Abstract
Designing organic fluorescent and phosphorescent materials based on various core fluorophore has gained great attention, but it is unclear whether similar luminescent units exist for inorganic materials. Inspired by the BX6 octahedral structure of luminescent metal halide perovskites (MHP), here we propose that the BX6 octahedron may be a core structure for luminescent inorganic materials. In this regard, excitation-dependent color-tunable phosphorescence is discovered from α-AlF3 featuring AlF6 octahedron. Through further exploration of the BX6 unit by altering the dimension and changing the center metal (B) and ligand (X), luminescence from KAlF4, (NH4)3AlF6, AlCl3, Al(OH)3, Ga2O3, InCl3, and CdCl2 are also discovered. The phosphorescence of α-AlF3 can be ascribed to clusterization-triggered emission, i.e., weak through space interaction of the n electrons of F atoms bring close proximity in the AlF6 octahedra (inter/intra). These discoveries will deepen the understanding and contribute to further development of BX6 octahedron-based luminescent materials.
Similar content being viewed by others
Introduction
Luminescent materials are indispensable for our daily life, especially in lighting, displaying, and imaging-related applications1,2. Therefore, luminescent materials design (either organic or inorganic) is of great importance and attracts great attention. It is widely accepted that luminescence from organic materials can be ascribed to their core structure in most cases (Fig. 1, together with the substituents), for example, fluorescent materials from xanthene3,4,5,6,7 and phosphorescent materials based on carbazole8,9,10,11. Such core structure endow organic fluorophores with great flexibility and processability. While for inorganic luminescent materials, although structurally diverse and mostly acting as host materials for doping of transition- or rare-earth metal ions (e.g., ZnS12 and SrAl2O413), core structure as the light-emitting unit has seldom been reported and explored like organics. So, is there similar core structure for the luminescent inorganic materials?
All-inorganic metal halide perovskites (MHPs), a type of semiconductor materials with excellent photoelectric properties14, have been widely used in solar cells15, LED16, and thermoelectric modules17. The core structure of luminescent MHPs can be described as the BX6 octahedron (Fig. 1), which is constituted by the central cation (B, hexa-coordinated) and six halide ligands (X=Cl, Br, I). Normally, the BX6 octahedron is organized in an all-corner-sharing 3D network. Due to the adjustable octahedral connectivity, a series of lower dimensional metal halide-based luminescent perovskite derivatives have been reported18. On the other hand, the central cation and ligand halides could be altered, leading to tunable luminescence performance from 3D and lower dimension metal halides19,20. Therefore, the BX6 octahedron is an important structure for the luminescence of MHPs, but whether such unit can be generalized for other luminescent inorganic materials remains unexplored.
In this work, we find that inorganic materials constructed by the BX6 octahedron exhibit interesting long-lived room temperature phosphorescence (RTP), i.e., the BX6 octahedron may be regarded as a basic unit for the luminescent inorganic materials. For example, α-AlF3, the material constructed by AlF6 (the lightest BX6 octahedron) with 3D perovskite-like structure21, shows color-tunable RTP (up to 7 s for the blue emission). When lowering the dimension of the AlF6 octahedron, luminescence from KAlF4 (2D) and (NH4)3AlF6 (0D) is also discovered. Moreover, by changing B and X in the octahedral emissive unit of BX6, luminescence from AlCl3, Al(OH)3, Ga2O3, InCl3, and CdCl2 are also obtained. Similar to BX6 octahedron-based MHPs, the luminescence from AlF3 exhibit typical self-trapped exciton (STE) emission. Besides, the octahedron also brings close proximity of F atoms, resulting in weak through-space interaction of the n electrons in F atoms for clusterization-triggered emission (CTE, recently found in n electron-rich organics for excitation-dependent color-tunable phosphorescence22,23,24). It should be noted that it is normally the heavy atoms that drives the formation of triplet and phosphorescence in inorganics (e.g., previous Al-based luminescent materials, Supplementary Table 1). However, α-AlF3 contains only light elements. Therefore, the discovery here is interesting for both organic and inorganic phosphors. In addition, the intriguing color-tunable phosphorescence without extra sophisticated molecular design can be explored for facile UV light detection with visible colored afterglow emission as readout.
