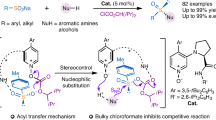
Abstract
Over the past decade, the catalysis of N-heterocyclic carbenes has achieved significant advances. In this area, aldehydes, enals, and esters, are commonly employed as starting materials through various catalytic activation modes. However, NHC-activated strategy of amide and its derivatives remains elusive. Described herein is the realization of asymmetric desymmetrization of N-Cbz glutarimides with alcohols through an imide C-N bond cleavage under NHC organocatalysis. A structurally diverse set of enantioenriched 4-amido esters is generated with acceptable yields and high enantioselectivities. This method features mild reaction conditions, excellent substrate scope, and excellent atom economy. DFT calculations have been performed to explore the detailed reaction mechanism and the origin of the enantioselectivity, which indicate that the strength of the C-H···O hydrogen bond and C–H⋯π interactions should be responsible for the stereoselectivity. The current strategy could open a door for efficient construction of (R)-Rolipram with excellent stereoselectivity.
Similar content being viewed by others
Introduction
Over the past decade, N-heterocyclic carbene (NHC) catalysis has proven to be one of the most privileged organocatalytic methods. It enables the construction of a structurally diverse set of synthetically valuable scaffolds from simple raw materials1,2,3,4,5. Since Breslow intermediate was revealed6, umpolung reactions of aldehydes under NHC catalysis have been frequently investigated, most notably on benzoin condensations7,8,9,10,11 and Stetter reactions12,13,14,15. The Glorius16 and Bode17 groups independently realized the seminal discovery that NHCs could further reverse the reactivity of β-carbon of enals to form homoenolate intermediates18,19,20, which greatly promoted the development of NHC chemistry. Subsequently, the Studer group21 reported in a pioneering work that NHCs could activate enals to generate α, β-unsaturated acyl azoliums22,23,24 under oxidative conditions. In addition to aldehydes (or enals), stable and relatively less reactive esters could also be activated creatively by NHCs, which was discovered by the groups of Chi and Lupton25,26,27,28, significantly expanding the scope of NHC chemistry. In addition, α-activation (enolate intermediates)29,30,31,32,33 and remote activation (such as γ34,35,36,37, δ38,39, and ε-activation40,41) of the aforementioned substrates, have also been achieved significantly under NHC catalysis. Beside commonly classical two-electron pathways, NHC-catalyzed radical reactions via a single electron transfer (SET) pathway of aldehydes or esters, and their surrogates have also received continuous attention42,43,44,45,46. Besides the aforementioned use of NHC as Lewis base catalyst, the asymmetric Bronsted base catalysis with NHC has also made impressive advances, in which NHC can activate 1,3-dicarbonyl compounds, amines, and thiols to realize asymmetric reactions via noncovalent interactions47,48,49,50,51. In sharp contrast to the aforementioned well-developed NHC-activation of aldehydes, enals, and esters, NHC-activation of valuable amide and its derivatives has been largely underdeveloped (Fig. 1a).
Amide bonds are not only essential functional groups in organic chemistry but also fundamental units of polypeptides and proteins. Direct activation of an amide bond is very challenging due to the high stability of the amide linkage. Notably, NHC-catalyzed amide C–N bond activation remains to be established. As a part of our ongoing interest in organocatalysis52,53,54,55,56, we envisioned the imides, containing a more reactive amide bond and unknown in the area of NHC, might be activated by NHC and further realize asymmetric reactions57,58,59. If successful, this methodology will expand the scope of carbene chemistry. Specifically, easily available cyclic imides were selected to investigate their asymmetric desymmetrization via amide bond activation by NHCs36,60,61,62,63. The success of the current strategy is determined by two key issues, namely: (i) increasing the reactivity of the imides to ensure C–N imide cleavage under NHC catalysis; (ii) both avoiding the background reaction and controlling the reaction conditions to ensure excellent enantioselectivities. Notably, the chiral center is far away from the reactive site, which makes enantiocontrol difficult.
