Abstract
Two-dimensional (2D) semiconducting monolayers such as transition metal dichalcogenides (TMDs) are promising channel materials to extend Moore’s Law in advanced electronics. Synthetic TMD layers from chemical vapor deposition (CVD) are scalable for fabrication but notorious for their high defect densities. Therefore, innovative endeavors on growth reaction to enhance their quality are urgently needed. Here, we report that the hydroxide W species, an extremely pure vapor phase metal precursor form, is very efficient for sulfurization, leading to about one order of magnitude lower defect density compared to those from conventional CVD methods. The field-effect transistor (FET) devices based on the proposed growth reach a peak electron mobility ~200 cm2/Vs (~800 cm2/Vs) at room temperature (15 K), comparable to those from exfoliated flakes. The FET device with a channel length of 100 nm displays a high on-state current of ~400 µA/µm, encouraging the industrialization of 2D materials.
Similar content being viewed by others
Introduction
For high-performance electronics in advanced technology nodes, the thickness of transistor channels needs to be as thin as possible to ensure sufficient gate control with the gate length scaling1. Therefore, the transition metal dichalcogenide (TMD) monolayer around 1 nm thick has been considered as a promising channel material for future nodes2,3. The extraordinary properties of atomically thin 2D TMDs are profoundly influenced by the presence of imperfections4,5. It has been widely accepted that the electrical quality of mechanically exfoliated TMD monolayer flakes is superior to those from synthetic processes4,6; however, the non-scalability impedes their practical applications. TMD monolayers from scalable synthetic approaches like chemical vapor deposition (CVD) method usually contain abundant of imperfections including grain boundaries, point defects and strain5,6. Recently, oxygen substituted sulfur vacancy (OS) has been demonstrated as the dominant point defect in CVD samples by scanning tunneling microscope (STM) measurements7. Although some efforts have been made to reduce the point defects, for example, thiol chemistry8 and chalcogen gas annealing9 for ‘repairing’ the chalcogen vacancy, it remains a formidable challenge to passivate other substitutional point defects. Hence, minimizing the defect density of synthetic 2D TMDs is crucial for achieving high electronic properties for practical applications.
Among the typical TMD monolayers, WS2 exhibits high mobilities and saturation velocities for both electrons and holes based on the full-band Monte Carlo analysis of the Boltzmann transport equation10,11. Conventional CVD methods can provide scalable WS2 monolayers through the direct sulfidation of either tungsten trioxide (WO3) or other oxygen-containing precursors12,13,14. Although single crystal WS2 flakes can be achieved, abundant defects are still present15,16, which in turn leads to insufficient performance for advanced electronic devices. Transport agents like water17,18,19 and oxygen20,21 have been used to enhance the volatilization of metal source for improving the growth; however, their impact on materials have seldom been explored.
In this work, we discover that hydroxide vapor phase deposition (OHVPD) enables the growth of WS2 monolayers with a significantly lower density of structural defects. The simulation results prove that W-OH bond in the hydroxide intermediates provides an energy favorable route for the sulfurization process. By analyzing the statistical photoluminescence (PL) and Raman results, OHVPD-WS2 shows superior optical quality compared to conventional CVD-WS2. STM measurements for the OHVPD-WS2 monolayers transferred onto conducting substrates present the total defect density in the order of 1012 cm−2 which is one order magnitude lower than that of CVD-WS2. As-grown low-defect-density WS2 monolayer show prominent electrical performance including high electron mobility of ~200 cm2/Vs (~800 cm2/Vs) at room temperature (15 K), and high current density of ~400 µA/µm for short channel device.
Results and discussion
Hydroxide vapor phase deposition for WS2 monolayers
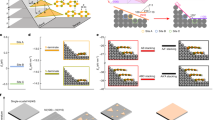
In contrast to the direct sulfidation of WO3, the OHVPD method utilizes water vapors to transport high-purity W metal to reduce the incorporation of oxygen and other impurities (such as Mo atoms in the WO3 source) into the deposited WS2 films, where the growth is schematically depicted in Fig. 1a. The W metal undergoes a few oxidation steps with water vapors to form tungsten hydroxide WO2(OH)2 at an elevated temperature22,23,24 as evidenced by the X-ray Diffraction (XRD) results (see Supplementary Note 1 and Supplementary Figs. 1 and 2 for details). The volatile WO2(OH)2 intermediates transported to the target substrates at the downstream area are reduced in the presence of sulfur vapors and H2 gases to form WS2 monolayer crystals. Although WO2(OH)2 and conventionally used WO3 may undergo similar reduction paths to form WS2, the sulfidation kinetics is distinctly different in both cases25,26. We construct molecular models to understand the difference in sulfurization of the oxygen and hydroxide bonded in WS2 (details in Supplementary Fig. 3) and the key reaction step is depicted in Fig. 1b. Our simulation shows that the W-O bond length of the bonded oxygen W-O (2.061 Å) is shorter than that of the bonded hydroxide W-OH (2.152 Å), and the kinetic barrier for breaking W-O (1.440 eV) is higher than the 0.936 eV for W-OH. Also, it needs two H atoms to form H2O for the removal of the bonded O, while only one H atom is required for the bonded hydroxide. The results indicate that the presence of W-OH bonds provides a more energy favorable route to perform the sulfidation26. Figure 1c, d shows the typical optical and atomic force microscopy (AFM) images of the OHVPD-grown WS2 monolayers. Their domain size can reach several microns and the inch-scale continuous WS2 monolayer film is also achievable (Fig. 1e). PL and Raman mapping results in Supplementary Fig. 4 present a homogeneous and high-quality OHVPD-WS2 film.
