Abstract
Microglia are derived from primitive myeloid cells and gain their early identity in the embryonic brains. However, the mechanism by which the brain milieu confers microglial maturation signature remains elusive. Here, we demonstrate that the baxcq55 zebrafish and Baxtm1Sjk mouse embryos exhibit similarly defective early microglial maturation. BAX, a typical pro-apoptotic factor, is highly enriched in neuronal cells and regulates microglial maturation through both pro-apoptotic and non-apoptotic mechanisms. BAX regulates dlb via the CaMKII-CREB axis calcium-dependently in living neurons while ensuring the efficient Notch activation in the immigrated pre-microglia by apoptotic neurons. Notch signaling is conserved in supporting embryonic microglia maturation. Compromised microglial development occurred in the Cx3cr1Cre/+Rbpjfl/fl embryonic mice; however, microglia acquire their appropriate signature when incubated with DLL3 in vitro. Thus, our findings elucidate a BAX-CaMKII-CREB-Notch network triggered by the neuronal milieu in microglial development, which may provide innovative insights for targeting microglia in neuronal disorder treatment.
Similar content being viewed by others
Introduction
As resident macrophages in the central nervous system (CNS), microglia guard and maintain CNS homeostasis by classic phagocytic performances1. Microglia are implicated in neural development and function2. They facilitate the integration of new neurons into neuronal circuits and release neurotrophic factors, including neural growth factor (NGF)3,4 and brain-derived neurotrophic factor (BDNF)5, to support the proliferation of neural progenitor cells. Microglia sculpt the synapses through the complement system, which is composed of complement receptor 3 (CR3), located on microglia, and C3 expressed by neurons, in mediating the forgetting and erasure of stored memories from specific neuronal groups6. Anticipatively, microglia are intensively involved in the progression of neurological diseases and are a prospective therapeutic target.
Unlike other CNS cell types, which arise from the neuroectoderm, microglia derive from primitive myeloid cells of mesoderm origins7. They enter the embryonic brain via default pathways from around E9.5-E10 and 22–40 h post-fertilization (hpf) in mouse and zebrafish, respectively, until the formation of the blood-brain barrier8,9,10. A large number of neurons provide the signals to attract and locate microglial cells in the brains. For example, some apoptotic neurons induce microglial brain colonization by releasing “find me” signals, such as lysophosphatidylcholine or adenosine 5′-triphosphate (ATP)11,12. However, interleukin (IL) 34, a colony-stimulating factor 1 receptor (CSF1R) ligand expressed in neurons, functions independently in controlling microglial immigration13. In embryonic mouse brains, microglial progenitors express some genes associated with neural maturation and synaptic pruning since E14 and mature in a few postnatal weeks14. This process is accomplished through complex interactions with neurons, astrocytes, and other cell types15,16,17. However, the mechanism by which the brain milieu confers a specific microglia signature, particularly in the embryonic stages, remains elusive. Although transforming growth factor beta (TGF-β) and CSF1, which are mainly provided by astrocytes and neurons, have been documented to be indispensable in promoting certain microglia-specific gene expression in the postnatal stages18,19, the microglia signatures are rapidly lost in culture media containing CSF-1, TGF-β2, and cholesterol20. These data indicate that additional CNS-specific cues are required in microglial maturation. The optical transparency and considerable genetic screening advantages of zebrafish enable the discovery of additional factors involved in the conserved microglial development, particularly in their maturation during the embryonic stages. Upon settling in the brain, embryonic zebrafish macrophages undergo a phenotypic transition into early pre-microglia in the cephalic mesenchyme at 55–60 hpf10. These cells differentiate and mature rapidly to obtain the core microglia signature, with strong upregulation of specific genes, such as apoeb, and increasingly phagocytose cell debris from 3 days post-fertilization (dpf) onwards10,12.
Notch signaling participates in many cell–cell communication events via variable ligand-receptor pairing21, and is involved in microglia polarization and activation22,23. In mice, Notch1 knockdown causes a significant reduction in the number of activated microglia in the ipsilateral ischemic cortex and a corresponding decreased expression of pro-inflammatory cytokines24. In the BV-2 microglial cell line, Notch signaling is detected and involved in cell migration and morphological transformation upon stimulation25. However, the mechanism by which the brain microenvironment deploys Notch signaling to dictate embryonic microglial development is poorly understood. BCL2-associated X protein (BAX), a typical pro-apoptotic factor, participates in the efficient flux of Ca2+ between the cytosol and the endoplasmic reticulum (ER) to control neuronal Ca2+ homeostasis26,27. The genetic deletion of BAX in mice leads to cerebellar neurogenesis disruption, medulloblastoma formation, and a significantly reduced neuronal Ca2+ flux during the N-methyl-D-aspartate (NMDA) excitation period26,28. However, the roles of BAX in microglial development are poorly elucidated, although BAX-dependent neuronal apoptosis affects the disease-associated microglia (DAM)-related gene expression in postnatal retinal microglia in mice27.
The zebrafish (Danio rerio) baxa is homologous to the mammalian BAX and is conserved in apoptotic regulation29. In the present study, we identify a bax mutant zebrafish allele that compromises microglial maturation and reduces neuronal apoptosis in the embryonic stages. Combined with the results obtained using Baxtm1Sjk mice, our data reveal that BAX is highly enriched in neuronal cells and dictates the early microglia signature via Notch signaling. BAX regulates a specific neuronal activity to activate the calcium-calmodulin-dependent protein kinase II (CaMKII)-cAMP-response element-binding protein (CREB) axis, which controls dlb expression, to provide a Notch ligand in living neurons. BAX simultaneously provides attraction signals through the purinergic signaling of apoptotic neurons to positionally retain pre-microglia for an effective Notch signaling transmission. Notch signaling is conserved in early microglial maturation regulation, according to the compromised microglia signatures observed in embryonic Cx3cr1Cre/+Rbpjfl/fl mice brains and the apparent presentation of microglia characteristics in DLL3-containing in vitro cultures.
Results
Identification of a bax cq55 mutant zebrafish with a compromised microglial maturation in the embryonic stages
We imaged the process of embryonic microglial maturation in a Tg(apoeb:GFP;coro1a:DsRed) midbrain, in which apoeb labels microglia30 and coro1a indicates myeloid cells31. Approximately three long thin apoeb-GFP−coro1a-DsRed+ (a-G−c-D+) pre-microglia (PM) per midbrain with limited branches initially appeared at 2 dpf, which actively moved at a speed of 1.47 ± 0.20 µm/min and transiently touched 3–4 HuC-GFP+ (H-G+) neuronal cells (Fig. 1a-g, Supplementary Fig. 1a, b). The a-G−c-D+ PM rarely engulfed annexin V+/active Casp3+ signals, neutral red (NR)+ particles and DsRed+ bacteria (E. coli) (Fig. 1h, Supplementary Fig. 1c-g, Supplementary Movie 1). One day later, approximately 25 a-G−c-D+ cells per midbrain expressed apoeb, p2ry12 and p2ry6 and differentiated to apoeb-GFP+coro1a-DsRed+ (referred to as a-G+c-D+) cells (Fig. 1a–c, Supplementary Fig. 1a, b, h, i, j, Supplementary Movie 2). Compared to a-G−c-D+ counterparts, a-G+c-D+ cells were morphologically amoeboid and were thus named amoeboid microglia (AM). The size and mobility of a-G+c-D+ AM, which contacted much more with H-G+ cells, increased to 2,416.00 ± 178.50 μm3 and significantly decreased to 0.21 ± 0.04 µm/min, respectively (Fig. 1b, d–g). The phagocytic abilities of a-G+c-D+ AM were robustly enhanced to collect apoptotic cells, NR+ particles, and DsRed+ bacteria, and these cells expressed higher transcript levels of phagocytic-related genes (Fig. 1h, Supplementary Fig. 1c–g). Notably, zebrafish microglia could be derived from the a-G−c-D+ PM, which matured into a-G+c-D+ AM (Fig. 1a, b). Then, large portions of AM transformed into ramified microglia (RM) (86.21% at 6 dpf) and inhabited the brain (Fig. 1a–c).
a Representative images of midbrain microglial cells. b Zebrafish microglial development process. c The number and proportion of PM, AM and RM in midbrains (2 dpf: sib and cq55: n = 5; 3 dpf: sib and cq55: n = 6; 6 dpf: sib: n = 7, cq55: n = 6). Each dot denotes one fish. d The movement trajectory of c-D+ cells during 145 min. Each line represents one cell. The right panels are the enlarged images of boxed cells. The yellow circle and red square indicates start and end points, respectively. e The movement speed of c-D+ cells in d (n = 23 PM, 41 AM and 22 cq55 M from 6 fish per group). f Representative images (top) and 3D reconstructions (bottom) of c-D+ PM, AM or cq55 M (red) contacting HuC-GFP+ neurons (green and white colors in top and bottom panels). White arrows and blue asterisks mark the c-D+ cells and touched neurons. g The numbers of H-G+ neurons contacting PM, AM or cq55 M (n = 24 PM from 6 fish; n = 40 AM from 5 fish; n = 24 cq55 M from 6 fish). h The volume of phagocytized E. coli (n = 23 PM from 6 fish; n = 40 AM from 5 fish; n = 23 cq55 M from 6 fish). i Imagings of c-K+ PM (left panel). Coronal (right top) and transverse (right bottom) views of red c-K+ cells at 4 dpf. j The number and proportion of red c-K+ cells in different regions in (i) (n = 10 per group). k Schematic overview of microglia phenotypes, created with BioRender.com. sib siblings, M microglia, PM pre-microglia, AM amoeboid microglia, RM ramified microglia, c-D coro1a-DsRed, c-K coro1a-Kaede, MB midbrain, HB hindbrain, VB ventral brain, E eye. The numbers (c) and (j) indicate the average percentage. Each dot in (e), (g), and (h) represents one cell. Error bars, mean ± SEM. **P < 0.01; ***P < 0.001; ****P < 0.0001; ns no significant, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
Based on the co-staining results obtained using apoeb with p2ry1232 and p2ry633 (Supplementary Fig. 1i) and on previous reports10,34, we utilized whole-mount in situ hybridization (WISH) of apoeb to indicate the matured microglia in a forward genetic screening of an N-ethyl-N-nitrosourea (ENU)-treated zebrafish library35, to identify the instructive molecules in microglial maturation in the embryonic stages. A total of 203 crossing pairs were examined. The Chongqing number 55 pairs (cq55) were derived from the embryos exhibiting few apoeb+ signals and were maintained as a candidate mutant allele. The complementation tests indicated that the cq55 mutant was neither caused by csf1ra10, pu.136, nor irf837 and that they warranted further investigation. The careful characterization indicated that the cq55 mutant initially gave rise to comparable 3-4 a-G−c-D+ cells at 2 dpf (Fig. 1a, c, Supplementary Fig. 1a-b). Compared with that in siblings, the mutant a-G−c-D+ cells (cq55 M) failed in expressing the microglia signature genes, apoeb, slc7a738, p2ry12, and p2ry6, during embryonic development (Fig. 1a, Supplementary Fig. 1a, h). These cq55 M recapitulated the identical morphological appearance, closed transcript signature, similar movement and contact behaviors, and limited phagocytic abilities of PM (Fig. 1a, d-h, Supplementary Fig. 1e-g, k, Supplementary Movie 1). Additionally, cq55 M was not confined to the midbrain (Supplementary Movie 1). Over half of the cells entered the eyes, hindbrains, and ventral brains, causing a significant reduction in the c-D+ microglia pools (Fig. 1i, j). These data indicate compromised microglial recruitment and/or maturation in cq55 embryos (Fig. 1k).