Results
Luminescence of α-AlF3
To investigate the luminescent properties of the BX6 octahedron, hexa-coordinated AlF6 was chosen first. Considering its outermost electronic structure (3s23p1), aluminum is lightest atom to generate the octahedral structure. Meanwhile, F‒ is the smallest anion25 that can coordinate with Al3+. The corner shared octahedra of AlF6 results in the formation of three-dimensional network of α-AlF3 (Fig. 1). Normally, AlF3 is used as the electrolyte regulator in the aluminum smelting industry for increasing the melting point and conductivity. However, its photophysical properties are rarely studied.
Here, we found AlF3 exhibited exciting color-tunable luminescence at room temperature (Fig. 2a and Supplementary Fig. 3), irrespective of its origins (Supplementary Table 6 and Fig. 2). From the time-resolved emission spectra (TRES, Fig. 2b and Supplementary Fig. 11), the luminescence of α-AlF3 could be attributed to phosphorescence, with quantum yield (ΦP) of ~4.22% and lifetime up to ~0.9 s (λex = 280 nm, Fig. 2c). The room-temperature afterglow of α-AlF3 could last more than 7 s (naked eye observable), and the excitation-dependent blue to yellow afterglow could be visualized clearly after ceasing the excitation (Fig. 2d, Supplementary Figs. 12–13, and Supplementary Movies 1–3). The color-tunable emission was also clearly revealed by the Commission Internationale de l’Eclairage (CIE) chromaticity coordinates (Fig. 2e). In addition, pure white light emission could be obtained (approaching CIE of 0.33, 0.33) when changing λex from 270 nm to 390 nm (Fig. 2d and Supplementary Fig. 15).
a excitation- phosphorescence emission mapping of AlF3 (delay time: 40 ms); b time-resolved emission spectra (TRES, λex = 280 nm) of AlF3; c lifetime decay profiles of phosphorescent emission excited at 280, 355, and 390 nm, respectively; d photographs taken under different excitation (250 to 430 nm) off and afterglow emission images excited at 290, 350 and 390 nm, respectively (the excitation-dependent afterglow can be observed in the excitation range from 250 to 510 nm at room temperature condition); e CIE coordinates of AlF3 phosphorescence under different excitation (250 to 370 nm).
To exclude the potential influence from trace impurities, direct synthesis of AlF3 through exposing aluminum metal of the highest purity available to HF vapor was carried out. As expected, similar emission properties were also obtained (Supplementary Figs. 6-7), confirming that the luminescence was exactly from AlF3. Furthermore, the purchased and as-prepared samples were processed by calcination, ball milling, and acid-washing, and no appreciable change of the luminescence property was received (Supplementary Figs. 8-10).
Extending of the BX6 octahedral luminescent unit
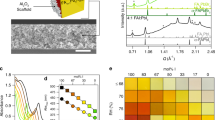
Since the 3D perovskite-like structure of α-AlF3 is constituted by the AlF6 octahedron, the basic unit of BX6 was further explored by adjusting the dimension of the octahedra (Fig. 3a). As shown in Figs. 3b and 3c, phosphorescence from KAlF4 (2D, layers of corner-sharing AlF6 octahedra and BCC coordinated K+) and (NH4)3AlF6 (0D, isolated AlF6 octahedra surrounded by NH3 ligands) was also collected (Supplementary Figs. 22 and 25), accompanied with similar excitation-dependent emission. The crystalline structure information (Supplementary Table 7) and XRD patterns confirmed that both KAlF4 and (NH4)3AlF6 were composed by the AlF6 octahedral unit and belonged to 2D and 0D metal halides structures (Supplementary Fig. 20), respectively. It should be noted that lowering the dimension of the octahedra resulted in largely decreased phosphorescence intensity and shortened lifetime (Fig. 3c), indicating that the linkage of the AlF6 octahedra also contributed to the observed phosphorescence.
a, schematic diagram of chemical structure of AlF3 and engineering of the octahedral basic unit; b and c, relative phosphorescence intensity and lifetime decay profiles (λex = 280 nm) of the phosphorescence emission at 456, 480, 446 nm of AlF3, KAlF4, and (NH4)3AlF6, respectively; d, e, relative phosphorescence intensity and lifetime decay profiles (λex = 280 nm) of the phosphorescence emission at 456, 486, 450, 496 nm of AlF3, AlCl3, Al(OH)3, and AlBr3, respectively; f, g, relative phosphorescence intensity, and lifetime decay profiles (λex = 280 nm) of the phosphorescence emission at 486, 468, 460, 500 nm of AlCl3, InCl3, CdCl2, and Ga2O3, respectively; and h, summary of the diverse crystalline structures of the luminescent comprising BX6 octahedra. Error bars represent standard deviation (n = 3).