a Schematic of hydroxide vapor phase deposition (OHVPD) growth of WS2 monolayers. b Nudged elastic band (NEB) simulation of kinetic energy barriers (ΔE) for bonded OH and O dissociating from the edge of WS2. c, d Optical Image c and AFM image d of the OHVPD-WS2 monolayer. e Photo of a 2-inch OHVPD-WS2 monolayer film grown on a sapphire substrate.
High optical qualities of OHVPD-WS2 monolayers
Raman spectra of the as-grown WS2 monolayers prepared by conventional sulfidation of WO3 (labeled as CVD) and the proposed OHVPD are compared in Fig. 2a, where many characteristic modes are identified, including the in-plane vibration mode \({E}_{2{{{{{\rm{g}}}}}}}^{1}\,\left({{{{{\boldsymbol{\Gamma }}}}}}\right)\) (~354 cm−1), two defect-sensitive modes27 out-of-plane \({A}_{1{{{{{\rm{g}}}}}}}\) (~416 cm−1) and longitudinal acoustic at M point in the Brillouin zone LA(M) (~173 cm−1), and others28. To qualitatively compare the defect level, 50 Raman spectra from various single crystals were collected for each type of samples. Figure 2b shows that the statistical average of \({A}_{1{{{\rm{g}}}}}\) peak width of 5.5 cm−1 for CVD-WS2 is broader than the 4.2 cm−1 for OHVPD-WS2; meanwhile, the normalized intensity of LA(M) peak of CVD-WS2 is larger than that of OHVPD-WS2 (Fig. 2c). These results indicate that OHVPD-WS2 exhibits superior quality27,29. The room temperature photoluminescence (PL) spectra of monolayer OHVPD-WS2 typically exhibit a higher peak energy and a narrower full width at half maximum (FWHM) compared with the CVD-WS2 (see Supplementary Fig. 5 for details), indicating its superior quality30. Figure 2d shows the PL measurement for both samples at 4 K can be better deconvoluted by Gaussian functions, where the high energy mode is assigned to neutral exciton (X0), the peak with a lower energy by ~30 meV is assigned to trion (XT), and the broad peak with the lowest energy is assigned to defect-bound exciton (XD). The significantly lower intensity of XT and XD peaks for OHVPD-WS2 corroborate a lower defect density on its basal plane. We also applied OHVPD for MoS2 growth; similarly, OHVPD-MoS2 shows a higher peak energy and narrower FWHM compared with CVD-MoS2 (see Supplementary Fig. 6).
a Typical Raman spectra showing the characteristic modes of OHVPD- and CVD-WS2 monolayers excited by 532 nm wavelengths. The hollow circles and coloured lines are the experimental and Lorentzian fit curves respectively. b, c Statistic distribution of out-of-plane mode A1g Raman peak width and normalized intensity of longitudinal acoustic at M point in the Brillouin zone LA(M) Raman peak for OHVPD- and CVD-WS2 monolayers. The dashed lines represent the normal distribution curves. d Low-temperature PL spectra of OHVPD- and CVD- WS2 monolayers at 4 K. The solid lines and dashed ones are the experimental and fitted peaks respectively. The fitted peaks can be assigned to neutral exciton (X0), trion (XT), and defect-bound exciton (XD).
Defect analysis of WS2 monolayers by STM
To investigate the structural defects in as-grown WS2 monolayers, we perform scanning tunneling microscopy (STM) measurements to characterize their types and densities following Schuler et al.7. Figure 3a, b shows the STM images of CVD-WS2 and OHVPD-WS2 monolayers directly grown on conductive highly oriented pyrolytic graphite (HOPG) substrates. It is conspicuous that CVD-WS2 exhibits a larger number of structural defects than OHVPD-WS2. These typically observed defects can be categorized into a few types, including oxygen substituting upper (OS(top)) and bottom sulfur (OS(bottom)), molybdenum substituting tungsten (MoW), other negatively charged defects (NCD), and positively charged defects (PCD), as featured in the magnified STM images in Fig. 3c–g. Only a very small number of sulfur vacancies are found (Supplementary Fig. 7). To estimate the area number density of various defects, at least more than 20 STM images (40 nm by 40 nm) for each CVD-WS2 and OHVPD-WS2 are analyzed, and the estimated densities of different structural defects are shown in Fig. 3h. It is noteworthy that we do not use high-resolution scanning transmission electron microscopy for quantitative characterization of defects owing to the potential damages by electron beams during the imaging and its difficulty in distinguishing OS from S-vacancy.
a, b STM images of a CVD-WS2 (Bias Voltage (V) = 1.35 V, Current (I) = 40 pA) and b OHVPD-WS2 monolayer (V = 1.15 V, I = 30 pA). c–f STM images (V = 1.1 V, I = 30 pA) of the commonly observed point defects in CVD- and OHVPD-WS2: oxygen substituting sulfur (Os) in the c top and d bottom sulfur plane; e Mo substitutional tungsten (MoW); f Positively charged defect (PCD) and g Negatively charged defect (NCD). h, Histograms table of observed point defect density in different OHVPD- and CVD- WS2.
