Abstract
Considerable attention has been drawn to tune the geometric and electronic structure of interfacial catalysts via modulating strong metal-support interactions (SMSI). Herein, we report the construction of a series of TiO2−x/Ni catalysts, where disordered TiO2−x overlayers immobilized onto the surface of Ni nanoparticles (~20 nm) are successfully engineered with SMSI effect. The optimal TiO2−x/Ni catalyst shows a CO conversion of ~19.8% in Fischer–Tropsch synthesis (FTS) process under atmospheric pressure at 220 °C. More importantly, ~64.6% of the product is C2+ paraffins, which is in sharp contrast to the result of the conventional Ni catalyst with the main product being methane. A combination study of advanced electron microscopy, multiple in-situ spectroscopic characterizations, and density functional theory calculations indicates the presence of Niδ−/TiO2−x interfacial sites, which could bind carbon atom strongly, inhibit methane formation and facilitate the C-C chain propagation, lead to the production of C2+ hydrocarbon on Ni surface.
Similar content being viewed by others
Introduction
CO hydrogenation to high value-added chemicals via Fischer–Tropsch synthesis (FTS) process has attracted growly attention, which has become the most frontier field in academia and industry1,2,3,4,5,6. Despite of great success in industry, there is still much debate over reaction mechanism and governing structure-activity relationship, which mainly originates from extensive studies on Fe or Co-based FTS. It is of vital importance to find an alternative to Co or Fe catalysts and thereafter develop mechanistic understanding of structure-activity relationship from a new perspective7,8,9,10,11. Ni-based catalysts had been considered as a vital candidate for FTS for a long time as they displayed high activities for CO hydrogenation under relatively mild operation conditions compared to those in Co or Fe-catalyzing FTS, prevailing methanation and fast deactivation due to the formation of Ni-carbonyl severely hinder their practical applications12,13,14. However, some experiments and calculations reported the metal-support interactions and its effect on product distributions in Ni-catalyzing FTS15,16, which suggests that the optimal Ni-based catalysts may display similar FTS activity to Co catalysts17. Therefore, it is of great interest to develop a new type of Ni-based catalyst to efficiently inhibit the formation of nickel carbonyl and simultaneously enhance the C–C chain propagation, so as to increase the selectivity of CO hydrogenation towards high hydrocarbons. As indicated by the aforementioned works18,19,20,21, modulating the geometric and electronic structures of Ni particles should be an effective way to tune the catalytic performance of CO hydrogenation towards long-chain hydrocarbons.
Strong metal-support interaction (SMSI) was firstly put forward by Tausters et al. in 197822,23, which alters the geometric and electronic structures of supported metal particles, especially for the interfacial properties24. Suboxides migrated on the surface of supported metal particles with strong electron transfers at the interface is a typical characteristic of SMSI, resulting in the re-arrangement of atoms or charge redistribution of the interface25,26,27,28. Actually, many SMSI-type catalysts have been developed to substantially enhance the catalytic activity and selectivity of CO hydrogenation towards desirable long-chain hydrocarbons in C1 chemistry29,30. De Jong et al. designed a reduction-oxidation-reduction (ROR)-SMSI type Co-based catalyst with high exposure of active interfacial sites and metal dispersion to efficiently increase the catalytic performance of FTS reaction31. In addition, the catalytic properties of Ru/TiO2 catalysts could be also efficiently improved via SMSI during FTS, owing to promoting the activation and cleavage of CO bond by TiO2−x overlayers located at Ru/TiO2 interface32. Compared to traditional impregnation method, in situ structural transformation of Layered double hydroxides (LDHs) could provide an efficient cutting-edge approach to construct new-type SMSI catalyst, which has been explored in C1 catalytic chemistry in recent years33,34. These results inspire us to synthesize a new-type Ni-based SMSI catalyst with enhanced FTS performance with respect to its academic and practical significance.
In this work, SMSI-type TiO2−x overlayers decorated Ni nanoparticle catalysts (TiO2−x/Ni) were successfully synthesized via the reduction of calcinated ultrathin NiTi-LDHs precursor. Abundant Niδ−/TiO2−x interfacial sites can be found on TiO2−x/Ni catalyst, which were validated by quasi in situ XPS, in situ EXAFS, and in situ DRIFTS in detail. The optimal TiO2−x/Ni catalyst exhibits a CO conversion of ~19.8% with ~64.6% selectivity to C2+ paraffin at 220 °C under atmosphere pressure. Both experimental and theoretical results evidence that the Niδ−/TiO2−x interfacial sites at TiO2−x/Ni catalysts can facilitate CO activation and C-C chain propagation to produce C2+ paraffin but hinder methane formation. The well-designed LDHs transformation is proved to be an efficient approach to construct the new-type SMSI catalysts with the controllable exposure of abundant active interfacial sites, which paves a new way to the rational design of high-performance heterogeneous catalysts.
Results
Synthesis of TiO2−x overlayers decorated Ni nanoparticles (TiO2−x/Ni) catalysts
The NiTi-LDHs nanosheets were successfully synthesized by the hydrothermal synthesis approach described in the previous work35. The XRD pattern shows a series of diffraction peaks at 2θ of 12.0°, 24.6°, 33.4°, 37.9°, and 59.8°, which are corresponded to the (003), (006), (009), (012), and (110) planes of NiTi-LDHs phase (Supplementary Fig. 1a)36. The as-synthesized NiTi-LDHs nanoplates display a mean diameter of ~200 nm with an average thickness of ~6 nm (Supplementary Fig. 1b–d). After calcination at 500 °C for 4 h, mixed oxides (NiTi-MMO) composed of NiO and TiO2−x are obtained (Supplementary Fig. 2). The primary diffraction peaks at 37.3°, 43.5°, 63.2° can be ascribed to the cubic NiO crystal phase37 (Supplementary Fig. 2a). The anatase phase of TiO2−x with weak and broad reflection could be observed with respect to its poor crystal structure. As seen in the TEM image (Supplementary Fig. 2b–d), small NiO nanoparticles are well-dispersed into the oxide composite. Afterwards, the oxide mixture (NiO and TiO2-x) was reduced by H2 at 300 °C, 350 °C, 400 °C, 450 °C, 500 °C, 550 °C, and 600 °C, respectively. The reduced samples are designated as TiO2−x/Ni-T, with T denoting the reduction temperature. Inductively coupled plasma atomic emission spectrometry (ICP-AES) measurements confirm around 50 wt% of nickel loadings in the reduced samples (Supplementary Table 1). Notably, formation of metallic nickel could be observed after reduction at 350 °C according to the XRD data (Fig. 1a). By increasing the reduction temperature, the XRD signals was getting increased and narrower, it indicates the growth of Ni particles when raising up the temperature (Fig. 1). According to the Scherrer equation, the particle sizes increase from ~15 nm to ~20 nm within the temperature raising from 400 °C to 600 °C (Supplementary Table 1). TEM was carried out to further explore the metal dispersion (Supplementary Fig. 3 and Supplementary Table 1), which agrees well with the XRD characterizations.