Herein, we present the successful development of a strategy for NHC-catalyzed desymmetrization of cyclic imides with alcohols via an imide C–N bond cleavage under mild conditions (Fig. 1b). Notably, the resulting amido esters as key motifs are frequently found in natural products associated with a variety of promising biological activities (Fig. 1c). Importantly, the resulting product could be efficiently converted into (R)-Rolipram.
Results
Reaction optimization
Initial attempts were performed with prochiral cyclic imide 1a or N-Bn cyclic imide 1b, and ethanol 2a, one of the simplest alcohols, in the presence of the achiral triazolium N-Mes pre-catalyst A with K2CO3 as the base in DCM at room temperature. Unfortunately, no reaction occurred, probably because of the insufficient reactivity of the imide C–N bond (entries 1–2). Next, prochiral N-Cbz 1c (Cbz: an electron-withdrawing and good leaving group), was investigated under the same reaction conditions. To our delight, the desired ethyl 5-(((benzyloxy)carbonyl)amino)-5-oxo-3-phenylpentanoate 3a was obtained in 75% yield.
Gratifyingly, the desired enantioenriched 3a, with much structural difference of the remote third atom from the chiral center, was obtained in 78% yield with 84% ee when the aminoindanol-derived triazolium pre-catalyst B was used. This result confirmed the feasibility of NHC-activation of the imide C–N bond and catalytic asymmetric desymmetrization of prochiral cyclic imides. Next, several bases were investigated, with the inorganic base K2CO3 proving to be the best choice (entries 3–12). Subsequent solvent screening revealed that DCM was the most effective (entries 13–16). Lowering the temperature could improve the enantioselectivity considerably (entries 17–19). The product 3a was obtained in 85% yield with 95% ee when the temperature was dropped to −30 °C. Reducing the catalyst loading led to a decrease in the yield and enantioselectivity considerably (entries 20 and 21). Low enantioselectivity (9% ee) was observed when the NHC C was employed (entry 22). In the absence of the catalyst, no reaction occurred (entry 23). The absolute configuration of products 3 was determined via X-ray of 3i.
Substrate scope
With acceptable optimized conditions in hand (Table 1, entry 19), we then investigated the scope of the reaction for prochiral cyclic imide substrates by using ethanol 2a as a model substrate (Fig. 2). R1 groups bearing substituents with a variety of electronic and steric properties on the aromatic ring were well tolerated, which resulted in the formation of the corresponding products 3b-3j in good to excellent yields (73–92%) with excellent enantioselectivities (92–95% ees). Substrates with both 1-naphthyl and 2-naphthyl groups reacted smoothly to deliver the products 3k and 3l, respectively, with excellent enantioselectivities. The 2-thienyl group is well tolerated, yielding the product 3m in 84% yield with 85% ee. Notably, alkyl groups, such as Me, n- Pr, i-Pr, t-Bu, i-Bu, and Cy, were efficient substrates for this reaction to provide the products 3n-3s with good to excellent enantioselectivities (82–95% ee values). To demonstrate the generality of this catalytic asymmetric desymmetrization, the scope of the alcohols was further investigated (Fig. 2). Besides ethanol, other alkyl alcohols, such as MeOH, i-PrOH, n-PrOH, phenethyl alcohol, 1-octanol, i-BuOH, cyclohexylmethanol, cyclopentylmethanol, cyclopropylmethanol, and benzyl alcohol worked efficiently to access the desired products 3t-3af with good to excellent enantioselectivities (80–97% ee). Pleasingly, several other functionalized groups, such as terminal alkenyl, double bond, and even NHBoc groups were well-tolerated, affording the products 3ag-3ai in good to excellent yields (76–91%) with excellent enantioselectivities (91–98% ee). The protective group on the imide moiety was investigated; when Cbz was replaced with Boc, no reaction occurred under the optimized conditions, perhaps due to the greater steric hindrance of the Boc group. To our delight, raising the temperature to 30°C afforded the N-Boc product 3ad in 72% yield with 71% ee. Notably, in addition to alcohols, H2O is also compatible with this transformation to form 3ak in 68% yield with 79% ee. Unfortunately, other nucleophiles, such as benzyl mercaptan, and amine, did not give satisfying outcomes under the current conditions (see SI for details). Delightingly, cyclic imides with 3,5-substituents, such as benzyl 2,4-dioxo-3-azabicyclo[3.3.1]nonane-3-carboxylate, reacted smoothly to give the desired product 3al in 65% yield with 46% ee. Mild reaction conditions, excellent functional group tolerance, and broad substrate scope significantly increased the utility of this method for further synthetic transformations.