The OS(top) and OS(bottom) in CVD-WS2 are at a similar density level, estimated as 3.52 × 1012 cm−2 and 3.46 × 1012 cm−2, respectively. These predominant defects are significantly lower in OHVPD-WS2, 1.2 × 1012 cm−2 for OS(top) and 1.18 × 1012 cm−2 for OS(bottom). These major defects, including OS(top) and OS(bottom), do not introduce in-gap charged states, and their measured dI/dV spectrums (See Supplementary Fig. 8) are close to that in pristine WS2 regions, agreeing well with previous reports7,31,32. We also observe another neutral defect MoW with the density in the order of 1012 cm−2 in CVD-WS2, which is likely caused by the presence of Mo impurity in the W-precursors (~6.5ppm in WO3 according to the material provider). Recent DFT simulation argues that OS does not introduce in-gap charged states and only marginally affects WS2 electronic structures owing to the isoelectronic feature of S and O7. Also, the band structure of WS2 with a MoW closely resembles that of pristine WS27. Hence, we suspect that the electron mobility of WS2 may not be critically affected by these neutral OS and MoW defects in particularly at the density level lower than 1013 cm−2. We note that other recent reports have suggested that electron mobility in MoS2 may be increased with the band gap narrowing effects from the incorporation of high density charged S vacancies33 (up to 1014 cm−2) or with the screening effect by heavy oxygen doping34, where these approaches are different from the low-defect density requirement for scalable electronics. Charged defects scatter carriers through Coulomb interaction that can also lead to significant band bending and possibly a local potential change around the defects7. A recent report by Yu et al. has demonstrated the electron mobility of MoS2 monolayer can be significantly enhanced by the passivation of charged S-vacancies using thiol molecules8. Therefore, the number of charge defects should be minimized as possible. Our STM results show that the density of NCDs in CVD-WS2 (3.9 × 1010 cm−2) is almost five times of that in OHVPD-WS2 (8 × 109 cm−2). The measured dI/dV spectra for NCDs (See Supplementary Fig. 9) is consistent with the reference30 and the NCDs can be assigned as the S vacancies substituted with CH, C, or N atoms.
For device fabrication, the WS2 monolayers typically need to be transferred from the sapphire growth substrates onto target substrates. To estimate the defect levels of WS2 after the mechanical transfer processes, both samples are transferred from sapphire substrates onto HOPG substrates for STM analysis, shown as t-CVD and t-OHVPD in Fig. 3h. In this study, we adopt the polydimethylsiloxane (PDMS)-assisted transfer method (See methods)35. The overall defect density of t-OHVPD WS2 (2.5 × 1012 cm−2) is one order of magnitude lower than that in t-CVD (2.1 × 1013 cm−2). Also, the total charge impurity in t-OHVPD WS2 (2.0 × 1010 cm−2) is roughly one order of magnitude lower than t-CVD (2.5 × 1011 cm−2).
Interestingly, the density of OS(top) is much larger than OS(bottom) in t-CVD WS2, which could be due to the differences in growth substrate (sapphire rather than HOPG). Similar features have also been observed in other systems, such as WS2 on graphitized-SiC substrates7,31 (OS (bottom) > OS(top)) and oxygen-doped MoS2 on HOPG (OS(top) > OS(bottom))34. The remarkably larger OS(top) than OS(bottom) and the increase in NCDs for t-CVD samples indicate that the WS2 monolayers grown by conventional CVD are prone to damage from the subsequent transfer processes. It is suspected that the substitution of S atoms by environmental oxygen to form neutral OS(top) defects and the reaction carbon or nitrogen impurity species to form NCDs occur during the transfer. Our simulation (Supplementary Fig. 10) suggests that the removal of S atoms adjacent to the OS on the same side tends to be thermodynamically and kinetically favorable by oxidation, agreeing with the observation that further O substitution is easier in the sample with a higher density OS (i.e., CVD samples). It is anticipated that top-side S atoms exposed to the chemicals and air should be oxidized easier compared to the bottom side. Thus, the OS(top) density is larger than the OS(bottom) density after transfer as revealed by the experiments. Hence, the growth of low-defect-density TMD films and the development of mild transfer methods warrant intense efforts and should be the focus of 2D electronics.
