Abstract
Poleroviruses, enamoviruses, and luteoviruses are icosahedral, positive sense RNA viruses that cause economically important diseases in food and fiber crops. They are transmitted by phloem-feeding aphids in a circulative manner that involves the movement across and within insect tissues. The N-terminal portion of the viral readthrough domain (NRTD) has been implicated as a key determinant of aphid transmission in each of these genera. Here, we report crystal structures of the NRTDs from the poleroviruses turnip yellow virus (TuYV) and potato leafroll virus (PLRV) at 1.53-Å and 2.22-Å resolution, respectively. These adopt a two-domain arrangement with a unique interdigitated topology and form highly conserved dimers that are stabilized by a C-terminal peptide that is critical for proper folding. We demonstrate that the PLRV NRTD can act as an inhibitor of virus transmission and identify NRTD mutant variants that are lethal to aphids. Sequence conservation argues that enamovirus and luteovirus NRTDs will follow the same structural blueprint, which affords a biological approach to block the spread of these agricultural pathogens in a generalizable manner.
Similar content being viewed by others
Introduction
Poleroviruses (Family: Solemoviridae), enamoviruses (Family: Solemoviridae) and luteoviruses (Family: Tombusviridae), formerly described as luteovirids but herein referred to as P/E/L viruses, are insect vector-borne, icosahedral viruses capable of infecting most major crop and biofuel plants. Their positive-sense RNA viral genomes are roughly 5.8 kb in size and share a conserved arrangement of open reading frames that spawn five to nine known gene products1 (Fig. S1a). These in turn orchestrate plant infection and insect transmission through a series of temporally and spatially regulated protein interactions2. P/E/L viruses are transmitted almost exclusively by sap-feeding aphid vectors1,3. P/E/L virions circulate throughout the aphid body, interacting with proteins in the aphid’s gut and accessory salivary glands prior to transmission to a new host plant. The aphid gut represents the first barrier for transmission, providing selectivity for the uptake of P/E/L viruses. Virus replication is limited to the plant phloem and no replication occurs in the insect vector4. This mode of transmission is deemed circulative, non-propagative.
P/E/L viruses encode two structural proteins5 (Fig. S1a). The coat protein (CP), derived from ORF3, constitutes the major component of the viral capsid6. Stochastic ribosomal readthrough of the CP stop codon generates a second minor capsid component termed the readthrough protein (RTP), which contains an additional readthrough domain (RTD) encoded by ORF5 that is fused to the CP C-terminus6,7,8,9. The leakiness of the CP stop codon has been maintained throughout evolution and ensures that the RTP is incorporated into the capsid sub-stoichiometrically10: mutant viruses that lack the stop codon and make only the full-length RTP cannot assemble proper virions, infect plants, or be transmitted by aphid vectors11,12,13. A soluble form of the RTP that is not associated with the capsid plays a role in phloem limitation and movement within the plant host13,14. The readthrough domain itself can be subdivided into a globular N-terminal portion (NRTD) and an unstructured C-terminal portion (CRTD) that undergoes proteolytic processing as part of the normal viral lifecycle15,16. Mutant viruses lacking the RTD are not aphid transmissible but form functional capsids capable of protecting the RNA genome and can infect plants at a reduced titer11,16,17,18. In contrast, engineered RTP truncations that remove only the CRTD incorporate efficiently into virions13, retain the ability to interact with aphid proteins19 and can be transmitted to new hosts10. These observations implicate the NRTD as a key determinant of P/E/L virus transmission and necessary for traversing aphid gut epithelial cells during viral uptake.
Previous structural studies have detailed the underlying organization of P/E/L capsids. Cryo-electron microscopy (cryo-EM) and crystallographic characterization of polerovirus CP constructs lacking the RTD confirmed that P/E/L viruses assemble with T = 3 icosahedral symmetry20,21, which arranges 180 quasiequivalent monomers into closed particles that display two-fold, three-fold, and five-fold symmetry22,23 (Fig. S2a, b). Despite these efforts, nothing is known about the structure of the RTD, how it is presented on the capsid, and why it is limited within the mature virion. Deciphering these details is essential for understanding how P/E/L viruses interact with and are transmitted by their aphid vectors.
Here we present atomic-resolution crystal structures of polerovirus NRTDs, which define a two-domain architecture with a unique, interdigitated topology. Our structures rationalize phenotypes observed in previous mutagenesis studies and provide new insights into the organization of the RTD on the capsid surface and the factors limiting its incorporation within mature virions. We also uncover an unexpected evolutionary connection to non-aphid transmissible tombusviruses, which informs how the presence or absence of specific structural features correlate with different requirements for transmission to a new host. Functional experiments establish that the PLRV NRTD can act as an inhibitor of virus transmission and identify NRTD mutant variants that function as potent bioinsecticides. We demonstrate several effective methods for delivering the NRTD to aphids, paving the way for a generalized management strategy to prevent the spread of destructive P/E/L pathogens. Molecular approaches to block virus transmission are of major interest for the development of novel disease control technologies2 and our findings represent a significant advance toward achieving this in an agricultural setting.
Results & discussion
Structural organization of polerovirus NRTDs provides insights into the evolution of aphid transmission
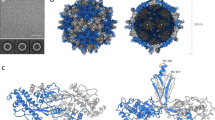
To understand the molecular interactions regulating polerovirus acquisition and transmission by aphids, we generated soluble versions of the NRTD regions from PLRV (residues 230-458 of the complete RTP fusion) and TuYV (residues 224-459) that could be expressed in E. coli and purified on the milligram scale for structural and biochemical studies (Fig. S1b, c). Size exclusion chromatography coupled to multi-angle light scattering (SEC-MALS) shows that these constructs form stable dimers in solution (Fig. S1d, e). Both readily crystallized, and we solved the structure of the PLRV NRTD at 2.22 Å by single wavelength anomalous diffraction (SAD) phasing24 using selenomethionine-labeled protein (Fig. S3a, b and Table S1). The TuYV NRTD structure was subsequently solved by molecular replacement25, yielding a more complete model that was refined to 1.53-Å resolution (Fig. 1 and Table S1).
a, b Structure (a) and topology (b) of TuYV NRTD with jelly roll domain (orange), cap domain (purple) and C peptide (marine) labeled. Cap domain loops are labeled L1-L5. See Fig. S5 for correspondence between the secondary structure elements and the TuYV sequence. c Superposition of TuYV NRTD with tomato bushy stunt virus (TBSV) coat protein (PDB: 2TBV; sequence identity: 9% (across the P domain); DALI26 Z score: 7.0; RMSD: 2.7 Å; P domain, light green; S domain, teal). d Topology of TBSV P domain.
Each TuYV NRTD monomer folds into a two-domain protein comprised of a total of 16 β-strands. Eleven of these strands form two anti-parallel β-sheets—ordered β12-13-7-16-1-4-5 (sheet 1) and β5-6-14-10 (sheet 2)—that sandwich together into a jellyroll fold (Fig. 1, orange). β5 adopts a twisted conformation that runs orthogonal to the plane of the sandwich and connects the two sheets along one edge (Fig. 1a). The short β11 strand connects the sheets on the opposite edge. The additional five strands form an anti-parallel β-sheet (β9-8-15-2-3) that curves into a small barrel with a short a helix (α1) flanking the edge of β3 (Fig. 1, purple). A series of well resolved loops (L1-L5) connect these segments, with L4 folding over and acting as a lid. We designate this barrel the ‘cap domain’, as it sits above the jellyroll base. The DALI alignment algorithm26 indicates that the cap domain barrel is present in several unrelated proteins, including the dimerization domains of aminopeptidases, the N-terminal region of the F1-ATPase rotary subunits, the Aeropyrum pernix lF5B initiation factor, and mammalian Norovirus spike proteins (Fig. S4). Though the topology differs within these proteins, they each maintain a spatially conserved fold (Fig. S4b, c). The TuYV cap domain, however, remains an outlier among this group in that its secondary structure elements are not contiguous, being interspersed throughout the jellyroll domain rather than being connected sequentially to form an isolated globular unit (Fig. 1b and S4). PLRV NRTD monomers adopt the same specific domain arrangement and topology (Fig. S3a, b), suggesting this unique organization is a characteristic feature of poleroviruses and, due to sequence conservation in this region, likely enamo- and luteoviruses as well (Fig. S5).
