Abstract
We investigated thrombocytopenic, thromboembolic and hemorrhagic events following a second dose of ChAdOx1 and BNT162b2 using a self-controlled case series analysis. We used a national prospective cohort with 2.0 million(m) adults vaccinated with two doses of ChAdOx or 1.6 m with BNT162b2. The incidence rate ratio (IRR) for idiopathic thrombocytopenic purpura (ITP) 14–20 days post-ChAdOx1 second dose was 2.14, 95% confidence interval (CI) 0.90–5.08. The incidence of ITP post-second dose ChAdOx1 was 0.59 (0.37–0.89) per 100,000 doses. No evidence of an increased risk of CVST was found for the 0–27 day risk period (IRR 0.83, 95% CI 0.16 to 4.26). However, few (≤5) events arose within this risk period. It is perhaps noteworthy that these events all clustered in the 7–13 day period (IRR 4.06, 95% CI 0.94 to 17.51). No other associations were found for second dose ChAdOx1, or any association for second dose BNT162b2 vaccination. Second dose ChAdOx1 vaccination was associated with increased borderline risks of ITP and CVST events. However, these events were rare thus providing reassurance about the safety of these vaccines. Further analyses including more cases are required to determine more precisely the risk profile for ITP and CVST after a second dose of ChAdOx1 vaccine.
Similar content being viewed by others
Introduction
The three Coronavirus Disease 2019 (COVID-19) vaccines currently being administered in the United Kingdom (UK), namely ChAdOx1 nCoV-19 (Oxford-AstraZeneca; henceforth ChAdOx1), BNT162b2 mRNA (Pfizer-BioNTech; henceforth BNT162b2) and mRNA-1273 (Moderna), have been shown to reduce COVID-19 infection, hospitalization and death1,2,3,4. The mRNA-1273 vaccine was first given in Scotland on 7 April 2021 and remains a less commonly used vaccine (169,842 second doses as of 13 January 2022)5.
There have been reports of post-vaccination exacerbation of chronic idiopathic or immune thrombocytopenic purpura (ITP) amongst those receiving mRNA vaccines (including BNT162b2 and ChAdOx1)6,7,8,9. ITP associated with first dose ChAdOx1 vaccine has previously been reported using the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II) surveillance platform10,11,12 and based on our analysis and other evidence, ITP was added to the product information as a side-effect of ChAdOx1 on 6 October 202113. There have also been reports of adverse events following first dose ChAdOx1 vaccination including vaccine induced immune thrombotic thrombocytopenia (VITT) and thrombosis with thrombocytopenia syndrome (TTS) resulting in venous or arterial thrombosis, including cerebral venous sinus thrombosis (CVST), and thrombocytopenia7,8,9.
There is the need for evidence on the safety of the second dose COVID-19 vaccines and, in particular, any association with ITP, venous thromboembolic (including CVST), arterial thromboembolic and hemorrhagic events described in case reports7. To investigate this, we used a national prospective COVID-19 surveillance cohort in Scotland, which consisted of linked databases containing individual-level patient data relating to vaccination status, virological real-time reverse transcriptase polymerase chain reaction (RT-PCR) COVID-19, laboratory tests, and clinical and mortality records.
Results
Between 8 December 2020 and 7 November 2021, 2.0 million people (39,682 females and 33,588 males under 30 years of age) were vaccinated with a second dose of ChAdOx1 and 1.6 million people (294,511 females and 276,476 males under 30 years of age) with a second dose of BNT162b2 (Extended Data Fig. 1).
Thrombocytopenia
No increased risk of thrombocytopenia was found following second dose ChAdOx1 (0–27 days incidence rate ratio (IRR) = 0.84; 95% CI 0.62–1.13) (Table 1) or BNT162b2 vaccine (0–27 days IRR = 0.95; 95% CI 0.65–1.38) during any of the post-vaccination time periods (Table 2).
Idiopathic thrombocytopenic purpura
The incidence of ITP post-second dose ChAdOx1 was 0.59 per 100,000 doses (95% CI 0.37–0.89). The IRR 14–20 days post-vaccination for second dose ChAdOx1 was 2.14 (95% CI 0.90–5.08) (Table 1). The 0–27 days IRR was 1.15 (95% CI 0.61–2.15).