Electrical performance of OHVPD-WS2 monolayers
For evaluating the electrical performance of the low-defect OHVPD-WS2 monolayers, we fabricated field-effect transistors with the back-gate configuration and characterized their electrical properties in a high vacuum (~ \({10}^{-6}\) Torr) using a standard four-probe technique. Figure 4a presents the four-probe conductivity \(\sigma =\,({I}_{{{{{{\rm{d}}}}}}}/\Delta V)\,\times \,({L}_{{CH}}/{W}_{{CH}})\) as a function of back-gate voltage \({V}_{{{{{{\rm{g}}}}}}}\)at various temperatures, where Id is the source-drain current; ΔV, LCH, and WCH are the voltage difference, length, and width between the two voltage probes, respectively. The OHVPD-WS2 sample exhibits at least 10X higher conductivity than the typical CVD-WS2 (in Supplementary Fig. 11). The OHVPD-WS2 shows an apparent metal–insulator transition (MIT) at around \({V}_{{{{{{\rm{g}}}}}}}\) = 60 V (corresponding to the carrier density \(n={C}_{{{{{{\rm{OX}}}}}}}{V}_{{{{{{\rm{g}}}}}}}\, \sim \,4.3\times {10}^{12}\)cm–2), where \({C}_{{{{{{\rm{OX}}}}}}}\) (\(1.15\times {10}^{-8}\) F cm–2) is the geometric gate capacitance per unit area for a 300 nm SiO2 dielectric. The MIT has been observed in the low charge-trap-state sample, i.e., as-exfoliated or vacancy-passivated samples8,36,37. Using the model proposed in reference38, we estimate the trap density (Ntr) and charge impurity density (NCI) in OHVPD-WS2 as ~3.6 × 1012 cm−2 and ~8.7 × 1010 cm−2 (see Methods and Supplementary Fig. 12), which are the lowest compared to the reported MoS2 and WS2 monolayers (see Supplementary Fig. 13 and Supplementary Table 1). The extracted NCI is around four times of the charge defects observed by STM (~2 × 1010 cm−2 for t-OHVPD in Fig. 2h), suggesting that some NCI may come from other sources such as the WS2-SiO2 interfaces and e-beam lithographic processes during the metal line patterning. We have also extracted the Ntr and NCI for CVD-WS2 as ~ 8.2 × 1012 cm−2 and ~2.2 × 1012 cm−2 (based on the results in Supplementary Fig. 12), where the trap density (from both the interface and channel defects) is comparable to the OHVPD-WS2. However, the extracted NCI is ~ 25 times higher than that in OHVPD-WS2. Note that the extracted NCI for CVD-WS2 (2.2 × 1012 cm−2) is much higher than the STM charge defect density of 2.5 × 1011 cm−2, suggesting that the WS2 with more structural defects may incur more charge impurities during the device fabrication processes. Supplementary Fig. 14 shows the typical dual-sweep transfer curves of our devices. The normalized hysteresis width is 40 mV/MV cm−1, which is on par with reported hysteresis values and indicates the presence of low border traps and interface states39.
a Four-probe conductivity as a function of Vg for OHVPD-WS2 monolayer device on the 300 nm SiOx substrate at different temperatures. Insect shows the device structure. (Scale bar: 5 μm) b Field-effect mobility as a function of temperature for OHVPD- and CVD-WS2 monolayers. c Comparison of mobility distribution for our OHVPD-WS2 results (orange), mechanical exfoliation WS2 monolayers (ME, green), and conventional CVD-WS2 (cyan) from literatures. d FET transfer curve of an OHVPD-WS2 monolayer for the short channel device (LCH = 100 nm).
We adopt the expression \({\mu }_{{{{{{\rm{FE}}}}}}}=({{{{{\rm{d}}}}}}\sigma /{{{{{\rm{d}}}}}}{V}_{{{{{{\rm{g}}}}}}})\,\times \,(1/{C}_{{{{{{\rm{OX}}}}}}})\)to extract the field-effect mobility \({\mu }_{{{{{{\rm{FE}}}}}}}\) for OHVPD-WS2 in four-probe measurements (at the carrier concentration of n = ~ 4.7 × 1012 cm−2) and the \({\mu }_{{{{{{\rm{FE}}}}}}}\) reaches 198 cm2V−1s−1 (789 cm2V−1 s−1) at room temperature (15 K) as shown in Fig. 4b, recognized as the highest value among the reported synthetic monolayer WS2. The \({\mu }_{{{{{{\rm{FE}}}}}}}\) for CVD-WS2 is significantly lower, ~ 17 cm2 V−1 s−1 (105 cm2 V−1 s−1) at room temperature (15 K). Figure 4c and Supplementary Fig. 15 compare the statistical results of electron mobility for the transistors based on OHVPD-WS2, and CVD-WS2 and exfoliated WS2 from literature38,40,41,42,43, where the electron mobility of OHVPD-WS2 is comparable to the exfoliated WS2 but obviously superior to CVD-WS2. In addition, Fig. 4d demonstrates that the short-gate-length FET based on OHVPD-WS2 monolayer can reach a maximum Ion ≈ 403 µA/μm and Ion/Ioff current ratio ~ 108 at \({V}_{{{{{{\rm{ds}}}}}}}\) = 1 V, significantly higher than that made from CVD-WS2 monolayer using the same device fabrication processes. Supplementary Fig. 16 shows the output characteristics of this short-gate-length FET, which demonstrates promising current control and saturation. These facts point out that the density of charged defects is a critical factor that limits the performance of 2D monolayers. In conclusion, the as-grown CVD films with higher defect densities are susceptible to transfer and device fabrication processes. Our proposed OHVPD approach provides a route to largely reduce the defects directly from growth, which makes synthetic 2D TMDs potential for electronic applications.