Further structural comparison using DALI26 reveals that the jellyroll folds of the PLRV and TuYV NRTDs are structurally related to the P domains of tombusviruses, with the nearest structural homologs being tomato bushy stunt virus (TBSV) and cucumber necrosis virus. Tombusviruses share key biological properties with P/E/L viruses—including a small, positive-sense single-stranded RNA genome, similar host range, and a non-enveloped icosahedral capsid with T = 3 symmetry comprised of 180 copies of the CP (Fig. S2a, c)—but are distinct in that they are not aphid transmissible and lack an analogous readthrough domain. Instead, the viral CP contains two domains (S and P) that are constitutively expressed as a single polypeptide27,28, with the S domain forming the icosahedral capsid shell and the P domain extending from each monomer via a flexible linker at all points of two-fold rotational symmetry within the assembled virion (Fig. S2c, d). While previous cryo-EM studies demonstrated structural homology between polerovirus CPs and tombusvirus S domains (Fig. S2e), structural superposition here shows that the TBSV P domain aligns with the jelly roll domain of each NRTD but lacks the corresponding segments that make up the cap domain (Fig. 1c, d and Fig. S3c). The conserved topologies between both families (Fig. 1b, d) and the intricate distribution of cap domain segments throughout the primary sequence (Fig. S5) suggest that polerovirus structural proteins may be ancestral to those of tombusviruses, with tombusvirus capsids likely evolving via the gradual loss of cap domain elements and truncation of loops L1-L5 rather than through the concerted acquisition of these segments in a manner that would be constrained by the proper folding of both domains.
We also observe a largely unstructured peptide (the ‘C peptide’) extending from β16 in the TuYV NRTD, which transverses sheet 1 and terminates in a final strand (β17) that packs against β12 in an antiparallel orientation (Fig. 1a, b, marine). From the electron density, we can define the sequence of this segment unambiguously as the C-terminal portion of the construct (residues 431-459) (Fig. S6a). A disconnected fragment of the C peptide (residues 442-445) is resolved in the PLRV structure (Fig. S6b), likely owing to partial proteolytic cleavage and dissociation of the liberated fragment during purification and/or crystallization.
The C peptide stabilizes the NRTD dimer interface
PLRV and TuYV NRTDs crystallize as dimers (Fig. S1f, g), consistent with their stoichiometry in solution. Individual monomers superimpose with an overall RMSD ranging from 1.12-1.42 Å across all atoms, with the L2, β4-β5, and β13-β14 loops and portions of the C peptide exhibiting the greatest degree of structural variability (Fig. S3d, e). Within each dimer, NRTD monomers are oriented parallel to the dimer symmetry axis with the sheet 1 side of the jelly roll facing inward (Fig. 2a, b and Fig. S7a, b). The dimensions of the TuYV dimer are 63 Å by 62 Å by 42 Å (Fig. S8).
a, b Cartoon (a) and surface (b) representations of the TuYV NRTD dimer. Individual structural segments are labeled in each monomer and colored as follows: Jelly roll domains, orange and light orange; cap domains, purple and light pink; C peptides, marine and light blue. c, d Slice sections through the dimer at the levels indicated by the solid lines in (a) highlighting stabilizing interactions at the dimer interface. Dashed black lines denote hydrogen bonds. Key residues are labeled with a superscript (A or B) to indicate from which monomer they originate. Secondary structure elements (see Fig. 1) are labeled where applicable. e C peptide interactions. Residues contributing hydrogen bonding (dashed black lines) and hydrophobic contacts are labeled.
Cap domain loops L4 and L5 form the upper portion of the TuYV dimer interface, with main chain atoms and residues E315, H356, E360, N362, and Y410 (H362, E366, N368, S369, and Y415 in PLRV) making hydrogen bonds in trans (Fig. 2c, d and Fig. S7c, d). Interacting side chains from β12, L2, and β9 provide additional contacts at the edges of the dimer (Fig. 2d and Fig. S7d). The C peptides snake up from the bottom of the TuYV jelly roll, filling the large cavity beneath the cap domains before exiting in opposite directions to wrap around sheet 2 (Fig. S6c). The R440 and R443 side chains anchor an extensive network of stabilizing hydrogen bonds and hydrophobic interactions along the interior of the structure while β17 serves a similar role on the exterior (Fig. 2e and Fig. S6d). Together, the C peptides increase the total buried surface area from 908 Å2 to 3615 Å2, constituting a major driving force of dimerization. Although we only resolve a partial fragment from one C peptide in the PLRV NRTD dimer structure (Figs. S6b and S7a, b), this piece forms similar stabilizing interactions with both monomers (Figs. S6d and Fig. S7e). Deletion of the C peptide from either NRTD expression construct renders the resulting proteins insoluble. ConSurf analysis29 shows that residues directly contacting the TuYV C peptides are highly conserved across all P/E/L viruses (Figs. S9 and S5), signifying the general importance of these interactions as they are maintained throughout the evolution and adaptation of all three genera. Interestingly, a C-terminal truncation of the CABYV RTP terminating immediately after the C peptide can be efficiently incorporated into mature virions whereas mutants disrupting the anchoring arginines cannot13 (Fig. S6e). Together these data underscore the critical role the C peptide plays in the proper folding and stability of the NRTD dimer.
Disruption of NRTD folding and stability impairs transmission of mutant viruses
Viral mutants have played an integral part in advancing our understanding of P/E/L virus biology, particularly with regard to movement, uptake, and transmission. Our data afford the opportunity to re-examine these perturbations in a structural context and, consequently, reinterpret the associated phenotypes. Systematic mutation of conserved residues throughout the PLRV NRTD, including a series of triplet residue deletions (Fig. S10), previously yielded some mutants where the RTP was not incorporated into the assembled virion and other mutants that were incorporated but were not aphid transmissible14 (Table S2). Many of the mutated side chains are buried and form stabilizing hydrogen bonding and hydrophobic interactions (Fig. S10a, b), suggesting that the triplet deletions interfere with the structural integrity and folding of the NRTD dimer. To test this hypothesis, we introduced a subset of the triple deletion and triple alanine mutations from previous studies into our PLRV NRTD construct (unincorporated: 241PML243, 409YNY411; incorporated: 233RFI235, 268EDE270, 315SST317) and examined the solubility of the resulting proteins. Western blot analysis was used to detect the NRTD in the soluble (supernatant) and insoluble (pellet) fractions following recombinant expression in E. coli. The two non-incorporated mutants (PML and YNY) were insoluble in this context, as neither was detected in the supernatant (Fig. S10d, e). The incorporated EDE mutants were similarly insoluble while the SST mutants were partially soluble. Only the ΔRFI mutant remained soluble though a triple alanine mutant at this same position was not. These observations argue that the in vivo defects associated with mutant viruses arise from disruption of the proper folding and/or stability of the NRTD, which is critical for aphid transmission.
Point mutations in the RFI, EDE, and YNY motifs produced similar transmission defects in TuYV30 (Table S2). An alanine substitution at R227 (R233 in PLRV RFI triplet) reduced transmission while a double alanine mutant at E262 and D263 (E268 and D269 in PLRV EDE triplet) was only transmissible after microinjection, meaning the mutant virus was unable to traverse the aphid gut. As in PLRV, these side chains participate in a network of hydrogen bonds that stretches between β1, β4 and β6 and buttresses sheets 1 and 2 at the bottom of the jellyroll domain (Fig. S10b, c). Interestingly, a compensatory second site mutation converting proline 235 to a leucine restores both the infectivity and transmission of the R227A and E262A/E263A TuYV mutants30 (Table S2). P235 sits in an unstructured segment between β1 in the jelly roll domain and β2 in the cap domain, facing into a hydrophobic pocket lined with F259, I288, I421, and I424 (Fig. S10c). We speculate that a leucine substitution at this position would alter the overall secondary structure and/or strengthen the existing hydrophobic interactions to keep the cap domain in place, ultimately overcoming any instability in the distal portions of the fold.
The K403A/Y404D (Y409 in PLRV YNY triplet) double mutant also showed reduced TuYV transmission, which could be improved by microinjection30. These conserved side chains lie on the edge of the cap domain in both structures where K403 helps anchor L4 and Y404 lines the wall of a cavity on the surface (Figs. S9d, S10c). Revertant mutations switching Y404D back to a tyrosine or structurally similar phenylalanine rescue the transmission defect, suggesting a pi-cation interaction with Q399 is important for the stabilization of this region of the protein31.
