (10X Genomics) according to the manufacturer’s recommendations. Suspensions containing ∼16,000 cells were loaded on the instrument. RNA from each sample was subsequently reverse-transcribed in a Veriti Thermal Cycler (Thermo Fisher Scientific), and all subsequent steps to generate single-cell libraries were performed according to the manufacturer’s protocol, with 14 cycles used for cDNA amplification. Then ∼50 ng of cDNA was used for gene expression library amplification by 14 cycles in parallel with cDNA enrichment and library construction for T cell libraries. Fragment size of the libraries were confirmed with the Agilent 2100 Bioanalyzer (Agilent). Libraries were sequenced on an Illumina NovaSeq 6000 as paired-end mode (read1: 28 bp; read2: 91 bp). The raw reads were processed by cellranger 3.1.0 (10X Genomics). Clonotype analysis was done using Loupe Cell Browsers provided by 10x Genomics (https://support.10xgenomics.com/single-cell-gene-expression/software/downloads/latest#loupetab). After identification of TCR clonotypes by the loupe V(D)J browser, transcriptome analysis of these clusters was further performed by the loupe browser.
Bulk RNA-sequencing analysis
Single-cell suspensions from splenocytes were dissolved in the TRIzol (Cat. No.15596026, Thermo Fisher Scientific) and applied for bulk RNA-sequencing analysis was applied for TCR repertoire analysis using the SMARTer Mouse TCR a/b Profiling Kit (Takara Bio USA Inc).
Pseudotime analysis
For inferring cell lineages and pseudotimes, a Bioconductor package “slingshot57” and their lineage inference workflow to predict cell lineage (s) and pseudotime (s) were used. The output value “filtered_feature_bc_matrix” from the Cell Ranger was used as count data for slingshot. An R clustering package “mclust” was applied to the t-SNE matrix generated by Cell Ranger to infer clusters, yielding a final repertoire of 7 clusters (“chartreuse”, “cyan”, “khaki”, “plum”, “orange”, “orangered”, “sienna”). The “getLineages” function of slingshot was used to obtain lineage structure of the t-SNE matrix and their pseudotime (s). We specified the initial cluster as chartreuse based on gene expression of Mki67. We also used the “getCurves” function of slingshot to construct smooth curve in each lineage which inferred by getLineages function using default parameter setting except for extend = “n” and stretch = 0. For gene expression in the t-SNE dimension, the natural logarithm of the given value plus one (log1p) of the count data of each gene was plotted by plotGeneCount functions of a Bioconductor package “tradeSeq58”. We customized the plotGeneCount script to change color and position of the log1p color scales (Supplementary_code.txt).
Preparation of fractionated tissue homogenates
After snap-frozen tissues in liquid nitrogen were smashed in a mortar, homogenates were centrifuged (15,000 × g for 20 min at 4 °C). The homogenates were resuspended in water with 0.1% TFA and fractionated by HPLC (JASCO LC-NetII/ADC) using COSMOSIL 5C18-MS-II 4.6 mmID × 250 mm (Cat. No. 38020-41, Nakalai tesque). After loading, 70 fractions were collected by increasing Acetonitrile to 80%. Fractions were dried and stored at −80 °C until use.
Reporter assay
Reporter cell lines were cocultured with mouse MR1-expressing NIH3T3 cells in the presence of homogenates59. One day after the coculture, GFP expressions in reporter cells were analyzed by flowcytometry after gating on CD3+ reporter cells.