Reaction conditions as in Table 1, entry 19; yields (after SiO2 chromatography purification) were based on 1. [b] Reaction was performed at −40 °C. [c] Reaction was performed at 30 °C.
After the establishment of this catalytic desymmetrization strategy, 4,4-disubstituted glutarimides 4 was further tested (Fig. 3). A variety of 4,4-disubstituted glutarimides were investigated, and 2,3-dihydrospiro[indene-1,4'-piperidine]-2',6'-diones were identified as the best skeleton to give the desired products 5a-5h in 62–80% yields with 84–94% ee values. The absolute configruation of the product 5 was detemined via X-ray of 10, which was derived from the product 6b. However, other frameworks, such as tetrahydronaphthalenyl or acyclic structures could not give the desired products when NHC B was used. Instead, decarbobenzoxylation product was observed. Then, although several reaction conditions have been profoundly investigated (For details, see Supporting Information), products 5j-5o were obtained in 55–87% yields with 9–66% ee. The obtained results indicated that NHC B has proven to be the more effective for 4-substituted glutarimides and indenyl glutarimides, and NHC C has proven to be the more effective for other 4,4-disubstituted glutarimides in most cases, probably due to more steric hindrance of 4,4-disubstituted glutarimides. Furthermore, prochiral center far away from the reaction site and similar two substituted groups on the prochiral center led to difficulty realize considerable enantiomeric control for 4,4-disubstituted glutarimides.
Reaction conditions as in Table 1, entry 19; yields (after SiO2 chromatography purification) were based on 4. [b] Reaction was performed at −30 °C. [c] NHC precursor C was used. Reaction was performed at −30 °C.
Mechanistic studies
To shed light on the mechanism and origin of stereoselectivity for these efficient desymmetrizations of cyclimides in an excellent enantioselecitivity manner, we have DFT calculations by using Gaussian program64, and more computational details can be found in Supporting Information. As shown in Fig. 4, four steps including nucleophilic addition via transition states TS1R (14.1 kcal/mol) and TS1S (15.9 kcal/mol), which is followed by C–N bond cleavage via transition states TS2R (18.7 kcal/mol) and TS2S (20.1 kcal/mol). Subsequently, nucleophilic addition coupled with a proton transfer undergoes transition states TS3R (14.0 kcal/mol) and TS3S (17.2 kcal/mol). The final step is the dissociation of NHC with products via transition states
TS4R (14.4 kcal/mol) and TS4S (15.9 kcal/mol). The R-isomer pathway locates below the S-isomer pathway in the Gibbs free energy file. Particularly, the energy of TS3R is 2.8 kcal/mol lower than that of TS3S, indicating the pathway associated with R-isomer should be more energetically favorable in kinetics. This conclusion is in agreement with the experimental results.
To probe the reaction pathway, control experiment was performed as shown in Fig. 5. Treatment of 1c with 1.0 equiv. of NHC B was performed in the presence of K2CO3 in DCM for 8 h. Immediately, the high-resolution mass spectroscopy (HRMS-ESI) analysis of the reaction mixture was carried out. And one signal peak was detected at m/z 655.2922. M+ value combined with the isotope distribution pattern on HRMS analysis of the NHC-substrate adduct which probably corresponded to the intermediate Int-1.