Methods
Materials synthesis and transfer
CVD-WS2 monolayer samples were synthesized on sapphire substrates by the typical CVD method with tungsten oxide (WO3, Sigma-Aldrich, 99.995%) powders and sulfur (S, Sigma-Aldrich, 99.99%) powders as precursors. Generally, the S powders, WO3 powders and sapphire substrates were placed on the upper stream, center and downstream of the furnace, respectively. After the chamber pressure went down to 1mtorr, Ar/H2 gas was purged into the chamber and kept the chamber pressure at 10 torr. The temperature was elevated to 900 °C and kept for 15 min for growth.
OHVPD-WS2 monolayer samples were achieved in a homemade 3-inch CVD system. High purity tungsten foil (W, 99.95%) and S powders were used as precursors. Moisture was delivered into the growth chamber by Ar gas flow (180 s.c.c.m.) at atmospheric pressure. The S powders were placed upstream of the tube and the temperature was controlled by an additional heating belt at 180 °C. The W foil was placed on the center of the furnace at 1050 °C while the sapphire substrates were placed downstream at 950-800 °C. During the growth, H2 gas (20 s.c.c.m.) was delivered into the chamber for the reaction. The growth was kept for 15 min and followed by natural cooling to room temperature with the same carrier gas (Ar/H2 180/20 s.c.c.m.) without the presence of water vapors. More details on the growth process and results are provided in Supplementary Note 3 and Supplementary Figs. 17–18.
The as-grown monolayer WS2 samples were transferred onto the target substrates by a polydimethylsiloxane (PDMS)-assisted transfer method35. The PDMS and hardener were mixed at a ratio of 10:1 (Sungyoung, PDMS 184 AB) in a clean beaker. The mixed solution was poured into the plastic container until 2 mm in thickness. The container was then put in a vacuum chamber for 2 h to remove the bubbles and the PDMS film was cured at 50 °C for 24 h. To perform the transfer, the PDMS film was smoothly placed on the top of as-grown WS2 on sapphire and soaked the whole stacked films into 1 M KOH(aq) for 5 min to weaken the interaction between WS2 and sapphire substrates. Next, the PDMS/WS2 film was slowly peeled off from sapphire and rinsed with DI water to remove the residue. The sample was then transferred to the target substrate and annealed at 70 °C for 20 min to remove the residue water and increase the adhesion. Finally, the PDMS film was slowly peeled off to get a clean WS2 sample on the target substrate.
Device fabrication and electrical measurements
For the short channel device, the monolayer WS2 films were transferred onto the commercial SiNx film (thickness = 100 nm) on p++-Si substrates as back gated field-effect transistors (FET). Then Helium-ion beam lithography (ORION NanoFab, Zeiss) with the ion-beam-resist, PMMA (Allresist, AR-P 672-Serie, spin-coated with 4000 rpm for 40 s and baked at 180 °C for 3 min.) was used to pattern the source/drain (S/D) metal contacts, which defined the channel length (LCH) from 100 nm to 400 nm and was developed by using 1:3 mixture of 4-methyl-2-pentanone (MIBK) and isopropyl alcohol (IPA). For the contact metals, 20 nm of Bi followed by 15 nm of gold (Au) encapsulating layer were deposited on the WS2 using e-gun evaporation at a high vacuum chamber (~1 × 10−7 torr). The metal lift-off process was carried out in warm acetone (60 oC) and then rinsed by IPA. Finally, the WS2 electrical characteristics were measured in a vacuum system (10−5–10−6 Torr) in a Lakeshore probe station using a Keithley 4200-SCS parameter analyzer.
For the four-terminal device measurement, the heavily doped Si substrate was used as a back gate and the 300 nm SiO2 was used as a gating dielectric. The devices were patterned using PMMA masks and electron beam lithography. 5 nm Al and 65 nm Au electrodes were deposited using e-beam evaporation. The electrical characterization of monolayer WS2 FETs was carried out under vacuum (<10−4 Torr) in a JANIS CCS350 closed-cycle refrigerator (10–500 K). Our four-terminal measurements were performed from 15 to 300 K, starting from the lowest temperature. The gate and drain biases are provided by the Keithley Model K-6430 Sub-Femtoamp Remote Source Meters, which are also used to monitor the leakage current and drain current. And the Keithley Model K-2182 is used to sense the voltage difference as a voltmeter. In our structure, the voltage sensed probes minimally affect the current flow in the channel material and thus act like perfect voltmeters.