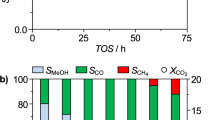
a XRD patterns of NiTi-MMO and a series of TiO2−x/Ni catalysts reduced at different temperatures. b The conversion and product distribution as well as (c) reaction rate and activity over various TiO2−x/Ni catalysts. d The stability evaluation of TiO2−x/Ni-450 at 220 °C. Reaction conditions: catalysts (120 mg), 1 bar, syngas (CO/H2/Ar = 32/64/4; space velocity: 10000 mL gcat−1 h−1).
Evaluation of catalytic performance
The catalytic performances of TiO2−x/Ni catalysts reduced at different temperatures were evaluated at 220 °C with a weight hourly space velocity (WHSV) of 10,000 ml gcat−1 h−1, as shown in Fig. 1b and Supplementary Table 2. Promisingly, the product distributions demonstrate that C2+ paraffin is a predominant product rather than methane (CH4) for CO hydrogenation over TiO2−x/Ni catalysts. The six TiO2−x/Ni-T catalysts show similar C2+ paraffin selectivity at the same conditions, indicating all the catalysts contain similar active sites. CO conversion increases initially but decreases subsequently when the catalyst activation temperature increases from 350 to 600 °C, suggesting that the decrease of active sites during reduction at higher temperature. The optimal TiO2−x/Ni-450 catalyst exhibits the highest CO conversion (~19.8%) at 220 °C under atmosphere pressure with the selectivity of C2+ paraffin up to ~64.6% (Supplementary Fig. 4). The selectivity of C5+ products can attain ~21.5% at the relatively mild conditions. When switching to a lower reaction temperature of 200 °C, CO conversion of the optimum TiO2−x/Ni-450 catalyst is decreased to ~6.8% associated with a higher selectivity of C2+ paraffin (~72.3%, Supplementary Fig. 5 and Supplementary Table 3). Obviously, the activity and selectivity of CO hydrogenation are significantly dependent on the reaction temperature. The catalytic reaction rates and activities of various TiO2−x/Ni catalysts show a volcano-like trend with the increment of catalyst reduction temperature in Fig. 1c. The optimum TiO2−x/Ni-450 catalyst shows the highest reaction rate (0.053 molCO gNi−1 h−1) and catalytic activity (0.085 molCO molsuface-Ni−1 s−1) at 220 °C. Additionally, TiO2−x/Ni-450 catalyst displays a good stability (20% activity decrement) within 50 h at 220 °C (Fig. 1d).
As a comparison, the 15% Ni/SiO2 catalyst (Supplementary Figs. 6 and 7) was also evaluated. However, the 15% Ni/SiO2 exhibit a poor CO conversion (2.4%) and much higher selectivity of CH4 (67.9%) with a reaction rate (0.031 molCO gNi−1 h−1) and catalytic activity (0.007 molCO molsuface-Ni−1 s−1) at the same condition. Obviously, the selectivity of C5+ paraffin for TiO2−x/Ni-450 is ~21.5%, which is much higher than that of 15% Ni/SiO2 (C5+ paraffin selectivity: 1.4%) in Supplementary Fig. 8 and Supplementary Table 4. In addition, it is worth noting that the catalytic activity of TiO2−x/Ni-450 is ~12-fold than that of Ni/SiO2. When the Ni loading increases to 30 wt% (Supplementary Fig. 9), the 30% Ni/SiO2 catalyst exhibits an even worse CO conversion (1.0%) and much higher selectivity of CH4 (80.3%). The catalytic activity of 30% Ni/SiO2 is lower than that of 15% Ni/SiO2 at 220 °C with higher selectivity of CH4. It suggests that there is no SMSI in the SiO2 supported catalysts, or the SMSI effect present in the Ni/SiO2 catalyst system is significantly different from that in the TiO2−x/Ni-450 system. In order to explore the influence of different support, catalytic performance of the conventional Ni/TiO2 prepared by a conventional impregnation method was also examined (Supplementary Fig. 10). Clearly, the catalyst displays much worse catalytic performance at 220 °C (10.1% conversion and 7.3% selectivity to C5+ paraffin) in contrast to TiO2−x/Ni-450 (19.8% conversion and 21.5% selectivity to C5+ paraffin). Therefore, the support effect between Ni and TiO2 play an important role in improving the catalytic activity and selectivity towards to C2+ paraffin.
In order to understand the active sites, the apparent activation barrier (Ea) of CO hydrogenation was measured over various TiO2−x/Ni catalysts in Supplementary Fig. 11. The kinetic studies show that apparent activation barrier of CO hydrogenation is similar over various TiO2−x/Ni catalysts, i.e., the intrinsic active sites is similar, resulting the similar product selectivity. It is obvious that the catalytic activity first raises and then declines even when normalized per surface Ni as shown in Supplementary Fig. 12. This is attributed to the surface Ni concentration decreases with the increment of reduction temperature, owing to the influence by the SMSI effect at the high temperature, i.e., the sub-oxide (TiO2−x) migrated gradually on the surface of metal Ni particles driven by SMSI. Simultaneously, the active interfacial Niδ−/TiO2−x sites decrease gradually with the increment of reduction temperature after 450 °C. This indirectly indicates that the interfacial Niδ−/TiO2−x sites were a key factor governing the catalytic efficiency whilist the surface Ni concentration.
The identification of Niδ−/TiO2−x interfacial sites over various TiO2−x/Ni catalysts
Quasi in-situ XPS was carried out to reveal the surface electronic structures of various TiO2−x/Ni samples activated at different temperatures (Fig. 2a, b). For NiTi-MMO11,38 prepared via the calcination of NiTi-LDHs precursor, a 2p3/2 peak at 853.4 eV is assigned to Ni2+ species39,40 and a peak at ∼856.1 eV is indexed to Ni3+ species due to the formation of abundant VNi species in NiTi-MMO37,41, both of which demonstrate that Ni3+ and Ni2+ species exist in NiTi-MMO. Whereas a weak 2p3/2 peak at 852.5 eV can be observed in TiO2−x/Ni-300 catalyst, which is indexed to Ni0 species42. With the reduction temperature raising up to 400 °C, a stronger 2p3/2 peak at 852.0 eV can be found and attributed to the Niδ− species17,21. When the reduction temperature rises to 600 °C, the 2p3/2 peak located at 852.0 eV becomes broader and stronger, indicating a higher content of Niδ− species in the further reduced catalyst. The corresponding amount of Niδ− species could be quantified over various TiO2−x/Ni catalysts with a deconvolution by Gaussian peak fitting method (Supplementary Fig. 13 and Supplementary Table 5). According to Ti 2p XPS spectra, presence of Ti3+ species43,44 can be observed at 458.0 eV over various TiO2−x/Ni catalysts in Fig. 2b. It is very imperative to monitor the structure change of TiO2−x/Ni catalysts during the reaction, so as to confirm the stability of catalysts under the realistic reaction condition. It is obviously that the electronic structure of metal Ni species for TiO2−x/Ni-450 catalyst unchanged under the reaction condition at 220 °C as shown in Supplementary Fig. 14.