For patients who had ITP post-second dose ChAdOx1, five of thirteen patients (39%) were hospitalized with a median length of stay of one day (IQR 1–2 days). In comparison to the unvaccinated, these post-second dose patients were equally likely to have been hospitalized at the time of the event (39% vs. 25%, p = 0.493) and more likely to have at least one clinical risk condition (75% vs. 37%, p = 0.035). There were no deaths reported after post-second dose ITP. The sex and age distributions were similar for ITP after second dose ChAdOx1 vaccination compared to those who were unvaccinated: females 58% vs. 53%, p = 0.961) with a median age of 63 years (interquartile range (IQR) 53–76) vs. 54 years old (IQR 35–71).
All patients with a post-second dose vaccination ITP had platelet counts available, all 13 (100%) had counts below 150,000/µl and nine (69%) had counts below 100,000/µl. Sixty-five percent of post-second dose ChAdOx1 ITP cases had prior prescriptions that could induce drug dependent thrombocytopenia or ITP, compared with 51% of those who were unvaccinated at the time of their ITP event. Twenty four percent of ITP cases were prescribed ITP therapies by general practitioners in the community after vaccination with ChAdOx1.
No positive association was found between the BNT162b2 vaccine and ITP (Table 2) at any time-period (0–27 days: 1.68; 95% CI 0.80–3.52).
Venous thromboembolic events
No increase in the IRR of venous thromboembolic events was found during any of the post-second dose vaccination time periods analyzed for ChAdOx1 with a 0–27 day IRR = 0.83 (95% CI 0.69–1.00) (Table 3) or BNT162b2 with a 0–27 day IRR = 0.80; 95% CI 0.69–0.94 (Table 4).
Cerebral venous sinus thrombosis
When focusing on CVST after second dose ChAdOx1, the incidence of CVST was 0.05 per 100,000 doses (95% CI 0.01–0.19). The IRR 7–13 days post-vaccination was 4.06 (95% CI 0.94–17.51) (Table 3). For second dose ChAdOx1 vaccination associated CVST, all individuals were female, their mean age was 62 (IQR 58–65) and all had a hospital admission recorded at the time of the event with a median length of stay of 8 days (IQR 4–11 days). No individuals with a second dose vaccination CVST event died. Platelet count results (available for all individuals) were >150,000/µL.
No increased risk of CVST events was found for the BNT162b2 vaccine during any of the post-vaccination time periods (0–27 days IRR = 0.00) (Table 4).
Deep vein thrombosis and pulmonary embolism
An a priori subgroup analysis of deep vein thrombosis (DVT) and pulmonary embolism (PE) found no clear association with ChAdOx1 (Tables 3, 5) or BNT162b2 vaccination (Tables 4, 6) at any time post-second dose.
Arterial thromboembolic events
The IRR for second dose ChAdOx1 and arterial thromboembolic events 0–27 days post-vaccination was 0.92 (95% CI 0.87–0.97) (Table 7). There was also no 0–27 days post-vaccination increased risk of arterial thromboembolic events associated with BNT162b2 vaccination (IRR = 0.86; 95% CI 0.79–0.93) or during any of the post-vaccination time periods analyzed for either vaccine (Tables 7, 8).
Hemorrhagic events
We found no clear evidence of associations between ChAdOx1 (0–27 days IRR = 0.83; 95% CI 0.69–1.00) and hemorrhagic events (Table 1). There was no 0–27 day post-vaccination increased risk of hemorrhagic events associated with BNT162b2 vaccination (IRR = 0.80; 95% CI 0.61–1.04) or during any of the post-vaccination time periods analyzed for either vaccine (Tables 7, 8).
Discussion
This population-based analysis has found borderline increased risks of ITP and CVST following second dose ChAdOx1. There was no evidence of an association with any of the adverse events of interest after second dose BNT162b2. Several IRRs suggest a statistically significantly protective effect for ChAdOx1 and BNT262b2 vaccines. This includes VTE, PE, arterial thromboembolic, and hemorrhagic events for ChAdOx1; and VTE, DVT, PE, and arterial thromboembolic events for BNT262b2. This is most likely due to well-vaccinee bias, as people who are unwell defer vaccination. Alternatively, this might conceivably be a by-product of vaccine protection against COVID-19, if COVID-19 is a cause of these events14.