Genetic recovery analysis indicated that the mutation was located in linkage group 3 (LG3) (Supplementary Fig. 2a). A T-to-A mutation in the baxa gene (ENSDARG00000020623), the homolog of BAX, was identified (Supplementary Fig. 2a, b). The mutation caused an Ile to Asn substitution in the conserved BH1 domain and significantly disrupted the BAX protein levels (Supplementary Fig. 2b–d). Two newly generated bax null alleles with 1- and 58-bp deletions exhibited identical microglia deficiency phenotypes (Supplementary Fig. 2b, d, e). Widespread supplementation of WT, but not mutant bax mRNA, or a controllable supply of bax in heat-shock-induced Tg(hsp70:bax;cryaa:Cerulean) in the baxcq55 mutants significantly induced bax transcription and recovered the apoeb+ and NR+ signals (Supplementary Fig. 2f–j).
Bax is expressed in neurons and non-cell-autonomously regulates embryonic microglial maturation
Bax transcripts were clearly detected in the brain, eyes, and digestive organs of zebrafish embryos using WISH (Fig. 2a). In the brain, bax was much more enriched in the Sox2+ and H-G+ cells than in the c-D+ cells (Fig. 2b-e). Transplantation assays and transgenic rescue strategies were conducted to explore the mechanisms by which bax regulates microglial development. The H-G+ cells were sorted from 55 hpf WT or baxcq55/Tg(HuC:GFP) brains and transplanted into the midbrains of recipient baxcq55 mutant embryos (Fig. 2f). Consequently, the transplantation of isolated WT rather than mutant H-G+ cells into the baxcq55 mutant brains led to a reappearance of apoeb+ signals at 17 h post-transplantation (hpt, Fig. 2g, h). Furthermore, Tg(NBT:bax) or Tg(coro1a:bax) were generated, in which the bax coding sequence was controlled by the neuronal cell-specific (NBT) or macrophages/microglia (coro1a) regulatory elements. The complete recovery of apoeb+ and NR+ phenotypes and an increase in the AM and RM populations were observed in baxcq55/Tg(NBT:bax) (Fig. 2i, j). However, no rescue effects were discernable in baxcq55/Tg(coro1a:bax) (Fig. 2i, j). These results collectively indicate that neuronal BAX non-cell-autonomously regulates embryonic microglial maturation (Fig. 2k).
a WISH of bax in 30 hpf, 2 dpf, and 3 dpf zebrafish embryos. b Schematic illustration of the anatomical position and observational region (red square) in (c) and (d), created with photoshop CS6. c, d Immunofluorescence of zBax (z) with Sox2 (S) and HuC-GFP (H) (c) or coro1a-Dsred (d) in 3 dpf OT region. e qPCR results of the bax transcriptional levels in the sorted H-G+ and c-D+ cells from 3dpf WT midbrains. The data are from three independent experiments. Each dot represents an independent experiment. f The transplantation workflow created with photoshop CS6. g Fluorescent images of H-G+ neurons (top) and WISH of apoeb (bottom) in baxcq55 recipient midbrains at 17 hpt. h The percentage of baxcq55 recipients showing rescued apoeb+ signals in (g). The number in each histogram indicates the average percentage. Data are pooled from three independent experiments. i Representative images of apoeb WISH and NR staining signals in the midbrains of siblings, baxcq55, baxcq55/Tg(NBT:bax) and baxcq55/Tg(coro1a:bax). j The number and percentage of PM, AM and RM in 3 dpf midbrains (n = 6 fish in each group). k Schematic diagram of neuronal Bax regulating microglial maturation, created with BioRender.com. cb cerebellum, mhb mid-hindbrain boundary, IHC immunohistochemistry, D dorsal, V ventral, E eye, OT optic tectum, zBax zebrafish Bax, hpt hours post transplantation, WISH whole-mount in situ hybridization, NR neutral red. The number in each histogram of (h) and (j) indicates the average percentage. Numbers in the right corners in (g) and (i) indicate the counts of embryos with a typical appearance (first number) in the total examined fishes (last number). Error bars, mean ± SEM. ****P < 0.0001, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
Classical pro-apoptotic effects of BAX are required for the brain residency of PM
BAX is a canonical pro-apoptotic factor27. The counts of active Casp3+ and acridine orange+ (AO+) signals were significantly reduced; however, the HuC-GFP+ pool expanded obviously in the baxcq55 mutants compared with that in their WT counterparts (Supplementary Fig. 3a–c). The AO+ signals were effectively recovered upon bax replenishment in the baxcq55/Tg(hsp70:bax) (Supplementary Fig. 3b). Apoptotic neurons have a crucial function in microglial development11,12. To weigh the pro-apoptotic roles of BAX in this scenario, we generated a Tg(NBT:DenNTR) line, in which the fluorescent protein Dendra2 was fused to NTR, enabling the easy visualization of NBT+ cells. The application of MTZ to Tg(NBT:DenNTR) led to increased active Casp3+ signals and reduced NBT+ pools relative to those in the DMSO controls (Fig. 3a). This is because NTR catalyzed the conversion of a nontoxic prodrug into a cytotoxic agent in the NBT+ cells39. Consequently, a significant increment of mpeg1+ and c-D+ cells was observed in the MTZ- rather than in the DMSO-treated WT and baxcq55 mutants bearing the Tg(NBT:DenNTR) background (Fig. 3a, b). Counterintuitively, no apoeb+ and NR+ signals were simultaneously detected in baxcq55 mutant brains despite the higher intensity of the apoeb+ and NR+ signals in the MTZ-treated WT than in the controls (Fig. 3a). Similarly, the application of PAC-1, a procaspase-activating compound, induced obvious AO+ signals in the baxcq55 mutant brains (Supplementary Fig. 3d). No apoeb+ signals presented again in the baxcq55 mutants despite the amoeboid appearance of c-D+ cells (Supplementary Fig. 3d). These data indicated that the pro-apoptotic role of bax is crucial for the brain location of mpeg1+ and c-D+ PM; nonetheless, accomplishing their maturation in the baxcq55 mutants was insufficient. To provide more evidence, we focused on the nucleotides, such as ATP, employed by dead neurons to attract and hold microglial cells12,32. We injected apyrase, an ATP-hydrolyzing enzyme, directly into the midbrain of 3 dpf WT embryos. The c-D+ cells actively moved away, causing a marked decrement in the coro1a-Kaede+ (c-K+) and apoeb+ populations in the treated brain (Supplementary Fig. 3e, Supplementary Movie 3). Similarly, the injection of probenecid and carbenoxolone (CBX), the pannexin-1 (panx1) gap junction inhibitors that block ATP release, caused apoeb+ cells to robustly vanish (Supplementary Fig. 3f). These phenotypes mirrored those observed in the baxcq55 mutants. We then supplied ATP to the baxcq55/Tg(coro1a:Kaede) brains. Despite a significant accretion of c-K+ cells, these cells acquired apoeb+ signals to a limited extent (Supplementary Fig. 3g). These results collectively indicate that solely increasing the apoptotic cell number or signals in baxcq55 mutant brains failed to accomplish microglial maturation.
a The images of NBT-DenNTR+, active Casp3+, coro1a-DsRed+, mpeg1 WISH, apoeb WISH and NR staining signals in DMSO and/or MTZ-treated midbrains at 12 h post-treatment (hpt). b Quantification of mpeg1+ signals in (a) (DMSO: sib: n = 9, baxcq55: n = 10; MTZ: sib: n = 9, baxcq55: n = 9). c Heat-map of altered genes in 3 dpf H-G+ cells. The altered Calcium/Calmodulin, CREB, and Notch signaling pathway were indicated by different colors. d KEGG pathways of the down-regulated genes in 3 dpf red c-K+ cells. Enrichment P values were calculated with Fisher’s Exact test. Multiple testing was corrected using FDR correction. e Representative images of DsRed+ and GFP+ signals. f Representative images of a-G+ signals after supplying coro1a:NICD1a. g Representative images of apoeb WISH and NR staining after supplying HuC:NICD1a or coro1a/mfap4:NICD1a. h Quantification of apoeb+, NR+ and c-D+ signals in 3 dpf midbrains of Tg(coro1a:DN-MAML-FLAG) (c-DN) (apoeb: control: n = 7, c-DN: n = 15; NR: control: n = 7, c-DN: n = 15; c-D: n = 6 per group). i, j Quantification of apoeb+ and NR+ signals in 3 dpf midbrains treated with DAPT (i, apoeb: control: n = 14, DAPT: n = 13; NR: n = 12 per group) or notch1a MO (j, apoeb: control: n = 10, notch1a MO: n = 11; NR: n = 15 per group). k Quantification of coro1a+, apoeb+ and NR+ signals in 3 dpf midbrains (coro1a: sib: n = 10, mibta52b: n = 6; apoeb: sib: n = 10, mibta52b: n = 9; NR: n = 10 per group). l A diagram of neuronal Bax dictating microglial development via Notch signaling, created with BioRender.com. Each dot in (b, h–k) denotes one fish. Numbers in the right corners in (a) and (g) indicate the counts of embryos with a typical appearance (first number) in the total examined fishes (last number). Error bars, mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns no significant, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
BAX promotes embryonic microglial maturation via Notch signaling
To identify the additional molecules induced by BAX in embryonic microglial maturation in the baxcq55 mutants, H-G+ cells (neurons) and photo-conversion labeled red c-K+ cells (microglial cells) were collected from 3 dpf siblings and baxcq55 mutant brains to perform parallel RNA sequencing (RNA-seq). The data revealed that a total of 783 genes presented significant transcriptional differences between the sibling and baxcq55 mutant H-G+ cells, and 1,112 genes exhibited transcriptional differences in c-K+ cells (Supplementary Fig. 4a, b). Differences were also observed in a set of biological processes between the baxcq55 mutant H-G+ cells and the controls (Supplementary Fig. 4c). Notably, the levels of the Notch signaling factors concomitantly exhibited pronounced disparities in both baxcq55 mutant H-G+ and c-K+ cells compared to the corresponding controls (Fig. 3c, d). Concordantly, the imaging of the Notch activity in the Tg(coro1a:DsRed;Tp1bglob:eGFP) and/or Tg(apoeb:GFP;Tp1bglob:hmgb1-mCherry) reporter lines revealed that the GFP+ and/or mCherry+ signals were clearly observed in 3 dpf c-D+ and/or a-G+ cells (Fig. 3e, Supplementary Fig. 4d). However, these signals were sparingly detected in the baxcq55 mutant counterparts (Fig. 3e). The western blot results of a validated Notch1 antibody indicated a significant decline in the NICD1 level in the baxcq55 mutants compared to that in their siblings (Supplementary Fig. 4e). These data indicate that the Notch signaling, which might be indispensable in microglial development, was compromised in baxcq55 mutants. To this end, the rescue experiments were conducted by supplying the functional Notch1a intracellular domain (NICD1a) to macrophages/microglia (coro1a/mfap4:NICD1a) and neurons (HuC:NICD1a). As expected, the transient supplementation of coro1a/mfap4:NICD1a, but not HuC:NICD1a, to the baxcq55 mutants effectively promoted the reappearance of apoeb+ signals in c-D+ populations in approximately 40% of the treated embryos (Fig. 3f, g). These restored c-D+ cells phagocytosed the dead cells and NR+ dyes (Fig. 3f, g).