Quasi in situ XPS spectra of (a) Ni 2p and (b) Ti 2p for various TiO2−x/Ni catalysts. c In situ CO-DRIFTS spectra of TiO2−x/Ni catalysts at room temperature. d The normalized Ni K-edge XANES spectra of various TiO2−x/Ni catalysts, respectively. e The corresponding normalized Ni K-edge Fourier-transform EXAFS spectra. f The Normalized Ti K-edge XANES spectra of various TiO2−x/Ni catalysts. WT analysis of (g) Ni foil, (h) NiTi-MMO, and (i) TiO2−x/Ni-450 catalyst.
To further confirm the presence of electron-enriched Niδ− species over various TiO2−x/Ni catalysts, in situ CO-DRIFTS chemisorption experiments were carried out (Fig. 2c). The IR bands at ~2074, ~2047, and ~2035 cm−1 at 25 °C can be assigned to linear CO molecules on Ni defect sites, Ni0 atop sites, and Niδ− atop sites, respectively45,46. With the temperature rising up from 350 °C to 600 °C, the bands decrease owing to better crystallinity and coverage of TiO2−x suboxides, thereafter inhibiting the formation of nickel carbonyl17. It is well known CO adsorption can be strongly modulated by the electronic structure of supported metals. Therefore, the electron-enriched Niδ− species with much more d-electron can enhance the back-donation into 2π* antibonding orbital of CO molecules21, and then strengthen CO chemisorption. It is observed that, when the temperature treatment increased from 300 °C to 450 °C, the band at ~1970 cm−1 ascribed to bridged CO adsorbed on Ni0 sites shifted to ~1940 cm−1 accompanied with the formation of three-fold carbonyl C = O stretching vibrations at ~1900 cm−1, it is due to the increment of Niδ− species which bonds the CO molecules stronger than Ni0 45. According to our previous work47, the linear and bridge adsorbed CO can desorb from the surface of Ni0 sites after flow in the Ar atmosphere for 30 min above elevated temperatures. Whilst the linear and bridge chemisorbed CO species on Niδ− sites are even stable at 250 °C on TiO2−x/Ni-450 after Ar flow for 30 min (Supplementary Fig. 15). It further confirms the presence of significant amounts of Niδ− sites with a higher affinity for CO.
In situ XAFS spectra were obtained to reveal the electronic state, geometric structure, and coordination environment of various TiO2−x/Ni samples. In terms of NiTi-MMO, the normalized Ni K-edge XANES (Fig. 2d) displays a higher white line and the adsorption edge shifts toward the higher photon energy compared to the NiO reference, indicating that Ni3+ species present in the NiTi-MMO precursor in accordance with the result of XPS spectra. After the precursor was activated at 400 °C in 10% H2/He atmosphere at 400 °C, the XANES spectrum of TiO2−x/Ni-400 exhibits a slightly stronger white line compared to other TiO2−x/Ni catalysts, but is very close to that of Ni foil, further demonstrating that the predominant phase is the metallic nickel with a slight oxidation, i.e., the co-existence of Ni metal and NiO phase. Within the reduction temperature varying from 450 to 600 °C, the adsorption edge displays a shift toward lower energy than that for Ni foil and the white line is close to the Ni foil. Combined with the XPS and CO-DRIFTS characterizations, we can further confirm the formation of electron-enriched Niδ− species over the TiO2−x/Ni-400, TiO2−x/Ni-450, TiO2−x/Ni-500, and TiO2−x/Ni-600 catalysts. The electron-enriched Niδ− species is due to the strong electron donation from TiO2−x to the interfacial Ni atoms by SMSI. In the case of R space plot for TiO2−x/Ni within the reduction temperature varying from 400 to 600 °C in Fig. 2e, the Ni-Ni shell of the four catalysts shows the similar coordination number in Supplementary Fig. 16, indicating they have similar particle size in line with the result of XRD and HRTEM images. The coordination number, bond distance, Debye-Waller factor, and other parameters determined by EXAFS characterizations are listed in the Supplementary Table 6. Wavelet transform (WT) of Ni K-edge EXAFS oscillations of Ni foil (Fig. 2g), NiTi-MMO (Fig. 2h) and TiO2−x/Ni-450 (Fig. 2i) indicate that NiTi-MMO was reduced to metallic particles. A prominent peak can be observed at 2.2 Å for Ni particles, which can be ascribed to the Ni-Ni scattering. The similar phenomenon can be observed for TiO2−x/Ni-400 (Supplementary Fig. 17), TiO2−x/Ni-500 (Supplementary Fig. 18), and TiO2−x/Ni-600 (Supplementary Fig. 19).
In situ Ti K-edge XANES were carried out to explore the Ti−O coordination environment. In terms of post-edge feature above 4984 eV, the white line and resolvable peaks of TiO2−x/Ni-450 show a broad and less pronounced peak than that for the anatase reference, indicating the severely disordered Ti−O coordination environment nature. As for the pre-edge region below 4984 eV, three pre-peaks labeled as P1, P2, and P3 are attributed to the transition of the core electron to the hybridized states of Ti 3d4p4s orbitals43,48,49 in Fig. 2f. In the case of TiO2−x/Ni-450, the P2 peak at 4970.6 eV increased significantly and distinctly shifted to lower photon energy compared to the standard anatase (4972.2 eV). According to previous works34, the peak at 4970.6 eV is ascribed to the five coordinated Ti atoms, which is closely related with the formation of Ov − Ti3+ species. The strong electronic metal-support interaction can occur at the interface accompanied with the electron transfer from Ov − Ti3+ species to the adjacent interfacial Ni atoms to form Niδ− sites. Therefore, abundant Niδ−/TiO2−x interfacial sites can be efficiently fabricated and stabilized over the TiO2−x/Ni-450 catalyst. The raising of reduction temperature enhances Niδ− species content, owing to the SMSI between Ni particles and TiO2−x support, resulting in the formation of more Niδ−/TiO2−x interfacial sites.