Estimation of the Ntr and NCI in monolayer WS2
The theoretical model we used was proposed by Wang’s group38. In brief, the band mobility (the mobility for free carriers) of monolayer WS2 was calculated according to Matthiessen’s rule, which is expressed as \({\mu }_{0}{(n,T)}^{-1}={\mu }_{{ph}}{(T)}^{-1}+{\mu }_{{CI}}{(n,T)}^{-1}\). Here we ignore phonon-limited mobility as a result that theoretical phonon-limited mobility is much higher than the experimental values over the entire temperature range. The CI-limited electron mobility μCI can be defied by
where e, ℏ, kB, T and f (E) are the electron charge quantum, the Planck constant divided by 2π, the Boltzmann constant, the temperature and the Fermi-Dirac distribution, respectively. Moreover, the experimental mobility μ is not exactly equal to the band mobility μ0, due to the charge traps, which reduce the free carrier population and is responsible for the MIT. The density of conducting electrons in the extended states can be calculated by
Finally, we can calculate the experimental mobility μ by
STM measurement
Our STM experiments were conducted in the commercial ultra-high vacuum LT-STM system (CreaTec) with a base pressure of 1.0 × 10−10 mBar. All STM images were acquired at 77 K in the constant-current mode by using a chemically etched tungsten tip and the bias voltages refer to the sample with respect to the STM tip. Before measurement, the samples were annealed at ~550 K for over 3 h to remove possible adsorbates. Note that such a low annealing temperature is used to avoid the transition to sulfur vacancies31. The dI/dV spectra were acquired at 5.3 K by using a lock-in technique with the bias modulation of 20 meV at 717.3 Hz.
Kinetic simulation of sulfurization process
It is important to understand the role of H2O in the formation of WS2 monolayer during growth. Here, we applied nudged elastic band (NEB) method44,45,46 to model the energy barrier of the sulfurization process with and without H2O. Without H2O, the precursor used in conventional CVD is WO3. Therefore, there are W-O bonds at the edges (or the growth-front) of CVD-WS2. On the other hand, the edge of OHVPD-WS2 possibly contains W-OH bonds due to the H2O supply. A monolayer of 5 × 4 × 1 supercell of WS2 with the zigzag edge is used. The vacuum of 15 and 20 Å along y and z directions are applied to avoid interaction between their replica images because of periodic conditions. A gamma-centered 1 × 1 × 1 k-mesh is employed for ion relaxation and NEB calculation. Supplementary Fig. 3a shows the kinetic energy barriers of transformation of W-OH to W-SH group. The three major barriers are 0.41, 0.94 and 0.71 eV, respectively. The first barrier is 0.41 eV, which is related to one H atom from H2S molecular to S atom near O atom at the WS2 edge. The second barrier is 0.94 eV, which corresponds to the H on S transfer to O atom and then the formation of H2O. The third barrier is 0.71 eV, relating to the detachment of H2O from the WS2 edge. Supplementary Fig. 3b shows the kinetics of transformation of W-O to W-S group. There are also three barriers. The first barrier is 0.17 eV, which is related to the transfer of one H from H2S molecular to one S atom on WS2 edge. However, the second and third barriers are 1.44 and 1.39 eV, respectively, which are much larger than the case of transformation from W-OH to W-SH. The second barrier is related to the two H atoms moving to O at the edge. The third barrier corresponds to the leaving of H2O from edge of WS2. The difference of energy barriers for two different scenarios indicates W-O bond is much more difficult in transforming to W-S group compared to the W-OH to W-SH bond.
Simulation of OS defect formation
It has been proposed that the reaction of O2 with WS2 is one origin of OS defect formation47. The formation energy for one OS defect generation in pristine WS2 can be described as the following equation:
Where \({E}^{{form}}\)is the formation energy of the OS in WS2, \({E}^{{def}}\) and \({E}^{{pristine}}\)are total energies of defective and pristine WS2, \({{\mu }}_{{{{{{\rm{O}}}}}}}\)is the chemical potential of oxygen, \({E}^{{SO}2}\) is the total energy of SO2 gas molecular, n and m are the number of substitutional O and formed SO2. A monolayer of 6 × 6 × 1 supercell of WS2 is used to investigate the oxidation process. A vacuum of 20 Å is applied to avoid interaction between their replica images because of periodic conditions. A gamma centered 2 × 2 × 1 k-mesh is employed for ion relaxation and NEB calculation.
Supplementary Fig. 10a shows the formation energies of OS in WS2. OS defects are thermodynamically favorable under O-rich conditions. Moreover, the more OS defects in WS2, the much lower the formation energy. This indicates once more OS are formed, it is much easier to further have O substitution in the WS2 system from a thermal dynamics point of view.
It is also vital to investigate the kinetics of O2 dissociation and the formation of OS in WS2. The NEB method was applied to model the energy barrier for the two steps. Different from the previously proposed two steps formation of OS in MoS248, where the S vacancy on MoS2 forms firstly, and then the O occupies the S vacancy position. Supplementary Fig. 10b shows the kinetics and energetics of the O2 dissociation and formation of OS in the pristine WS2. There are two major barriers. The first one is O2 molecular absorption on top of the S atom in the WS2 plane. The energy barrier is 1.20 eV, which is for the breaking of O–O bond. One O atom gets close to one W atom, and the other connects with one S atom. Such configuration is about 1.5 eV lower than the initial configuration. The second barrier is 1.075 eV for the break of one W–S bond, leading to the S atom lifted up. In the end, the SO group is out of the plane and can be taken by other O2 or H2O molecules. The two barriers are over 1.0 eV, suggesting the oxidation process is extremely slow. Supplementary Fig. 10c–d shows the kinetics and energetics of the further O substitution step in the WS2 with one OS defect at the flipside near OS and the same side near OS. Both barriers are lowered compared with Supplementary Fig. 10b, indicating the presence of OS defects will accelerate the oxidation process.