Histological analysis
Histological analysis was performed after freshly isolated tissue samples were fixed with formalin and then embedded in paraffin or OCT compound (Cat No.45833, Sakura Finetek). Sections were stained with Hematoxylin and Eosin (H&E) for detecting inflammatory cells or with Sirius red for the assessment of collagen contents using Picrosirius Red Stain Kit (Cat. No.24901, Polyscience). OCT compound-embedded sections of mouse pancreatic tissues were stained with antibodies against mIgG1 (0.5 μg/ml, A85-1, Cat. No.553443, BD Biosciences), mCD4 (10 μg/ml, GK1.5, Cat. No.100407, BioLegend) or B220 (5 μg/ml, RA3-6B2, Cat. No.103225, BioLegend) or biotinylated PNA (20 μg/ml, Cat. No.BK-1000, Vector laboratories) overnight at 4 °C. For PNA staining, Alexa Fluor 647-conjugated Streptavidin (1 μg/ml, Cat. No.405237, BioLegend) was applied on the tissues and incubated for 2 h at 37 °C. Stained tissues were visualized using an FV3000 confocal microscope (Olympus). Inflammatory score was determined as described previously60. The degrees of cell infiltration judged by histology using H&E staining of pancreatic tissues were scored as follows: 0, no detectable infiltration; 1, a focus of perivascular infiltration; 2, several foci of perivascular infiltration; 3, cellular infiltration in more than 50% of vasculature; 4, cellular infiltration in more than 80% of vasculature; and 5, massive infiltration. For quantification of the percentage of Picrosirius Red-positive area, images of pancreatic tissue were binarized using a fixed threshold by ImageJ and positive pixel ratio was calculated.
Determination of isotype-specific immunoglobulins, blood glucose levels, and lipase activities in serum
Concentrations of polyclonal mIgM (Cat. No. E99-101), mIgG1 (Cat. No. E99-105), IgG2a (Cat. No. E99-107), mIgG3 (Cat. No. E99-111) and mIgA (Cat. No. E99-103) were analyzed by isotype-specific enzyme-linked immunosorbent assay (Bethyl Laboratories, Inc). Blood glucose level was analyzed by ACCU–CHEK Aviva System Blood Glucose Meter (Roche). Lipase activity was analyzed with Lipase Color Liquid (Roche).
Immunoblot analysis
For analysis of sorted CD4+ αβ T cells, cells were lysed for 5 min at 95 ˚C in SDS lysis buffer (20 mM EDTA, 10 mM Tris HCl, 150 mM NaCl and 1% NP40). For analysis of pancreatic homogenates, tissues from Rag1-deficient mice were sonicated and then suspended in RIPA buffer. Proteins were resolved by SDS-PAGE and transferred to Immobilon–P membranes. Membranes were blocked by Blocking One (Cat. No. 03953-66, Nakalai tesque) and were detected by immunoblot analysis. The rabbit anti-mBcl11b polyclonal Ab was kind gift from Yukio Mishima (Niigata University). Anti-β-actin (2F1-1) mAb (Cat. No.622101, BioLegend) was used as an internal control. For pancreatic homogenates, serums from indicated mice were used as the first antibody. The first antibody was detected by peroxidase-labeled Goat anti-mIgG (H + L) (Cat. No.115-035-003, Jackson ImmunoResearch Laboratories Inc.). Signals were detected using LAS4000mini (GE healthcare).
Electrospray ionization-quadrupole Orbitrap high-resolution tandem mass spectrometry (ESI-HRMS/MS) analysis and data mining
Soluble fractions were dissolved in 50 μl of methanol/water (95:5, vol/vol). Flow injection analysis was performed using a Nexera LC system (Shimadzu Co., Kyoto, Japan) coupled with Q Exactive, a high performance bentchtop quadrupole Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA), equipped with an electron spray ionization source. The flow injection conditions in the positive and negative ionization modes were described previously61. Data mining procedure was described previously27. In short, Compound Discoverer 3.0 software (Thermo Fisher Scientific) was used for data processing, involving peak detection, data grouping, and filling gaps. Differential analysis (fraction #54 vs. other fractions, fold change > 5) was performed using an XLSM extension developed with Visual Basic for applications in Microsoft Excel 2013. Among more than 20,000 ion peaks (including isotopes and adducts detected in the positive and negative ionization), 22 candidates were detected selectively and reproducibly present in fraction #54.