The revelation of structure and morphology for various TiO2−x/Ni Catalysts
In situ environment scanning transmission electron microscope (ESTEM)50 was employed to identify the structure and surface topography of the optimal TiO2−x/Ni-450 and TiO2−x/Ni-600 catalysts. The detailed schematic diagram of ESTEM can be found in Supplementary Fig. 20. It is generally known that Ni nanoparticles could be oxidized to form superficial NiO layers upon exposure to air at ambient conditions, which inevitably ruined original surface configurations of the reduced catalyst. In order to reveal the intrinsic physicochemical property of Ni-based catalysts, environmental electron microscopy is necessary so as to characterize reduced Ni nanoparticles and their surroundings. In a typical process, the passivated TiO2−x/Ni-450 catalyst was reduced in H2 atmosphere (pressure: 10 Pa) at 450 °C for 1 h. The FCC microstructure of the reduced metallic Ni nanoparticle was determined by the inset FFT pattern (Fig. 3a).
a STEM-BF (b) STEM-SE and (c) STEM-ADF images of TiO2−x/Ni-450 catalyst after reduction in H2 atmosphere (10 Pa) at 450 °C. d Schematic diagram of Ni particle as shown in b. In situ electron energy-loss spectroscopy (EELS) collected at spot I and II. e Ni L-edge spectra collected at Spot I and II, respectively. f Ti L-edge spectra collected at Spot I and II, respectively.
In particularly, the advanced ESTEM equipped with a SE (secondary electron) detector enables us to deeply explore surface and morphology information of TiO2−x/Ni catalyst under the realistic chemical environment. Figure 3b discloses that the reduced Ni particle with a well-ordered shape is partially covered by TiO2−x overlayers after the passivated TiO2−x/Ni-450 reduction at 450 °C. A schematic diagram (Fig. 3d) rationalizes that the surface crystalline plane of the Ni NP is only terminated by low-indexed crystalline planes51 and visualizes surface TiO2−x deposits on the crystalline surface of the Ni nanoparticles (also seeing Supplementary Fig. 21). In order to evidence the specific interaction between the metal nanoparticle and the oxide, EELS Ni L-edge and Ti L-edge were also obtained at Area I (Ni particle), Area II (interface), and Area III (TiO2 support), so as to distinguish the electronic properties of bulk of Ni NP, interfacial Ni atoms and Ti atoms, and Ti atoms of TiO2 support in Fig. 3e, f. It is obvious that the L-edge of Ni species at the interface (Area II) show a slight shift (0.6 eV) toward the lower energy compared to the surface Ni species of metal particle (Area I), indicating that the presence of Niδ− species. Abundant Ti3+ species52,53 can be observed at the interface whilst is absent at the TiO2 support (Area III). ESTEM was also carried out over the TiO2−x/Ni-600 catalyst. After activation in H2 atmosphere at 600 °C, the Ni particles were close to be fully covered by several atomic thickness of TiO2−x overlayers (Supplementary Fig. 22). STEM-EDS mapping data further confirm the full coverage of TiO2−x over the Ni particle (Supplementary Fig. 23). The full coverage significantly hinders the active sites (interfacial Niδ−/TiO2−x species), resulting in the reduction of catalytic performance (Fig. 1b). It indicates that the higher reduction temperature promotes TiO2−x overlayers migrate on the surface of Ni particle via SMSI, this is consistent to the surface Ni concentration analysis from CO titration (Supplementary Table 1).
The investigation of CO hydrogenation process via IR spectroscopy
In situ time-resolved DRIFTS were carried out to monitor the dynamic evolution of reactive molecules, including intermediates and products, at different conditions for CO hydrogenation over the TiO2−x/Ni-450 catalyst (Fig. 4). The primary IR bands at ~2079 cm−1 and ~1924 cm−1, assigned to linear adsorbed CO species on Ni defect sites and bridge adsorbed species on Niδ− sites, respectively, are observed at 160 °C under the reaction condition in Fig. 4a, which is in consistent with the result in CO atmosphere in Supplementary Fig. 24. The IR bands at ~2926 cm−1 and ~2855 cm−1, which are ascribed to the asymmetric vas(C − H) and symmetric vs(C − H) stretching vibration of CH2* species54, increase gradually with the extension of exposure time (Fig. 4b), indicating the formation of C2+ paraffin products. However, no obvious IR band at ~3016 cm−1 (assigned to the v(C−H) stretching vibration32,55 of CH4 molecules) can be found for the TiO2−x/Ni-450 catalyzing CO hydrogenation. This demonstrates that C2+ hydrocarbon products are the primary product rather than CH4 for CO hydrogenation at 160 °C. When the reaction temperature raises up to 180 °C, two types of IR bands at ~2079 cm−1 and ~1924 cm−1 show inconspicuous change (Fig. 4c). A very weak IR band at ~3016 cm−1 was observed assigned to the C − H stretching mode of CH4 molecules. The IR bands at ~2926 cm−1 and ~2855 cm−1 display obvious increment as shown in Fig. 4d, indicating the enhancement of catalytic performance of FTS with the formation of much more C2+ paraffin product. The similar phenomenon could also be found at 200 °C in Fig. 4e, f. With the reaction temperature sequentially increasing to 220 °C, the IR bands at ~2068 cm−1 and ~1916 cm−1 in Fig. 4g decreased remarkably due to the substantial increment of catalytic performance. The C − H stretching mode of CH4 molecules at ~3016 cm−1 increase substantially accompanied with the presence of abundant CH2* species at ~2926 cm−1 and ~2855 cm−1 in Fig. 4h, indicating the simultaneous enhancement of catalytic activity for the methanation and C2+ paraffin products via FTS, which is in good agreement with the experiment results (Supplementary Table 3).
In situ time-resolved DRIFTS spectra were carried out over the TiO2−x/Ni-450 catalyst at a, b 160 °C, c, d 180 °C, e, f 200 °C, g, h 220 °C, respectively. The corresponding spectra were recorded in 2250 − 1600 cm−1 and 3100 − 2700 cm−1 at different temperature with the time stream on. From left to right: upon exposure to syngas (CO: H2:Ar = 5:10:85; 30 mL/min) at different temperatures as a function of time.
Reaction mechanisms identification by DFT calculations
Density functional theory (DFT) calculations were performed to understand CO hydrogenation mechanism over TiO2−x/Ni(110) catalyst. Here, Ti6O11/Ni(110) surface, where one layer Ti6O11 cluster kept the features of TiO2(101) surface was deposited on Ni(110) surface, were used to model interfacial TiO2−x/Ni(110) catalysts (Supplementary Fig. 25). The open Ni(110) surface was selected here because it exposes the Ni particles and contains many under-coordinated surface Ni atoms exhibiting high activity for CO activation20. All Ti atoms and eight O atoms in Ti6O11 cluster tend to bind Ni(110) surface resulting in a great charge transfer between Ti6O11 cluster and Ni(110) surface (Fig. 5a). Two Ti3+ cations always generate accompanied with the formation of one oxygen vacancy (Ov) at the interface of Ti6O11/Ni(110) surface. Bader charge analysis indicates that the interficial Ni atoms at VO nearby have negative charge states (Supplementary Fig. 25 and Supplementary Table 7), which is corroborated by our experimental characterizations. The formation of interfacial Niδ−/TiO2−x site over Ti6O11/Ni(110) surface could show different catalytic performance of CO hydrogenation as compared with Ni(110) surface attributed from their different geometric and electronic structures discussed below.