Next, the ligand (X) of the BX6 basic unit was changed with other halogens, namely AlCl3 and AlBr3 (Fig. 3a). As shown in Fig. 3d and Supplementary Fig. 23, AlCl3 (cubic layered AlCl6 octahedra) still exhibited appreciable long-lived phosphorescence (Fig. 3e and Supplementary Fig. 26 and Supplementary Movie 4). Through altering halide composition from F to Br, their emission spectra are readily tunable from blue to yellow (Insets of Fig. 3d), which was similar with the emission tunable perovskite materials through halide engineering26. Although the outer space of Al is unable to accommodate six Br atoms to form stable hexadentate structure due to their relatively large atom radius discrepancy, AlBr3 (tetrahedral) is still luminescent, but with significantly reduced intensity and shortened lifetime (Fig. 3d, 3e). When altering the ligand from F to O, phosphorescence from the AlO6 (octahedral) derivatives (Al(OH)3 or Al2O3, 2D sheets of edge sharing Al(OH)6 octahedra) was also collected (Fig. 3d and Supplementary Fig. 23).
Upon changing the metal center of BX6, octahedral structure from Ga2O3 (mixture of GaO6 octahedral and GaO4 tetrahedral), InCl3 (distorted InCl6 octahedra in the 1D chains), and CdCl2 (2D sheets of edge-sharing CdCl6 octahedra) can be expected. Again, phosphorescence from these species was successfully collected (Fig. 3f and Fig. 3g, Supplementary Figs. 23 and 27, and Supplementary Movie 5 for Ga2O3 afterglow). It should be noted that there are diverse hexa-coordinate structures evolved from different bond angles (X-B-X), thus varied phosphorescence properties (excitation, emission, intensity, and lifetime, Fig. 3h)27.
Luminescence mechanism of α-AlF3
The luminescence mechanism of the octahedral unit was investigated with the 3D network AlF6 (α-AlF3). Compared with MHPs, α-AlF3 showed the 3D perovskite-like structure, but without the insertion of alkali metal cations. These cations only contribute to lattice stabilization and does not participate in the formation of the frontier molecule orbitals28. The intrinsic emission of MHPs could be originated from free, bound and self-trapped excitons29. Among them, self-trapped exciton (STE) emission is a well-accepted mechanism to account for the broadband and large-stokes shift emission30,31,32,33. For α-AlF3, similar broad-band excitation-dependent blue to yellow emission was emerged, with full width at half-maximum (FWHM) of 120 nm and Stokes shift up to ~180 nm (Fig. 4a, emission spectra from λex = 250 nm). In addition, a high energy narrow emission at 336 nm could be identified in the broad PL spectra (Fig. 4b), with lifetime of ~2.05 ns (Fig. 4c). According to the previous reports34,35, such emission could be ascribed to free excitons, which can be captured by the lattice distortion due to strong electron-phonon interaction in metal halides, resulting in the generation of STE.
a Absorbance and phosphorescence spectra (λex = 280, 350, and 390 nm, respectively.); b Normalized PL spectra (λex = 250 nm, the dotted line was measured without long pass filters, while the dashed line with 341 nm long pass filter placed at the emission exit); c lifetime of AlF3 monitored at 336 nm (λex = 280 nm); d plot of PL intensity as a function of excitation power density (λex = 405 nm); e plots of FWHM and intensity of phosphorescence as a function of temperature (λex = 280 nm; delay time: 40 ms).