Notch signaling is indispensable in microglial development
To validate the roles of Notch in microglial development, we isolated 24,742 coro1a-DsRed+ (c-D+) cells from 4 dpf zebrafish brains to conduct single-cell RNA sequencing (scRNA-seq, Supplementary Fig. 4f). Two subtypes of microglia signatures were annotated using the t-distributed stochastic neighbor embedding (tSNE) method (Supplementary Fig. 4g, h). The microglia-specific genes (apoeb, p2ry12, and slc7a7) were intensively detected in cluster 2 (Supplementary Fig. 4i), which presented mature microglia signatures including immune response (GO:0006955), phagocytosis (GO:0006909), microglia/macrophage differentiation (GO:0030225, GO:0014004) (Supplementary Fig. 4j). However, the cells of cluster 1 presented a high expression of epd, atp5mc1, calm1a, and hmgb1a, which are associated with an active metabolism and translation, including the isocitrate metabolic process (GO:0006102), cytoplasmic translation (GO:0002181), and translational elongation (GO:0006414) (Supplementary Fig. 4i, j). notch1a, notch2, and her9 were significantly detected in both clusters, particularly in cluster 2 (Supplementary Fig. 4i), implying the involvement of Notch in physical microglial development. To provide further evidence, we developed a Tg(coro1a:DN-MAML-FLAG) strain, in which a dominant-negative (DN) isoform of the murine mastermind-like (MAML) protein was fused to FLAG and controlled by the coro1a promoter to specifically inhibit Notch activities in microglia (referred to as c-DN). FLAG fluorescence was evident in the Lcp1+a-G+ cells (Supplementary Fig. 5a). The transcript levels of her4.1, a typical Notch target, were significantly reduced in the c-D+ cells of Tg(coro1a:DN-MAML-FLAG) compared with those in the controls (Supplementary Fig. 5b). Consequently, obvious suppression of apoeb+, NR+, and c-D+ cells were observed in Tg(coro1a:DN-MAML-FLAG) embryos compared with that in the controls (Fig. 3h, Supplementary Fig. 5c). Furthermore, the proportions of the AM and RM populations were simultaneously and significantly reduced (Supplementary Fig. 5d). Concordantly, the transient global inhibition of Notch signaling via interference with the γ-secretase inhibitor (DAPT) and notch1a morpholino oligos (MOs), or the heat-shocking Tg(hsp70:DN-MAML-GFP) line, led to a marked reduction in the apoeb+ and NR+ pools (Fig. 3i, j, Supplementary Fig. 5e, f). mibta52b, a Notch signaling-depleted mutant, displayed a limited number of apoeb+ and NR+ cells; however, the coro1a+ population was comparable to that of its siblings (Fig. 3k, Supplementary Fig. 5g). The supplication of coro1a:NICD1a efficiently rescued the phenotypes of apoeb+ and NR+ cells in mibta52b (Supplementary Fig. 5g). These data jointly demonstrate the integral roles of Notch activation by BAX in promoting early microglial maturation (Fig. 3l).
Neuronal DeltaB is required for microglial Notch activation
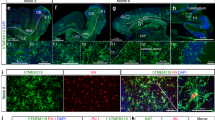
To capture the neuronal ligands involved in triggering Notch activation in microglia, the expression profiles of delta and jagged members, including dla, dlb, dlc, dld, jag1a, jag1b, and jag2b were examined using WISH (Fig. 4a, Supplementary Fig. 5h). All of these ligands were apparent in the midbrain. However, the intensity of the dlb signals declined impressively in the baxcq55 mutants compared to that of their siblings (Fig. 4a). We examined the cellular localization of DeltaB using a validated antibody of the mammalian homolog DLL3 (Supplementary Fig. 5i). The double staining results indicated that the DeltaB signals mainly appeared in the H-G+; however, they were sparingly detected in the c-D+ cells (Fig. 4b, c) and were neither presented in the TUNEL+ nor the engulfed cells (Fig. 4b, c). In the H-G+ cells, the DeltaB signals were merged with the β-catenin+ signals of the fringe membrane (Fig. 4d). However, their intensities and levels diminished significantly in the baxcq55 mutants compared to those in their siblings (Fig. 4e, f). A dlb mutant allele that produced an unstable truncated form of DeltaB was created (Fig. 4g, Supplementary Fig. 5j). The dlb-/- mutants manifested a substantial absence of apoeb+ and NR+ signals; nonetheless, they exhibited no evident alterations in the active Casp3+, coro1a+, and mpeg1+ signals (Fig. 4h, i, Supplementary Fig. 5k). Similar results reappeared in dlb morphants (Fig. 4j, Supplementary Fig. 5l). Transiently providing HuC:dlb evidently prompted the recovery of apoeb+ and NR+ cells in more than 29% of the baxcq55 mutant embryos (Fig. 4k, l). However, these effects were significantly reduced to less than 13% upon suppressing the Notch activities in coro1a+ microglial cells by additionally supplying coro1a:DN-MAML-FLAG to the baxcq55 mutants (Fig. 4k, l). Notably, we observed that HuC:dlb supplementation promoted the advanced appearance of apoeb+ and NR+ signals in 21.98% of the WT midbrains at 2 dpf, one day earlier than its physiological appearance. This effect was significantly increased to 31.20% after the synergistic application of ATP and HuC:dlb (Supplementary Fig. 5m, n). At the same time, ATP depletion decreased the efficiency to 10.77% (Supplementary Fig. 5m, n). These data indicate the important role of neuronal DeltaB in triggering Notch activity in embryonic microglial maturation upon their proper positioning via purinergic signaling (Fig. 4m).
a WISH of dlb in 3 dpf midbrains. b–d Immunofluorescence of HuC-GFP (green) with DeltaB (red) and TUNEL (magenta) (b) or coro1a-DsRed (red) with DeltaB (gray) (c) or DeltaB (red) with β-catenin (green) (d) in the transverse sections of 3 dpf midbrains. DeltaB mainly co-localized with the GFP+ signals in membrane (white arrowhead), rarely with DsRed+, neither TUNEL signals (white arrow), nor non-phagocytized cells (green arrowhead). e Immunofluorescence of DeltaB in the transverse sections of 3 dpf midbrains. f, g WB of DeltaB, β-catenin and GAPDH in baxcq55 (f) and dlb-/- (g). The experiments were repeated three times and the results were similar. h Representative images of apoeb WISH, NR staining, and active Casp3 immunofluorescence in 3 dpf midbrains. i, j Quantification of active Casp3+, coro1a+, mpeg1+, apoeb+, and NR+ signals in siblings and dlb-/- (i, active Casp3, coro1a: n = 9 per group; mpeg1, apoeb, NR: sib: n = 9, dlb-/-: n = 10 per group) and apoeb+ and NR+ signals in control and dlb MO group (j, apoeb: n = 10 per group; NR: control: n = 10, dlb MO: n = 14). Each dot denotes one fish. k Representative images of apoeb WISH and NR staining in the midbrains after supplying HuC:dlb or synergistic application of HuC:dlb and coro1a:DN-MAML. l The percentage of baxcq55 mutants with rescued apoeb+ signals in (k). The number in each histogram indicates the average percentage. Data are pooled from three independent experiments. m A diagram of neuronal DeltaB inducing Notch activation in microglia, created with BioRender.com. WB, western blot. The inserted panels in (b–e) indicate the positions sectioned (blue line). Numbers in the right corners in (a, h, and k) indicate the counts of embryos with a typical appearance (first number) in the total examined fishes (last number). Error bars, mean ± SEM. ***P < 0.001; ****P < 0.0001; ns no significant, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
BAX regulates dlb via spontaneous neuronal activity
We then explored the molecular clues of BAX in neuronal dlb regulation by re-analyzing the RNA-seq profile. Transcriptional changes associated with several molecular pathways that are potentially engaged by neuronal activity, including the calcium/calmodulin-related pathways, G-protein coupled receptor, and protein kinase pathways, were observed between the baxcq55 HuC-GFP+ neurons and the WT controls (Fig. 3c, Supplementary Fig. 4c). We monitored the spontaneous neural activities by imaging a Tg(HuC:GCaMP6s) larva, in which neurons express the genetically encoded calcium indicators GCaMP6s. Annexin V (AV) was administered to exclude apoptotic cells. The embryonic neuronal activity appeared from 2.5 dpf and was significantly detected at 3 dpf, as indicated by the neurons with calcium events quantified between 2–5 dpf in the unilateral midbrain (Supplementary Fig. 6a). Six WT and six baxcq55 mutant embryos were stochastically selected to perform the imaging at 3 dpf. One side of each midbrain was serially captured for 47 min by using the same protocol. In total, 110 and 56 neurons were snapped in WT and baxcq55 mutant embryos, respectively and they demonstrated different GCaMP6s+ transient fluorescence dynamics. Continual and strong intensities of the GCaMP6s+ signals were observed in the AV+ apoptotic neurons in the WT group; however, these signals were rare in the baxcq55 mutants (Fig. 5a, b). In GCaMP6s+AV- neurons, 1,195 calcium transients were identified in 99 WT neurons and presented at different frequencies. We primarily classified the calcium events into two types according to a GCaMP6s+ fluorescence transient of nine, which appeared at a limited level in the calcium events of frequent present groups (Supplementary Fig. 6b). The number of neurons with a high frequency (HF) of GCaMP6s+ transients (≥ nine times during the imaging period) was significantly reduced from 53, in the siblings, to 17, in the baxcq55 mutants (Fig. 5a–d, Supplementary Movie 4). The calcium changes, which were quantified by the ΔF/F0 peak, declined notably in the HF GCaMP6s+ transients of baxcq55 mutants compared with those of the siblings (Fig. 5e). However, no apparent changes were detected in the population with a low frequency (LF, GCaMP6s+ transients occurring less than nine times) between the siblings and baxcq55 mutants (Fig. 5d, e, Supplementary Fig. 6c, and Supplementary Movie 4).
a Confocal time-lapse imaging of GCaMP6s+ signals (green) in HF (top), LF (middle) and AV+ apoptotic neurons (bottom, magenta) in the midbrains. The number in the up-right corner indicated the time pointes. b Representative patterns of Ca2+ events in the HF, LF, and AV+ neurons during 1000 seconds of imaging. c GCaMP6s+ traces of HF neurons in the unilateral midbrains of 3 dpf siblings and baxcq55 mutants (n = 6 per group). d The number of HF, LF, and total active neurons in the unilateral midbrains of 3 dpf siblings and baxcq55 mutants. Each dot denotes one fish (n = 6 per group). e Quantification of the peak amplitude of calcium events (ΔF/F0) of HF, LF, and total calcium events in the unilateral midbrains of 3 dpf siblings and baxcq55 mutants (n = 6 fish in each group). Each dot denotes one calcium event. f qPCR result of the dlb transcriptional level at 5 hpu. The data are from three independent experiments. Each dot represents an independent experiment. g Representative images of apoeb WISH, NR and TUNEL staining in the midbrains of baxcq55 mutant at 5 hpu. h Quantification of apoeb+ and NR+ signals after application of nemadipine and nivadipine. Each dot denotes one fish (apoeb: n = 10 per group; NR: DMSO: n = 5, nemadipine: n = 7, nilvadipine: n = 6). i Schematic presentation of Bax regulating dlb via neuronal activity, created with BioRender.com. HF high frequency, LF low frequency, AV Annexin V, IP3-un IP3 uncaging, hpu hours post-uncaging. Numbers in the right corners in (g) indicate the counts of embryos with a typical appearance (first number) in the total examined fishes (last number). Error bars, mean ± SEM. *P < 0.05; **P < 0.01; ****P < 0.0001; ns no significant, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
We next determined whether recovering the neuronal activities of Ca2+ signaling could restore the dlb expression and microglia phenotypes in the baxcq55 mutants. To this end, uncaging experiments were conducted by administrating cage IP3 or glutamate to the baxcq55 mutant midbrains (Supplementary Fig. 6d). The neuronal GCaMP6s+ dynamics, particularly those with HF, were recovered in the baxcq55 mutants upon uncaging (Supplementary Fig. 6e, f, Supplementary Movie 5). Simultaneously, the transcript levels of dlb and the apoeb+ and NR+ signals were effectively boosted in the uncaged baxcq55 mutant brains (Fig. 5f, g, Supplementary Fig. 6g). However, no evident intervention in the TUNEL+ signals was observed simultaneously (Fig. 5g). To perform a better estimation, nemadipine and nilvadipine, two calcium channel inhibitors, were utilized to disrupt the neuronal activities in WT embryos. Both chemicals perturbed the GCaMP6s+ dynamics and induced significant reductions in the dlb+, apoeb+, and NR+ signals (Fig. 5h, Supplementary Fig. 6h, i, Supplementary Movie 6). Notably, the c-D+ cells exhibited active and random motility in response to nemadipine, similar to the results observed in the baxcq55 mutants (Supplementary Fig. 6j, k, Supplementary Movie 7). These results indicate the instructive role of neuronal activity, which is regulated by BAX, in dlb expression and microglial development (Fig. 5i).