Expression and purification of clonotype #1 ectodomain
Expression and purification of clonotype #1 αβ ectodomain was performed as described recently62. In brief, both clonotype #1 α and β ectodomains were expressed as fusion proteins with N-terminal hexahistidine tag and Tabaco Etch Virus (TEV) protease cleavage site. The clonotype #1 α and β ectodomains were separately expressed in Escherichia coli as inclusion bodies. The inclusion bodies were solubilized with 6 M guanidine hydroxychloride. The solubilized clonotype #1 α and β ectodomains were mixed, refolded, and then dialyzed. Clonotype #1 αβ heterodimer was applied to Ni-NTA agarose. After the removal of histidine tag by TEV protease treatment, the proteins were applied to size exclusion and anion change chromatography. The purities of proteins were assessed by SDS-PAGE.
Crystallization, data collection, and structure determination of clone #1 ectodomain
All crystallization trials were performed by sitting drop vapor diffusion method as described previously62. Initial crystallization conditions were searched using Index (Hampton Research) and SG1 Screen (Molecular Dimensions). Diffraction-quality crystals were obtained under the condition of 0.1 M Bis-tris (pH 5.5), 0.2 M lithium acetate and 17% (w/v) polyethylene glycol 10,000 at 293 K. Crystals were soaked into reservoir containing 10% (v/v) glycerol and rapidly frozen in liquid nitrogen. X-ray diffraction data were collected on the beamline BL-1A at Photon Factory (Tsukuba, Japan). All data sets were integrated with program XDS and scaled with the program Scala. The phases of the crystal were determined by molecular replacement with program Molrep using TCR-017 ectodomain (PDB code: 7EA6) as a search model. Further model building was manually performed with program Coot. Refinement was initially conducted using program Refmac5 and then Phenix.refine for final model. The stereochemical quality of the final model was assessed by Molprobity. Data collection and refinement statistics were summarized in Supplementary Table 2. Structural factors and coordinates have been deposited on Protein Data Bank under accession code 7F5K. All structural figures were depicted by program PyMOL2.0 (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.). Structural superposition was performed with program SUPERPOSE.
Statistical analyses
Statistical significance was calculated using Prism software (GraphPad, San Diego, CA). Differences with values for p < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Bulk RNA-sequencing and single-cell RNA-sequencing data have been deposited at the DNA Data Bank of Japan (DDBJ) database under the accession number DRA011320 within the Bioproject PRJDB10983. Structure factor and 3D coordinates of clone #1 TCR ectodomain have been deposited in the Protein Data Bank under the accession code, 7F5K. Source data are provided with this paper.
Code availability
The customized plotGeneCount script and source data for Fig. 3k are provided as Supplementary Code. The script code provided in the supplementary material is available under the MIT License (Copyright 2022, Takahide Hayano). The original code was included in the package “tradeSeq” (https://bioconductor.org/packages/release/bioc/html/tradeSeq.html) which is distributed under the MIT License (Copyright 2019, Koen Van den Berge; Hector Roux de Bezieux). See https://opensource.org/licenses/mit-license.php for the full text of the MIT license.
References
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Corbett, A. J. et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014).
Legoux, F., Salou, M. & Lantz, O. Unconventional or preset alphabeta T cells: evolutionarily conserved tissue-resident T cells recognizing nonpeptidic ligands. Annu. Rev. Cell Dev. Biol. 33, 511–535 (2017).
Rahimpour, A. et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 212, 1095–1108 (2015).
Godfrey, D. I., Koay, H. F., McCluskey, J. & Gherardin, N. A. The biology and functional importance of MAIT cells. Nat. Immunol. 20, 1110–1128 (2019).
Ikawa, T. et al. An essential developmental checkpoint for production of the T cell lineage. Science 329, 93–96 (2010).
Li, L., Leid, M. & Rothenberg, E. V. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 329, 89–93 (2010).
Li, P. et al. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science 329, 85–89 (2010).
Turchinovich, G. & Hayday, A. C. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity 35, 59–68 (2011).
Shibata, K. et al. IFN-gamma-producing and IL-17-producing gammadelta T cells differentiate at distinct developmental stages in murine fetal thymus. J. Immunol. 192, 2210–2218 (2014).
Ciofani, M., Knowles, G. C., Wiest, D. L., von Boehmer, H. & Zuniga-Pflucker, J. C. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity 25, 105–116 (2006).