The luminescence intensity of α-AlF3 exhibited a linear dependence on the excitation power density (more than three orders of magnitude, Fig. 4d), indicating that the emission is originated from photogenerated exciton (self-trapped) rather than permanent defect, the latter of which would show saturated PL intensity upon increasing the excitation power density36. Also, the emission band was not changed upon altering the excitation power density (Supplementary Fig. 16), further excluding the possibility of other emissive defects. Moreover, temperature-dependent phosphorescence spectra and cryogenic lifetime were collected (Supplementary Figs. 17, 18). The emission intensity was enhanced upon lowering the temperature (294 K→77 K), accompanied by a decrease in the FWHM (Fig. 4e), which was consistent with the characteristics of electron-lattice coupling37. Therefore, all these above spectral features agreed well with STE.
On the other hand, although α-AlF3 owns distorted 3D perovskite-like structure, its room-temperature phosphorescence exhibited interesting excitation-dependent feature. To further illustrate the mechanism, the transitions of AlF3 was investigated through theoretical calculations. Considering that the BX6 octahedra can be luminescent in isolated, corner-shared, and distorted structures, a single unit of AlF6 was calculated with the time-dependent density functional theory (TD-DFT)38,39. The calculated excitation energy with the highest oscillator strength and the emission energy from the lowest triplet state (T1) to the ground state (S0) are 4.76 eV and 3.36 eV, respectively, indicating potential large Stokes shift. Next, the natural transition orbitals (NTO) were analyzed with Multiwfn40. As shown in Fig. 5a and Supplementary Fig. 29, the transition with highest oscillator strength happened from the un-bonding n electron of F to the antibonding orbitals composed by the s orbital of Al and the p orbital of F. Meanwhile, such transition exhibited a typical n→σ* character, which is consistent with the deep UV absorption of AlF3. For phosphorescence transition (T1→S0), the frontier orbitals comprise F 2p (HOMO) as well as Al 3p and F 2p (LUMO). According to the selection rule for electronic spectra41, the electron transition of p-p (similar to f-f transition of lanthanides) orbitals is parity-forbidden, which is essential for the long-lived phosphorescence of AlF3. For the other BX6 octahedra, similar transitions (n→σ*) could also be identified and their T1→S0 transitions agreed well with experimental results (Supplementary Figs. 32–36).
a the TD-DFT calculated isosurfaces of occupied and unoccupied orbitals of excited singlet state with maximum oscillator strength and lowest triplet excited state in AlF6 octahedral unit. b structural analysis of α-AlF3 (crystalline structure from ISCD 68826). c excitation spectra (λem = 456, 500, and 535 nm, respectively.) of α-AlF3 d schematic energy level diagram of AlF3 from one octahedron to network. e Photograph of AlF3 afterglow with a flower pattern, consisting of untreated and pressure-treated α-AlF3, respectively.
Recently, a number of excitation-dependent color-tunable phosphors featuring n→σ* transitions were reported, in which weak through-space interaction (TSI) was identified from the rich n electrons of the heteroatoms in the phosphors (cluster-induced emission, CIE)22,23,24. Structurally, α-AlF3 is a nonconjugated system without through-bond conjugation, but F atoms with n electrons are abundant to form corner sharing networks (Fig. 5b). Importantly, the distances between the adjacent F atoms in and between the AlF6 octahedra (e.g., intra/inter: ~2.544 and ~2.549 Å; inter: ~3.052 Å) generally fall in the van der Waals radii of F atom (1.47 Å)42. Therefore, there is possible van der Waals F···F interaction, thus leading to effective electron cloud overlap of the n electrons for n→σ* transition23. It should be noted that F atoms also exist in NaF and KF, but no such phosphorescence could be found (Supplementary Fig. 24), further highlighting the importance of BX6 octahedron.
Spectrally, the excitation spectra of α-AlF3 contains two peaks, the short wavelength of which matches with its absorption, while those at longer wavelengths red shifted upon increasing the emission wavelengths (Fig. 5c). Besides, the excitation peaks at wavelength longer than the absorption were generally not detectable as a significant feature in the absorption spectra (Fig. 3a), which is also a typical sign of CTE. Moreover, as further calculated with the quantum mechanics and molecular mechanics (QM/MM) method, the energy gap decreased as increasing the number of AlF6 octahedra (increasing the sizes of the clusters, Supplementary Fig. 30). Therefore, different clustering states may result in varied energy gaps (Fig. 5d), thereby excitation-dependent and color-tunable phosphorescence.
