Abstract
The RNF43_p.G659fs mutation occurs frequently in colorectal cancer, but its function remains poorly understood and there are no specific therapies directed against this alteration. In this study, we find that RNF43_p.G659fs promotes cell growth independent of Wnt signaling. We perform a drug repurposing library screen and discover that cells with RNF43_p.G659 mutations are selectively killed by inhibition of PI3K signaling. PI3K/mTOR inhibitors yield promising antitumor activity in RNF43659mut isogenic cell lines and xenograft models, as well as in patient-derived organoids harboring RNF43_p.G659fs mutations. We find that RNF43659mut binds p85 leading to increased PI3K signaling through p85 ubiquitination and degradation. Additionally, RNA-sequencing of RNF43659mut isogenic cells reveals decreased interferon response gene expression, that is reversed by PI3K/mTOR inhibition, suggesting that RNF43659mut may alter tumor immunity. Our findings suggest a therapeutic application for PI3K/mTOR inhibitors in treating RNF43_p.G659fs mutant cancers.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality and the third most commonly diagnosed cancer worldwide with a growing incidence rate among younger patients1,2. Despite advances in treatment strategies for CRC, the outcomes for patients with CRC have only modestly improved3. Thus, there is a pressing need to develop novel treatment options for patients with this disease. Despite extensive genomic profiling of CRC4,5,6, the role of CRC genetic variants in therapeutic decisions remains limited largely due to the fact that the carcinogenic role of relevant mutations is inadequately characterized7,8,9. Therefore, determining the specific function of key genetic variants is critical to facilitate new targeted therapeutic approaches, and ultimately improve patient outcomes.
RNF43 (Ring Finger Protein 43) encodes a Ring finger E3 ubiquitin ligase which acts as a negative regulator of the Wnt signaling pathway through the degradation of the Wnt receptor, Frizzled10. Thus, RNF43 loss-of-function renders tumor cells dependent on secreted Wnt ligands for survival10,11,12. Inactivating mutations in RNF43 were reported to confer susceptibility to pharmacologic inhibitors that target the Wnt-specific acyltransferase porcupine (PORCN) in pancreatic ductal adenocarcinoma13, gastric cancer14 and CRC15. Intriguingly, two truncating mutations, RNF43_p.G659fs and RNF43_p.R117fs, have been observed with high frequency in CRC6,16,17. The more frequent G659fs mutation is found in ~8% of patients with CRC and is enriched in microsatellite-instability high cancers16. However, the functional consequences of RNF43 mutations on Wnt signaling as well as colorectal carcinogenesis remain controversial, especially for p.G659fs. Previous studies have showed that the RNF43_p.G659fs mutation remains fully functional in promoting Frizzled degradation and attenuating Wnt signaling and is thus unlikely to confer Wnt-dependency onto CRC18,19. In line with this, Wetering et al. showed that a CRC organoid which harbors a RNF43_p.G659fs mutation was insensitive to two selective PORCN inhibitors, WNT974 and IWP215. Indeed, PORCN inhibitors are currently in clinical trials, however, patients with RNF43_p.G659fs mutations are not eligible due to insufficient evidence supporting Wnt ligand-dependency for these tumors. Consequently, although RNF43_p.G659fs truncation is a frequent mutational event in CRC, there are currently no therapeutics targeting it. Thus, mechanistic insights and identification of novel therapeutics for the treatment of patients whose tumors harbor the RNF43_p.G659fs mutation are crucial.
In this study, we provide molecular evidence that the RNF43_p.G659fs mutation drives CRC in a Wnt-independent manner. We find that RNF43_p.G659fs mutant colorectal tumor cells activate PI3K/AKT signaling and that PI3K inhibitors selectively target RNF43_p.G659fs mutant tumor cells. Mechanistically, we discover that RNF43_p.G659fs interacts with p85 (Phosphoinositide-3-Kinase Regulatory Subunit 1) to increase PI3K (Phosphoinositide-3-Kinase)/AKT signaling. Additionally, we associate RNF43_p.G659fs mutations with decreased interferon response. Our results support PI3K inhibition as a therapeutic option for patients whose tumors harbor the RNF43_p.G659fs mutation.
Results
RNF43 659mut has a Wnt-independent carcinogenic role
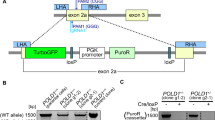
RNF43 is recurrently mutated at two residues, p.R117fs and p.G659fs in CRC16. To evaluate whether these RNF43 hot-spot frameshift mutations functionally contribute to tumor progression, we selected several CRC cell lines with wildtype RNF43 and CTNNB1 and either wildtype or mutant APC20, namely C10 (APCWT), LS513 (APCWT) and HT29 (APCmut) and utilized CRISPR-Cas9 to genetically edit the target sites of RNF43-117aa (RNF43117mut) and RNF43-659aa (RNF43659mut) (Supplementary Fig. 1a). Cell growth was initially assessed through colony formation assays with and without addition of Wnt-3a ligand. Compared to sgRNA controls, the addition of Wnt-3a demonstrated increased cell growth in RNF43117mut cells in both APCWT cell lines (C10 and LS513) (Fig. 1A). However, no significant difference was observed in the APCWT cells without Wnt-3a or in the APCmut cell line (HT29) with intrinsic Wnt activation (Fig. 1A) indicating the function of RNF43117mut was Wnt context and ligand-dependent. Intriguingly, we found that RNF43659mut significantly increased colony formation irrespective of the presence of Wnt-3a or APC mutation status across all three CRC lines (Fig. 1A). To further assess Wnt activation in RNF43117mut and RNF43659mut cell growth, we carried out a TOP/FOP luciferase reporter assay to measure Wnt signaling activity. As illustrated in Fig. 1B, RNF43117mut significantly increased Wnt signaling upon Wnt-3a supplementation in the APCWT C10 and LS513 cells. However, RNF43659mut had no increased Wnt activity compared to control regardless of APC status or Wnt-3a supplementation suggesting intact suppression of Wnt signaling by RNF43659mut (Fig. 1B). Consistent with this finding, we found no change in the expression and phosphorylation of LRP6, GSK3β, CK1, or CTNNB1 in isogenic HT29 RNF43659mut cells (Supplementary Fig. 2). Colony formation assays of nine distinct RNF43659mut single clones derived from HT29 and LS513 confirmed increased cell growth (Fig. 1C, D and Supplementary Fig. 1b, c). Indicated single clones were used in subsequent experiments.