To our knowledge, this is the one of the first real-world contemporaneous studies identifying all individuals vaccinated with a second dose of COVID-19 vaccine within a national population assessing a range of hematological and vascular events. Previous studies have observed increased rates of thrombocytopenia, venous thromboembolic events, including CVST and intracerebral hemorrhage, after a first dose14,15,16. One French study found no significant increased risk in those over 75 years 14 days following first or second dose of BNT162b2 vaccine17. The incidence of ITP post 0–28 day second dose ChAdOx1 was lower than post first dose (0.59 vs. 1.13 per 100,000 doses)12. Post-second dose ChAdOx1 incident CVST events were similar (0.05 per 100,000 doses vs. 0.44 to 0.59 incident CVST cases per million people)16.
Our study has several strengths, including our ability to rapidly access and analyze data on vaccination status and medical and death records from linked national databases10, 18. This study is therefore less susceptible to recall or misclassification bias than studies of samples of the population. We believe our findings are likely to have generalizability across countries using these vaccines as part of national vaccination programs (with the proviso that further work is needed to determine if low adverse events rates in low- and middle-income countries is a result of underreporting or different susceptibility).
Study limitations include our reliance on diagnostic coding from the electronic health record (EHR) with possible under ascertainment of CVST19. Major diagnoses are likely to be recorded in a timely way, be accurate and have high completeness20. Information on the PPV and accuracy of timing for Read codes was not available. If low PPV occurs, it is likely to have biased results towards the null. Also, the EAVE II platform is a national public health surveillance platform that was established at the request of the Scottish Government to help inform the public health response to the pandemic. As the policy aim was for national coverage, it was not feasible to obtain individual patient consent. This therefore restricted our ability to interrogate and report on more detailed aspects of the clinical record. Due to delays in reporting of coded data, we did not use hospital coded data for our primary analysis21. Although there was a priori evidence that ITP might be clustered between 14 to 20 and 21 to 27 days (with incidence rate ratios of 7.81 and 14.07 respectively12) and unusual venous thrombosis events take place 5 to 16 day post ChAdOx17, low numbers distributed across the time periods mean that this distribution could be a chance effect. Further investigation in studies with greater power is therefore warranted. SARS-CoV-2 infections were included in the analysis as time-varying confounders. It was also not possible to determine whether VITT/TTS was the underlying cause of CVST as we do not have access to anti-PF4 antibody data nor are we able to access platelet counts from the hospital data (all CVST were hospitalized for at least a week at the time of the event). The timing of reported ITP side-effects (on 9 June 2021)12 and age restrictions for ChAdOx1 (restricted to >30 years old on 7 April 2021 and >40 years old on 7 May 2021) during the period when second doses were being given may have led to the exclusion of higher risk individuals from second dose ChAdOx1. Alternatively, those experiencing first dose adverse effects may not have proceeded with a second dose of ChAdOx1. Further work is required to understand if these reports or restrictions, or first dose adverse events had any role in the lower incidence we observed for second dose adverse events. We only included cases who received both vaccine doses. This will potentially oversample events occurring post-dose two, and hence may have tended to inflate the IRRs. We also assumed that the events of interest did not cause early deaths and thus censored the observation period. There were no deaths 0–27 days post-second dose ITP and CVST. The scenario of CVST being observed in the clearance period prior to second dose ChAdOx1 is possible. This is because CVST during the clearance period will not be due to the first dose ChAdOx1 vaccination (due to the gap in vaccination doses) and vaccination is recommended for individuals, even with past clotting episodes, as they remain at risk of COVID-19 disease22.
The increased risk found means further monitoring of CVST and ITP amongst those receiving second doses of adenovirus-based SARS-CoV-2 vaccines is warranted. Replication of our study in other countries is needed to confirm our results. We plan to update our analysis for booster doses and mRNA-1273 vaccines.
In conclusion, second dose BNT162b2 vaccination was not found to be associated with increased risks of thromboembolic, hemorrhagic or thrombocytopenic events. Second dose ChAdOx1 vaccination was associated with borderline increased risk of ITP and CVST events, however these events were rare and usually short-lived, thus providing reassurance about the safety of this vaccine. Further analyses including more cases are required to determine more precisely the risk profile for ITP and CVST after a second dose of ChAdOx1 vaccine.