a Differential charge densities of Ti6O11/Ni(110) surface. The light blue and yellow contours (isosurface = 0.006 e/Å3) represent the charge depletion and charge accumulation, respectively. b Key transition states configurations for CO hydrogenation towards CH4 and C2H6 products over Ni(110) (Top panel) and Ti6O11/Ni(110) (Bottom panel) surfaces. The solid blue line is drawn to signify the bond dissociation or formation. The bond distances at the transition states for CO hydrogenation are indicated in Å. The blue, light gray, gray, red and white spheres are Ni, Ti, C, O and H atoms, respectively. c, d Potential energy diagrams for CO methanation and C2H6 formation via CH-CH coupling and hydrogenation over Ni(110) and Ti6O11/Ni(110) surfaces, respectively. The elementary reaction barriers are indicated in eV and all the energies in (c) and (d) are calculated with respect to CO + 2H2 and 2CO + 3H2 in the gas phase, respectively.
CO hydrogenation is initialized by CO activation and hydrogenation in the generation of CHx (x = 0–3) monomers, which can be further hydrogenated to methane (CH4) and/or coupled with each other forming C2+ alkanes/olefines (Fig. 5b). Ti6O11/Ni(110) and Ni(110) surfaces exhibit different CO activation and further hydrogenation mechanisms (Supplementary Fig. 26). The overall activation barrier for hydrogen-assisted CO dissociation has the highest activation barrier (Ea = 1.80 eV) and can be regarded as the rate-determining step (RDS) for CH4 and C2H6 formation over Ni(110) surface. The elementary reaction steps in CH hydrogenation towards CH4 are neutral with the highest activation barrier of 0.82 eV on Ni (110) surface. However, C2H6 formation via CH-CH, CH-CH2 and CH2-CH2 coupling have comparable overall higher activation barriers of 1.21–1.26 eV over Ni (110) surface (Fig. 5c, d and Supplementary Fig. 26). Therefore, CH4 rather than C2H6 is often the dominant product for CO hydrogenation on Ni-based surface in line with our experimental observations.
Different from Ni(110) surface, direct CO dissociation path (Ea = 1.14 eV) is more feasible than hydrogen-assisted CO activation via formyl (HCO) route monomer by 0.43 eV over Ti6O11/Ni(110) surface. Therefore, Ti6O11/Ni(110) surface has a much higher activity than Ni(110) surface by 0.55/0.54 eV for CO activation via COH/HCO intermediates more favorable for Ni catalyst (Supplementary Fig. 26). The higher activity of Ti6O11/Ni(110) for CO activation can be attributed to the strong binding strength between Ti atom and O atom lowering the corresponding transition state energy and reaction energy of direct CO dissociation.
The hydrogenation of strongly adsorbed dissociated C atoms towards CH4 at the interfacial Niδ−/TiO2−x site of Ti6O11/Ni(110) is highly endothermic thus resulting in a low activity for CH4 formation with high activation barrier of 2.05 eV (Fig. 5c). In contrast, C2H4 formation via CH-CH coupling step is much more favorable in the generation of C2H4 intermediate than CH4 product at the interfacial Niδ−/TiO2−x site of Ti6O11/Ni(110) surface with an activation barrier of 1.06 eV (Fig. 5d and Supplementary Fig. 27). The hydrogenation of formed C2H4 intermediate in the generation of C2H6 has to overcome a high activation barrier of 1.67 eV at the the interfacial Niδ−/TiO2−x site of Ti6O11/Ni(110) surface (Supplementary Fig. 27) that C2H4 intends to migrate to Ni (110) surface for further hydrogenation towards C2H6 with a lower overall activation barrier of 1.18 eV. In other words, Niδ−/TiO2−x site of Ti6O11/Ni(110) can be used for CO activation and CH-CH coupling in the formation of C2H4, whereas Ni(110) surface serves as the hydrogenation platform in the formation of C2H6 product. Both CO activation and C2H6 formation control CO hydrogenation activity at the interfacial Niδ−/TiO2−x site of Ti6O11/Ni(110) surface with the activation barriers lower than those on Ni(110) surface by 0.62 eV at least. Ultimately, Niδ−/TiO2−x site of Ti6O11/Ni(110) surface shows higher activity and selectivity of CO hydrogenation towards C2H6 as compared with Ni(110) surface with CH4 as the main product. Our theoretical calculations are corroborated by experimental measurements that the interfacial site at TiO2−x/Ni-450 catalyst plays an important role in CO hydrogenation towards ethane. TiO2−x/Ni-450 is more active than 15% Ni/SiO2 for CO hydrogenation towards ethane and TiO2−x/Ni-450 catalyst has a higher TOF and lower apparent activation barrier (Supplementary Table 1 and Supplementary Fig. 11).
Discussion
In summary, an interfacial TiO2−x/Ni catalyst was fabricated derived from LDHs precursor displaying a high CO conversion of ~19.8% and ~64.6% selectivity for C2+ paraffin at atmosphere pressure for FTS process. Advanced electron microscopy and in situ characterization were executed to verify that disordered titania overlayers modulated the surface of Ni particles to generate abundant Niδ−/TiO2−x interfacial sites by SMSI. DFT calculations reveal that TiO2−x/Ni catalyst exhibits extraordinary high activity and selectivity for CO hydrogenation towards carbon coupling products originated from catalytic synergistic effect, namely, the interfacial Niδ−/TiO2−x site enhances CO dissociation and facilitates the growth of C-C chain. The present work not only discovers a new-type SMSI catalyst with the unique interfacial structure for syngas conversion at atmosphere pressure, but also insight into the interfacial synergistic catalysis driven by SMSI effect.
Methods
Catalyst preparation
Firstly, the ultrathin NiTi-LDHs precursor was prepared via the revised hydro-thermal synthesis approach according to our previous work34. In a typical process, Ni(NO3)2·6H2O (0.006 mol), HCl (0.25 mL), TiCl4 (0.25 mL), and urea (0.1 mol) were dissolved into deionised water (100 mL) under vigorous stirring for 24 h at 95 °C; The resulting solid was centrifuged washed thoroughly and dried at 60 °C overnight; Afterward, NiTi-LDHs nanoplates were calcinated under air with the heating rate of 2 °C/min in the muffle furnace to obtain the mixed oxide NiTi-MMO (the mixture of NiO and TiO2). Subsequently, the as-obtained NiTi-MMO mixed oxides were reduced under 10%H2/Ar with a flow rate of 20 mL/min and a heating rate of 2 °C/min for 4 h at different temperature, such as 300, 350, 400, 450, 500, 550, and 600 °C, which were denoted as TiO2−x/Ni-300, TiO2−x/Ni-350, TiO2−x/Ni-400, TiO2−x/Ni-450, TiO2−x/Ni-500, TiO2−x/Ni-550, and TiO2−x/Ni-600, respectively. For comparison, the 15% Ni-SiO2 catalyst was fabricated by the traditional impregnation approach. In a typical process, an aqueous solution containing of 0.55 mmol Ni(NO3)2·6H2O was mixed with the SiO2 support (1 g), the suspension liquid kept stirring for 3 h, and then was transformed into the drying oven at 120 °C overnight. The as-synthesized NiO-SiO2 was reduced under 10% H2/Ar with a flow rate of 20 mL/min for 4 h at 450 °C (a heating rate: 5 °C/min), so as to obtain the 15% Ni/SiO2 catalyst. The loading of Ni was determined via inductively coupled plasma atomic emission spectrometry (ICP).