NRTD architecture does not limit stoichiometry in the context of the mature virion
Stochastic ribosomal readthrough of the CP stop codon sub-stoichiometrically limits the amount of RTD that is translated, and, hence, present for incorporation into mature, infectious virions32. Why the stop codon has been evolutionarily maintained in the virus genome despite the critical role for the NRTD in aphid transmission is unknown. Leveraging the observed homology with tombusvirus S and P domains (Fig. 1, Figs. S2, and S3c), we modelled the organization of the NRTD on the capsid surface to identify possible restraints on virion assembly (Fig. 3). Tombusvirus P domains are constitutively translated and tethered to each S domain via an unstructured linker (Fig. 3a). When assembled, the P domains occupy every two-fold symmetry axis in the T = 3 icosahedral capsid (Fig. 3b and Fig. S2c). We anticipate that intact RTPs encoded by P/E/L viruses will follow the same architectural design but with the added constraint of head-to-head NRTD dimerization imposed. A composite model combining the TuYV NRTD and CP (PDB: 6RTK) coordinates suggests a similar overall connectivity (Fig. 3c), with the NRTD dimer situated about the two-fold symmetry axis but rotated approximately 15° relative to the position of the TBSV P domains (Fig. 3d–f). This organization confirms previous predictions that special interactions stabilize the association of icosahedral asymmetric units (Fig. 3f, black triangles) across the two-fold axis of symmetry and may contribute to the overall pathway of virion assembly21. Importantly, we note no steric clashing if this RTP model is placed at each position in the T = 3 icosahedral asymmetric unit (Fig. 3g). This implies that the NRTD could feasibly occupy every two-fold position in a polerovirus capsid (yielding a total of 90 NRTD dimers) and that the architecture of the NRTD itself does not intrinsically limit its stoichiometry. The proximity of this arrangement, however, might be problematic in that it could promote aggregation and/or collision between the disordered C-terminal region of the RTD in neighbouring subunits, ultimately destabilizing the structure or masking segments of the RTD that may interact with aphid receptors. We speculate that the leaky CP stop codon is therefore preserved to ensure a low concentration of this bulky C-terminal extension on the virion surface. NRTD dimerization might also impose kinetic constraints that further limit RTP incorporation into the capsid if the timescale of folding is slower than the rate of CP assembly.
a Domain connectivity in TBSV capsid proteins. Unstructured linker that connects the C-terminus of the S domain (light blue) to the N-terminus of the P domain (dark blue) highlighted in yellow. b Arrangement of TBSV capsid proteins at the two-fold symmetry axis (dashed arrow) in the assembled virion (see Fig. S2) shown in two orientations. S and P domains associated with individual monomers are colored orange and olive (monomer A) and slate and dark blue (monomer B). c Predicted connectivity in TuYV capsid proteins based on structural modeling. Dashed yellow line denotes the predicted trajectory linking the C-terminus of the TuYV CP (light blue, PDB: 6RTK) to the N-terminus of the TuYV NRTD (dark blue). d Composite model of the polerovirus RTP built from the crystallized TuYV NRTD dimer and CP monomers taken from the cryo-EM reconstruction of modified TuYV virion devoid of the readthrough domain (PDB: 6RTK). RTP dimer is organized around two-fold symmetry axis analogous to the arrangement in (b) (see Fig. S2). e View of subunit associations in (b, d) looking down the two-fold axis of symmetry in the direction of the dashed arrow in (b). f Side (left) and top down (right) views of TuYV RTP modeled at the two-fold symmetry axis of the icosahedral virion. Two-, three-, and five-fold symmetry axes are marked with a yellow ellipse, yellow triangles, and yellow pentagons, respectively. RTP is colored as in (d) with the rest of the capsid subunits colored wheat. g Model illustrating the feasible positioning of NRTD dimers (olive and dark blue) around icosahedral asymmetric unit of TuYV VLP assuming the structural organization of the RTP in (d). Associated CP monomers are colored orange and slate with the rest of the capsid surface colored wheat.
NRTD can function as an inhibitor of viral transmission
A soluble version of the tomato spotted wilt virus (Genus: Orthotospovirus; Family: Tospoviridae) membrane surface glycoprotein GN was shown in feeding experiments to inhibit viral transmission by its insect vector, the western flower thrips, Frankliniella occidentalis33,34. Given the important role of the NRTD in aphid transmission, we asked whether the purified PLRV NRTD dimer could similarly compete with the mature virus for binding to aphid tissues and subsequently hinder its uptake and transmission by its primary vector, the green peach aphid, Myzus persicae. To test this, we first exposed M. persicae to the purified NRTD in an artificial diet feeding system prior to PLRV acquisition and then monitored the subsequent transmission to healthy plants (Fig. S11). Transmission of PLRV to potato was significantly decreased under these conditions as compared to the no protein control (Fig. 4a, Tables S3, S4, P = 0.046, Wald z test), despite the fact that oral delivery of the same concentration of the purified bovine serum albumin (BSA) control significantly increased transmission (P = 0.035 compared to the no protein control, Wald z test; P < 0.0001 compared to NRTD, Wald χ2 test), a well-described phenotype in the literature observed for proteins unrelated to aphids or virus transmission, including BSA, casein, lysozyme and cytochrome C35.
a The PLRV transmission efficiency of Myzus persicae is significantly different after feeding on various artificial diet treatments (see Fig. S11a): no protein control (n = 101 inoculated plants), BSA (n = 116), purified WT PLRV NRTD (n = 124), or PLRV NRTD point mutant H321A (n = 43). b PLRV transmission efficiency of M. persicae aphids is reduced after transient in planta delivery of the PLRV NRTD (n = 37 inoculated plants for all treatments, see Fig. S12). c Aphid acquisition of PLRV is reduced after exposure to the PLRV NRTD transgenic (n = 4 pools of 5 insects) compared to empty vector control (n = 2) potato plants as quantified via droplet digital PCR (see Fig. S13). Mean ± one standard error for all replicates (dots) is shown. Letters above each bar represent significantly different treatments (P = 0.038) by an unpaired one-sided Student’s t test. d, PLRV transmission efficiency of M. persicae aphids is reduced after exposure to the PLRV NRTD transgenic (n = 60 inoculated plants) compared to empty vector control (n = 26) potato plants (P = 0.044, see Fig. S13). For panels (a), (b), (d), the mean ± one standard error for all independent repeats of the experiment (dots) is shown. Each inoculated plant was considered a biological replicate. Letters above each bar represent significantly different treatments (P < 0.05) by logistic regression analysis using a one-sided Wald χ2 test of model coefficients. Full models, model diagnostics, test statistics, degrees of freedom, and exact P-values are reported in Supplementary Tables S4 (a), S6 (b), and S8 (d).
Next, we tested whether transient expression of the NRTD in planta using Agrobacterium tumefaciens would also block virus transmission (Fig. S12). Expression tests and western blot analysis showed that the PLRV NRTD requires a small protein tag to facilitate folding in planta (Fig. S12a–d), an unsurprising finding as native NRTD would be fused to the CP on its N-terminus and the CRTD on its C-terminus. M. persicae were allowed to feed on Nicotiana benthamiana leaves transiently expressing YFP-tagged PLRV NRTD before testing their ability to transmit virus (Fig. S12e). We tested both N-terminal and C-terminal YFP tags and found that aphids pre-exposed to the YFP-NRTD by this delivery method also had a decreased ability to transmit virus (Fig. 4b, Tables S5 and S6, P = 0.011 compared to uninfiltrated control, Wald z test).
Considering the promising results of transient in planta expression, we generated transgenic potato plants constitutively expressing YFP-NRTD under the control of the cauliflower mosaic virus (CaMV) 35 S constitutive promoter. Expression of YFP-NRTD in these plants was confirmed via western blot analysis, RT-PCR, and fluorescence confocal microscopy (Fig. S13a–c). YFP signal was observed along the cell periphery and in the nucleus of the YFP-NRTD transgenics but not in the empty vector controls (Fig. S13c). M. persicae were exposed to transgenic leaves for 48 hours as in previous experiments and then PLRV titer in aphids was quantified by droplet digital PCR (Fig. S13d) and their ability to transmit PLRV was assessed (Fig. S13e). Aphids exposed to YFP-NRTD acquired significant fewer copies of PLRV (Fig. 4c, P = 0.038, unpaired one-sided Student’s t test) and had a reduced ability to transmit PLRV (Fig. 4d, Tables S7 and S8, P = 0.044, Wald z test). These results are consistent with the outcomes from the other delivery strategies and show that the reduction in PLRV transmission is likely due to the reduced ability of aphids to acquire virions across the midgut barrier.
A meta-analysis of our data found that pre-treatment of aphids with the PLRV NRTD significantly reduced the chances of a plant from becoming infected by nearly half (risk ratio of 0.55) with the 95% confidence interval ranging from a 25% reduction in infection (risk ratio of 0.75) to a 60% reduction (risk ratio of 0.40, Fig. S14) regardless of delivery route. There was remarkably low heterogeneity between experiments and even among delivery methods (I2 = 0%, τ2 = 0, P = 0.42, Cochran’s Q test), indicating that this effect is highly reproducible even when using different delivery strategies. These results argue that the isolated NRTD can function as an inhibitor of viral transmission.