To reveal the origin and determining factor of enantioselectivity, the energy decomposition has been performed by using PSI465,66. The energy decomposition analysis summarized in Table S2 of SI indicates that the interim dispersion should be responsible for the favorability of R-isomer pathway via TS2R. Non-covalent interaction (NCI) and atoms-in-molecules (AIM) analyses have been performed by using Multiwfn67, and the results have been shown in Fig. 6. As depicted in Fig. 6, there are five C–H⋯O (1.90, 2.57, 2.54 2.70, and 2.45 Å), one C–H⋯π (2.87 Å), and one C–H⋯N (2.76 Å) interactions in TS2R, while there are six C–H⋯O (2.79, 1.90, 2.55, 2.57, 2.52, and 2.48 Å) interactions in TS2S, indicating the strength of C–H⋯π and C–H⋯N hydrogen-bond interactions should be responsible for the favorability of R-isomer pathway. Inspired by the good examples68,69,70, we have also additionally performed the NCI analysis by employing NCIplot71,72, and similar weak interactions have been identified and depicted in the Supplementary Fig. 301 of SI. More computational details and results can be found in Supporting Information.
Synthetic transformations
Subsequently, further synthetic transformations of the resulting products were conducted as shown in Fig. 7. Treatment of 3c with Pd/C under 1 atm H2, followed by the reduction with LiAlH4 furnished product 6 in 74% yield with 91% ee. Especially, 3i underwent decarbobenzyloxylation followed by hydrolysis to result in the formation of 7 in excellent yield, which was then converted into the skeletal muscular relaxants (R)-Baclofen 8 via a known process73. More importantly, (R)-Rolipram 9, which is a phosphodiesterase inhibitor with antidepressant properties74, was prepared in 50% overall yields with 95% ee from 3j via a stepwise sequence of decarbobenzyloxylation, decarbonylation of the amide moiety and an intramolecular lactamization. The compound 6b was smoothly converted into product 10 in 45% yield with 97% ee after recrystallization by using a similar strategy.
Discussion
In summary, we have developed an NHC-enabled desymmetrization of N-Cbz glutarimides with alcohols under mild conditions via imide C–N bond activation. A diverse set of enantioenriched 4-amido esters was obtained in good yields with good to excellent enantioselectivities. This reaction features mild reaction conditions, wide substrate scope, and an excellent atom economy. DFT calculations and NCI analysis indicate that the C–H⋯O hydrogen bond and C–H⋯π interactions between the NHC catalyst and substrate should be the key for controlling the enantioselectivity. Importantly, the resulting product was converted into (R)-Rolipram in 50% overall yields with 95% ee. In summary, this case work combined with experimental and theoretical studies should be valuable for understanding the similar organocatalytic desymmetrization reactions of cyclic imides with imide C–N bond activation. Further investigations and exploration of this catalytic process are underway in our laboratory.
Methods
General procedure for the synthesis of 3
To a suspension of starting materials 1 (0.10 mmol), catalyst B (7.3 mg, 20 mol%) and K2CO3 (0.15 mmol, 20.7 mg) in DCM (1.0 mL, 0.10 M) was added the appropriate alcohols (0.15 mmol, 1.5 equiv.). The mixture was stirred for 72 h at −30 °C. Upon completion of the reaction (monitored by TLC), the reaction mixture was directly purified by column chromatography on silica gel to afford the desired product 3.
Data availability
The X-ray crystallographic coordinates for structures of 3i and 10 reported in this study have been deposited in the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2115884(3i), and 2115883(10). These data can be obtained free of charge from http://www.ccdc.cam.ac.uk/data_request/cif. The experimental procedures, characterization of the new compounds, and computational studies in this study are provided in Supplementary Information and Supplementary Data 1. All other data are available from the authors upon request.
References
Chen, X. Y., Liu, Q., Chauhan, P. & Enders, D. N-heterocyclic carbene catalysis via azolium dienolates: an efficient strategy for remote enantioselective functionalizations. Angew. Chem. Int. Ed. 57, 3862 (2018).