Methods
Ethical permission for this study was granted from South-East Scotland Research Ethics Committee 02 [12/SS/0201]. The Public Benefit and Privacy Panel Committee of Public Health Scotland approved the linkage and analysis of the de-identified datasets for this project [1920-0279].
Study setting and population
The National Health Service in Scotland (NHS Scotland) provides comprehensive health services that are free at the point-of-care for all residents. Our base population for this study was 3.6 m (78.2% of eligible population) registered with a general medical practice (GP) in Scotland who had received a second dose of either ChAdOx1 or BNT162b2 vaccine. The baseline period comprised the remaining time from 1 December 2020 to 7 November 2021 or date of death, whichever came first, excluding clearance periods and risk periods (number of person-days).
Study design
Following a pre-specified analysis plan, we carried out a self-controlled case series (SCCS) using the EAVE II prospective cohort. The GP clinical data contained records of the specified safety outcomes from 1 September 2019 through to 7 November 2021. A case was defined as anyone with a clinician recorded incident event of thrombocytopenia, venous thromboembolism, arterial thromboembolism or hemorrhage following the start of the COVID-19 vaccination program in Scotland. An incident case was defined as the first event in the 28-day period after a second dose.
Data sources
Almost all residents in Scotland are registered with a GP and have a unique Community Health Index (CHI) number used by NHS Scotland. We used the CHI number to deterministically link all datasets with vaccination records in Public Health Scotland (PHS) (Extended Data Fig. 2). Vaccination information was extracted from the GP records and the Turas Vaccination Management Tool (TVMT) system; together these captured all vaccination records, including those vaccinated in general practices, community vaccination hubs and other settings such as care homes and hospitals in Scotland23. Further details on the data sources used in this study are available in a published project protocol18.
Exposure definition
We studied second doses of the BNT162b224 and ChAdOx1 vaccines25. We did not report Moderna second dose results due to the small number given in Scotland at the time of analysis. An individual was defined as exposed to each vaccine if they received their second dose vaccine from 11 January 2021.
Outcomes
The outcomes analyzed in this study were one or more of: (i) ITP and other thrombocytopenic; (ii) venous or arterial thromboembolic; or (iii) hemorrhagic (excluding traumatic, gastrointestinal and genitourinary bleeding) events. We undertook additional a priori subgroup analyses focused on ITP, CVST, deep vein thrombosis (DVT) and pulmonary embolism (PE)26. Read codes (Version 2) were used to determine adverse incident events recorded in the primary care EHR data (Supplementary Table 1), which were then followed up for whether hospitalizations or deaths occurred in the linked Rapid Preliminary Inpatient Data (RAPID) dataset and National Records Scotland for hospitalization and mortality outcomes, respectively. The code lists used in this study were drawn up by the EAVE II clinical team and validated by experts in neurology and hematology. Incidence was the outcome of interest during the post vaccine exposure periods after vaccination; the primary incidence covered the 28 day period post-second dose and is expressed as number of events per 100,000 vaccinated.
Statistical analysis
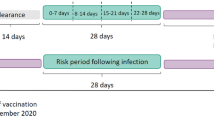
Incidence rates of the outcomes in the different exposure periods were calculated as the number of events in the exposure period divided by the person time in the exposure period. The SCCS models were fitted using a conditional Poisson regression model with an offset for the length of the risk period27. Incidence rate ratios (IRR) for each outcome of interest comparing risk periods relative to baseline periods (Fig. 1) were estimated by the SCCS model adjusting for week number as time-varying covariates. Exposure terms for each vaccine were included in the same model.
People were eligible for each study cohort if they had received at least two vaccine doses (either ChAdOx1 and BNT162b2), were at least 16 years old, and had a recording in the GP electronic health record (EHR) for the outcome of interest from the beginning of patient follow-up (i.e., 1 December 2020) to the earliest of the end of the study period (i.e., 7 November 2021) or when they died. If a patient had more than one adverse event of interest, only the first one was used.