Data availability
Relevant data supporting the key findings of this study are available within the article and the Supplementary Information file. All raw data generated during the current study are available from the corresponding authors upon request.
Code availability
The code used to plot the data is available from the corresponding authors upon request.
References
Li, M.-Y., Su, S.-K., Wong, H.-S. P. & Li, L.-J. How 2D semiconductors could extend Moore’s law. Nature 567, 169–170 (2019).
Ferain, I., Colinge, C. A. & Colinge, J.-P. Multigate transistors as the future of classical metal–oxide–semiconductor field-effect transistors. Nature 479, 310–316 (2011).
Chhowalla, M., Jena, D. & Zhang, H. Two-dimensional semiconductors for transistors. Nat. Rev. Mater. 1, 16052 (2016).
Hong, J. et al. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. 6, 6293 (2015).
Chu, Z. et al. Unveiling defect-mediated carrier dynamics in monolayer semiconductors by spatiotemporal microwave imaging. Proc. Natl Acad. Sci. USA 117, 13908 (2020).
Rhodes, D., Chae, S. H., Ribeiro-Palau, R. & Hone, J. Disorder in van der Waals heterostructures of 2D materials. Nat. Mater. 18, 541–549 (2019).
Schuler, B. et al. How substitutional point defects in two-dimensional WS2 induce charge localization, spin–orbit splitting, and strain. ACS Nano 13, 10520–10534 (2019).
Yu, Z. et al. Towards intrinsic charge transport in monolayer molybdenum disulfide by defect and interface engineering. Nat. Commun. 5, 5290 (2014).
Lin, Y.-C. et al. Realizing large-scale, electronic-grade two-dimensional semiconductors. ACS Nano 12, 965–975 (2018).
Liu, L., Kumar, S. B., Ouyang, Y. & Guo, J. Performance limits of monolayer transition metal dichalcogenide transistors. IEEE Trans. Electron Devices 58, 3042–3047 (2011).
Jin, Z., Li, X., Mullen, J. T. & Kim, K. W. Intrinsic transport properties of electrons and holes in monolayer transition-metal dichalcogenides. Phys. Rev. B 90, 045422 (2014).
Lan, C., Li, C., Ho, J. C. & Liu, Y. 2D WS2: from vapor phase synthesis to device applications. Adv. Electron. Mater. n/a, 2000688, https://doi.org/10.1002/aelm.202000688 (2020).
Lee, Y.-H. et al. Synthesis and transfer of single-layer transition metal disulfides on diverse surfaces. Nano Lett. 13, 1852–1857 (2013).
Briggs, N. et al. A roadmap for electronic grade 2D materials. 2D Mater. 6, 022001 (2019).
Rosenberger, M. R., Chuang, H.-J., McCreary, K. M., Li, C. H. & Jonker, B. T. Electrical characterization of discrete defects and impact of defect density on photoluminescence in monolayer WS2. ACS Nano 12, 1793–1800 (2018).
Chubarov, M. et al. Wafer-scale epitaxial growth of unidirectional WS2 monolayers on sapphire. ACS Nano 15, 2532–2541 (2021).
Kastl, C. et al. The important role of water in growth of monolayer transition metal dichalcogenides. 2D Mater. 4, 021024 (2017).
Zhao, Y. & Jin, S. Controllable water vapor assisted chemical vapor transport synthesis of WS2–MoS2 heterostructure. ACS Mater. Lett. 2, 42–48 (2020).
Cohen, A. et al. Growth-etch metal–organic chemical vapor deposition approach of WS2 atomic layers. ACS Nano 15, 526–538 (2021).
Chen, W. et al. Oxygen-assisted chemical vapor deposition growth of large single-crystal and high-quality monolayer MoS2. J. Am. Chem. Soc. 137, 15632–15635 (2015).
Yu, H. et al. Wafer-scale growth and transfer of highly-oriented monolayer MoS2 continuous films. ACS Nano 11, 12001–12007 (2017).
Kilpatrick, M. & Lott, S. K. Reaction of flowing steam with refractory metals: III. Tungsten (1000–1700 C). J. Electrochem. Soc. 113, 17 (1966).
Sahoo, P. K., Memaran, S., Xin, Y., Balicas, L. & Gutiérrez, H. R. One-pot growth of two-dimensional lateral heterostructures via sequential edge-epitaxy. Nature 553, 63–67 (2018).
Belton, G. R. & McCarron, R. L. The volatilization of tungsten in the presence of water vapor. J. Phys. Chem. 68, 1852–1856 (1964).
Van der Vlies, A., Kishan, G., Niemantsverdriet, J., Prins, R. & Weber, T. Basic reaction steps in the sulfidation of crystalline tungsten oxides. J. Phys. Chem. B 106, 3449–3457 (2002).
van der Vlies, A. J., Prins, R. & Weber, T. Chemical principles of the sulfidation of tungsten oxides. J. Phys. Chem. B 106, 9277–9285 (2002).
Li, J. et al. Atypical defect-mediated photoluminescence and resonance raman spectroscopy of monolayer WS2. J. Phys. Chem. C. 123, 3900–3907 (2019).
Shi, W. et al. Raman and photoluminescence spectra of two-dimensional nanocrystallites of monolayer WS 2 and WSe 2. 2D Mater. 3, 025016 (2016).