BAX regulates dlb transcription through the CaMKII-CREB axis
CaMKII, the Ca2+/calmodulin-dependent protein kinase type II, is an enzyme enriched in the central nervous system and was selected as a candidate target of Ca2+ signals in modulating dlb. The qualified p-CaMKII antibody was applied (Supplementary Fig. 7a). The autophosphorylated form of CaMKII was more obviously observed in H-G+ than in c-D+ cells (Fig. 6a). However, its activity diminished robustly in the baxcq55 mutants compared to that of their siblings (Fig. 6c, Supplementary Fig. 7b). KN-93 is a p-CaMKII inhibitor that specifically suppresses p-CaMKII activity but maintains the CaMKII global levels of the inactivated form (unphosphorylated)40. Treating WT embryos with KN-93 caused a significant reduction in dlb expression and the simultaneous abortive generation of a-G+, apoeb+ and NR+ cells (Supplementary Fig. 7c–f). CREB is typically thought to be phosphorylated by activated p-CaMKII. The transcription of the factors in the CREB pathway was notably suppressed in the baxcq55 mutants (Fig. 3c). The widespread application of creb1a, rather than creb1b mRNA, or the CREB agonist Forskolin, in the baxcq55 mutants promoted the reappearance of apoeb+ and NR+ cells (Supplementary Fig. 7g). CREB is highly conserved41, and the p-CREB antibody was observed to function effectively (Supplementary Fig. 7h). The immunofluorescence staining and western blot results revealed that p-CREB was highly expressed in the H-G+ cells (Fig. 6b), and that its activity was significantly erased in the baxcq55 mutants (Fig. 6c, Supplementary Fig. 7i). Disrupting the p-CREB activity by applying a specific inhibitor (PF-CBP1 HCl) or the newly designed creb1a MOs caused a significant depletion in both the dlb expression and c-D+ pools, as well as the a-G+c-D+ and NR+ populations (Fig. 6d, e, Supplementary Fig. 7j–o). Furthermore, the introduction of NBT:creb1a in the baxcq55 mutant NBT+ cells significantly elevated the dlb transcript levels and effectively rescued the apoeb+ and NR+ cells in approximately 41% of the embryos (Fig. 6f). Two potential CREB binding sites in the dlb promoter (Site 1: TGACGTCA; Site 2: TGACATCA; Fig. 6g) were predicted using bioinformatics. Concordantly, the DNA fragments containing the suggested binding elements were immunoprecipitated using an anti-p-CREB antibody (Fig. 6g). The overexpression of a full-length creb1a coding sequence (CDS) in HEK293T cells significantly elevated the luciferase activity of the dlb promoter harboring two p-CREB binding sites (Fig. 6h). Conversely, the luciferase activity was reduced when the p-CREB binding sites, especially that of site 2, were mutated (Fig. 6h), supporting that dlb was directly targeted by activated CREB. Thus, BAX regulates dlb transcription via the CaMKII-CREB axis in embryonic neurons (Fig. 6i).
a Immunofluorescence of p-CaMKII (magenta), H-G (green) and c-D (red) in the transverse sections of 3 dpf WT midbrain. p-CaMKII co-stained with GFP+ (white arrowhead) but not DsRed+ (white arrow) signals. b Immunofluorescence of p-CREB (magenta), H-G (green) and c-D (red) in the transverse sections of 3 dpf WT midbrain. p-CREB merged with GFP+ (white arrowhead) but not DsRed+ (white arrow) signals. c WB of p-CaMKII, CaMKII (pan), p-CREB, CREB (pan), and Tubulin in the brain cells from 3 dpf siblings and baxcq55 mutants. The experiments were repeated three times and the results were similar. d WISH of dlb in 3 dpf midbrains after treated with DMSO or PF-CBP1 HCl. e The number of c-D+ and a-G+c-D+ signals in 3 dpf midbrains after DMSO and PF-CBP1 HCl treatment. Each dot denotes one fish (n = 6 in each group). f Representative images of dlb and apoeb WISH, and NR staining in the baxcq55 midbrains after supplying NBT:creb1a. g Top panel: the predicted schematic structure of dlb promoter with two potential CREB binding sites. Bottom panel: the gel results of ChIP-PCR. p-CREB binding sites 1 and 2 were amplified by the dlb-F1/R1 and dlb-F2/R2 primers, respectively. dlb-NF/NR primers were used as the negative control. h Quantification of fold alterations in the luciferase activities after transfected with different vectors. Data were pooled from three independent experiments. Each dot represents an independent experiment. i A diagram of the CaMKII-CREB axis in controlling dlb expression, created with BioRender.com. MU, mutation. Numbers in the right corners in (d) and (f) indicate the counts of embryos with a typical appearance (first number) in the total examined fishes (last number). Error bars, mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns no significant, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
Bax tm1Sjk mutant mice exhibit compromised microglial maturation and Notch signaling in embryonic stages
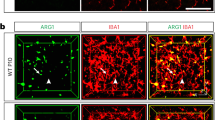
The effective mitigation of microglia deficiency via the supply of mouse, or even human BAX, to baxcq55 mutant zebrafish embryos suggested a conserved regulatory axis (Supplementary Fig. 2f). Correspondingly, the mouse BAX level was higher in E14.5 embryonic SOX2+ than in F4/80+ cells in the mesencephalic region (Fig. 7a, Supplementary Fig. 8a). We analyzed the microglia density, morphology, and identity in the brain tissues of E14.5 Baxtm1Sjk mice, which presented a prominent absence of active Casp3+ signals (Supplementary Fig. 8b, c). Like those in baxcq55 mutant zebrafish, the density and population of either F4/80+ and IBA1+ or CD11bhiCD45lo cells were significantly reduced in the E14.5 Baxtm1Sjk mesencephalon (Fig. 7b, Supplementary Fig. 8d–g). The limited IBA1+ cells in Baxtm1Sjk embryos presented a long thin appearance, and their sizes were much smaller than those of the amoeboid WT counterparts (Fig. 7c, d). Furthermore, the examination of the microglial homeostatic signature genes (MHSGs) (P2ry12, Hexb, C1qa, Slc2a5, and Fcrls)16 revealed a significant decline in their transcript levels in the remaining CD11bhiCD45lo cells from Baxtm1Sjk mice (Fig. 7e). p-CaMKII, p-CREB, and DLL3, which were more easily discerned in HuC/D+ neurons than in F4/80+ microglia, were significantly decreased in the Baxtm1Sjk brains compared with their amount in the controls (Fig. 7f–i, Supplementary Fig. 8h, i). Concurrently, the levels of NICD1 subsided significantly in Baxtm1Sjk mice (Fig. 7j). Hes1, a Notch target gene, was detected in approximately 55.65% of the F4/80+ cells in the E14.5 WT mesencephalon (Fig. 7k, l). However, it was sparsely found in 19.58% of the remaining counterparts in Baxtm1Sjk (Fig. 7k, l). The transcripts level of Hes1 was significantly reduced in the CD11bhiCD45lo cells in the E14.5 Baxtm1Sjk brains (Supplementary Fig. 8j). These findings imply the conserved regulatory mechanisms of the BAX-Notch axis in the developmental programming of microglia (Fig. 7m).
a Immunofluorescence of BAX (red) with SOX2 (gray) and F4/80 (green) in the sagittal sections of E14.5 mice brains. b Quantification of IBA1+ and F4/80+ cell density in E14.5 WT and Baxtm1Sjk mice midbrains. Each dot denotes one mouse (n = 5 mice in each group). Three sections per mouse were quantified. c, d 3D reconstructions of confocal z-stacks (c) and measured volume (d) of IBA1+ cells in E14.5 WT and Baxtm1Sjk mice mesencephalon (n = 3 mice in each group). Each dot in (d) denotes one cell. Ten cells per mouse were quantified. e Transcriptional levels of P2ry12, Hexb, C1qa, Slc2a5, and Fcrls in CD11bhiCD45lo cells from E14.5 WT and Baxtm1Sjk mice mesencephalon. The data are from three independent experiments. Each dot represents an independent experiment. f Immunofluorescence of p-CaMKII (left) and p-CREB (right) in brain sagittal sections of E14.5 WT and Baxtm1Sjk mice. g WB of p-CaMKII, p-CREB and GAPDH in the brain cells from E14.5 WT and Baxtm1Sjk mice. The experiments were triply repeated. The results were similar. h Immunofluorescence of HuC/HuD (green) and DLL3 (red) in the brain sagittal sections of E14.5 WT and Baxtm1Sjk mice. i, j WB of DLL3 and β-catenin (i), NICD1 and Hes1 (j) in the brain cells from E14.5 WT and Baxtm1Sjk mice. The experiments were triply repeated. The results were similar. k Immunofluorescence of F4/80 (green) and Hes1 (red) in the brain sagittal sections of E14.5 WT and Baxtm1Sjk mice. White arrows indicated the co-stained signals. White arrowheads presented the F4/80+ signals only. l The percentage of F4/80+ microglia with or without Hes1+ signals in (k) (n = 4 mice in each group). Three sections per mouse were quantified. The number in each histogram indicates the average percentage. m A diagram of the Bax-Notch axis in regulating microglial maturation, created with BioRender.com. The small images in the right corners of (a, h, and k) are the enlargement regions (white dotted box). Error bars, mean ± SEM. **P < 0.01; ****P < 0.0001, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
Microglia-specific Notch deficiency impairs embryonic microglial maturation
To validate the role of Notch activity in mouse microglial development, we reviewed the bioinformatics data regarding the CD45lowCx3cr1highCD11bhigh cells from E14.5 mice42. The unsupervised clustering of these cells revealed 13 unique clusters (Supplementary Fig. 8k). In the twelve subpopulations of microglia signatures, the transcript levels of the Notch signaling factors were impressively higher than those of their border-associated macrophages partners (Supplementary Fig. 8l, m). We disrupted the Notch activity in mouse microglia by generating Cx3cr1Cre/+Rbpjfl/fl mice via crossing conditional alleles of Rbpj with a microglia-specific Cre-recombinase line. The activities of RBPJ and Hes1 decreased significantly in the F4/80+ and IBA1+ cells from Cx3cr1Cre/+Rbpjfl/fl mice compared with those of the controls at E14.5 (Fig. 8a, b, Supplementary Fig. 9a–c). No visible changes in individual development were observed in Cx3cr1Cre/+Rbpjfl/fl embryonic mice. We excluded the influence of Cx3cr1-Cre43 in embryonic microglial development, according to the paralleled populations of IBA1+ and F4/80+ cells, the percentage of CD11bhiCD45lo microglia, and their gene expression in the mesencephalic regions among Rbpjfl/fl, Cx3cr1Cre/+, and WT mice (Fig. 8c, d, Supplementary Fig. 9d–h). However, the populations of IBA1+ and F4/80+ cells were notably diminished in the mesencephalic regions of E14.5 Cx3cr1Cre/+Rbpjfl/fl mice compared to those of either their Rbpjfl/fl or Cx3cr1Cre/+ siblings (Fig. 8c, d, Supplementary Fig. 9d, e). Consistently, flow cytometry results revealed a lower portion of the CD11bhiCD45lo pools in the brains of Cx3cr1Cre/+Rbpjfl/fl mice than in the Rbpjfl/fl controls (Supplementary Fig. 9i, j). The residual IBA1+ cells in E14.5 Cx3cr1Cre/+Rbpjfl/fl mice presented a typical amoeboid appearance; however, they were smaller than their Rbpjfl/fl counterparts (Supplementary Fig. 9k, l). Of note, the MHSGs (Sall1, Tgfb1, Fcrls, Slc2a5, and Gpr34) transcript levels were significantly lower in the CD11bhiCD45lo cells of Cx3cr1Cre/+Rbpjfl/fl mice than in the Rbpjfl/fl controls (Fig. 8e).