The exposure variable was a second dose of the ChAdOx1 or BNT162b2 vaccines. We defined the exposure risk intervals as 0–6, 7–13, 14–20, and 21–27 days after the date of second vaccine dose. A 14-day clearance period was used prior to the exposure date (Fig. 1). To avoid overlapping risk periods, we assumed that later exposures (second dose) took precedence over the earlier one (first dose), except for the 14-day clearance period for the second dose.
Platelet counts for individuals were extracted from the GP EHR data. We extracted prescriptions related to ITP therapy and which may cause thrombocytopenia (Supplementary Table 2). These were used to determine duration of disease.
Analyses were carried out by one statistician (J.P.) and independently checked by an additional statistician (C.R.). All analyses were carried out with R software, version 3.6.128.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
A data-dictionary covering the datasets used in this study can be found at https://github.com/EAVE-II/EAVE-II-data-dictionary. Patient-level data underlying this article is controlled and cannot be shared publicly due to data protection and confidentiality requirements. Data can be made available to approved researchers for analysis after securing relevant permissions from the data holders via the Public Benefit and Privacy Panel (PBPP). Enquiries regarding data availability should be directed to phs.edris@phs.scot. The normal expectation is that the application process will be concluded within 30 working days of submission, not including any time that has elapsed while waiting for a response.
Code availability
All code used in this study is publicly available at https://github.com/EAVE-II/Covid-vaccine-safety-haemo.
References
Lopez Bernal, J. et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 373, n1088 (2021).
Vasileiou, E. et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 397, 1646–1657 (2021).
Simpson, C. R. et al. Evaluating the effectiveness, impact and safety of live attenuated and seasonal inactivated influenza vaccination: protocol for the Seasonal Influenza Vaccination Effectiveness II (SIVE II) study. BMJ Open 7, e014200 (2017).
Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 384, 403–416 (2021).
GOV.UK. COVID-19 mRNA vaccine BNT162b2 product information sheet, <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/967861/Temporary_Authorisation_HCP_Information_BNT162_7_0_UK_.pdf (2020).
Australian Government Department of Health. COVID-19 vaccine weekly safety report—08-07-2021, https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-08-07-2021 (2021).
Greinacher, A. et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 384, 2092–2101 (2021).
Schultz, N. H. et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384, 2124–2130 (2021).
Scully, M. et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384, 2202–2211 (2021).
Simpson, C. R. et al. The UK’s pandemic influenza research portfolio: a model for future research on emerging infections. Lancet Infect. Dis. 19, e295–e300 (2019).
Simpson, C. R. et al. The UK hibernated pandemic influenza research portfolio: triggered for COVID-19. Lancet Infect. Dis. 20, 767–769 (2020).
Simpson, C. R. et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 27, 1290–1297 (2021).
European Medicines Agency. COVID-19 vaccine safety update VAXZEVRIA AstraZeneca AB, https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-6-october-2021_en.pdf (2021).
Hippisley-Cox, J. et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 374, n1931 (2021).
Pottegård, A. et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 373, n1114 (2021).
Kerr, S. et al. First dose ChAdOx1 and BNT162b2 COVID-19 vaccinations and cerebral venous sinus thrombosis: A pooled self-controlled case series study of 11.6 million individuals in England, Scotland, and Wales. PLOS Med. 19, e1003927 (2022).
Jabagi, M. J. et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA 327, 80–82 (2022).
Simpson, C. R. et al. Early pandemic evaluation and enhanced surveillance of COVID-19 (EAVE II): protocol for an observational study using linked Scottish national data. BMJ Open 10, e039097 (2020).
McKeigue, P. M. et al. Association of cerebral venous thrombosis with recent COVID-19 vaccination: case-crossover study using ascertainment through neuroimaging in Scotland. BMC Infect. Dis. 21, 1275 (2021).
Royal College of General Practitioners. GP Summary Component of the NHS Summary Care Record. https://www.scimp.scot.nhs.uk/wp-content/uploads/documents/RCGP_SCR__Final_dated.pdf (2007).
Public Health Scotland. SMR Timeliness. https://www.isdscotland.org/products-and-Services/Data-Support-and-Monitoring/SMR-Timeliness/ (2021).
UK Government. COVID-19: the green book, chapter 14a. https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a (2022).