Cap domain mutants are lethal to aphids
Our modelling suggests that the NRTD protrudes from the virion surface with the cap domain directed outward (Figs. 2a, 3d, f), poised to make direct contact with aphid receptors that contribute to viral transmission19. We reasoned that mutating surface-exposed side chains within this domain would disrupt critical interactions needed for viral uptake and thus could impair the ability of the NRTD to function as an inhibitor in our transmission assays. To test this hypothesis, we introduced a series of alanine substitutions into the PLRV NRTD, including point mutations at non-conserved residues H321, E366, H371, E374, and a “cluster” mutant containing three mutations at N368, C370, and Y411, which form a highly conserved pocket (Fig. 5a and Fig. S15). These positions were chosen as they do not interfere with NRTD dimerization and folding. NRTD mutants were purified and delivered to aphids via artificial diet feeding prior to PLRV acquisition and then viral transmission to healthy plants was measured by ELISA (Fig. S11a). As predicted, the H321A mutation interfered with the inhibitor function of the NRTD and did not significantly decrease PLRV transmission compared to the no protein control (P = 0.817; unpaired two-tailed Student’s t test) (Fig. 4a). This behavior was distinct from the increase in transmission observed with the BSA control (Fig. 4a, compared to BSA, P = 0.140, unpaired two-tailed Student’s t test), providing further support that the observed phenotypes are specific for the NRTD and not a general consequence of ingesting protein.
a Top view of TuYV NRTD dimer illustrating positions of PLRV cap domain mutants. Coloring reflects sequence conservation among polero-, enamo-, and luteoviruses (legend below, see also Figs. S5 and S9) and was generated using the ConSurf Server29. See Fig. S15 for further representation of where the mutants localize within the PLRV NRTD structure. b Myzus persicae mortality after feeding on 0.1 mg/mL of BSA (n = 232 insects), purified WT PLRV NRTD (n = 247), PLRV NRTD point mutants H321A (n = 183), E366A (n = 118), H371A (n = 264), E374A (n = 161), a PLRV NRTD cluster mutant (containing mutations N368A, C370A, and Y411A; n = 256) or no protein controls (n = 244) for 48 h and then moved to an uninfected (light gray) or PLRV-infected (dark gray) detached hairy nightshade leaf (See Fig. S11b). Mean ± one standard error for all independent repeats of the experiment (dots) is shown. Each individual aphid was considered a biological replicate. Different letters represent significantly different treatments (P < 0.05) by quasibinomial regression analysis using a one-sided Wald χ2 test of model coefficients. Full models, model diagnostics, test statistics, degrees of freedom, and exact P-values are reported in Supplementary Table S10.
While attempting to assay the other cap domain mutants, we noted that aphids died at a significant rate. To quantify this mortality, aphids were fed the NRTD mutants via an artificial diet and then moved to either PLRV-infected or uninfected leaves for 24 h after which the numbers of live and dead insects were counted (Fig. S11b, Table S9). E366A, H371A, E374A, and the cluster mutant all caused significant mortality (Fig. 5b, Table S10, P < 0.001 for all four mutants, respectively, as compared to no protein control), with E374A and the cluster mutant having the strongest effects. Aphids died even when transferred from the NRTD mutant laden diet treatments to uninfected leaves (Fig. 5b), showing that the observed mortality was linked exclusively to the purified NRTD variants and independent of infectious virus.
The H321A mutant did not cause significant aphid death when compared to no protein and BSA controls (P > 0.44, Fig. 5b). The fact that this mutation localizes to a different region of the cap domain (Fig. 5a) and has different effects with respect to viral transmission and aphid mortality hints that the NRTD interacts with the aphid gut in a structure-specific manner. Differential binding of the WT NRTD and the mutants to various aphid proteins is one hypothesis that may explain both the transmission assay and aphid mortality data. Regardless of the mechanism, the ability of these mutants to kill aphids means they can be deployed as biopesticides, either through transgenic plant delivery as described above for the wild-type NRTD or via some other delivery strategy.
Mechanistic and translational implications of our findings
Our work here defines the basic structural organization of polerovirus NRTDs and provides a model for how the RTP is incorporated into the mature virion. Polerovirus NRTDs adopt an interdigitated two-domain architecture and form dimers that are stabilized by a C-terminal peptide. The requirement for dimerization suggests that the NRTD is situated on the two-fold symmetry axis of the icosahedral viral capsid. Our modeling, however, suggests that this arrangement does not intrinsically limit the stoichiometry of the RTD within the mature virus, which instead may be a consequence of potential CRTD aggregation and/or kinetic constraints imposed by NRTD folding and dimerization. Our data also rationalize the effects of various RTD mutants that have been reported over the last several decades16,18,36,37,38. We now can attribute the observed transmission defects associated with different deletions and truncations to the production of insoluble forms of the NRTD.
Previous studies have used the leaky CP stop codon as the starting point for the readthrough domain. Our work here, however, establishes the NRTD as a defined structural unit that begins downstream of this juncture (at residue 230 in PLRV and 224 in TuYV) and is conserved across all P/E/L viruses (Fig. S5). The intervening sequence that lies between the leaky stop and the beginning of our NRTD crystallographic models is variable and predicted to be unstructured when analyzed by a variety of modelling algorithms (e.g., Phyre, RaptorX, ITASSer, JPRED, GlobPlot, etc.). Given these observations, we propose that the NRTD be defined based on the boundaries elucidated here and that the intervening sequence immediately following the leaky CP stop codon be henceforth referred to as a “variable linker” (Fig. S5). We anticipate these definitions will be more consistent for the field moving forward, especially as we begin to explore the interactions and applications of NRTD constructs deriving from other P/E/L viruses.
Structural comparisons revealed an unexpected evolutionary connection to tombusviruses, intimating that poleroviruses more closely resemble a common ancestor and that loss of the cap domain decouples a virus from its obligate vector and coincides with the ability to be transmitted in other ways. In 2021, the International Committee on Taxonomy of Viruses abolished the previous designation of P/E/L viruses as a single family, Luteoviridae, recategorizing luteoviruses as Tombusviridae and poleroviruses and enamoviruses as Solemoviridae based solely on differences in their respective RNA-dependent RNA polymerases39. Our data argue that P/E/L viruses are in fact structurally and mechanistically distinct from other members of these families and should therefore be treated as a separate group when considering the assembly and organization of the capsid and the mode of transmission.
There are presently no treatments to cure plants of polerovirus infections and current methods to breed viral disease-resistant crops or to control aphid vector populations have proven ineffectual to manage these viruses in the field. Our functional experiments demonstrate that the purified PLRV NRTD can act as an inhibitor to reduce its vector transmission, not only in the context of artificial diet feeding in the laboratory but also when but delivered transgenically in planta in the greenhouse. This provides a biologically based strategy for controlling these insect-transmitted viruses that can be deployed for widespread field application pending regulatory approval. The high degree of sequence conservation across the jelly roll and cap domains, particularly in regions contacting the C peptide, implies that enamovirus and luteovirus NRTDs will follow the same structural blueprint and that this inhibitor-based approach could be extended to mitigate these agricultural pathogens as well. Moreover, we show that certain point mutations in the cap domain are lethal to the insect vector, indicating we have discovered a potent biopesticide with many possible permutations and the use of transgenic plants as an already proven delivery strategy. The deployment of a genetically encoded insecticidal protein that also blocks virus transmission in crop plants lies at the forefront of agricultural technology40,41 and may one day eliminate the need for environmentally harmful and costly pesticide applications. Our work here represents a major step forward toward achieving this end.
Taken together, our findings advance our fundamental understanding of plant virology and vector biology and impart new tools that can be used to thwart both vector-borne phytoviruses and an economically damaging group of insects.
Methods
Cloning, expression, and purification of polerovirus NRTD constructs
DNA encoding the N-terminal portion of the potato leaf roll virus readthrough domain (PLRV NRTD; residues 230-458) and the N-terminal portion of the readthrough domain of turnip yellows virus (TuYV NRTD; residues 224-459) were amplified by PCR and cloned into pCAV4, a modified T7 expression vector that introduced an N-terminal 6xHis-NusA tag followed by a HRV3C protease site upstream of the inserted sequence. Constructs were transformed into BL21(DE3) cells, grown at 37 °C in Terrific Broth to an OD600 of 0.7-0.9, and then induced with 0.3 mM IPTG overnight at 19 °C. Cells were pelleted, washed with nickel loading buffer (20 mM HEPES pH 7.5, 500 mM NaCl, 30 mM imidazole, 5% glycerol (v:v), and 5 mM β-mercaptoethanol), and pelleted a second time. Pellets were typically frozen in liquid nitrogen and stored at −80 °C.