A Representative image (upper panel) and quantification (lower panel) of colony formation assays in sgRNA-control, RNF43117mut and RNF43659mut CRISPR-edited HT29, C10 and LS513 CRC cells in the absence (−) or presence (+) of exogenous Wnt-3a ligand. B Luciferase reporter assay of Wnt signaling activity in indicated CRISPR-edited cells. C Representative images (left panel) and quantification (right panel) of colony formation assay in two single RNF43659mut clones in each of HT29 and LS513 cell lines, compared to non-targeting sgRNA-control. D Cell viability assay for control and RNF43659mut edited single clones of HT29 and LS513 cells. E Representative images and quantification of colony formation assays with RNF43-wildtype (WT) and RNF43-659fs*41 overexpression (OE) in HCT116 and HT29 cells. Data are the mean ± SD. Two-sided Student’s t test (n = 3 independent experiments in triplicate). Source data are provided as a Source Data file.
To assess for a gain of function with RNF43_p.G659Vfs, we overexpressed V5-tagged RNF43-wildtype and RNF43_p.G659Vfs protein into the HCT116 (RNF43117mut) and HT29 (RNF43WT) cell lines. Consistent with the observation in the CRISPR-edited isogenic cell lines, overexpression of RNF43_p.G659Vfs caused a significant growth increase compared to either RNF43-wildtype or the non-transfected control in both HCT116 and HT29 cell lines, confirming the oncogenic role for RNF43659mut (Fig. 1E). Collectively, these results provide evidence that RNF43_p.G659Vfs has a functional role in carcinogenesis via a Wnt-independent manner.
RNF43 659mut activates PI3K/AKT signaling and is vulnerable to PI3K/mTOR inhibitors
In order to discover potential therapeutic vulnerabilities of RNF43659mut CRC cells, we performed a comprehensive drug screen to identify small molecules that selectively inhibit RNF43659mut cells in HT29 CRISPR-edited isogenic cell lines. We tested the Broad Institute’s Repurposing Library21, which contained a set of 5363 compounds at different phases of preclinical and clinical development. For the primary screen, we identified 612 compounds that reduced the viability of RNF43659mut with a z-score < -3 compared to DMSO-treated controls (Fig. 2A and Supplementary Data 1). Out of these, 221 compounds had >5% killing in RNF43659mut compared to RNF43WT isogenic cells (Fig. 2A, B). Remarkably, among the 612 and 221 compound lists, we identified 30 and 22 drugs, respectively, targeting the PI3K/mTOR pathway (Fig. 2A and Supplementary Table 1). This pathway was significantly enriched among the repurposing library compounds that selectively target RNF43659mut over DMSO-treated and isogenic controls (Fig. 2C). We selected PI3K/mTOR compounds (n = 30) for a secondary screen at nine doses (0 to 10 uM) in LS513 RNF43659mut CRISPR-edited cells and HCT116 RNF43G659fs-overepressing (OE) cells (Fig. 2B). We observed that five compounds, alpelisib, PF-04691502, PKI-179, Torin-1, and Torin-2, led to significant selective killing in all three cell lines (Fig. 2B, D).
A Flowchart of Repurposing Library compound screening and selection criteria for candidate drug identification. B Quantification of selective inhibition of library compounds in HT29 (top), and validation of PI3K/mTOR inhibitor compounds in LS513 RNF43659mut edited cells and HCT116 RNF43G659fs overexpressing (OE) cells (bottom). Red data points indicate PI3K/mTOR inhibitors. C Pathway enrichment analysis of repurposing library compound screening in the initial screening population of HT29 RNF43659mut with a Z value less than −3 (top, among 612 significant drugs) and compounds that had >5% killing between HT29 RNF43659mut and HT29 ctrl (bottom, among 221 significant drugs). Two-sided Pearson’s χ2 test. D Venn diagram illustration of PI3K/mTOR significant candidate compound overlap (red) in HT29, LS513 RNF43659mut edited cells and HCT116 RNF43659mut overexpressing cells with n the number of compounds from the primary screen that validated in the secondary screen for each cell line. Source data are provided as a Source Data file.
We further characterized and optimized the dose-response curve with alpelisib, PF-04691502, PKI-179, Torin-1, and Torin-2 in RNF43659mut HT29 and LS513 cells, as well as HCT116 RNF43G659fs-OE cells. All five compounds exhibited potent and selective toxicity toward RNF43659mut cells and overexpressing RNF43659mut cells, but not toward sgRNA-control cells or wildtype-overexpressing cells (Fig. 3A and Supplementary Fig. 3). Further testing of alpelisib, which is FDA-approved for breast cancer treatment, and PF-04691502, currently in clinical trials, showed selective killing of CRC organoids harboring the RNF43_p.G659Vfs mutation (Fig. 3B). Through analysis of TCGA CRC whole-transcriptome (RNA-Seq) data, we also observed that tumors with RNF43659mut had an increased PI3Ki sensitivity signature22 (Supplementary Fig. 4). We further evaluated the effectiveness of PI3K/mTOR inhibition against the RNF43_p.G659fs mutation in HT29 cell line-derived xenograft (CDX) models using sg_Ctrl mice and RNF43659mut mice. PF-04691502 treatment over 28 days was able to reduce tumor growth in RNF43659mut CDX mice compared to the placebo group while there was no significant change in the sg_Ctrl mice (Fig. 3C, D). These results provide evidence that PI3K/mTOR inhibitors can target tumors harboring RNF43659mut mutations.
A Alpelisib and PF-04691502 dose-response curves in either RNF43659mut edited cells (HT29, LS513) or ectopically expressing (OE) RNF43_G659Vfs*41 cells (HCT116) compared to controls. Experiments were performed in triplicate. B Dose-response curve of alpelisib and PF-04691502, along with an MDM2 antagonist and MEK, tankyrase, and PORCN inhibitors, in patient-derived CRC organoids harboring RNF43_G659fs and normal colon organoids. C Image representation of mouse tumor size after placebo or PF-04691502 treatment in cell-derived xenografts harboring either HT29 sg_Ctrl or HT29 sg_RNF43659mut. D Quantification of tumor weight after placebo or PF-04691502 treatment in cell-derived xenograft harboring either HT29 sg_Ctrl or HT29 sg_RNF43659mut. Data are the mean ± SD. Two-sided Student’s t test (n = 5 mice/group). E Representative immunohistochemistry images of p-AKT (S473), Ki-67 and TUNEL-positive cells in HT29_sg-Ctrl or HT29_RNF43659mut-C1 tumors from mice treated with vehicle or PF-04691502. Scale bar 10 μm. Source data are provided as a Source Data file.