Mondal, S., Yetra, S. R., Mukherjee, S. & Biju, A. T. NHC-catalyzed generation of alpha,beta-unsaturated acylazoliums for the enantioselective synthesis of heterocycles and carbocycles. Acc. Chem. Res. 52, 425 (2019).
Chen, X. Y., Gao, Z. H. & Ye, S. Bifunctional N-heterocyclic carbenes derived from l-pyroglutamic acid and their applications in enantioselective organocatalysis. Acc. Chem. Res. 53, 690 (2020).
Ohmiya, H. N-heterocyclic carbene-based catalysis enabling cross-coupling reactions. ACS Catal. 10, 6862 (2020).
Chen, X., Wang, H., Jin, Z. & Chi, Y. R. N‐heterocyclic carbene organocatalysis: activation modes and typical reactive intermediates. Chin. J. Chem. 38, 1167 (2020).
Breslow, R. On the mechanism of thiamine action. IV.1 Evidence from studies on model systems. J. Am. Chem. Soc. 80, 3719 (1958).
Jia, M.-Q. & You, S.-L. N-Heterocyclic carbene-catalyzed enantioselective intramolecular N-tethered aldehyde–ketone benzoin reactions. ACS Catal. 3, 622 (2013).
Zhang, G. et al. Dynamic kinetic resolution enabled by intramolecular benzoin reaction: synthetic applications and mechanistic insights. J. Am. Chem. Soc. 138, 7932 (2016).
Wu, J., Zhao, C. & Wang, J. Enantioselective intermolecular enamide-aldehyde cross-coupling catalyzed by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 138, 4706 (2016).
Hornillos, V. et al. Dynamic kinetic resolution of heterobiaryl ketones by zinc-catalyzed asymmetric hydrosilylation. Angew. Chem. Int. Ed. 57, 3777 (2018).
Yan, J. et al. N-heterocyclic carbene-catalyzed asymmetric benzoin reaction in water. J. Org. Chem., 83, 7547 (2018).
Zhang, J., Xing, C., Tiwari, B. & Chi, Y. R. Catalytic activation of carbohydrates as formaldehyde equivalents for Stetter reaction with enones. J. Am. Chem. Soc. 135, 8113 (2013).
Rafiński, Z., Kozakiewicz, A. & Rafińska, K. (−)-β-pinene-derived N-heterocyclic carbenes: application to highly enantioselective intramolecular stetter reaction. ACS Catal. 4, 1404 (2014).
Janssen-Müller, D. et al. NHC-catalyzed enantioselective dearomatizing hydroacylation of benzofurans and benzothiophenes for the synthesis of spirocycles. ACS Catal. 6, 5735 (2016).
Zhao, M., Liu, J. L., Liu, H. F., Chen, J. & Zhou, L. Construction of bisbenzopyrone via N-heterocyclic carbene catalyzed intramolecular hydroacylation-stetter reaction cascade. Org. Lett. 20, 2676 (2018).
Burstein, C. & Glorius, F. Organocatalyzed conjugate umpolung of α, β‐unsaturated aldehydes for the synthesis of γ‐butyrolactones. Angew. Chem. Int. Ed. 43, 6205 (2004).
Sohn, S. S., Rosen, E. L. & Bode, J. W. N-heterocyclic carbene-catalyzed generation of homoenolates: γ-butyrolactones by direct annulations of enals and aldehydes. J. Am. Chem. Soc. 126, 14370 (2004).
Guo, C. et al. Cooperative N-heterocyclic carbene/palladium-catalyzed enantioselective umpolung annulations. J. Am. Chem. Soc. 138, 7840 (2016).
Chen, J. et al. Enantioselective β-protonation of enals via a shuttling strategy. J. Am. Chem. Soc. 139, 7045 (2017).
Singha, S. et al. Diastereodivergent synthesis of enantioenriched α,β-disubstituted γ-butyrolactones via cooperative N-heterocyclic carbene and Ir catalysis. Nat. Catal. 3, 48 (2020).