a, b Immunofluorescence (a) and fluorescent intensity emission values (b) of RBPJ (red) in F4/80+ (green) cells in the sagittal sections of E14.5 Rbpjfl/fl and Cx3cr1cre/+Rbpjfl/fl mice (n = 3 mice in each group). Each dot in (b) denotes one cell. Ten cells per mouse were quantified. The white arrow indicates the co-localized signals of RBPJ and F4/80, whereas white arrowhead point to the limited signals of RBPJ. c, d Immunofluorescence (c) and quantified cell density (d) of IBA1+ cells in the sagittal sections of E14.5 Rbpjfl/fl, Cx3cr1cre/+ and Cx3cr1cre/+Rbpjfl/fl mice (n = 6 mice in each group). Each dot denotes one mouse. Three slices per mouse were quantified. e Transcriptional levels of Sall1, Tgfb1, Fcrls, Slc2a5, and Gpr34 in CD11bhiCD45lo cells from E14.5 Rbpjfl/fl and Cx3cr1cre/+Rbpjfl/fl mice mesencephalon. The data are from three independent experiments. Each dot represents an independent experiment. f Immunofluorescence of IBA1 and Hes1 in the embryonic microglia in vitro furnished with MCSF or additional DLL3 for 5 days. g Quantification of IBA1+ cell density in (f). Data were pooled from three independent experiments. Cultured IBA1+ cells in each experiment were from twenty E12.5 mice mesencephalon. Each dot represents individual experiment. h The percentage of Hes1-IBA1+ or Hes1+IBA1+ cells after cultured 5 days in (f). The number in each histogram indicates the average percentage. Data are pooled from three independent experiments. i Transcriptional levels of Hes1, Mcm5, Dab2, Pros1, Tmem119, Gpr34, and Sall1 in embryonic microglia after cultured for 5 days. The data were from three independent experiments. Each dot represents an independent experiment. j Schematic diagram of Notch activation in mouse microglial differentiation, created with BioRender.com. Error bars, mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns no significant, Unpaired two-tailed Student’s t test. Source data are provided as a Source Data file.
Notch activation contributes to the microglia signature in vitro
To further corroborate the prominence of Notch activity, we sorted the CD11b+ cells from E12.5 embryonic mice cerebral cortexes (largely immature microglia at embryonic stages) and cultured them in a microglia nutrition medium containing MCSF or MCSF plus DLL3 (Supplementary Fig. 10a, b). The addition of MCSF alone led to a noticeable increase in the survival and proliferation of IBA1+ cells after two days (Supplementary Fig. 10c–e). After five days, these IBA1+ cells cultured with MCSF presented lower expression levels of early microglia genes (Mcm5 and Dab2) but elevated levels of Hes1 and MHSGs (Pros1 Tmem119, and Gpr34) (Fig. 8f–i). Notably, the additional supplementation with DLL3 led to a drastic expansion of the Hes1+ signals in the similarly enlarged IBA1+ population compared with that of those cultured with MCSF alone. However, these cells presented a much more significant elevation of the MHSGs (Pros1, Tmem119, Gpr34, and Sall1) levels than their counterparts cultured in a medium supplemented with MCSF alone (Fig. 8i). These in vivo and in vitro results together substantiate the important role of Notch signaling in triggering embryonic microglia differentiation in mice (Fig. 8j).
Discussion
The optical transparency of zebrafish embryos provides advantages for the live imaging and precise delineation of nascent microglial maturation. The initial long thin PM actively moved within the brain. They attained the microglia signatures to differentiate into AM and engulfed apoptotic neurons. The body enlargement and mobility reduction resulting from phagocytosis plausibly facilitate the intimate and effective conversation between microglial cells and the brain milieu to gain their resident identity during maturation (Supplementary Fig. 10f). A similar phenomenon was noticed in mice. Transcriptomic and functional studies revealed that the mouse microglia underwent distinct phases of differentiation that relied on the expression of milieu cytokines, including but not restricted to Cxcl14, Csf1, and Il-3415,19,44, which are derived from neurons. These observations support the sheared cellular features of environmental components in promoting microglial development in zebrafish and higher vertebrates.
The exposure of microglia to astrocyte/neuron co-cultures conditioned medium (AN-CM) promotes their maturation with an increased ramification and a significant upregulation of MHSGs. However, microglia signatures subside rapidly in culture, evidencing that microglial maturation requires additional instructions. Notch activity was detected in the embryonic microglia in both zebrafish and mice. The specific intervention of Notch activity in zebrafish and mice significantly compromised microglial maturation in the embryonic stages, substantiating the sufficient roles of Notch signaling in early microglia differentiation. The Notch pathway functioned in the cell–cell contact scenario. The enlarged size of microglia and the enhanced neuronal density during embryonic development contributed to the increase in the number of neurons connecting with microglia, which agreed with the role of Notch in the microglial maturation process. In the macrophage and/or microglial development, Notch signaling fosters macrophage maturation when challenged with pro-inflammatory stimuli45. Notch signaling is also involved in microglia activation in neuro-inflammatory diseases23. Our results extended the understanding of the indispensable roles of Notch in nascent microglial maturation. Concordantly, the in vitro culture of the isolated embryonic mice microglia with DLL3 and MCSF aggregated the Notch activity, which could increase the expression of several MHSGs. However, we did not estimate the influence of TGF-β, a verified factor in harnessing adult microglia signatures and functions20, on this condition. Future studies should primarily focus on the comparison and cooperation of TGF-β with Notch pathways in microglial development, which might be valuable in optimizing microglia culture.
The dlb level decreased the most among those of the Notch ligands in the baxcq55 mutant neurons. Further investigation indicated that dlb was expressed in the living neuron membranes and was transcriptionally controlled by p-CREB, which is regulated by the neuronal activity-dependent calcium-CaMKII axis46. The neuronal activity attracts microglia and regulates their behaviors. When the voltage-gated sodium channel blocker tetrodotoxin (TTX) is injected into the zebrafish OT, it suppresses neural activity and reduces the contact of the microglia bulbous with neurons47. The current imaging results of the neuronal activity, which were reflected by the presentation of calcium events, appeared at 2.5–3 dpf in zebrafish, suggesting the appearance of functional neurons at this stage. This process was consistent with microglial development. Several types of neuronal activities showing different calcium signal frequencies and aptitudes were primarily classified. The HF subtype of neuronal activity was altered in the baxcq55 mutant brains, suggesting that a specific neuronal subtype or condition plays a functional role in early microglial development. However, whether the alterations in neuronal activity affect the behavior of baxcq55 mutant fish and their underlying cellular and molecular basis requires further investigation. The membrane localization of dlb facilitated the transactivation of Notch signaling through effective contact with microglia for their identity acquisition. A compromised dlb function in the mutant or the MO assay led to a significant reduction, but not total loss of, apoeb+ microglia compared to that observed in the mibta52b embryos. This difference was probably ascribed to the redundant roles of other ligands in the baxcq55 mutant.
A pronounced reduction in the density and expression of disease-associated microglia (DAM)-related genes was observed in the retinal microglia of Bax mutant mice, implying a major driver of the neuronal apoptosis in the disease-related profile in microglia27. However, our study extended the involvement of BAX in embryonic microglial maturation. BAX regulates neuronal apoptosis through its classical functions27. Dead cells are engulfed and cleared by microglia, which advances neural development by the refinement of neuronal components, networks, and even environments. However, microglia also benefit from this process. Their movement is reduced, and their bodies become enlarged from their active premature conditions, which advances their conditions to interact with living neurons and accept the instructive signals (Notch ligands). Moreover, BAX equips living neuronal cells with Notch ligands by regulating the calcium-CaMKII-CREB axis. The dual nature of BAX aligns well with embryonic microglial development and neurogenesis. Recognizing the molecular basis of the neuronal milieu in regulating one early step of microglial maturation will facilitate an extensive understanding of microglial development and provide clues regarding the pathogenesis of neurodegenerative diseases. This conclusion also suggests the application of microglia to perfect the existing therapeutic strategies.
Methods
Zebrafish and Mice
The wild-type (WT) AB and WIK strains were used in this study. The mibta52b mutant48, Tg(coro1a:DsRed)31, Tg(coro1a:Kaede)49, Tg(mfap4:GFP)50, Tg(apoeb:GFP)30, Tg(ubiq:secAnnexinV-mVenus)51; Tg(Tp1bglob:eGFP)52, Tg(Tp1bglob:hmgb1-mCherry)52, Tg(hsp70:DN-MAML-GFP)53, Tg(HuC:GCaMP6s)54 were employed. Zebrafish were bred and maintained under standard laboratory conditions according to Institutional Animal Care and Use Committee protocols. Embryos were raised in egg water at 28.5 °C and treated with 0.003% 1-phenyl-2-thiourea (PTU, Sigma-Aldrich, P7629) to inhibit pigment formation. Mice (C57BL/6J background) were maintained in a specific pathogen-free (SPF) condition in accordance with the guidelines of Institutional Animal Care and Use Committee protocols. All mice used were socially housed under a 12 h light/dark cycle with 40-60% relative humidity at 23 °C. The following mice were obtained from the Jackson laboratory: Bax+/tm1Sjk mice (B6.129×1-Baxtm1Sjk/J, 002994), Rbpjfl/fl (C57BL/6J-Rbpjem2Lutzy/J, 034200), Cx3cr1-Cre (B6J.B6N(Cg)-Cx3cr1tm1.1(cre)Jung/J, 025524). Mice carrying Cx3cr1-Cre transgene were crossed with Rbpjfl/fl mice to obtain Cx3cr1-Cre, Rbpjfl/fl, and Cx3cr1cre/+Rbpjfl/fl mice. Mice were genotyped using polymerase chain reaction (PCR). And the toe DNA was used as a template. The genotyping primers’ information is listed in Supplementary Table 1. All animal experiments were approved by the Institutional Review Board of Southwest University (Chongqing, China).
Forward genetic screening and genetic mapping
The ENU mutagenesis screening and positional cloning were performed as described previously35. Briefly, adult WT zebrafish males of AB background were mutagenized with N-ethyl-N-nitrosourea (ENU) and then outcrossed to generate F1 and F2 families to perform the screening. A total of 203 crossing pairs from 42 F2 families were examined by apoeb WISH. Among the identified candidate mutant pairs with notable apoeb phenotypes at 3 dpf, the 55th pair was a recessive mutant allele showing loss of apoeb signals. Initial complementary assay indicated that this candidate mutant was not caused by csf1ra, pu.1, and irf8 mutation and was nominated as cq55 (Chongqing (cq) NO. 55) mutant. The cq55 mutants were mated with WT female of WIK background to perform the positional cloning. We first mapped the mutation to linkage group (LG) 3 by bulked segregation analysis, which indicated a close correlation of the cq55 mutant pools with the sequence-length polymorphism (SSLP) and single nucleotide polymorphism (SNP) markers in LG3. Based on the recombination events in cq55 mutant embryos, high-resolution mapping was conducted by using additional polymorphic SSLPs and SNPs to narrow cq55 mutation to a restricted region. Sequencing was eventually conducted to identify a T to A mutation in exon 4 of baxa gene (Ensemble: ENSDARG00000020623), which causes the alteration of Ile (I) to Asn (N) in the conserved BH1 domain in cq55 mutants.