RNF43 659mut activates PI3K/AKT signaling through RNF43659mut-p85 interaction
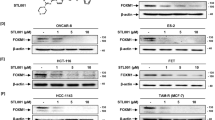
Overexpression of RNF43_p.G659fsV*41 in 293T, HCT116, and HT29 cells increased levels of p-AKT, p-S6, and p-4EBP1 (Supplementary Fig. 5). HT29 and LS513 RNF43659mut-edited single clones also showed increased expression levels of p-AKT, p-S6, and p-4EBP1 (Fig. 4A and Supplementary Fig. 6). Treatment with alpelisib and PF-04691502 specifically attenuated PI3K signaling in HT29-RNF43659mut cells in a dose-dependent manner and in RNF43659mut CDX models (Figs. 4A and 3E). RNF43 knockout or RSPO1 treatment reversed the phosphorylation of p-AKT, p-S6 and p-4EBP1 in isogenic HT29 RNF43659mut cells (Fig. 4B and Supplementary Fig. 6). In addition, RNF43659mut knockout, RSPO1 treatment, or siRNA mediated AKT silencing reduced the increased cell growth conferred by RNF43659mut (Supplementary Figs. 7 and 8). Importantly, in the Cancer Cell Line Encyclopedia and TCGA CRCs23,24, we also found that p-AKT and p-S6 levels were increased in RNF43659mut cell lines and tumors, respectively (Supplementary Fig. 9a, b).
A Expression of p-AKT, p-S6, and p-4EBP1 in RNF43659mut edited and sg_Ctrl isogenic HT29 and LS513 cells after 48 h treatment with PF-04691502 (left) or alpelisib (right). β-Actin was used as a loading control. B p-AKT (S473), AKT, p-S6 (S235/236), S6, and p85 in sg-Ctrl and RNF43659mut cells alone or with sg-RNF43-KO or RSPO1 (500 ng/ml) treatment. C Schematic representation of the quantitative proteomics workflow used to analyze RNF43 p.G659Vfs protein–protein interactions. Schematic created with BioRender.com. D Volcano plot illustrating the significance (y-axis) and magnitude (x-axis) of protein–protein interactions enriched in RNF43-p.G659Vfs*41 relative to RNF43-wildtype (WT) as detected by immunoprecipitation–mass spectrometry. E p85 expression in RNF43659mut edited isogenic and sg_Ctrl CRC cells with and without MG132 treatment. F Ubiquitin immunoprecipitation assay assessing p85 ubiquitination in vector, RNF43_WT (WT), and RNF43-G659Vfs*41 (G659Vfs) expressing 293T cells. G Binding between p85 and RNF43-WT (WT), RNF43-G659Vfs*41 (G659Vfs) and RNF43-N terminal from 1aa to 659aa truncation (659_stop). H p85 expression in 293T cells transfected with RNF43-WT (WT), RNF43_G659Vfs (G659Vfs), RNF43_659_stop (659_stop), or RNF43_659ΔRING (ΔRING). Source data are provided as a Source Data file.
To understand the molecular link between RNF43659mut and PI3K/AKT signaling, we sought to identify proteins that interact with RNF43659mut by affinity proteomics, and that could mediate PI3K/AKT activation. V5-tag-RNF43_p.G659Vfs and V5-tag-RNF43_WT were overexpressed in 293T cells, and immunoprecipitated under non-denaturing conditions. The immunoprecipitated proteins were digested, labeled with tandem mass tag reagents (TMT) for quantification and analyzed by LC-tandem mass spectrometry (MS) (Fig. 4C). Interestingly, the PI3K regulating subunit p85 (also known as PIK3R1) emerged as a novel interacting partner of RNF43_p.G659Vfs but not RNF43_WT (Fig. 4D and Supplementary Data 2). Indeed, we showed reduced p85 levels in RNF43659mut HT29 cells which were restored with RSPO1 supplementation or RNF43 knockout (Fig. 4B). p85 reduction was due to post-translational degradation and its expression was restored upon proteasome inhibition in RNF43659mut HT29, LS513, and 293T, cells, but not upon lysosome inhibition in 293T cells (Supplementary Figs. 10 and 11a and Fig. 4E). In addition, p85 knockdown in HT29 and C10 cells showed increased p-AKT expression and led to increased colony formation in HT29 cells (Supplementary Fig. 11b, c).
We next sought to investigate whether p85 is ubiquitinated by RNF43659mut. We assessed p85 ubiquitination in cells expressing vector, RNF43_WT and RNF43_p.G659Vfs*41 by immunoprecipitating ubiquitinated proteins and observed increased p85 ubiquitination with RNF43_G659Vfs (Fig. 4F). Similar results were obtained with the reciprocal immunoprecipitation (Supplementary Fig. 12) and Tandem Ubiquitin Binding Entities (TUBEs) immunoprecipitation in isogenic RNF43659mut HT29 cells (Supplementary Fig. 13). To further study the interaction between RNF43 and p85 we used RNF43-WT (WT), RNF43 from 1aa to 659aa (659_Stop), RNF43_G659Vfs (G659Vfs) and RNF43_659 fs containing a RING domain deletion (ΔRING)25,26. We observed that p85 binds with G659Vfs and 659_Stop but not the wildtype RNF43 (Fig. 4G). RNF43_G659Vfs and RNF43_659_Stop decreased p85 expression and this decrease was reversed by deletion of the RNF43_659fs RING domain, further supporting RNF43-mediated ubiquitination as the mechanism (Fig. 4H). To understand the structural basis of the RNF43_659 fs–p85 interaction, we computationally modeled27,28 RNF43 and observed that the C-terminus of the RNF43 model is negatively charged (residues 736–781), folding back to a positively charged region (residues 551–578), which could block binding of potential partners (Supplementary Fig. 14a). Thus, the truncated structure of RNF43659mut may expose a positively charged site, allowing for increased binding and ubiquitination of p85 (Supplementary Fig. 14b). Overall, these results demonstrate that p85 interacts with RNF43659mut and that ubiquitination and degradation of p85 leads to increased PI3K/AKT signaling in RNF43659mut cells.
RNF43659mut modulates interferon activity
RNF43659mut frequently occurs in CRCs with deficient DNA mismatch repair (dMMR)/high microsatellite instability (MSI-H) that are under immune pressure16, while the PI3K/AKT pathway is involved in immunomodulation and immunosurveillance escape in CRC29. Having found that RNF43659mut activates the PI3K/AKT pathway, we sought to identify other genes that are modulated in RNF43659mut and may contribute to immune evasion. We performed whole-transcriptome analysis (RNA-Seq) and Gene Set Enrichment Analysis (GSEA)30 in the RNF43659mut and RNF43WT isogenic HT29 cell lines. RNF43659mut cells demonstrated features that negatively correlated with interferon-alpha and interferon-gamma response gene sets that contribute to inflammatory and innate immune responses, respectively (Fig. 5A, B and Supplementary Fig. 15). Consistently, in the TCGA Colorectal Adenocarcinoma dataset (n = 500 CRCs), the mRNA expression of interferon pathway genes were significantly lower in the samples with RNF43_p.G659fs mutation than those with wildtype RNF43 (Fig. 5C). Lastly, treatment with PF-04691502 restored expression of interferon pathway genes in RNF43659mut HT29 cells (Fig. 5D).