Selenomethionine labeled protein was expressed in T7 Express Crystal E. coli (BL21 derivative, New England Biolabs) according to the manufacturer’s recommended protocol. Briefly, a 2 L culture of minimal media (1X M9 salts, 0.4% glucose, 0.1 mM CaCl2, 2 mM MgSO4, 0.0002% ferric ammonium citrate) supplemented with methionine (50 µg/mL) was grown to an OD600 of 0.7. The media was then removed following centrifugation and replaced with minimal media lacking methionine. After three hours of incubation at 37 °C, the media was supplemented with 50 µg/mL L-selenomethionine (Sigma) and induced overnight at 19 °C with 0.3 mM IPTG (Millipore).
Thawed 500 ml pellets of native and SeMet NRTD constructs were resuspended in 30–40 ml of nickel loading buffer supplemented with DNAse (0.25 mg/ml, Roche), 1 mM MgCl2, 10 mM PMSF, and a Roche complete protease inhibitor cocktail tablet. Lysozyme was added to a concentration of 1 mg/ml and the mixture was incubated for 10 minutes rocking at 4 °C. Cells were disrupted by sonication and the lysate was cleared via centrifugation at 13,000 rpm (19,685 × g) for 30 min at 4 °C. The supernatant was filtered, loaded onto a 5 ml HiTrap chelating column charged with NiS04, and then washed with nickel loading buffer. Native and SeMet NRTD were eluted by an imidazole gradient from 30 mM to 500 mM. Peak fractions were pooled and HRV3C protease was added to a final concentration of 0.5 units/mg of fusion to remove the 6xHis-NusA. Pooled fractions and protease were dialyzed overnight at 4 °C into Q loading buffer (20 mM TRIS pH 8.0, 50 mM NaCl, 1 mM EDTA, 5% glycerol (v:v), and 1 mM DTT). The dialyzed sampled was applied to 5 ml HiTrap Q HP column equilibrated with Q loading buffer, washed in the same buffer, and eluted with a NaCl gradient from 50 mM to 500 mM. Peak fractions were pooled, concentrated, and further purified by size exclusion chromatography (SEC) using a Superdex 75 10/300 column. Native and SeMet PLRV NRTD were exchanged into a final buffer of 20 mM HEPES pH 7.5, 150 mM KCl, and 1 mM DTT during SEC and concentrated to 20–30 mg/ml. Native TuYV NRTD was purified in the same manner.
All PLRV NRTD mutants were generated by QuikChange mutagenesis (Agilent Technologies) and confirmed by sequencing. Cap domain mutants for aphid feeding experiments (H321A, H366A, H371A, H374A, N368A/C370A/Y411A) were expressed and purified as described above for the native WT PLRV NRTD. Triple mutant NRTD constructs (Fig. S9) were further cloned into pET15b for expression tests and western blot analysis (see below).
Size exclusion chromatography coupled to multiangle light scattering
The oligomeric state of PLRV and TuYV NRTD was determined by size-exclusion chromatography coupled to multi-angle light scattering (SEC-MALS). The NRTD proteins were loaded at 4 mg/mL onto a Superdex 200 10/300 Increase column (GE) in size exclusion buffer (20 mM HEPES pH 7.5, 150 mM KCl, and 1 mM DTT) at a flow rate of 0.5 ml/min. Eluent from the sizing column flowed directly to a static 18-angle light scattering detector (DAWN HELEOS-II) and a refractive index detector (Optilan T-rEX) (Wyatt Technology) with data collected every second. Molar mass was determined using the ASTRA VI software. Monomeric BSA (Sigma) was used for normalization of light scattering.
Crystallization, X-ray data collection, and structure determination
SeMet PLRV NRTD at 6 mg/ml was crystallized by sitting drop vapor diffusion at 20 °C in 0.1 M Tris-HCl pH 8.5, 0.25 M Ammonium sulfate, 14% polyethylene glycol 8000, 3% ethylene glycol, and 5 mM DTT. Crystals were of the space group P21221 with unit cell dimensions a = 63.229 Å, b = 65.147 Å, c = 109.681 Å and α = 90°, β = 90°, γ = 90° and contained a dimer in the asymmetric unit. Samples were cryoprotected with Parabar 10312 prior to freezing in liquid nitrogen. Crystals were screened and optimized remotely using NE-CAT beamlines at the Advanced Photon Source. Single-wavelength anomalous diffraction (SAD) data24 were collected remotely on the 24-ID-E NE-CAT beamline at the selenium edge (λ = 0.9792 Å) at 100 K to a resolution of 2.21 Å (Table S1). Data were integrated and scaled via the NE-CAT RAPD pipeline, using XDS42 and AIMLESS43, respectively. Experimental phasing was carried out remotely from a beach in Malta using the RAPD pipeline and a surprisingly strong hotel Wi-Fi signal. A total of 13 selenium sites were found by SHELX44 and used for initial phasing with a figure of merit of 0.29. Density modification and initial model building was carried out using the Autobuild routines of the PHENIX package45. Further model building and refinement was carried out manually in COOT46 and PHENIX45. The final model was refined with Rwork/Rfree values of 0.20/0.25 and contained residues 230-436 in monomer A, residues 232-435 in monomer B, a short peptide (C peptide) consisting of residues 442-458, one sulfate ion, and 16 water molecules. The model contains 96.2% Ramachandran favored resides, 3.6% allowed, and 0.2% outliers.
Native TuYV NRTD at 12 mg/ml was crystallized by sitting drop vapor diffusion at 20 °C in 0.1 M HEPES pH 7.7, 1.4 M AmSO4, 0.1 M NaCl. Crystals were of the space group P212121 with unit cell dimensions a = 46.4 Å, b = 74.9 Å, c = 130.8 Å and α = 90°, β = 90°, γ = 90° and contained a dimer in the asymmetric unit. Samples were cryoprotected by transferring the crystal directly to Parabar 10312 (Hampton Research) prior to freezing in liquid nitrogen. Diffraction data were collected remotely on the NE-CAT 24-ID-C beamline at the Advanced Photon Source at the selenium edge (λ = 0.9791 Å) at 100 K to a resolution of 1.53 Å (Table S1). Data was processed as above. Molecular replacement was carried out with Phaser-MR in the PHENIX package45 using residues 230-436 of the PLRV N-RTD crystal structure (PDB: 7ULO). PHENIX Autobuild45 was used for the initial model building; manual model building and refinements were carried out in COOT46 and PHENIX45. The final model was refined with Rwork/Rfree values of 0.17/0.20 (Table S1). Both monomers were modelled with residues 225-460, along with 587 water molecules. The final model has 98.5% Ramachandran favored residues and 1.5% allowed.
Structural superpositions and viral capsid modeling were carried out in Chimera47 and surface electrostatics were calculated using APBS48. All structural models were rendered using Pymol (Schrodinger). Visual mapping of conserved residues was carried out using the ConSurf server29.
Expression tests and western blot analysis of NRTD triple mutants
To avoid any confounding effects from the NusA solubility tag, wildtype and triple alanine or deletion mutant PLRV NRTD constructs (Fig. S9) were cloned into the pET15b expression plasmid, introducing an N-terminal 6xHis tag. Constructs were transformed into BL21(DE3) cells and grown in 50 mL of LB broth at 37 °C to an OD600 of 0.6. Protein expression was induced with 0.3 mM IPTG overnight at 19 °C. Cells were harvested and resuspended in 30 mL of lysis buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 1 mM DTT). Following a 10-minute incubation with 0.1 mg/mL lysozyme at 4 °C, cells were lysed via sonication. Insoluble material was pelleted by centrifugation at 13,000 rpm (19,685 × g) for 30 min at 4 °C. The soluble fraction was collected and the insoluble pellet was resuspended in lysis buffer. Samples from the soluble and insoluble protein fractions were combined with Laemmli buffer (BioRad) and separated by electrophoresis through a 12% SDS polyacrylamide gel. Proteins were wet transferred to a nitrocellulose membrane and blocked with 5% milk in Tris-buffered saline (TBST, 25 mM Tris, 150 mM NaCl, 0.05% Tween-20) for two hours at 22 °C, followed by incubation overnight at 4 °C with 1:5000 dilution of a polyclonal rabbit antibody raised against the purified PLRV NRTD, diluted in TBST. After washing with TBST, blots were incubated with 1:3000 HRP-conjugated goat anti-rabbit IgG (Promega W4011) for 1 h at 22 °C. After washing with TBST, blots were developed with the ECL Prime Western Blot Detection Reagent (New England Biolabs) and visualized with a ChemiDoc (BioRad).