Lee, C. et al. Unveiling defect-related raman mode of monolayer WS2 via tip-enhanced resonance raman scattering. ACS Nano 12, 9982–9990 (2018).
Reale, F. et al. High-mobility and high-optical quality atomically thin WS2. Sci. Rep. 7, 14911 (2017).
Schuler, B. et al. Large spin-orbit splitting of deep in-gap defect states of engineered sulfur vacancies in monolayer WS2. Phys. Rev. Lett. 123, 076801 (2019).
Barja, S. et al. Identifying substitutional oxygen as a prolific point defect in monolayer transition metal dichalcogenides. Nat. Commun. 10, 3382 (2019).
Zhang, X. et al. Hidden vacancy benefit in monolayer 2D semiconductors. Adv. Mater. 33, 2007051 (2021).
Tang, J. et al. In situ oxygen doping of monolayer MoS2 for novel electronics. Small 16, 2004276 (2020).
Meitl, M. A. et al. Transfer printing by kinetic control of adhesion to an elastomeric stamp. Nat. Mater. 5, 33–38 (2006).
Radisavljevic, B. & Kis, A. Mobility engineering and a metal–insulator transition in monolayer MoS2. Nat. Mater. 12, 815–820 (2013).
Liu, M. et al. in 2018 IEEE 13th Nanotechnology Materials and Devices Conference (NMDC). 1-4.
Cui, Y. et al. High-performance monolayer WS2 field-effect transistors on high-κ dielectrics. Adv. Mater. 27, 5230–5234 (2015).
Huang, J.-K. et al. High-κ perovskite membranes as insulators for two-dimensional transistors. Nature 605, 262–267 (2022).
Iqbal, M. W. et al. High-mobility and air-stable single-layer WS2 field-effect transistors sandwiched between chemical vapor deposition-grown hexagonal BN films. Sci. Rep. 5, 10699 (2015).
Ovchinnikov, D., Allain, A., Huang, Y.-S., Dumcenco, D. & Kis, A. Electrical transport properties of single-layer WS2. ACS Nano 8, 8174–8181 (2014).
Alharbi, A. & Shahrjerdi, D. Electronic properties of monolayer tungsten disulfide grown by chemical vapor deposition. Appl. Phys. Lett. 109, 193502 (2016).
Kang, K. et al. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 520, 656 (2015).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Pető, J. et al. Spontaneous doping of the basal plane of MoS2 single layers through oxygen substitution under ambient conditions. Nat. Chem. 10, 1246–1251 (2018).
KC, S., Longo, R. C., Wallace, R. M. & Cho, K. Surface oxidation energetics and kinetics on MoS2 monolayer. J. Appl. Phys. 117, 135301 (2015).
Acknowledgements
V.T., and Y.W. are indebted to the support from the King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under Award No: OSR-2018-CARF/CCF-3079. E.L. and N.L. acknowledge the support of Hong Kong UGC (C6012-17E). H.W., M.Y.L., and A.S.C. thanks the support from Taiwan Semiconductor Manufacturing Company (TSMC). W.H.C. acknowledges the supports from the Ministry of Science and Technology of Taiwan (MOST-108-2119-M-009-011-MY3, MOST-107-2112-M-009-024-MY3) and from the CEFMS of NCTU supported by the Ministry of Education of Taiwan. L.J.L. and Y.W. acknowledge the support from the University of Hong Kong. Special thanks to Kate Chuang for her assistance.
Author information
Authors and Affiliations
Contributions
Y.W., L.J.L. and V.T. conceived the project. Y.W., J.-K.H. and M.-Y.L. performed the synthesis of CVD- and OHVPD-WS2, and carried out Raman, PL, and AFM characterizations. Z.Y., A.-S.C., M.-Y.L., J.-J. L., S.-P.C., Y.-T.L. and X.W. conducted fabrication of field-effects transistors and associated calculations. E.L., H.-C.H., Y.-P.C. and N.L. performed and analyzed STM/STS characterization. C.-J.L. and W.-H.C. performed and analyzed the low-temperature PL measurement. Q.Z. and Y.C. performed the first-principles and nudged elastic band calculations. H.W., A.A., J.-H.F. and Y.S. provided constructive opinions and suggestions. All authors discussed and contributed the results. Y.W., L.J.L. and Y.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Humberto Gutierrez, Jun He, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, Y., Li, E., Yu, Z. et al. Low-defect-density WS2 by hydroxide vapor phase deposition. Nat Commun 13, 4149 (2022). https://doi.org/10.1038/s41467-022-31886-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-31886-0
This article is cited by
-
Growth of millimeter-sized 2D metal iodide crystals induced by ion-specific preference at water-air interfaces
Nature Communications (2024)
-
Transistor engineering based on 2D materials in the post-silicon era
Nature Reviews Electrical Engineering (2024)
-
Robust multiferroic in interfacial modulation synthesized wafer-scale one-unit-cell of chromium sulfide
Nature Communications (2024)
-
Vapour-phase deposition of two-dimensional layered chalcogenides
Nature Reviews Materials (2023)
-
Synthetic two-dimensional electronics for transistor scaling
Frontiers of Physics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.