The weak interaction between AlF6 octahedra in α-AlF3 was further investigated with Atoms in Molecules (AIM) analysis43. As shown in Supplementary Fig. 31, in addition to traditional bond path in the octahedron (Al-F), there is also plenty of through-space interaction path (F···F interaction) as the AlF6 octahedra system extended. Experimentally, upon high pressure treatment (900 MPa) to strengthen the weak intermolecular Van-der Waals interaction44, the emission brightness of α-AlF3 was increased somewhat (Fig. 5e, ΦP increase from ~4.22% to ~5.46%), while the multicolor emission profiles of α-AlF3 were not disturbed (Supplementary Fig. 19). Therefore, such interactions are expected to facilitate electron communications between the n electrons of F atoms and rigidify the system for efficient phosphorescence.
On the basis of the above analysis, similar excitation-dependent but decreased phosphorescence intensity from KAlF4 and (NH4)3AlF6 (and also other n electrons rich compounds featuring BX6 octahedra, Fig. 3h) can thus be expected. Lowering the dimension of the AlF6 octahedra from 3D corner-sharing (α-AlF3) to layered (KAlF4) and isolated ((NH4)3AlF6) would decrease the inter-octahedra F···F interaction, thus weakening the through-space interaction (Supplementary Fig. 21). Moreover, the 3D corner-sharing structure may also rigidify the AlF6 octahedra, which is beneficial for stabilization of the excited triplet states. Therefore, compared with α-AlF3, lowering the dimension of the AlF6 octahedra would result in sharply decreased phosphorescence intensity and lifetime in KAlF4 and (NH4)3AlF6. For other BX6 octahedron-containing materials, different phosphorescence performances would also be expected, due to their differences in B, X, and connection of the octahedra (Fig. 3h).
RTP of α-AlF3 for UV wavelength detection
Considering that the long-lived phosphorescence from AlF3 is color-tunable in the visible range and excitation-dependent (particularly in the UV range), it was simply and conveniently explored for ultraviolet wavelength detection in a testing paper manner. As demonstrated in Fig. 6a, after mixed with Aloe vera gel for fixing, AlF3 was coated on the filter paper. Upon UV irradiation, white luminescence from AlF3 was excited (Fig. 6b). After ceasing of UV excitation, visible afterglow images could be obtained (Fig. 6c and Supplementary Movie 6). Notably, upon excited with UV light of different wavelengths, the afterglow emission varied from blue to orange, which could be further compared with standard color chart to confirm UV excitation wavelength (Fig. 6d and Fig. 6e). Such application provided a method for unknown UV wavelength detection and offered rapid and simple standard screening and testing of commercially UVA and UVB ultraviolet lamps available.
a illustration of the testing paper coated with AlF3; b a beam of UV excitation irradiated on the testing paper; c AlF3-testing paper phosphorescence demonstration after ceasing 365 nm excitation; d multicolor phosphorescence from the AlF3-tesing paper after ceasing a series of UV excitation; e color chart of UV wavelength testing paper.
Discussion
In this work, perovskite-like octahedral BX6 was proposed a basic unit for luminescent inorganic materials. In this regard, we found that α-AlF3 (constituted by vertex sharing AlF6 octahedral unit) exhibited long-lived color-tunable phosphorescence emission, which could last up to 7 s and be observed by naked eyes. Besides, by lowering the dimension of the AlF6 octahedron and changing B and X in the BX6 octahedron, luminescence from KAlF4, (NH4)3AlF6, AlCl3, Al(OH)3, Ga2O3, InCl3, and CdCl2 were also obtained. The phosphorescence of AlF3 could also be explained with the well-accepted STE mechanism of perovskite BX6 octahedra, together with the clusterization-triggered emission of n electron-rich phosphors. Therefore, BX6 octahedron may be a universal structure motif for inorganic luminescent materials (Fig. 7).