The NRTD polyclonal antibody was generated by Cocalico Biologicals by injecting a rabbit with crystallography-grade purified NRTD protein. The antibody was cross absorbed against E. coli, N. benthamiana, and M. persicae protein homogenates to remove non-specific antibodies. The antibody was purified via sodium sulfate precipitation and specificity was verified by western blot analysis against E. coli, N. benthamiana and M. persicae protein homogenates, using the purified NRTD as a positive control.
Artificial diet delivery of the PLRV NRTD
The potato leafroll virus sequence used for recombinant protein expression is from a cDNA infectious clone developed by Franco-Lara, et al.49. This infectious clone was used to inoculate hairy nightshade (Solanum sarrachoides, HNS)50, for use as a source of inoculum for all virus experiments50. The parthenogenetic clone of Myzus persicae Sulz used in these experiments, originally collected from New York state, was maintained on Physalis floridana. Fourth instar and adult insects were used for all experiments unless otherwise specified.
Artificial diet delivery of WT PLRV NRTD, H321A, and the control protein bovine serum albumin (BSA, BioRad), was achieved by diluting the proteins to 0.1 mg/mL or 1 mg/mL in an artificial sucrose diet for M. persicae supplemented with amino acids51. Diet with no added protein was used as a control. After starving for 1–2 h, M. persicae aphids were placed in dishes that were sealed by stretching Parafilm over the top and sandwiching the diet beneath a second piece of Parafilm. Aphids were allowed 48 h of feeding on the diet treatments.
Immediately following artificial diet feeding, aphids were transferred to detached PLRV-infected HNS leaves for a 48-hour acquisition access period (AAP, eight artificial diet feeding experiment only) or 24-hour AAP (all other experiments). After the AAP, 3 aphids per plant (the first two oral delivery experiments) or 5 aphids per plant (all other experiments) were transferred to uninfected potato seedlings (Solanum tuberosum cv. Red Maria, 6–15 plants/treatment) for a 72-hour inoculation access period (IAP). Potato seedlings were treated with pymetrozine (Endeavor) and bifenthrin (Talstar P) after the IAP to remove aphids. Systemic PLRV infection was accessed three weeks later by double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) using a 1:200 dilution of a polyclonal antibody generated towards purified PLRV (Agdia SRA30002). Each inoculated plant represents a replicate. Exact sample size for each experiment is given in Tables S3 and S5. The artificial diet experiment was repeated nine times independently.
Transient in planta delivery of the PLRV NRTD
For in planta delivery, transient expression constructs were generated by cloning the PLRV NRTD sequence into pEarlyGate binary expression vectors pEarleyGate101 and pEarleyGate10452, creating untagged and YFP-tagged versions of the PLRV NRTD, with YFP adhered to the N- or C-terminus. Expression and solubility of these constructs was tested in planta via agroinfiltration into N. benthamiana. Three leaves per plants, 3 plants per construct were infiltrated, and leaf discs taken at 2-, 3-, and 4-days post inoculation (dpi) for subsequent protein extraction and western blot analysis. Protein was extracted by cryogenic grinding of leaf discs for 6 min at 25 Hz in a Mixer Mill 440 (Retsch) followed by resuspension in extraction buffer (0.1 M Trist pH 8.0, 150 mM NaCl, 20 mM HEPES pH 7.0). Protein extracts were combined with Laemmli buffer (BioRad), separated by SDS-PAGE and analyzed by western blot with the anti-NRTD antibody as described above. The integrity of the YFP tag was confirmed by western blot analysis with 1:5000 anti−GFP polyclonal antibody (Abcam ab6556). Expression tests for each construct were repeated independently at least twice.
To test the ability of aphids to transmit PLRV after exposure to the NRTD via in planta expression, N. benthamiana leaves were infiltrated with 35:YFP-NRTD, 35 S:NRTD-YFP, or 35 S:GFP (control) constructs. At 2 dpi, aphids were caged on the protein-expressing leaves as well an uninfiltrated (control) leaves for 48 h. Then aphids were moved to PLRV-infected detached HNS leaves for 24 h, and health potato seedlings for 72 h (5 aphids/plant, 10–15 plants/treatment), as in the artificial diet experiments described above. Aphids were removed by a pesticide application and systemic PLRV infection of the potato plants was assessed via DAS-ELISA 2–4 weeks post inoculation. Each inoculated plant represents a replicate (n = 37 for all treatments). The experiment was repeated three times independently.
Transgenic potato delivery of the PLRV NRTD
To generate a plasmid for transformation and expression in potato, the cassette containing 35 S:YFP-NRTD from the pEarleyGate104 backbone used for transient expression in N. benthamiana was cloned between the ClaI and DraIII restriction sites in pBI121. Western blot analysis (as described above) was used to assess the in planta expression of the pBI121 35 S:YFP-NRTD construct. This plasmid was transformed into Agrobacterium tumefaciens strain AGL1 for potato transformation. Potato plants cv. Desiree were transformed as follows: stem internode segments of 0.5–1 cm in length were excised from six-week-old in vitro-grown plants and incubated with A. tumefaciens for 10 min before being moved to callus induction medium. After 48 h, the internode segments were transferred to a selective plant regeneration medium containing kanamyacin. Explants were transferred every 7–14 days to fresh selective plant regeneration medium. When regenerants were approximately 0.5–1 cm in length, they were excised and transferred to a selective rooting medium. Successfully rooted transgenic plants were then transferred to soil.
RT-PCR and confocal microscopy were used to confirm the expression of YFP-NRTD in transgenic potato plants. For RT-PCR, briefly, leaf discs were disrupted in a Mixer Mill 400 (Retsch) and total RNA was extracted using the Zymo Quick-DNA/RNA Miniprep kit. cDNA was synthesized using the iScript Select kit (Bio-Rad) and PCR was carried out using Q5 Hot Start High-Fidelity Polymerase (New England Biolabs), a YFP-specific forward primer (5′ AAGGGCATCGACTTCAAGGA 3′) and NRTD-specific reverse primer (5′ ATTGTAGCGTCCCGTTCAAG 3′). RT-PCR analysis was repeated independently at least three times. YFP fluorescence was visualized in epidermal peels using a Leica TCS-SP5 laser scanning confocal microscope running LAS AF software version 2.6.0. YFP was excited with the 514-nm line of a multiline argon laser with emission spectra collected by a hybrid detector (HyD) in the range of 530–569 nm. Chlorophyll was excited by the 514-nm line of a multiline argon laser with emission spectra collected by a HyD in the range of 600–642 nm. All scans were conducted sequentially with line averaging of 8. Empty vector transgenics and wild-type potato plants were imaged with the same settings as controls. The microscopy experiment was repeated independently three times.
To test the effect of the transgenic potato plants on aphid acquisition and transmission of PLRV, M. persicae were transferred to YFP-NRTD and empty vector transgenic plants and allowed to feed for 48 h, followed by a 48-hour AAP on PLRV-infected HNS plants. For acquisition, aphids were flash frozen (five aphids/tube, two tubes for empty vector control and two tubes for two independent YFP-NRTD transgenic line) and RNA was extracted and cDNA synthesized as described above. Data for transgenic lines were pooled to calculate standard error for that treatment (Fig. 4c). PLRV titer in aphids was quantified using the QX200 digital droplet PCR system (Bio-Rad)53. Briefly, 2 uL of aphid cDNA (normalized to 100 ng/uL) was added to the ddPCR reaction mixture along with 10 uL of 2x EvaGreen SuperMix (Bio-Rad), 7uL of RT-grade H20, and 0.25 uM of each of the following primers specific to the PLRV CP: FP 5′ TGTCCTTTGTAAACACGAATGTC 3′ and RP 5′ CTAACAGAGTTCAGCCAGTGG 3′. The reactions were thermocycled and read on the QX200 ddPCR machine according to manufacturer’s instructions for EvaGreen SuperMix (Bio-Rad). Droplet counts were analyzed using QuantaSoft (Bio-Rad). For transmission, after the AAP, aphids were moved to uninfected potato plants for a 72-hour IAP. Aphids were devitalized with pymetrozine (Endeavor) and bifenthrin (Talstar P), and systemic PLRV infection of the potato plants was assessed via DAS-ELISA 2–4 weeks post inoculation. Each inoculated plant represents a replicate (n = 60 for YFP-NRTD and n = 26 for empty vector control). The experiment was repeated twice independently.