Generation and genotyping of bax and dlb mutants
The baxΔ1/Δ1, baxΔ58/Δ58 and dlb-/- mutant alleles were generated by the CRISPR/Cas9 system55. The guide RNAs targeting the “gRNA1: 5′-GGACATCGTCCAGCTTATGA-3′ and gRNA2: 5′-GGATCAGGGAACAGGGTGGA-3′” in bax exon5 and 5′-GGAGAGTGCAAGTGTCGTCT-3′” in dlb exon5 were in vitro synthesized respectively using the T7 transcription kit (Thermo Fisher, AM1334). 1 nL of the mixed solution containing Cas9 mRNA (300 ng/µl) and gRNA (100 ng/µl) was injected into the fertilized embryos at one-cell stage. Two bax mutant (baxΔ1/Δ1 and baxΔ58/Δ58) and one dlb mutant (2 bp deletion with a 10 bp insertion) alleles were identified by high-resolution melt analysis of PCR and DNA sequencing using the designed primers (seen in Supplementary Table 1).
Whole-mount in situ hybridization (WISH), fluorescence in situ hybridization (FISH), neutral red (NR), acridine orange (AO), TUNEL, and immunofluorescence staining
The RNA probes were synthesized in vitro by using the DIG RNA Labelling kit SP6/T7 (Roche, 11175025910). WISH and FISH were performed following the standard protocols49. For the chemical staining assays, zebrafish embryos were incubated with NR solution10 (Santa Cruz, sc-281691, 1:400) for 4 h and AO solution12 (Sangon Biotech, A642007, 1:100) for 1 h. They were then washed with fresh E3 buffer before imaging. To perform the immunofluorescence staining, vibratome section of zebrafish embryos was applied using a Leica VT1000S machine according to a previous study56. Briefly, 3 dpf embryos were fixed by 4% paraformaldehyde (PFA) at room temperature (RT) for 1 h. They were dehydrated in 30% (M/V) sucrose at RT for 30 min and embedded in 4% low-melting-point agarose gel (LMA) for the followed section. The samples were sectioned to about 100 μm in thickness. Sagittal frozen section of E14.5 mouse brain was done as that described previously57 by using a Leica CM1950 machine. The thickness of one slice was 10 μm. For immunofluorescence staining, the tissue pieces were rinsed in PBDT (PBS with 1% BSA, 1% DMSO, 0.5% TrintonX-100) and blocked with buffer (2% FBS and 0.1% Tween20 in PBS) at RT for 1 h. Primary antibodies were utilized at 4 °C overnight: goat anti-GFP (Abcam, ab6658, 1:400), mouse anti-DsRed (Santa Cruz, sc-101526,1:400), rabbit anti-DsRed (Takara Bio Clontech, 632496, 1:400), rabbit anti-Lcp1 (GTX124420, GeneTex, 1:400), mouse anti-zBax (This paper, 1:400), mouse anti-Bax (B-9) (Santa Cruz, sc-7480, 1:200), rabbit anti-activated Caspase-3 (BD Biosciences, 559565, 1:400), rabbit anti-SOX2 (Abcam, ab94959, 1:200), rabbit anti-CaMKII (pan) (Cell Signalling technology, 4436, 1:400), rabbit anti-phospho-CaMKII (Thr286) (Cell Signalling technology, 12716, 1:400), rabbit anti-CREB (Abcam, ab32515,1:400), rabbit anti-CREB (phospho S133) (Abcam, ab32096,1:400), rabbit anti-DLL3 (Abcam, ab103102, 1:200), rabbit β-catenin (Merck Millipore, 06-734, 1:400), Rat anti-F4/80 (Abcam, ab6640, 1:100), goat anti-IBA1 (Abcam, ab5076, 1:200), mouse anti-Hes1 (Abcam, ab119776, 1:400), rabbit anti-activated Notch1 (Abcam, ab8925, 1:400), rabbit anti-Rbpj (BOSTER, A00767-1, 1:400), mouse anti-FLAG (Sigma-Aldrich, F1804, 1:400). Afterwards, the samples were washed with PBDT and then stained with AF488/555/647-conjugated secondary antibodies including 488 Goat Anti-Rat (Abcam, ab150165), 488 Donkey Anti-Goat (Thermo Fisher Scientific, A-11055), 555 Donkey Anti-Rabbit (Thermo Fisher Scientific, A-31572), 647 Donkey Anti-Mouse (Thermo Fisher Scientific, A-31571) or TUNEL by its specific kit (Roche, 12156792910) at 37 °C for 3 h. The WISH pictures were captured by a Carl Zeiss Discovery.V20 microscope. FISH and immunofluorescence staining images were taken by Zeiss LSM700 and LSM880 confocal microscopes.
Uncaging of IP3 and Glutamate
1 mM NPE-caged-IP3 (Invitrogen, I23580) or 5 mM MNI-caged-L-glutamate (Tocris Bioscience, 1490) was mixed with pluronicF127 (final concentration 0.2%) (Invitrogen, p-6866) and Alexa Fluor 647 dextran (Invitrogen, D22914, 1:1000). The mixture was injected into the midbrain of anesthetized 3dpf baxcq55/Tg(HuC:GCaMP6s) larvae. Uncaging was carried out at the desired regions by using 405 nm laser under a Zeiss LSM700 confocal microscope, according to that described previously32. Afterwards, Ca2+ signals were imaged to evaluate the effects.
3D reconstruction, photo-conversion, and in vivo time-lapse imaging
The 3D reconstruction of selected images and cell tracking analysis were performed by the Imaris software. To quantify the cell size, the surface renderings of each Z-stack image of coro1a-Dsred+ cells or IBA1+ cells were created by Imaris software. Then surface-rendered images were used to calculate the 3D volume. The coro1a-Kaede+ PM cells in 52 hpf zebrafish larval optic tectum (OT) were selected with the ROI mode and stimulated via a 405 nm laser. The colonization and number of red Kaede+ cells were quantified at 4 dpf. In vivo time-lapse imaging was carried out according to a previous report49. Briefly, the embryos were anesthetized in 0.01% tricaine (Sigma-Aldrich, MS-222) and mounted in 1% low-melting agarose (Thermo Fisher Scientific, 16520050). Then, the midbrain was imaged under an LSM700 confocal microscope with ×20 objective. Images were processed with ZEN or ImageJ software. The calcium signals imaging was performed on Tg(HuC:GCaMP6s). The Annexin V-Cy5 (Biovision, K103) was loaded to the brain to label the apoptotic cells30. Acquisitions were performed by scanning at 1-2 Hz at an excitation of 488 nm58. The resolution was designed by 256 ×256 pixels on Tg(HuC:GCaMP6s). Imaging data were analyzed by using the ImageJ software and the drift fluorescence images were aligned by StackReg software59. The fluorescence intensity of each calcium event in the regions of interest (ROIs) was calculated by (F-F0)/F0. F0 was the average intensity of the ROI accordingly60. A spontaneous calcium event was composed of varied calcium transients61. The calcium transient with the amplitude exceeding the baseline noise was counted. Only one neuronal calcium event showed nine transients during the imaging time, which was set as the standard to classify the higher and lower frequencies of calcium events. Based on the ΔF/F0, we defined the max amplitude of calcium transients in each event as the peak amplitude58.
Single-cell RNA-sequencing (scRNA-seq)
The collected coro1a-DsRed+ cells were loaded onto the channels of Single Cell G Chip (v3.1 chemistry, PN-1000120). The 10x libraries were constructed using Chromium single-cell gene expression platform and Chromium Single Cell 3′ Reagent Kits (v3.1 chemistry PN-1000121) by AccuraMed Medical Technology Co., LTD (Shanghai, China). Briefly, single-cell suspensions in each channel of the chip were loaded on a Chromium Controller (10x Genomics, Pleasanton, CA) to generate single-cell GEMs (gel beads in emulsion). Then the 10x libraries of each channel were prepared using the Chromium Single Cell 3′Gel Bead and Library Kit v3.1 (PN-1 −1000123, 1000157, 1000129; 10x Genomics). Libraries were sequenced by the Illumina NovaSeq 6000 S4 Reagent Kit v1.5 (illumine PN-20028312).
scRNA-seq data analysis
The scRNA-seq data were aligned to the zebrafish reference genome (Danio rerio, GRCz11) by Cell Ranger 5.0.0 (https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger). The resulting matrix was inputted into Seurat v3 for quality control, filtration, dimensionality reduction, clustering analysis and differential expressed genes analysis62. In brief, the cells with 200 <nFeature_RNA < 6,000 and percent.mito <30% were obtained for downstream analysis. Cellcyclesorting and ScaleData were used to eliminate the effects of cell cycle heterogeneity63. After identifying the significantly principal components by conducting principal component analysis (PCA), the first 20 principal components were used to compute the k.param nearest neighbors and generated cell clusters. Non-linear dimensional reduction (UMAP) was followed to visualize clustering results. FindAllMarkers (min.pct = 0.1, logfc.threshold = 0.25, test.use = “wilcox” (Wilcoxon Rank Sum test)) was employed to filtrate differentially expressed genes (DEGs) across each cluster. Immunocytes, microglia and cluster specific/focused genes were visualized using Featureplot and Dotplot. Then DAVID Bioinformatics Resources 6.8 were engaged to explain the gene ontology-biological processes64,65. The summary terms were listed.
Bulk RNA Sequencing and data analysis
The cell suspensions were collected from zebrafish larval brains according to the protocols previously described66. About 500 HuC-GFP+ neurons and 100 photo-converted red coro1a-Kaede+ cells in brains were sorted by flow cytometry (Moflo XDP, Beckman) to establish the cDNA libraries by Anoroda corporation. The RNA sequencing was carried out by using the PE100 strategy (HiSeq 2500, Illumina) and the data were aligned to the zebrafish reference genome (Danio rerio, GRCz11). Then, genes with P.adjust <0.1 and log2FoldChange > 0 were identified as differentially expressed genes (DEGs) using the DESeq (v1.32.0) software. Finally, DAVID Bioinformatics Resources 6.8 were engaged to explain the gene ontology-biological processes of these DEGs64,65.
Transplantation
The cell transplantation was performed according to a previous description67. The donor brains of 55 hpf WT and baxcq55/Tg(HuC:GFP) larvae were dissected according to the method mentioned above. WT and baxcq55 GFP+ cells were sorted by flow cytometry. After briefly centrifuging (1,000 x g) and washing, the donor HuC-GFP+ cells were resuspended with 5% FBS and injected into 55 hpf baxcq55 recipient larval midbrains by using a micromanipulator (Eppendorf, 5196000030). GFP+ signals were examined to estimate the transplantation efficiency after 17 h post transplantation (hpt).
Bacterial phagocytosis
The DsRed labeled E. coli (DH5a) bacteria30 were injected into the midbrain of 2 dpf Tg(mfap4:GFP), 3 dpf Tg(mfap4:GFP) and 3 dpf baxcq55/Tg(mfap4:GFP) fish. 30 min later, the larvae were anesthetized and mounted in 1% low-melting agarose. Then the fluorescence of bacteria and mfap4-GFP+ cells was captured by a Zeiss LSM700 confocal microscope. Imaris software was used to quantify the bacterial volume in the mfap4-GFP+ cells.