For a long time, it has been well-accepted that there are core structures for organic luminescent materials, and their general photophysical properties can thus be interpreted. For inorganic luminescent materials, such core structure remains elusive. Our results here indicated that the boundary between the above two may be somewhat vague. Inorganic AlF3 (also AlCl3, Ga2O3, and etc.), featured with perovskite-like octahedral BX6 basic unit, can emit long-lived phosphorescence very much similar to CTE from non-conjugated organic luminophores22. Moreover, typical n→σ* transition was found in the single unit of AlF6, which is also the typical character of CTE. Therefore, future development of luminescent materials integrating of both organic and inorganic luminescence mechanisms is expected to be appealing, particularly in MOFs containing both inorganic and organic units.
Last, in the long history of the afterglow phosphor family, the IIIA group elements contributed heavily (Supplementary Table 1, 2), particularly Al- and Ga-containing materials (e.g., the well-known SrAl2O4:Eu2+-Dy3+13 and ZnGa2O4:Cr3+ 45). However, the luminescence center are mostly lanthanides or transition metal ions46 (e.g., Mn2+, and Cr3+). Here, Al- and Ga-containing afterglow phosphors with ~4% QY (e.g., AlF3 and Ga2O3) are discovered. Particularly, the luminescence is come from the Al- and Ga-involved octahedral unit, not the dopants. Therefore, there is still much room for the exciting IIIA group chemistry in luminescent materials design.
Methods
Preparation of AlF3
AlF3 was prepared through calcination of AlF3·3H2O (99.9%, Macklin) at 350 °C for 3 h. To eliminate potential organic impurities, other AlF3 samples were also subjected to the same calcination process as above. In addition, direct synthesis of AlF3 was carried out through exposing aluminum metal (Aladdin, 99.999%, the highest purity available) to HF vapor from hydrofluoric acid (Macklin, 49wt. % in H2O, 99.99998%) for 3 h, followed by calcination at 350 °C for 3 h.
Phosphorescence measurements
Phosphorescence spectra were collected on HORIBA FluoroMax-4P with a delay time of 40 ms. For the temperature-dependent emission spectra, a model Optistat CF2 liquid nitrogen chamber (Oxford Instruments) was used and coupled with the FluoroMax-4P spectrofluorometer. Phosphorescence lifetime and the time-resolved emission spectra (TRES) were collected on HORIBA FluoroLog-3 spectrofluorometer with 280, 355, and 390 nm spectraLED as the excitation sources, respectively. The absorption spectra of solid sample were measured on Shimadzu UV-3600 with an integrating sphere unit. The phosphorescence quantum yield (Фp) of samples were measured in an integrating sphere (IS80, Labsphere) using 2-fluorophenylboronic acid (Фp = 0.98%47) as the reference 48.
Afterglow images
The samples were excited with light selected from the Xe lamp in the Fluolog-3 spectrofluorometer (250 to 510 nm)48. The shutting of the excitation was controlled by instrumental software. The camera started to record video for 10 s after the excitation was turned off immediately.
Theoretical calculation
Theoretical calculations for the octahedral unit were performed on Orca program package (Revision 4.1.1). The ground states (S0) were fully optimized by M062X with ma-def2-TZVP basis set. The excitation energies in the n-th singlet (Sn) and n-th triplet (Tn) states were obtained using the time-dependent density functional theory (TD-DFT) method based on an optimized molecular structure. In order to explore detailed excited state properties, NTO (Natural transition orbitals) analysis and hole-electron analysis method were further employed using Multiwfn program. For different cluster sizes of the AlF6 octahedra, QM/MM method was applied to obtain optimized structure of isolated, layered and cubic corner sharing AlF6 octahedra performed in the Gaussian 09 package49. The central AlF6 octahedra were treated at the (TD) M062X/6-31G+ (d,p) level, while the surrounding molecules were treated with universal force field (UFF).
For the weak interaction between AlF6 octahedra, AIM analysis was carried out, which dictates the form of atoms in molecules and analyzes the topology of electron density. Typically, the results could be characterized by “critical points” of, namely bond (BCP), ring (RCP) and cage (CCP) critical points, representing the extreme points of electron density on the bond paths, centers of rings, and enclosed space formed by rings, respectively. It was further employed using Multiwfn program. The molecular orbitals (MO) and AIM models were all displayed using VMD.