Catalyst characterization
Powder XRD experiments were carried out on a Rigaku XRD-6000 diffractometer with Cu Kα radiation (Condition: λ = 0.15418 nm, 40 kV, and 30 mA; Scan rate: 5° min−1) and a 2θ angle ranging from 3° to 90°. The crystal structures of components could be identified based on JCPDS standard cards. Scanning electron microscope (SEM; Zeiss SUPRA 55) was carried out with an accelerating voltage of 20 kV. High-resolution transmission electron microscopy (HRTEM) was performed on a JEM-3010 at an accelerating voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were conducted on an Axis Ultra Imaging Photoelectron Spectrometer equipped with Al Kα (1486.7 eV) quartz monochrometer source. The freshly prepared catalysts were transferred to the measurement chamber without exposure to air. The binding energy was corrected by setting active carbon sp2 C 1 s of 284.5 eV as the reference. The XAFS spectra of Ni K-edge (8333 eV) and Ti K-edge was collected at 1W1B beamline of Beijing Synchrotron Radiation Facility (BSRF), Institute of High Energy Physics (IHEP), Chinese Academy of Sciences (CAS). The beam was tuned by the Si (111) double-crystal monochromators. The energies were calibrated according to the absorption edge of pure Ni foil and Ti foil. The typical energy of the storage ring was 2.5 GeV with a maximum current of 250 mA. The powdered sample was first pressed into the sheet and loaded into a reactor cell equipped with polyimide windows. The sample sheet was reduced in a H2/He stream at different temperatures ranging from 400 to 600 °C in the reactor cell, followed by flushing (50 mL min−1) with high purity He for 1.0 h, and then the EXAFS spectra at the Ni K-edge and Ti K-edge were collected respectively. In situ diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS) were carried out in a modified in situ reaction cell on a VERTEX 70 spectrometer equipped with a MCT narrow-band detector, with a resolution of 4 cm−1.
CO DRIFTS experiment
In situ DRIFTS spectra were performed to reveal the CO chemisorption state at different Ni sites over various TiO2−x/Ni catalysts (Fig. 2c). As for CO-DRIFTS on TiO2−x/Ni-450 catalyst, the powdered sample (NiTi-MMO) was filled into the reactor, and was pre-reduced in 10%H2 (Ar balance) at 450 °C for 4 h and flushed with high purity Ar for 30 min. Then, 5% CO (Ar balance) was introduced at 25 °C, respectively, for 30 min. Afterward, CO-DRIFTS spectra were collected after flushing with Ar for another 30 min. The CO-DRIFTS experiments for TiO2−x/Ni-300, TiO2−x/Ni-350, TiO2−x/Ni-400, TiO2−x/Ni-500, TiO2−x/Ni-550, and TiO2−x/Ni-600 samples were carried out via the similar experimental processes.
In situ time-resolved DRIFTS experiments
(a) In situ time-resolved DRIFTS were also carried out to further monitor the dynamic evolution of reactive molecules, active intermediates, and products at different conditions for CO hydrogenation over the fresh TiO2−x/Ni-450 catalyst (Fig. 4). As for TiO2−x/Ni-450 catalyst, the powdered sample (NiTi-MMO) was filled into the reactor, and was pre-reduced in 10%H2 (Ar balance) at 450 °C for 4 h and flushed with high purity Ar for 30 min. Afterwards, the temperature was decreased to 160 °C. Then, syngas (CO:H2:Ar = 5:10:85; 30 mL/min) was introduced into this system at the temperature for 30 min. Simultaneously, in situ time-resolved DRIFTS spectra were collected with the time stream on. In addition, the temperature was increased to 180 °C, 200 °C, and 220 °C, respectively. The corresponding time-resolved DRIFTS spectra were collected at different temperatures for 30 min as a function of the time. (b) In situ time-resolved CO-DRIFTS spectra were also carried out at 160 °C, 180 °C, 200 °C, and 220 °C, respectively, according to the above similar experiment processes.
ESTEM experiments
The sample was directly dispersed in a mems chip used for an in-situ environmental STEM experiment (not dispersion in ethanol). After inserting a heating holder equipped with the prepared chip inside the TEM, H2 gas was introduced into the TEM column and the pressure was maintained at 2 Pa. The temperature was gradually increased from 200 to 450 °C. The structural evolution was recorded under the STEM mode. All EELS spectra were collected during the in-situ experiment (in presence of H2). Reference spectra (TiO2 and metallic Ni) were recorded at 450 °C under H2 atmosphere. EELS spectrum imaging (SI) under STEM mode was performed in the targeted area to examine the ELNES fine structure of Ni and titania. Instrumental information: Hitachi HF5000 probe-corrected environmental S/TEM, cold FEG, accelerating voltage 200 kV. The instrument enables simultaneously imaging ABF, ADF and SE images of an identical particle. EELs collected Gatan 965 energy filter.
CO chemisorption
This experiment was performed in a quartz tube reactor on an Automated Catalyst Characterization System (AutoChem 2920) from MICROMERITICS equipped with an on-line mass spectrometry (MS). In a typical process, 100 mg of sample was firstly pre-reduced at 300 °C, 350 °C, 400 °C, 450 °C, 500 °C, 550 °C, and 600 °C for 4 h, respectively, followed by flushing with high purity He for 1.0 h and then the temperature was decreased to 200 K. Subsequently, successive pulses of CO were introduced, using He as the carrier gas (50 mL min–1), until a stable mass signal of CO was obtained. The dispersion of metal Ni (D%, Eq. 1) was calculated on the basis of CO chemisorption value34:
Where MNi, ms, Vad, and Ws are the molecular weight of Ni, the weight of sample (g), the volume (mL) of CO chemisorption, and the weight fraction of Ni, respectively. Vm is the molar volume of CO (22414 mL mol−1) at the standard temperature and pressure (STP); dr is the reduction degree of Ni; SF is the stoichiometric factor, which is assumed as 1:1 for CO:Ni.