Vector construction and transgenic lines generation
The coro1a, HuC, NBT, mfap4 and hsp70 regulatory elements were used. The 7 kb sequence of coro1a promoter31, 3.2 kb sequence of HuC promoter68, 3.8 kb sequence of NBT promoter30, and 1.6 kb sequence of mfap4 promoter elements50 were amplified according to previous studies respectively. These promoters were cloned to the PT2AL2 backbone for further utilization. The coding sequences of bax, dlb, zebrafish notch1a intracellular domain (zNICD1a) and creb1a were amplified by the corresponding primers that are listed in Supplementary Table 1. To construct the coro1a:DN-MAML-FLAG plasmid, the coding sequences for amino acids 13-74 of murine MAML (dominant-negative mastermind-like 1; DN-MAML) were obtained from Tg(hsp70:DN-MAML-GFP)53 by the listed primers in Supplementary Table 1. This element was then fused to the FLAG epitope at the N terminus. All the successfully amplified elements were then cloned to the pT2AL2 vectors that contained several tissue-specific regulatory elements and the eye-marker cassette, Cryaa-Cerulean49, was reversely inserted to facilitate screening. pT2AL2-hsp70-bax was constructed by sub-cloning the coding sequence of bax downstream of the zebrafish hsp70 promoter53 and a Cryaa-Cerulean was reversely inserted at the same time. The successfully constructed vectors (30 pg) were mixed with Tol2 transposase mRNA (250 pg) and injected into one-cell embryos to perform the transient rescue assays. Transgenic lines Tg(HuC:GFP), Tg(HuC:dlb), Tg(HuC:bax), Tg(HuC:NICD1a), Tg(NBT:DenNTR), Tg(NBT:creb1a), Tg(hsp70:bax), Tg(coro1a:NICD1a), Tg(mfap4:NICD1a) and Tg(coro1a:DN-MAML-FLAG) were generated in this study. To acquire these stable lines, the constructs, together with transposase mRNA, were injected into one-cell stage embryos. The treated embryos were selected by the transient fluorescence signals and raised into the adulthood F0 founder. The stable hereditary transgenic lines were identified in the F1 generation based on the genetic sequence and the fluorescence signals. The 3 dpf WT or baxcq55/Tg(hsp70:bax) and 4 dpf WT Tg(hsp70:DN-MAML-GFP) was identified by the cerulean+ (eyes) and AO+ (brain) signals after heat-shock at 37 °C for 30 min.
Chemical, mRNA, and morpholinos (MOs) treatments
The embryos were treated at designed time points by using chemicals: Nemadipine-A (10 μM, 2.5 dpf, 12 h; Santa Cruz, sc-202727); Nilvadipine (40 μM, 2.5dpf, 12 h; Selleck, S2721); DAPT (50 μM, 2.5 dpf, 12 h; Sigma-Aldrich, D5942); KN-92 phosphate (15 μM, 2.5 dpf, 12 h; ApexBio, A3531); KN-93 phosphate (15 μM, 2.5 dpf, 12 h; Selleck, S7423); PF-CBP1 HCl (35 μM, 2.5 dpf, 12 h; Selleck, S8180); Forskolin (35 μM, 2.5 dpf, 12 h; Selleck, S2449). The apyrase (100 U/ml; Sigma-Aldrich, A6410), ATP (100 mM; Sigma-Aldrich, A6559), carbenoxolone (CBX, 25 μM; Sigma-Aldrich, C4790), Probenecid (70 mM; Sigma-Aldrich, P8761), and PAC-1 (35 μM; Tocris Bioscience, 2581/10) were mixed with Alexa Fluor 647 Dextran (1:1000) and injected into the OT region of 2.5 dpf or 3 dpf anesthetized WT zebrafish embryos. To induce neuronal cell ablation of zebrafish midbrain, 2.5 dpf Tg(NBT:DenNTR) embryos were treated with metronidazole (MTZ, 15 mM; Sigma-Aldrich, M3761) for 12 h. Then these embryos were rinsed in E3 buffer and analyzed further. The mRNAs of Zebrafish (Z)-bax (mut), Zebrafish (Z)-bax (WT), Mouse (M)-bax, Human (H)-bax and creb1a were synthesized in vitro through the SP6 kit (Thermo Fisher Scientific, AM1340) by the primers (seen in Supplementary Table 1). The dlb MO and notch1a MO were used following previous reports69,70. The creb1a morpholino targets and its control (seen in Supplementary Table 1) were designed by Gene Tools LLC. Both mRNA and MOs were injected into the fertilized embryos at one-cell stage.
Bax monoclonal antibody production
A monoclonal antibody against zebrafish Bax antigen was generated by Chongqing Biomean Technology Co., Ltd company. Briefly, the designed appropriate Bax antigen segments “GAALLNNFVYERVRRHGDRDAEVTRSQLGGVELCDPSHKRAQCLQQIGDELDGNAQLQSMLNNSNLQPTQDVFIRVAREIFSDGKFN (20-108)” were recombinantly expressed. Next, combined with complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA), Bax antigen was subcutaneously injected into Balb/c mice. The high titer mice were selected after immunized for 1 month or 2 months. Subsequently, we collected the spleen cells of immunized mice to fuse with SP2/0 myeloma cells. The high-affinity and specific anti-Bax antibody strains, such as 3E10, were then screened to infect mice to produce ascites. Finally, the ascites was concentrated and purified with protein A/G. The purified antibody was anti-Bax monoclonal antibody at 3.3 mg/ml. To make an evaluation, WB of Bax antibody (1:400) was performed on the total protein of 12 hpf zebrafish brain after injection of bax mRNA.
Western blotting (WB)
The total proteins of 3 dpf zebrafish brain or E14.5 mouse mesencephalon were collected to perform WB. The antibodies utilized in this study: mouse anti-zBax, rabbit anti-CaMKII (pan), rabbit anti-phospho-CaMKII (Thr286), rabbit anti-CREB, rabbit anti-CREB (phospho S133), rabbit anti-NICD1a, rabbit anti-activated Notch1, mouse anti-Hes1, mouse anti-GAPDH. To detect the protein level of dlb or DLL3 in the plasma membrane of 3 dpf zebrafish brain or E14.5 mouse mesencephalon, the membrane was firstly isolated by Minute™ Plasma Membrane Protein Isolation and Cell Fractionation Kit (Invent, SM-005). Mouse anti-β tubulin and rabbit anti-Dll3 were then utilized to perform WB. Detections were completed by Goat anti-mouse or anti-rabbit HRP-conjugated secondary antibodies and enhanced chemiluminescent (ECL Plus) reagent. Each protein sample contains approximately 100 zebrafish brains or 2 mice mesencephalon. Each WB result was repeated three times independently with similar results.
Validation of the NICD1a, DLL3, p-CaMKII, and p-CREB antibodies
WB was conducted to validate the NICD1a antibody in zebrafish. A specific fragment of approximately 80 kDa was appeared, matching the predicted molecular weight. Further, WT zebrafish embryos were treated with DAPT from 52 hpf to 3 dpf. The total protein of treated zebrafish brains was collected to perform WB. The data indicated an obvious reduction in NICD1a expression in DAPT group. To validate whether the DLL3 antibody authentically binds DeltaB in zebrafish, pCS2-dla-flag, pCS2-dlb-flag, pCS2-dlc-flag and pCS2-dld-flag plasmids were constructed. The primers were listed in Supplementary Table 1. These four plasmids and pCS2 empty vector (control) were transfected into HEK293T cell lines with Lipo8000 (beyotime, C0533). Two days later, the cells were collected. The cellular proteins of the cultured cells were harvested to perform WB by using DLL3 and FLAG antibodies. The concurrent detection of the bands of 70 kDa, the weight of DeltaB (pCS2-dlB-flag), by both FLAG and DLL3 antibody validated the specificity of DLL3 to DeltaB. The molecular sizes of the presumed other Delta proteins are: 87 kDa (pCS2-dla-flag), 74 kDa (pCS2-dlc-flag), and 81 kDa (pCS2-dld-flag). p-CaMKII and p-CREB antibodies were similarly validated. The total proteins of 3 dpf zebrafish brains were immunoprecipitated (IP) with p-CaMKII and p-CREB, respectively. WB assays indicated that the weight of protein labeled by p-CaMKII antibody was the predicted molecular weight (60 kDa) and the weight of protein labeled by p-CREB was approximate 43 kDa of the predicted volume.
ChIP and luciferase reporter assay
Chromatin immunoprecipitation (ChIP) assay was performed as described previously67. 3dpf WT zebrafish DNA was extracted by chromatin immunoprecipitation kit (Sigma-Aldrich, 17-295) and then precipitated by immunoglobulin G (lgG as controls) (Thermo Fisher Scientific, 31460) and p-CREB antibodies. PCR was performed by using the designed primers (see Supplementary Table 1) that targeted the predicted CREB binding sites. The luciferase assays were performed by using the Dual-Luciferase Reporter Assay System as previously described. In brief, luciferase reporter constructs (PGL3) containing WT (site1: TGACGTCA; site2: GACATCA) and mutant (deletion) binding sites in the dlb promoter regions were generated. Then together with pCS2-creb1a (160 ng) and pRL-CMV (40 ng) plasmid, the PGL3-dlb (WT, 200 ng) or PGL3-dlb (MU1, 200 ng), PGL3-dlb (MU2, 200 ng), PGL3-dlb (MU1 + 2, 200 ng) plasmids were transfected into HEK293T cell lines via Lipo8000. In this assay, PGL3-basic plasmid was used as a control. After cultured for 2 days, the cells were lysed and the supernatant was collected after centrifugation at 12,000 × g. Finally, the supernatant was used to determine the fluorescence intensity.
Mouse microglia isolation and culture
Primary microglia were directly isolated from C57BL/6J E12.5 embryonic mice. After perfused with ice-cold PBS, mouse brains were rapidly dissected and placed into ice-cold dPBS. Cerebella and spine cords were discarded. The brains were minced to ~1 mm3 with scissors under a dissecting microscope and digested with papain at 37 °C for around 40 min. Then, the digested cell pellets were centrifuged with 800 rpm at 4 °C for 5 min, washed by HBSS, resuspended in 5 mL 5% FBS and passed through 1.2 μm and 0.5 μm glass. Finally, cell suspension was pushed through a 40 μm cell strainer to gain the single-cell suspension. Cell suspensions were incubated with FITC anti-mouse/human CD11b antibody (Elabscience, E-AB-F1081C, 5:100 in blocking buffer containing 5% FBS in dPBS) at 4 °C for 2 h. Cell sorting was performed by flow cytometry. Sorted CD11b+ cells were cultured at 24-well plate (6 × 104 cells per well) coated with poly-D-lysine, and grown in microglia culture medium containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml recombinant mouse MCSF (R&D Systems, 416-ML-010/CF) with or without the presence of 1 μg/ml recombinant human DLL3 (R&D Systems, 9749-DL-050) for 5 days.
Immunofluorescence staining of primary microglia
After a briefly washing with PBS, the primary microglia were fixed in 4% PFA for 10 min at room temperature and washed with PBS. Then the cells were treated with 0.2% Triton X-100 for 15 min, blocked with 5% FBS for 30 min, and incubated with primary antibodies, including goat anti-IBA1, mouse anti-Hes1, rabbit anti-activated Caspase-3, and mouse anti-PCNA (Sigma-Aldrich, P8825, 1:800), at 4 °C overnight. The next day, the cells were washed with PBS for 3 times and incubated with Alexa Fluor 488 donkey anti-rabbit IgG, Alexa Fluor 647 donkey anti-rabbit IgG and Alexa Fluor 555 donkey anti-mouse IgG at room temperature for 2 h. DAPI (Sigma-Aldrich, 28718-90-3) was used to stain the nucleus. Immunofluorescence staining was imaged under LSM880 confocal microscope.