UV wavelength testing paper
α-AlF3 was mixed with Aloe vera gel with mass ratio of ~1: 1, followed by fixing on the filter paper to make the mixture evenly distributed and drying. For UV wavelength detection, the testing paper was first subjected to UV excitation for 5 seconds, then the afterglow images were either naked eye observed or photo taken with a camera.
Data availability
The data supporting the findings of this study are available within the paper and the Supplementary Information. Source data are provided with this paper.
References
O’Hara, P. B., Engelson, C. & St Peter, W. Turning on the light: Lessons from luminescence. J. Chem. Edu. 82, 49–52 (2005).
Xu, J. & Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumines 205, 581–620 (2019).
Chen, X. Q., Pradhan, T., Wang, F., Kim, J. S. & Yoon, J. Fluorescent chemosensors based on spiroring-opening of Xanthenes and related derivatives. Chem. Rev. 112, 1910–1956 (2012).
Luo, X. et al. A diversity-oriented rhodamine library for wide-spectrum bactericidal agents with low inducible resistance against resistant pathogens. Nat. Commun. 10, 258 (2019).
Beija, M., Afonso, C. A. M. & Martinho, J. M. G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 38, 2410–2433 (2009).
Grimm, J. B. et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 12, 244–250 (2015).
Wang, Y. et al. Halo-Fluorescein for photodynamic bacteria inactivation in extremely acidic conditions. Nat. Commun. 12, 526 (2021).
Jain, K. et al. Density functional investigation and some optical experiments on dye-sensitized quantum dots. Phys. Chem. Chem. Phys. 17, 28683–28696 (2015).
Chen, C. et al. Carbazole isomers induce ultralong organic phosphorescence. Nat. Mater. 20, 175–180 (2021).
Yang, Z. Y. et al. Intermolecular electronic coupling of organic units for efficient persistent room-temperature phosphorescence. Angew. Chem. Int. Ed. 55, 2181–2185 (2016).
Jin, J. B. et al. Thermally activated triplet exciton release for highly efficient tri-mode organic afterglow. Nat. Commun. 11, 842 (2020).
Wu, P. & Yan, X.-P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 42, 5489–5521 (2013).
Matsuzawa, T., Aoki, Y., Takeuchi, N. & Murayama, Y. A New long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+,Dy3+. J. Electrochem. Soc. 143, 2670–2673 (1996).
Fu, Y. et al. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 4, 169–188 (2019).
Tai, Q., Tang, K.-C. & Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 12, 2375–2405 (2019).
Lu, M. et al. Metal halide perovskite light-emitting devices: promising technology for next-generation displays. Adv. Funct. Mater. 29, 1902008 (2019).
Haque, M. A., Kee, S., Villalva, D. R., Ong, W.-L. & Baran, D. Halide perovskites: thermal transport and prospects for thermoelectricity. Adv. Sci. 7, 1903389 (2020).
Chen, P. et al. Progress and perspective in low-dimensional metal halide perovskites for optoelectronic applications. Sol. RRL 2, 1700186 (2018).
Li, M., Molokeev, M. S., Zhao, J. & Xia, Z. Optical functional units in zero-dimensional metal halides as a paradigm of tunable photoluminescence and multicomponent chromophores. Adv. Opt. Mater. 8, 1902114 (2020).
Benin, B. M. et al. Highly emissive self-trapped excitons in fully inorganic zero-dimensional tin halides. Angew. Chem., Int. Ed. 57, 11329–11333 (2018).
Krahl, T. & Kemnitz, E. Aluminium fluoride–the strongest solid Lewis acid: structure and reactivity. Catal. Sci. Technol. 7, 773–796 (2017).
Zhang, H. et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 32, 275–292 (2020).
Tang, S. et al. Nonconventional luminophores: characteristics, advancements and perspectives. Chem. Soc. Rev. 50, 12616–12655 (2021).
Zhang, H. & Tang, B. Z. Through-space interactions in clusteroluminescence. JACS Au 1, 1805–1814 (2021).
Goesten, M. G., Hoffmann, R., Bickelhaupt, F. M. & Hensen, E. J. M. Eight-coordinate fluoride in a silicate double-four-ring. Proc. Natl Acad. Sci. 114, 828 (2017).