Catalyst performance measurements
The reactions were carried out in a multichannel fixed-bed reactor with a 10 mm inner diameter quartz tube inside. 120 mg catalyst sieved into 40–60 mesh was diluted with quartz sand. Prior to catalytic test, the catalyst was activated with 10% H2/N2 (20 mL min−1) at 300, 350, 400, 450, 500, 550, 600 oC for 4 h. The reaction was conducted in bar syngas (32%CO, 64%H2, 4%Ar) at a flow rate of 20 mL min−1 at 200 and 220 oC, respectively. The FTS products were analyzed by Agilent 7890 A gas chromatography. Permanent gas and CO2 were analyzed by 5 A molecular sieve column and Plot Q column with thermal conductivity detector and low hydrocarbons were analyzed by Al2O3 capillary column with hydrogen flame ionization detector.
The CO conversion, productive selectivity, reaction rate, and catalytic activity were calculated based on the following formula:
Where F is the moles of CO and product Ci (CO2 and hydrocarbon) containing i carbon atoms; GHSV is the hourly space velocity (10000 mL gcat−1 h−1), and ωNi is the mass fraction of Ni, which was detected by ICP-OES; MNi is the atomic mass of Ni (58.69 g·mol−1); Nidispersion is the dispersion of metal Ni for different Ni-based catalysts calculated from the Eq.1 above.
Data availability
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Pan, X., Jiao, F., Miao, D. & Bao, X. Oxide-zeolite-based composite catalyst concept that enables syngas chemistry beyond fischer-tropsch synthesis. Chem. Rev. 121, 6588–6609 (2021).
Zhang, Q., Kang, J. & Wang, Y. Development of novel catalysts for fischer-tropsch synthesis: tuning the product selectivity. ChemCatChem 2, 1030–1058 (2010).
Galvis, H. M. T. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 355, 835–838 (2012).
Torres Galvis, H. M. & de Jong, K. P. Catalysts for production of lower olefins from synthesis gas: a review. ACS Catal. 3, 2130–2149 (2013).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Luk, H. T., Mondelli, C., Ferre, D. C., Stewart, J. A. & Perez-Ramirez, J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 46, 1358–1426 (2017).
Yang, C., Zhao, H., Hou, Y. & Ma, D. Fe5C2 nanoparticles: a facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 134, 15814–15821 (2012).
Zhong, L. et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 538, 84–87 (2016).
Yang, C. et al. Construction of synergistic Fe5C2/Co heterostructured nanoparticles as an enhanced low temperature fischer–tropsch synthesis catalyst. ACS Catal. 7, 5661–5667 (2017).
Li, W. Z. et al. Chemical insights into the design and development of face-centered cubic ruthenium catalysts for fischer-tropsch synthesis. J. Am. Chem. Soc. 139, 2267–2276 (2017).
Asundi, A. S. et al. Understanding structure-property relationships of MoO3-Promoted Rh catalysts for syngas conversion to alcohols. J. Am. Chem. Soc. 141, 19655–19668 (2019).
Munnik, P., Velthoen, M. E., de Jongh, P. E., de Jong, K. P. & Gommes, C. J. Nanoparticle growth in supported nickel catalysts during methanation reaction−larger is better. Angew. Chem., Int. Ed. 53, 9493–9497 (2014).
Mihaylov, M., Hadjiivanov, K. & Knözinger, H. Formation of Ni(CO)4 during the interaction between CO and silica-supported nickel catalyst: an FTIR spectroscopic study. Catal. Let. 76, 59–63 (2001).
Wang, J., Yao, N., Liu, B., Cen, J. & Li, X. Deposition of carbon species on the surface of metal: As a poison or a promoter for the long-term stability of Ni/SiO2 methanation catalyst? Chem. Eng. J. 322, 339–345 (2017).
Enger, B. C. & Holmen, A. Nickel and fischer-tropsch synthesis. Catal. Rev. 54, 437–488 (2012).
Vannice, M. A. & Garten, R. L. Metal-support effects on the activity and selectivity of Ni catalysts in CO/H2 synthesis reactions. J. Catal. 56, 236–248 (1979).
Hernández Mejía, C., Vogt, C., Weckhuysen, B. M. & de Jong, K. P. Stable niobia-supported nickel catalysts for the hydrogenation of carbon monoxide to hydrocarbons. Catal. Today 343, 56–62 (2020).
Lee, I., Delbecq, F., Morales, R., Albiter, M. A. & Zaera, F. Tuning selectivity in catalysis by controlling particle shape. Nat. Mater. 8, 132–138 (2009).
Barrientos, J., González, N., Lualdi, M., Boutonnet, M. & Järås, S. The effect of catalyst pellet size on nickel carbonyl-induced particle sintering under low temperature CO methanation. Appl. Catal. A: Gen. 514, 91–102 (2016).
Liu, J. X., Zhang, B. Y., Chen, P. P., Su, H. Y. & Li, W. X. CO dissociation on face-centered cubic and hexagonal close-packed nickel catalysts: a first-principles study. J. Phys. Chem. C. 120, 24895–24903 (2016).
Wang, Y. et al. Manganese oxide modified nickel catalysts for photothermal CO hydrogenation to light olefins. Adv. Energy Mater. 10, 1902860 (2019).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal-support interactions. group 8 noble metals supported on TiO2. J. Am. Chem. Soc. 100, 170–175 (1978).
Tauster, S. J., Fung, S. C., Baker, R. T. K. & Horsley, J. A. Strong interactions in supported-metal catalysts. Science 211, 1121–1125 (1981).
Wang, L. X., Wang, L., Meng, X. J. & Xiao, F. S. New strategies for the preparation of sinter-resistant metal-nanoparticle-based catalysts. Adv. Mater. 31, 1901905 (2019).
van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal−support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Matsubu, J. C. et al. Adsorbate-mediated strong metal-support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Zhang, S. et al. Dynamical observation and detailed description of catalysts under strong metal-support interaction. Nano lett. 16, 4528–4534 (2016).
Tang, H. et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 3, e1700231 (2017).
Mejía, C. H., van der Hoeven, J. E. S., de Jongh, P. E. & de Jong, K. P. Cobalt−nickel nanoparticles supported on reducible oxides as fischer−tropsch catalysts. ACS Catal. 10, 7343–7354 (2020).
Smith, J. S., Thrower, P. A. & Vannice, M. A. Characterization of Ni/TiO2, catalysts by TEM, X-ray diffraction, chemisorption on techniques. J. Catal. 68, 270–285 (1981).
Mejía, C. H., van Deelen, T. W. & de Jong, K. P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Com. 9, 4459–4466 (2018).
Zhang, Y. R. et al. Tuning reactivity of Fischer–Tropsch synthesis by regulating TiOx overlayer over Ru/TiO2 nanocatalysts. Nat. Com. 11, 3185–3192 (2020).
Zhao, Y. F. et al. Oxide-modified nickel photocatalyst for the production of hydrocarbons in visible light. Angew. Chem. Int. Ed. 55, 1–6 (2016).