A RNA-seq GSEA results in HT29_RNF43659mut reveal decreased expression of interferon alpha and interferon gamma response genes. NES normalized enrichment score. B Volcano plot of differentially expressed genes in HT29_RNF43659mut vs. HT29_sgCtrl. Interferon alpha or gamma response genes are displayed in red. C Differential expression of interferon alpha or gamma response genes in G659fs vs. WT MSI-H CRC cell lines in the Cancer Cell Line Encyclopedia (CCLE). Two-sided t-tests were used to compare RNF43_WT (n = 5) and RNF43_G659fs (n = 7) cells. D Comparison of interferon response genes between HT29_sgCtrl and HT29_RNF43659mut-C1 before and after treatment with PF-04691502. Data are the mean ± SD. Two-sided Student’s t test (n = 3 independent experiments in triplicate). Source data are provided as a Source Data file.
Discussion
RNF43 is a recurrently mutated gene in CRC and other malignancies, but the functional effects of its hot-spot variants warrants further investigation. Previous studies by our group and others have pointed at functional heterogeneity among RNF43 mutations found in patients31,32. RNF43 deleterious N-terminal domain mutations have been shown to activate Wnt signaling pathways, which in turn confers susceptibility to Wnt-ligand inhibitors such as PORCN inhibitors13,15. On the other hand, onco-RNF43 mutations (504aa-568aa) lead to impaired Wnt receptor endocytosis and increased Wnt signaling but decreased susceptibility to PORCNi32. However, the role of C-terminal mutations of RNF43 in Wnt signaling and oncogenesis remains controversial33. Two previous reports demonstrated that RNF43_p.G659fs mutations do not compromise RNF43 activity, suggesting that it is a passenger event18,19. Nevertheless, the frequency of the RNF43_p.G659fs truncation mutant was shown to be significantly greater than predicted by chance in colorectal and endometrial tumors indicating positive selection16.
In this work, we demonstrated that RNF43_p.G659fs mutations have tumorigenic properties independent of Wnt signaling. In agreement with our findings, previous research showed that treatment with PORCN inhibitors had no effect on CRC organoids with 659fs mutations15. In order to evaluate specific therapeutics against tumors harboring the RNF43_p.G659fs mutation, we utilized the Broad Institute’s drug repurposing library21 and identified a significant enrichment in PI3K/mTOR inhibitors such as PF-04691502 and alpelisib. PF-04691502 is a dual PI3K/mTOR inhibitor in clinical trials, while alpelisib is an FDA-approved drug for the treatment of advanced breast cancer34. In this work, we showed that alpelisib and PF-04691502 inhibit the growth of preclinical tumor models harboring the RNF43_p.G659fs mutation. We demonstrated that this mutation activates PI3K in vitro and in vivo and showed that this activation occurs through degradation of PI3K regulatory subunit p85. A caveat of these studies is that since, to the best of our knowledge, there are no patient-derived isogenic cells for the RNF43 p.G659fs mutation, we used CRISPR-Cas9 genomic editing to generate isogenic models.
p85 has previously been found to inhibit PI3K/AKT signaling through the inhibition of p11035, while knockdown of p85 promotes oncogenic activation of PI3K/AKT effector signaling36,37,38,39. Similarly, inactivating mutations of p85 increased p-AKT levels, while wildtype p85 had no effect on p-AKT levels40. We find that RNF43_p.G659fs removes p85 inhibition of PI3K/AKT signaling through binding, ubiquitinating and degrading p85, and that RNF43_p.G659fs-induced activation of PI3K/AKT signaling can be reversed by PI3K/mTOR inhibitors.
RNF43_p.G659fs mutations are predominantly present in MSI‐H CRCs, which are characterized by increased immune cell infiltration and have ~40–50% response rate to immune checkpoint inhibition34. We showed that RNF43659mut leads to decreased expression of interferon-alpha and gamma response genes in vitro and in TCGA CRCs. This diminished activity was restored in CRC cells through PI3K/mTOR inhibition. Given this role of RNF43_p.G659fs in immunomodulation, it is intriguing to speculate about its potential effect on immune checkpoint blockade resistance and the possibility of combination immunotherapy trials with PIK3/mTOR inhibitors. Additional studies are needed, however, to further investigate the role of RNF43_p.G659fs in immunotherapy failure in MSI-H CRC.
Overall, our findings provide new insights into the role of RNF43_p.G659fs in tumor development and suggest therapeutic uses for PI3K/mTOR inhibitors in patients whose tumors harbor this mutation.
Methods
Cell culture
HT29 (RRID:CVCL_0320), HCT116 (RRID:CVCL_0291), LS513 (RRID:CVCL_1386), HEK293T (RRID:CVCL_QW54), L-Wnt3a (RRID:CVCL_0635) cell lines were obtained from the American Type Culture Collection, 293T-HA-RspoI-Fc (RRID:CVCL_RU08) was obtained from Trevigen (# 3710-001-K) and C10 cell line (ECACC Cat# 12022901, RRID:CVCL_5245) was obtained from the European Collection of Authenticated Cell Cultures (ECACC). All cell lines were cultured under standard conditions recommended by the manufacturer supplemented with 10% FBS (Sigma-Aldrich, #F2442) and 1% penicillin/streptomycin (Thermo Fisher Scientific, #BW17603E) and kept at 37 °C in a humidified atmosphere with 5% CO2. All cell lines were authenticated by validating short tandem repeat fingerprinting and were free of mycoplasma contamination.