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Filip, M. R., Eperon, G. E., Snaith, H. J. & Giustino, F. Steric engineering of metal-halide perovskites with tunable optical band gaps. Nat. Commun. 5, 5757 (2014).
Meloni, S., Palermo, G., Ashari-Astani, N., Grätzel, M. & Rothlisberger, U. Valence and conduction band tuning in halide perovskites for solar cell applications. J. Mater. Chem. A 4, 15997–16002 (2016).
Smith, M. D., Connor, B. A. & Karunadasa, H. I. Tuning the luminescence of layered halide perovskites. Chem. Rev. 119, 3104–3139 (2019).
Li, S., Luo, J., Liu, J. & Tang, J. Self-trapped excitons in all-inorganic halide perovskites: fundamentals, status, and potential applications. J. Phys. Chem. Lett. 10, 1999–2007 (2019).
Smith, M. D. & Karunadasa, H. I. White-light emission from layered halide perovskites. Acc. Chem. Res. 51, 619–627 (2018).
Zhou, L. et al. A highly red-emissive lead-free indium-based perovskite single crystal for sensitive water detection. Angew. Chem., Int. Ed. 58, 5277–5281 (2019).
Guo, Q., Zhao, X., Song, B., Luo, J. & Tang, J. Light emission of self-trapped excitons in inorganic metal halides for optoelectronic applications. Adv. Mater. https://doi.org/10.1002/adma.202201008 (2022).
Yuan, Z. et al. One-dimensional organic lead halide perovskites with efficient bluish white-light emission. Nat. Commun. 8, 14051 (2017).
Smith, M. D., Jaffe, A., Dohner, E. R., Lindenberg, A. M. & Karunadasa, H. I. Structural origins of broadband emission from layered Pb–Br hybrid perovskites. Chem. Sci. 8, 4497–4504 (2017).
Wu, G. et al. A one-dimensional organic lead chloride hybrid with excitation-dependent broadband emissions. ACS Energy Lett. 3, 1443–1449 (2018).
Dohner, E. R., Jaffe, A., Bradshaw, L. R. & Karunadasa, H. I. Intrinsic white-light emission from layered hybrid perovskites. J. Am. Chem. Soc. 136, 13154–13157 (2014).
Neese, F. The ORCA program system. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2, 73–78 (2012).
Neese, F. Software update: the ORCA program system, version 4.0. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 8, e1327 (2018).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Lin, R. et al. X-ray radiation excited ultralong (>20,000 seconds) intrinsic phosphorescence in aluminum nitride single-crystal scintillators. Nat. Commun. 11, 4351 (2020).
Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964).
Matta, C. F. & Boyd, R. J. An Introduction to the Quantum Theory of Atoms in Molecules. In The Quantum Theory of Atoms in Molecules p1–34 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007).
Wang, Q. et al. Reevaluating protein photoluminescence: remarkable visible luminescence upon concentration and insight into the emission mechanism. Angew. Chem., Int. Ed. 58, 12667–12673 (2019).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Wu S., Pan Z., Chen R., Liu X. Long Afterglow Phosphorescent Materials; Springer.
Li, M. et al. Prolonging ultralong organic phosphorescence lifetime to 2.5 s through confining rotation in molecular rotor. Adv. Opt. Mater. 7, 1800820 (2019).
Zheng, H. Y., Cao, P. S., Wang, Y. Y., Lu, X. M. & Wu, P. Ultralong room-temperature phosphorescence from boric acid. Angew. Chem. Int. Ed. 60, 9500–9506 (2021).
Frisch, M. J. T. et al. Inc., Wallingford CT: 2013.
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21522505) and Sichuan Science and Technology Program (No. 2021YFH0124). Detailed characterizations supported by the public Platform of Analytical & Testing Center, Sichuan University, are greatly appreciated.
Author information
Authors and Affiliations
Contributions
P.W. supervised this work. P.C. and H.Z. carried out the experimental and theoretical investigations. P.C. and P.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, P., Zheng, H. & Wu, P. Multicolor ultralong phosphorescence from perovskite-like octahedral α-AlF3. Nat Commun 13, 5712 (2022). https://doi.org/10.1038/s41467-022-33540-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33540-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.