Smrkar, S. D. & Studer, A. NHC-catalyzed Michael addition to α,β-unsaturated aldehydes by redox activation. Angew. Chem. Int. Ed., 49, 9266 (2010).
Zhang, C., Hooper, J. F. & Lupton, D. W. N-heterocyclic carbene catalysis via the α,β-unsaturated acyl azolium. ACS Catal. 7, 2583 (2017).
Zhao, C. et al. Enantioselective [3+3] atroposelective annulation catalyzed by N-heterocyclic carbenes. Nat. Commun. 9, 611 (2018).
Chen, K. Q., Gao, Z. H. & Ye, S. (Dynamic) kinetic resolution of enamines/imines: enantioselective N‐heterocyclic carbene catalyzed [3+3] annulation of bromoenals and enamines/imines. Angew. Chem. Int. Ed. 58, 1183 (2019).
Hao, L. et al. Enantioselective activation of stable carboxylate esters as enolate equivalents via N-heterocyclic carbene catalysts. Org. Lett. 14, 2154 (2012).
Candish, L. & Lupton, D. W. N-heterocyclic carbene-catalyzed Ireland–coates Claisen rearrangement: synthesis of functionalized β-lactones. J. Am. Chem. Soc. 135, 58 (2013).
Fu, Z. et al. β-Carbon activation of saturated carboxylic esters through N-heterocyclic carbene organocatalysis. Nat. Chem. 5, 835 (2013).
Zhang, Y. et al. Carbene-catalyzed enantioselective synthesis of γ-keto-β-silyl esters and amides. Org. Lett. 22, 9545 (2020).
Li, F., Wu, Z. & Wang, J. Oxidative enantioselective α‐fluorination of aliphatic aldehydes enabled by N‐heterocyclic carbene catalysis. Angew. Chem. Int. Ed. 54, 656 (2015).
Gao, Z. et al. N-Heterocyclic carbene-catalyzed [2+3] cyclocondensation of α-chloroaldehydes with azomethine imines. Chem. Commun. 51, 9328 (2015).
Yuan, S. et al. Oxidative asymmetric [2 + 3] annulation of aldehydes with azomethine imines enabled by N-heterocyclic carbene catalysis. Org. Lett. 19, 6100 (2017).
Zhang, Z. F. et al. N‐heterocyclic carbene‐catalyzed annulation of α‐chloroaldehydes with γ‐/δ‐amino‐α,β‐unsaturated ketones: enantioselective synthesis of pyrrolidones and piperidones. Chem. Eur. J. 24, 8302 (2018).
Singha, S. et al. Highly enantioselective [5 + 2] annulations through cooperative N-heterocyclic carbene (NHC) organocatalysis and palladium catalysis. J. Am. Chem. Soc. 140, 3551 (2018).
Mo, J., Chen, X. & Chi, Y. R. Oxidative γ-addition of enals to trifluoromethyl ketones: enantioselectivity control via lewis acid/N-heterocyclic carbene cooperative catalysis. J. Am. Chem. Soc. 134, 8810 (2012).
Wu, Z., Li, F. & Wang, J. Intermolecular dynamic kinetic resolution cooperatively catalyzed by an N‐heterocyclic carbene and a lewis acid. Angew. Chem. Int. Ed. 54, 1629 (2015).
Zhu, T. et al. Carbene‐catalyzed desymmetrization and direct construction of arenes with all‐carbon quaternary chiral center. Angew. Chem. Int. Ed. 58, 15778 (2019).
Zhang, C.-L. et al. Enantioselective synthesis of axially chiral benzothiophene/benzofuran‐fused biaryls via N‐heterocyclic carbene catalyzed arene formation. Angew. Chem. Int. Ed. 60, 13918 (2021).
Zhu, T. et al. N-heterocyclic carbene-catalyzed δ-carbon LUMO activation of unsaturated aldehydes. J. Am. Chem. Soc. 137, 5658 (2015).