Organoid culture
Colon tissues in this study were obtained and used for research purposes under protocols approved by Dana-Farber Cancer Institute’s IRB with informed consent from patients. For establishment of CRC patient-derived organoids, tumor tissues were obtained from patient-derived RNF43_G659fs xenograft models. For establishment of normal patient-derived colorectal organoids, tissue was obtained from surgical specimens. The organoid samples were dissociated and embedded in Matrigel (Corning #356231). After Matrigel solidification for 10 min at 37 °C, cells were cultured in human intestinal organoid medium composed of IntestiCult™ Organoid Growth Medium (STEMCELL Technologies, #06010) with 100 U/ml penicillin-streptomycin (Thermo Fisher Scientific, #15140122) and supplemented with Y-27632 for 48 h after establishment or passage. Confluent organoids were passaged by incubating in Gentle Cell Dissociation Reagent (STEMCELL Technologies, #100-0485). Organoids were resuspended in a 1:1 mixture of Matrigel and basal media composed of DMEM/F12 (Thermo Fisher Scientific, #11320033), 10 mM HEPES (Life Technologies, #15630080), 2 mM GlutaMax (Life Technologies, #35050061), and 100 U/ml penicillin-streptomycin.
Human specimens
Human Colonic tissues and clinical data were obtained from CRC patients at Dana-Farber Cancer Institute following informed consent and IRB-approval. Clinical data corresponding to patient-derived specimens is listed in Supplementary Table 2. TCGA datasets (lluminaHiSeq_RNASeqV2data and DNA mutations using Affymetrix Genome-Wide Human SNP 6.0 array data) were obtained from the cBioPortal (https://www.cbioportal.org/). TCGA proteomic/Reverse phase protein array data were obtained from The Cancer Proteome Atlas (http://tcpaportal.org/tcpa/).
Plasmids and transfection
For overexpression experiments, RNF43 wildtype open-reading frame constructs (ORFs) were obtained from the human ORFeome library version (http://horfdb.dfci.harvard.edu) and used as templates for site-directed mutagenesis to generate RNF43_G659fs*41 mutated cDNAs in the pDONR223 Gateway entry vector followed by Sanger sequencing verification. Ectopic expression of V5-tagged RNF43 wildtype, G659fs*41 and indicated truncated fragments were cloned into the LentiV_Puro expression vector (gifted from Christopher Vakoc; RRID:Addgene_111886) with the HIFI DNA assembly kit (New England Biolabs #E2621S) according to manufacturer specifications. The RNF43_659fsΔRING plasmid was constructed by VectorBuilder (www.vectorbuilder.com). CRISPR-Cas9 genome editing, single guide RNAs were designed to target DNA sequences corresponding to amino acid residues 117 (sgRNA_RNF43117mut: 5′-TAGCCAGTGACAGGCAGGGG-3′) and 659 (sgRNA_RNF43659mut: 5′-CCACAGAGGAAAAGGCGGGG-3′) of RNF43 using GPP sgRNA Designer (https://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design) and introduced into the lentiGuide_Puro backbone (gift from Feng Zhang; RRID:Addgene_52963) following Lenti_Cas9-2A-Blast (RRID:Addgene_73310) transfection. Additionally, the TCF/LEF sequence was PCR amplified and inserted into a pGL4.15 vector (Progema, #E6701) via HIFI DNA assembly to create a TOP luciferase reporter vector. Plasmid constructs were sequenced and confirmed by Sanger sequencing (Quintara Bioscience). All primers for plasmid construction and sgRNA target sequences are listed in Supplementary Table 3.
Lentivirus was produced in HEK293T cells as per the low throughput viral production protocol on the shRNA/sgRNA/ORF Low Throughput Viral Production Consortium Portal (see https://portals.broadinstitute.org/gpp/public/). Briefly, the HEK293T cells were plated at 70% confluence in 6-well plates 24 h prior to transduction. Lentivirus was produced by transfecting HEK293T cells with previously described plasmids as well as psPAX2 (RRID: Addgene_12260) and VSV-G (RRID: Addgene_8454) packaging plasmids. Stable transfection was performed in indicated cell lines by spin-infection lentivirus transduction and 48 h incubation in the presence of 5 μg/ml polybrene prior to the addition of puromycin (1 ug/ml) selection for 7 days. For transient transfection of plasmids or oligonucleotides into most mammalian cell lines, Lipofectamine LTX reagent (Thermo Fisher Scientific, #15338100) was used following the manufacturer instructions while HEK293T cell transfection was performed with the Calphos mammalian transfection kit (Takara Bio, #631312) as per manufacturer instructions.
CRISPR/Cas9 genome editing
To generate isogenic RNF43 mutant cell lines, cells were transduced with Lenti_Cas9-2A-Blast and an indicated Lenti-Guide-puro construct containing the sgRNA inserts. Blasticidin (2 ug/ml) and puromycin (1 µg/ml) selection treatments were applied for 7 days. To confirm efficient CRISPR cutting at the target loci, gDNA was extracted using QIAamp DNA mini kit (Qiagen, #51104) and PCR primers were designed flanking the sgRNA cut site by 100–150 base pairs on either side. DNA from the bulk resistant population of cells transduced with sgRNAs targeting RNF43 117 and RNF43 659 was amplified with the primers to yield an amplicon roughly 200–300 bp in length using Q5 High-Fidelity 2X Master Mix (New England Biolabs, #M0492S) and confirmed by next generation sequencing to determine editing efficiency (Massachusetts General Hospital DNA Core). Mutation efficiency was assessed as the percentage of reads containing a frameshift caused by an indel compared to the total read count (Supplementary Fig. 1a). Single colonies were selected by serial dilution and screened for frameshift mutations by Sanger sequencing of the PCR products generated using the primers that flanked the targeted site (Supplementary Fig. 1b). Editing efficiency was assessed by determining the percentage of sequences with frameshift mutations compared to the total number of polymers sequenced. The gDNA PCR primer sequences corresponding to the regions targeted by indicated sgRNAs are shown in Supplementary Table 4.
Colony formation assays
Cells (1000 cells per well) were seeded in 12-well plates for 14 days. Cells were fixed with 100% methanol and then stained with 0.5% crystal violet for 5 min. Plates were imaged with an Epson Workforce GT-1500 scanner (Epson, B11B190011). Viable cellular colonies larger than the indicated diameter were counted. Colony formations assays were performed in triplicate.
Cell and organoid viability and treatment assays
Cell viability was assessed using the CellTiter-Glo Luminescent cell viability assay (Promega #G9242) according to the manufacturer’s protocol and measured on a Spectramax M5 microplate luminometer (Molecular Devices). For the cell proliferation experiment, cells were plated (1000 cells per well) in 96 well plates and assayed daily for 6 days to determine cell proliferation. Cell treatments were conducted with alpelisib (Selleck #S2814), PF-04961502 (Selleck #S2743), Trametinib (Selleck #2673), Nutlin-3 (Selleck #8059), XAV939 (Santa Cruz Biotechnology #SC-296704), LGK974 (Selleck #S7143), IWP2 (Selleck #7085), PKI-179 (EMDBio #526561), Torin-1 (Selleck #S2827), and Torin-2 (Tocris #4248) with 7-point 2-fold dilution series for 96 h before the viability was assayed. For the organoid drug treatment experiments, organoids were dissociated to single cells (1000/well) and resuspended in Wnt-ligand free organoid growth media containing 10% Matrigel. Organoid viability was assessed using 3D CellTiter-Glo (Promega #G9681) after 7 days of drug treatment. Each assay was measured in triplicate using at least three technical replicates (two for the normal organoids) and luminescent output was normalized to cells treated with DMSO. The Linear regression analysis and dose-response inhibition (Ln(inhibitor) vs. normalized response—variable slope) or (Ln(inhibitor) vs. normalized response) were calculated by GraphPad PRISM (GraphPad).