National Services Scotland. National Data Catalogue. Rapid Preliminary Inpatient Data (RAPID), https://www.ndc.scot.nhs.uk/National-Datasets/data.asp?SubID=37 (2020).
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Paul Ehrlich Institut. COVID-19 Vaccine AstraZeneca—Safety Assessment Result: The Vaccine is Safe and Effective in the Fight against COVID-19, https://www.pei.de/EN/newsroom/hp-news/2021/210319-covid-19-vaccine-astrazeneca-safety-assessment-result-vaccine-safe-and-effective.html (2021).
Glanz, J. M. et al. Four different study designs to evaluate vaccine safety were equally validated with contrasting limitations. J. Clin. Epidemiol. 59, 808–818 (2006).
R Code Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2020).
Acknowledgements
EAVE II is funded by the Medical Research Council (MR/R008345/1) with the support of BREATHE—The Health Data Research Hub for Respiratory Health [MC_PC_19004], which is funded through the UK Research and Innovation Industrial Strategy Challenge Fund and delivered through Health Data Research UK. This research is part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (grant ref MC_PC_20058). Additional support has been provided through Public Health Scotland and Scottish Government DG Health and Social Care. FDRH acknowledges part support as Director of the NIHR Applied Research Collaboration (ARC) Oxford Thames Valley, and Theme Lead of the NIHR OUH BRC. We thank Dave Kelly from Albasoft Ltd for his support with making primary care data available and Frances Burns, Mathhew Thankur, Wendy Inglis-Humphrey, Vicky Hammersley, Dom Balharry and Laura Brook, Morag Edwards and Laura Gonzalez Rienda for their support with project management and administration. We thank the EAVE II Public Advisory Group. Our thanks to Prof. Jenni Quint, Prof. Rustam Al-Shahi Salman and Dr. Quentin Hill for their help with reviewing code lists.
Author information
Authors and Affiliations
Contributions
C.R.S., A.S., C.R., L.R., S.V.K. conceived this study. C.R.S., A.S., S.K., S.V.K., and C.R. commented on the paper, oversaw the analysis and edited the final paper. C.R.S., A.S., C.R., and S.V.K. led the writing of the paper. C.R. and J.P. cleaned and analysed the data. All authors contributed to the study design. C.R.S., S.K., S.V.K., C.M., L.D.R., J.P., S.J.S., I.R., R.S.M.T., S.d.L., F.D.R.H., A.A., R.A.L., C.R., A.S. contributed to drafting the paper and revised the paper for important intellectual content. All authors gave final approval of the version to be published.
Corresponding authors
Ethics declarations
Competing interests
A.S. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group and its Standing Committee on Pandemics; the UK Government’s New and Emerging Respiratory Virus Threats (NERVTAG) Risk Stratification Subgroup; and was a member of AstraZeneca’s COVID-19 Thrombocytopenia Taskforce. All roles are remunerated to A.S. or his institution. C.R.S. declares funding from the MRC, NIHR, CSO, and New Zealand Ministry for Business, Innovation and Employment and Health Research Council during the conduct of this study. SVK is co-chair of the Scottish Government’s Expert Reference Group on COVID-19 and ethnicity, is a member of the Scientific Advisory Group on Emergencies (SAGE) subgroup on ethnicity and acknowledges funding from a NRS Senior Clinical Fellowship (SCAF/15/02), MRC (MC_UU_00022/2) and CSO (SPHSU17). C.R. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group, SPI-M, MHRA Vaccine Benefit and Risk Working Group. All other authors report no competing interests.
Peer review
Peer review information
Nature Communications thanks Paddy Farrington and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simpson, C.R., Kerr, S., Katikireddi, S.V. et al. Second-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Commun 13, 4800 (2022). https://doi.org/10.1038/s41467-022-32264-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32264-6
This article is cited by
-
Herpes zoster vaccine safety in the Aotearoa New Zealand population: a self-controlled case series study
Nature Communications (2023)
-
Risk of death following COVID-19 vaccination or positive SARS-CoV-2 test in young people in England
Nature Communications (2023)
-
Safety of mRNA COVID-19 Vaccines in Patients with Inborn Errors of Immunity: an Italian Multicentric Study
Journal of Clinical Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.