Kreslavsky, T., Garbe, A. I., Krueger, A. & von Boehmer, H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J. Exp. Med. 205, 1173–1186 (2008).
Wakabayashi, Y. et al. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat. Immunol. 4, 533–539 (2003).
Drashansky, T. T. et al. BCL11B is positioned upstream of PLZF and RORgammat to control thymic development of mucosal-associated invariant T cells and MAIT17 program. iScience 24, 102307 (2021).
Albu, D. I. et al. BCL11B is required for positive selection and survival of double-positive thymocytes. J. Exp. Med. 204, 3003–3015 (2007).
Lopez-Sagaseta, J. et al. The molecular basis for mucosal-associated invariant T cell recognition of MR1 proteins. Proc. Natl Acad. Sci. USA 110, E1771–E1778 (2013).
Patel, O. et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat. Commun. 4, 2142 (2013).
Keller, A. N., Corbett, A. J., Wubben, J. M., McCluskey, J. & Rossjohn, J. MAIT cells and MR1-antigen recognition. Curr. Opin. Immunol. 46, 66–74 (2017).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Ding, Y. et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J. Immunol. 191, 1614–1624 (2013).
Hu, J. et al. Sclerostin domain-containing protein 1 is dispensable for the differentiation of follicular helper and follicular regulatory T cells during acute viral infection. Am. J. Transl. Res. 11, 3722–3736 (2019).
Deshpande, V. et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 25, 1181–1192 (2012).
Shiokawa, M. et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci. Transl. Med. 10 (2018).
Kamisawa, T. et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 58, 1504–1507 (2009).
Koay, H. F. et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 17, 1300–1311 (2016).
Legoux, F. et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366, 494–499 (2019).
Takahashi, M. et al. Highly accurate detection and identification methodology of xenobiotic metabolites using stable isotope labeling, data mining techniques, and time-dependent profiling based on LC/HRMS/MS. Anal. Chem. 90, 9068–9076 (2018).
Harriff, M.J. et al. MR1 displays the microbial metabolome driving selective MR1-restricted T cell receptor usage. Sci. Immunol. 3, eaao2556 (2018).
Lepore, M. et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. Elife 6, e24476 (2017).
Crowther, M. D. et al. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 21, 178–185 (2020).
Yan, J. et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov. 10, 124–141 (2020).
Salio, M. et al. Ligand-dependent downregulation of MR1 cell surface expression. Proc. Natl Acad. Sci. USA 117, 10465–10475 (2020).
Tsuda, J. et al. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human gammadelta T cells. J. Immunol. 186, 2003–2012 (2011).
Vanvalkenburgh, J. et al. Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J. Exp. Med. 208, 2069–2081 (2011).
Bennett, M. S., Trivedi, S., Iyer, A. S., Hale, J. S. & Leung, D. T. Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J. Leukoc. Biol. 102, 1261–1269 (2017).
Salou, M. et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 216, 133–151 (2019).
Lee, Y. J. et al. Lineage-specific effector signatures of invariant NKT cells are shared amongst gammadelta T, innate lymphoid, and Th cells. J. Immunol. 197, 1460–1470 (2016).
Nunez-Cruz, S. et al. LAT regulates gammadelta T cell homeostasis and differentiation. Nat. Immunol. 4, 999–1008 (2003).
Schmitt, N., Bentebibel, S. E. & Ueno, H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 35, 436–442 (2014).
Califano, D. et al. Diverting T helper cell trafficking through increased plasticity attenuates autoimmune encephalomyelitis. J. Clin. Invest. 124, 174–187 (2014).
Rouxel, O. et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat. Immunol. 18, 1321–1331 (2017).
Lessel, D. et al. BCL11B mutations in patients affected by a neurodevelopmental disorder with reduced type 2 innate lymphoid cells. Brain 141, 2299–2311 (2018).
Dolens, A. C. et al. Distinct Notch1 and BCL11B requirements mediate human gammadelta/alphabeta T cell development. EMBO Rep. 21, e49006 (2020).