Xu, K. W., Li, W., Zhu, S. T. & Zhu, T. Atroposelective arene formation by carbene‐catalyzed formal [4+2] cycloaddition. Angew. Chem. Int. Ed., 58, 17625 (2019).
Dai, L. et al. Visible‐light‐driven n‐heterocyclic carbene catalyzed γ- and ϵ-alkylation with alkyl radicals. Angew. Chem. Int. Ed. 58, 18124 (2019).
Dai, L. & Ye, S. NHC-catalyzed ε-umpolung via p-quinodimethanes and its nucleophilic addition to ketones. ACS Catal. 10, 994 (2020).
Meng, Q.-Y., Döben, N. & Studer, A. Cooperative NHC and photoredox catalysis for the synthesis of β‐trifluoromethylated alkyl aryl ketones. Angew. Chem. Int. Ed. 59, 19956 (2020).
Shibutani, S. et al. Organophotoredox-catalyzed decarboxylative C(sp3)–O bond formation. J. Am. Chem. Soc. 142, 1211 (2020).
Zhang, B., Peng, Q., Guo, D. & Wang, J. NHC-catalyzed radical trifluoromethylation enabled by Togni reagent. Org. Lett. 22, 443 (2020).
Ren, S.-C. et al. Carbene-catalyzed alkylation of carboxylic esters via direct photoexcitation of acyl azolium intermediates. ACS Catal. 11, 2925 (2021).
Liu, K. & Studer, A. Direct α-acylation of alkenes via N-heterocyclic carbene, sulfinate, and photoredox cooperative triple catalysis. J. Am. Chem. Soc. 143, 4903 (2021).
Chen, J. & Huang, Y. Asymmetric catalysis with N-heterocyclic carbenes as non-covalent chiral templates. Nat. Commun. 5, 3437 (2014).
Wang, L., Chen, J. & Huang, Y. Highly enantioselective Aza-Michael reaction between alkyl amines and β-trifluoromethyl β-aryl nitroolefins. Angew. Chem. Int. Ed. 54, 1514 (2015).
Santra, S. et al. N-Heterocyclic carbenes as chiral bronsted base catalysts: a highly diastereo- and enantioselective 1,6-addition reaction. Chem. Sci. 9, 6446 (2018).
Santra, S., Maji, U. & Guin, J. Enantioselective alpha-amination of acyclic 1,3-dicarbonyls catalyzed by n-heterocyclic carbene. Org. Lett. 22, 468 (2020).
Guo, F. F., Chen, J. & Huang, Y. A bifunctional N-heterocyclic carbene as a noncovalent organocatalyst for enantioselective Aza-Michael addition reactions. ACS Catal. 11, 6163 (2021).
Wang, G. J., Fu, Z. Q. & Huang, W. Access to amide from aldimine via aerobic oxidative carbene catalysis and LiCl as cooperative Lewis acid. Org. Lett. 19, 3362 (2017).
Gao, Y. et al. Potassium 2-oxo-3-enoates as effective and versatile surrogates for α, β‐unsaturated aldehydes in NHC‐catalyzed asymmetric reactions. Adv. Synth. Catal. 360, 479 (2018).
Zhang, Y. et al. Access to enantioenriched organosilanes from Enals and β‐silyl enones: carbene organocatalysis. Angew. Chem. Int. Ed. 57, 4594 (2018).
Wang, G. et al. Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids. Nat. Commun. 11, 946 (2020).
Wang, G. et al. Asymmetric carbene‐catalyzed oxidation of functionalized aldimines as 1,4‐dipoles. Angew. Chem. Int. Ed. 60, 7913 (2021).
Masato, I. et al. Chemoselective hydrogenation of imides catalyzed by Cp*Ru(PN) complexes and its application to the asymmetric synthesis of paroxetine. J. Am. Chem. Soc. 129, 290 (2007).
Masato, I. et al. Highly enantioselective hydrogenative desymmetrization of bicyclic imides leading to multiply functionalized chiral cyclic compounds. J. Am. Chem. Soc. 132, 11414 (2010).