Aphid mortality assays
Mortality of M. persicae on PLRV NRTD mutants delivered via artificial diet was assessed using age-synchronized, fourth instar nymphs and adults. Aphids were synchronized by placing adult M. persicae aphids on P. floridana leaves for two days to lay nymphs. Adults were removed and nymphs were allowed to develop for a week (reaching fourth instar and adulthood) before being used in mortality assays. Purified BSA (n = 232), WT PLRV NRTD (n = 247), point mutants of the PLRV NRTD H321A (n = 183), E366A (n = 118), H371A (n = 264), E374A (n = 161) or one cluster mutant of the PLRV NRTD (containing the three mutations N367, C369, Y410, n = 256) were diluted to 0.1 mg/mL in artificial sucrose diet. After starving for 1–2 h, age-synchronized M. persicae were placed on artificial diet sachets containing the PLRV NRTD mutants or diet only control for 48 h before being moved to a PLRV-infected or uninfected detached HNS leaf. After 24 h on the HNS leaves, mortality of M. persicae was tallied for each treatment and leaf combination. Each individual aphid is considered a replicate. The experiment was repeated three times independently.
Statistical analysis
PLRV transmission data (Fig. 4, Tables S3, S5, and S7) was analyzed by logistic regression to predict whether an inoculated plant would become infected using the different treatments as predictors. For the transgenic potato transmission experiments (Fig. 4e, d), a one-tailed likelihood ratio test showed that experiment could be removed from the model as a predictor (P = 0.7184, P = 0.435, respectively) leaving a single predictor: treatment. For artificial diet and transient in planta delivery (Fig. 4a, b), there was a significant effect of experiment (one-tailed likelihood ratio test, P = 0.002, P = 0.0018, respectively), so a two-predictor model was used: treatment and experiment. This allowed us to observe if there was any effect of treatment on the infection state of the plants after accounting for the variation between experiments. The direction of the effect of a predictor is indicated by the sign of its coefficient (β): a positive coefficient means the predictor increases the chance of a plant becoming infected, negative denotes a decrease. Statistical grouping was determined by comparing the effects (β) of each treatment with one another (one-sided Wald z test for comparison to the control, Wald χ2 for all other comparisons), using a P = 0.05 cut-off. Full models, model diagnostics, test statistics, degrees of freedom, and P-values are reported in Tables S4, S6, and S8. PLRV titer in aphids after feeding on transgenic or empty vector control potato plants (Fig. 4d) was compared using an unpaired, one-tailed Student’s t test (P = 0.038), since the data met the assumptions of normality (Shapiro-Wilk test, P = 0.142) and homoscedasticity (Bartlett’s test, P = 0.253).
Meta-analysis was used to determine the overall effect of aphid pre-exposure to NRTD on PLRV transmission as compared to the no protein control. Subgroup meta-analysis was conducted using the fixed-effects model for between-subgroup-differences using the Mantel-Haenszel method to pool effect sizes and calculate τ2 since the output data was binary (whether or not a plant became infected with PLRV). The meta-analysis examined all nine artificial diet delivery experiments (pooling the two concentrations used in experiments 1 and 2) and all five in planta delivery experiments (transient and transgenic expression). The output of the analysis was used to generate a forest plot which graphically the displays the risk ratio and 95% confidence intervals for each experiment, subgroup, and overall (Fig. S14).
Aphid mortality data (Fig. 5b) was analyzed by quasibinomial regression (ϕ = 0.041, underdispersed) to predict whether an aphid would die using the different treatments and infection status of the leaf they were moved to (PLRV-infected or uninfected) as predictors. A one-tailed likelihood ratio test showed that experiment could be removed from the model (P = 0.996) leaving two predictors: treatment and infection status (Table S10). Statistical grouping was determined by comparing the effects of each treatment with one another (unpaired two-tailed Student’s t test), using a P = 0.05 cut-off. Full models, model diagnostics, test statistics, and P-values are reported in Table S10. All statistical analyses were conducted with R54 version 3.6.3.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The atomic coordinates of the TuYV and PLRV NRTD structures are deposited in the Protein Data Bank with accession numbers 7ULN and 7ULO, respectively. The atomic coordinates for the tomato bushy stunt virus coat protein, the turnip yellow virus coat protein, potato leafroll virus coat protein, S. pneumoniae PepA, T. brucei F1-ATPase, Norovirus Saga GII-4 P domain, and A. pernix IF5B that were used for structural comparisons are publicly available from the Protein Data Bank under the accession numbers 2TBV, 6RTK, 6SCO, 3KL9, 65FD, 6H9V, and 5FG3, respectively. All amino acid sequences used for alignment and structural conservation mapping were obtained from the publicly accessible Kyoto Encyclopedia of Genes and Genomes (KEGG) database [https://www.genome.jp/kegg/] (see Fig. S5 for individual sequence IDs). Raw data and images generated in this study associated with PLRV transmission efficiency (Fig. 4) and aphid mortality (Fig. 5) are available in the Source Data file or in the Supplementary Information. All reagents are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Wilson, J. R., DeBlasio, S. L., Alexander, M. M. & Heck, M. Looking through the lens of ‘omics technologies: Insights into the transmission of insect vector-borne plant viruses. Curr. Issues Mol. Biol. 34, 113–144 (2019).
Heck, M. & Brault, V. Targeted disruption of aphid transmission: a vision for the management of crop diseases caused by Luteoviridae members. Curr. Opin. Virol. 33, 24–32 (2018).
Gray, S., Cilia, M. & Ghanim, M. Circulative, “nonpropagative” virus transmission: an orchestra of virus-, insect-, and plant-derived instruments. Adv. Virus Res. 89, 141–199 (2014).
Pinheiro, P. V. et al. Plant viruses transmitted in two different modes produce differing effects on small RNA-mediated processes in their aphid vector. Phytobiomes (2019).
Miller, W. A., Dinesh, K. S. P. & Paul, C. P. Luteovirus gene expression. Crit. Rev. Pl. Sci. 14, 179–211 (1995).
Dinesh-Kumar, S. P., Brault, V., Allen & Miller, W. Precise mapping and in vitro translation of a trifunctional subgenomic RNA of barley yellow dwarf virus. Virology 187, 711–722 (1992).
Bahner, I., Lamb, J., Mayo, M. A. & Hay, R. T. Expression of the genome of potato leafroll virus: readthrough of the coat protein termination codon in vivo. J. Gen. Virol. 71, 2251–2256 (1990).
Cheng, S. L., Domier, L. L. & D’Arcy, C. J. Detection of the readthrough protein of barley yellow dwarf virus. Virology 202, 1003–1006 (1994).
Filichkin, S. A., Lister, R. M., McGrath, P. F. & Young, M. J. In vivo expression and mutational analysis of the barley yellow dwarf virus readthrough gene. Virology 205, 290–299 (1994).
Wang, J. Y., Chay, C., Gildow, F. E. & Gray, S. M. Readthrough protein associated with virions of barley yellow dwarf luteovirus and its potential role in regulating the efficiency of aphid transmission. Virology 206, 954–962 (1995).
Liang, D., Gray, S. M., Kaplan, I. & Palukaitis, P. Site-directed mutagenesis and generation of chimeric viruses by homologous recombination in yeast to facilitate analysis of plant-virus interactions. Mol. Plant Microbe Interact. 17, 571–576 (2004).
Lee, L. et al. A surface loop of the potato leafroll virus coat protein is involved in virion assembly, systemic movement, and aphid transmission. J. Virol. 79, 1207–1214 (2005).
Boissinot, S., Erdinger, M., Monsion, B., Ziegler-Graff, V. & Brault, V. Both structural and non-structural forms of the readthrough protein of cucurbit aphid-borne yellows virus are essential for efficient systemic infection of plants. PLoS ONE 9, e93448 (2014).
Peter, K. A., Liang, D., Palukaitis, P. & Gray, S. M. Small deletions in the potato leafroll virus readthrough protein affect particle morphology, aphid transmission, virus movement and accumulation. J. Gen. Virol. 89, 2037–2045 (2008).
Alexander, M. M. et al. Insights in luteovirid structural biology guided by chemical cross-linking and high resolution mass spectrometry. Virus Res. 241, 42–52 (2017).
Brault, V. et al. Aphid transmission of beet western yellows luteovirus requires the minor capsid read-through protein P74. Embo J. 14, 650–659 (1995).
Reutenauer, A. et al. Identification of beet western yellows luteovirus genes implicated in viral replication and particle morphogenesis. Virology 195, 692–699 (1993).
Chay, C. A., Gunasinge, U. B., Dinesh-Kumar, S. P., Miller, W. A. & Gray, S. M. Aphid transmission and systemic plant infection determinants of barley yellow dwarf luteovirus-PAV are contained in the coat protein readthrough domain and 17-kDa protein, respectively. J. Virol. 219, 57–65 (1996).