Dual-luciferase reporter assay
Cells were seeded in triplicate 1 day prior to transfection. In total, 50 ng of the previously assembled TOP/Wnt luciferase reporter vector or M50 Super TOPFlash (gift from Dr. Randall Moon; Addgene, #12456) were transfected into cells alongside 1 ng of pRL-TK Renilla plasmid (Promega, #E2241) by using lipofectamine 3000 (Invitrogen, #L3000001) according to the manufacturer specifications. Forty-eight hours post-transfection, firefly and Renilla luciferase activities were measured by dual-luciferase assay kit (Promega, #E1910) on a Spectramax M5 (Molecular Devices) according to the manufacturer’s instructions. The firefly luciferase activity for each sample was normalized to the Renilla luciferase activity to account for transfection efficiency. Each assay was measured in triplicate using at least two technical replicates.
Small molecule compound profiling
The Broad Institute Repurposing Library screen was performed using 5363 small molecules arrayed in 384-well plates at a final concentration of 5 uM. Assay Ready Plates (Corning Cat# 3765) were generated by dispensing 10 mM DMSO compound stocks using the Labcyte Echo acoustic liquid handler. HT29-sgCtrl and HT29-RNF43659mut isogenic cell lines were seeded in duplicate at a density of 2500 cells per well in 50 ul culture medium supplemented with 10% FBS. Assay plates were incubated for 96 h and cell viability was measured using CellTiter-Glo per manufacturer recommendation. Quality control measures were met for screening plates with a Z-factor score >0.5 comparing negative (DMSO) and positive control compound MG132 (a proteasome inhibitor toxic to most cell lines at 1 µM) and %CV < 15%. Compounds which reliably inhibited cell growth (<10% difference between replicates; z-score < −3) and exhibited at least 5% more growth inhibition in HT29-RNF43659mut than in HT29-RNF43_sgCtrl were considered primary hits. These primary hits were used to generate a secondary screen consisting of an 8-point 2-fold dilution series to generate a concentration-response curve and assess drug potency and sensitivity.
Mouse experiments
All animal experiments and study protocols were reviewed and approved by the Dana-Farber Cancer Institute’s Animal Care and Usage Committee (protocol 20-013), in compliance with the Animal Welfare Act and the Office of Laboratory Welfare (OLAW) of the National Institutes of Health. Laboratory mice are housed in solid-bottom, polysulfone 75 sq. in. microisolator cages. The cages are used in conjunction with the Optimice® rack systems with integrated automatic watering. Temperature and humidity in the rodent facilities are controlled at 72 ± 2 °F and a target range of 35–55% relative humidity. A standard photoperiod of 12 h light/12 h dark is controlled by an automated system. For the cell-derived xenograft assay, 2 × 106 cells of HT29-sgCtrl or HT29-RNF43659mut in 100 µl of a 50% PBS/50% Matrigel mixture were injected subcutaneously into flanks bilaterally in Taconic NCr-Nude (CrTac:NCr-Foxn1nu) female mice at 7 weeks of age. When tumors reached ~150 mm3 after 7 day of tumor cells inoculation, mice (n = 5/group) were randomized into vehicle control (5%DMSO + 40%PEG300 + 5%Tween 80 + 50%ddH2O) and PF-04961502 treatment (50 mg/kg/day by oral gavage) groups for 5 days on and 2 days off. On day 28 of treatment, mice were euthanized. The endpoints for mice were tumor volume exceeding 2000 mm3, interference with basic/vital bodily functions or persistent ulceration. At no point did any mice exceed maximal tumor burden. Blinding and randomization to the treatment arm was performed. When endpoints were reached, tumors were removed from mice, washed, and stored overnight in 4% paraformaldehyde. Histological analysis and immunostaining of paraffin-embedded tumor-samples was then performed by iHisto Inc. Anti-Ki67 (Abcam #15580), anti-p-AKT (Cell Signaling Technologies #4060T) were used for immunohistochemistry analysis, while TUNEL staining was provided and performed by iHisto Inc.
Western blot analysis
Whole cell extracts for western blotting were obtained by applying RIPA buffer (Sigma-Aldrich, #R0278) supplemented with protease inhibitor (Cell Signaling Technology, #5872S) onto a cell monolayer and incubating on ice for 5 min. Following centrifugation (>16,000 × g) for 10 min), protein concentrations were quantitated using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, #23225). Equal amounts of protein were resolved and transferred according to manufacturer specifications, followed by overnight incubation at 4 °C with the primary antibodies listed in Supplementary Table 5. Chameleon Duo Prestained Protein Ladder (Licor, #928-60000) or PageRuler Prestained Protein Ladder (Thermo Fisher Scientific, #26616) were used as a molecular weight marker. Densitometry analysis of each blot was quantitated using ImageJ. Uncropped immunoblots are provided in Source Data.
Proteasome and lysosome inhibition assays
To detect p85 expression in LS513 and HT29 cells, cells were incubated with 10 μM MG132 (Sigma-Aldrich) for 6 h before analysis. Additionally, HEK293T expressing RNF43_G659Vfs were treated with MG132 (Selleck, #S2619), Aloxistatin (E64d) (Selleck, #S7393) or NH4Cl (Sigma-Aldrich, #S7393). Then, cells were lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM Na3VO4, 20 mM NaF, 1 mM PMSF and complete protease inhibitor cocktail) and subjected to immunoblotting. Expression levels of p85 were quantified and normalized to beta-actin using ImageJ.
Cycloheximide chase assay
HEK293T cells were transfected with RNF43 (wildtype or G659Vfs mutant) for 24 h and further incubated with protein synthesis inhibitor 5 μM Cycloheximide (CHX, Sigma-Aldrich, #C104450) for the indicated amount of time before analysis.