Ibrahim, U. K. & Simon, J. Enantioselective desymmetrization of glutarimides catalyzed by oxazaborolidines derived from cis-1-Amino-indan-2-ol. J. Org. Chem. 80, 11468 (2015).
Huang, Z. et al. Access to P-stereogenic phosphinates via N-heterocyclic carbene-catalyzed desymmetrization of bisphenols. J. Am. Chem. Soc. 138, 7524 (2016).
Zhuo, S. et al. Access to all‐carbon spirocycles through a carbene and thiourea cocatalytic desymmetrization cascade reaction. Angew. Chem. Int. Ed. 58, 1784 (2019).
Shee, S. et al. Enantioselective synthesis of tricyclic β-lactones by NHC-catalyzed desymmetrization of cyclic 1,3-diketones. Org. Lett. 22, 5407 (2020).
Barik, S. et al. NHC‐catalyzed desymmetrization of n‐aryl maleimides leading to the atroposelective synthesis of N‐Aryl succinimides. Angew. Chem. Int. Ed. 60, 12264 (2021).
Frisch, M. J. et al. GAUSSIAN 09 (Revision D.01) (Gaussian, Inc., Wallingford, CT, 2013).
Parker, T. M. et al. Levels of symmetry adapted perturbation theory (SAPT). I. Efficiency and performance for interaction energies. J. Chem. Phys. 140, 094106 (2014).
Smith, D. G. A. et al. PSI4 1.4: open-source software for high-throughput quantum chemistry. J. Chem. Phys. 152, 184108 (2020).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580 (2011).
Monika, P. & Raghavan, B. S. Mechanism and stereoselectivity in an asymmetric N-heterocyclic carbene-catalyzed carbon-carbon bond activation reaction. Org. Lett. 18, 5932 (2016).
Wang, Y. et al. DFT perspective toward [3 + 2] annulation reaction of enals with α-ketoamides through NHC and Brønsted acid cooperative catalysis: mechanism, stereoselectivity, and role of NHC. Org. Chem. Front. 3, 190 (2016).
Ryuji, K. et al. β,γ-trans-selective γ-butyrolactone formation via homoenolate cross-annulation of enals and aldehydes catalyzed by sterically hindered N-heterocyclic carbene. Tetrahedron 91, 132191 (2021).
Julia, C. G. et al. NCIPLOT: a program for plotting noncovalent interaction regions. J. Chem. Theory Comput. 7, 625–632 (2011).
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498 (2010).
Ji, L. et al. An efficient synthesis of (R)- and (S)-baclofen via desymmetrization. Tetrahedron Lett. 50, 6166 (2019).
Butler, C. et al. Evidence of the effects of intrathecal baclofen for spastic and dystonic cerebral palsy. Dev. Med. Child Neurol. 42, 634 (2000).
Acknowledgements
We acknowledge financial support by the National Key R&D Program of China (2017YFA0204704), National Natural Science Foundation of China (21602105 & 22174065), Natural Science Foundation of Jiangsu Province (BK20171460), the General Program of Chongqing Natural Science Foundation Project (cstc2020jcyj-msxmX0712), and Ningbo Natural Science Foundation (202003N4063).
Author information
Authors and Affiliations
Contributions
Z.F. conceived the idea and designed the experiments. Z.H., C.W., and X.H. conducted most of the experiments. X. Zhou, J.H., W.C., and A.G. prepared some of starting materials. Q.S. performed DFT study supervised by D.W.Z.F. drafted the manuscript with the assistance from J.L., X. Zhang, and W.H. All authors contributed to the discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Z., Wei, C., Shi, Q. et al. Desymmetrization of N-Cbz glutarimides through N-heterocyclic carbene organocatalysis. Nat Commun 13, 4042 (2022). https://doi.org/10.1038/s41467-022-31760-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-31760-z
This article is cited by
-
Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Nature Communications (2024)
-
N-Heterocyclic carbene-catalyzed enantioselective (dynamic) kinetic resolutions and desymmetrizations
Science China Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.