Xu, M. et al. TiO2–x-Modified Ni nanocatalyst with tunable metal–support interaction for water–gas shift reaction. ACS Catal. 7, 7600–7609 (2017).
Zhao, Y. et al. NiTi-Layered double hydroxides nanosheets as efficient photocatalysts for oxygen evolution from water using visible light. Chem. Sci. 5, 951–958 (2014).
Xu, M. & Wei, M. Layered double hydroxide-based catalysts: recent advances in preparation, structure, and applications. Adv. Funct. Mater. 28, 1802943 (2018).
Zhao, Y. et al. Ultrafine NiO Nanosheets Stabilized by TiO2 from Monolayer NiTi-LDH precursors: an active water oxidation electrocatalyst. J. Am. Chem. Soc. 138, 6517–6524 (2016).
Shu, X., An, Z., Wang, L. & He, J. Oxide-sensitized TiO2 and TiO2−xNx with efficient charge transport conduits. Chem. Com. 39, 5901–5903 (2009).
Yuan, K. et al. Dynamic oxygen on surface: catalytic intermediate and coking barrier in the modeled CO2 reforming of CH4 on Ni (111). ACS Catal. 6, 4330–4339 (2016).
Yang, Y. et al. Enhanced electrocatalytic hydrogen oxidation on Ni/NiO/C derived from a nickel-based metal-organic framework. Angew. Chem. Int. Ed. 58, 10644–10649 (2019).
Zhou, B. et al. Platinum modulates redoxproperties and 5-hydroxymethylfurfural adsorption kinetics of Ni(OH)2 for biomass upgrading. Angew. Chem. Int. Ed. 60, 22908–22914 (2021).
Liu, Q. et al. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 337, 221–232 (2016).
Wan, J. et al. Defect effects on TiO2 nanosheets: stabilizing single atomic site Au and promoting catalytic properties. Adv. Mater. 30, 1705369 (2018).
Zhang, J. et al. Wet-chemistry strong metal–support interactions in titania-supported Au catalysts. J. Am. Chem. Soc. 141, 2975–2983 (2019).
Vogt, C. et al. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 1, 127–134 (2018).
Vogt, C. et al. Understanding carbon dioxide activation and carbon-carbon coupling over nickel. Nat. Com. 10, 5330–5339 (2019).
Xu, M. et al. Insights into interfacial synergistic catalysis over Ni@TiO2−x catalyst toward water-gas shift reaction. J. Am. Chem. Soc. 140, 11241–11251 (2018).
Angelome, P. C. et al. Mesoporous anatase TiO2 films: use of Ti K XANES for the quantification of the nanocrystalline character and substrate effects in the photocatalysis behavior. J. Phys. Chem. C. 111, 10886–10893 (2007).
Carta, D. et al. X-ray absorption spectroscopy study of TiO2–x thin films for memory applications. J. Phys. Chem. C. 119, 4362–4370 (2015).
Liu, X. et al. Environmental transmission electron microscopy (ETEM) studies of single iron nanoparticle carburization in synthesis gas. ACS Catal. 7, 4867–4875 (2017).
Tran, R. et al. Anisotropic work function of elemental crystals. Surf. Sci. 687, 48–55 (2019).
Liu, S. et al. Ultrastable Au nanoparticles on titania through an encapsulation strategy under oxidative atmosphere. Nat. Com. 10, 5790–5798 (2019).
Zhang, Y. et al. Structure sensitivity of Au-TiO2 strong metal–support interactions. Angew. Chem. Int. Ed. 60, 2–10 (2021).
Kazansky, V. B. & Pidko, E. A. Intensities of IR stretching bands as a criterion of polarization and initial chemical activation of adsorbed molecules in acid catalysis. Ethane adsorption and dehydrogenation by zinc ions in ZnZSM-5 zeolite. J. Phys. Chem. B 109, 2103–2108 (2005).
Wang, F. et al. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 138, 6298–6305 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC: 21725301, 21932002, 22232001, 22102007, 21991150, 22172150, 22272106, and 22072090 to D.M., M.X., J.L., M.W., X.L.), the National Key R&D Program of China (Grant No. 2021YFA1501102, 2021YFB3800300, 2021YFA1500303, 2022YFE0114900, 2021YFA1500300, and 2021YFA1502804 to D.M., M.W., X.L, J.L.), and the Fundamental Research Funds for the Central Universities (Grant No. buctrc202112 to M.X.), Certificate of China Postdoctoral Science Foundation Grant (2019M650306 and 2020T130010 to M.X.), USTC Research Funds of the Double First-Class Initiative (YD2060002012 to J.L.), K.C. Wong Education (GJTD-2020-15 to J.L.). The authors are thankful for the support of the BSRF (Beijing Synchrotron Radiation Facility) during the XAFS measurements at the beamline 1W1B. We also appreciate technical support from Mr Hiroaki Matsumoto and Mr Chaobin Zeng, Hitachi High-Technologies (Shanghai) Co. Ltd, for HR-STEM characterization. High-performance computational resources were provided by the University of Science and Technology of China (http://scc.ustc.edu.cn) and Hefei advanced computing center.
Author information
Authors and Affiliations
Contributions
D.M. designed the study. M.X. conducted material synthesis and carried out the catalytic performance test. Y.X. and X.Z. help the analysis of catalytic activity. M.X. carried out the in situ DRIFTS experiment and data analysis. M.X., X.Q., and L.Z. conducted the X-ray absorption fine structure spectroscopy measurements and analyzed the data. Y.X. carried out quasi in situ XPS experiments and data analysis. X.L. contributed to the measurement and analysis of environmental STEM experiment. J.X.L. performed the DFT calculations. M.X., X.Q., J.X.L., X.L., M.W., and D.M. wrote the paper. All authors performed certain experiments, discussed, and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yong Tae Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, M., Qin, X., Xu, Y. et al. Boosting CO hydrogenation towards C2+ hydrocarbons over interfacial TiO2−x/Ni catalysts. Nat Commun 13, 6720 (2022). https://doi.org/10.1038/s41467-022-34463-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34463-7
This article is cited by
-
Engineering ZrO2–Ru interface to boost Fischer-Tropsch synthesis to olefins
Nature Communications (2024)
-
Direct conversion of CO and H2O to hydrocarbons at atmospheric pressure using a TiO2−x/Ni photothermal catalyst
Nature Energy (2024)
-
Comparative Study of Different Mesostructured Silica-supported Nano-iron Catalysts for Fischer–Tropsch Synthesis
Catalysis Letters (2024)
-
Copper-based catalysts for carbon monoxide electroreduction to multicarbon products
Science China Materials (2024)
-
Polymethyl Methacrylate assisted mesoporous TiO2 supported Ni–Co alloy catalysts for the in situ hydrodeoxygenation of methyl palmitate
Reaction Kinetics, Mechanisms and Catalysis (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.