Alam, C. et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 54, 1398–1406 (2011).
Kim, K. S. et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351, 858–863 (2016).
Kitajima, S., Morimoto, M., Sagara, E., Shimizu, C. & Ikeda, Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp. Anim. 50, 387–395 (2001).
Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624 (2019).
Kurashima, Y. et al. Pancreatic glycoprotein 2 is a first line of defense for mucosal protection in intestinal inflammation. Nat. Commun. 12, 1067 (2021).
Adolph, T. E., Mayr, L., Grabherr, F., Schwarzler, J. & Tilg, H. Pancreas-microbiota cross talk in health and disease. Annu. Rev. Nutr. 39, 249–266 (2019).
Loh, L. et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc. Natl Acad. Sci. USA 113, 10133–10138 (2016).
Sheu, T. T. & Chiang, B. L. Lymphopenia, lymphopenia-induced proliferation, and autoimmunity. Int. J. Mol. Sci. 22, 4152 (2021).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004).
Murali-Krishna, K. & Ahmed, R. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165, 1733–1737 (2000).
Walsh, M. C. et al. IL-18 synergizes with IL-7 to drive slow proliferation of naive CD8 T cells by costimulating self-peptide-mediated TCR signals. J. Immunol. 193, 3992–4001 (2014).
Kamisawa, T. et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J. Gastroenterol. 49, 961–970 (2014).
Nowicka, M. et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res. 6, 748 (2017).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Van den Berge, K. et al. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 11, 1201 (2020).
Shibata, K. et al. The intracellular pathogen Francisella tularensis escapes from adaptive immunity by metabolic adaptation. Life Sci. Alliance 5, e202201441 (2022).
Sekai, M., Hamazaki, Y. & Minato, N. Medullary thymic epithelial stem cells maintain a functional thymus to ensure lifelong central T cell tolerance. Immunity 41, 753–761 (2014).
Nagata, M. et al. Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc. Natl Acad. Sci. USA 114, E3285–E3294 (2017).
Lu, X. et al. Identification of conserved SARS-CoV-2 spike epitopes that expand public cTfh clonotypes in mild COVID-19 patients. J. Exp. Med. 218, e20211327 (2021).
Acknowledgements
We thank S. Iwai, M. Kurata, S. Mondoon, M. Kawano, M. Shiokawa, K. Toyonaga, A. Kubota, X. Lu, Y. Harima, A. Tanaka, E. Ito, T. Oono, K. Kawabe, D. N. Azizah and T. Ito for technical support; S. Inuki, KH. Sonoda and Y. Yoshikai for discussions; M. Tanaka and K. Kaseda for embryonic engineering; the Cooperative Research Project Program of the Medical Institute of Bioregulation, Kyushu University and Science Research Center of Yamaguchi University for technical support. We also thank the beamline staff at the Photon Factory for crystallographic data collection. This research was supported by AMED (JP20gm0910010, JP20ak0101070, and JP20fk0108075), JSPS KAKENHI (JP20H00505 and JP16K08740), the Kurozumi Medical Foundation (K.S.), and the Takeda Science foundation (M.F.S.). The MR1 tetramer technology was developed jointly by Dr. James McCluskey, Dr. Jamie Rossjohn, and Dr. David Fairlie, and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne.
Author information
Authors and Affiliations
Contributions
K.S. and S.Y. conceptualized research; K.S., C.M., T.S., E.I., Y.I., M.T., and M.N. did investigation; N.F. and Y.O. provided resources; K.S., C.M., D.M., D.O., J.B.W., T.H., Y.A., and T.B. did data curation; M.F.S., M.S., and S.Y. supervised the research; K.S. and S.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Liza Konnikova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shibata, K., Motozono, C., Nagae, M. et al. Symbiotic bacteria-dependent expansion of MR1-reactive T cells causes autoimmunity in the absence of Bcl11b. Nat Commun 13, 6948 (2022). https://doi.org/10.1038/s41467-022-34802-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34802-8
This article is cited by
-
MR1 antigen presentation to MAIT cells and other MR1-restricted T cells
Nature Reviews Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.