Quantitative RT-PCR
Zebrafish and mouse embryonic quantitative RT-PCR (qPCR) were conducted according to a previous method49. Zebrafish embryonic total RNA isolation (Roche, 11667165001) and cDNA synthesis (Thermo Fisher Scientific, 18080051) were performed as previous described49. HuC-GFP+ and coro1a-DsRed+ cells were sorted from 3 dpf zebrafish midbrains by flow cytometry. Mouse mesencephalon cell suspensions were incubated with FITC anti-mouse/human CD11b antibody (Elabscience, E-AB-F1081C, 5:100 in blocking buffer containing 5% FBS in dPBS) and Alexa Fluor 700 anti-mouse CD45 antibody (BioLegend, 103128, 2:100 in blocking buffer containing 5% FBS in dPBS) at 4 °C for 1 h. Then, CD11bhiCD45lo cells were sorted by flow cytometry. These harvested cells and primary microglial cells were applied to generate cDNA libraries by using REPLI-g WTA Single Cell Kit (QIAGEN, 150063). Relative mRNA levels were determined using the Eppendorf qPCR machine and gapdh or ef1α was used as the control reference. Samples were run in duplicate or triplicate, and the data are presented as mean ± SEM. Genes primers for qPCR assay were listed in Supplementary Table 1.
Statistics and reproducibility
The positive signals were manually scored and double confirmed blindly. The quantified data were analyzed by GraphPad Prism 8.3.0. Statistical analysis was performed using unpaired two-tailed Student’s t test. All error bars represented mean ± SEM. All “n” and “P” values and statistical tests were indicated in figures and corresponding legends. The experiments in this study, including live-imaging and uncaging, immunofluorescent and neutral red staining, transplantation, WISH and FISH, drugs treatment, WB and CHIP PCR, luciferase and apoptosis detection, were independently repeated at least three times. Similar results were obtained.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The RNA-seq and scRNA-seq data had been deposited in the Sequence Read Archive under accession code PRJNA660936 (RNA-seq of H-G+ neurons), PRJNA661142 (RNA-seq of photo-converted red c-K+ microglia) and Gene Expression Omnibus under accession code GSE183340 (scRNA-seq), respectively. Previous published scRNA-seq that were re-analyzed here were available under accession codes GSE12165445. Raw data were provided with this paper. Source data were provided as a Source Data file with this paper. All data that support the findings of this study, including experimental animals, antibodies, vector constructions, and chemicals, are available within the article, its Supplementary Information, or from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Damisah, E. C. et al. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv. 6, eaba3239 (2020).
Frost, J. L. & Schafer, D. P. Microglia: architects of the developing nervous system. Trends Cell Biol. 26, 587–597 (2016).
Heese, K., Fiebich, B. L., Bauer, J. & Otten, U. Nerve growth factor (NGF) expression in rat microglia is induced by adenosine A2a-receptors. Neurosci. Lett. 231, 83–86 (1997).
Cattaneo, E. & McKay, R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature 347, 762–765 (1990).
Zhang, J. et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci. Adv. 7, eabb9888 (2021).
Wang, C. et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 367, 688–694 (2020).
Rio-Hortega, P. THE MICROGLIA. Lancet 233, 1023–1026 (1939).
Ginhoux, F. & Prinz, M. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 7, a020537 (2015).
Xie, J., Farage, E., Sugimoto, M. & Anand-Apte, B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 10, 76 (2010).
Herbomel, P., Thisse, B. & Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol. 238, 274–288 (2001).
Xu, J., Wang, T., Wu, Y., Jin, W. & Wen, Z. Microglia colonization of developing zebrafish midbrain is promoted by apoptotic neuron and lysophosphatidylcholine. Dev. Cell 38, 214–222 (2016).
Casano, A. M., Albert, M. & Peri, F. Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Rep. 16, 897–906 (2016).
Wu, S. et al. Il34-Csf1r pathway regulates the migration and colonization of microglial precursors. Dev. Cell 46, 552–563 (2018). e554.
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Li, Z., Li, Y. & Jiao, J. Neural progenitor cells mediated by H2A.Z.2 regulate microglial development via Cxcl14 in the embryonic brain. Proc. Natl Acad. Sci. USA 116, 24122–24132 (2019).
Baxter, P. S. et al. Microglial identity and inflammatory responses are controlled by the combined effects of neurons and astrocytes. Cell Rep. 34, 108882 (2021).
Pasciuto, E. et al. Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell 182, 625–640 (2020). e624.
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Wegiel, J. et al. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 804, 135–139 (1998).
Bohlen, C. J. et al. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759–773.e758 (2017).
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Cheng, Z. et al. Inhibition of Notch1 signaling alleviates endotoxin-induced inflammation through modulating retinal microglia polarization. Front Immunol. 10, 389 (2019).
Grandbarbe, L. et al. Notch signaling modulates the activation of microglial cells. Glia 55, 1519–1530 (2007).
Wei, Z. et al. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke 42, 2589–2594 (2011).
Yuan, Y. et al. Scutellarin regulates the Notch pathway and affects the migration and morphological transformation of activated microglia in experimentally induced cerebral ischemia in rats and in activated BV-2 microglia. J. Neuroinflammation 12, 11 (2015).
D’Orsi, B. et al. Bax regulates neuronal Ca2+ homeostasis. J. Neurosci. 35, 1706–1722 (2015).
Anderson, S. R. et al. Developmental apoptosis promotes a disease-related gene signature and independence from CSF1R signaling in retinal microglia. Cell Rep. 27, 2002–2013.e2005 (2019).
Garcia, I. et al. Bax deficiency prolongs cerebellar neurogenesis, accelerates medulloblastoma formation and paradoxically increases both malignancy and differentiation. Oncogene 32, 2304–2314 (2013).
Kratz, E. et al. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 13, 1631–1640 (2006).
Peri, F. & Nüsslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927 (2008).
Li, L., Yan, B., Shi, Y. Q., Zhang, W. Q. & Wen, Z. L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 287, 25353–25360 (2012).
Sieger, D., Moritz, C., Ziegenhals, T., Prykhozhij, S. & Peri, F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev. Cell 22, 1138–1148 (2012).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007).
Ferrero, G. et al. Embryonic microglia derive from primitive macrophages and are replaced by cmyb-dependent definitive microglia in zebrafish. Cell Rep. 24, 130–141 (2018).
Mullins, M. C., Hammerschmidt, M., Haffter, P. & Nüsslein-Volhard, C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr. Biol. 4, 189–202 (1994).
Xu, J. et al. Temporal-spatial resolution fate mapping reveals distinct origins for embryonic and adult microglia in zebrafish. Dev. Cell 34, 632–641 (2015).
Li, L., Jin, H., Xu, J., Shi, Y. & Wen, Z. Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood 117, 1359–1369 (2011).
Rossi, F., Casano, A. M., Henke, K., Richter, K. & Peri, F. The SLC7A7 Transporter Identifies Microglial Precursors prior to Entry into the Brain. Cell Rep. 11, 1008–1017 (2015).
Curado, S. et al. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev. Dyn. 236, 1025–1035 (2007).
Anderson, M. E. et al. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J. Pharm. Exp. Ther. 287, 996–1006 (1998).
Dworkin, S. et al. CREB activity modulates neural cell proliferation, midbrain-hindbrain organization and patterning in zebrafish. Dev. Biol. 307, 127–141 (2007).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271.e256 (2019).
Sahasrabuddhe, V. & Ghosh, H. S. Cx3Cr1-Cre induction leads to microglial activation and IFN-1 signaling caused by DNA damage in early postnatal brain. Cell Rep. 38, 110252 (2022).
Guan, Z. et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 19, 94–101 (2016).
Krishnasamy, K. et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat. Commun. 8, 952 (2017).
Ma, H. et al. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159, 281–294 (2014).
Li, Y., Du, X. F., Liu, C. S., Wen, Z. L. & Du, J. L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 23, 1189–1202 (2012).
Zhang, C., Li, Q. & Jiang, Y. J. Zebrafish Mib and Mib2 are mutual E3 ubiquitin ligases with common and specific delta substrates. J. Mol. Biol. 366, 1115–1128 (2007).
Huang, Y. et al. Ikzf1 regulates embryonic T lymphopoiesis via Ccr9 and Irf4 in zebrafish. J. Biol. Chem. 294, 16152–16163 (2019).
Yu, T. et al. Tripartite-motif family protein 35-28 regulates microglia development by preventing necrotic death of microglial precursors in zebrafish. J. Biol. Chem. 295, 8846–8856 (2020).
Morsch, M. et al. In vivo characterization of microglial engulfment of dying neurons in the zebrafish spinal cord. Front Cell Neurosci. 9, 321 (2015).
Parsons, M. J. et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 126, 898–912 (2009).
Zhao, L. et al. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl Acad. Sci. USA 111, 1403–1408 (2014).
Vladimirov, N. et al. Light-sheet functional imaging in fictively behaving zebrafish. Nat. Methods 11, 883–884 (2014).
Chang, N. et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res. 23, 465–472 (2013).
Abdelaal, H. M. et al. Comparison of vibratome and compresstome sectioning of fresh primate lymphoid and genital tissues for in situ MHC-tetramer and immunofluorescence staining. Biol. Proced. Online 17, 2 (2015).
Masuda, T. et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604, 740–748 (2022).
Zhang, R. W., Li, X. Q., Kawakami, K. & Du, J. L. Stereotyped initiation of retinal waves by bipolar cells via presynaptic NMDA autoreceptors. Nat. Commun. 7, 12650 (2016).
Thévenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process 7, 27–41 (1998).
Cong, L. et al. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). Elife 6, e28158 (2017).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019). e1821.
Barron, M. & Li, J. Identifying and removing the cell-cycle effect from single-cell RNA-Sequencing data. Sci. Rep. 6, 33892 (2016).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Liu, C. et al. Macrophages mediate the repair of brain vascular rupture through direct physical adhesion and mechanical traction. Immunity 44, 1162–1176 (2016).
Xue, Y. et al. The vascular niche regulates hematopoietic stem and progenitor cell lodgment and expansion via klf6a-ccl25b. Dev. Cell 42, 349–362.e344 (2017).
Park, H. C. et al. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev. Biol. 227, 279–293 (2000).
Geudens, I. et al. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb. Vasc. Biol. 30, 1695–1702 (2010).
Lorent, K. et al. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development 131, 5753–5766 (2004).
Acknowledgements
The authors thank Z. Wen and J. Du for plasmids, antibody and fish lines discussions, H. Huang, H. Li, and D. Wang for technical assistances. This work was supported by National Key Research and Development Project (2019YFA802703); National Natural Science Foundation of China Grants (32270873, 32000568, 31822033); Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0104), and Fundamental Research Funds for the Central Universities Grant (XDJK2020C041).
Author information
Authors and Affiliations
Contributions
F.Z., J.H., J.T., L.Li, and L.Luo designed the experiments. F.Z., J.H., and J.T. designed and conducted most experiments. L.Luo performed the ENU mutagenesis. Y.S. conducted the mutant screening. N.C., Z.L., and S.L. performed mouse microglia isolation and culture. Y.W., C.Z., M.M., and L.Li provided technical assistance and discussed the results, F.Z. and L.Li wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks F. Bennett, Cody Smith and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, F., He, J., Tang, J. et al. Brain milieu induces early microglial maturation through the BAX-Notch axis. Nat Commun 13, 6117 (2022). https://doi.org/10.1038/s41467-022-33836-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33836-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.