Co-immunoprecipitation assays
HEK293T cells were transiently transfected with purified V5-tagged-RNF43-WT or -RNF43_G659Vfs*41 plasmids using the Calphos Mammalian Transfection kit (Takara Bio, #631312) and incubated for 24–72 h before protein is harvested. Cells were washed with cold PBS and lysed with IP lysis buffer (Thermo Fisher Scientific, #87787) or NP40 cell lysis buffer (Thermo Fisher Scientific, #FNN0021) containing Halt Protease Inhibitor Single-use Cocktail (Thermo Fisher Scientific, #PI78430) and incubated 10 min on ice. After pelleting the cell debris by centrifugation at 16,000 × g for 10 min at 4 °C, supernatants (cell extracts) were incubated with 2 ug of anti-ubiquitin antibody FK2 (Sigma-Aldrich, #04-263) or 1 ug anti-V5 antibody and 10 ul each of Protein A/G Dynabeads (Thermo Fisher Scientific, #88802) at 4 °C overnight while rotating. After four times extensive washing with IP buffer, the immunoprecipitated bead conjugates were heated in 2 × NuPAGE LDS Sample Buffer (Thermo Fisher Scientific, #NP0007) supplemented with 20 mmol/l DTT at 70 °C for 10 min and subjected to NUPAGE 4–12% BT GEL (Thermo Fisher Scientific, #NP032B), followed by immunoblotting probed with an antibody against P85. TUBE immunoprecipitations were carried out similarly; cells were treated with MG132 and 1 mg of HT29 or HT29-RNF43659mut cell lysate was loaded onto 20 uL TUBE1 agarose (LifeSensors, #UM401). Antibodies are listed in Supplementary Table 5.
Proteomic analysis
Sample were processed for quantitative MS assays following anti-V5 immunoprecipitation by on-bead digestion (add: PMID 33139955). Briefly, for protein interaction analyses, samples were reduced, alkylated, and digested on-bead with trypsin as previously described (add: PMID 33139955). Desalted peptides were labeled with TMT (8-plex) reagents (Thermo Fisher Scientific), combined and analyzed by online nanoflow liquid chromatography-tandem MS using a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific) coupled online to a Proxeon Easy-nLC 1200 (Thermo Fisher Scientific) using a 260 min method. Briefly, the Q Exactive plus MS was operated in the data-dependent mode, acquiring HCD-MS/MS scans at a resolution of 35,000 on the top-12 most abundant precursor ions in each full MS scan (70,000 resolution). The automatic gain control target was set to 3 × 106 ions for MS1 and 5 × 104 for MS2, and the maximum ion time was set to 120 ms for MS2. The collision energy was set to 28, peptide matching was set to “preferred,” isotope exclusion was enabled, and dynamic exclusion time was set to 20 s. All data were analyzed using Spectrum Mill software package. Spectra from the same precursor or within a retention time window of ±60 s and an m/z range of ±1.4 were merged. Spectra were filtered to include only those with a precursor mass range of 750 to 6000 Da and a sequence tag length greater than 0. MS/MS searching was performed against a human UniProt database. Digestion enzyme conditions were set to “Trypsin allow P” for the search, allowing up to 4 missed cleavages within a matched peptide. Fixed modifications were carbamidomethylation of cysteine and TMT10 on the N-terminus and internal lysine. Variable modifications were oxidized methionine and acetylation of the protein N-terminus. Peptide-level matches were validated below a 1.0% FDR threshold and within a precursor charge range of 2–6. A second round of validation was performed at the protein level, requiring a minimum protein score of 0. Protein data were filtered to consider only proteins with two or more unique peptides and median normalized. Data were median normalized and subjected to a 1-sample moderated t-test using an internal R-Shiny package based in the limma R library. Correction for multiple testing was performed using the Benjamini–Hochberg FDR method. Proteins were selected based on increased binding in RNF43_G659Vfs*41 compared to RNF43_WT and a significant p value <0.05.
RT-qPCR and RNA-sequencing
Total cellular RNA was isolated using the RNeasy plus mini kit (Qiagen, #74104) and subsequently underwent reverse transcription using the iScript cDNA synthesis kit (Bio-Rad Laboratories, #1708891) according to manufacturer instructions. RT-PCR was performed with Bio-Rad CFX96 qPCR System using PowerUp SYBR Green cDNA kit (Thermo Fisher Scientific, #A25741). GAPDH was used as an internal control for normalization, and the relative expression level was calculated by the comparative CT (ΔΔCT) method. Each assay was measured in triplicate using at least two technical replicates. Primer sequences are listed in Supplementary Table 6.
RNA-Sequencing of the isogenic cell lines was performed by Novogene Corporation Inc Service using the Illumina NovaSeq 6000 with an average read depth of at least 20 million reads per replicate. In total, 150 bp paired-end sequencing reads were aligned to the human genome reference build hg19 using STAR v2.5. Differential expression analysis was performed using the DESeq2 R package (2_1.6.3) and thresholds for significant expression gene were defined as q < 0.05 and |log2 fold-change| > 0.5. Pathway enrichment analysis was performed using GSEA 2.0.9 software with the hallmark gene sets database (https://www.gsea-msigdb.org/gsea/msigdb/collections.jsp). Gene sets with a q < 0.25 false discovery rate and p value <0.05 were selected for significant pathway enrichment. All p values were adjusted for multiple testing using the Benjamini–Hochberg method (false discovery rate).
Statistics and reproducibility
Statistical analyses were performed using Graphpad Prism software (version 9.0.1 (128) (San Diego, CA). Student’s t test was used to determine the statistical differences between two groups. Experiments were performed in triplicate using at least three independants replicates unless otherwise indicated. All experiments were presented as the means ± standard deviation and all tests are two-sided.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available within the article, the Supplementary Information files, and the Source Data file. The Cancer Genome Atlas (TCGA, PanCancer Atlas) and Cancer Cell Line Encyclopedia (Broad, 2019) datasets were accessed via CBioPortal (https://www.cbioportal.org/). Uniprot human database (https://www.uniprot.org/) was used for identification of proteins by mass spectrometry. Proteomic GSEA experiments were performed using GSEA 2.0.9 software with the hallmark gene sets database (https://www.gsea-msigdb.org/gsea/msigdb/collections.jsp). The RNA-seq data for this study have been deposited to the GEO public dataset under the series GSE179464. The original mass spectra and the protein sequence database used for searches have been deposited in the public proteomics repository MassIVE (http://massive.ucsd.edu) and are accessible at under accession code MSV000089343. Source data are provided with this paper.
References
Siegel, R. L. et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J. Natl Cancer Inst. 109. https://doi.org/10.1093/jnci/djw322 (2017).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2020).