Mulot, M. et al. Transmission of turnip yellows virus by Myzus persicae is reduced by feeding aphids on double-stranded RNA targeting the ephrin receptor protein. Front. Microbiol. 9, 457 (2018).
Byrne, M. J. et al. Combining transient expression and cryo-EM to obtain high-resolution structures of luteovirid particles. Structure 27, 1761–1770.e1763 (2019).
Adams, M. C., Schiltz, C. J., Heck, M. L. & Chappie, J. S. Crystal structure of the potato leafroll virus coat protein and implications for viral assembly. J. Struct. Biol. 214, 107811 (2022).
Johnson, J. E. & Speir, J. A. Quasi-equivalent viruses: a paradigm for protein assemblies. J. Mol. Biol. 269, 665–675 (1997).
Prasad, B. & Schmid, M. F. Principles of virus structural organization. Viral Mol. Mach. 17–47 (2012).
Hendrickson, W. A. Anomalous diffraction in crystallographic phase evaluation. Q Rev. Biophys. 47, 49–93 (2014).
Bunkóczi, G. et al. Phaser. MRage: automated molecular replacement. Acta Crystallogr. Sect. D: Biol. Crystallogr. 69, 2276–2286 (2013).
Holm, L. & Rosenstrom, P. DALI server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010).
Harrison, S. C., Olson, A. J., Schutt, C. E., Winkler, F. K. & Bricogne, G. Tomato bushy stunt virus at 2.9 A resolution. Nature 276, 368–373 (1978).
Olson, A. J., Bricogne, G. & Harrison, S. C. Structure of tomato busy stunt virus IV. The virus particle at 2.9 A resolution. J. Mol. Biol. 171, 61–93 (1983).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Brault, V. et al. Effects of point mutations in the readthrough domain of the beet western yellows virus minor capsid protein on virus accumulation in planta and on transmission by aphids. J. Virol. 74, 1140–1148 (2000).
Burley, S. K. & Petsko, G. A. Amino-aromatic interactions in proteins. FEBS Lett. 203, 139–143 (1986).
Xu, Y. et al. A stem-loop structure in potato leafroll virus open reading frame 5 (ORF5) is essential for readthrough translation of the coat protein ORF stop codon 700 bases upstream. J. Virol. 92, https://doi.org/10.1128/JVI.01544-17 (2018).
Whitfield, A. E., Ullman, D. E. & German, T. L. Expression and characterization of a soluble form of tomato spotted wilt virus glycoprotein GN. J. Virol. 78, 13197–13206 (2004).
Whitfield, A. E. et al. A soluble form of the tomato spotted wilt virus (TSWV) glycoprotein GN (GN-S) inhibits transmission of TSWV by Frankliniella occidentalis. Phytopathology® 98, 45–50 (2008).
Bencharki, B. et al. Phloem protein partners of Cucurbit aphid borne yellows virus: possible involvement of phloem proteins in virus transmission by aphids. Mol. Plant Microbe Interact. 23, 799–810 (2010).
Gildow, F. E. et al. Aphid acquisition and cellular transport of potato leafroll virus-like particles lacking P5 readthrough protein. Phytopathology 90, 1153–1161 (2000).
Reinbold, C. et al. Studies on the role of the minor capsid protein in transport of Beet western yellows virus through Myzus persicae. J. Gen. Virol. 82, 1995–2007 (2001).
Bruyere, A. et al. Effects of mutations in the beet western yellows virus readthrough protein on its expression and packaging and on virus accumulation, symptoms, and aphid transmission. Virology 230, 323–334 (1997).
Walker, P. J. et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021). Arch. Virol. 166, 2633–2648 (2021).
Shatters, R. G. et al. Compositions and methods for modifying a plant characteristic without modifying the plant genome. United States of America patent (2021).
Torti, S. et al. Transient reprogramming of crop plants for agronomic performance. Nat. Plants 7, 159–171 (2021).
Kabsch, W. Xds. Acta Crystallogr D. Biol. Crystallogr 66, 125–132 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr D. Biol. Crystallogr 69, 1204–1214 (2013).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr A 64, 112–122 (2008).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of COOT. Acta Crystallogr D. Biol. Crystallogr 66, 486–501 (2010).
Pettersen, E. F. et al. UCSF Chimera - a visualization system for exploratory research and analysis. J. Comput Chem. 25, 1605–1612 (2004).
Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 27, 112–128 (2018).
Franco-Lara, L. F. et al. Transformation of tobacco and potato with cDNA encoding the full-length genome of potato leafroll virus: evidence for a novel virus distribution and host effects on virus multiplication. J. Gen. Virol. 80, 2813–2822 (1999).
DeBlasio, S. L. et al. Insights into the polerovirus-plant interactome revealed by coimmunoprecipitation and mass spectrometry. Mol. Plant-Microbe Interact. 28, 467–481 (2015).
Mittler, T. E. & Dadd, R. H. Artificial feeding and rearing of the aphid, Myzus persicae (Sulzer), on a completely defined synthetic diet. Nature 195, 404–404 (1962).
Earley, K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006).
Pinheiro, P. V. et al. Host plants indirectly influence plant virus transmission by altering gut cysteine protease activity of aphid vectors. Mol. Cell Proteomics, https://doi.org/10.1074/mcp.M116.063495 (2016).
R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2017).
Acknowledgements
We thank the Northeastern Collaborative Access Team (NE-CAT) beamline staff at the Advanced Photon Source (APS) for assistance with remote X-ray data collection, Dr. Lynn Johnson from the Cornell Statistical Consulting Unit for assistance with statistical methodologies and the meta-analysis of NRTD inhibitor functions, Maria Gutierrez-Feliciano (BTI) for maintenance of plants and insects, and Dr. Mamta Srivastava (BTI) for technical assistance with imaging. We also thank Kerry Swartwood (BTI) for potato transformation and Dr. Mariko Alexander (Cornell) for helping to initiate the collaboration that led to this work. Research conducted at the NE-CAT beamlines (24-ID-C and 24-ID-E) is supported by NIH [P41 GM103403, S10 RR029205] and carried out under the general user proposal GUP-51113 (PI: J.S.C.). This research also used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Confocal microscopy images were taken at the BTI Plant Cell Imaging Center, which is supported by the NSF (DBI-0618969). This work was supported by United States Department of Agriculture Grant 2020-67013-31917 (to M.L.H. and J.S.C.) and USDA ARS CRIS Project 8062-22410-007-000-D (to M.L.H.). J.S.C. is a Meinig Family Investigator in the Life Sciences. J.R.W. was supported by a NSF Graduate Research Fellowship (DGE-1650441) and a NIFA Predoctoral Fellowship (2019-67011-29610). M.C.A. is supported by a NIFA Predoctoral Fellowship (2020-67034-31750). Funding for this research was also provided by the Schmittau Novak Small Grants Program from the Cornell School of Integrative Plant Sciences (to J.R.W. and C.J.S.).
Author information
Authors and Affiliations
Contributions
C.J.S., J.R.W., M.L.H. and J.S.C. designed the study and analysed data. C.J.S., C.J.H., and M.C.A. purified and crystallized all NRTD constructs and collected X-ray diffraction data. C.J.S. solved the NRTD structures and built the models with assistance from M.C.A. and J.S.C. J.S.C. carried out computational modelling. C.J.S. generated and purified all NRTD mutants. M.C.A. performed additional NRTD protein purifications. M.L.H. designed the aphid transmission experiments with J.R.W., H.J.M. and S.E.P. J.R.W. conducted aphid microinjection, artificial diet assays, and transient in planta delivery transmission assays as well as the aphid mortality assays. M.L.H. and J.R.W. designed and generated all expression constructs for transient in planta delivery and potato transformation. J.V.E. supervised the generation of the transgenic potato plants. S.E.P. validated and performed experiments on the transgenic potato plants with microscopy assistance from S.L.D. H.J.M. completed and analysed data for additional transmission assays. J.R.W. and M.L.H. conducted statistical analysis on all aphid experiments. C.J.S., J.R.W., M.L.H., and J.S.C. wrote the manuscript. M.L.H., J.S.C., J.R.W., C.J.S. and M.C.A obtained funding to support the research.
Corresponding authors
Ethics declarations
Competing interests
M.L.H., J.S.C., J.R.W., C.J.S., and M.C.A. have a patent filing related to the technologies described in this paper (U.S. Provisional Patent Application Serial No. 63/289,790). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiltz, C.J., Wilson, J.R., Hosford, C.J. et al. Polerovirus N-terminal readthrough domain structures reveal molecular strategies for mitigating virus transmission by aphids. Nat Commun 13, 6368 (2022). https://doi.org/10.1038/s41467-022-33979-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33979-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.