Yu, I. S. & Cheung, W. Y. Metastatic colorectal cancer in the era of personalized medicine: a more tailored approach to systemic therapy. Can. J. Gastroenterol. Hepatol. 2018, 9450754 (2018).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Giannakis, M. et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 15, 857–865 (2016).
Yaeger, R. et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 33, 125–136 e123 (2018).
Mohd Yunos, R. I., Ab Mutalib, N. S., Tieng, F. Y. F., Abu, N. & Jamal, R. Actionable potentials of less frequently mutated genes in colorectal cancer and their roles in precision medicine. Biomolecules 10. https://doi.org/10.3390/biom10030476 (2020).
Inamura, K. Colorectal cancers: an update on their molecular pathology. Cancers 10. https://doi.org/10.3390/cancers10010026 (2018).
Garraway, L. A. & Lander, E. S. Lessons from the cancer genome. Cell 153, 17–37 (2013).
Koo, B. K. et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012).
Koo, B. K., van Es, J. H., van den Born, M. & Clevers, H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc. Natl Acad. Sci. USA 112, 7548–7550 (2015).
Hao, H. X. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 (2012).
Jiang, X. et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 12649–12654 (2013).
Nanki, K. et al. Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis. Cell 174, 856–869 e817 (2018).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Giannakis, M. et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 46, 1264–1266 (2014).
Yan, H. H. N. et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut 66, 1645–1656 (2017).
Li, S. et al. Commonly observed RNF43 mutations retain functionality in attenuating Wnt/beta-catenin signaling and unlikely confer Wnt-dependency onto colorectal cancers. Oncogene 39, 3458–3472 (2020).
Tu, J. et al. The most common RNF43 mutant G659Vfs*41 is fully functional in inhibiting Wnt signaling and unlikely to play a role in tumorigenesis. Sci. Rep. 9, 18557 (2019).
Mouradov, D. et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res 74, 3238–3247 (2014).
Corsello, S. M. et al. The drug repurposing hub: a next-generation drug library and information resource. Nat. Med 23, 405–408 (2017).
Zhang, Y. et al. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell 31, 820–832 e823 (2017).
Li, J. et al. Characterization of human cancer cell lines by reverse-phase protein arrays. Cancer Cell 31, 225–239 (2017).
Akbani, R. et al. A pan-cancer proteomic perspective on The Cancer Genome Atlas. Nat. Commun. 5, 3887 (2014).
Burslem, G. M. et al. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem. Biol. 25, 67–77.e63 (2018).
Meng, X. et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature 564, 130–135 (2018).
Yang, J. & Zhang, Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 43, W174–181 (2015).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Long, X. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol 4, 2319–2330 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Thornton, A. M., Fang, L., O’Brien, C., Berger, A. H. & Giannakis, M. eVIP2: expression-based variant impact phenotyping to predict the function of gene variants. BioRxiv (2019).
Spit, M. et al. RNF43 truncations trap CK1 to drive niche-independent self-renewal in cancer. EMBO J. 39, e103932 (2020).
Yu, J. et al. The functional landscape of patient-derived RNF43 mutations predicts sensitivity to Wnt inhibition. Cancer Res. 80, 5619–5632 (2020).
Sahin, I. H. et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 121, 809–818 (2019).
Thorpe, L. M., Yuzugullu, H. & Zhao, J. J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 15, 7–24 (2015).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 (2002).
Luo, J. & Cantley, L. C. The negative regulation of phosphoinositide 3-kinase signaling by p85 and it’s implication in cancer. Cell Cycle 4, 1309–1312 (2005).
Thorpe, L. M. et al. PI3K-p110alpha mediates the oncogenic activity induced by loss of the novel tumor suppressor PI3K-p85alpha. Proc. Natl Acad. Sci. USA 114, 7095–7100 (2017).
Wei, Y. et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc. Natl Acad. Sci. USA 110, 6829–6834 (2013).
Cheung, L. W. et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Disco. 1, 170–185 (2011).
Acknowledgements
This work was supported by the Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (SU2C-AACR-DT22-17 to M.Gi.), administered by the American Association for Cancer Research, a scientific partner of SU2C. Stand Up To Cancer is a division of the Entertainment Industry Foundation. This work was also supported by the Project P Fund, the Crush Colon Cancer Fund, the Cancer Research UK Grand Challenge Award (UK C10674/A27140 to K.N. and M.Gi.), and in part by grants from the National Cancer Institute (NCI) Clinical Proteomic Tumor Analysis Consortium grants NIH/NCI U24-CA210986 and NIH/NCI U01 CA214125 (to S.A.C.). A Conquer Cancer Foundation of ASCO Career Development Award and a High Pointe Investigatorship in Gastrointestinal Oncology supported M.Gi. The National Institutes of Health (K08CA230220) supported S.M.C. We also thank the Center for the Development of Therapeutics and Repurposing Hub at the Broad (https://clue.io/repurposing).
Author information
Authors and Affiliations
Contributions
L.F., D.F.R., M.R., and C.O. designed and created various CRISPR constructs and edited cell lines. L.F. and C.O. performed WNT assays. L.F., D.F.R., M.R., C.O., C.G., S.R., S.M.C., and J.B. aided in drug screening experimental preparation and/or analysis. L.F., D.F.R., M.R., and C.O. performed PI3K/mTOR drug validation assays. L.F., D.F.R., M.R., and E.S. performed and analyzed all mouse experiments. L.F., D.F.R., M.R., and C.O. performed all cell culture, western blotting, immunoprecipitation, and colony formation assays. L.F., D.F.R., M.R., and M.Gr. established organoid models and performed organoid related experiments. L.F., S.A.C., N.D.U., and M.Gi. generated and analyzed proteomic data. X.X. modeled protein structures. L.F. prepared samples for RNA-sequencing and L.F., M.R., C.G., and M.Gi. analyzed RNA-sequencing results. L.F. and D.F.R. analyzed all CCLE and TCGA data. K.N. and M.Gi. provided resources and models for the study. L.F. and M.Gi. designed the study. L.F., D.F.R., M.R., and M.Gi. wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.Gi. received research funding from Bristol-Myers Squibb, Merck, Servier and Janssen. S.R. holds equity in Amgen. S.M.C. received research funding from Bayer and Calico Life Sciences. S.A.C. is a member of the scientific advisory boards of Kymera, PTM BioLabs, Seer and PrognomIQ. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, L., Ford-Roshon, D., Russo, M. et al. RNF43 G659fs is an oncogenic colorectal cancer mutation and sensitizes tumor cells to PI3K/mTOR inhibition. Nat Commun 13, 3181 (2022). https://doi.org/10.1038/s41467-022-30794-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30794-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.